Abstract
Agricultural organic dust exposures trigger harmful airway inflammation, and workers experiencing repetitive dust exposures are at increased risk for lung disease. Mesenchymal stem/stromal cells (MSCs) regulate wound repair processes in the lung, and may contribute to either proresolution or -fibrotic lung responses. It is unknown how organic dust exposures alter lung-resident MSC activation and proinflammatory versus prorepair programs in the lung. To address this gap in knowledge, we isolated human lung-resident MSC from lung tissue. Cells were stimulated with aqueous extracts of organic dusts (DE) derived from swine confinement facilities and were assessed for changes in proliferative and migratory capacities, and production of proinflammatory and prorepair mediators. Through these investigations, we found that DE induces significant release of proinflammatory mediators TNF-α, IL-6, IL-8, and matrix metalloproteases, while also inducing the production of prorepair mediators amphiregulin, FGF-10, and resolvin D1. In addition, DE significantly reduced the growth and migratory capacities of lung-resident MSC. Together, these investigations indicate lung-resident MSC activation and wound repair activities are altered by organic dust exposures. These findings warrant future investigations to assess how organic dusts affect lung-resident mesenchymal stem/stromal cell function and impact airway inflammation, injury, and repair during agricultural aerosol exposures.
Keywords: organic dust, agriculture, mesenchymal stem cell, lung inflammation
Workers in agricultural industries are exposed to a wide variety of organic dusts. Inhalation of these dusts leads to airway inflammation, and history of long-term exposure increases the risk for numerous airway inflammatory diseases, including bronchitis, nonatopic asthma, and pulmonary fibrosis (American Thoracic Society, 1998; Kirkhorn and Garry, 2000; Langley, 2011; Von Essen and Romberger, 2003). Few therapeutic options exist to alleviate symptoms or prevent lung function decline due to these illnesses (Mitchell and Schenker, 2008; Szczyrek et al., 2011). By understanding the cellular response of the lung milieu to these irritant exposures, new treatments can be devised to improve the endogenous capacity of the lung to respond to these inflammatory exposures with productive healing responses.
Mesenchymal stem/stromal cells (MSCs) are a progenitor cell population that can be found in many different tissues, including the lung (Ma et al., 2014; Pittenger et al., 1999; Sinclair et al., 2013). Examination of MSC in lung transplant patients confirms that the MSC population retains the donor’s gene signature years after transplant, indicating that this MSC population is not derived from circulating MSC that engraft in the lung, but are a long-term resident population (Badri et al., 2011a; Rolandsson et al., 2014). MSC play vital roles in immunomodulation and tissue repair processes, making them an attractive therapeutic target (Javazon et al., 2007; Khatri et al., 2015; Ma et al., 2014; Wu et al., 2007; Yoon et al., 2010). Although the potential of MSC to orchestrate wound-healing processes during lung inflammation and injury is recognized (Inamdar and Inamdar, 2013), no studies to date have assessed how exposures to organic dusts affect the activation or function of MSC.
To address this gap in knowledge, we investigated the effects of organic dust exposure on human lung-resident MSC growth and function. To do so, we isolated lung-resident MSC from human lung tissue. Cells were stimulated with extracts derived from swine confinement facility dusts (DE), and assayed for their proliferative and migration capacities, and proinflammatory and prorepair mediator production. Our observations indicate MSC are activated by DE exposure, and present an important cell type to consider when devising therapeutic schemes designed to improve airway inflammatory diseases associated with organic dust exposures.
MATERIALS AND METHODS
Isolation, and culture of primary human lung-resident MSCs
Lung-resident MSCs were isolated as previously published (Rolandsson et al., 2014). De-identified normal human lung tissue was obtained from the Nebraska Organ Retrieval Service and the National Disease Registry. We excluded donors with a >20 pack-year history of smoking, smoking in the last 10 years, lung disease (i.e. cystic fibrosis, chronic obstructive pulmonary disease, asthma, pulmonary fibrosis, acute respiratory distress syndrome), pulmonary infection (pneumonia, bronchitis, sepsis), use of oral or inhaled steroids, alcohol abuse (defined as >2 drinks/d for women, 3 for men) or lung cancer. Briefly, a 2 × 2 cm section of parenchymal lung tissue was digested in Protease XIV (Sigma, St Louis, Missouri) for 48 h at 4 °C. The resulting suspensions were then pipetted to loosen tissue clumps and filtered through a 70-μm strainer. Isolated cells were plated in T75 tissue culture-treated flasks in MesenPro RS medium (Thermo Fisher Scientific, Waltham, Massachusetts) containing penicillin/streptomycin and L-Glutamine (Thermo Fisher Scientific, Waltham, Massachusetts). Cell culture medium was changed 24 h after initial plating, and every 3 days thereafter. After 7–10 days, numerous colonies of adherent cells displaying a typical MSC phenotype were evident. Cultures were passaged prior to reaching complete confluence, and experiments were performed on cells below 10 passages.
Confirmation of lung-resident MSC phenotype
To ensure that this technique was effective in the isolation of MSC, we assessed the capacity of the lung-resident MSC to differentiate into osteocytes, chondrocytes, and adipocytes. To do so, we utilized the Gibco StemPro Chondrogenesis, Osteogenesis, and Adipogenesis Differentiation Kits (Thermo Fisher Scientific; Waltham, Massachusetts) and performed differentiation assays according to kit directions. For chondrogenesis differentiation, cell cultures were maintained in the differentiation medium for 14–17 days prior to staining with Alcian Blue dye solution (Sigma-Aldrich; St Louis, Missouri). In the osteogenesis assays, alkaline phosphatase activity in the cell cultures was assessed at 10–14 days of culturing in the differentiation medium using a Vector Blue Alkaline Phosphatase Substrate kit (Vector Laboratories; Burlingame, California). For assessing adipogenesis, cell cultures were stained with Oil Red O (Sigma-Aldrich; St Louis, Missouri) after 10–14 days in the differentiation medium.
To assess for the characteristic surface marker expression of lung-resident MSC, cells were incubated with Alexa Fluor 647 antihuman CD90 (clone 5E10; Biolegend; San Diego, California), Alexa Fluor 488 antihuman CD105 (clone 43A3; Biolegend), or Alexa Fluor 488 antihuman CD45 (clone H130; Biolegend) at 1:200 dilution for 30 min at 4 °C. Cells were washed and resuspended in 4% paraformaldehyde and kept in the dark at 4 °C prior to being analyzed with a BD LSRII multicolor laser flow cytometer by the University of Nebraska Medical Center Flow Cytometry Research Core Facility.
To assess the ability of the isolated MSC cultures to produce colonies from a single cell, we performed colony-forming unit-fibroblast (CFU-F) assays as previously described in (Rolandsson et al., 2014). Cells were resuspended in MesenPro RS growth medium or DMEM-low glucose (Thermo Fisher Scientific, Waltham, Massachusetts) with 10% fetal bovine serum (Thermo Fisher Scientific, Waltham, Massachusetts) at a final concentration of 200 cells/ml, and 1 ml of cell solution was added to each 100-mm tissue culture dish containing 12 ml medium. After 14 days, cultures were washed with PBS and stained using 0.5% crystal violet solution/methanol (BioQuest, Cockeysville, Maryland). The number of colonies on each dish was enumerated using a dissecting microscope. A minimum threshold of 40 cells per colony was required for a colony to be included in the counts.
