Abstract
Background
Combination therapy with oral fluoropyrimidine and irinotecan has not yet been established as first-line treatment of metastatic colorectal cancer (mCRC). We carried out a randomized, open-label, phase III trial to determine whether S-1 and irinotecan plus bevacizumab is noninferior to mFOLFOX6 or CapeOX plus bevacizumab in terms of progression-free survival (PFS).
Patients and methods
Patients from 53 institutions who had previously untreated mCRC were randomly assigned (1 : 1) to receive either mFOLFOX6 or CapeOX plus bevacizumab (control group) or S-1 and irinotecan plus bevacizumab (experimental group; a 3-week regimen: intravenous infusions of irinotecan 150 mg/m2 and bevacizumab 7.5 mg/kg on day 1, oral S-1 80 mg/m2 twice daily for 2 weeks, followed by a 1-week rest; or a 4-week regimen: irinotecan 100 mg/m2 and bevacizumab 5 mg/kg on days 1 and 15, S-1 80 mg/m2 twice daily for 2 weeks, followed by a 2-week rest). The primary end point was PFS. The noninferiority margin was 1.25; noninferiority would be established if the upper limit of the 95% confidence interval (CI) for the hazard ratio (HR) of the control group versus the experimental group was less than this margin.
Result
Between June 2012 and September 2014, 487 patients underwent randomization. Two hundred and forty-three patients assigned to the control group and 241 assigned to the experimental group were included in the primary analysis. Median PFS was 10.8 months (95% CI 9.6–11.6) in the control group and 14.0 months (95% CI 12.4–15.5) in the experimental group (HR 0.84, 95% CI 0.70–1.02; P < 0.0001 for noninferiority, P = 0.0815 for superiority). One hundred and fifty-seven patients (64.9%) in the control group and 140 (58.6%) in the experimental group had adverse events of grade 3 or higher.
Conclusion
S-1 and irinotecan plus bevacizumab is noninferior to mFOLFOX6 or CapeOX plus bevacizumab with respect to PFS as first-line treatment of mCRC and could be a new standard treatment.
Clinical trials number
UMIN000007834
Keywords: IRIS, SIRB, FOLFOX, XELOX, mCRC
Key Message
To our knowledge, the TRICOLORE trial is the first randomized phase III study to demonstrate the efficacy of combination therapy using oral fluoropyrimidine and irinotecan as first-line treatment of metastatic colorectal cancer. We have shown that S-1 and irinotecan plus bevacizumab is noninferior to mFOLFOX6 or CapeOX plus bevacizumab in terms of progression-free survival.
Introduction
FOLFOX, CapeOX, or FOLFIRI plus bevacizumab are extensively used as first-line treatment of metastatic colorectal cancer (mCRC) [1, 2]. Oxaliplatin-based combination regimens such as FOLFOX and CapeOX are commonly used in general clinical practice in many countries, including Japan and the United States, in preference to FOLFIRI, because the resulting alopecia and gastrointestinal toxicity are milder. However, peripheral neuropathy induced by oxaliplatin often leads to treatment withdrawal, negatively affecting treatment continuity. Furthermore, peripheral neuropathy is usually prolonged, interfering with the daily lives of patients and reducing their quality of life (QOL) [1, 3]. Meanwhile, combination therapy with an oral fluoropyrimidine and irinotecan as first-line treatment of mCRC has not yet been established [4].
The oral fluoropyrimidine S-1 has been approved in Japan; the European Medicines Agency has approved S-1 for gastric cancer. S-1 is a combined preparation consisting of tegafur, a prodrug of 5-fluorouracil (5-FU), and the modulators gimeracil and oteracil potassium [5]. S-1 plus irinotecan was shown to be noninferior to FOLFIRI as second-line chemotherapy for mCRC [6, 7]. Two phase II studies have evaluated 3-week and 4-week regimens of S-1 combined with irinotecan plus bevacizumab as first-line chemotherapy for mCRC; promising outcomes were obtained [8, 9].
On the basis of these studies, we examined whether S-1 and irinotecan plus bevacizumab was noninferior in terms of progression-free survival (PFS) to mFOLFOX6 or CapeOX plus bevacizumab as first-line chemotherapy for mCRC.
Methods
Study design
The TRICOLORE trial was an open-label, multicenter, randomized phase III trial conducted in Japan in patients who previously untreated mCRC. This study was conducted in accordance with the ethical principles of the Declaration of Helsinki and complied with the Japanese ethical guidelines for clinical studies. The study was approved by the institutional review board of each participating institution.
