Abstract
Epidemiological studies show that the incidence and mortality rates of prostate cancer (PCa) are significantly higher in African-American (AA) men when compared with Caucasian (CA) men in the United States. Transforming growth factor β (TGFβ) signaling pathway is linked to health disparities in AAs. Recent studies suggest a role of TGFβ3 in cancer metastases and its effect on the migratory and invasive behavior; however, its role in PCa in AA men has not been studied. We determined the circulating levels of TGFβ3 in AA and CA men diagnosed with PCa using ELISA. We analyzed serum samples from both AA and CA men diagnosed with and without PCa. We show that AA PCa patients had higher levels of TGFβ3 protein compared with AA controls and CA patients. In fact, TGFβ3 protein levels in serum were higher in AA men without PCa compared with the CA population, which may correlate with more aggressive disease seen in AA men. Studies on AA-derived PCa cell lines revealed that TGFβ3 protein levels were also higher in these cells compared with CA-derived PCa cell lines. Our studies also reveal that TGFβ does not inhibit cell proliferation in AA-derived PCa cell lines, but it does induce migration and invasion through activation of PI3K pathway. We suggest that increased TGFβ3 levels are responsible for development of aggressive PCa in AA patients as a consequence of development of resistance to inhibitory effects of TGFβ on cell proliferation and induction of invasive metastatic behavior.
Circulating levels of TGFβ3 are much higher in AA PCa patients compared with those in CA patients. AA-derived PCa cell lines also express high levels of TGFβ3 and exert significant stimulatory effects on cell migration and invasion.
Introduction
Prostate cancer (PCa), the most frequently diagnosed malignancies among men worldwide, remains the second leading cause of cancer-related deaths in the United States. Among the known risk factors associated with PCa, such as age (over 65 years), family history, race, environmental factors and diet, current research studies indicate that race/ethnicity plays a major role in men developing PCa (1). Incidence and mortality rates for African Americans (AAs) are 1.6 times and 2.5 times higher than Caucasian (CA) men, respectively (2). Recent studies show that the determinants of this high incidence and aggressiveness of PCa seen in AA are associated with the differences at the genetic and molecular level that result in racial disparities in PCa incidence and outcomes seen in AA men (2–4). Recent studies show that molecular factors such as genetic modifications (5,6), epigenetic changes (7–9), altered microRNAs (10,11) and signaling pathways, including hormone receptor, growth factor receptor and inflammation signaling pathways, are associated with PCa racial disparities (2,12–14).
Transforming growth factor β (TGFβ) signaling pathway plays a pivotal role in diverse cellular processes and has been implicated as a factor in cancer formation and progression (15,16). TGFβ functions as a tumor suppressor in normal epithelial cells and early-stage cancer by inhibiting proliferation, inducing apoptosis and inhibiting cell immortalization to maintain and regulate a cell’s normal state (15,17,18). However, in later stages of the disease, the growth inhibitory function of TGFβ is lost, and TGFβ functions as a tumor promoter and is associated with aggressive forms of cancers due to its effects on survival and growth, epithelial–mesenchymal transition, migration, invasion, angiogenesis and metastasis of cancer cells (15,17–19). TGFβ isoforms (TGFβ1, -β2 and -β3), when activated, bind to and bring together transmembrane, serine threonine kinase receptors designated as TGFβ receptors type I (TGFβRI) and type II (TGFβRII), to form a ligand–receptor complex that propagates the signal to the nucleus leading to several intracellular processes (15,16). Previous studies in our laboratory have investigated the role of TGFβ in PCa cells representing specific stages of PCa progression and showed that TGFβ1 was ubiquitously expressed in all prostate cells (15). On the other hand, TGFβ3 was expressed at very low levels in normal epithelial cells and early-stage PCa but was highly expressed in more metastatic PCa cell lines (15). In addition, TGFβ3 (versus TGFβ1) exerted a greater effect on cell migration and invasion via activation of PI3K pathway in PCa cells (15). Several studies in human breast carcinoma (20,21), endometrial cancer (22), head and neck cancer (23) have suggested an important role of TGFβ3 (versus TGFβ1) in cancer metastases. These studies show that TGFβ3 is specifically unregulated in later stage in metastatic cancer cells and that this isoform is indeed involved in tumor cell migration, invasion and promoting epithelial–mesenchymal transition in these cells. These effects of TGFβ3 on migratory and invasive behavior in these cells were mediated via PI3-kinase-dependent pathway. There is sufficient evidence linking TGFβ3 to more metastatic disease; however, there is no information linking TGFβ3 specifically to health disparities in AA men with PCa.
Several studies have linked the overexpression of TGFβ1 with health disparities in AAs. Significant overexpression of TGFβ1 levels was observed in AA patients with hypertension (24,25), diabetes (26),burden of renal disease (27), breast cancer (28) and systemic sclerosis (29,30) but not in CA patients. Furthermore, additional studies showed that high levels of TGFβ1 found in AA individuals without the disease suggest that there are evident molecular differences between AA and CA populations that play a role in health disparities (29). To date, several research studies have been conducted to investigate the molecular and biological bases of TGFβ1’s role in health disparities in AAs in several diseases, yet the underlying source of PCa health disparity seen in AA men remains largely unclear. The current study was carried out to determine the possible role of TGFβ in PCa health disparity in AA men.
Materials and methods
Chemicals and reagents
Recombinant human TGFβ1 and TGFβ3 were purchased from PeproTech (Rocky Hill, NJ). Inhibitors to TGFBRI (SB431542) and TGFBRII (LY2157299) were purchased from Tocris Biosciences (Ellisville, MO) and Xcess Biosciences (San Diego, CA), respectively. Antibodies against AR (Cat. # sc-816), TGFβ1 (Cat. # sc-130348), TGFβ3 (Cat. # sc-82), TGFβRI (Cat. #s c-398), TGFβRII (Cat. # sc-400), MMP2 (Cat. #), MMP2 (Cat. # sc-10736) and MMP9 (Cat. # 21733) were all purchased from Santa Cruz Biotechnology (Dallas, TX). The antibodies against pSmad3 (Cat. # 9520), Smad2/3 (Cat. # 3102), pAKT (Cat. # 4060) and pan AKT (Cat. # 4685) were purchased from Cell Signaling Technology (Danvers, MA). Anti-α-Tubulin (Cat. # T5168) antibody was purchased from Sigma–Aldrich (St. Louis, MO). Anti-mouse IgG-HRP and Goat anti-rabbit IgG-HRP (immunoglobulin-horseradish peroxidase) were purchased from GE Healthcare (Piscataway, NJ) and Promega (Madison, WI), respectively.
Human subjects: sample collection
We used serum samples from an ongoing case-control study of men undergoing radical prostatectomy at Fox Chase Cancer Center, Philadelphia, PA. The study involving serum samples from patients and from controls was approved by Institutional Review Boards at Fox Chase Cancer Center and Clark Atlanta University. Subjects were recruited between 2003 and 2015. This study included AA and CA men between the ages of 21 and 80 years. In addition to the group of patients with PCa disease, this study included serum samples from healthy volunteers to serve as controls. As detailed in Table 1, the 200 patients with prostate disease who were selected included 150 AA and 50 CA men. Frozen serum samples received from Fox Chase Cancer Center were stored at −80°C until further analysis to maintain the integrity of the serum. The clinic-pathological data from patients including age, races, serum prostate-specific antigen (PSA) levels, pathological stage and Gleason score were collected from patients by Fox Chase Cancer Center.
Table 1.
