Abstract
Vitamin D is a fat-soluble vitamin that is synthesized in the skin with exposure to sunlight or is ingested from dietary supplements or food. There has been a dramatic increase in research on vitamin D, linking it with health outcomes as varied as reproductive function, infection, cardiovascular disease, and cancer. The study of vitamin D has generated much excitement, partly because there is an ideal intervention: Low levels may be common and can be remedied with widely available supplements. Determination of vitamin D status is complex and has advanced dramatically in the past 5 years. In this paper, we begin by describing important considerations for measurement of total 25-hydroxyvitamin D (25(OH)D), the biomarker traditionally assessed in epidemiologic studies. While 25(OH)D remains the most commonly measured biomarker, emerging evidence suggests that other related analytes may contribute to the characterization of an individual’s vitamin D status (e.g., vitamin D-binding protein, bioavailable and free 25(OH)D, the C-3 epimer of 25(OH)D, 1,25-dihydroxyvitamin D, and 24,25-dihydroxyvitamin D). The measurement of these analytes is also complex, and there are important considerations for deciding whether their measurement is warranted in new research studies. Herein we discuss these issues and provide the reader with an up-to-date synthesis of research on vitamin D measurement options and considerations.
Keywords: biomarkers, epimers, 25-hydroxyvitamin D, immunoassays, mass spectrometry, validity, vitamin D
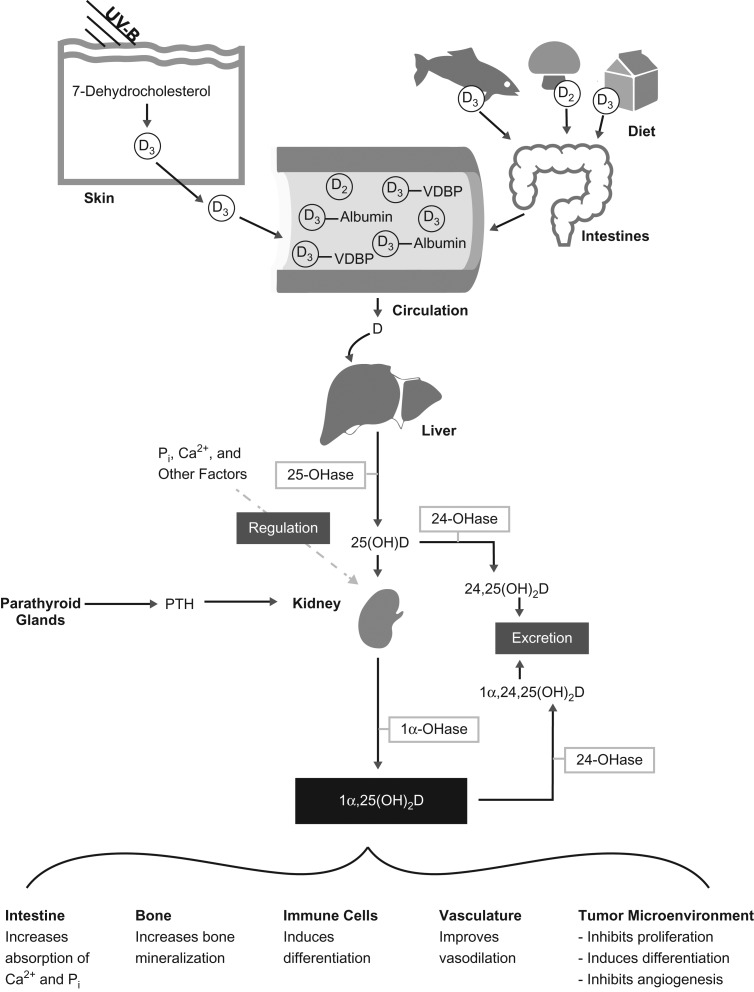
Vitamin D is a fat-soluble vitamin that is synthesized in the skin with exposure to sunlight. It can also be obtained from the diet, either from supplements or from food (such as dairy products) (Figure 1). The “active” form of vitamin D, 1,25-dihydroxyvitamin D (1,25(OH)2D), is the form that binds to the vitamin D receptor. However, the relatively short half-life of 1,25(OH)2D (11–21 hours) (1) makes it a poor biomarker for assessing vitamin D status in epidemiologic studies. Instead, circulating total 25-hydroxyvitamin D (25(OH)D) concentration has traditionally been used to measure vitamin D status (2, 3).
Figure 1.
Vitamin D metabolism and functions. Vitamin D can be obtained through either sun exposure (top left) or diet (top right). When ultraviolet-B (UV-B) radiation strikes the skin, it stimulates the conversion of a cholesterol precursor into vitamin D3 (D3), which then enters the circulation. Vitamin D3 can be absorbed from foods such as fatty fish and fortified milk products. Vitamin D2 (D2) is found in fungi, such as mushrooms. Vitamin D supplements can contain either vitamin D2 or vitamin D3. Most (85%–90%) of the vitamin D in blood is carried by vitamin D-binding protein (VDBP), although it can also bind to albumin (10%–15%) or be unbound or “free” (<1%). Both vitamin D2 and vitamin D3 follow the same metabolic pathway and are therefore represented with a “D” in the lower portion of the figure. Vitamin D is hydroxylated in the liver by the enzyme 25-hydroxyvitamin D 1-α-hydroxylase (25-OHase), to make 25-hydroxyvitamin D (25(OH)D), the metabolite most frequently used as a measure of vitamin D status. 25(OH)D can be further hydroxylated to 24,25-dihydroxyvitamin D (24,25(OH)2D), which is then excreted, or to 1α,25-dihydroxyvitamin D (1α,25(OH)2D), which is also known as the active form of vitamin D; this form can bind the vitamin D receptor with the greatest affinity. Parathyroid hormone (PTH), calcium (Ca2+), and phosphate (Pi) all act on the kidney to regulate calcium balance, partly through the conversion of 25(OH)D to its active form. The conversion of 25(OH)D to its active form can occur in a variety of tissues but is most well-known to occur in the kidney. The active form of vitamin D is responsible for several physiological changes across a variety of cell and tissue types. The effects of the active form are down-regulated by its conversion to 1α,24,25-dihydroxyvitamin D (1α,24,25(OH)2D), which is excreted from the body. (Adapted with permission from Macmillan Publishers Ltd.: Nature Reviews Cancer (119), copyright 2007).
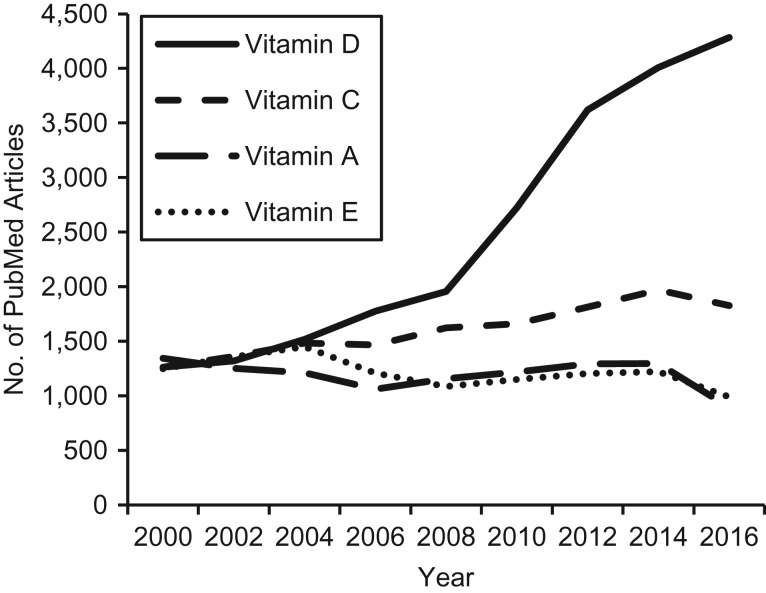
Since 2000, there has been a dramatic increase in vitamin D research (Figure 2), linking it with health outcomes as varied as reproductive function, infection, cardiovascular disease, and cancer (4). The study of vitamin D has generated excitement, in part because there is an ideal intervention: Low levels may be common (5–9), they are amenable to correction through supplementation or sun exposure, and supplements are inexpensive and widely available. Moreover, the discovery that 25(OH)D is being converted to 1,25(OH)2D within various tissues, including the brain, the uterus and placenta, and vascular smooth muscle cells (10–13), suggests that vitamin D is relevant to those tissues independently of the well-established calcium homeostasis pathway (5, 14).