Participants
The main inclusion criteria were as follows: histologically confirmed colorectal adenocarcinoma; unresectable mCRC; age 20 years or older; ECOG performance status of 0 or 1; no previous chemotherapy or radiotherapy; adequate oral intake; and adequate organ function. The main exclusion criteria were as follows: sensory neuropathy; serious diarrhea; gastrointestinal obstruction; symptomatic peritoneal metastasis; and a history of gastrointestinal perforation within the 6 months before enrollment. All patients provided written informed consent before enrollment. The details of the criteria have been reported previously [10].
Randomization and masking
Participants were randomly assigned (1 : 1) to receive either mFOLFOX6 or CapeOX plus bevacizumab (control group) or to receive either a 3-week or a 4-week regimen of S-1 and irinotecan plus bevacizumab (experimental group). Each participating institution could select either mFOLFOX6 plus bevacizumab or CapeOX plus bevacizumab and either a 3-week or 4-week regimen of S-1 and irinotecan plus bevacizumab. After reporting to the data center (AC Medical Inc., Tokyo, Japan), patient enrollment was initiated (supplementary Figure S1, available at Annals of Oncology online). Randomization was performed centrally using the minimization method with the following stratification factors: institution; adjuvant chemotherapy (none, including oxaliplatin, or not including oxaliplatin); and the number of metastatic organs (0 or 1 versus ≥2).
Procedures
The mFOLFOX6 plus bevacizumab regimen consisted of bevacizumab (5 mg/kg) given as an intravenous infusion on day 1 of each 2-week cycle, followed by a simultaneous intravenous infusion of oxaliplatin (85 mg/m2) plus l-leucovorin (200 mg/m2), an intravenous bolus 5-FU (400 mg/m2), and a continuous intravenous infusion of 5-FU (2400 mg/m2). The CapeOX plus bevacizumab regimen consisted of bevacizumab (7.5 mg/kg) given as an intravenous infusion on day 1 of each 3-week cycle, followed by an intravenous infusion of oxaliplatin (130 mg/m2). Capecitabine (1000 mg/m2) was taken orally twice daily, from after dinner on day 1 to after breakfast on day 15, followed by a 7-day rest. The 3-week S-1 and irinotecan plus bevacizumab regimen consisted of bevacizumab (7.5 mg/kg) given as an intravenous infusion on day 1 of each 3-week cycle, followed by an intravenous infusion of irinotecan (150 mg/m2). S-1 (40 mg/m2) was taken orally twice daily, from after dinner on day 1 to after breakfast on day 15, followed by a 7-day rest. The 4-week S-1 and irinotecan plus bevacizumab regimen consisted of bevacizumab (5 mg/kg) given as an intravenous infusion on day 1 and day 15 of each 4-week cycle, followed by an intravenous infusion of irinotecan (100 mg/m2). S-1 (40 mg/m2) was taken orally twice daily, from after dinner on day 1 to after breakfast on day 15, followed by a 14-day rest. Cycles were repeated for each patient until criteria for withdrawal of the study treatment were met. For the mFOLFOX6 plus bevacizumab and CapeOX plus bevacizumab regimens, oxaliplatin-induced sensory neuropathy was taken into consideration, and treatment could be skipped if patients had received at least 600 mg/m2 of oxaliplatin overall. The details of dose modifications have been reported previously [10].
Tumor assessment by means of diagnostic imaging was carried out every 8 weeks, and tumor responses were assessed according to RECIST version 1.1. Observed adverse events were evaluated according to CTCAE v4.0. QOL was assessed according to FACT-C TOI scale and FACT/GOG-Ntx scale before the start of treatment and at 16 and 24 weeks.
Outcomes
The primary end point was PFS, defined as the period from the date of enrollment to the date of disease progression or of death from any cause without progression, whichever came first. Secondary end points were overall survival, time to treatment failure (TTF), response rate, adverse events, QOL, quality-adjusted life years (QALYs), cost-effectiveness, and biomarker analysis.
Statistical analysis
On the basis of the results of previous studies, the median PFS was estimated to be 11 months for the control group and 12 months for the experimental group [hazard ratio (HR), 0.917]. Given that the permissible limit for the HR was 1.25, with a statistical power of 85%, an alpha level of 0.025 (one-sided), an enrollment period of 36 months, and a follow-up period of 18 months for the primary end point of PFS, we estimated that 434 patients would be required (required number of events, 374). To compensate for ineligible patients, the target number of patients was set at 450. Thus, noninferiority would be established if the upper limit of the 95% confidence interval (CI) for the HR of the control group versus the experimental group was <1.25. If noninferiority was demonstrated in the study, superiority would be tested. The primary analysis was conducted using the full analysis set on an intention-to-treat basis.
We estimated time-dependent events using the Kaplan–Meier method. We calculated HRs and their CIs with Cox proportional-hazards models and adjusted for stratification factors (excluding institutions) and treatment groups as covariates. Patients who received at least one dose of the assigned study drugs were included in the analyses of dose intensity and safety. QOL analysis was conducted using data from patients in the safety analysis population for whom the pretreatment QOL could be evaluated.