Study set and patient clinical profiles and pathological characteristics.
| Characteristics (mean ± SE) | All (N = 350) | CA controls (N = 50) | CA cases (N = 50) | AA controls (N = 100) | AA cases (N = 150) | P-value |
|---|---|---|---|---|---|---|
| Median age (years) | 57 ± 0.5 | 45 ± 1.9 | 64 ± 1.2 | 55 ± 0.6 | 56.5 ± 1.0 | 0.001* |
| PSA levels | 4.2 ± 0.96 | 1.1 ± 0.2 | 5.4 ± 0.64 | 1.57 ± 0.38 | 10.97 ± 1.7 | 0.001* |
| Gleason score | 0.224 | |||||
| <7 | – | 19 (38%) | – | 53 (35%) | ||
| =7 | – | 21 (42%) | – | 53 (35%) | ||
| >7 | – | 10 (20%) | – | 44 (29%) | ||
| Pathological stage group | 0.414 | |||||
| I | – | 1 (2%) | – | 1 (0.68%) | ||
| II | – | 22 (44%) | – | 77 (52.4%) | ||
| III | – | 11 (22%) | – | 25 (17%) | ||
| IV | – | 1 (2%) | – | 6 (4.1%) | ||
| Unstageable (99) | – | 10 (20%) | – | 40 (25.8) |
P-values given for all parameters are Mann–Whitney U-test or Kruskal–Wallis one-way analysis of variance.
*Denotes significance in reference to P < 0.05.
Enzyme-linked immunosorbent assay
Human serum samples from 150 AA and 50 CA PCa patients, 100 AA and 50 CA normal men were analyzed for circulating levels of TGFβ3 using a sandwich type ELISA (Cat # DY243) purchased from R & D Systems (R & D, Minneapolis, MN). Plate preparation and assay procedures were followed according to the manufacturer’s instructions with minor modifications. In brief, 96-well plates were coated with TGFβ3 primary antibody and incubated overnight at 4°C. Standards were prepared from 170 ng/ml of recombinant human TGFβ3 stock solution in concentrations ranging from 0 to 2000 pg/ml using 2-fold serial dilutions in reagent diluent [1% BSA in phosphate-buffered saline (PBS), pH 7.5]. Serum samples were diluted (1:4) in reagent diluent to account for very high concentrations and were corrected in final analyses. TGFβ3 was activated in the diluted human serum samples using HCl prior to assay. The standards, 100 µl, and diluted serum samples were transferred into wells of an ELISA plate (Clear Microplate Cat # DY990, R&D) for duplicate analysis. Absorbance was read at 450 nm with the reference filter set to 540 nm using a microplate reader (Bio-Rad). After plotting the independent human TGFβ3 standard curve, TGFβ3 concentrations of the serum samples were calculated using the formula C = Sa/Sv pg/ml, where Sa is the sample absorbance of unknown (in pg) from standard curve, and Sv is the sample volume (ml) added to each well. All analyses were performed in duplicates, and the amounts of TGFβ3 protein measured by the ELISA are expressed as TGFβ3 pg/ml of proteins. The intra-assay coefficient of variation was 5.9%.
Cell lines and cell culture conditions
PCa cell lines derived from an AA patient, E006AA and E006AA-HT, kindly provided by Dr Shahriar Koochekpour (Roswell Park Cancer Institute, Buffalo, NY), were cultured and maintained in Dulbecco's modified Eagle's medium supplemented with 5% fetal bovine serum (FBS) (31,32). The established E006AA cell line, a pure epithelial cell line, was obtained from the prostate tissue of a 50-year-old AA patient who underwent radical retrapubic prostatectomy for treatment of clinically localized PCa at LSU-Health Sciences Center (31). E006AA-HT cells (highly tumorigenic) were generated by stably transfecting E006AA (parental cell line) with pcDNA3.1-NeoR vector and propagated the entire pool of G418-resistant cells (32). Both cell lines were generated and extensively characterized by Dr Koochekpour using western blots, RT-PCR/real-time PCR, cytodifferentiation and prostate-specific markers, spectral karyotyping, cell line authentication assays, cell proliferation, migration and invasion assays (31,32). Following cell line authentication, E006AA cell lines were provided to our lab for further analysis on PCa metastases. MDA-2A and MDA-2B cells were kindly provided by Dr Ming-Fong Lin (University of Nebraska Medical Center, Omaha, NE) and were cultured in BRFF-HPC1 medium (Cat # 0403, Athena ES Company, Baltimore, MD) supplemented with 20% FBS (33). MDA-2A and MDA-2B cells were both established from a bone metastasis (from different areas of the tumor) of a 63-year-old AA patient diagnosed with metastatic adenocarcinoma and underwent spinal decompression and tumor resection followed by bilateral orchiectomy at University of Texas MD Anderson Cancer Center in 1995 (33). Both MDA cell lines were generated and extensively characterized by Nora M. Novone et al. giving evidence that these cells originated from human prostatic cancer by using karyotyping, growth assay, RT-PCR and western blots (33). Human PCa cell lines LNCaP and DU145 were purchased from the American Type Culture Collection (ATCC, Manassas, VA) and cultured in RPMI or MEM, respectively, and supplemented with 5% FBS (15). All cells were grown and maintained at 37°C, 5% CO2 with 100% humidity.
RT-PCR and quantitative real time analysis
Total RNA was isolated from PCa cell lines using Trizol reagent (Invitrogen, Carlsbad, CA). The concentration and purity of all RNA samples were measured using Nanodrop 2000c Spectrophotometer (Thermo Fisher Scientific, Waltham, MA). The optical density reading was at 260 nm, and the OD260/OD280 ratios for RNA samples were between 1.8 and 2.0. Total RNA (2 µg) was reverse transcribed into cDNA as described previously (15). RT-PCR reactions were performed using the Master Cycler PCR Systems (Eppendorf) as described previously (15). Quantitative real-time PCR (qRT-PCR) was performed using GoTaq Master Mix (Promega) on Bio-Rad CFX Connect Real-Time PCR System (Bio-Rad, Hercules, CA). qRT-PCR was performed in triplicate, and the results were calculated using the ΔΔCT method, whereby each sample (gene of interest) is normalized to its corresponding GAPDH values, and the average of each individual ΔCt values was used for comparison between groups (34). Data from RT-PCR and qPCR were normalized to internal controls, L-19 and GAPDH expression levels, respectively. All human gene-specific primers were designed with Beacon-Designer 5.0 as described previously (15). All primers with their respective sequences are presented in Supplementary Table 1, available at Carcinogenesis Online. For RT-PCR analysis, PCR mixtures ran between 30 and 35 cycles with annealing temperature with respect to the melting temperature of specific primers. The PCR products were visualized on 1–1.5% agarose gels stained with ethidium bromide (Amresco, Solon, OH) and captured using Bio-Rad Image Lab Software (Bio-Rad). Analyses of PCR products were carried out in at least three independent experiments.
Western blot analysis
PCa cells from several independent experiments were washed in ice-cold PBS and harvested in lysis buffer (Cell Signaling Technology, Beverly, MA) containing 20 mM Tris–HCl (pH 7.5), 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 μg/ml leupeptin and 1× protease inhibitor cocktail (Calbiochem, La Jolla, CA). Protein concentrations were measured by the Lowry protein assay using the Bio-Rad DC Protein Assay kit (Bio-Rad) according to the manufacturer’s protocol. Equal amounts of the protein (50 µg) were denatured in sample buffer and then electrophoresed on 10% SDS-PAGE gels, transferred to the polyvinylidene fluoride membranes (Millipore, Billerica, MA) at 100 V for 1.5 h and blocked in 5% milk in 1× TBST (50 mM Tris, pH 7.5, containing 0.15 M NaCl and 0.05% Tween 20) and incubated with the following primary antibodies overnight at 4°C with specific dilutions (1:800 dilution for anti-AR, anti-TGFβRI, anti-TGFβRII, anti-TGFβI, anti-TGFβ, anti-MMP2 and anti-MMP9; 1:000 dilution for anti-pSmad3, anti-Smad2/3, anti-pAKT and anti-pan AKT; 1:3000 dilution of anti-α-Tubulin). The blots were washed with 1× TBST and incubated with appropriate immunoglobulin coupled to horseradish peroxidase (dilution 1:10 000) for 1 h at room temperature with shaking. The blots were then developed in Millipore Luminata Forte (EMD Millipore) for 5 min and visualized by Syngene PXI 6 imagining system (Syngene, Frederick, MD). Western blots for α-Tubulin were probed on previously probed blots and used as loading controls.
