Abstract
Background
We previously demonstrated that brentuximab vedotin (BV) used as second-line therapy in patients with Hodgkin lymphoma is a tolerable and effective bridge to autologous hematopoietic cell transplantation (AHCT). Here, we report the post-AHCT outcomes of patients treated with second-line standard/fixed-dose BV and an additional cohort of patients where positron-emission tomography adapted dose-escalation of second-line BV was utilized.
Patients and methods
Patients on the dose-escalation cohort received 1.8 mg/kg of BV intravenously every 3 weeks for two cycles. Patients in complete remission (CR) after two cycles received two additional cycles of BV at 1.8 mg/kg, while patients with stable disease or partial response were escalated to 2.4 mg/kg for two cycles. All patients, regardless of treatment cohort, proceeded directly to AHCT or received additional pre-AHCT therapy at the discretion of the treating physician based on remission status after second-line BV.
Results
Of the 20 patients enrolled to the BV dose-escalation cohort, 8 patients underwent BV dose-escalation. BV escalation was well-tolerated, but no patients who were escalated converted to CR. Of 56 evaluable patients treated across cohorts, the overall response rate (ORR) to second-line BV was 75% with 43% CR. Twenty-eight (50%) patients proceeded directly to AHCT without post-BV chemotherapy, and a total of 50 patients proceeded to AHCT. Thirteen patients received consolidative post-AHCT therapy with either radiation, BV, or a PD-1 inhibitor. After AHCT, the 2-year progression-free survival (PFS) and overall survival were 67% and 93%, respectively. The 2-year PFS among patients in CR at the time of AHCT (n = 37) was 71% compared with 54% in patients not in CR (p = 0.12). The 2-year PFS in patients who proceeded to AHCT directly after receiving BV alone was 77%.
Conclusions
Second-line BV is an effective bridge to AHCT that produces responses of sufficient depth to provide durable remission in conjunction with AHCT (clinicaltrials.gov: NCT01393717).
Keywords: Hodgkin lymphoma, autologous stem-cell transplantation, brentuximab vedotin
Key Message
We report the outcomes after autologous hematopoietic cell transplantation (AHCT) in patients with relapsed/refractory Hodgkin lymphoma treated on a trial of second-line brentuximab vedotin (BV), including a cohort evaluating positron-emission tomography (PET)-adapted BV dose-escalation. BV as initial salvage therapy followed by AHCT produced durable remissions. PET-adapted BV-escalation was tolerable but did not deepen responses.
Introduction
Standard of care treatment for patients with relapsed or refractory (rel/ref) classical Hodgkin lymphoma (cHL) is salvage chemotherapy followed by autologous hematopoietic cell transplantation (AHCT) in chemosensitive patients [1–3]. Various second-line salvage chemotherapy regimens are utilized before AHCT, including platinum-based and gemcitabine-based regimens, and are associated with comparable response rates [4–9].
Brentuximab vedotin (BV) is an antibody drug conjugate of an anti-CD30 monoclonal antibody with the microtubule-disrupting agent monomethyl auristatin E, which selectively induces apoptosis of CD30-positive cHL cells. We and others have previously demonstrated that BV used as second-line therapy can be an effective bridge to AHCT, with 27% to 35% of patients achieving a complete response (CR) and proceeding directly to AHCT without additional chemotherapy [10, 11]. Overall, a high proportion of patients (86%–98%) proceeded to AHCT following a second-line BV strategy, either directly after BV alone or after receiving additional chemotherapy. Moskowitz and colleagues demonstrated excellent outcomes after a positron-emission tomography (PET)-adapted sequential second-line BV followed by ifosfamide, carboplatin, etoposide salvage approach with 2-year post-AHCT progression-free survival (PFS), and overall survival (OS) of 80% and 95%, respectively [11].
