Abstract
Although diffusion magnetic resonance imaging (dMRI) has been widely used to characterize the effects of repetitive mild traumatic brain injury (rmTBI), to date no studies have investigated how novel geometric models of microstructure relate to more typical diffusion tensor imaging (DTI) sequences. Moreover, few studies have evaluated the sensitivity of different registration pipelines (non-linear, linear and tract-based spatial statistics) for detecting dMRI abnormalities in clinical populations. Results from single-subject analyses in healthy controls (HC) indicated a strong negative relationship between fractional anisotropy (FA) and orientation dispersion index (ODI) in both white and gray matter. Equally important, only moderate relationships existed between all other estimates of free/intracellular water volume fractions and more traditional DTI metrics (FA, mean, axial and radial diffusivity). These findings suggest that geometric measures provide differential information about the cellular microstructure relative to traditional DTI measures. Results also suggest greater sensitivity for non-linear registration pipelines that maximize the anatomical information available in T1-weighted images. Clinically, rmTBI resulted in a pattern of decreased FA and increased ODI, largely overlapping in space, in conjunction with increased intracellular and free water fractions, highlighting the potential role of edema following repeated head trauma. In summary, current results suggest that geometric models of diffusion can provide relatively unique information regarding potential mechanisms of pathology that contribute to long-term neurological damage.
Keywords: Diffusion tensor imaging, NODDI, Concussion, Repetitive injury, Edema, Water fractions
Background
Studies investigating the pathophysiology of mild traumatic brain injury (mTBI) typically include patients who experienced a single, temporally isolated injury (smTBI), patients who suffered repetitive injuries in a temporally proximal matter (rmTBI; typically contact sport athletes) or a combination of both. Growing evidence suggests that rmTBI and repetitive sub-concussive blows may lead to long-term neurological (chronic traumatic encephalopathy; CTE) and neuropsychiatric impairment (Didehbani et al. 2013; McKee et al. 2013; Omalu et al. 2005). However, the pathology associated with CTE is primarily investigated at autopsy (McKee et al. 2013; Omalu et al. 2005) or late in the disease course (Barrio et al. 2015; Hart et al. 2013), when the only currently available intervention (i.e., discontinuation of risky activities) is ineffective. Thus, there has been great interest in developing objective, reliable and sensitive biomarkers that capture dynamic changes in neurophysiology early in the disease course rather than relying purely on clinical definitions.
Diffusion magnetic resonance imaging (dMRI) is arguably the most widely utilized method for investigating mTBI pathology in vivo. To date, the majority of dMRI mTBI studies have assumed a Gaussian distribution of diffusion displacement (Jensen et al. 2005), measuring diffusion with a single b-value and modeling diffusion as a mono-exponential decay with tensors (diffusion tensor imaging; DTI). The most commonly utilized DTI metric is fractional anisotropy (FA), which measures the preferential diffusion of water based on cellular microstructure properties (Beaulieu 2002). As reviewed in detail elsewhere (Dodd et al. 2014; Eierud et al. 2014; Hulkower et al. 2013; Shenton et al. 2012), there have been several cross-sectional and longitudinal studies examining FA changes in smTBI and rmTBI patients. Results from the acute (i.e., days) to sub-acute (i.e., weeks to month) phases of mTBI have been mixed, with rmTBI studies reporting both increased (Borich et al. 2013; Henry et al. 2010; Virji-Babul et al. 2013) and decreased (Murugavel et al. 2014) FA, as well as no significant findings (Zhang et al. 2010; Zhu et al. 2015). In contrast, the majority of studies report decreased FA for mTBI patients in the chronic injury phase, similar to findings observed in more severely injured patients (Dodd et al. 2014; Eierud et al. 2014; Hulkower et al. 2013).
Biological tissue is fundamentally complex, and diffusion is differentially restricted or hindered dependent on tissue complexity within different compartments (Jensen et al. 2005; Wu and Cheung 2010). Mean (MD), axial (AD) and radial (RD) diffusivity are frequently used in conjunction with FA to provide additional information about cellular microstructure following trauma (Song et al. 2003). However, the same pattern of findings (e.g., reduced FA with increased RD) could potentially arise from several pathophysiological mechanisms following trauma (e.g., edema, disrupted axonal membranes and/or changes in myelin). Multi-shell (i.e., multiple b-values) sampling schemes with high diffusion values (e.g., b = 7000 s/mm2) and hundreds of directions were originally proposed to model the full complexity of cellular microstructure (Jensen and Helpern 2010; Wedeen et al. 2005). Although these sequences undoubtedly provided a more accurate characterization of non-Gaussian diffusion processes, they have only recently become clinically feasible with the development of simultaneous multi-slice technology.
Diffusion kurtosis imaging (DKI; Jensen et al. 2005) and more recent geometric models of diffusion (Zhang et al. 2012) have been proposed as a further compromise for examining deviations from Gaussian diffusion with clinically feasible data acquisition schemes (two b-values less than 2500 s/mm2 with 30–50 diffusion weighted images in each shell). However, to date there have only been a handful of studies that examined deviations from Gaussian diffusion in mTBI, or have attempted to characterize diffusion within different tissue compartments (Grossman et al. 2012, 2013; Pasternak et al. 2014). Two DKI studies reported abnormal mean kurtosis in the thalamus and white matter in separate cohorts of mTBI patients within the first few months of injury or at the chronic injury stage (Grossman et al. 2012, 2013). Other groups have estimated free water using a single shell sequence in conjunction with DTI analyses (Pasternak et al. 2014). Results indicated a reduced free-water volume following mTBI, as well as reduced AD/FA following elimination of free-water from the volume (Pasternak et al. 2014). Although various geometric models (Daducci et al. 2015; Zhang et al. 2012) have recently been applied to the study of stroke (Adluru et al. 2014), and a very limited TBI sample (Koay et al. 2015), to our knowledge no one has examined differences in microstructure composition and water fractions following sub-concussive head blows typically experienced in rmTBI.