All statistical analyses were carried out using SAS, version 9.4 (SAS Institute, Cary, NC). This trial is registered with UMIN-CTR (http://www.umin.ac.jp/ctr/) (000007834).
Results
From 1 June 2012 through 16 September 2014, 487 patients from 53 institutions were randomly assigned, 244 patients to the control group and 243 patients to the experimental group (supplementary Figure S2, available at Annals of Oncology online). Two patients who were confirmed to have no colorectal adenocarcinoma after randomization and one patient who withdrew consent were excluded from the primary analysis. The cut-off date for primary analysis of the primary end point was 30 April 2016. Demographic characteristics were similar in both groups (Table 1).
Table 1.
Baseline characteristics
| mFOLFOX6 or CapeOX plus bevacizumab | S-1 and irinotecan plus bevacizumab | |||
|---|---|---|---|---|
| (n = 243) |
(n = 241) |
|||
| n | (%) | n | (%) | |
| Sex | ||||
| Male | 143 | (58.8) | 151 | (62.7) |
| Female | 100 | (41.2) | 90 | (37.3) |
| PS (ECOG) | ||||
| 0 | 205 | (84.4) | 204 | (84.6) |
| 1 | 38 | (15.6) | 37 | (15.4) |
| Age | ||||
| Median [range] | 65 [29–85] | 64 [22–87] | ||
| ≥65 | 134 | (55.1) | 118 | (49.0) |
| CCr at enrollment | ||||
| Median [range] | 80.9 [60.0–153.1] | 82.7 [60.0–182.8] | ||
| ≥70 | 181 | (74.5) | 185 | (76.8) |
| Complications | ||||
| Yes | 107 | (44.0) | 108 | (44.8) |
| No | 136 | (56.0) | 133 | (52.2) |
| Adjuvant chemotherapy for colorectal cancer | ||||
| Yes | 31 | (12.8) | 32 | (13.3) |
| No | 212 | (87.2) | 209 | (86.7) |
| Differentiation assessed by histology | ||||
| Well or moderate | 212 | (87.2) | 209 | (86.7) |
| Poorly | 14 | (5.8) | 14 | (5.8) |
| Other | 17 | (7.0) | 18 | (7.5) |
| Primary lesion | ||||
| Colon | 122 | (50.2) | 130 | (53.9) |
| Rectosigmoid | 39 | (16.0) | 32 | (13.3) |
| Rectum | 82 | (33.7) | 79 | (32.8) |
| Primary lesion resection | ||||
| Yes | 164 | (67.5) | 156 | (64.7) |
| No | 79 | (32.5) | 85 | (35.3) |
| Metastatic organs | ||||
| 0–1 | 124 | (51.0) | 127 | (52.7) |
| ≥2 | 119 | (49.0) | 114 | (47.3) |
| Target lesion | ||||
| Yes | 221 | (90.9) | 214 | (88.8) |
| No | 22 | (9.1) | 27 | (11.2) |
| RAS status | ||||
| Wild type | 99 | (40.7) | 105 | (43.6) |
| Mutant type | 65 | (26.7) | 58 | (24.1) |
| Not definable | 6 | (2.5) | 3 | (1.2) |
| Missing data | 73 | (30.0) | 75 | (31.1) |
CCr, creatinine clearance.