In our initial study of second-line BV in cHL, all patients who achieved a CR did so after two cycles of BV. No patients who had a partial response (PR) or stable disease (SD) after two cycles converted to CR after two additional cycles at the standard dose (1.8 mg/kg), and, in fact, some patients who had PR/SD experienced progressive disease (PD) before AHCT. Although the approved BV dose is 1.8 mg/kg, higher doses have been safely administered in clinical trials, including 2.4 mg/kg, administered in a phase II study of patients with CD30-negative nonlymphomatous malignancies[12], and 2.7 mg/kg, which demonstrated a 25% dose-limiting toxicity rate in a phase I study [13]. The achievement of CR before AHCT is a key determinant of post-AHCT outcome [14–17]. We hypothesized that BV dose-escalation in patients with PR/SD after two cycles may convert patients to CR before AHCT and prevent PD in subsequent cycles. With the goal of maximizing the proportion of patients in CR after second-line BV, we added a cohort to our multicenter phase II study evaluating the efficacy of second-line PET-adapted BV dose-escalation in patients with rel/ref cHL. Here, we present the findings of our PET-adapted BV dose-escalation approach, as well as the post-AHCT outcomes in all of the rel/ref cHL patients (standard and dose-escalated BV cohorts) enrolled onto our multicenter phase II study of second-line BV.
Materials and methods
Patients and treatment plan
The patients included in this analysis were enrolled onto a prospective, multicenter (City of Hope, Weill Cornell) phase II trial of second-line BV after failure of initial therapy in patients with transplant-eligible rel/ref cHL. The study was approved by the Institutional Review Board at both centers according to the Declaration of Helsinki.
The eligibility criteria for patients enrolled onto the study have been previously described [10]. In brief, patients over 10 years of age with biopsy-proven cHL that had relapsed or was primary refractory (lack of CR or PD within 3 months of upfront therapy) after standard initial therapy were eligible. Patients enrolled onto the standard BV dose cohort (cohort 1) were treated with 1.8 mg/kg of BV intravenously every 3 weeks for two cycles followed by computed tomography with PET (CT/PET) evaluation. Patients in SD/PR/CR after two cycles received an additional two cycles of BV at 1.8 mg/kg followed by an end-of-treatment (EOT) CT/PET. The eligibility criteria for enrollment onto the PET-adapted BV dose-escalation cohort (cohort 2) were the same as for cohort 1. Patients enrolled onto cohort 2 were treated with 1.8 mg/kg of BV intravenously every 3 weeks for two cycles followed by CT/PET. Patients in CR after two cycles received two additional cycles of BV at 1.8 mg/kg. Patients in PR/SD after two cycles received 2.4 mg/kg BV for two cycles. Responses were assessed by investigators according to the 2007 Cheson criteria (primary end point) and were also assessed using the 2014 Lugano classification.
The decision to administer, the type, and number of cycles of additional salvage therapy before AHCT was at the discretion of the treating physician. AHCT was performed according to institutional practices. CD68 assessment by immunohistochemistry was performed as previously described [10] when tumor tissue (preferentially obtained at the time of relapse) was available.
Statistical analysis
The primary end point of this analysis was 2-year PFS. OS was a secondary end point. Baseline characteristics were reported descriptively. PFS and OS were estimated using the Kaplan–Meier method and the log-rank test was used to compare OS and PFS between subgroups. P-values were two-sided with a significance level of 0.05. Univariate and multivariable logistic regression models were used to assess associations between clinical characteristics and use of additional chemotherapy after BV. Cox regression models were used to identify factors associated with post-AHCT PFS and OS. For multivariable analyses, factors significant at the P < 0.10 level univariately were included. All data were analyzed using SAS version 9.3 (Cary, NC).
Results
PET-adapted escalation of brentuximab vedotin
Twenty patients were enrolled onto cohort 2 between 9/2014 and 3/2016 and all were evaluable for safety and efficacy. The baseline characteristics of cohort 2 patients are described in Table 1. The median number of BV cycles received was 4 (range, 2–4). Eleven patients (55%) achieved CR after two cycles and were treated at 1.8 mg/kg for all four cycles, while eight patients had PR/SD after two cycles and then were treated at 2.4 mg/kg in cycles 3–4. One patient had PD after two cycles and discontinued BV. BV dose-escalation was well-tolerated, there were no grade 3 or 4 AEs among escalated patients. The most common adverse events (AEs) in patients who received escalated BV dosing were peripheral neuropathy (63%, Gr2 13%), rash (50%, Gr2 25%), elevated alanine aminotransferase (50%, all Gr1), hypertension (50%, all Gr1), and arthralgia (50%, Gr2 13%). No patients who had BV dose-escalation converted to CR. The overall best response rate was 85% (17/20) and the CR rate was 55% (11/20). One patient with PR after two cycles who underwent BV dose-escalation had PD at the EOT response assessment. Similarly, a patient in CR after two cycles who received two additional cycles of standard dose BV had PD at EOT.
Table 1.