Figure 2.
Numbers of publications related to vitamins A, C, D, and E in PubMed (National Library of Medicine, Bethesda, Maryland), 2000–2016.
In this paper, we describe the important considerations for measurement of total 25(OH)D, the most commonly assessed biomarker. Further, emerging evidence suggests that other vitamin D metabolites and related analytes may improve the characterization of an individual’s vitamin D status, which is a composite of his/her ability to access and utilize vitamin D in physiological processes (15). The measurement of vitamin D-related analytes is complex, and there are important issues to consider both for interpreting the published literature and for deciding whether their measurement is warranted in new research studies (Table 1). Herein we discuss these issues with the goal of providing the reader with an up-to-date synthesis of options and considerations for measuring vitamin D within the context of epidemiologic studies.
Table 1.
Biomarkers of Vitamin D Metabolism and Status
| Biomarker | Biospecimen | Measurement Options | Additional Considerations |
|---|---|---|---|
| Total 25(OH)D | Serum, plasma, whole blood, or blood spots | LC-MS/MS, immunoassay (such as an ELISA or chemiluminescent immunoassay) |
|
| Free 25(OH)D | Serum, plasma | Immunoassay Calculated |
|
| Bioavailable 25(OH)D | Serum, plasma | Calculated |
|
| Vitamin D-binding protein (VDBP) | Serum, plasma | Immunoassay LC-MS/MS |
|
| 3-epi-25(OH)D | Serum, plasma | LC-MS/MS |
|
| 1,25(OH)2D | Serum, plasma | Immunoassay LC-MS/MS |
|
| 24,25(OH)2D3 | Serum, plasma | LC-MS/MS |
|
Abbreviations: ELISA, enzyme-linked immunosorbent assay; 3-epi-25(OH)D, 3-epi-25-hydroxyvitamin D; LC-MS/MS, liquid chromatography–tandem mass spectrometry; 1,25(OH)2D, 1,25-dihydroxyvitamin D; 24,25(OH)2D, 24,25-dihydroxyvitamin D; 25(OH)D, 25-hydroxyvitamin D; VDBP, vitamin D binding protein.
TOTAL 25(OH)D
Clinical recommendations for vitamin D sufficiency are based on total 25(OH)D (4). Similarly, most epidemiologic studies evaluating the association between vitamin D and health outcomes have focused on total 25(OH)D. Total 25(OH)D concentration is the sum of levels of the metabolites 25-hydroxyvitamin D2 (25(OH)D2) and 25-hydroxyvitamin D3 (25(OH)D3). While these analytes are generally viewed as similar in terms of biological effect, they are derived from different sources, and there is some evidence suggesting that 25(OH)D3 has a longer half-life than 25(OH)D2 (15.1 days vs. 13.9 days) (16). In 2016, only 19% of the US population had detectable 25(OH)D2 levels (limit of detection, 2.05 nmol/L), with higher levels being seen among persons aged 60 years or more (17 nmol/L) in comparison with other age groups (<5 nmol/L) (17).
Total 25(OH)D can be measured in serum, plasma, whole blood, or blood spots. It has been shown to be extremely stable under a variety of laboratory preanalytical conditions (18–20) and with long-term storage (21–24). It can be measured using either liquid chromatography–tandem mass spectrometry (LC-MS/MS) or a ligand-binding assay (such as an immunoassay platform like a competitive enzyme-linked immunosorbent assay (ELISA) or a competitive chemiluminescent immunoassay, or competitive receptor-binding assays). In direct comparisons, immunoassay methods show bias (significant deviation from linearity) and increased variability relative to LC-MS/MS (25). In contrast to LC-MS/MS, immunoassays are not able to separately quantify the metabolites 25(OH)D2 and 25(OH)D3, and immunoassays are susceptible to cross-reactivity with 24,25-dihydroxyvitamin D (24,25(OH)2D), a metabolite of 25(OH)D (26). LC-MS/MS is not without fault, however. For example, under typical chromatographic conditions, the epimeric form of 25(OH)D3 is not resolved from the native form (Figure 3). If a longer chromatographic separation or more selective column is not used, the epimer will be included in the measurement of total 25(OH)D concentration. (The relevance of the 25(OH)D epimer is discussed below.) Other shortcomings of LC-MS/MS, versus immunoassay, are that it requires more expensive equipment and expert staff, though reagent costs for LC-MS/MS are substantially lower.
Figure 3.
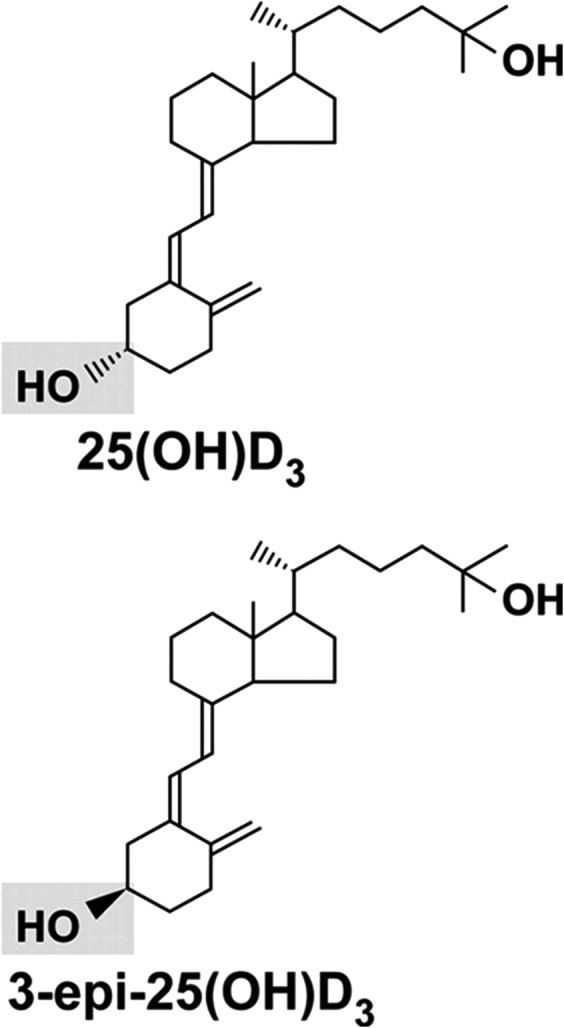
Chemical structure of 25-hydroxyvitamin D3 (25(OH)D3) and its epimeric form (3-epi-25(OH)D3). (Reprinted from Lensmeyer et al. (96) by permission of The Endocrine Society).
Regardless of whether LC-MS/MS or a ligand-binding assay is used to measure 25(OH)D, it is vital to select a laboratory with appropriate quality controls in place. An indicator of outstanding laboratory performance is traceability to the National Institute of Standards and Technology (Gaithersburg, Maryland) (27), Ghent University (Ghent, Belgium) (28), and/or Centers for Disease Control and Prevention (Atlanta, Georgia) reference measurement procedures. Substantial variability and bias can exist in laboratory measurements of 25(OH)D (29). This led to the development of the Vitamin D Standardization Program, which was initiated by the National Institutes of Health’s Office of Dietary Supplements. The Vitamin D Standardization Program aims to standardize vitamin D laboratory measurements such that results are accurate and comparable over time, location, and laboratory procedure (2, 3).
25(OH)D concentrations can be expressed in either ng/mL or nmol/L (1 ng/mL = 2.496 nmol/L). The Endocrine Society defines 25(OH)D sufficiency as 30–100 ng/mL, insufficiency as 21–29 ng/mL, and deficiency as <20 ng/mL (30). At the same time, the Institute of Medicine concluded that a 25(OH)D concentration of 16 ng/mL is adequate for bone health in 50% of the population, while 20 ng/mL is adequate for 97.5% of the population (4). Furthermore, as highlighted in a recent editorial (31), 97.5% of individuals require 25(OH)D concentrations less than 20 ng/mL. Although this is the intention of the Institute of Medicine report, in both research and clinical settings this cutpoint of 20 ng/mL is frequently misinterpreted as the minimum concentration for adequacy in any one individual, rather than the concentration at which 97.5% of the population has replete vitamin D stores (31). To additionally complicate the issue, questions have been raised about the appropriateness of these cutpoints for different racial/ethnic groups (9, 32–43).