Cell proliferation assay
Cells were plated at a density of 2 × 104 cells/well in 24-well plates in specific media + 5% FBS overnight. Cells were then serum starved for 24 h and treated with TGF-β1 or -β3 (5 ng/ml) in the presence of 0.1% FBS for 5 days. Cells were allowed to grow for 5 days and then trypsinized and counted using a Cellometer Auto X4 Cell Counter (Nexcelom Bioscience, Lawrence, MA). Cell growth assays were performed in at least three independent experiments.
Migration and invasion assay
Cell migration was performed using transwell insert chambers (BD Biosciences) as described previously with minor modifications (32). In brief, for migration assay, transwell inserts were coated on both sides with 50 µg/ml Type I Collagen (Cat # 354236) purchased from Corning (Glenview, IL). For the cell invasion assays, the upper transwell membranes were precoated with 50 μl of 1:4 Matrigel/Medium dilutions (BD Sciences, San Jose, CA). AA-derived PCa cells, E006AA and E006AA-HT suspension of cells (2 × 104 cells/insert for migration and 4 × 104 cells/insert for invasion) in 100 or 400 ul medium suspension containing 0.1% BSA + 1% FBS, respectively, were added to the upper chamber of the insert. The lower chamber was filled with 400 ul medium + 5% FBS containing chemoattractants. The cells were allowed to migrate or invade at 37°C for 24 or 48 h, respectively. TGFβ1 and -β3 (5 ng/ml) were used as chemoattractants, and EGF (10 ng/ml) was used as a positive control. DMEM with 0.1% BSA + 5% FBS was used for the control cells in migration and invasion assays, respectively. The nonmigratory cells were then removed using a cotton swab, inserts and were washed gently with PBS and then fixed with 3.7% paraformaldehyde and then stained with 5 mg/ml 4′,6-diamidino-2-phenylindole (DAPI) for 30 min. Migrated or invaded cells were viewed under the Zeiss inverted microscope (Zeiss). Migrated or invaded cells in transwell inserts were counted in five randomly selected fields from two independent inserts. The migration or invasion index was calculated and graphed (average treated/average control). Each experiment was performed in duplicates and was repeated at least three times using an independent cell preparation.
Statistical analysis
Data are representative of at least three independent experiments. Data were plotted using Sigma Plot. The significance of the differences was evaluated by one-way ANOVA followed by the Student’s t-test. Error bars represent the standard error of the mean, and the values of P < 0.05 were considered to indicate statistically significant differences.
Results
Patient characteristics
Patients’ clinical profile and pathological characteristics are presented in Table 1. For the entire group (N = 350), the median age for collection of serum was 57 ± 0.5 years (range: 21–80); the median age at diagnosis of PCa for AA men was 55.5 ± 0.6 years (range: 44–71) and for CA men diagnosed with PCa was 64 ± 1.2 years (range: 47–80). The majority of patients with high PSA levels at PCa diagnosis were AA men with a mean PSA of 10.97 ± 1.7, whereas the mean PSA levels at PCa diagnosis for CA men were 5.4 ± 0.64. Of the cases, there were a total of 72 low grade (Gleason score < 7), CA (n = 19, 38%) and AA (n = 53, 35%); 74 Intermediate grade (Gleason score = 7), CA (n = 21, 42%) and AA (n = 53, 35%) and 53 high grade (Gleason score 7–10), CA (n = 10, 20%) and AA (n = 44, 29%). In addition, the majority of patients diagnosed with PCa were in pathological stage group 2 for each ethnic group; AA (n = 77, 52.4%) versus CA (n = 22, 44%).
Circulating levels of TGFβ3 in men diagnosed with and without PCa
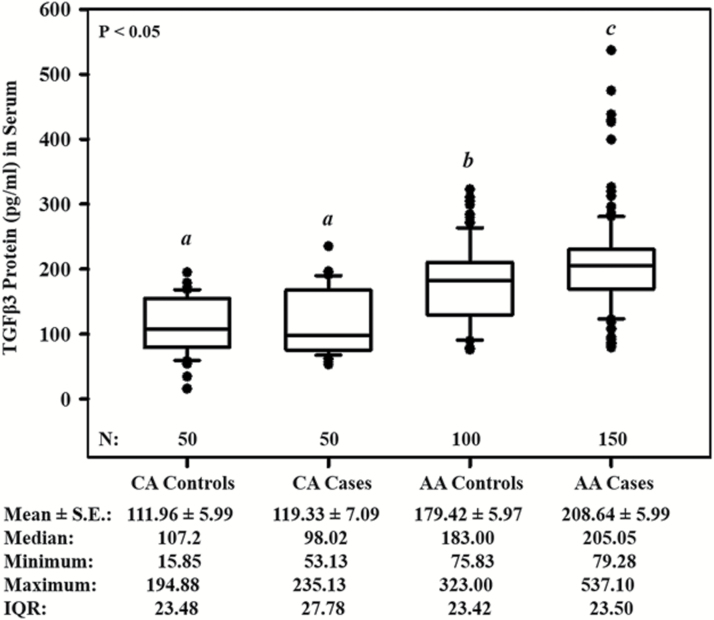
We found significant differences (P < 0.05) in circulating TGFβ3 protein levels in serum between AA groups and CA groups (Figure 1). AA PCa patients had markedly higher circulating levels of TGFβ3 compared with AA men without PCa and CA men with or without PCa. Interestingly, AA men without PCa had higher circulating levels of TGFβ3 compared with both CA men with and without PCa. Furthermore, there was no significant difference between CA men with and without PCa. The mean circulating levels of TGFβ3 protein in serum samples from CA controls were 111.96 ± 5.99 pg/ml (range: 15.85–194.88 pg/ml; 95% CI, 100.22–123.70), CA PCa cases were 119.33 ± 7.09 pg/ml (range: 53.13–235.13; 95% CI, 105.44–133.22), AA men without PCa were 179.42 ± 5.97 pg/ml (range: 75.83–323.00; 95% CI, 167.71–191.13) and AA cases were 208.64 ± 5.99 ng/ml (range: 79.28–537.10; 95% CI, 196.89–220.39).
Figure 1.
Circulating levels of TGFβ3 in men diagnosed with and without PCa (control group) in AA and CA men. Data represents the concentrations of TGFβ3 (pg/ml) determined in each serum sample by ELISA analysis. In the box plots, the horizontal black line marks the median. The total number (N) of participants per group are shown at the bottom within the graph. The Mean ± S.E., median, minimum, maximum and IQR are presented at the bottom outside the graph. ‘a–c’ denote significant (P < 0.05) differences of TGFβ3 levels between the indicated groups. IQR, interquartile range; SE, standard error.