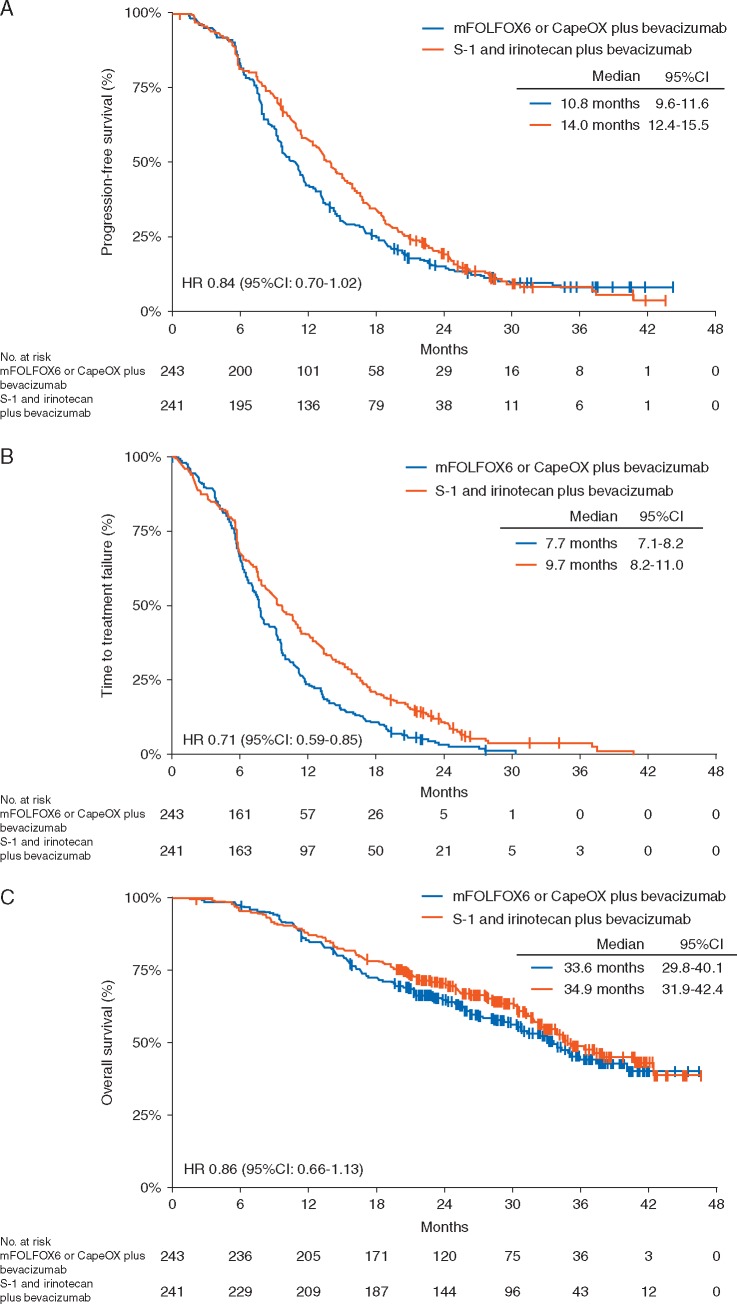
The median follow-up period was 32.4 months (range 1.5–46.6). During this period, PFS events occurred in 426 (88%) of 484 patients. While the median PFS was 10.8 months (95% CI 9.6–11.6) in the control group, it was 14.0 months (95% CI 12.4–15.5) in the experimental group (HR 0.84, 95% CI 0.70–1.02). The upper limit of the HR for PFS was lower than the prespecified noninferiority margin of 1.25 (P < 0.0001 for noninferiority, P = 0.0815 for superiority; Figure 1A). The details of PFS for each chemotherapy regimen are given in supplementary Figure S3, available at Annals of Oncology online. In the subgroup analysis of PFS, significant interactions were observed between the allocated groups and age (supplementary Figure S4, available at Annals of Oncology online). Median TTF in the control group and the experimental group was 7.7 months (95% CI 7.1–8.2) and 9.6 months (95% CI 8.2–11.0), respectively (HR 0.71, 95% CI 0.59–0.85, P = 0.0002; Figure 1B). Treatment status in each group is presented in supplementary Table S1, available at Annals of Oncology online. The response rate of target lesions was 70.6% in the control group and 66.4% in the experimental group (P = 0.34; supplementary Table S2, available at Annals of Oncology online). The curative resection rate was 8.6% in the control group and 12.4% in the experimental group (P = 0.17). Overall survival analysis was conducted on the basis of 218 deaths (45%) among 484 patients. The median survival time in the control group and the experimental group was 33.6 months (95% CI 29.8–40.1) and 34.9 months (95% CI 31.9–42.4), respectively (HR 0.86, 95% CI 0.66–1.13, P = 0.2841; Figure 1C).
Figure 1.
Kaplan–Meier curves for (A) progression-free survival, (B) time to treatment failure, and (C) overall survival.
Adverse events are summarized in Table 2. The incidences of grade 3 or higher leukopenia, neutropenia, febrile neutropenia, thromboembolism, and diarrhea were significantly higher in the experimental group than in the control group. In post hoc analyses, the incidences of grade 3 or higher diarrhea in patients with a creatinine clearance (CCr) of 70 ml/min or higher and patients with a CCr of <70 ml/min at enrollment were, respectively, 6.7% and 6.5% in the control group as compared with 11.5% and 19.6% in the experimental group. The incidences of grade 3 or higher sensory neuropathy, hand–foot syndrome, and paralytic ileus were significantly higher in patients receiving the control treatment than in those receiving the experimental treatment. Further information on the types of adverse events occurring in each treatment regimen is given in supplementary Table S3, available at Annals of Oncology online. There was one treatment-related death among patients given the CapeOX regimen and four treatment-related deaths among patients given the S-1 and irinotecan plus bevacizumab regimen.
Table 2.