Previous work indicates high fidelity between estimates of geometric microstructure with both ground-truth simulations (Daducci et al. 2015; Zhang et al. 2012) and ex vivo histology (Sepehrband et al. 2015). However, the relationship between estimates of microstructure geometry and more traditional DTI metrics (FA, MD, AD and RD) is unknown from in vivo human samples, which is an important step for establishing validity. Second, it is well known that voxel-based analyses (VBA) are highly sensitive to registration errors that result in false positives (Ashburner and Friston 2000), especially in the context of dMRI (Chung et al. 2008). One study (Schwarz et al. 2014) recently suggested the superiority of nonlinear algorithms for registering individual subject data to a normalized space relative to tract-based spatial statistics (TBSS). Non-linear normalization algorithms also generally perform better than linear registration algorithms when expert tracings are used as a gold standard (Klein et al. 2009). However, to our knowledge a direct comparison of registration pipelines during VBA analyses has not been performed in mTBI participants, with the majority of VBA studies defaulting to TBSS registration instead (Smith et al. 2006).
The current study therefore investigated whether biophysical models of diffusion would differentiate a high-risk rmTBI sample (mixed-martial artists; MMA) from healthy controls (HC) without a history of contact sport participation. Based on theoretical analyses of the literature (Eierud et al. 2014; Ling et al. 2012b, our a priori prediction was that rmTBI would be associated with decreased FA relative to HC and that novel microstructure metrics would reveal separate regions of pathology relative to traditional DTI metrics. Methodological goals included examining how diffusion-estimated microstructure measures related to more typical DTI metrics (FA, MD, AD and RD), and a comparison of the sensitivity of different registration pipelines (non-linear, linear and TBSS) for detecting dMRI abnormalities.
Methods
Participants
A total of 14 MMA were recruited from a local training gym in Albuquerque, New Mexico. MMA were required to be between the ages of 18 and 45, have no contraindications for MRI scanning and be participating in a regular training routine, including professionally organized fighting events as well as weekly sparring. A total of 16 matched HC were recruited to match patients in terms of sex, age (±4 years) and education (±4 years). As a result of the matching process, the majority of HC data were acquired following completion of the study by MMA participants. All HC were required to be physically active (strenuous exercise at least 2 times per week), and have no history of regular, organized contact-sport participation to reduce the likelihood of previous sub-concussive blows. All participants were evaluated clinically and with an extensive neuroimaging battery. Clinical and imaging assessments occurred on the same day for the majority (>90 %) of visits across both groups of participants. 1H-MRS and structural atrophy data from this cohort have already been presented (Mayer et al. 2015). As such, behavioral and clinical results are only briefly presented in the current manuscript.
Participants were excluded for a history of mental illness, history of neurological disorders, history of psychiatric disorders, a previous traumatic brain injury with more than 30 min of loss of consciousness and recent history of substance abuse. One MMA fighter was diagnosed with a pre-existing neurological disorder independent of the current study and was subsequently excluded, leaving a total of 13 MMA (11 males, 2 females; 28.2 ± 4.9 years old; 13.9 ± 1.7 years of education). Two HC were excluded due to failures with data acquisition (e.g., MRI-induced claustrophobia), leaving a total of 14 matched (12 males, 2 females) HC (28.1 ± 5.1 years old; 15.8± 2.3 years of education). Participants provided informed consent based on institutional guidelines at the University of New Mexico School of Medicine.
Clinical assessment
Neuropsychological test scores were combined into composite indices for the domains of attention, working memory, processing speed, executive function and memory (see Mayer et al. 2015 for details). Balance was assessed with the Balance Error Scoring System (BESS). The Wechsler Test of Adult Reading (WTAR) provided an estimate of overall pre-morbid intellectual functioning and the Test of Memory and Malingering (TOMM) allowed assessment of testing cooperation. Self-report for measures of somatic, cognitive and emotional complaints common in concussion (Neurobehavioral Symptom Inventory; NBSI), history of previous concussions and the degree of symptoms associated with previous concussions (a modified version of the Rivermead Post-Concussion Symptoms Questionnaire; RPSQ) and general emotional status (State-Trait Anxiety Index and Beck Depression Inventory-Second Edition; STAI and BDI-II, respectively) were also assessed. The RPSQ was specifically modified to provide retrospective self-report of symptoms associated with up to 4 different head injuries, age at each insult and the presence/absence of loss of consciousness and post-traumatic amnesia. For all measures, raw data were converted to t-scores using published age-specific norms whenever possible. Effect sizes are reported using Cohen’s d.