An additional consideration for the measurement and interpretation of 25(OH)D concentrations is their inherent seasonality; typically a peak is observed in summer and a trough in winter, corresponding to usual variations in ultraviolet light exposure. For exposure-outcome relationships where the biological association is believed to be acute (e.g., 25(OH)D and sex hormone concentrations), the measured 25(OH)D concentration may be most appropriate. For exposure-outcome relationships where the influence of vitamin D on the outcome is believed to occur over a longer time frame (e.g., cancer and cardiovascular disease), estimating the annual average 25(OH)D concentration may be most appropriate (44). Several approaches have been used to account for seasonal variation in 25(OH)D concentrations. One approach is adjusting the exposure-outcome association for season or month of blood draw. Another approach is to use the measured 25(OH)D concentrations to estimate the annual average 25(OH)D concentration using a cosinor model (44) or a residuals-based approach (45).
A final issue for 25(OH)D measurement is the suggestion that it should be accompanied by measurement of parathyroid hormone (PTH) level. However, the Institute of Medicine considers this approach controversial due to inconsistencies in the relationship between 25(OH)D and PTH, and because no clear threshold has been established for defining “sufficiency” using both 25(OH)D and PTH (4).
FREE AND BIOAVAILABLE 25(OH)D
It is possible that bioavailable or free 25(OH)D may better quantify vitamin D status than total 25(OH)D (15). For many hormones, such as testosterone, the free (unbound) hormone is considered the biologically relevant fraction (46, 47) because free hormones, particularly lipophilic steroid hormones, may passively diffuse across the cell membrane (48). However, for 25(OH)D the relevance of the free fraction is an open research question (49), the answer to which may depend on the outcome, or organ, or tissue of interest. Free 25(OH)D may be most relevant in populations that are expected to show variation in vitamin D-binding protein (VDBP) concentrations, such as pregnant women, women using estrogens, or persons with liver or kidney disease (50). On the other hand, when 25(OH)D is bound to VDBP, the entire complex can enter a cell by binding the transmembrane protein, megalin. Megalin is expressed in the kidney, but messenger RNA and/or protein expression has also been found in the parathyroid, placenta, epididymis, mammary epithelium, and thyroid (48). It is possible that extrarenal tissues that contain vitamin D receptors can also acquire 25(OH)D that is not bound to VDBP—in other words, free or bioavailable 25(OH)D (49, 51–53). In sum, it is possible that both free and bound 25(OH)D play a role in vitamin D signaling, depending on the organ system of interest.
Laboratory assays typically measure total 25(OH)D, which includes 25(OH)D that is bound to VDBP (which is the largest proportion—approximately 85%–90%), 25(OH)D that is bound to albumin (10%–15%), and 25(OH)D that is unbound (<1%) (54). Because 25(OH)D binds weakly to albumin (Ka = 6 × 105 M−1 vs. Ka = 7 × 108 M−1 for VDBP) (54, 55), it is thought that 25(OH)D dissociates from albumin during tissue perfusion (56). Thus, the albumin-bound and free fractions together are called “bioavailable” 25(OH)D.
Quantifying free 25(OH)D requires either an assay that targets the unbound 25(OH)D directly (54, 57, 58) or the measurement of 25(OH)D, VDBP, and albumin, which together provide the inputs necessary for calculating free and bioavailable 25(OH)D. Equations for the calculation of free and bioavailable 25(OH)D using VDBP and albumin have been modified from the equations used to calculate free testosterone (54, 59). There has been some discussion regarding 1) whether equations for testosterone are applicable to vitamin D (46) and 2) whether these equations should account for genotypic differences in VDBP (49).
To our knowledge, it is not possible to directly measure bioavailable 25(OH)D. Doing so would require a cell-based biological assay, which is technically challenging and hard to interpret. On the other hand, free 25(OH)D can be directly measured. In the past, this was challenging because free 25(OH)D is present at a very low concentration and the laboratory techniques were demanding and expensive (46). A recently developed immunoassay can directly measure free 25(OH)D (58); however, given its novelty, additional validation is needed.
To date, only a few publications have investigated the relationships between directly measured free 25(OH)D and calculated free 25(OH)D. In this literature (limited to studies that measured VDBP with either a polyclonal antibody or LC-MS/MS), the correlation between calculated free 25(OH)D and directly measured free 25(OH)D has been reported as strong (r = 0.6–0.8) (60–62), low (r = 0.41) (63), and nonsignificant (no point estimate reported) (64). While directly measured free 25(OH)D and calculated free 25(OH)D are correlated, the two methods do not consistently arrive at the same concentrations. In studies with both calculated and directly measured 25(OH)D, average calculated free 25(OH)D level has been higher (60–63), sometimes twice as high (60, 63), although Denburg et al. (65) reported that it was higher only among white participants and lower among black participants. In total, it is unclear whether calculated and directly measured free 25(OH)D are in fact measuring the same quantity. Further research is needed to explore the differences in calculated versus directly measured free 25(OH)D.
A logical question, given the importance of vitamin D for calcium balance, might be: Which correlates more strongly with markers of bone health—free 25(OH)D or total 25(OH)D? Some of the previous studies aimed at answering this question were flawed because their calculation of free 25(OH)D was based on a VDBP measure that was estimated with a monoclonal antibody (46), which has been shown to be biased (see further discussion below) (66). Thus, only more recent studies that incorporated a direct measurement of free 25(OH)D, or that quantified VDBP using either LC/MS-MS or a polyclonal immunoassay, can be leveraged to answer this question.
Of these studies, 6 (62, 63, 67–70) have reported correlations between PTH and both total 25(OH)D and free 25(OH)D (Table 2). Four of these (62, 67–69) reported correlations of similar magnitude between free 25(OH)D, total 25(OH)D, and PTH. Aloia et al. (63) reported that total 25(OH)D is more strongly correlated with PTH, and only among white women, not black women. The final study found a stronger correlation with free 25(OH)D than with total 25(OH)D (although the magnitude of the correlation between total 25(OH)D and PTH was similar to that seen in other studies) (70). In 1 additional study, Johnsen et al. (71) reported that among postmenopausal women, free, bioavailable, and total 25(OH)D were associated with PTH but only free and bioavailable 25(OH)D were correlated with bone mineral density. In a supplementation trial, change in intact PTH was associated with the change in directly measured free 25(OH)D but not total 25(OH)D (72). Finally, a case-control study showed that osteoporotic men had lower free 25(OH)D but not lower total 25(OH)D, suggesting that free 25(OH)D may be a more useful measure of biological activity (73). Thus, based on the limited available data, there is no clear preference for free or total 25(OH)D when considering PTH or bone health. Further research is needed to explore the functional differences between free and total 25(OH)D and the racial/ethnic differences observed in these studies.
Table 2.
Published Correlations Between Parathyroid Hormone and Either Free or Total 25-Hydroxyvitamin Da
| First Author, Year (Reference No.) | Method of Free 25(OH)D Measurement | Correlation of PTH With Free 25(OH)Db | Correlation of PTH With Total 25(OH)Db | Which Is/Are More Strongly Correlated With PTH? | ||
|---|---|---|---|---|---|---|
| Correlation Coefficient (r) | P Value | Correlation Coefficient (r) | P Value | |||
| Aloia, 2015 (63) | Directly measured | Total 25(OH)D (free 25(OH)D is weakly correlated), and only among white women | ||||
| White women | −0.16 | >0.05 | −0.33 | <0.05 | ||
| Black women | 0.01 | >0.05 | −0.002 | >0.05 | ||
| Dastani, 2014 (68) | Calculated (54) | −0.26 | 1.9e−33 | −0.29 | 1.3e−39 | Both equally |
| Jemielita, 2016 (67) | Calculated (59) | Both, but total 25(OH)D is stronger in white women | ||||
| White women | −0.12 | 0.14 | −0.24 | 0.004 | ||
| Black women | −0.32 | <0.0001 | −0.30 | 0.0002 | ||
| Schwartz, 2014 (69) | Directly measured | −0.19 | <0.02 | −0.15 | <0.05 | Both |
| Schwartz, 2016 (70) | Directly measured | −0.28 | 0.02 | −0.17 | 0.15 | Free 25(OH)D (total 25(OH)D is weakly correlated) |
| Sollid, 2016 (62) | Directly measured | −0.17 | <0.001 | −0.21 | <0.001 | Both equally |
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; PTH, parathyroid hormone.
a One additional study reported the associations between free, bioavailable, and total 25(OH)D with PTH as standardized β estimates, not correlations, and is reviewed in the text (71).
b Confidence intervals were not reported.