Preparation of organic dust extract
The DE utilized in these investigations was prepared as previously described in Romberger et al. (2002). Briefly, organic dusts were collected from swine concentrated animal feeding operations, incubated in Hanks Buffered Saline Solution (Thermo Fisher Scientific, Waltham, Massachusetts), centrifuged at high speed to remove particulates, and sterile-filtered through a 0.22 μm pore filter. The resulting solution (100% DE) was stored at −20 C prior to being used in experiments, where it was diluted vol/vol to reach 0.1%–1% solutions in medium. We chose these doses based on previously published work from our group assessing DE-induced inflammatory responses in cell cultures, where 1%–5% DE are typically tolerated in various cell types (Bailey et al., 2008; Poole et al., 2011; 2012; Romberger et al., 2002). Notably, when we performed a dose response from 0% to 5% DE in the lung-resident MSC cultures, doses of 2.5%–5% DE were toxic to the lung-resident MSC. For this reason, we chose doses ranging from 0% to 1% DE, which resulted in minimal cytotoxicity. At these doses, cells continued to grow as confirmed by visual inspection. For most experiments (except for wound/migration assays where cells were grown to confluency), cells were treated at approximately 80% confluency and over 48 h (the longest length of time for experiments performed) would near or reach confluency. In each experiment, treated cells were compared with a medium control group consisting of cells grown in normal growth medium alone (MesenPro RS). For experiments utilizing protease-depleted DE, undiluted DE was incubated in 4 mM AEBSF protease inhibitor (Thermo Scientific, Waltham, Massachusetts) for 2 h at 37°C prior to use, as previously described in Romberger et al. (2015). For experiments utilizing lipopolysaccharide (LPS)-depleted DE, undiluted DE was run twice through a Bioworld endotoxin affisorbent (polymyxin B) separopore column (Dublin, Ohio). Flow-through DE was used for treatments. Two different lots of organic dusts collected from the same swine confinement facility at different times were utilized in these investigations. The active components of DE have been previously characterized for major plant, bacterial, and other active components as well as elemental analyses identifying trace metals (Boissy et al., 2014; Poole et al., 2010; Romberger et al., 2015). Studies of bacterial content of the dusts prior to preparation of the extract reveal primarily (>90%) gram positive bacteria, with <10% gram negative bacteria, and significant amounts of peptidoglycan, LPS, and ergosterol in the dust extracts.
Determination of mediator/cytokine production
Cell-free supernatant fractions of cell cultures were assayed for mediator levels using commercially available enzyme immunoassays. The TNF-α, IL-6, IL-8, and amphiregulin (AREG) levels were measured using R&D Systems DuoSet enzyme-linked immunosorbant assay development kits (ELISAs; Minneapolis, Minnesota). Resolvin D1 (RvD1) was measuring using an ELISA kit from Cayman Chemical (Ann Arbor, Michigan), and human FGF10 was measured using an ELISA kit from Antigenix (Huntington Station, New York). Assays were run according to manufacturer’s instructions.
Determination of supernatant matrix metalloprotease activities
Gelatin zymography was performed on supernatant fractions of cell cultures, as previously described in Lantz and Ciborowski (1994), Romberger et al. (2015), and Sweetman and Ornstein (1974). Briefly, 8% polyacrylamide gels were prepared containing 1% gelatin. Supernates from cells mixed in a nondenaturing sample buffer were loaded and run on the gels. Gels were then incubated in zymogram renaturing buffer for 30 min, followed by incubation for 30 min in zymogram developing buffer. The developing buffer was replaced, and gels were incubated overnight in fresh developing buffer. Gels were then stained with 0.5% Coomassie Brilliant Blue R-250 (Thermo Fisher Scientific, Waltham, Massachusetts) for 30 min, and were destained in a methanol-acetic acid solution prior to scanning for cleared areas, where matrix metalloproteases had digested the gelatin. ImageJ Software was utilized to determine fold changes in protease activities.
Determination of MSC proliferation and migratory capacities
To determine the proliferation/metabolic activity of the lung-resident MSC cultures, cells were plated at 20 000 cells/well in 96-well plates. After 2 days in culture, cells were stimulated with 0.1%–1% DE in fresh medium (100 μl/well). At 44 h following the addition of DE, 25 μl of a 5 mg/ml solution of MTT (Sigma-Aldrich; St. Louis, Missouri) was added to wells. At 48 h, the plate medium was decanted and 100 μl DMSO was added to each well. Absorbance at 590 nm was read using a VersaMax microplate reader (Molecular Devices; Sunnyvale, California).
To assess the migratory capacity of lung-resident MSC, cells were grown to confluence on 48-well tissue culture plates. Cultures were incubated in medium ± 1% DE for 18 h. Following DE pretreatment, the center of the confluent monolayer was scratched with a pipet tip to produce a circular clearing depleted of cells. Closure of this clearing was assessed at 24 h. Percent closure was determined by measuring the size of the denuded area immediately following scratching, and 24 h later using NIH ImageJ software. Only clearings initially <100 000 pixels in size were included in the analyses.
Statistical analyses
Data are graphed as means ± SEM. Results are obtained from at minimum, n = 3 experiments for each investigation. Statistical analyses were performed using Graphpad Prism Software employing1-way ANOVA with Tukey’s posthoc analyses for comparisons amongst groups. A resulting p value < .05 was considered significant.
RESULTS
Isolated Human Lung-Resident MSCs Exhibit Typical Cell Morphology and Differentiation Capacities
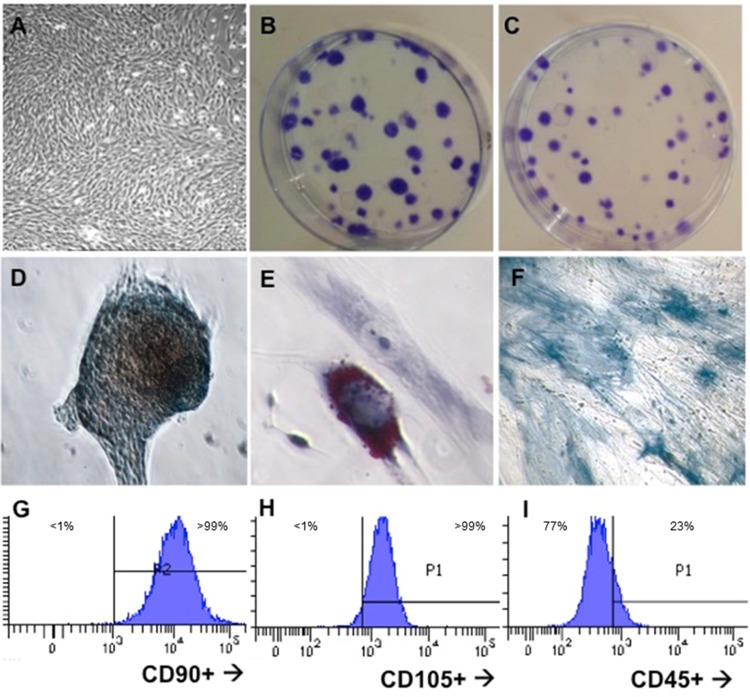
As set forth by the Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy, typical MSC have a fibroblast-like morphology, exhibit the capacity to form CFUs in the CFU-fibroblast assay, differentiate into numerous cell lineages including chondrocytes, adipocytes, and osteocytes, and are CD45−, CD90+, and CD105+ (Dominici et al., 2006). Following isolation and culture of MSC from human lung tissue, we assessed our MSC lines for these typical features. The isolated lung-resident MSC exhibited the expected fibroblastic appearance (Figure 1A) and formed colonies in the CFU-fibroblast assay when seeded at 200 cells per 100-mm dish and grown in either MesenPro RS medium (Figure 1B) or DMEM-F10 medium (Figure 1C). When cultured in differentiation media for 12–21 days, our lung-derived MSC also exhibited the capacity to differentiate into chondrocytes (Figure 1D), adipocytes (Figure 1E), and osteocytes (Figure 1F). As assayed by flow cytometry, the MSC populations exhibited over 99% positivity for CD90 and CD105, and were largely CD45− (Figs. 1G–I), although it is noted that using our isotype control-gating led to small portions of our cell population to gate as CD45low. These experiments demonstrate the efficacy of our lung-resident MSC isolation and propagation techniques, and corroborate the methodology utilized by Rolandsson et al. (2014) for the isolation of human lung-resident MSC.
Figure 1.