Image acquisition
Anatomical imaging sequences were collected on a Siemens 3 Tesla TrioTim scanner with a 12-channel head coil. High resolution T1-weighted [TE (echo time) = 1.64, 3.5, 5.36, 7.22, 9.08 ms, TR (repetition time) = 2.53 s, TI (inversion time)= 1.2 s, flip angle 7°, number of excitations (NEX)= 1, slice thickness=1 mm, FOV (field of view)=256 mm, resolution=256×256], T2-weighted [TE=77.0 ms, TR=1.55 s, flip angle 152°, NEX=1, slice thickness=1.5 mm, FOV=220 mm, matrix=192×192, voxel size=1.15×1.15×1.5 mm3], FLAIR [TE = 88.0 ms, TR = 10.4 s, flip angle 160°, NEX = 1, slice thickness = 3 mm, FOV = 256 mm, matrix = 320 × 320, voxel size = 0.8 × 0.8 × 3.0 mm3], susceptibility weighted imaging (SWI) [TE=20.0 ms, TR=28 ms, flip angle 15°, NEX=1, slice thickness=1.5 mm, FOV=240 mm, matrix=256×256, voxel size = 1.0 × 0.9 × 1.5 mm3] and MRA [TE = 3.59 ms, TR=20 ms, flip angle 18°, NEX=1, slice thickness=0.5 mm, FOV = 200 mm, matrix = 384 × 384, voxel size = 0.5 × 0.5 × 0.5 mm3] images were collected for all participants. Structural images from each scan were reviewed for any abnormal findings by a certified radiologist who was blinded to patient diagnosis.
A single high angular resolution dMRI scan (11:46 min) was acquired using a twice-refocused spin-echo sequence with 180 diffusion gradients (60 at b = 1000 s/mm2; 120 at b = 2500 s/mm2) and the b = 0 experiment repeated 6 times [66 interleaved slices; TE = 110 ms; TR = 3600 s; 84° flip angle; NEX = 1; voxel resolution = 2.2 × 2.2 × 2.2 mm3; multi-band factor = 3] on a 32-channel head coil. The gradient directions at each shell were selected based on previously published guidelines (Jones et al. 2002; Skare et al. 2000) and were ordered so that they were uniformly distributed over an electrostatic sphere at each b-value using the CAMINO software package (Jones et al. 1999; Jansons and Alexander 2003).
Image processing and statistical analyses
DTI data were processed with a variety of packages including AFNI (v2011_12_21_1014), FSL (v5.0.8), SPM (v12) and Matlab (vR2014a). Image distortions caused by eddy currents and head motion were first corrected by registering all diffusion weighted (DW) images to the first b = 0 s/mm2 image using a 12 degree-of-freedom (DOF) affine correction with correlation ratio as the cost function (FSL). For each DW image, the vector corresponding to the rotation component was then extracted from the resultant transformation matrix and applied to the corresponding gradient from the gradient table. Diffusion tensors and associated scalar measures were calculated from the resulting images using all DW images (from both shells). A non-linear method was adopted for tensor calculations to decrease tensor estimate errors caused by noise, especially in regions of high anisotropy (Cox and Glen 2006). DTI scalars were then blurred with a 3 mm FWHM Gaussian kernel.
Three different pipelines were evaluated for transforming each subject’s individual data to template space. In the primary pipeline, each subject’s first unblurred b0 image was registered to their T1-weighted image using an affine transformation, with T1 images subsequently normalized to Talairach space using a non-linear transformation (AFNI). Both matrices were concatenated to bring scalar data into stereotaxic space. The second pipeline was identical, with the exception that an affine, rather than non-linear, transformation was used to transform native T1 images to Talairach space followed by matrix concatenation. Finally, a standard TBSS analysis pipeline was also examined using default settings from FSL (Smith et al. 2006). Importantly, the default TBSS setting includes a non-linear alignment of single - subject FA data to the FMRIB58_FA normalized template.
The neurite orientation dispersion and density imaging (NODDI; Zhang et al. 2012) algorithm was used to investigate cellular microstructure. Importantly the rate of diffusion in the NODDI model is fixed at 3.0 × 10−3 mm2/s for isotropic diffusion and 1.7 × 10-3 mm2/s for intracellular diffusion, such that NODDI represents a purely geometric model of diffusion. In the NODDI framework (Zhang et al. 2012) the full normalized signal A is equal to:
| (1) |
where iso = isotropic, ic = intracellular, ec = extracellular, Vx = measurable volume fraction within each compartment and Ax = normalized signal for each compartment (Zhang et al. 2012). As described in the equation above, the two primary metrics of interest are the measureable intracellular volume fraction (Vic) and isotropic volume fraction (Viso), both of which are bounded from 0.0 to 1.0. The measurable extracellular volume fraction (Vec) is directly related to the intracellular component (Vec = 1 − Vic) and therefore does not provide any unique information. In addition, NODDI also calculates an orientation dispersion index (ODI), which also ranges from 0 (i.e., highly parallel neurites typified by white fibers in corpus callosum) to 1.0 (i.e., randomly oriented neurites typified by dendritic arborization in gray matter). These three metrics were then spatially normalized using the same concatenated matrices as above and blurred using a 3 mm FWHM Gaussian kernel.
Voxel-wise comparisons of FA, MD, Viso, Vic and ODI maps were conducted using ANCOVA. Image smoothness was estimated to be approximately 5.5 mm isotropic based on residuals from the FA data. Based on this estimate, a volume threshold of 469 μl was applied with a parametric threshold of p < 0.005 to correct for false positives at p < 0.05 based on 10,000 Monte Carlo simulations (AFNI). For descriptive and visualization (i.e., box-plots in figures) purposes only, clusters surviving the threshold were identified using a combination of the Johns Hopkins University white matter (WM) DTI atlas (Mori et al. 2008) and Desai gray matter (GM) maximum probability atlas, including both cortical (Destrieux et al. 2010) and sub-cortical labels (FreeSurfer parcellations). Labelling priority was given to the JHU atlas by removing any intersecting voxels from the Desai GM atlas. Significant clusters had to exhibit a minimum volume of spatial intersection with an atlas label, which varied dependent on the total volume of the atlas label. Specifically, a minimum intersection volume of 235 μl was used for large atlas regions (i.e., volume ≥required 469 μl), whereas a variable intersection volume was used for labels with smaller volumes (i.e., 50 % of total label value).