TGFβ3 expression in PCa cells derived from AA patients
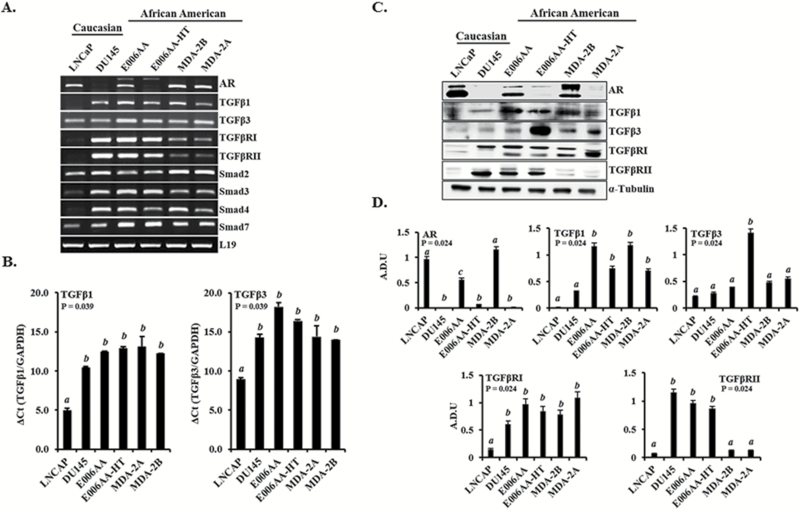
TGFβ3 has been shown to be highly expressed in more metastatic PCa cell lines and expressed at lower levels in normal prostate epithelial cell lines, all of which were derived from CA PCa patients (15). To further examine whether TGFβ3 protein is overexpressed in highly metastatic PCa cells derived from AA patients and to further assess the role of TGFβ signaling components in AA PCa, we analyzed and compared the expression of TGFβ isoforms and TGFβ signaling components in four AA-derived PCa cell lines, E006AA (parental and less metastatic), E006AA-HT(highly metastatic), MDA-2A (androgen sensitive, dependent) and MDA-2B (androgen sensitive, independent), and compared their expression with previously characterized CA-derived PCa cell lines, LNCaP (AR+) and DU145 (AR−). After RT-PCR analysis, mRNA levels of TGFβ isoforms and signaling components were observed to be differentially expressed in both AA- and CA-derived PCa cell lines, with AA-derived PCA cells expressing higher TGFβ3 mRNA (Figure 2A). We further analyzed TGFβ1 and TGFβ3 mRNA expression by real-time qPCR. This data shows a significant (P = 0.039) increase in DU145, E006AA, E006AA-HT, MDA-2A and MDA-2B compared with LNCaP cells (Figure 2B). Similarly, western blot analysis (Figure 2C and D) showed that metastatic AA-derived PCa cell lines (E006AA, E006AA-HT, MDA-2A and MDA-2B) expressed significantly higher levels of TGFβ1 proteins (P = 0.024) compared with CA-derived PCA cells lines (LNCaP and DU145) with a remarkable overexpression of TGFβ3 protein in E006AA-HT cells, which are highly tumorigenic and metastatic. Similar to LNCaP cells, the AA-derived PCa cell lines expressed AR mRNA and protein; however, the expression in E006AA-HT and MDA-2A was much lower than that in E006AA and MDA-2B cells. On the other hand, the expression levels of mRNA and protein of TGFβ signaling components were markedly lower in LNCaP cells compared with all other cell lines.
Figure 2.
Expression of AR and TGFβ signaling components in PCa cell lines derived from CA and AA patients. (A) Expression of TGFβ signaling components’ mRNA determined by RT-PCR in CA- (LNCaP and DU145) and AA- (E006AA, E006AA-HT, MDA-2A and MDA-2B) derived PCa cells. (B) Real-time qPCR analysis of TGFβ1 and TGFβ3 mRNA expression and (C) Expressions of AR and TGFβ signaling component proteins determined by western blot analysis. (D) Densitometry analysis of protein levels normalized to α-Tubulin. LNCaP cells were used as AR (+) control, and DU145 cells were used for AR (−) control. L19, GAPDH and α-Tubulin were used as the loading control for mRNA and protein analysis, respectively. Data represent at least three independent experiments. ‘a–c’ denote significant differences (P < 0.05) among different treatment groups.
To further elucidate the role of TGFβ signaling in AA-derived PCa cell lines and to confirm whether these cells respond to TGFβ stimulus via classical TGFβ signaling pathway, E006AA and E006AA-HT cells were treated with TGFβ1 and -β3 (5 ng/ml) for 30 min in the presence and absence of specific inhibitors of TGFβRI (SB431542) or -βRII (LY2157299) for 2 h. As shown in Supplementary Figure 1A and B, available at Carcinogenesis Online, TGFβ1 and -β3 activated TGFβ signaling pathway and significantly (P < 0.001) induced phosphorylation of SMAD3 (pSMAD3), which was blocked after pretreatment with TGFβRI and -βRII inhibitors.
Effects of TGFβ on proliferation, migration and invasion in AA-derived PCa cell lines
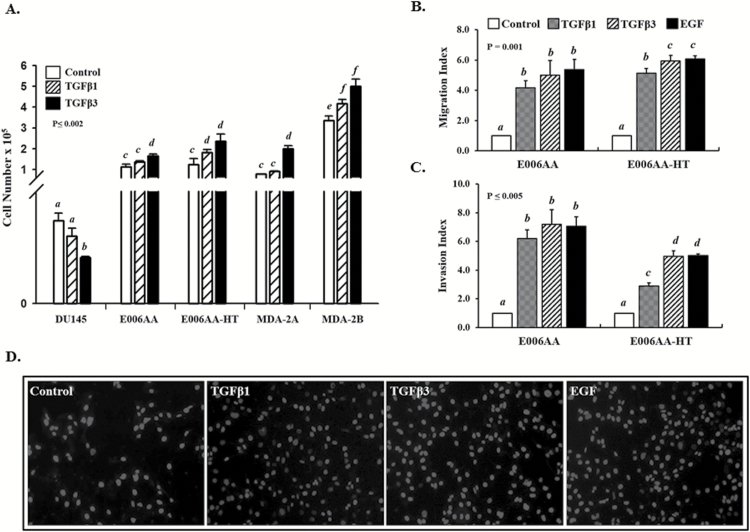
We have shown previously that TGFβ inhibits proliferation of RWPE-1 and DU145 cells but had no effect on proliferation of PC3 cells in the presence of functional TGFβ receptors and SMAD signaling (35). To examine the differential effects of TGFβ isoforms on proliferation of AA-derived PCa cell lines, E006AA, E006AA-HT, MDA-2A and MDA-2B cells, were first serum starved for 24 h and then treated with TGFβ1 or -β3 for 5 days. DU145 cells were used as controls. The results from the data represented in Figure 3A show that TGFβ caused a significant inhibition of proliferation in DU145 cells as previously seen (15) but had no effect on proliferation in E006AA and E006AA-HT, MDA-2A and MDA-2B cells and caused a slight increase in proliferation. These data suggest that AA-derived PCa cells are resistant to the growth inhibitory effects of TGFβ on cell proliferation.
Figure 3.
Effects of TGFβ1 and -β3 on cell proliferation, migration and invasion in PCa cell lines. (A) DU145, E006AA, E006AA-HT, MDA-2A and MDA-2B cells were treated with TGFβ1 and -β3 (5 ng/ml) for 5 days, and the cell number was determined using a Cellometer. Each bar represents the mean ± SEM from at least three independent experiments. ‘a–f’ denote significant differences (P ≤ 0.002) among various cell lines and treatments. (B, C) TGFβ stimulation promotes (B) migration and (C) invasion in E006AA and E006AA-HT cells. Cells were seeded at appropriate densities and allowed to migrate or invade for 24 or 48 h, respectively. TGFβ1 and -β3 (5 ng/ml) were used as chemoattractants, and EGF (10 ng/ml) was used as a positive control. DMEM with 0.1% BSA + 5% FBS was used for the control cells. Data represent at least three independent experiments. ‘a–d’ denote significant differences (P ≤ 0.05) among different treatments and cell lines. (D) Representative images of migrated E006AA cells depicted in (B).