Baseline characteristics of patients in PET-adapted BV escalation cohort (N = 20), all patients enrolled onto study (N = 57), and all patients who underwent AHCT (N = 50)
| Variable | PET-adapted BV escalation cohort (N = 20) | All patients (N = 57) | All AHCT patients (N = 50) |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Patient gender | |||
| Female | 12 (60) | 29 (51) | 24 (48) |
| Male | 8 (40) | 28 (49) | 26 (52) |
| Median age in years (range) | 25 (15–57) | 32 (11–67) | 31 (11–59) |
| KPS at enrollment | |||
| 70 | 0 (0) | 2 (3) | 1 (2) |
| 80 | 4 (20) | 14 (25) | 10 (20) |
| 90 | 9 (45) | 28 (49) | 26 (52) |
| 100 | 7 (35) | 13 (23) | 13 (26) |
| Stage at diagnosis | |||
| I | 1 (5) | 2 (4) | 2 (4) |
| II | 9 (45) | 27 (47) | 24 (48) |
| III | 7 (35) | 11 (19) | 9 (18) |
| IV | 3 (15) | 17 (30) | 15 (30) |
| B symptoms at diagnosis | |||
| No | 3 (15) | 14 (25) | 13 (26) |
| Yes | 12 (60) | 35 (61) | 29 (58) |
| Unknown | 5 (25) | 8 (14) | 8 (16) |
| Bulky disease at diagnosis | |||
| No | 4 (20) | 9 (16) | 9 (18) |
| Yes | 15 (75) | 47 (82) | 40 (80) |
| Unknown | 1 (5) | 1 (2) | 1 (2) |
| Bone marrow involvement at diagnosis | |||
| No | 13 (65) | 42 (74) | 37 (74) |
| Yes | 4 (20) | 8 (14) | 7 (14) |
| Unknown | 3 (15) | 7 (12) | 6 (12) |
| Initial treatment regimen | |||
| ABVD | 17 (85) | 51 (89) | 44 (88) |
| ABVE+PC | 3 (15) | 4 (7) | 4 (8) |
| ABVD/BEACOPP | 0 (0) | 2 (5) | 2 (4) |
| Prior radiation therapy | |||
| No | 15 (75) | 43 (75) | 36 (72) |
| Yes | 5 (25) | 14 (25) | 14 (28) |
| Relapsed or refractory status | |||
| Relapsed | 8 (40) | 22 (39) | 21 (42) |
| Primary refractory | 12 (60) | 35 (61) | 29 (58) |
| Disease status at AHCT | |||
| CR | 37 (74) | ||
| PR | 12 (24) | ||
| SD | 1 (2) | ||
| Conditioning regimen | |||
| BEAM | 33 (66) | ||
| CBV | 9 (18) | ||
| Y90-labeled anti-CD25/BEAM | 7 (14) | ||
| BeEAM (Be = bendamustine) | 1 (2) | ||
AHCT, autologous hematopoietic cell transplantation; CR, complete response; PR, partial response; SD, stable disease; CBV, carmustine, cyclophosphamide, and etoposide; BEAM, carmustine, etoposide, cytarabine, melphalan; Y90, yttrium-90; PET, positron-emission tomography; BV, brentuximab vedotin; KPS, Karnofsky Performance Status.
In total, eight patients received salvage combination chemotherapy after BV, and the overall best response rate to post-BV chemotherapy was 88% and CR rate was 50%. Eighteen (90%) cohort 2 patients (18/20) successfully proceeded to AHCT, with 14 in CR and 4 in PR at the time of AHCT. Of the two patients who did not undergo AHCT, one underwent allogeneic hematopoietic cell transplantation (alloHCT) after receiving multiple subsequent lines of therapy and one patient had initial PR following subsequent salvage therapy but had PD before AHCT and was not responsive to subsequent treatments (supplemental Figure S1, available at Annals of Oncology online).
Final response data in entire cohort
In total, 57 patients were accrued to cohorts 1 and 2, and 56 were evaluable for efficacy. The baseline characteristics for all enrolled patients are listed in Table 1. The overall best response rate to second-line BV was 75% (42/56) and CR rate was 43% (24/56). Response rates were identical regardless of the response criteria utilized. Across both cohorts, 28 patients required additional chemotherapy after BV. The overall best response rate to chemotherapy in these patients was 86% (24/28) and the CR rate was 50% (14/28). Univariate and multivariable logistic regression models were used to assess the possible association between clinical characteristics (Table 1) and need for post-BV salvage chemotherapy. Patients with poorer performance status (Karnofsky ≤ 80) [odds ratio (OR) 4.76, 95% confidence interval (95% CI): 1.19–19.08, P = 0.028] were more likely to receive additional chemotherapy after BV. A lack of prior radiation therapy during initial treatment was associated with need for post-BV chemotherapy in univariate analyses, but was not significant in multivariate analyses (OR: 3.80, 95% CI: 0.92–15.73, P = 0.066).