Comparison of microstructure and traditional DTI estimates
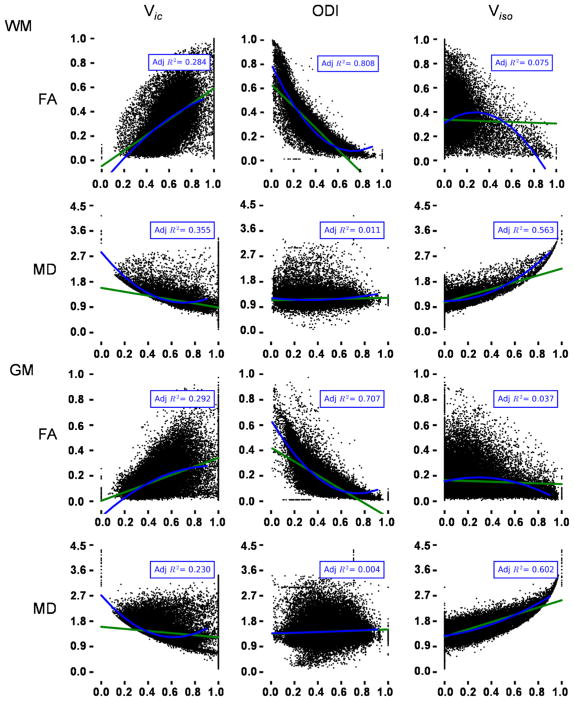
The primary methodological goal of the current study was to empirically evaluate the relationship between standard DTI metrics (FA, MD, AD and RD) and geometric microstructure estimates (Vic, Viso and ODI) derived from NODDI in HC only. Each participant’s T1 data were first segmented into gray and white matter masks in native space using SPM. Next, the relationship between each microstructure estimate (independent variable) and traditional DTI metrics (dependent variable) were evaluated using either a simple linear term (reduced model) or linear and quadratic terms (full model) for each subject in each tissue compartment (Fig. 1). Estimates of variance (adjusted R2) were transformed to Fisher’s z prior to statistical analyses using established methods (Harel 2009), with the inverse transformation occurring for reporting purposes. In this analytic framework, the reduced model captures the basic relationship between DTI scalars and newer geometric measures. The adjusted R2 value permits a quantitative comparison of the two models to see if the quadratic term accounts for significantly more variance relative to a reduced model. The strength of relationships between NODDI and DTI metrics were qualitatively classified as either moderate (Adjusted R2 ≥ 0.25) or strong (Adjusted R2 ≥ 0.64) for descriptive purposes (Ferguson 2009).
Fig. 1.
The scatterplots present relationships between microstructure estimates based on geometric models (Vic Intracellular volume fraction, ODI orientation dispersion index, Viso Isotropic volume fraction) and traditional DTI metrics (FA fractional anisotropy, MD mean diffusivity) separately for both gray (GM) and white (WM) matter. Data from each plot were randomly selected from a single healthy control athlete in the study. Data were modeled with either linear (green line; reduced model) or linear and quadratic (blue line) terms (full model). Adjusted R2 (Adj R2) values for each tissue type/metric are presented for the full model
Quality assurance protocol
Outliers were examined prior to any analyses. One HC was an outlier on their TOMM score and exhibited poor performance on other neuropsychological domains. However, other clinical measures were completed correctly (i.e., reverse scoring items) and their dMRI data passed all quality assurance metrics. This participant’s demographic, clinical (i.e., self-report) and imaging data were therefore retained.
Signal-to-noise ratios (SNR) were calculated separately for each b0 image and then averaged (Dietrich et al. 2007), with averaged SNR values compared across groups. The degree of head motion was also quantified on a per-subject basis. There were no MMA or HC who were outliers on head motion relative to their respective cohorts on more than a single parameter. Trained raters visually inspected all raw DW images to identify common artifacts with a binary rating system (i.e., 0 = no artifact; 1 = artifact). Finally, FA data were analyzed with and without the RESTORE algorithm (Chang et al. 2005) to eliminate any effects associated with gradient artifacts.
Results
Neuropsychological and clinical measures
Clinical results are identical to our previous publication (Mayer et al. 2015) and are therefore only briefly presented here. There were no differences between the two groups on age, sex, levels of testing effort, balance performance or hand preference (p > 0.10), with educational attainment (t25 = 2.47, p = 0.021) and estimated premorbid intelligence (t1,24 = 2.29, p = 0.031) greater for HC relative to MMA (Table 1). A MANCOVA comparing neurocognitive performance with WTAR as a covariate was not significant for the multivariate effect of group (p > 0.10), although significant univariate effects (MMA < HC) were observed for both memory (F1,23= 5.75, p = 0.025) and processing speed (F1,23 = 4.45, p = 0.046). A MANOVA comparing self-reports of somatic, cognitive and emotional issues (NBSI) was not significant for the multivariate effect of group (p > 0.10), with univariate tests indicating significantly elevated somatic complaints (F1,25= 4.39 p = 0.046) in MMA.