Isolated human lung-resident MSC exhibit markers typical MSC phenotype. A, Fibroblastic appearance in culture; B, CFUs in MesenPro RS medium; C, CFUs in DMEM + 10% serum; D, Chondrocyte differentiation stained with Alcian Blue; E, Adipocyte differentiation, stained with Oil Red O; F, Osteocytes differentiation, stained with alkaline phosphatase; G, expression of CD90; H, expression of CD105; I, lack of CD45 expression.
Organic Dust Extract Treatment Reduces Proliferation and Migratory Capacities of Lung-Resident MSC
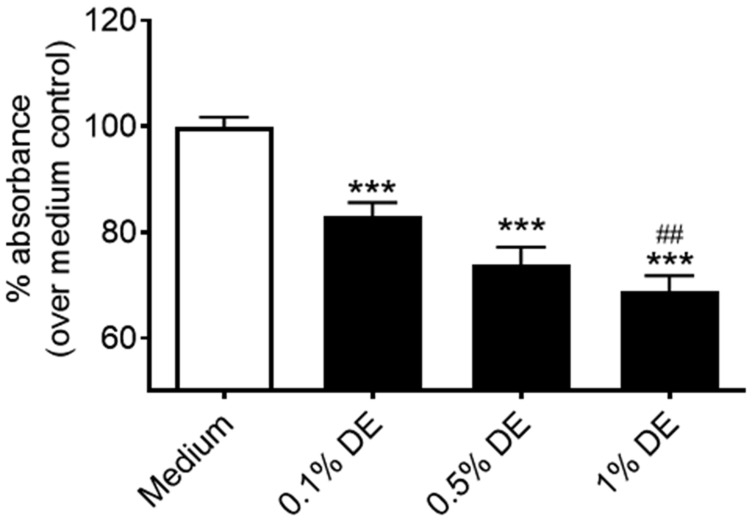
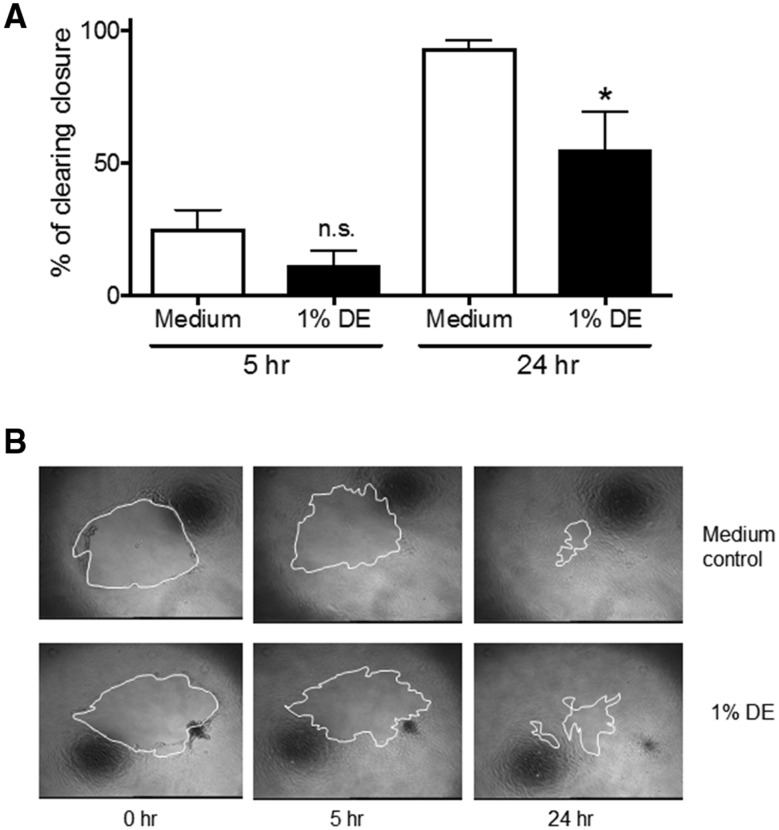
Previous investigations establish that lung-resident MSC mobilization is associated with protection from airway inflammatory/disease processes (Foronjy and Majka, 2012; Horie and Laffey, 2016; Lama et al., 2007; Ma et al., 2014). We assessed how DE exposure alters the proliferation and migratory capacities of human lung-resident MSC. When MSC were stimulated with DE, cell metabolic activity/proliferation as determined via MTT assay was significantly reduced compared with normal cell growth at 48 h (Figure 2). The growth of cells treated with 1% DE was also significantly reduced compared with the 0.1% DE-treated cells, suggesting a dose response. No cytotoxicity was observed at these doses; DE dosing at 2.5% and 5% were tested and found to be cytotoxic (data not shown). Furthermore, in our proliferation/migration scratch assay, lung-resident MSC pretreated with 1% DE for 18 h prior to being wounded demonstrated a significant decrease in their ability to close a circular decellularized clearing within 24 h when compared with control cultures (Figure 3).
Figure 2.
Effect of DE on lung-resident MSC proliferation/metabolic activity. Lung-resident MSC were treated for 48 h with 0%–1% DE. Proliferative capacity/metabolic activity was measured via MTT assay. ***p < .001 versus medium control. ##p < .01 versus 0.1% DE treatment.
Figure 3.
Effect of DE on lung-resident MSC migration. A clearing was made in confluent cultures of lung-resident MSC with a pipet tip. Migration/proliferation into the clearing was assessed at 5 and 24 h following wounding in the presence or absence of 1% DE given 18 h prior to wounding. A, Graphical summary of all experiments indicating the percent of clearing closure. B, Representative images of wounds with white outline demarcating wound boundaries. *p < .05 versus 24 h medium control.