Outcomes in all patients who underwent AHCT
Overall, 50 (88%) of 57 enrolled patients proceeded to AHCT after receiving second-line BV. Of the six evaluable patients who did not undergo AHCT, three patients ultimately proceeded to alloHCT and three patients were refractory to other salvage therapies. Twenty-eight patients (56%) proceeded directly to AHCT without post-BV chemotherapy, including one patient who received consolidative radiotherapy before AHCT. The remaining 22 patients (39%) received additional salvage chemotherapy, and only one patient required more than one type of salvage therapy regimen (supplemental Figure S2, available at Annals of Oncology online). Aside from the one patient, no other patients received pre-AHCT consolidative radiotherapy. Baseline characteristics of the patients who underwent AHCT are described in Table 1. Fifty-eight percent of patients had primary refractory disease after induction therapy and 80% of patients had bulky disease (defined here as >5 cm) at diagnosis. Thirty-seven patients (74%) were in CR, 12 (24%) were in PR, and 1 patient had SD at the time of AHCT. Following AHCT, seven patients underwent post-AHCT consolidative radiotherapy, two patients received consolidative BV, one patient received consolidative radiotherapy and BV, and three patients received consolidation with a PD-1 inhibitor as part of an investigational protocol.
Stem cell mobilization and collection information and engraftment data for cohort 1 were reported previously [10], and the findings in cohort 2 were similar. Among the cohort 2 patients, median cell dose collected was 7.96 × 106 CD34 cells/kg (range, 2.77–30.5), median time to neutrophil engraftment was 11 days (range, 10–12), and platelet engraftment was 17 days (range, 14–24).
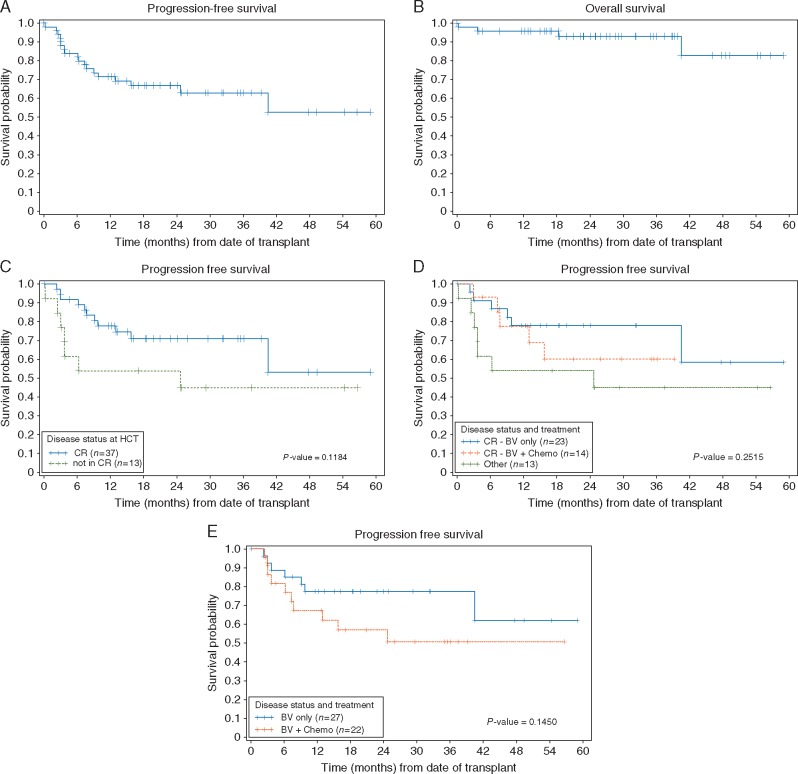
The median follow-up time in the cohort was 25.5 months (range, 0.2–58.9) in all patients, and 26.5 months (range, 3.9–58.9) in survivors. The 2-year PFS and OS in all patients who underwent AHCT were 66.8% (95% CI: 51.5–78.2) and 93.3% (95% CI: 80.2–97.8), respectively (Figure 1A and B). The 2-year nonrelapse mortality was 4.0% (95% CI: 1.0–15.6), and the rate of relapse/PD was 29.2% (95% CI: 18.8–45.6). Among patients in CR at the time of AHCT (n = 37), 2-year PFS was 70.9% (95% CI: 52.4–83.3) as compared with 53.8% (95% CI: 24.8–76.0) in patients who were not in CR (P = 0.12, Figure 1C). Patients who achieved CR with BV alone and proceeded directly to AHCT (n = 23), had a 2-year PFS of 77.8% (95% CI: 54.6–90.1). Patients who did not have a CR with BV, but achieved CR after receiving additional therapy (n = 14) had a 2-year PFS of 60.2% (95% CI: 28.8–81.3). Patients not in CR (n = 13) at the time of AHCT had a 2-year PFS of 53.8% (95% CI: 24.8–76.0, three-way P = 0.25, Figure 1D).