Table 1.
Neuropsychological and clinical summary measures
| MMA | HC | Cohen’s d | |
|---|---|---|---|
| Demographic | Mean (SD) | Mean (SD) | |
| Age | 28.23 (4.94) | 28.14 (5.08) | 0.02 |
| Education | 13.85 (1.68) | 15.79 (2.33) | −0.96 |
| HQ | 69.60 (52.01) | 82.08 (52.96) | −0.24 |
| Neuropsychological | |||
| Attention▲ | 52.36 (7.96) | 54.00 (5.17) | −0.25 |
| Memory▲ | 39.15 (8.90) | 47.34 (6.32) | −1.06 |
| WM▲ | 52.92 (9.34) | 52.58 (7.57) | 0.04 |
| PS▲ | 50.51 (5.97) | 56.57 (6.97) | −0.93 |
| EF▲ | 49.90 (7.95) | 48.98 (8.66) | 0.11 |
| WTAR | 52.26 (10.02) | 60.28 (7.71) | −0.90 |
| TOMM | 53.65 (5.10) | 51.21 (6.96) | 0.40 |
| BESS | 12.23 (5.46) | 12.64 (4.01) | −0.09 |
| Self Report-Emotional | |||
| BDI-II | 42.39 (4.78) | 41.14 (4.25) | 0.28 |
| STAI-S | 43.46 (9.73) | 40.50 (6.20) | 0.36 |
| Self Report-Concussion history | |||
| Lifetime mTBIs | 2.23 (1.09) | 0.43 (0.51) | 2.11 |
| Average RPSQ-3 | 2.90 (2.12) | 0.17 (0.41) | 1.79 |
| Average RPSQ-13 | 4.97 (7.01) | 0.00 (0.00) | 1.00 |
| NBSI-Som | 4.62 (5.11) | 1.50 (2.14) | 0.80 |
| NBSI-Cog | 2.62 (2.87) | 1.07 (1.77) | 0.65 |
| NBSI-Emot | 4.92 (4.50) | 2.57 (2.87) | 0.62 |
Abbreviations: HQ handedness quotient, WM working memory, PS processing speed, EF executive function, WTAR Wechsler Test of Adult Reading, TOMM Test of Memory Malingering, BESS Balance Error Scoring System, BDI-II Beck Depression Inventory – Second Edition, STAI-S State-Trait Anxiety Index – state, RPSQ Rivermead Post-Concussion Symptoms Questionnaire, NBSI-Cog Neurobehavioral Symptom Inventory cognitive complaints (Som Somatic complaints, Emot Emotional complaints), SD Standard deviation, N/A not applicable. All clinical (with the exception of the BESS) and self-reported emotional data are t-scores, with the remainder representing raw data. Cohen’s d values are interpreted as |0.20| = small, |0.50| = medium, |0.80| = large, and |1.30| = very large. These data are identical to previous publication on same cohort (Mayer et al. 2015).
Means, standard deviations and effect sizes for neuropsychological indices reported following correction for WTAR as covariate. HC RPSQ mean and SD include cases only HC with a history of concussion
A higher proportion of MMA reported a previous history of mTBI (χ2 = 10.56, p = 0.001; 6/14 HC; 13/13 MMA). MMA also experienced as significantly higher previous number of mTBIs (Mann–Whitney U = 170.00, z = 4.00; p < 0.001; HC = 0.43 ± 0.5; MMA = 2.23 ± 1.1). Blinded MRI reads (T1, T2, FLAIR, MRA and SWI) by a board-certified neuroradiologist indicated trauma induced pathology on 4/13 scans for MMA and 2/14 HC, a finding which was not statistically significant (p > 0.10). A MANOVA examining the average acute somatic and cognitive symptoms resulting from previous head injuries (modified version of RPSQ) returned a significant multivariate effect of group (F2,16 = 4.57, p = 0.027), with MMA reporting significantly more symptoms on the RPSQ-3 (F1,17 = 9.51, p = 0.007) from previous injuries.
dMRI quality metric comparison
There were no differences in estimated SNR between MMA and HC (p > 0.10). There were no group differences (p > 0.10) on the number of artifact images using a non-parametric Mann–Whitney U test. Finally, the results of group comparisons on FA data (see below) remained similar when the RESTORE algorithm was utilized to eliminate artifacts (Supplementary Fig. 1).
MANOVAs compared framewise displacement on translational and rotational head motion across the two groups. The multivariate effect of group was significant for translations (F3,23 = 5.01, p = 0.008) with a trend observed for rotations (F3,23 = 2.40, p = 0.094). Univariate tests indicated significantly greater motion among MMA for yaw (F1,25 = 4.37, p = 0.047) and y-axis displacement (F1,25 = 11.10, p = 0.003), with trend differences for roll (F1,25 = 3.86, p = 0.061). Effect sizes across all six motion parameters ranged from small to large (Cohen’s d range = 0.13–1.27; MMA > HC). As a result of these analyses, mean framewise displacement was used as a covariate for all group dMRI comparisons.
Relationship between NODDI estimates and FA measures in HC
Table 2 and Fig. 1 present the relationship between NODDI microstructure estimates (Vic, Viso and ODI) and traditional DTI (FA, MD, AD and RD) metrics for HC only. Multiple regression analyses confirmed that the full model (linear and quadratic terms) was statistically superior (all p < 0.05) to a reduced model (simple linear fit) for all models. In WM, there was a strong relationship between ODI and FA (84 % of captured variance with full model), with moderate relationships (Adjusted R2 range = 0.25–0.63; based on Ferguson 2009) also existing between ODI and AD/RD. Moderate relationships existed between Viso and MD/AD/RD, whereas Vic
Table 2.