The PI3K pathway plays an important role in cancer and is a critical effector of multiple growth factor receptors (36,37). We have previously shown that TGFβ1- and -β3-induced cell migration and invasion in metastatic PCa cells (PC3 and PC3M) are mediated via PI3K/AKT pathway (38). To examine the role of TGFβ in cell motility in AA-derived PCa cell lines, a transwell migration assay was performed. As shown in Figure 3B and D, both TGFβ1 and -β3 caused increased cell migration in E006AA and E006AA-HT cells (P = 0.001), while TGFβ3 effects were more pronounced. A moderate increase of cell migration is also observed in the presence of TGFβ in E006AA-HT, the more metastatic cell line (P < 0.05) as compared with E006AA, parental cell line (Figure 3B). To further examine the effects of TGFβ stimulation on the motility and metastasis in PCa in AA cell lines, a Matrigel invasion assay was performed to determine the potential effects of TGFβ on the invasive behavior of AA-derived PCa cells. As shown in Figure 3C, TGFβ isoforms dramatically promoted Matrigel invasion (P ≤ 0.05) of E006AA and E006AA-HT cells when compared with untreated cells.
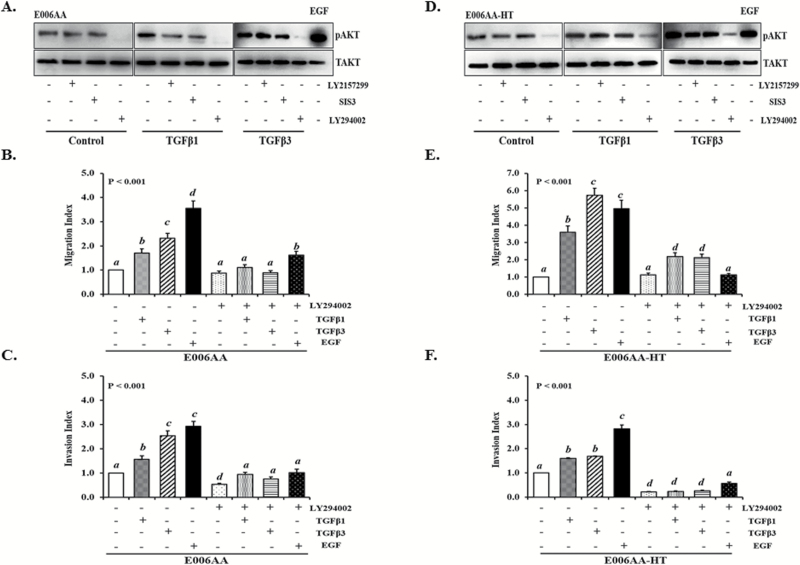
To determine whether TGFβ-activated PI3K/AKT signaling pathway promotes PCa cell motility and to confirm that this signaling is essential for PCa migration and invasion in AA-derived PCa cells, we examined the activation of PI3K signaling pathway and phosphorylation of AKTser473 (pAKTser473) after TGFβ treatment in E006AA and E006AA-HT cells. As shown in Figure 4A and D, both TGFβ1 and -β3 induced an increase in the levels of pAKTser473 in both E006AA and E006AA-HT cells. Moreover, a robust increase in pAKTser473 levels is seen after TGFβ3 treatment in both cells lines.
Figure 4.
The effects of TGFβ on the phosphorylation of AKTser473 and the migration and invasion of AA-derived PCa cells. (A, D) TGFβ1 and -β3 induces the phosphorylation of AKTser473 in E006AA and E006AA-HT cells. PI3K inhibitor blocks TGFβ-induced pAKTser473. Cells were treated with TGFβ1 and -β3 (5 ng/ml) in the presence or absence of specific inhibitors of βRII (LY2157299; 10 µM), Smad3 (SIS3; 3 µM) and PI3K (LY294002 10 µM). Cells treated with EGF were used as positive controls. Total (pan) AKT was used as a loading control. (B, C, E, F) TGFβ promotes cell migration and invasion of E006AA and E006AA-HT cells via the PI3K-signaling pathway. Cells were treated with TGFβ1 and -β3 in the presence or absence of the PI3K inhibitor (LY294002) and allowed to migrate or invade for 24 and 48 h, respectively. Data represent at least three independent experiments. ‘a–d’ denote significant differences (P < 0.001) among different treatment groups.
To further determine whether TGFβ-induced increased pAKTser473 levels are mediated by TGFβRII, SMAD3 or PI3K pathway, E006AA and E006AA-HT cells were pretreated with specific inhibitors of TGFβRII (LY2157299), SMAD3 (SIS3) and PI3K (LY294002) for 2 h, followed by stimulation with TGFβ1 or -β3. As shown in Figure 4A and D, both TGFβRII (LY2157299) and SMAD3 (SIS3) inhibitors blocked TGFβ-induced increase levels of pAKTser473 in E006AA and E006AA-HT cells. Additionally, pretreatment with PI3K inhibitor, LY294002, resulted in decrease in pAKTser473 levels in control and TGFβ-treated E006AA and E006AA-HT cells. Furthermore, PI3K inhibitor significantly (P < 0.001) blocked TGFβ1- and -β3-induced cell migration and invasion of AA-derived PCa cells (4B, 4C, 4E and 4F) indicating that TGFβ-induced PI3K/AKT activation is essential for cell migration and invasion in AA-derived PCa cells (15).
TGFβ induces MMP2 and MMP9 expression in AA-derived PCa cells
Previous studies have shown that matrix metalloproteinases (MMPs) are involved in cancer metastases (39) and that TGFβ induces the expression of MMPs, specifically MMP2 and MMP9 in several malignancies (40,41). To examine MMPs expression levels in PCa cells derived from AA patients, we analyzed the expression levels of MMP2 and MMP9 mRNA and protein via RT-PCR and western blot analysis in E006AA, E006AA-HT, MDA-AS and MDA cells and compared their expressions with CA-derived PCa cell lines, LNCaP and DU145 cells. As shown in Supplementary Figure 2A, available at Carcinogenesis Online, MMP2 and MMP9 mRNA is highly expressed in AA-derived PCa cell lines compared with CA-derived PCA cell lines with lower mRNA levels in LNCaP cells. MMP2 and MMP9 proteins were also ubiquitously expressed in all PCa cell lines. Moreover, MMP2 was significantly expressed (P < 0.001) in E006AA-HT, MDA-2A and MDA-2B, compared with LNCaP, DU145 and E006AA cell. Additionally, MMP9 was highly expressed (P < 0.001) in E006AA, E006AA-HT and MDA-2B, compared with LNCaP and DU145 cells (Supplementary Figure 2B and C, available at Carcinogenesis Online).