Similarly, the literature that compares free 25(OH)D and total 25(OH)D for other health endpoints is sparse. Only 1 study has examined free 25(OH)D in relation to preeclampsia, and those authors found no association (74). Free or bioavailable 25(OH)D may be more relevant for immune function. For example, one study has shown that dendritic cells can convert 25(OH)D to active vitamin D, but this process is hindered in the presence of VDBP (75). Further, monocytes cultured with 25(OH)D and increasing doses of VDBP showed lower production of cathelicidin, an antimicrobial protein (reviewed by Chun et al. (49)). Thus, for immune endpoints, free or bioavailable 25(OH)D may be the most relevant measure.
Calculated free and bioavailable 25(OH)D were also associated with development of end-stage renal disease in a nested case-control study of participants from the Atherosclerosis Risk in Communities (ARIC) cohort, but total 25(OH)D was unrelated (76). In a series of studies from a Finnish population (where the questionable monoclonal VDBP assay was used but the population was assumed to be mostly Caucasian), investigators have reported that the associations between 25(OH)D and several cancers (prostate cancer (77), renal cell carcinoma (78), colorectal cancer (79), and pancreatic cancer (80)) are modified by VDBP levels, suggesting that VDBP levels should be taken into account when examining associations between 25(OH)D and cancer. Finally, in a supplementation trial of vitamin D (in combination with statins; n = 49 men and women aged ≥60 years), at the end of the trial there was no correlation between total 25(OH)D and lipid levels; however, free 25(OH)D was inversely correlated with triglycerides, low-density lipoprotein cholesterol, and total cholesterol (81).
Interest in studying free and bioavailable 25(OH)D is recent, and studies examining the associations of these measures with health outcomes are rare. The literature that does exist is intriguing and warrants further investigation of free or bioavailable 25(OH)D, especially among persons with health conditions which may influence VDBP (i.e., pregnancy) and across populations that are diverse in terms of age and racial/ethnic background.
VITAMIN D-BINDING PROTEIN
Until recently, VDBP was measured relatively infrequently. However, as noted above, data on VDBP concentrations are needed to calculate levels of free and bioavailable 25(OH)D. The desire to measure VDBP has grown in parallel with interest in calculating bioavailable and free 25(OH)D concentrations.
VDBP assays have recently been the source of controversy. Within the past decade, numerous studies have used a monoclonal 2-site sandwich immunoassay in a 96-well ELISA format, which has been shown to be flawed. Two independent research groups have documented that the monoclonal ELISA binds differentially by VDBP genotype (50, 61, 66, 82); specifically, it improperly binds to the Gc1F/Gc1F haplotype of the gc-globulin (group-specific component) gene (GC) (i.e., rs7041 G and rs4588 A alleles), resulting in uniformly low values for persons with this haplotype. Blacks are much more likely to have the Gc1F haplotype than are whites (e.g., in the community-based ARIC sample, it was present in 53.5% of blacks and 2.4% of whites (authors’ unpublished data (P.L.L.))). When it is measured using an assay that is not biased by genotype, blacks and whites have similar concentrations of VDBP (50, 61, 63, 66, 82–84). To its credit, the manufacturer of the monoclonal ELISA (R&D Systems, Minneapolis, Minnesota) provided researchers (including two of us, P.L.L. and A.N.H.) with free assays for comparing the monoclonal ELISA with the gold-standard LC-MS/MS, and it has since removed the monoclonal ELISA from the market. However, in recent years the monoclonal ELISA was used regularly to measure VDBP, and numerous publications have reported findings based on this assay (either VDBP itself, or free and bioavailable 25(OH)D calculated using VDBP measured by this assay). This literature should be viewed with extreme skepticism. An open question is whether results from the monoclonal ELISA among populations where the Gc1F haplotype is infrequent can be considered valid.
Alternate ways to measure VDBP include polyclonal immunoassays and LC-MS/MS, both of which are less likely to be biased by genotype. The LC-MS/MS approach was developed recently, and it has been shown to possess all of the fundamental characteristics of a valid assay—namely precision, linearity, specificity, and stability (85). However, few laboratories are presently measuring VDBP by means of LC-MS/MS, and as noted above, LC-MS/MS requires expensive equipment and expert staff. Several polyclonal immunoassays for measuring VDBP have become commercially available. It will be important to rigorously validate these assays against the gold-standard LC-MS/MS.
3-EPI-25-HYDROXYVITAMIN D
All vitamin D metabolites can be epimerized (86). The C-3 epimer of vitamin D, 3-epi-25-hydroxyvitamin D (3-epi-25(OH)D or 3-epi), is formed when the hydroxyl group at position C-3 of the A-ring is converted from an α orientation to a β orientation (Figure 3) (86). When measured by mass spectrometry, because of their identical molar weight, the epimeric forms are included with the nonepimeric forms in the total 25(OH)D level, unless care is used to chromatographically separate them. When measured by immunoassay, the epimeric form of 25(OH)D is not included in the total 25(OH)D level, as the antibody does not recognize the epimeric form.
The biological significance of 3-epi-25(OH)D is unclear (17, 86, 87). The epimer is detectable across several diverse populations (17, 45, 87–101; also partially reviewed by Bailey et al. (86)), although levels of it appear to be higher in infants and children (17, 87, 88, 91–95, 99). 3-epi-25(OH)D is positively correlated with 25(OH)D (17, 45, 87, 92, 96–106). Most studies report correlations between 0.6 and 0.8 (17, 88, 92, 98–100, 102–106), but lower values (as low as 0.2 (87)) have also been reported, and Lutsey et al. (45) reported a slightly lower coefficient among blacks (r = 0.36) than among whites (r = 0.54). Study results are divided as to whether the increase of 3-epi with 25(OH)D is linear (87, 92, 98, 103) or whether the proportion of 3-epi increases with increasing 25(OH)D (96, 100, 104, 105). While levels of 3-epi and total 25(OH)D are correlated, the absolute quantity of 3-epi-25(OH)D in adults is small: a median concentration of 3.4 nmol/L in the National Health and Nutrition Examination Surveys (17). Similarly, in a recent review of 8 studies, Bailey et al. (86) reported a weighted mean 3-epi-25(OH)D concentration of 4.3 nmol/L for adults (1.7 ng/mL); however, for infants, the mean was 18.2 nmol/L (7.3 ng/mL), and the corresponding percentage of total 25(OH)D that was 3-epi was 21.
Although it is clear that 3-epi can be detected in a variety of populations, the source of the epimer is still unknown. For the most part, 3-epi-25(OH)D has not been found in vitamin D supplements (91, 105, 107) (Baily et al. (88) did find 3-epi-25(OH)D in a supplement). In randomized trials of vitamin D in pregnant women (88, 106) and preterm infants (107), levels of 3-epi increase with vitamin D treatment, which suggests endogenous formation. There is some evidence that vitamin D treatment increases levels of 3-epi-25(OH)D in lactating women and nonpregnant women (106), but the magnitude of the increase is small. Some have suggested that 3-epi-25(OH)D levels are higher in infants due to liver immaturity; however, this may not be the case. As Bailey et al. described, 1) in vitro studies have found that epimerization is tissue-specific, 2) epimers are absent in the adult liver disease population, and 3) cytochrome P-450 enzymes are not involved in epimerization (86). This was supported by a recent study that found, in a hypervitaminosis D population, that 3-epi-25(OH)D was not associated with liver function (105). The developmental advantages of increased 3-epi-25(OH)D in infants should be further explored (107).
The epimeric forms of vitamin D show reduced binding of the vitamin D receptor and VDBP compared with the nonepimeric forms; however, the epimeric forms are similar to the nonepimeric forms in suppressing PTH secretion (86). Given these inconsistencies, the decision to separate 3-epi-25(OH)D from its nonepimeric counterparts will depend on the biological pathways of interest. For example, for infants in whom bone growth may be of primary concern, it has been suggested that clinical decision-making should be based on a 25(OH)D measure that excludes 3-epi (86, 108, 109). Similarly, some authors suggest that the presence of 3-epi may conceal low 25(OH)D levels that may be relevant for clinical decisions or public health (92, 110), while other authors find very little difference in the classification of vitamin D deficiency when the epimer is included versus when it is excluded (45, 90, 111).