Mean Adjusted R2 and standard deviation values between NODDI and traditional DTI metrics in HC only for both the reduced and full model
| NODDI | Model | WM | |||
|---|---|---|---|---|---|
| FA | MD | AD | RD | ||
| Vic | Reduced | 0.26 (0.000) | 0.11 (0.003) | 0.01 (0.002) | 0.22 (0.003) |
| Full | 0.29 (0.001) | 0.33 (0.002) | 0.06 (0.003) | 0.41 (0.001) | |
| Viso | Reduced | 0.00 (0.001) | 0.45 (0.001) | 0.32 (0.001) | 0.23 (0.002) |
| Full | 0.08 (0.000) | 0.57 (0.003) | 0.32 (0.001) | 0.40 (0.002) | |
| ODI | Reduced | 0.76 (0.001) | 0.00 (0.001) | 0.43 (0.002) | 0.24 (0.002) |
| Full | 0.84 (0.002) | 0.02 (0.001) | 0.60 (0.003) | 0.26 (0.001) | |
| GM | |||||
| FA | MD | AD | RD | ||
| Vic | Reduced | 0.26 (0.001) | 0.08 (0.011) | 0.02 (0.008) | 0.14 (0.011) |
| Full | 0.30 (0.001) | 0.29 (0.006) | 0.15 (0.002) | 0.34 (0.007) | |
| Viso | Reduced | 0.00 (0.001) | 0.54 (0.004) | 0.61 (0.006) | 0.45 (0.003) |
| Full | 0.03 (0.001) | 0.55 (0.004) | 0.62 (0.006) | 0.47 (0.004) | |
| ODI | Reduced | 0.66 (0.002) | 0.01 (0.001) | 0.07 (0.001) | 0.09 (0.001) |
| Full | 0.74 (0.003) | 0.01 (0.001) | 0.11 (0.001) | 0.09 (0.001) | |
Abbreviations: NODDI neurite orientation dispersion and density imaging, WM white matter, GM gray matter, FA fractional anisotropy, MD mean diffusivity, AD axial diffusivity, RD radial diffusivity, Vic intracellular volume fraction, Viso isotropic volume fraction, ODI orientation dispersion index exhibited a moderate relationship with FA/MD/RD. In GM voxels, a strong relationship was also present between ODI and FA (74 % of variance). Moderate relationships were observed between Viso and MD/AD/RD, as well as between Vic and FA/MD/RD.
dMRI results
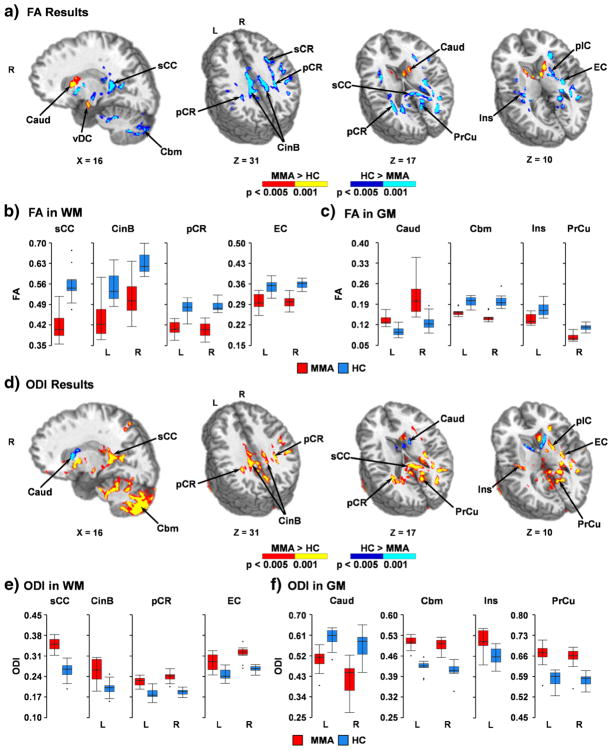
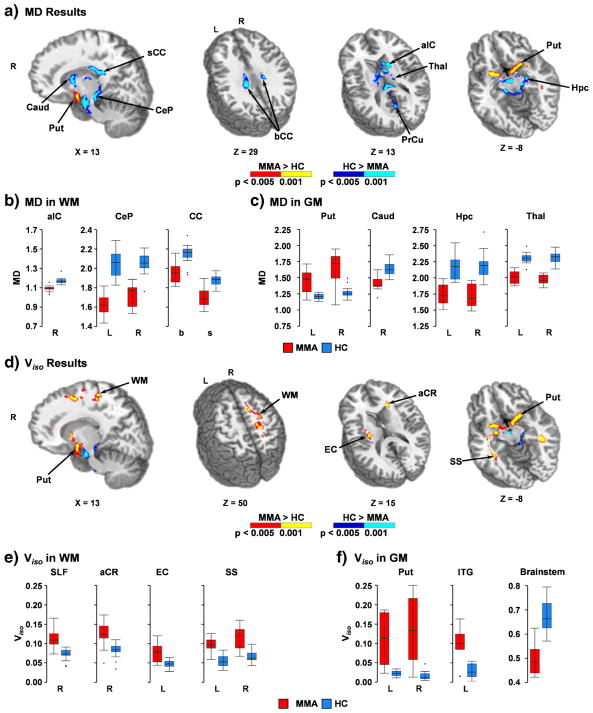
Group comparisons of traditional DTI metrics indicated widespread areas of reduced FA in WM (predominantly in corpus callosum, internal/external capsule, corona radiata, cingulum and superior longitudinal fasciculus), GM and in tissue junctions (e.g., gray-white, gray-CSF) for MMA relative to HC (Fig. 2a–c and Supplementary Table 1). In addition, areas of increased FA for MMA relative to HC were observed within the junction between the ventral diencephalon and cerebellar peduncle, bilateral caudate and the junction between the caudate and internal capsule. In general, the findings of reduced FA were more pronounced in medial rather than lateral white and gray matter. Reduced MD was also observed in the bilateral cerebral peduncles, cerebellar peduncle, corpus callosum, internal capsule, caudate, deep GM nuclei, visual cortex, brainstem and cerebellum for MMA relative to HC (Fig. 3a–c and Supplementary Table 2). Increased MD for MMA was also observed within the inferior temporal gyrus, portions of the ventral diencephalon and inferior putamen.