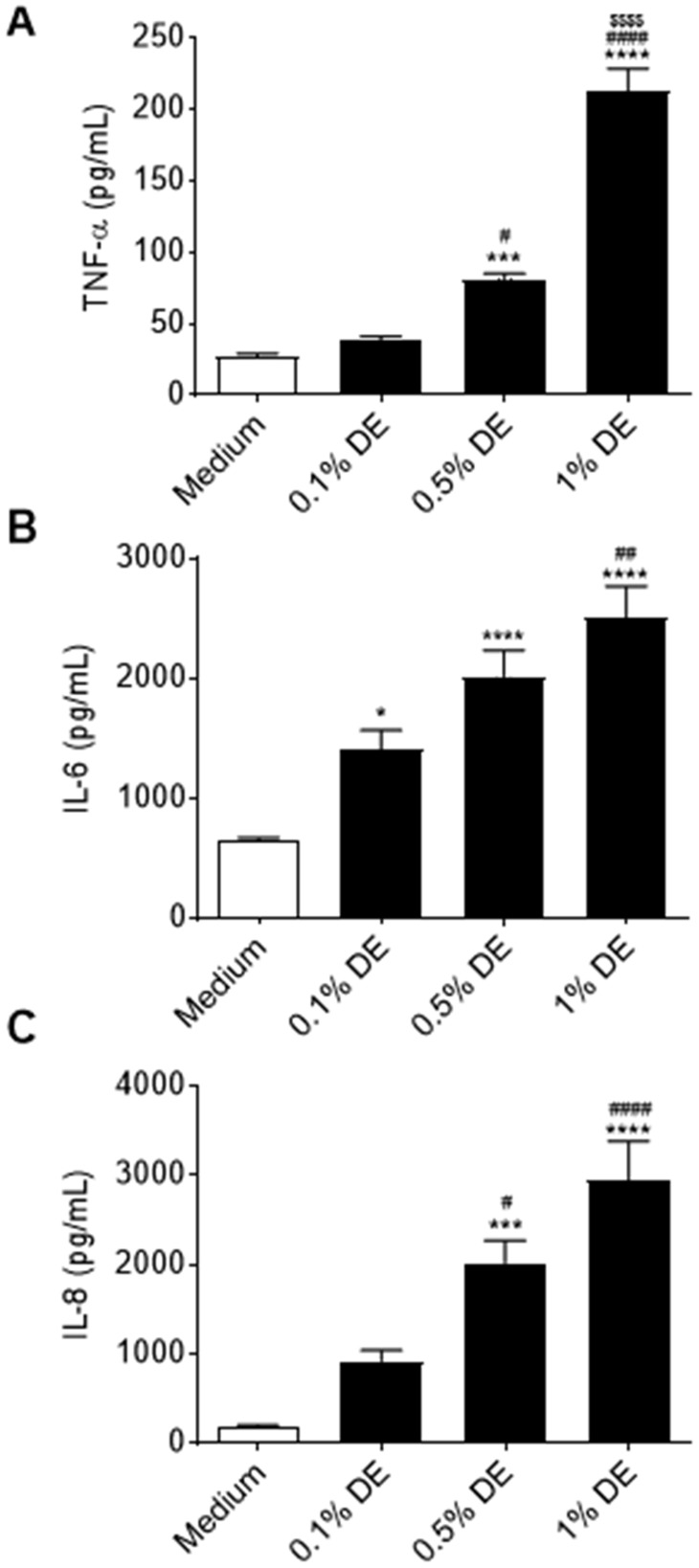
Organic Dust Extract Treatment Stimulates the Release of Inflammatory Mediators From Human Lung-Resident MSCs
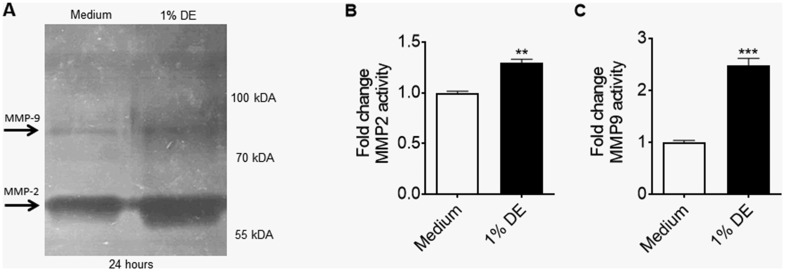
Numerous studies indicate that the beneficial actions of MSC within inflamed or injured tissue are a result of mediator production that influences the tissue response to inflammation and/or wounding (Hayes et al., 2015; Petrella et al., 2015; Schilders et al., 2016). MSC exposed to 1% DE for 24 exhibited a significant dose-dependent release of proinflammatory cytokines, including TNF-α, IL-6, and IL-8 (Figs. 4A–C). Gelatin proteolytic activities in the supernatant fractions of DE-stimulated MSC were also increased, as evidenced by gel zymography (Figure 5A); the most evident proteolytic band occurred at approximately 65 kDa, corresponding to the typical location of MMP-2, while bands at approximately 85 kDa, corresponding to the typical location of MMP-9, were also evident. At the 1% concentration, DE alone in the absence of cells does not exhibit this proteolytic banding (data not shown). Average fold change in proteolytic activities in the MMP-2 and MMP-9 zymogram bands at 24 h of DE treatment as determined by densitometry in lung-resident MSC are shown in Figures 5B and 5C, respectively.
Figure 4.
Effects of DE on proinflammatory mediator production by human lung-resident MSC. Lung-resident MSC were treated with 0%–1% DE. Supernates were assayed for IL-6 (A), and IL-8 (B), and TNF-α (C) release at 24 h. *p < .05, ***p < .001, ****p < .0001 versus medium control; ##p < .01, ####p < .0001 versus .1% DE treatment; $$$$ p < .0001 versus .5% DE treatment.
Figure 5.
Effect of DE on matrix metalloprotease activities in supernates of lung-resident MSC. Lung-resident MSC were treated with 0% or 1% DE for 24 h. Supernates were tested via gelatin zymography to assess for proteolytic activities. A, Inverse-coloration visualizing bands of gelatin clearance with ladder demarcating approximate molecular weights of responsible matrix metalloproteases. B, Fold-change of bands corresponding to the molecular weight of MMP2. C, Fold-change of bands corresponding to the molecular weight of MMP9. **p < .01, ***p < .001 versus medium control.
Organic Dust Extract Treatment Stimulates the Production of Prorepair Mediators From Human Lung-Resident MSCs
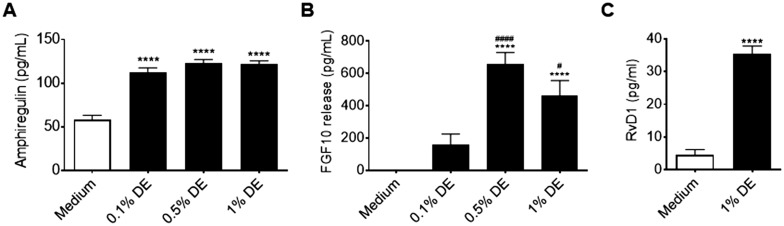
Lung-resident MSC have been shown to facilitate wound-healing processes via mesenchymal-epithelial signaling (Inamdar and Inamdar, 2013; Javazon et al., 2007; Ma et al., 2014; McQualter et al., 2013; Wu et al., 2007; Yoon et al., 2010). We thus sought to determine how DE treatment might alter lung-resident MSC prorepair signaling. In lung-resident MSC cultures treated for 24 h with DE, we identified increased release of ARGE and FGF10—proproliferative/repair mediators for epithelial cells (Figs. 6A and 6B) as well as increased production of the proresolution/prorepair lipid mediator, RvD1 (Figure 6C).
Figure 6.
Effects of DE on prorepair mediator production by human lung-resident MSC. Lung-resident MSC were treated with 0%–1% DE. Supernates were assayed for AREG (A), FGF-10 (B), and RvD1 (C) release at 24 h. ****p < .0001 versus medium control; #p < .05, ####p < .0001 versus 1% DE treatment.
Activation of Lung-Resident MSCs by Organic Dust Extract Is Mediated in Part by Proteases in the Organic Dust Extract
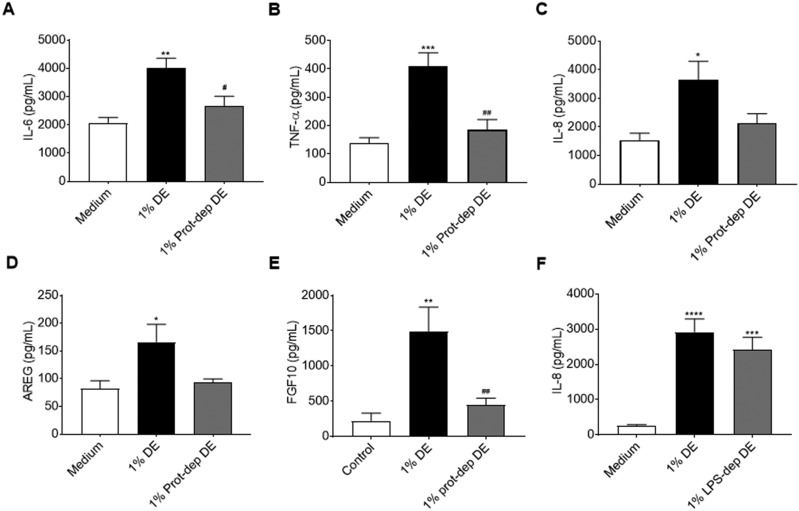
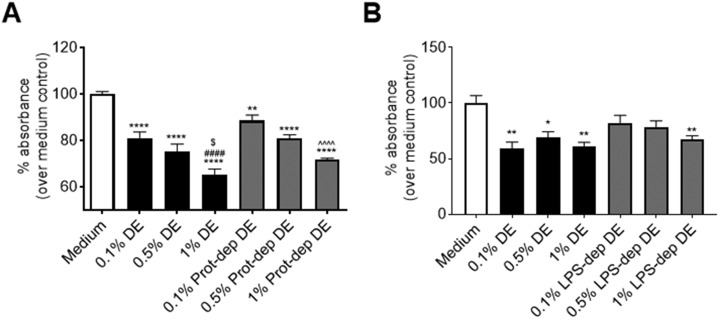
We have previously identified that active proteases in DE mediate the activation of proinflammatory responses in bronchial epithelial cells, and promote airway inflammation in vivo (Romberger et al., 2015). To assess the role of DE proteases in the activation of lung-resident MSC, we incubated DE with the AEBSF protease inhibitor prior to treating lung-resident MSC cell cultures. As shown in Figures 7A–E, Protease depletion of DE abrogated the DE-induced IL-6, TNF-α, IL-8, AREG, and FGF10 production by lung-resident MSC, suggesting an important role for proteases in promoting activation of the MSC. Interestingly, no significant change was seen when LPS was removed from DE, as evidenced by the lack of a significant difference in IL-8 production by MSC cultures treated with DE versus LPS-depleted DE (Figure 7F). In addition, when proliferative capacity/metabolic activity of lung-resident MSC was assessed, neither protease inhibition nor LPS depletion impacted DE-mediated reduced proliferative capacity (Figure 8). As shown in Figure 8, DE treatment, protease-depleted DE treatment, and LPS-depleted DE treatment all led to significant reductions in metabolic/proliferative activities in the lung-resident MSC. There were no significant differences found between the protease- or LPS-depleted DE at 0.1%, 0.5%, or 1% concentrations as compared with the corresponding DE concentrations.