Adverse events
| mFOLFOX6 or CapeOX plus bevacizumab | S-1 and irinotecan plus bevacizumab | P valuea | |||||||
|---|---|---|---|---|---|---|---|---|---|
| (n = 242) |
(n = 239) |
||||||||
| Any |
≥Grade 3 |
Any |
≥Grade 3 |
||||||
| n | (%) | n | (%) | n | (%) | n | (%) | ||
| Patients with at least 1 AE | 242 | (100.0) | 157 | (64.9) | 236 | (98.7) | 140 | (58.6) | 0.16 |
| Laboratory findings | |||||||||
| Leukopenia | 154 | (63.6) | 6 | (2.5) | 157 | (65.7) | 21 | (8.8) | <0.01 |
| Neutropenia | 139 | (57.4) | 33 | (13.6) | 150 | (62.8) | 58 | (24.3) | <0.01 |
| Thrombocytopenia | 151 | (62.4) | 4 | (1.7) | 74 | (31.0) | 2 | (0.8) | 0.69 |
| Anemia | 92 | (38.0) | 5 | (2.1) | 121 | (50.6) | 12 | (5.0) | 0.09 |
| Bilirubin | 80 | (33.1) | 6 | (2.5) | 104 | (43.5) | 8 | (3.3) | 0.60 |
| AST | 119 | (49.2) | 8 | (3.3) | 80 | (33.5) | 5 | (2.1) | 0.58 |
| ALT | 82 | (33.9) | 6 | (2.5) | 84 | (35.1) | 5 | (2.1) | 1.00 |
| Creatinine | 30 | (12.4) | 2 | (0.8) | 30 | (12.6) | 2 | (0.8) | 1.00 |
| Proteinuria | 107 | (44.2) | 7 | (2.9) | 103 | (43.1) | 6 | (2.5) | 1.00 |
| Clinical findings | |||||||||
| Mucositis/stomatitis | 104 | (43.0) | 4 | (1.7) | 128 | (53.6) | 7 | (2.9) | 0.38 |
| Anorexia | 149 | (61.6) | 16 | (6.6) | 143 | (59.8) | 16 | (6.7) | 1.00 |
| Nausea | 119 | (49.2) | 9 | (3.7) | 136 | (56.9) | 8 | (3.3) | 1.00 |
| Vomiting | 37 | (15.3) | 4 | (1.7) | 59 | (24.7) | 5 | (2.1) | 0.75 |
| Diarrhea | 109 | (45.0) | 16 | (6.6) | 149 | (62.3) | 32 | (13.4) | 0.02 |
| Rash/desquamation | 39 | (16.1) | 1 | (0.4) | 50 | (20.9) | 0 | (0.0) | 1.00 |
| Hyperpigmentation | 99 | (40.9) | – | – | 100 | (41.8) | – | – | – |
| Hand–foot syndrome | 125 | (51.7) | 15 | (6.2) | 59 | (24.7) | 2 | (0.8) | <0.01 |
| Fatigue | 149 | (61.6) | 12 | (5.0) | 142 | (59.4) | 9 | (3.8) | 0.66 |
| Peripheral sensory neuropathy | 223 | (92.1) | 53 | (21.9) | 47 | (19.7) | 0 | (0.0) | <0.01 |
| Alopecia | 30 | (12.4) | – | – | 143 | (59.8) | – | – | – |
| Watery eye | 2 | (0.8) | 0 | (0.0) | 18 | (7.5) | 3 | (1.3) | 0.12 |
| Hypertension | 86 | (35.5) | 29 | (12.0) | 76 | (31.8) | 20 | (8.4) | 0.23 |
| Paralytic ileus | 8 | (3.3) | 7 | (2.9) | 2 | (0.8) | 0 | (0.0) | 0.02 |
| Febrile neutropenia | 0 | (0.0) | 0 | (0.0) | 8 | (3.3) | 8 | (3.3) | <0.01 |
| Thromboembolism | 5 | (2.1) | 2 | (0.8) | 10 | (4.2) | 9 | (3.8) | 0.04 |
| Hemorrhage, nose | 28 | (11.6) | 0 | (0.0) | 40 | (16.7) | 0 | (0.0) | – |
| Gastrointestinal perforation | 3 | (1.2) | 3 | (1.2) | 0 | (0.0) | 0 | (0.0) | 0.25 |
Comparison of the frequency of adverse events of grade 3 or higher in the two groups.