Fig. 2.
This figure presents group differences in fractional anisotropy (FA; Panel a) and orientation dispersion indices (ODI; Panel d) between mixed-martial artists (MMA) and healthy controls (HC). Directionalities of abnormalities are color-coded (MMA > HC: p < 0.005 = red; p < 0.001 = yellow and HC > MMA: p < 0.005 = blue; p < 0.001 = cyan) and locations of sagittal (X) and axial (Z) slices are given according to the Talairach atlas for the left (L) and right (R) hemispheres. White matter (WM) regions exhibiting differences included splenium of the corpus callosum (sCC), cingulum bundle (CinB), superior (sCR) and posterior (pCR) corona radiata, external capsule (EC), and anterior and posterior (pIC) limb of the internal capsule. Gray matter (GM) regions exhibiting differences included caudate (Caud), cerebellum (Cbm), insula (Ins), precuneus (PrCu), and ventral diencephalon (vDC). FA (Panels b and c) and ODI (Panels e and f) values for selected regions are displayed with box-and-whisker plots for each group (MMA = red; HC = blue)
Fig. 3.
This figure presents group differences in mean diffusivity (MD; Panel a) and isotropic volume fractions (Viso; Panel d) between mixed-martial artists (MMA) and healthy controls (HC). Directionalities of abnormalities are color-coded (MMA > HC: p < 0.005 = red; p < 0.001 = yellow and HC > MMA: p < 0.005 = blue; p < 0.001 = cyan) and locations of sagittal (X) and axial (Z) slices are given according to the Talairach atlas for the left (L) and right (R) hemispheres. White matter (WM) regions exhibiting significantly different MD (Panel a) included the anterior limb of the internal capsule (aIC), cerebral peduncle (CeP), and body (bCC) and splenium (sCC) of the corpus callosum. Gray matter (GM) regions exhibiting significantly different MD included the precuneus (PrCu) and several subcortical structures (putamen [Put], caudate [Caud], hippocampus [Hpc], thalamus [Thal]). WM regions with significant group differences in Viso (Panel d) were similar to MD in deep brain structures, but also included additional WM structures such as the superior longitudinal fasciculus (SLF), anterior corona radiata (aCR), external capsule (EC), and sagittal striatum (SS). GM differences in Viso included the inferior temporal gyrus (ITG), putamen and brainstem. MD (Panels b and c) and Viso (Panels e and f) values for selected regions are displayed with box-and-whisker plots for each group (MMA = red; HC = blue)
As expected based on regression results, group differences in ODI were largely overlapping with FA findings but with an inverted relationship (Fig. 2d–f and Supplementary Table 3). Additional areas of increased ODI were also present within cerebellum, inferior temporal lobes and midline GM structures along the sagittal sinus. Abnormalities in free water estimates (Viso) mirrored MD findings within the cerebellar peduncle, brainstem and diencephalon (Fig. 3d–f and Supplementary Table 4), with MMA exhibiting reduced Viso relative to HC. Additionally, increased Viso for MMA was also observed within the inferior temporal gyrus, ventral diencephalon and inferior putamen. However, MMA also exhibited evidence of increased Viso within several other WM tracts (predominantly within internal/external capsule, corona radiata, sagittal striatum, posterior thalamic radiations and superior longitudinal fasciculus) that were absent on comparisons using traditional DTI metrics (MD or FA).
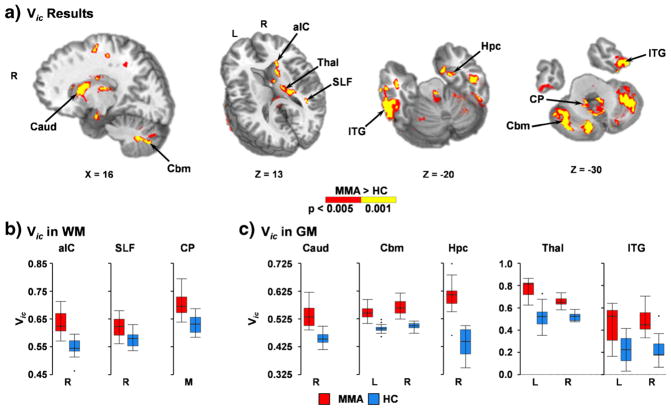
Finally, increased intracellular water fraction (Vic) was observed within several WM tracts (cerebellar peduncles, internal capsule and superior longitudinal fasciculus), brainstem, cerebellum, inferior/middle temporal lobes, deep GM nuclei and the caudate for MMA (Fig. 4; Supplementary Table 5). There was minimal overlap between Vic and either FA or MD although abnormalities also tended to have a more medial distribution.