Figure 7.
Effects of protease or LPS depletion on mediator production by human lung-resident MSC. Lung-resident MSC were treated for 24 h with 0 or 1% DE, or 1% DE that had been depleted of protease activity (Prot-dep DE) or LPS content (LPS-dep DE). Supernates from DE protease depletion experiments were assayed for IL-6 (A), TNF-α (B), IL-8 (C), AREG (D), and FGF-10 (E), while supernates from DE LPS depletion studies were assayed for IL-8 (F). *p < .05, **p < .01, ***p < .001, ****p < .0001 versus medium control; #p < .05, ##p < .01 versus 1% DE treatment.
Figure 8.
Effects of protease or LPS depletion on lung-resident MSC proliferation/metabolic activity. Lung-resident MSC were treated for 48 h with 0%–1% DE, or 0%–1% DE depleted of protease activity (Prot-dep DE) (A), or 0%–1% DE depleted of LPS (B). Proliferative capacity/metabolic activity was measured via MTT assay. *p < .05, **p < .01, ****p < .0001 versus medium control; ####p < .0001 versus .1% DE treatment; $p < .05 versus .5% DE treatment; ∧∧∧∧p < .0001 versus .1% prot-dep DE treatment.
DISCUSSION
Agricultural workplace exposures to organic dusts lead to airway inflammatory events that increase risk for lung disease (American Thoracic Society, 1998). Livestock farmers have increased risk for chronic obstructive pulmonary disease, and chronic bronchitis when compared with crop farmers (Eduard et al., 2009; Szczyrek et al., 2011). Although it is clear that inhalational exposures can lead to lung inflammation and injury, it remains unknown how certain inflammatory responses are resolved while others lead to chronic lung disease. Lung-resident MSCs are a long-term resident progenitor cell population that are implicated in controlling lung repair mechanisms including epithelial wound repair and inflammation resolution (Ma et al., 2014; Pittenger et al., 1999; Sinclair et al., 2013; Wu et al., 2007; Yoon et al., 2010). The milieu of the lung is pivotal in controlling MSC function, promoting either proresolution or -fibrotic MSC responses depending on the local lung microenvironment (Bernardo and Fibbe, 2013; Kota et al., 2014; Waterman et al., 2010). Our current results suggest that organic dust exposures alter the activities of these cells, leading to activation of proinflammatory and prorepair mediator production, while also inducing growth and migratory deficits. To our knowledge, these are the first published reports assessing the impact of environmental dust exposures on the function of lung-resident MSC. The differential activation of lung-resident MSC by organic dust exposure may be a contributing factor impacting the protective versus negative airway inflammatory responses to these workplace exposures.
MSC are recognized for their ability to reduce inflammation and injury in numerous tissues, including the lung (Inamdar and Inamdar, 2013; Javazon et al., 2007; Ma et al., 2014; Wu et al., 2007; Yoon et al., 2010;). Numerous micro-environmental factors can affect MSC function, however, promoting either proinflammatory or antiinflammatory/immunomodulatory MSC responses (Bernardo and Fibbe, 2013; Kota et al., 2014; Waterman et al., 2010). In general, it is thought that robust inflammatory responses trigger a protective, proresolution or immunomodulatory MSC phenotype, while low-grade inflammation can elicit a profibrotic MSC phenotype (Bernardo and Fibbe, 2013; Ma et al., 2014). Interestingly, recent reports categorize an MSC polarization process resulting in a proinflammatory “MSC1” phenotype versus an antiinflammatory/immunosuppressive “MSC2” phenotype (Bernardo and Fibbe, 2013; Kota et al., 2014; Waterman et al., 2010). In these investigations, TLR signaling was found to play a key role in determining the polarization dynamics, whereby TLR4 activation stimulates MSC1 polarization, while TLR3 activation stimulates MSC2 polarization (Bernardo and Fibbe, 2013; Kota et al., 2014; Waterman et al., 2010). Previous work by our group and others demonstrate that TLR ligands including LPS (TLR4 ligand) and peptidoglycan (TLR2 ligand) are present in substantial quantities in environmental dusts and potently stimulate lung inflammation (Bailey et al., 2008; Bauer et al., 2013; Poole et al., 2010, 2011; Wang et al., 1997). In our current studies, lung-resident MSC treated with DE produced significant quantities of proinflammatory mediators, including TNF-α, IL-6, IL-8, MMP-2, and MMP-9 (Figs. 4 and 5). Although these mediator profiles suggest an activated MSC phenotype, DE treatment also induced growth and/or migratory deficits in the cells (Figs. 2 and 3), suggesting an impairment of protective functions in the DE-treated MSC. It is important to note that the scratch wound assay and other in vitro assays do require growth of cell monolayers, and this in vitro culturing methodology does not replicate how lung-resident MSC are found in vivo; conclusions regarding the migratory and other capacities of MSC in the presence of DE must therefore be considered with this limitation in mind. It is also important to note that the MTT assay is not a direct measurement of proliferation, and may also indicate DE-induced increased cellular stress or reduced metabolic activity in the cells. Further investigations are warranted to better understand how organic dust exposures are impacting the typical activation profiles of lung-resident MSC particularly within in vivo physiological settings.
We have previously found that DE-induced inflammatory responses are initiated by numerous DE components, including TLR agonists and active proteases (Bauer et al., 2013; Poole et al., 2010, 2011; Romberger et al., 2015). When we assessed the role of active DE components in eliciting the proinflammatory and prorepair responses in lung-resident MSC, we found that active proteases in DE are responsible for much of the proinflammatory cytokine release observed (Figure 7). Interestingly, while protease depletion led to significant reductions in both proinflammatory and prorepair mediator release by lung-resident MSC, depletion of the proteases in DE had no impact on DE-mediated reductions in MSC proliferative/metabolic activities (Figure 8). These findings imply that the DE-mediated deficits in lung-resident MSC proliferation are likely driven by other active DE components and not the proteases. Nonetheless, as active environmental and endogenous proteases are recognized for their involvement in numerous lung diseases, including cystic fibrosis, allergic asthma, and chronic obstructive pulmonary disease (Brehm et al., 2014; Jacquet, 2011; Page et al., 2003; Post et al., 2014; Thibodeau and Butterworth, 2013), additional studies are warranted to identify how proteases from DE are mediating their proinflammatory actions in lung-resident MSC. In contrast to the effect of protease depletion, LPS depletion of the DE did not significantly impact IL-8 release (Figure 7F), nor did LPS depletion impact DE-induced reduced metabolic activities (Figure 8B). It is feasible that the levels of endotoxin in the dust may not be sufficient to modify the comparatively large influence of active proteases for the activation of these cells (endotoxin’s 3-hydroxy fatty acid content has been found to be approximately 200 pmol/ml in 1% DE; Poole et al., 2010). Alternatively, lung-resident MSC may not be stimulated by, or responsive to, the TLR4 agonist in the same manner as other MSC populations that have been previously assessed for TLR4-mediated activation and polarization (Bernardo and Fibbe, 2013; Kota et al., 2014; Waterman et al., 2010). Indeed, the roles of LPS and other TLR agonists in the polarization process of lung-resident MSC, as compared with other MSC populations (described earlier), remain unclear.