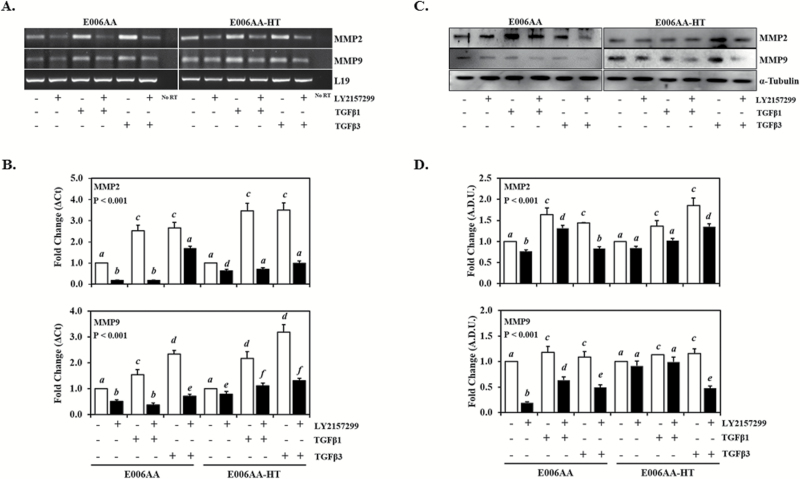
To assess the effect of TGFβ on MMP2 and MMP9 expression in AA-derived PCa cells, E006AA and E006AA-HT cells were treated with TGFβ1 and -β3 for 24 h. As seen in Figure 5A and B, TGFβ1 and -β3 significantly (P < 0.001) induces MMP2 and MMP9 mRNA expression compared with control cells in both E006AA and E006AA-HT cells, with TGFβ3 having a greater effect on MMP9 mRNA expression (Figure 5B). Additionally, our data show a significant increase (P < 0.001) in MMP2 and MMP9 protein levels in these cells following TGFβ1 and -β3 treatment as shown in Figure 5C and D. We also examined MMP2 and MMP9 expression in E006AA and E006AA-HT cells treated with TGFβ1 and -β3 in the presence of TGFβRII inhibitor (LY2157299). Our data show that LY2157299 significantly blocked (P < 0.001) TGFβ-induced MMP2 and MMP9 expression at both mRNA and protein levels (Figure 5).
Figure 5.
The effect of TGFβ on MMP2 and MMP9 expression in AA-derived PCa cells. E006AA and E006AA-HT cells were treated with TGFβ1 and -β3 in the presence or absence of TGFβRII inhibitor (LY2157299) for 24 h. (A) RT-PCR and (B) qPCR analysis of MMP2 and MMP9 mRNA expression and (C) western blot analysis of MMP2 and MMP9 protein levels in AA-derived PCa cells. (D) Densitometry analysis of protein levels normalized to α-Tubulin. Data represent at least three independent experiments. ‘a–d’ denote significant differences (P < 0.001) among different treatment groups.
Discussion
The primary goal of this study was to address the molecular basis of PCa health disparity in AA men. The present study investigated the potential role of TGFβ signaling, specifically TGFβ3 involvement in the development and progression of metastatic PCa. We investigated for the first time circulating levels of TGFβ3 in AA PCa patients and present evidence linking high circulating levels of TGFβ3 protein to PCa in AA men. We also show for the first time the role of TGFβ signaling in cell proliferation, migration and invasion of PCa cells derived from AA patients.
TGFβ signaling pathway has been studied for many years, and its involvement in cell proliferation, differentiation, survival, apoptosis and several other cellular processes has been well documented (15,17–19). TGFβ signaling pathway has also been implicated to play a role in cancer (15,17–19,42). Recent studies have correlated the overexpression of TGFβ1 with health disparities in AAs. These studies showed that increased TGFβ1 levels in peripheral blood were observed in AA patients diagnosed with hypertension (24,25), diabetes (26) and burden of renal disease (27) but not in CA patients. It has also been suggested that in addition to several signaling pathways, TGFβ signaling pathway is associated with PCa racial disparity (2,43,44). Previous studies from our lab determined the expression of TGFβ isoforms in several prostate cell lines representing normal epithelial cells and various stages of PCa progression and revealed that TGFβ1 was expressed in all prostate cell lines at a similar level; however, TGFβ3 was expressed at very low levels in normal prostate epithelial cells but was expressed at much higher levels in the more metastatic PCa cell lines (15). Earlier studies from several labs have also suggested an important role of TGFβ3 (versus TGFβ1) in cancer metastases such as endometrial cancer (22), breast cancer (20,21) and head and neck cancer (23). Despite homology in amino acid sequence and the ability of both TGFβ1 and -β3 to bind directly to type II receptor (TGFβRII), TGFβ3 differs significantly from TGFβ1 structurally (45). This structural difference between the isoforms affects the ligand–receptor complex that may engage the downstream signaling pathways in different ways, thus leading to different biological outcomes (45).Although there is sufficient evidence linking TGFβ3’s role in cancer metastases and other diseases, there was no previous data linking TGFβ3 specifically to health disparities in AA men with PCa. Therefore, in the current study, we focused on elucidating the role of TGFβ signaling, specifically TGFβ3 in PCa in AA men. Our results showed that circulating levels of TGFβ3 protein are higher in AA PCa patients compared with CA patients. In fact, AA men without PCa had higher circulating levels of TGFβ3 compared with CA men with and without PCa. Although this study showed a trend and statistical differences of hypersecretion of TGFβ3 association in AA men with and without PCa compared with the CA men, we did not have sufficient number of samples representing particular stages of cancer in both groups to show significant associations or correlations of high circulating levels of TGFβ3 with clinicopathological profile. To make the correlation between TGFβ3 protein and PCa health disparities, future studies must be completed to evaluate the differences in TGFβ3 protein levels in PCa progression in patients from each ethnic group. These comparative analyses must be based on the PSA levels, Gleason score and pathological stages of PCa. Overall, this data strongly support the overexpression of TGFβ-signaling pathway with health disparities seen in AAs (26–28,46). This study also provides evidence that disparities in cancer biology are evident between AA and CA populations; however, further assessments must be made to evaluate the possible implications of genetic ancestry on the observed differences in the expression of TGFβ3 between the two populations. These findings are very similar to previous studies that show AAs in general, regardless of being ‘normal’ or diagnosed with the disease having increased levels of TGFβ1 compared with CAs (28–30). Our data also support previous findings that suggest that molecular differences in tumor biology contribute to PCa health disparity (28,43,44). TGFβ has a dual role in human cancer progression (19,35). In normal epithelial cells and early carcinomas, TGFβ functions as a tumor suppressor, which includes the inhibition of cell growth, induction of apoptosis and genomic stability or inhibition of cell immortalization to maintain and regulate a cell’s normal state (15,17,18). However, when cells become abnormal or cancerous, TGFβ switches its role and function as a tumor promoter. At this point, cancerous cells become resistant to the growth inhibitory effects of TGFβ but instead grow uncontrollably; TGFβ induces epithelial–mesenchymal transition, and cells gain invasive and migratory behavior leading to metastasis (15,17–19). Our previous studies and present results show that TGFβ inhibits the proliferation of DU145 cells (35,47), while AA-derived PCa cells, E006AA, E006AA-HT, MDA-2A and MDA-2B cells, are resistant to these growth inhibitory effects in the presence of functional TGFβ receptors and SMAD signaling. Interestingly, our data showed a slight stimulatory effect of TGFβ isoforms on cell proliferation. These results indicate differences in signaling mechanisms downstream of receptor-dependent SMAD activation, which are responsible for differential effects of TGFβ on cell proliferation (35). Furthermore, we showed previously that the inhibitory effects of TGFβ on proliferation of prostate epithelial cells depend on proteosomal degradation of JunD, and the lack of TGFβ effects on degradation of JunD may be responsible for the resistance to growth inhibitory effects of TGFβ in advanced stages of PCa (35). Whether or not JunD plays a role in the lack of TGFβ effects on AA-derived cell lines remains to be established.