Figure 1.
(A) Progression-free survival (PFS) in all patients treated with second-line brentuximab vedotin (BV) who underwent autologous hematopoietic cell transplantation (AHCT). (B) Overall survival in all patients treated with second-line BV who underwent AHCT. (C) Post-AHCT PFS in patients who were in complete remission (CR) at the time of AHCT compared with patients not in CR at the time of AHCT. (D) Post-AHCT PFS in patients who achieved CR after BV alone compared with patients who entered CR with BV followed by chemotherapy compared with patients not in CR at the time of AHCT. (E) Post-AHCT PFS in patients who received BV alone compared with patients who received BV followed by chemotherapy before AHCT.
Among all 27 patients who proceeded directly to AHCT after receiving BV alone, including 4 patients in PR, the 2-year PFS was 77.4% (95% CI: 56.5–89.2) when compared with 57.0% (95% CI: 33.4–75.0) in patients (n = 22) who received post-BV chemotherapy (P = 0.15, Figure 1E). Although the number of patients is small (n = 4), the 2-year PFS in patients who proceeded to AHCT in PR after BV alone was 75% (95% CI: 13–96). In a univariate Cox model, there were no baseline characteristics significantly associated with post-AHCT PFS or OS, therefore no multivariate analyses were performed. In the subset of patients with CD68 testing available (n = 23), CD68 expression was not prognostic for post-AHCT outcome (P = 0.66, data not shown).
Discussion
In our study of second-line BV in patients with rel/ref cHL, we demonstrate that limited duration BV dose-escalation is tolerable and that BV is an effective first salvage therapy and bridge to AHCT with acceptable post-AHCT outcomes. In the small number of patients who underwent BV dose-escalation, no signal of increased toxicity was observed compared with patients treated with standard doses of BV. However, BV dose-escalation did not convert any patients from PR/SD to CR and there was a PD event in a patient receiving dose-escalated BV. BV dose-escalation is not a recommended approach.
Using an initial regimen of BV alone, a high proportion of patients proceeded to AHCT (88%). Similar to the findings by Moskowitz et al. [11], the overall 2-year post-AHCT PFS (67%) and OS (93%) observed in the cohort were excellent. In univariate and multivariate models, poorer performance status was associated with a higher likelihood of requiring post-BV salvage chemotherapy. There were no factors significantly associated with post-AHCT outcomes. Patients in a PET-negative CR at the time of AHCT, achieved either with BV alone or with BV plus additional salvage therapy, had higher a rate of 2-year post-AHCT PFS, though not significantly so. Although the result was not statistically significant likely due to the small number of patients not in CR at the time of AHCT, the trend we observed is consistent with the known prognostic value of pre-AHCT PET on post-AHCT outcomes [14–19]. Our analysis of predictive factors differs from the findings reported by Moskowitz and colleagues, who found no factors significantly associated with response to second-line BV, but observed that pre-AHCT PET was significantly associated with AHCT outcome in their cohort. The differences are likely due to the relatively small sizes of the cohorts.