AE, adverse events; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
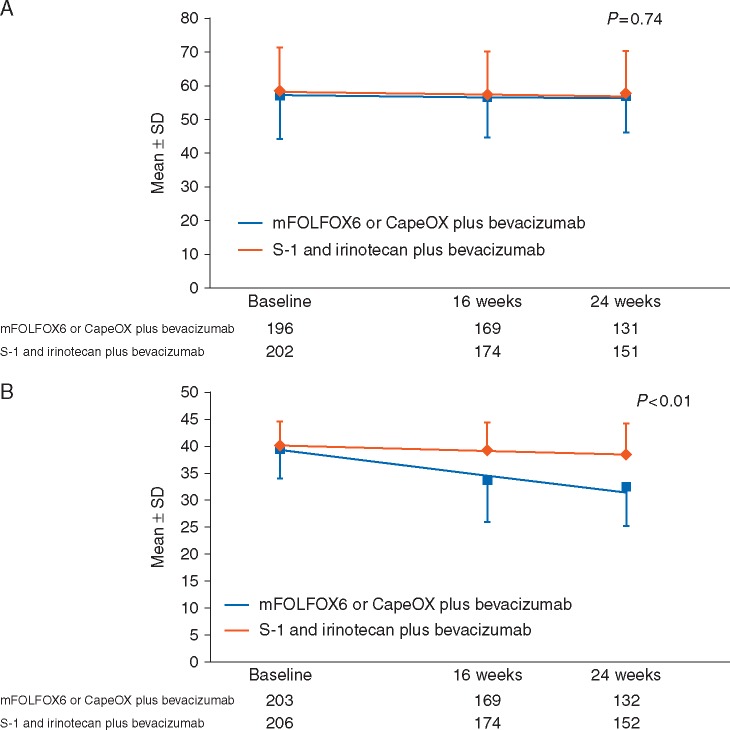
Before treatment, 436 (90.6%) patients completed the QOL questionnaire. There was no statistically significant difference in the FACT-C TOI score trends over time between the control group and the experimental group (P = 0.74; Figure 2A). However, the FACT/GOG-Ntx scores showed a significantly more favorable trend over time in the experimental group (P < 0.01; Figure 2B).
Figure 2.
Quality of life assessed by (A) FACT-C TOI and (B) FACT/GOG-Ntx. The line on the graph is a straight line drawn from average values using a mixed-effects model. FACT-C TOI, the Functional Assessment of Cancer Therapy-Colorectal Trial Outcome Index scale; FACT/GOG-Ntx, the neurotoxicity subscale of the FACT/Gynecology Oncology Group-Neurotoxicity.
The number of patients in whom the study treatment was discontinued by the data cut-off date was 235 in the control group and 226 in the experimental group. Among the patients whose study treatment was discontinued, second-line treatment was given to 206 patients (87.7%) in the control group and 198 patients (87.6%) in the experimental group. Oxaliplatin, irinotecan, bevacizumab, and EGFR antibodies were, respectively, administered to 12 (5.8%), 125 (60.7%), 111 (53.9%), and 26 (12.6%) patients in the control group and 112 (56.6%), 22 (11.1%), 106 (53.5%), and 20 (10.1%) patients in the experimental group. In addition, 106 (53.5%) patients in the experimental group were given an oral fluoropyrimidine.
A more detailed analysis of QOL will be reported separately. QALY, cost-effectiveness, and biomarker analysis will be reported after the conclusion of follow-up in September 2017.
Discussion
To the best of our knowledge, the TRICOLORE trial is the first randomized phase III study to demonstrate the effectiveness of combination therapy with an oral fluoropyrimidine and irinotecan as first-line treatment of mCRC. In this study, we established the noninferiority of S-1 and irinotecan plus bevacizumab in terms of PFS to the standard regimen of mFOLFOX6 or CapeOX plus bevacizumab as first-line treatment of mCRC. Although superiority could not be proven, the median PFS was 3.2 months longer in the experimental group than in the control group (HR 0.84, 95% CI 0.69–1.01), and the QOL results were favorable. In addition, S-1 and irinotecan plus bevacizumab prolonged median TTF by 1.9 months (HR 0.71, 95% CI 0.59–0.85), with statistical significance.
The incidences of adverse events associated with each regimen were similar to the results of previous studies [2, 8, 9]. The incidence of grade 3 or higher diarrhea (13.4%) in the S-1 and irinotecan plus bevacizumab group was similar to that previously reported for first-line treatment with FOLFIRI plus bevacizumab (approximately 10%–14%), which was considered to demonstrate its tolerability [11, 12]. In patients with reduced renal function, the clearance of gimeracil in S-1 is decreased, causing an increase in the blood concentration of FU as well as an increased incidence of related adverse reactions. In our study, in patients given S-1 and irinotecan plus bevacizumab whose CCr was <70 ml/min at enrollment, the incidence of grade 3 or higher diarrhea was 19.6%, which was higher than the incidence in patients whose CCr was 70 ml/min or higher (11.5%). UGT1A1 gene polymorphism, one of the risk factors for irinotecan-induced serious adverse reactions, was not assessed in our study; however, this factor may have affected the incidences of diarrhea and neutropenia [13]. We believe that the safety of S-1 and irinotecan plus bevacizumab can be further enhanced by modifying the initial S-1 dosage depending on renal function, modifying the initial irinotecan dosage depending on UGT1A1 gene polymorphism, providing patient education, and appropriately managing adverse events.