Few studies have examined the association of 3-epi-25(OH)D with health endpoints. In adults, there is some evidence that 3-epi-25(OH)D is inversely related to serum low-density lipoprotein cholesterol (while 25(OH)D is positively correlated) and positively associated with triglycerides (but negatively associated with 25(OH)D) (112). In a study of hypervitaminosis D patients, 3-epi was unrelated to C-reactive protein, calcium, liver and renal function, creatinine, and PTH (105). Finally, in preterm infants, 3-epi was correlated with gestational age at birth and negatively correlated with head circumference; this was true for both the absolute level of 3-epi and the level relative to total 25(OH)D (107). Additionally, infants who were receiving breast milk had a higher percentage of 3-epi-25(OH)D than infants who received formula exclusively (107).
The investigation of 3-epi-25(OH)D is in its infancy, and research is needed to clarify its importance. In future studies, researchers should aim to determine the origin and function of 3-epi-25(OH)D. At this time, not all laboratories that measure 25(OH)D can separate 3-epi-25(OH)D, and researchers interested in the epimer should be sure to clarify this with their intended laboratory. Epidemiologic studies will be important for describing the health outcomes associated with 3-epi-25(OH)D and the populations that exhibit higher levels. Stored samples from randomized trials in disparate populations can be used to determine whether vitamin D supplementation increases levels of 3-epi-25(OH)D relative to its nonepimeric form and, if so, at what dose. The epimeric form of vitamin D metabolites may be more relevant for some organ systems and not others, but this remains to be seen. Finally, etiological studies of 3-epi-25(OH)D with endpoints that have been inconsistently associated with total 25(OH)D may help to clarify those inconsistencies.
BIOMARKERS OF THE FUTURE?—1,25(OH)2D and 24,25(OH)2D3
The analytes with the greatest potential to expand our understanding of the relevance of vitamin D for human health are total 25(OH)D, VDBP, 3-epi-25(OH)D, and bioavailable and free 25(OH)D (calculated or directly measured). However, other biomarkers, such as 1,25(OH)2D, 24,25(OH)2D3, and cholecalciferol, are also of potential interest. Of course, biomarker discovery is actively under way as well.
Historically, 1,25(OH)2D has rarely been measured for assessing vitamin D status in research, as it is tightly regulated by serum calcium, phosphate, and PTH, has a relatively short half-life, and has been difficult to measure accurately (113). However, it is now possible to measure 1,25(OH)2D using a “gold-standard” mass spectrometry approach, thereby overcoming the analytical challenges of the past. Given its tight regulation and the fact that 1,25(OH)2D can be synthesized locally without circulating throughout the body, the added benefit of measuring 1,25(OH)2D in research settings remains uncertain.
24,25(OH)2D3 is the most abundant product of 25(OH)D3 catabolism and may serve as an indicator of functional tissue-level 1,25(OH)2D activity (36, 114–117). It has been more strongly correlated with PTH than 25(OH)D or 1,25(OH)2D (115, 117). Additionally, the ratio of 24,25(OH)2D3 to 25(OH)D has been hypothesized to be a novel potential biomarker of tissue-level 1,25(OH)2D3 deficiency (116, 117). Genetic analyses have identified polymorphisms in the cytochrome P-450, family 24, subfamily A, member 1, gene (CYP24A1) as an important predictor of serum/plasma PTH concentrations in large populations (118).
In conclusion, vitamin D signaling is complex, may involve VDBP and/or multiple metabolites, and may vary by tissue or organ system. Investigation of the relevance of vitamin D for human health is evolving and advancing as researchers attempt to better characterize this complex pathway. While many studies of vitamin D exist, the literature is fraught with inaccurate measurements. Moreover, recent technological advances have led to the ability to measure other vitamin D metabolites and related compounds, but there are, as of yet, few studies of these novel biomarkers. Given these unresolved issues, further vitamin D research is critical.
ACKNOWLEDGMENTS
Author affiliations: Department of Chronic Disease Epidemiology, Yale Center for Perinatal, Pediatric, and Environmental Epidemiology, Yale School of Public Health, New Haven, Connecticut (Anne Marie Z. Jukic); Department of Laboratory Medicine, School of Medicine, University of Washington, Seattle, Washington (Andrew N. Hoofnagle); and Division of Epidemiology and Community Health, School of Public Health, University of Minnesota, Minneapolis, Minnesota (Pamela L. Lutsey).
This research was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (award R00HD079659) and by the National Institute on Aging (grant (R01AG041776), the National Heart, Lung, and Blood Institute (grant R01HL103706), and the Office of Dietary Supplements, National Institutes of Health (grant R01HL103706-S1).
We thank Gabriela Leskur for her contributions to the design of Figure 1.
The University of Washington (A.N.H.) receives grant funding from Waters Corporation (Milford, Massachusetts), a manufacturer of mass spectrometry equipment.
Abbreviations
- ARIC
Atherosclerosis Risk in Communities
- ELISA
enzyme-linked immunosorbent assay
- 3-epi 25(OH)D
3-epi-25-hydroxyvitamin D
- LC-MS/MS
liquid chromatography–tandem mass spectrometry
- 25(OH)D
25-hydroxyvitamin D
- 25(OH)D2
25-hydroxyvitamin D2
- 25(OH)D3
25-hydroxyvitamin D3
- 1
25(OH)2D, 1,25-dihydroxyvitamin D
- 24
25(OH)2D, 24,25-dihydroxyvitamin D
- PTH
parathyroid hormone
- VDBP
vitamin D-binding protein
REFERENCES
- 1. Fakih MG, Trump DL, Muindi JR, et al. A phase I pharmacokinetic and pharmacodynamic study of intravenous calcitriol in combination with oral gefitinib in patients with advanced solid tumors. Clin Cancer Res. 2007;13(4):1216–1223. [DOI] [PubMed] [Google Scholar]
- 2. Sempos CT, Vesper HW, Phinney KW, et al. Vitamin D status as an international issue: national surveys and the problem of standardization. Scand J Clin Lab Invest Suppl. 2012;243:32–40. [DOI] [PubMed] [Google Scholar]
- 3. Sempos CT, Durazo-Arvizu RA, Binkley N, et al. Developing vitamin D dietary guidelines and the lack of 25-hydroxyvitamin D assay standardization: the ever-present past. J Steroid Biochem Mol Biol. 2016;164:115–119. [DOI] [PubMed] [Google Scholar]
- 4. Ross AC, Taylor CL, Yaktine AL, et al. Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: National Academies Press; 2011. [PubMed] [Google Scholar]
- 5. Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. [DOI] [PubMed] [Google Scholar]
- 6. Looker AC, Pfeiffer CM, Lacher DA, et al. Serum 25-hydroxyvitamin D status of the US population: 1988–1994 compared with 2000–2004. Am J Clin Nutr. 2008;88(6):1519–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ginde AA, Liu MC, Camargo CA Jr. Demographic differences and trends of vitamin D insufficiency in the US population, 1988–2004. Arch Intern Med. 2009;169(6):626–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Martins D, Wolf M, Pan D, et al. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: data from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2007;167(11):1159–1165. [DOI] [PubMed] [Google Scholar]
- 9. Gutiérrez OM, Farwell WR, Kermah D, et al. Racial differences in the relationship between vitamin D, bone mineral density, and parathyroid hormone in the National Health and Nutrition Examination Survey. Osteoporos Int. 2011;22(6):1745–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zehnder D, Bland R, Williams MC, et al. Extrarenal expression of 25-hydroxyvitamin D3-1α-hydroxylase. J Clin Endocrinol Metab. 2001;86(2):888–894. [DOI] [PubMed] [Google Scholar]
- 11. Hewison M, Burke F, Evans KN, et al. Extra-renal 25-hydroxyvitamin D3-1α-hydroxylase in human health and disease. J Steroid Biochem Mol Biol. 2007;103(3-5):316–321. [DOI] [PubMed] [Google Scholar]
- 12. Hewison M. Vitamin D and the intracrinology of innate immunity. Mol Cell Endocrinol. 2010;321(2):103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Somjen D, Weisman Y, Kohen F, et al. 25-hydroxyvitamin D3-1α-hydroxylase is expressed in human vascular smooth muscle cells and is upregulated by parathyroid hormone and estrogenic compounds. Circulation. 2005;111(13):1666–1671. [DOI] [PubMed] [Google Scholar]
- 14. Bikle D. Nonclassic actions of vitamin D. J Clin Endocrinol Metab. 2009;94(1):26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. LeFevre ML; US Preventive Services Task Force . Screening for vitamin D deficiency in adults: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;162(2):133–140. [DOI] [PubMed] [Google Scholar]