Notably, traditional salvage chemotherapy was avoided before AHCT in half of the patients treated with a second-line BV approach. These patients were able to avoid inpatient admissions for the administration of chemotherapy, treatment-related cytopenias, transfusions, and other organ toxicities associated with combination salvage chemotherapy. Additionally, high doses of chemotherapy are associated with late organ and bone marrow toxicity, and secondary malignancies, so although these patients ultimately received high-dose chemotherapy as part of their AHCT conditioning regimen, there is theoretical benefit to having received less chemotherapy overall. We have not yet observed any post-AHCT secondary malignancies in our cohort, but the current follow-up time is not sufficiently long and there is no control group with which to make any comparison. A striking finding is the favorable post-AHCT 2-year PFS (77%) in patients who proceeded to AHCT after BV alone. It is important to note that the median follow-up time after AHCT in our study is only ∼26 months. However, in patients treated with standard salvage chemotherapy, the great majority of post-AHCT relapses occur within 2 years [10]. Thus far, there does not appear to be an increase in post-AHCT relapse in patients who received BV alone before AHCT. These results suggest that the depth of response achieved with second-line BV alone appears comparable with a chemotherapy-induced remission, and appears to be of sufficient depth to provide durable remission in conjunction with AHCT.
In patients who required additional therapy after second-line BV, the delay of traditional salvage chemotherapy did not appear to impact the response rate to subsequent chemotherapy (86% ORR, 50% CR). It might have been expected that the population of patients who failed or had an insufficient response to second-line BV might have been a particularly high risk group selected for resistance to therapy. However, among patients who proceeded to AHCT after receiving additional post-BV salvage chemotherapy, the delay of chemotherapy did not appear to negatively impact the overall post-AHCT PFS. Overall, only four patients (7% of overall cohort) were unresponsive to subsequent salvage chemotherapy. Our findings suggest that patients who require additional therapy after second-line BV are readily salvaged with third-line chemotherapy and chemosensitive patients have acceptable post-AHCT outcomes.
Although our findings are promising, the prognostic importance of PET-negativity before AHCT mandates further improvement to the second-line therapy CR rate to achieve the goal of supplanting combination salvage chemotherapy as the standard of care. However, our findings and those of Moskowitz and colleagues [11] demonstrate the plausibility of studying alternative, nonchemotherapy-containing second-line salvage regimens and support the ongoing development of BV-based salvage therapy approaches. There is an ongoing study evaluating second-line BV plus nivolumab, which has demonstrated promising tolerability and efficacy in preliminary analyses [20]. In addition, other trials have demonstrated high response and CR rates when BV has been added to traditional salvage chemotherapy, including studies adding BV to etoposide, methylprednisone, cytarabine, and cisplatin (ESHAP), dexamethasone, cytarabine, cisplatin (DHAP), or bendamustine [21–23]. Although BV-based combination regimens have produced higher CR rates, a BV-first sequential approach as reported here and by Moskowitz and colleagues [11] allowed many patients to avoid chemotherapy before AHCT. This may be an especially desirable approach in older patients and patients with comorbidities who may be able to better tolerate second-line BV rather than salvage chemotherapy followed by AHCT. Finally, it should be noted that our study only included patients who were naïve to BV. If BV ultimately becomes a part of front-line therapy, additional studies will be necessary to understand the role of BV-based salvage therapy in patients with prior BV exposure.
Conclusion
In summary, our study demonstrated the tolerability of PET-adapted BV dose-escalation and the encouraging post-AHCT outcomes after second-line BV in patients with transplant-eligible rel/ref cHL. It is notable that half of the patients who were enrolled and treated with a second-line BV approach were able to proceed directly to AHCT, avoiding traditional combination chemotherapy without sacrificing efficacy. Reducing total chemotherapy exposure may have long-term benefits for a population of primarily young patients, potentially minimizing long-term risk of organ toxicity and secondary cancers. Although not a currently FDA-approved indication for the use of BV, our findings support the use of BV as second-line therapy of rel/ref cHL and continued study of salvage regimens that incorporate novel agents.
Supplementary Material
Acknowledgements
The authors would like to thank the patients who participated in the clinical trial and their families. The authors thank Dr Eileen Smith, Dr Chatchada Karanes, Dr Ryotaro Nakamura, Dr Pablo Parker, Dr Margaret O’Donnell, Dr Amrita Krishnan, Dr Ricardo Spielberger, and Dr Samer Khaled for their dedication to treating these complex patients. The authors also thank Michelle Mott, Tanya Paris, and Bernie Pulone for their assistance with collecting and managing the study data.