Our previous studies also showed that TGFβ3 is expressed in highly metastatic PCa cell lines (PC3 and PC3M) and is involved in the induction of invasive and migratory behavior in these cells via the PI3-kinase/Akt/mTOR pathway (15,38). Our present study shows that AA-derived PCa cells expressed higher levels of TGFβ3 protein compared with PCa cells derived from CA patients, similar to our findings on differences in serum TGFβ3 levels. Furthermore, we demonstrated that TGFβ acts as an extracellular signal that activates migration via PI3K pathway in a TGFβ/PI3K dependent manner in AA-derived PCa E006AA and E006AA-HT cells. We confirmed that the interaction between TGFβ and PI3k pathway is necessary for the activation of AKT in response to TGFβ stimulation in these cells. Consequently, TGFβ-stimulated AKT activation promotes the migratory and invasive behavior of AA-derived PCa cells.
The metastatic behavior of PCa cells derived from AA patients is further supported by the increased expression of MMP2 and MMP9 RNA and protein levels compared with PCa cells derived from CA patients. Moreover, TGFβ induced the expression of MMP2 and MMP9, which was blocked in the presence of TGFβRII inhibitor (LY2157299). Decreased levels of MMP2 and MMP9 mRNA expression and protein levels indicate that TGFβ signaling leads to the expression of these proteins and degradation of the extracellular matrix, thus resulting in cell migration and invasion. Similar to the majority of solid tumors, the main mortality factor attributed to PCa is the process of cell spreading (metastasis) from the primary tumor to secondary sites (48,49). The metastatic potential of cancerous tumors depends on the expression/function of a number of genes including MMPs, which are involved in regulating tumor growth, motility of tumor cells, angiogenesis and the invasiveness of the tumor cells (39). MMPs, specifically MMP2 and MMP9, have been implicated in several malignancies such as breast (50–52), lung (53), colorectal (54) and PCa (55,56) and have been shown to be highly expressed in these metastatic cancers. In addition, as tumors grow and progress, they generally produce and secrete a large amount of autocrine TGFβ that is released in the tumor microenvironment (17,19). These increased TGFβ levels not only affect the tumor cells themselves but also the surrounding stroma by inhibiting cell adhesion, inducing immunosuppression and angiogenesis, and by promoting the degradation of the extracellular matrix by activating MMPS, further contributing to the metastatic process (40,41).
Overall, the key findings in this study are that (i) There are higher levels of TGFβ3 in AA men in blood circulation compared with CA men, and there are higher protein levels of TGFβ3 in all AA-derived PCa cell lines, (ii) PCa cell lines derived from AA patients have developed resistance to antiproliferative effects of TGFβ and (iii) TGFβ does not inhibit cell proliferation in AA-derived PCa cell lines, but it does induce migration and invasion through activation of PI3K pathway in these cells. These findings support evidence that TGFβ3 levels may be responsible for development of more advanced and aggressive PCa in AA patients as a consequence of development of resistance to inhibitory effects of TGFβ on cell proliferation and induction of invasive metastatic behavior. Additional studies are required to determine whether TGFβ3 proteins levels are also high in the PCa patient tissues.
Though the changes in intracellular signaling pathways leading to differential effects of TGFβ during stages of cancer progression are poorly understood, the dual role played by TGFβ and particularly its prometastatic effects make it an attractive target for the development of novel therapies aimed at specifically blocking the prometastatic arm of its signaling pathway (19).
Supplementary material
Supplementary data are available at Carcinogenesis online.
Funding
These studies were supported by the National Institutes of Health (grant numbers: G12MD007590 and 5P20MD002285).
Acknowledgements
We are grateful to Dr Shahriar Koochekpour of Roswell Park Cancer Institute, in Buffalo, NY for providing E006AA and E006AA-HT cells and Dr Ming-Fong Lin of University of Nebraska Medical Center in Omaha, Nebraska for providing MDA-2A and MDA-2B cells, all PCa cell lines derived from AA patients. We would like to thank Mrs Ana Cecilia Millena for her technical assistance and Dr Silvia Caggia for her help in establishing MDAs cell culture in our lab.
Conflict of Interest Statement: None declared.
Abbreviations
- AA
African-American
- CA
Caucasian
- FBS
fetal bovine serum
- MMPs
matrix metalloproteinases
- PCa
prostate cancer
- PSA
prostate-specific antigen
- TGFβRI
TGFβ receptors type I
- TGFβRII
TGFβ receptors type II
References
- 1. Siegel R.L., et al. (2017)Cancer statistics, 2017. CA. Cancer J. Clin., 67, 7–30. [DOI] [PubMed] [Google Scholar]
- 2. Bhardwaj A., et al. (2017)Racial disparities in prostate cancer: a molecular perspective. Front. Biosci. (Landmark Ed.), 22, 772–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pietro G.D., et al. (2016)Racial differences in the diagnosis and treatment of prostate cancer. Int. Neurourol. J., 20(Suppl 2), S112–S119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Farrell J., et al. (2013)Genetic and molecular differences in prostate carcinogenesis between African American and Caucasian American men. Int. J. Mol. Sci., 14, 15510–15531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bensen J.T., et al. (2013)Genetic polymorphism and prostate cancer aggressiveness: a case-only study of 1,536 GWAS and candidate SNPs in African-Americans and European-Americans. Prostate, 73, 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ledet E.M., et al. (2012)Suggestive evidence of linkage identified at chromosomes 12q24 and 2p16 in African American prostate cancer families from Louisiana. Prostate, 72, 938–947. [DOI] [PubMed] [Google Scholar]
- 7. Devaney J.M., et al. (2015)Genome-wide differentially methylated genes in prostate cancer tissues from African-American and Caucasian men. Epigenetics, 10, 319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kwabi-Addo B., et al. (2010)Identification of differentially methylated genes in normal prostate tissues from African American and Caucasian men. Clin. Cancer Res., 16, 3539–3547. [DOI] [PubMed] [Google Scholar]
- 9. Long M.D., et al. (2017)The genomic impact of DNA CpG methylation on gene expression; relationships in prostate cancer. Biomolecules, 7(1), 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Theodore S.C., et al. (2010)MiRNA 26a expression in a novel panel of African American prostate cancer cell lines. Ethn. Dis., 20(1 Suppl 1), S1–S96. [PMC free article] [PubMed] [Google Scholar]
- 11. Yang Y., et al. (2016)Dysregulation of miR-212 promotes castration resistance through hnRNPH1-mediated regulation of AR and AR-V7: implications for racial disparity of prostate cancer. Clin. Cancer Res., 22, 1744–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang B.D., et al. (2013)Androgen receptor-target genes in african american prostate cancer disparities. Prostate Cancer, 2013, 763569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sreenath T.L., et al. (2011)Oncogenic activation of ERG: a predominant mechanism in prostate cancer. J. Carcinog., 10, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nakai Y., et al. (2013)Inflammation and prostate carcinogenesis. Int. J. Urol., 20, 150–160. [DOI] [PubMed] [Google Scholar]
- 15. Walker L., et al. (2013)Expression of TGFβ3 and its effects on migratory and invasive behavior of prostate cancer cells: involvement of PI3-kinase/AKT signaling pathway. Clin. Exp. Metastasis, 30, 13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nagaraj N.S., et al. (2010)Targeting the transforming growth factor-beta signaling pathway in human cancer. Expert Opin. Investig. Drugs, 19, 77–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bellomo C., et al. (2016)Transforming growth factor β as regulator of cancer stemness and metastasis. Br. J. Cancer, 115, 761–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Caja L., et al. (2015)Transforming growth factor β and bone morphogenetic protein actions in brain tumors. FEBS Lett., 589, 1588–1597. [DOI] [PubMed] [Google Scholar]