- 16. Jones KS, Assar S, Harnpanich D, et al. 25(OH)D2 half-life is shorter than 25(OH)D3 half-life and is influenced by DBP concentration and genotype. J Clin Endocrinol Metab. 2014;99(9):3373–3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schleicher RL, Sternberg MR, Looker AC, et al. National estimates of serum total 25-hydroxyvitamin D and metabolite concentrations measured by liquid chromatography-tandem mass spectrometry in the US population during 2007–2010. J Nutr. 2016;146(5):1051–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wielders JP, Wijnberg FA. Preanalytical stability of 25(OH)-vitamin D3 in human blood or serum at room temperature: solid as a rock [letter]. Clin Chem. 2009;55(8):1584–1585. [DOI] [PubMed] [Google Scholar]
- 19. Antoniucci DM, Black DM, Sellmeyer DE. Serum 25-hydroxyvitamin D is unaffected by multiple freeze-thaw cycles. Clin Chem. 2005;51(1):258–261. [DOI] [PubMed] [Google Scholar]
- 20. Lissner D, Mason RS, Posen S. Stability of vitamin D metabolites in human blood serum and plasma [letter]. Clin Chem. 1981;27(5):773–774. [PubMed] [Google Scholar]
- 21. Stamp TC, Round JM. Seasonal changes in human plasma levels of 25-hydroxyvitamin D [letter]. Nature. 1974;247(5442):563–565. [DOI] [PubMed] [Google Scholar]
- 22. Ellis G, Dixon K. Sequential-saturation-type assay for serum 25-hydroxyvitamin D. Clin Chem. 1977;23(5):855–862. [PubMed] [Google Scholar]
- 23. Zerwekh JE. The measurement of vitamin D: analytical aspects. Ann Clin Biochem. 2004;41(4):272–281. [DOI] [PubMed] [Google Scholar]
- 24. Bodnar LM, Catov JM, Wisner KL, et al. Racial and seasonal differences in 25-hydroxyvitamin D detected in maternal sera frozen for over 40 years. Br J Nutr. 2009;101(2):278–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Roth HJ, Schmidt-Gayk H, Weber H, et al. Accuracy and clinical implications of seven 25-hydroxyvitamin D methods compared with liquid chromatography-tandem mass spectrometry as a reference. Ann Clin Biochem. 2008;45(2):153–159. [DOI] [PubMed] [Google Scholar]
- 26. Wallace AM, Gibson S, de la Hunty A, et al. Measurement of 25-hydroxyvitamin D in the clinical laboratory: current procedures, performance characteristics and limitations. Steroids. 2010;75(7):477–488. [DOI] [PubMed] [Google Scholar]
- 27. Phinney KW, Bedner M, Tai SS, et al. Development and certification of a standard reference material for vitamin D metabolites in human serum. Anal Chem. 2012;84(2):956–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mineva EM, Schleicher RL, Chaudhary-Webb M, et al. A candidate reference measurement procedure for quantifying serum concentrations of 25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 using isotope-dilution liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2015;407(19):5615–5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Binkley N, Dawson-Hughes B, Durazo-Arvizu R, et al. Vitamin D measurement standardization: the way out of the chaos. J Steroid Biochem Mol Biol. 2017;173:117–121. [DOI] [PubMed] [Google Scholar]
- 30. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–1930. [DOI] [PubMed] [Google Scholar]
- 31. Manson JE, Brannon PM, Rosen CJ, et al. Vitamin D deficiency—is there really a pandemic? [editorial]. N Engl J Med. 2016;375(19):1817–1820. [DOI] [PubMed] [Google Scholar]
- 32. Aloia JF. African Americans, 25-hydroxyvitamin D, and osteoporosis: a paradox. Am J Clin Nutr. 2008;88(2):545S–550S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cauley JA, Danielson ME, Boudreau R, et al. Serum 25-hydroxyvitamin D and clinical fracture risk in a multiethnic cohort of women: the Women’s Health Initiative (WHI). J Bone Miner Res. 2011;26(10):2378–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kalkwarf HJ, Zemel BS, Gilsanz V, et al. The Bone Mineral Density in Childhood Study: bone mineral content and density according to age, sex, and race. J Clin Endocrinol Metab. 2007;92(6):2087–2099. [DOI] [PubMed] [Google Scholar]
- 35. Barrett-Connor E, Siris ES, Wehren LE, et al. Osteoporosis and fracture risk in women of different ethnic groups. J Bone Miner Res. 2005;20(2):185–194. [DOI] [PubMed] [Google Scholar]
- 36. van Ballegooijen AJ, Robinson-Cohen C, Katz R, et al. Vitamin D metabolites and bone mineral density: the Multi-Ethnic Study of Atherosclerosis. Bone. 2015;78:186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Michos ED, Misialek JR, Selvin E, et al. 25-Hydroxyvitamin D levels, vitamin D binding protein gene polymorphisms and incident coronary heart disease among whites and blacks: the ARIC Study. Atherosclerosis. 2015;241(1):12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Michos ED, Reis JP, Post WS, et al. 25-Hydroxyvitamin D deficiency is associated with fatal stroke among whites but not blacks: the NHANES-III linked mortality files. Nutrition. 2012;28(4):367–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Reis JP, Michos ED, von Mühlen D, et al. Differences in vitamin D status as a possible contributor to the racial disparity in peripheral arterial disease. Am J Clin Nutr. 2008;88(6):1469–1477. [DOI] [PubMed] [Google Scholar]
- 40. Reis JP, Michos ED, Selvin E, et al. Race, vitamin D-binding protein gene polymorphisms, 25-hydroxyvitamin D, and incident diabetes: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Clin Nutr. 2015;101(6):1232–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lutsey PL, Michos ED, Misialek JR, et al. Race and vitamin D binding protein gene polymorphisms modify the association of 25-hydroxyvitamin D and incident heart failure: the ARIC (Atherosclerosis Risk in Communities) Study. JACC Heart Fail. 2015;3(5):347–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Robinson-Cohen C, Hoofnagle AN, Ix JH, et al. Racial differences in the association of serum 25-hydroxyvitamin D concentration with coronary heart disease events. JAMA. 2013;310(2):179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Scragg R, Sowers M, Bell C, et al. Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care. 2004;27(12):2813–2818. [DOI] [PubMed] [Google Scholar]
- 44. Sachs MC, Shoben A, Levin GP, et al. Estimating mean annual 25-hydroxyvitamin D concentrations from single measurements: the Multi-Ethnic Study of Atherosclerosis. Am J Clin Nutr. 2013;97(6):1243–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lutsey PL, Eckfeldt JH, Ogagarue ER, et al. The 25-hydroxyvitamin D3 C-3 epimer: distribution, correlates, and reclassification of 25-hydroxyvitamin D status in the population-based Atherosclerosis Risk in Communities Study (ARIC). Clin Chim Acta. 2015;442:75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bouillon R. Free or total 25OHD as marker for vitamin D status? J Bone Miner Res. 2016;31(6):1124–1127. [DOI] [PubMed] [Google Scholar]
- 47. Faix JD. Principles and pitfalls of free hormone measurements. Best Pract Res Clin Endocrinol Metab. 2013;27(5):631–645. [DOI] [PubMed] [Google Scholar]
- 48. Lundgren S, Carling T, Hjälm G, et al. Tissue distribution of human gp330/megalin, a putative Ca2+-sensing protein. J Histochem Cytochem. 1997;45(3):383–392. [DOI] [PubMed] [Google Scholar]
- 49. Chun RF, Peercy BE, Orwoll ES, et al. Vitamin D and DBP: the free hormone hypothesis revisited. J Steroid Biochem Mol Biol. 2014;144(A):132–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nielson CM, Jones KS, Chun RF, et al. Free 25-hydroxyvitamin D: impact of vitamin D binding protein assays on racial-genotypic associations. J Clin Endocrinol Metab. 2016;101(5):2226–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Esteban C, Geuskens M, Ena JM, et al. Receptor-mediated uptake and processing of vitamin D-binding protein in human B-lymphoid cells. J Biol Chem. 1992;267(14):10177–10183. [PubMed] [Google Scholar]
- 52. Nykjaer A, Dragun D, Walther D, et al. An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell. 1999;96(4):507–515. [DOI] [PubMed] [Google Scholar]
- 53. Zella LA, Shevde NK, Hollis BW, et al. Vitamin D-binding protein influences total circulating levels of 1,25-dihydroxyvitamin D3 but does not directly modulate the bioactive levels of the hormone in vivo. Endocrinology. 2008;149(7):3656–3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bikle DD, Gee E, Halloran B, et al. Assessment of the free fraction of 25-hydroxyvitamin D in serum and its regulation by albumin and the vitamin D-binding protein. J Clin Endocrinol Metab.1986;63(4):954–959. [DOI] [PubMed] [Google Scholar]
- 55. Bikle DD, Gee E, Halloran B, et al. Free 1,25-dihydroxyvitamin D levels in serum from normal subjects, pregnant subjects, and subjects with liver disease. J Clin Invest. 1984;74(6):1966–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ferrini RL, Barrett-Connor E. Sex hormones and age: a cross-sectional study of testosterone and estradiol and their bioavailable fractions in community-dwelling men. Am J Epidemiol. 1998;147(8):750–754. [DOI] [PubMed] [Google Scholar]
- 57. Bikle DD, Halloran BP, Gee E, et al. Free 25-hydroxyvitamin D levels are normal in subjects with liver disease and reduced total 25-hydroxyvitamin D levels. J Clin Invest. 1986;78(3):748–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. DIASource Free 25OH vitamin D, ELISA, 96 tests (RUO). http://www.vitamin-d-diagnostics.com/Vitamin-D/Free-25OH-Vitamin-D/Free-25OH-Vitamin-D-ELISA-96-tests-RUO. Accessed November 4, 2016.