Funding
This research was directly supported by a grant from Seattle Genetics and indirectly by the National Cancer Institute of the National Institutes of Health (P30 CA33572) and the Tim Nesvig Lymphoma Research Fund (no grant number applies). RC was supported by the National Cancer Institute of the National Institutes of Health under award number K12CA001727 and CCITLA (no grant number applies). AFH was supported by the Lymphoma Research Foundation Larry and Denise Mason Clinical Investigator Career Development Award (no grant number applies) and the National Cancer Institute of the National Institutes of Health under award numbers NIH K12CA001727 and P50CA107399. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosure
AFH: Consulting or Advisory Role: Pharmacyclics, Bristol-Myers Squibb, Genentech, Merck; Research Funding: Seattle Genetics (Inst), Pharmacyclics (Inst), Genentech (Inst), Immune Design (Inst), Sequenta (Inst), Merck (Inst), Bristol-Myers Squibb (Inst), KiTE Pharma (Inst), Rhizen Pharmaceuticals S.A. (Inst), AstraZeneca (Inst). TS: Speakers’ Bureau: Pharmacyclics, Seattle Genetics; Research Funding: Pharmacyclics (Inst), Juno Therapeutics (Inst), Kite Pharma (Inst), Acerta Pharma (Inst), MedImmune (Inst), Genentech (Inst), TG Therapeutics (Inst), Merck (Inst), Boehringer Ingelheim (Inst), Karyopharm Therapeutics (Inst). LP: Honoraria: Cardinal Health. STR: Honoraria: Celgene, Genentech, Seattle Genetics; Consulting or Advisory Role: Celgene, Genentech Health Practices Consulting, Seattle Genetics; Speakers’ Bureau: Celgene, Seattle Genetics. LWK: Stock or Other Ownership: XEME BioPharma, Antigenics; Consulting or Advisory Role: XEME BioPharma, Celltrion, Sella Life Sciences. APN: Consulting or Advisory Role: Seattle Genetics, Gilead Sciences; Speakers’ Bureau: Seattle Genetics. RC: Consulting or Advisory Role: Seattle Genetics, Merck, Genentech; Speakers’ Bureau: Seattle Genetics, Genentech, Millennium Pharmaceuticals; Research Funding: Seattle Genetics, Pharmacyclics, Merck, Millennium Pharmaceuticals. All remaining authors have declared no conflicts of interest.
References
- 1. Kuruvilla J, Keating A, Crump M.. How I treat relapsed and refractory Hodgkin lymphoma. Blood 2011; 117(16): 4208–4217. [DOI] [PubMed] [Google Scholar]
- 2. Perales MA, Ceberio I, Armand P. et al. Role of cytotoxic therapy with hematopoietic cell transplantation in the treatment of Hodgkin lymphoma: guidelines from the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant 2015; 21(6): 971–983. [DOI] [PubMed] [Google Scholar]
- 3. Schmitz N, Pfistner B, Sextro M. et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin's disease: a randomised trial. Lancet 2002; 359(9323): 2065–2071. [DOI] [PubMed] [Google Scholar]
- 4. Hertzberg MS, Crombie C, Benson W. et al. Outpatient-based ifosfamide, carboplatin and etoposide (ICE) chemotherapy in transplant-eligible patients with non-Hodgkin's lymphoma and Hodgkin's disease. Ann Oncol 2003; 14(90001): i11–i16. [DOI] [PubMed] [Google Scholar]
- 5. Josting A, Rudolph C, Reiser M. et al. Time-intensified dexamethasone/cisplatin/cytarabine: an effective salvage therapy with low toxicity in patients with relapsed and refractory Hodgkin's disease. Ann Oncol 2002; 13(10): 1628–1635. [DOI] [PubMed] [Google Scholar]
- 6. Aparicio J, Segura A, Garcera S. et al. ESHAP is an active regimen for relapsing Hodgkin's disease. Ann Oncol 1999; 10(5): 593–595. [DOI] [PubMed] [Google Scholar]
- 7. Bartlett NL, Niedzwiecki D, Johnson JL. et al. Gemcitabine, vinorelbine, and pegylated liposomal doxorubicin (GVD), a salvage regimen in relapsed Hodgkin's lymphoma: CALGB 59804. Ann Oncol 2007; 18(6): 1071–1079. [DOI] [PubMed] [Google Scholar]
- 8. Santoro A, Magagnoli M, Spina M. et al. Ifosfamide, gemcitabine, and vinorelbine: a new induction regimen for refractory and relapsed Hodgkin's lymphoma. Haematologica 2007; 92(1): 35–41. [DOI] [PubMed] [Google Scholar]