In addition to producing proinflammatory mediators in response to DE, lung-resident MSC also produced numerous prorepair mediators including AREG, FGF-10, and RvD1 (Figure 6). Lung-resident MSC contribute to wound-healing processes via mesenchymal-epithelial signaling (Inamdar and Inamdar, 2013; Javazon et al., 2007; Ma et al., 2014; McQualter et al., 2013; Wu et al., 2007; Yoon et al., 2010). For example, FGF10/FGFR2b signaling is a well-established pathway regulating lung development, and is reactivated during lung injury to allow wound repair responses through mesenchymal-epithelial crosstalk (Crosby and Waters, 2010; El Agha et al., 2014; Hines and Sun, 2014; Itoh, 2016; Volckaert et al., 2011). Expression of epithelial FGFR2b allows for mesenchymal-derived FGF10 to initiate wound repair, including transient epithelial-to-mesenchymal transition in epithelial cell subsets (Itoh, 2016; Volckaert et al., 2011). MSC also express FGFR2b, potentially allowing for further modulation by FGF10 (Tong et al., 2016). Interestingly, pretreatment with FGF10 in a murine model of acute lung injury demonstrates that FGF10 mobilizes MSC and protects against lung injury (Tong et al., 2016). Furthermore, in a small human cohort, individuals with a heterozygous loss-of-function FGF10 allele had reduced lung function comparable with chronic obstructive pulmonary disease (Klar et al., 2011). These findings implicate the importance of this signaling system in responding to lung inflammatory events to prevent lung injury and disease. Furthermore, AREG and RvD1 are similarly known to promote lung and epithelial repair processes and confer protection against negative outcomes from lung inflammatory exposures. When RvD1 was given as a treatment to mice exposed to cigarette smoke, or challenged with Nontypeable Haemophilus influenzae, lavage inflammatory markers, inflammatory cell infiltration, as well as pathologic changes associated with injury and/or disease were reduced (Croasdell et al., 2015, 2016; Hsiao et al., 2013). AREG has also been shown to promote lung epithelial cell repair processes, while also promoting resolution and recovery processes in the lung following inflammation and injury (Berasain and Avila, 2014; Hall et al., 2016; Krishnamoorthy et al., 2015 ; Wang et al., 2016; Xu et al., 2016). Thus, the production of these mediators by DE-exposed lung-resident MSC may be leading to protective, prorepair signaling by the MSC. As mentioned earlier, active proteases in the DE also appear to mediate the production of prorepair mediators (Figure 7). Whether this is directly mediated through the activation of prorepair signaling pathways by the proteases, or a secondary response initiated by the inflammatory signaling induced by the DE proteases is unknown. Altogether, additional investigations are needed to elucidate how the combined proinflammatory and repair signaling initiated in DE-treated lung-resident MSC impact the lung environment during and following organic dust exposures, and how individual DE components, including proteases, contribute to these responses.
MSC have the potential to be a valuable therapeutic tool; when appropriately activated, MSC have dramatic beneficial effects on cells and outcomes in a variety of inflammatory states, including in the lung (Horie and Laffey, 2016; Iyer et al., 2009; Khatri et al., 2015; Sinclair et al., 2013). Several drawbacks, however, lessen their clinical/therapeutic potential. To date, most evaluations with MSC in the lung are performed via adoptive transfer schemes, often using MSC derived from bone marrow or adipose tissue (Horie and Laffey, 2016; Inamdar and Inamdar, 2013; Iyer et al., 2009; Sinclair et al., 2013). In these investigations, most observed immunomodulatory and lung repair effects appear to be due to mediator release from MSC (Hayes et al., 2015; Petrella et al., 2015; Schilders et al., 2016). Unfortunately, these effects are relatively short in duration, as these adoptively transferred MSC do not efficiently engraft in the lung (Petrella et al., 2015; Schilders et al., 2016). Interestingly, lung-resident MSC appear to be mobilized during the onset of bronchiolitis obliterans syndrome, potentially as a tissue reparative response (Badri et al., 2011a). Preclinical models further reveal that lung-derived MSC can engraft in lungs (Badri et al., 2011b), and, as mentioned earlier, mobilization of this cell population by FGF10 provides protection from LPS-induced acute lung injury (Tong et al., 2016). Thus, the appropriate activation and mobilization of lung-resident MSC could present a novel therapeutic option for improving lung disease outcomes.
Little is known regarding the effects of environmental exposures on lung-resident MSC function. Although the data reported here indicate DE alters MSC activities, the necessary use of in vitro culturing methods do limit the translatability of these findings into a physiological setting. Additional studies investigating the role of these inflammatory exposures in modulating endogenous lung-resident MSC functions are needed; these data could allow us to better understand how these exposures alter an exposed individual’s ability to respond and repair damaged lung tissue over time. Our data indicate exposures from organic dusts do indeed alter the activities of these cells, warranting this line of investigation.
Taken together, our results indicate that primary human lung-resident MSC are activated by exposure to DE. In response to DE treatment, these cells increase production of both proinflammatory and prorepair mediators, while exhibiting significant growth and/or migratory defects. These findings suggest that exposure to organic dusts may alter the activation and function of lung-resident MSC in exposed livestock workers. These changes may alter the endogenous capacity of their lungs to respond to inflammatory exposures, leading to increased susceptibility to lung disease.
FUNDING
High Plains Intermountain Center for Agricultural Health and Safety funded through the Center for Disease Control National Institute for Occupational Safety and Health (U54OH008085; pilot grant sub-award proposal No. 5341105 to T.M.N.); and a K99/R00 award through the National Institute of Environmental Health Sciences (K99ES025819 and R00ES025819 to T.M.N). HICAHS subaward and K99 NIEHS award were awarded to TMN while at UNMC; R00 NIEHS award was received by T.M.N. after transitioning to UCR.