- 59. Powe CE, Ricciardi C, Berg AH, et al. Vitamin D-binding protein modifies the vitamin D-bone mineral density relationship. J Bone Miner Res. 2011;26(7):1609–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Alzaman NS, Dawson-Hughes B, Nelson J, et al. Vitamin D status of black and white Americans and changes in vitamin D metabolites after varied doses of vitamin D supplementation. Am J Clin Nutr. 2016;104(1):205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Nielson CM, Jones KS, Bouillon R, et al. Role of assay type in determining free 25-hydroxyvitamin D levels in diverse populations. N Engl J Med. 2016;374(17):1695–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sollid ST, Hutchinson MY, Berg V, et al. Effects of vitamin D binding protein phenotypes and vitamin D supplementation on serum total 25(OH)D and directly measured free 25(OH)D. Eur J Endocrinol. 2016;174(4):445–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Aloia J, Mikhail M, Dhaliwal R, et al. Free 25(OH)D and the vitamin D paradox in African Americans. J Clin Endocrinol Metab. 2015;100(9):3356–3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lee MJ, Kearns MD, Smith EM, et al. Free 25-hydroxyvitamin D concentrations in cystic fibrosis. Am J Med Sci. 2015;350(5):374–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Denburg MR, Hoofnagle AN, Sayed S, et al. Comparison of two ELISA methods and mass spectrometry for measurement of vitamin D-binding protein: implications for the assessment of bioavailable vitamin D concentrations across genotypes. J Bone Miner Res. 2016;31(6):1128–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hoofnagle AN, Eckfeldt JH, Lutsey PL. Vitamin D-binding protein concentrations quantified by mass spectrometry [letter]. N Engl J Med. 2015;373(15):1480–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Jemielita TO, Leonard MB, Baker J, et al. Association of 25-hydroxyvitamin D with areal and volumetric measures of bone mineral density and parathyroid hormone: impact of vitamin D-binding protein and its assays. Osteoporos Int. 2016;27(2):617–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Dastani Z, Berger C, Langsetmo L, et al. In healthy adults, biological activity of vitamin D, as assessed by serum PTH, is largely independent of DBP concentrations. J Bone Miner Res. 2014;29(2):494–499. [DOI] [PubMed] [Google Scholar]
- 69. Schwartz JB, Lai J, Lizaola B, et al. A comparison of measured and calculated free 25(OH) vitamin D levels in clinical populations. J Clin Endocrinol Metab. 2014;99(5):1631–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Schwartz JB, Kane L, Bikle D. Response of vitamin D concentration to vitamin D3 administration in older adults without sun exposure: a randomized double-blind trial. J Am Geriatr Soc. 2016;64(1):65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Johnsen MS, Grimnes G, Figenschau Y, et al. Serum free and bio-available 25-hydroxyvitamin D correlate better with bone density than serum total 25-hydroxyvitamin D. Scand J Clin Lab Invest. 2014;74(3):177–183. [DOI] [PubMed] [Google Scholar]
- 72. Shieh A, Han W, Ishii S, et al. Quantifying the balance between total bone formation and total bone resorption: an index of net bone formation. J Clin Endocrinol Metab. 2016;101(7):2802–2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Al-Oanzi ZH, Tuck SP, Raj N, et al. Assessment of vitamin D status in male osteoporosis. Clin Chem. 2006;52(2):248–254. [DOI] [PubMed] [Google Scholar]
- 74. Powe CE, Seely EW, Rana S, et al. First trimester vitamin D, vitamin D binding protein, and subsequent preeclampsia. Hypertension. 2010;56(4):758–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Jeffery LE, Wood AM, Qureshi OS, et al. Availability of 25-hydroxyvitamin D3 to APCs controls the balance between regulatory and inflammatory T cell responses. J Immunol. 2012;189(11):5155–5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Rebholz CM, Grams ME, Lutsey PL, et al. Biomarkers of vitamin D status and risk of ESRD. Am J Kidney Dis. 2016;67(2):235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Weinstein SJ, Mondul AM, Kopp W, et al. Circulating 25-hydroxyvitamin D, vitamin D-binding protein and risk of prostate cancer. Int J Cancer. 2013;132(12):2940–2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Mondul AM, Weinstein SJ, Moy KA, et al. Vitamin D-binding protein, circulating vitamin D and risk of renal cell carcinoma. Int J Cancer. 2014;134(11):2699–2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Anic GM, Weinstein SJ, Mondul AM, et al. Serum vitamin D, vitamin D binding protein, and risk of colorectal cancer. PLoS One. 2014;9(7):e102966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Weinstein SJ, Stolzenberg-Solomon RZ, Kopp W, et al. Impact of circulating vitamin D binding protein levels on the association between 25-hydroxyvitamin D and pancreatic cancer risk: a nested case-control study. Cancer Res. 2012;72(5):1190–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kane L, Moore K, Lütjohann D, et al. Vitamin D3 effects on lipids differ in statin and non-statin-treated humans: superiority of free 25-OH D levels in detecting relationships. J Clin Endocrinol Metab. 2013;98(11):4400–4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Henderson CM, Lutsey PL, Misialek JR, et al. Measurement by a novel LC-MS/MS methodology reveals similar serum concentrations of vitamin D-binding protein in blacks and whites. Clin Chem. 2016;62(1):179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lauridsen AL, Vestergaard P, Nexo E. Mean serum concentration of vitamin D-binding protein (Gc globulin) is related to the Gc phenotype in women. Clin Chem. 2001;47(4):753–756. [PubMed] [Google Scholar]
- 84. Bouillon R, Jones K, Schoenmakers I. Vitamin D-binding protein and vitamin D in blacks and whites [letter]. N Engl J Med. 2014;370(9):879. [DOI] [PubMed] [Google Scholar]
- 85. Grant RP, Hoofnagle AN. From lost in translation to paradise found: enabling protein biomarker method transfer by mass spectrometry [editorial]. Clin Chem. 2014;60(7):941–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Bailey D, Veljkovic K, Yazdanpanah M, et al. Analytical measurement and clinical relevance of vitamin D3 C3-epimer. Clin Biochem. 2013;46(3):190–196. [DOI] [PubMed] [Google Scholar]
- 87. Strathmann FG, Sadilkova K, Laha TJ, et al. 3-Epi-25 hydroxyvitamin D concentrations are not correlated with age in a cohort of infants and adults. Clin Chim Acta. 2012;413(1-2):203–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Bailey D, Perumal N, Yazdanpanah M, et al. Maternal-fetal-infant dynamics of the C3-epimer of 25-hydroxyvitamin D. Clin Biochem. 2014;47(9):816–822. [DOI] [PubMed] [Google Scholar]
- 89. Gallo S, Comeau K, Vanstone C, et al. Effect of different dosages of oral vitamin D supplementation on vitamin D status in healthy, breastfed infants: a randomized trial. JAMA. 2013;309(17):1785–1792. [DOI] [PubMed] [Google Scholar]