- 9. Baetz T, Belch A, Couban S. et al. Gemcitabine, dexamethasone and cisplatin is an active and non-toxic chemotherapy regimen in relapsed or refractory Hodgkin's disease: a phase II study by the National Cancer Institute of Canada Clinical Trials Group. Ann Oncol 2003; 14(12): 1762–1767. [DOI] [PubMed] [Google Scholar]
- 10. Chen R, Palmer JM, Martin P. et al. Results of a multicenter phase II trial of brentuximab vedotin as second-line therapy before autologous transplantation in relapsed/refractory Hodgkin lymphoma. Biol Blood Marrow Transplant 2015; 21(12): 2136–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moskowitz AJ, Schoder H, Yahalom J. et al. PET-adapted sequential salvage therapy with brentuximab vedotin followed by augmented ifosamide, carboplatin, and etoposide for patients with relapsed and refractory Hodgkin's lymphoma: a non-randomised, open-label, single-centre, phase 2 study. Lancet Oncol 2015; 16(3): 284–292. [DOI] [PubMed] [Google Scholar]
- 12. Borate U, Mehta A, Reddy V. et al. Treatment of CD30-positive systemic mastocytosis with brentuximab vedotin. Leuk Res 2016; 44: 25–31. [DOI] [PubMed] [Google Scholar]
- 13. Younes A, Bartlett NL, Leonard JP. et al. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med 2010; 363(19): 1812–1821. [DOI] [PubMed] [Google Scholar]
- 14. Castagna L, Bramanti S, Balzarotti M. et al. Predictive value of early 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) during salvage chemotherapy in relapsing/refractory Hodgkin lymphoma (HL) treated with high-dose chemotherapy. Br J Haematol 2009; 145(3): 369–372. [DOI] [PubMed] [Google Scholar]
- 15. Adams HJ, Kwee TC.. Prognostic value of pretransplant FDG-PET in refractory/relapsed Hodgkin lymphoma treated with autologous stem cell transplantation: systematic review and meta-analysis. Ann Hematol 2016; 95(5): 695–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Devillier R, Coso D, Castagna L. et al. Positron emission tomography response at the time of autologous stem cell transplantation predicts outcome of patients with relapsed and/or refractory Hodgkin's lymphoma responding to prior salvage therapy. Haematologica 2012; 97(7): 1073–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moskowitz CH, Matasar MJ, Zelenetz AD. et al. Normalization of pre-ASCT, FDG-PET imaging with second-line, non-cross-resistant, chemotherapy programs improves event-free survival in patients with Hodgkin lymphoma. Blood 2012; 119(7): 1665–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sirohi B, Cunningham D, Powles R. et al. Long-term outcome of autologous stem-cell transplantation in relapsed or refractory Hodgkin's lymphoma. Ann Oncol 2008; 19(7): 1312–1319. [DOI] [PubMed] [Google Scholar]
- 19. Jabbour E, Hosing C, Ayers G. et al. Pretransplant positive positron emission tomography/gallium scans predict poor outcome in patients with recurrent/refractory Hodgkin lymphoma. Cancer 2007; 109(12): 2481–2489. [DOI] [PubMed] [Google Scholar]
- 20. Herrera AF, Moskowitz AJ, Bartlett NL. et al. Interim results of brentuximab vedotin in combination with nivolumab in patients with relapsed or refractory Hodgkin lymphoma. Blood 2018; 131: 1183–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Garcia-Sanz R, Sureda A, Gonzalez AP. et al. Brentuximab vedotin plus ESHAP (BRESHAP) is a highly effective combination for inducing remission in refractory and relapsed Hodgkin lymphoma patients prior to autologous stem cell transplant: a trial of the Spanish Group of Lymphoma and Bone Marrow Transplantation (GELTAMO). Am Soc Hematol 2016; 128: Abstract 1109. [Google Scholar]
- 22. LaCasce AS, Bociek G, Sawas A. et al. Brentuximab vedotin plus bendamustine: a highly active salvage treatment regimen for patients with relapsed or refractory Hodgkin lymphoma. Blood 2015; 126: 3982–3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hagenbeek A, Zijlstra J, Lugtenburg P. et al. Transplant brave: combining brentuximab vedotin with DHAP as salvage treatment in relapsed/refractory Hodgkin lymphoma. A phase 1 dose-escalation study. Haematologica 2016; 101: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.