Peripheral neuropathy, an adverse event that interferes with daily life, occurred in 92.1% of the patients in the control group. QOL analysis has shown that peripheral neuropathy not only impairs QOL and interferes with daily life, but also renders the continuation of oxaliplatin administration impracticable and affects efficacy. Similar to previous studies using oxaliplatin as first-line treatment of mCRC, the administration period of oxaliplatin in our study was 24 weeks; however, it was possible to continue the administration of irinotecan for up to approximately 40 weeks or up to discontinuation of the study treatment, and we believe that this contributed to the prolongation of TTF and PFS in the experimental group [1].
The median PFS obtained with mFOLFOX6 or CapeOX plus bevacizumab in our study was similar to the median PFS of approximately 10–11 months obtained in a previous study of fluoropyrimidine and oxaliplatin plus bevacizumab [1, 2]; however, the reported median PFS in that study of fluoropyrimidine and irinotecan plus bevacizumab was 1.5 months longer than the median PFS obtained with fluoropyrimidine and oxaliplatin plus bevacizumab (HR approximately 0.9); moreover, in our study the median PFS obtained with S-1 and irinotecan plus bevacizumab was longer by 3.2 months (HR = 0.84) [14]. These results suggest that the combination regimen of S-1 and irinotecan plus bevacizumab can be an effective first-line treatment of mCRC.
PFS was found to have an interaction with age in our study. Although an interaction was not demonstrated, patients given S-1 and irinotecan plus bevacizumab who had a CCr of 70 ml/min or higher at enrollment tended to have favorable results. It is therefore possible that elderly patients with decreased renal function did not receive sufficient S-1 because of adverse events. On the other hand, non-elderly patients might benefit from aggressive S-1 and irinotecan plus bevacizumab regimens.
About 53.5% of patients who initially receive S-1 (an oral fluoropyrimidine) and irinotecan plus bevacizumab regimens are also given oral fluoropyrimidines as second-line chemotherapy; first-line and second-line treatments for mCRC given orally and without the use of central venous ports are considered very beneficial for both patients and medical practitioners.
Our study had several limitations. The benefit of using S-1 and irinotecan plus bevacizumab as compared with FOLFIRI plus bevacizumab as first-line therapy for mCRC was not confirmed, because we conducted a comparative phase III study in which the most commonly used regimen, namely mFOLFOX6 or CapeOX plus bevacizumab, was given to the control group. However, the noninferiority of S-1 and irinotecan to FOLFIRI as second-line therapy has already been demonstrated [7]. Furthermore, the median PFS (14.0 months) obtained with S-1 and irinotecan plus bevacizumab in our study is longer than in that in any previous randomized controlled trial and compares favorably not only with the results obtained in the control arm of FOLFIRI plus bevacizumab in TRIBE (9.7 months), but also with the results obtained in the trial arm of FOLFOXIRI plus bevacizumab (12.1 months). Another potential limitation is that we do not know whether our results can be simply extrapolated to a Western population, because the pharmacokinetics and pharmacodynamics of S-1 might vary, and the approved dose of irinotecan (150 mg/m2, every 2 weeks) in Japan is lower than that in Western countries. If S-1 combined with irinotecan plus bevacizumab is used as chemotherapy in Western patients, the dose should be carefully adjusted.
Conclusion
In conclusion, we consider S-1 and irinotecan plus bevacizumab to be an effective first-line therapy for mCRC and believe that it can be included as one of the recommended standard regimens.
Supplementary Material
Acknowledgements
We thank all the patients, their families, the investigators, and the medical staff; we thank Yuh Sakata, Hiroyuki Uetake, and Wasaburo Koizumi for the data and safety monitoring; we also thank Rei Goto as the advisor on medical economics. A list of participating institutions is given in the online supplementary Appendix S1, available at Annals of Oncology online.
Funding
Tokyo Cooperative Oncology Group with funding from Taiho Pharmaceutical Co. Ltd., Japan (no grant number applies) under the study contract.