- 90. Yu S, Zhou W, Zhang R, et al. Validation and comparison of a rapid liquid chromatography tandem mass spectrometry method for serum 25OHD with the efficiency of separating 3-epi 25OHD3. Clin Biochem. 2016;49(13-14):1004–1008. [DOI] [PubMed] [Google Scholar]
- 91. Yazdanpanah M, Bailey D, Walsh W, et al. Analytical measurement of serum 25-OH-vitamin D3, 25-OH-vitamin D2 and their C3-epimers by LC-MS/MS in infant and pediatric specimens. Clin Biochem. 2013;46(13-14):1264–1271. [DOI] [PubMed] [Google Scholar]
- 92. van den Ouweland JM, Beijers AM, van Daal H. Fast separation of 25-hydroxyvitamin D3 from 3-epi-25-hydroxyvitamin D3 in human serum by liquid chromatography–tandem mass spectrometry: variable prevalence of 3-epi-25-hydroxyvitamin D3 in infants, children, and adults [letter]. Clin Chem. 2011;57(11):1618–1619. [DOI] [PubMed] [Google Scholar]
- 93. Stepman HC, Vanderroost A, Stöckl D, et al. Full-scan mass spectral evidence for 3-epi-25-hydroxyvitamin D3 in serum of infants and adults. Clin Chem Lab Med. 2011;49(2):253–256. [DOI] [PubMed] [Google Scholar]
- 94. Singh RJ, Taylor RL, Reddy GS, et al. C-3 epimers can account for a significant proportion of total circulating 25-hydroxyvitamin D in infants, complicating accurate measurement and interpretation of vitamin D status. J Clin Endocrinol Metab. 2006;91(8):3055–3061. [DOI] [PubMed] [Google Scholar]
- 95. Liebisch G, Matysik S. Accurate and reliable quantification of 25-hydroxy-vitamin D species by liquid chromatography high-resolution tandem mass spectrometry. J Lipid Res. 2015;56(6):1234–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Lensmeyer G, Poquette M, Wiebe D, et al. The C-3 epimer of 25-hydroxyvitamin D3 is present in adult serum. J Clin Endocrinol Metab. 2012;97(1):163–168. [DOI] [PubMed] [Google Scholar]
- 97. Karras SN, Shah I, Petroczi A, et al. An observational study reveals that neonatal vitamin D is primarily determined by maternal contributions: implications of a new assay on the roles of vitamin D forms. Nutr J. 2013;12:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Cooke DJ, Cooke BR, Bell DA, et al. 25-Hydroxyvitamin D C3-epimer is universally present in neonatal Western Australian samples but is unlikely to contribute to diagnostic misclassification. Ann Clin Biochem. 2016;53(5):593–598. [DOI] [PubMed] [Google Scholar]
- 99. Clarke MW, Tuckey RC, Gorman S, et al. Optimized 25-hydroxyvitamin D analysis using liquid-liquid extraction with 2D separation with LC/MS/MS detection, provides superior precision compared to conventional assays. Metabolomics. 2013;9(5):1031–1040. [Google Scholar]
- 100. Cashman KD, Kinsella M, Walton J, et al. The 3 epimer of 25-hydroxycholecalciferol is present in the circulation of the majority of adults in a nationally representative sample and has endogenous origins. J Nutr. 2014;144(7):1050–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Aghajafari F, Field CJ, Rabi D, et al. Plasma 3-epi-25-hydroxycholecalciferol can alter the assessment of vitamin D status using the current reference ranges for pregnant women and their newborns. J Nutr. 2016;146(1):70–75. [DOI] [PubMed] [Google Scholar]
- 102. Baecher S, Leinenbach A, Wright JA, et al. Simultaneous quantification of four vitamin D metabolites in human serum using high performance liquid chromatography tandem mass spectrometry for vitamin D profiling. Clin Biochem. 2012;45(16-17):1491–1496. [DOI] [PubMed] [Google Scholar]
- 103. Carter GD, Jones JC, Shannon J, et al. 25-Hydroxyvitamin D assays: potential interference from other circulating vitamin D metabolites. J Steroid Biochem Mol Biol. 2016;164:134–138. [DOI] [PubMed] [Google Scholar]
- 104. Engelman CD, Bo R, Zuelsdorff M, et al. Epidemiologic study of the C-3 epimer of 25-hydroxyvitamin D3 in a population-based sample. Clin Nutr. 2014;33(3):421–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Granado-Lorencio F, Blanco-Navarro I, Pérez-Sacristán B, et al. Serum levels of 3-epi-25-OH-D3 during hypervitaminosis D in clinical practice. J Clin Endocrinol Metab. 2012;97(12):E2266–E2270. [DOI] [PubMed] [Google Scholar]
- 106. Park H, Brannon PM, West AA, et al. Vitamin D metabolism varies among women in different reproductive states consuming the same intakes of vitamin D and related nutrients. J Nutr. 2016;146(8):1537–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Hanson C, Jones G, Lyden E, et al. Vitamin D metabolism in the premature newborn: a randomized trial. Clin Nutr. 2016;35(4):835–841. [DOI] [PubMed] [Google Scholar]
- 108. Contreras JJ, Hiestand B, O’Neill JC, et al. Vitamin D deficiency in children with fractures. Pediatr Emerg Care. 2014;30(11):777–781. [DOI] [PubMed] [Google Scholar]
- 109. Tapan S, Sertoglu E. Importance of C-3 epimer of 25-hydroxyvitamin D to assess vitamin D deficiency in children with fractures. Pediatr Emerg Care. 2015;31(12):e22. [DOI] [PubMed] [Google Scholar]
- 110. Black LJ, Anderson D, Clarke MW, et al. Analytical bias in the measurement of serum 25-hydroxyvitamin D concentrations impairs assessment of vitamin D status in clinical and research settings. PLoS One. 2015;10(8):e0135478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Keevil B. Does the presence of 3-epi-25OHD3 affect the routine measurement of vitamin D using liquid chromatography tandem mass spectrometry? [letter]. Clin Chem Lab Med. 2012;50(1):181–183. [DOI] [PubMed] [Google Scholar]
- 112. Chailurkit LO, Aekplakorn W, Srijaruskul K, et al. Discrepant association of serum C-3 epimer of 25-hydroxyvitamin D versus non-epimeric 25-hydroxyvitamin D with serum lipid levels. Lipids Health Dis. 2016;15(1):157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Feldman D, Pike JW, Adams JS. Vitamin D. 3rd ed (2-volume set). Cambridge, MA: Academic Press, Inc.; 2011. [Google Scholar]
- 114. Jones G, Prosser DE, Kaufmann M. 25-Hydroxyvitamin D-24-hydroxylase (CYP24A1): its important role in the degradation of vitamin D. Arch Biochem Biophys. 2012;523(1):9–18. [DOI] [PubMed] [Google Scholar]
- 115. Bosworth CR, Levin G, Robinson-Cohen C, et al. The serum 24,25-dihydroxyvitamin D concentration, a marker of vitamin D catabolism, is reduced in chronic kidney disease. Kidney Int. 2012;82(6):693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. de Boer IH, Sachs MC, Chonchol M, et al. Estimated GFR and circulating 24,25-dihydroxyvitamin D3 concentration: a participant-level analysis of 5 cohort studies and clinical trials. Am J Kidney Dis. 2014;64(2):187–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Berg AH, Powe CE, Evans MK, et al. 24,25-Dihydroxyvitamin D3 and vitamin D status of community dwelling black and white Americans. Clin Chem. 2015;61(6):877–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Robinson-Cohen C, Lutsey PL, Kleber ME, et al. Genetic variants associated with circulating parathyroid hormone. J Am Soc Nephrol. 2017;28(5):1553–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Deeb KK, Trump DL, Johnson CS.. Vitamin D signaling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer. 2007;7(9):684–700. [DOI] [PubMed] [Google Scholar]