REFERENCES
- American Thoracic Society. (1998). Respiratory health hazards in agriculture. Am. J. Respir. Crit. Care Med. 158, S1–S76. [DOI] [PubMed] [Google Scholar]
- Badri L., Murray S., Liu L. X., Walker N. M., Flint A., Wadhwa A., Chan K. M., Toews G. B., Pinsky D. J., Martinez F. J. et al. , (2011a). Mesenchymal stromal cells in bronchoalveolar lavage as predictors of bronchiolitis obliterans syndrome. Am. J. Respir. Crit. Care Med. 183, 1062–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badri L., Walker N. M., Ohtsuka T., Wang Z., Delmar M., Flint A., Peters-Golden M., Toews G. B., Pinsky D. J., Krebsbach P. H. et al. , (2011b). Epithelial interactions and local engraftment of lung-resident mesenchymal stem cells. Am. J. Respir. Cell Mol. Biol. 45, 809–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey K. L., Poole J. A., Mathisen T. L., Wyatt T. A., Von Essen S. G., Romberger D. J. (2008). Toll-like receptor 2 is upregulated by hog confinement dust in an IL-6-dependent manner in the airway epithelium. Am. J. Physiol. Lung Cell. Mol. Physiol. 294, L1049–L1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer C., Kielian T., Wyatt T. A., Romberger D. J., West W. W., Gleason A. M., Poole J. A. (2013). Myeloid differentiation factor 88-dependent signaling is critical for acute organic dust-induced airway inflammation in mice. Am. J. Respir. Cell Mol. Biol. 48, 781–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berasain C., Avila M. A. (2014). Amphiregulin. Semin. Cell Dev. Biol. 28, 31–41.http://dx.doi.org/10.1016/j.semcdb.2014.01.005 [DOI] [PubMed] [Google Scholar]
- Bernardo M. E., Fibbe W. E. (2013). Mesenchymal stromal cells: Sensors and switchers of inflammation. Cell. Stem Cell 13, 392–402.http://dx.doi.org/10.1016/j.stem.2013.09.006 [DOI] [PubMed] [Google Scholar]
- Boissy R. J., Romberger D. J., Roughead W. A., Weissenburger-Moser L., Poole J. A., LeVan T. D., Ibekwe A. M. (2014). Shotgun pyrosequencing metagenomic analyses of dusts from swine confinement and grain facilities. PLoS One 9, e95578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm A., Geraghty P., Campos M., Garcia-Arcos I., Dabo A. J., Gaffney A., Eden E., Jiang X. C., D'Armiento J., Foronjy R. (2014). Cathepsin G degradation of phospholipid transfer protein (PLTP) augments pulmonary inflammation. faseb J. 28, 2318–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croasdell A., Lacy S. H., Thatcher T. H., Sime P. J., Phipps R. P. (2016). Resolvin D1 Dampens Pulmonary Inflammation and Promotes Clearance of Nontypeable Haemophilus influenzae. J. Immunol. 196, 2742–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croasdell A., Thatcher T. H., Kottmann R. M., Colas R. A., Dalli J., Serhan C. N., Sime P. J., Phipps R. P. (2015). Resolvins attenuate inflammation and promote resolution in cigarette smoke-exposed human macrophages. Am. J. Physiol. Lung Cell. Mol. Physiol. 309, L888–L901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosby L. M., Waters C. M. (2010). Epithelial repair mechanisms in the lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 298, L715–L731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D., Deans R., Keating A., Prockop D., Horwitz E. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8, 315–317. [DOI] [PubMed] [Google Scholar]
- Eduard W., Pearce N., Douwes J. (2009). Chronic bronchitis, COPD, and lung function in farmers: The role of biological agents. Chest 136, 716–725.http://dx.doi.org/10.1378/chest.08-2192 [DOI] [PubMed] [Google Scholar]
- El Agha E., Herold S., Al Alam D., Quantius J., MacKenzie B., Carraro G., Moiseenko A., Chao C. M., Minoo P., Seeger W. et al. , (2014). Fgf10-positive cells represent a progenitor cell population during lung development and postnatally. Development 141, 296–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foronjy R. F., Majka S. M. (2012). The potential for resident lung mesenchymal stem cells to promote functional tissue regeneration: Understanding microenvironmental cues. Cells 1, 874.http://dx.doi.org/10.3390/cells1040874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall O. J., Limjunyawong N., Vermillion M. S., Robinson D. P., Wohlgemuth N., Pekosz A., Mitzner W., Klein S. L., Schultz-Cherry S. (2016). Progesterone-based therapy protects against influenza by promoting lung repair and recovery in females. PLoS Pathog. 12, e1005840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes M., Curley G. F., Masterson C., Devaney J., O’Toole D., Laffey J. G. (2015). Mesenchymal stromal cells are more effective than the MSC secretome in diminishing injury and enhancing recovery following ventilator-induced lung injury. Intensive Care. Med. Exp. 3, 29-015-0065-y. Epub 2015 Oct 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines E. A., Sun X. (2014). Tissue crosstalk in lung development. J. Cell. Biochem. 115, 1469–1477.http://dx.doi.org/10.1002/jcb.24811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie S., Laffey J. G. (2016). Recent insights: Mesenchymal stromal/stem cell therapy for acute respiratory distress syndrome. F1000Res 5, 1532. 10.12688/f1000research.8217.1. eCollection 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao H. M., Sapinoro R. E., Thatcher T. H., Croasdell A., Levy E. P., Fulton R. A., Olsen K. C., Pollock S. J., Serhan C. N., Phipps R. P. et al. , (2013). A novel anti-inflammatory and pro-resolving role for resolvin d1 in acute cigarette smoke-induced lung inflammation. PLoS One 8, e58258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inamdar A. C., Inamdar A. A. (2013). Mesenchymal stem cell therapy in lung disorders: Pathogenesis of lung diseases and mechanism of action of mesenchymal stem cell. Exp. Lung Res. 39, 315–327.http://dx.doi.org/10.3109/01902148.2013.816803 [DOI] [PubMed] [Google Scholar]
- Itoh N. (2016). FGF10: A multifunctional mesenchymal-epithelial signaling growth factor in development, health, and disease. Cytokine Growth Factor Rev. 28, 63–69.http://dx.doi.org/10.1016/j.cytogfr.2015.10.001 [DOI] [PubMed] [Google Scholar]
- Iyer S. S., Co C., Rojas M. (2009). Mesenchymal stem cells and inflammatory lung diseases. Panminerva Med. 51, 5–16. [PubMed] [Google Scholar]
- Jacquet A. (2011). Interactions of airway epithelium with protease allergens in the allergic response. Clin. Exp. Allergy 41, 305–311.http://dx.doi.org/10.1111/j.1365-2222.2010.03661.x [DOI] [PubMed] [Google Scholar]
- Javazon E. H., Keswani S. G., Badillo A. T., Crombleholme T. M., Zoltick P. W., Radu A. P., Kozin E. D., Beggs K., Malik A. A., Flake A. W. (2007). Enhanced epithelial gap closure and increased angiogenesis in wounds of diabetic mice treated with adult murine bone marrow stromal progenitor cells. Wound Repair Regen. 15, 350–359. [DOI] [PubMed] [Google Scholar]
- Khatri M., O’Brien T. D., Chattha K. S., Saif L. J. (2015). Porcine lung mesenchymal stromal cells possess differentiation and immunoregulatory properties. Stem Cell. Res. Ther. 6, 222-015–0220-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkhorn S. R., Garry V. F. (2000). Agricultural lung diseases. Environ. Health Perspect. 108, 705–712.http://dx.doi.org/10.1289/ehp.00108s4705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klar J., Blomstrand P., Brunmark C., Badhai J., Hakansson H. F., Brange C. S., Bergendal B., Dahl N. (2011). Fibroblast growth factor 10 haploinsufficiency causes chronic obstructive pulmonary disease. J. Med. Genet. 48, 705–709.http://dx.doi.org/10.1136/jmedgenet-2011-100166 [DOI] [PubMed] [Google Scholar]