Disclosure
YY has received honoraria from Taiho, Chugai, and Yakult. MG has received honoraria from Taiho, Chugai, Yakult, Ono, Shionogi, Nippon Kayaku, and Eli Lilly. HS has received honoraria from Taiho, Eisai, Bayer, Chugai, Eli Lilly, and Yakult and research grants from Taiho, Eisai, and Bayer. AS has received honoraria from Taiho, Chugai, and Yakult and research grants from Taiho and Chugai. SM has received honoraria from Taiho, Chugai, and Daichi-Sankyo. ST has received honoraria from Merck Serono, Taiho, Asahi Kasei, Daichi-Sankyo, Medicon, Novartis, and Mochida and research grants from Merck Serono. YK has received honoraria from Taiho, Eli Lilly, Chugai, Novartis, Bayer, Merck Serono, Pfizer, Yakult, and Daichi-Sankyo and research grants from Taiho, Eli Lilly, MSD, Ono, Chugai, Novartis, Bayer, Yakult, and Daichi-Sankyo. CI has received honoraria from Taiho, Merck Serono, Mochida, Chugai, Daichi-Sankyo, Ono, Takeda, Novartis, Nippon Kayaku, Bayer, and Eli Lilly and research grants from Taiho, Merck Serono, Mochida, Chugai, Daichi-Sankyo, Yakult, Ono, Takeda, Kyowa Hakko Kirin, Eisai, Tsumura, Novartis, Nippon Kayaku, Astellas, Asahi Kasei, Kissei, and Bristol-Myers. All remaining authors have declared no conflicts of interest.
References
- 1. Yamazaki K, Nagase M, Tamagawa H. et al. Randomized phase III study of bevacizumab plus FOLFIRI and bevacizumab plus mFOLFOX6 as first-line treatment for patients with metastatic colorectal cancer (WJOG4407G). Ann Oncol 2016; 27(8): 1539–1546. [DOI] [PubMed] [Google Scholar]
- 2. Cassidy J, Clarke S, Diaz-Rubio E. et al. Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. J Clin Oncol 2008; 26(12): 2006–2012. [DOI] [PubMed] [Google Scholar]
- 3. André T, Boni C, Navarro M. et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 2009; 27(19): 3109–3116. [DOI] [PubMed] [Google Scholar]
- 4. Fuchs CS, Marshall J, Mitchell E. et al. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: results from the BICC-C Study. J Clin Oncol 2007; 25(30): 4779–4786. [DOI] [PubMed] [Google Scholar]
- 5. Satoh T, Sakata Y.. S-1 for the treatment of gastrointestinal cancer. Expert Opin Pharmacother 2012; 13(13): 1943–1959. [DOI] [PubMed] [Google Scholar]
- 6. Muro K, Boku N, Shimada Y. et al. Irinotecan plus S-1 (IRIS) versus fluorouracil and folinic acid plus irinotecan (FOLFIRI) as second-line chemotherapy for metastatic colorectal cancer: a randomised phase 2/3 non-inferiority study (FIRIS study). Lancet Oncol 2010; 11(9): 853–860. [DOI] [PubMed] [Google Scholar]
- 7. Schmoll HJ, Van Cutsem E, Stein A. et al. ESMO Consensus Guidelines for management of patients with colon and rectal cancer: a personalized approach to clinical decision making. Ann Oncol 2012; 23(10): 2479–2516. [DOI] [PubMed] [Google Scholar]
- 8. Yamada Y, Yamaguchi T, Matsumoto H. et al. Phase II study of oral S-1 with irinotecan and bevacizumab (SIRB) as first-line therapy for patients with metastatic colorectal cancer. Invest New Drugs 2012; 30(4): 1690–1696. [DOI] [PubMed] [Google Scholar]
- 9. Komatsu Y, Yuki S, Sogabe S. et al. Phase II study of combined chemotherapy with irinotecan and S-1 (IRIS) plus bevacizumab in patients with inoperable recurrent or advanced colorectal cancer. Acta Oncol 2012; 51(7): 867–872. [DOI] [PubMed] [Google Scholar]
- 10. Komatsu Y, Ishioka C, Shimada K. et al. Study protocol of the TRICOLORE trial: a randomized phase III study of oxaliplatin-based chemotherapy versus combination chemotherapy with S-1, irinotecan, and bevacizumab as first-line therapy for metastatic colorectal cancer. BMC Cancer 2015; 15(1): 626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Heinemann V, von Weikersthal LF, Decker T. et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol 2014; 15(10): 1065–1075. [DOI] [PubMed] [Google Scholar]
- 12. Loupakis F, Cremolini C, Masi G. et al. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med 2014; 371(17): 1609–1618. [DOI] [PubMed] [Google Scholar]
- 13. Minami H, Sai K, Saeki M. et al. Irinotecan pharmacokinetics/pharmacodynamics and UGT1A genetic polymorphisms in Japanese: roles of UGT1A1*6 and *28. Pharmacogenet Genomics 2007; 17(7): 497–504. [DOI] [PubMed] [Google Scholar]
- 14. Schmiegel W, Reinacher-Schick A, Arnold D. et al. Capecitabine/irinotecan or capecitabine/oxaliplatin in combination with bevacizumab is effective and safe as first-line therapy for metastatic colorectal cancer: a randomized phase II study of the AIO colorectal study group. Ann Oncol 2013; 24(6): 1580–1587. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.