- 19. Lebrun J.J. (2012)The dual role of TGFβ in human cancer: from tumor suppression to cancer metastasis. ISRN Mol. Biol., 2012, 381428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ghellal A., et al. (2000)Prognostic significance of TGF beta 1 and TGF beta 3 in human breast carcinoma. Anticancer Res., 20, 4413–4418. [PubMed] [Google Scholar]
- 21. Lam S., et al. (2014)Wild-type p53 inhibits pro-invasive properties of TGF-β3 in breast cancer, in part through regulation of EPHB2, a new TGF-β target gene. Breast Cancer Res. Treat., 148, 7–18. [DOI] [PubMed] [Google Scholar]
- 22. Van Themsche C., et al. (2007)Transforming growth factor-beta3 increases the invasiveness of endometrial carcinoma cells through phosphatidylinositol 3-kinase-dependent up-regulation of X-linked inhibitor of apoptosis and protein kinase c-dependent induction of matrix metalloproteinase-9. J. Biol. Chem., 282, 4794–4802. [DOI] [PubMed] [Google Scholar]
- 23. Qin X., et al. (2016)TGFβ3-mediated induction of Periostin facilitates head and neck cancer growth and is associated with metastasis. Sci. Rep., 6, 20587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. August P., et al. (2000)Hypertension-induced organ damage in African Americans: transforming growth factor-beta(1) excess as a mechanism for increased prevalence. Curr. Hypertens. Rep., 2, 184–191. [DOI] [PubMed] [Google Scholar]
- 25. Suthanthiran M., et al. (2000)Transforming growth factor-beta 1 hyperexpression in African-American hypertensives: a novel mediator of hypertension and/or target organ damage. Proc. Natl. Acad. Sci. USA, 97, 3479–3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huan Y., et al. (2010)Regulation of transforming growth factor-beta1 by insulin in prediabetic African Americans. Kidney Int., 78, 318–324. [DOI] [PubMed] [Google Scholar]
- 27. August P., et al. (2009)Transforming growth factor beta and excess burden of renal disease. Trans. Am. Clin. Climatol. Assoc., 120, 61–72. [PMC free article] [PubMed] [Google Scholar]
- 28. Quan L., et al. (2014)Cytokine and cytokine receptor genes of the adaptive immune response are differentially associated with breast cancer risk in American women of African and European ancestry. Int. J. Cancer, 134, 1408–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Silver R.M., et al. (2012)Racial differences between blacks and whites with systemic sclerosis. Curr. Opin. Rheumatol., 24, 642–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Reese C., et al. (2014)Caveolin-1 deficiency may predispose African Americans to systemic sclerosis-related interstitial lung disease. Arthritis Rheumatol., 66, 1909–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Koochekpour S., et al. (2004)Establishment and characterization of a primary androgen-responsive African-American prostate cancer cell line, E006AA. Prostate, 60, 141–152. [DOI] [PubMed] [Google Scholar]
- 32. Koochekpour S., et al. (2014)Establishment and characterization of a highly tumorigenic African American prostate cancer cell line, E006AA-hT. Int. J. Biol. Sci., 10, 834–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Navone N.M., et al. (1997)Establishment of two human prostate cancer cell lines derived from a single bone metastasis. Clin. Cancer Res., 3, 2493–2500. [PubMed] [Google Scholar]
- 34. Schmittgen T.D., et al. (2008)Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc., 3, 1101–1108. [DOI] [PubMed] [Google Scholar]
- 35. Millena A.C., et al. (2016)JunD is required for proliferation of prostate cancer cells and plays a role in transforming growth factor-β (TGF-β)-induced inhibition of cell proliferation. J. Biol. Chem., 291, 17964–17976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chalhoub N., et al. (2009)PTEN and the PI3-kinase pathway in cancer. Annu. Rev. Pathol., 4, 127–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liu P., et al. (2009)Targeting the phosphoinositide 3-kinase pathway in cancer. Nat. Rev. Drug Discov., 8, 627–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vo B.T., et al. (2013)TGF-β effects on prostate cancer cell migration and invasion are mediated by PGE2 through activation of PI3K/AKT/mTOR pathway. Endocrinology, 154, 1768–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Petruzelka L. (2001)[Diagnosis and treatment of tumor metastases]. Vnitr. Lek., 47, 555–560. [PubMed] [Google Scholar]
- 40. Chiang S.P., et al. (2016)Tumor cell intravasation. Am. J. Physiol. Cell Physiol., 311, C1–C14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Al-Raawi D., et al. (2011)Membrane type-1 matrix metalloproteinase (MT1-MMP) correlates with the expression and activation of matrix metalloproteinase-2 (MMP-2) in inflammatory breast cancer. Int. J. Clin. Exp. Med., 4, 265–275. [PMC free article] [PubMed] [Google Scholar]
- 42. Loomans H.A., et al. (2014)Intertwining of activin A and TGFβ signaling: dual roles in cancer progression and cancer cell invasion. Cancers (Basel), 7, 70–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kinseth M.A., et al. (2014)Expression differences between African American and Caucasian prostate cancer tissue reveals that stroma is the site of aggressive changes. Int. J. Cancer, 134, 81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wallace T.A., et al. (2008)Tumor immunobiological differences in prostate cancer between African-American and European-American men. Cancer Res., 68, 927–936. [DOI] [PubMed] [Google Scholar]
- 45. Laverty H.G., et al. (2009)TGF-beta3 and cancer: a review. Cytokine Growth Factor Rev., 20, 305–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Alam A., et al. (2015)A preliminary study on racial differences in HMOX1, NFE2L2, and TGFβ1 gene polymorphisms and radiation-induced late normal tissue toxicity. Int. J. Radiat. Oncol. Biol. Phys., 93, 436–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Strong N., et al. (2013)Inhibitor of differentiation 1 (Id1) and Id3 proteins play different roles in TGFβ effects on cell proliferation and migration in prostate cancer cells. Prostate, 73, 624–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gialeli C., et al. (2011)Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J., 278, 16–27. [DOI] [PubMed] [Google Scholar]
- 49. Gomes L.R., et al. (2012)TGF-β1 modulates the homeostasis between MMPs and MMP inhibitors through p38 MAPK and ERK1/2 in highly invasive breast cancer cells. BMC Cancer, 12, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jacob A., et al. (2013)Rab40b regulates trafficking of MMP2 and MMP9 during invadopodia formation and invasion of breast cancer cells. J. Cell Sci., 126, 4647–4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ranogajec I., et al. (2012)Prognostic value of matrix metalloproteinase-2 (MMP-2), matrix metalloproteinase-9 (MMP-9) and aminopeptidase N/CD13 in breast cancer patients. Med. Oncol., 29, 561–569. [DOI] [PubMed] [Google Scholar]
- 52. Scorilas A., et al. (2001)Overexpression of matrix-metalloproteinase-9 in human breast cancer: a potential favourable indicator in node-negative patients. Br. J. Cancer, 84, 1488–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. González-Arriaga P., et al. (2012)Genetic polymorphisms in MMP 2, 9 and 3 genes modify lung cancer risk and survival. BMC Cancer, 12, 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhao Q., et al. (2017)MFG-E8 overexpression promotes colorectal cancer progression via AKT/MMPs signalling. Tumour Biol., 39, 1010428317707881. [DOI] [PubMed] [Google Scholar]
- 55. Huo C., et al. (2015)Androgen receptor inhibits epithelial-mesenchymal transition, migration, and invasion of PC-3 prostate cancer cells. Cancer Lett., 369, 103–111. [DOI] [PubMed] [Google Scholar]
- 56. Wilson S.R., et al. (2004)Amplification of MMP-2 and MMP-9 production by prostate cancer cell lines via activation of protease-activated receptors. Prostate, 60, 168–174. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.