- Kota D. J., DiCarlo B., Hetz R. A., Smith P., Cox C. S. Jr, Olson S. D. (2015). Differential MSC activation leads to distinct mononuclear leukocyte binding mechanisms. Sci. Rep. 4, 4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamoorthy N., Burkett P. R., Dalli J., Abdulnour R. E., Colas R., Ramon S., Phipps R. P., Petasis N. A., Kuchroo V. K., Serhan C. N. et al. , (2015). Cutting edge: Maresin-1 engages regulatory T cells to limit type 2 innate lymphoid cell activation and promote resolution of lung inflammation. J. Immunol. 194, 863–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lama V. N., Smith L., Badri L., Flint A., Andrei A. C., Murray S., Wang Z., Liao H., Toews G. B., Krebsbach P. H. et al. , (2007). Evidence for tissue-resident mesenchymal stem cells in human adult lung from studies of transplanted allografts. J. Clin. Invest. 117, 989–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley R. L. (2011). Consequences of respiratory exposures in the farm environment. N. C. Med. J. 72, 477–480. [PubMed] [Google Scholar]
- Lantz M. S., Ciborowski P. (1994). Zymographic techniques for detection and characterization of microbial proteases. Methods Enzymol. 235, 563–594. [DOI] [PubMed] [Google Scholar]
- Ma S., Xie N., Li W., Yuan B., Shi Y., Wang Y. (2014). Immunobiology of mesenchymal stem cells. Cell Death Differ. 21, 216–225.http://dx.doi.org/10.1038/cdd.2013.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQualter J. L., McCarty R. C., Van der Velden J., O'Donoghue R. J., Asselin-Labat M. L., Bozinovski S., Bertoncello I. (2013). TGF-beta signaling in stromal cells acts upstream of FGF-10 to regulate epithelial stem cell growth in the adult lung. Stem Cell. Res. 11, 1222–1233. [DOI] [PubMed] [Google Scholar]
- Mitchell D. C., Schenker M. B. (2008). Protection against breathing dust: Behavior over time in Californian farmers. J. Agric. Saf. Health 14, 189–203.http://dx.doi.org/10.13031/2013.24350 [DOI] [PubMed] [Google Scholar]
- Page K., Strunk V. S., Hershenson M. B. (2003). Cockroach proteases increase IL-8 expression in human bronchial epithelial cells via activation of protease-activated receptor (PAR)-2 and extracellular-signal-regulated kinase. J. Allergy Clin. Immunol. 112, 1112–1118.http://dx.doi.org/10.1016/j.jaci.2003.08.050 [DOI] [PubMed] [Google Scholar]
- Petrella F., Rizzo S., Borri A., Casiraghi M., Spaggiari L. (2015). Current Perspectives in Mesenchymal Stromal Cell Therapies for Airway Tissue Defects. Stem Cells Int. 2015, 746392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger M. F., Mackay A. M., Beck S. C., Jaiswal R. K., Douglas R., Mosca J. D., Moorman M. A., Simonetti D. W., Craig S., Marshak D. R. (1999). Multilineage potential of adult human mesenchymal stem cells. Science 284, 143–147. [DOI] [PubMed] [Google Scholar]
- Poole J. A., Dooley G. P., Saito R., Burrell A. M., Bailey K. L., Romberger D. J., Mehaffy J., Reynolds S. J. (2010). Muramic acid, endotoxin, 3-hydroxy fatty acids, and ergosterol content explain monocyte and epithelial cell inflammatory responses to agricultural dusts. J. Toxicol. Environ. Health A 73, 684–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole J. A., Gleason A. M., Bauer C., West W. W., Alexis N., van Rooijen N., Reynolds S. J., Romberger D. J., Kielian T. L. (2012). CD11c+/CD11b+ Cells are Critical for Organic Dust-Elicited Murine Lung Inflammation. Am. J. Respir. Cell Mol. Biol. 475, 652–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole J. A., Wyatt T. A., Kielian T., Oldenburg P., Gleason A. M., Bauer A., Golden G., West W. W., Sisson J. H., Romberger D. J. (2011). Toll-like receptor 2 regulates organic dust-induced airway inflammation. Am. J. Respir. Cell Mol. Biol. 45, 711–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post S., Heijink I. H., Petersen A. H., de Bruin H. G., van Oosterhout A. J. M., Nawijn M. C., Fessler M. B. (2014). Protease-activated receptor-2 activation contributes to house dust mite-induced IgE responses in mice. PLoS One 9, e91206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolandsson S., Andersson Sjoland A., Brune J. C., Li H., Kassem M., Mertens F., Westergren A., Eriksson L., Hansson L., Skog I. et al. , (2014). Primary mesenchymal stem cells in human transplanted lungs are CD90/CD105 perivascularly located tissue-resident cells. BMJ Open Respir. Res. 1, e000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romberger D. J., Bodlak V., Von Essen S. G., Mathisen T., Wyatt T. A. (2002). Hog barn dust extract stimulates IL-8 and IL-6 release in human bronchial epithelial cells via PKC activation. J. Appl. Physiol. 93, 289–296. [DOI] [PubMed] [Google Scholar]
- Romberger D. J., Heires A. J., Nordgren T. M., Souder C. P., West W., Liu X. D., Poole J. A., Toews M. L., Wyatt T. A. (2015). Proteases in agricultural dust induce lung inflammation through PAR-1 and PAR-2 activation. Am. J. Physiol. Lung Cell. Mol. Physiol. 309, L388–L399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilders K. A., Eenjes E., van Riet S., Poot A. A., Stamatialis D., Truckenmuller R., Hiemstra P. S., Rottier R. J. (2016). Regeneration of the lung: Lung stem cells and the development of lung mimicking devices. Respir. Res. 17, 44. 44-016-0358-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair K., Yerkovich S. T., Chambers D. C. (2013). Mesenchymal stem cells and the lung. Respirology 18, 397–411.http://dx.doi.org/10.1111/resp.12050 [DOI] [PubMed] [Google Scholar]
- Sweetman F., Ornstein L. (1974). Electrophoresis of elastase-like esterases from human neutrophils. J. Histochem. Cytochem. 22, 327–339.http://dx.doi.org/10.1177/22.5.327 [DOI] [PubMed] [Google Scholar]
- Szczyrek M., Krawczyk P., Milanowski J., Jastrzebska I., Zwolak A., Daniluk J. (2011). Chronic obstructive pulmonary disease in farmers and agricultural workers - an overview. Ann. Agric. Environ. Med. 18, 310–313. [PubMed] [Google Scholar]
- Thibodeau P. H., Butterworth M. B. (2013). Proteases, cystic fibrosis and the epithelial sodium channel (ENaC). Cell Tissue Res. 351, 309–323.http://dx.doi.org/10.1007/s00441-012-1439-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong L., Zhou J., Rong L., Seeley E. J., Pan J., Zhu X., Liu J., Wang Q., Tang X., Qu J. et al. , (2016). Fibroblast Growth Factor-10 (FGF-10) Mobilizes Lung-resident Mesenchymal Stem Cells and Protects Against Acute Lung Injury. Sci. Rep. 6, 21642.http://dx.doi.org/10.1038/srep21642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volckaert T., Dill E., Campbell A., Tiozzo C., Majka S., Bellusci S., De Langhe S. P. (2011). Parabronchial smooth muscle constitutes an airway epithelial stem cell niche in the mouse lung after injury. J. Clin. Invest. 121, 4409–4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Essen S., Romberger D. (2003). The respiratory inflammatory response to the swine confinement building environment: The adaptation to respiratory exposures in the chronically exposed worker. J. Agric. Saf. Health 9, 185–196.http://dx.doi.org/10.13031/2013.13684 [DOI] [PubMed] [Google Scholar]
- Wang S., Zhang Y., Wang Y., Ye P., Li J., Li H., Ding Q., Xia J. (2016). Amphiregulin Confers Regulatory T Cell Suppressive Function and Tumor Invasion via the EGFR/GSK-3beta/Foxp3 Axis. J. Biol. Chem. 29140, 21085–21095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Larsson K., Palmberg L., Malmberg P., Larsson P., Larsson L. (1997). Inhalation of swine dust induces cytokine release in the upper and lower airways. Eur. Respir. J. 10, 381–387. [DOI] [PubMed] [Google Scholar]
- Waterman R. S., Tomchuck S. L., Henkle S. L., Betancourt A. M. (2010). A new mesenchymal stem cell (MSC) paradigm: Polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLoS One 5, e10088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Chen L., Scott P. G., Tredget E. E. (2007). Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells 25, 2648–2659.http://dx.doi.org/10.1634/stemcells.2007-0226 [DOI] [PubMed] [Google Scholar]
- Xu Y., Meng C., Liu G., Yang D., Fu L., Zhang M., Zhang Z., Xia H., Yao S., Zhang S. (2016). Classically activated macrophages protect against lipopolysaccharide-induced acute lung injury by expressing amphiregulin in mice. Anesthesiology 124, 1086–1099. [DOI] [PubMed] [Google Scholar]
- Yoon B. S., Moon J. H., Jun E. K., Kim J., Maeng I., Kim J. S., Lee J. H., Baik C. S., Kim A., Cho K. S. et al. , (2010). Secretory profiles and wound healing effects of human amniotic fluid-derived mesenchymal stem cells. Stem Cells Dev. 19, 887–902. [DOI] [PubMed] [Google Scholar]