Fig. 4.
This figure presents regions showing increased intracellular volume fraction (Vic) between mixed-martial artists (MMA) and healthy controls (HC). Abnormalities are color-coded (MMA > HC: p < 0.005 = red; p < 0.001 = yellow) and locations of sagittal (X) and axial (Z) slices are given according to the Talairach atlas for the left (L) and right (R) hemispheres, as well as for medial (M) regions. White matter (WM) regions exhibiting greater Vic for MMA included the anterior limb of the internal capsule (aIC), superior longitudinal fasciculus (SLF), and cerebellar peduncle (CP). Gray matter (GM) regions exhibiting greater Vic for MMA included the caudate (Caud), cerebellum (Cbm), hippocampus (Hpc), thalamus (Thal), and middle and inferior (ITG) temporal gyri. Vic values for selected regions are presented in Panels b and c using box-and-whisker plots (MMA = red; HC = blue)
Spatial normalization analyses
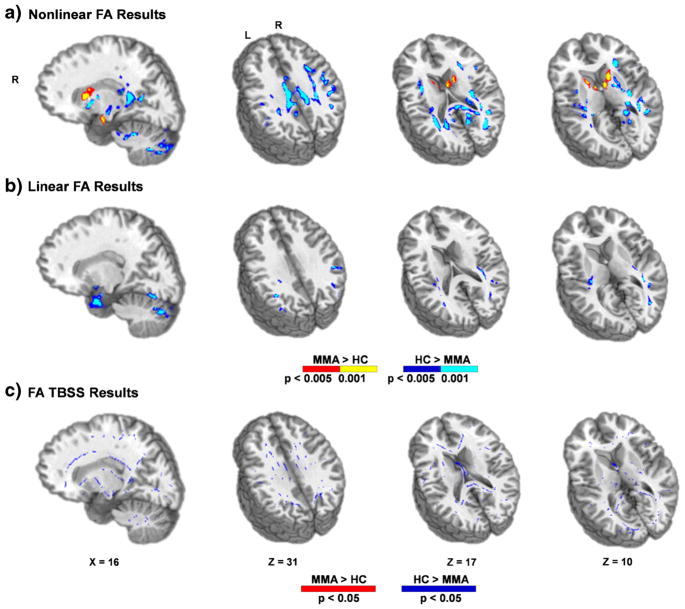
The second methodological aim of the current manuscript was to examine how typical dMRI results were affected by three different spatial normalization pipelines (non-linear normalization, linear normalization and TBSS). These analyses were restricted to FA and ODI. Importantly, all pipelines resulted in the same general pattern of findings (reduced FA and increased ODI for MMA). However, the regions in which these group differences were present were markedly different across analytic pipelines (Fig. 5 for FA; Supplementary Fig. 2 for ODI). Specifically, the non-linear alignment resulted in the largest amount of statistically significant differences between the two groups. These included bi-directional findings (i.e., MMA > HC and HC > MMA) for the non-linear analyses relative to the linear and TBSS pipelines for both FA and ODI. As expected, there were also several regions in GM and gray-white matter junctions that were present in either the linear or the non-linear pipeline that were absent for the TBSS pipeline. Finally, to ensure that current results were not specific to the AFNI algorithm, FA data were also spatially normalized to the same Talairach template using an independent non-linear registration algorithm from Advanced Normalization Tools v2.1.0 (ANTs; Avants et al. 2009). The bidirectional results observed in the AFNI non-linear pipeline were also significant for a majority of regions using the ANTs pipeline (Supplementary Fig. 3).
Fig. 5.
This figure presents differences in fractional anisotropy (FA) between mixed-martial artists (MMA) and healthy controls (HC) from three differential spatial normalization pipelines. Directionalities of abnormalities in Panels a and b are color-coded (MMA > HC: p < 0.005 = red; p < 0.001 = yellow and HC > MMA: p < 0.005 = blue; p < 0.001 = cyan) and locations of sagittal (X) and axial (Z) slices are given according to the Talairach atlas for the left (L) and right (R) hemispheres. The color coding scheme in Panel c (MMA > HC: p < 0.05 = red and HC > MMA: p < 0.05 = blue) is given according to tract-based spatial statistics (TBSS) defaults. The three pipelines included non-linear normalization (Panel a), linear normalization (Panel b) and TBSS (Panel c)
Discussion
The current experiment included both methodological and clinical aims for investigating the utility of newer geometric dMRI models for examining neuropathology related to rmTBI. Current results indicated that MMA exhibited a pattern of reduced FA in medial gray and white matter relative to HC, as well as increased FA within the bilateral cerebral peduncles and gray-white matter junctions in the striatum. Several previous studies have reported cross-sectional evidence of decreased FA in combat-sport athletes. For example, decreased FA has been observed in the corpus callosum/internal capsule of boxers (Zhang et al. 2006), and has been associated with previous number of knockouts (Shin et al. 2014) and history of heading the ball in soccer players (Lipton et al. 2013). Others have also documented progressive WM changes as a function of participation in a single season of contact sports (Bazarian et al. 2014; Koerte et al. 2012), further correlating DTI abnormalities with independent measures of head impact (Bazarian et al. 2014; Davenport et al. 2014). Finally, DTI abnormalities (FA and MD) in the corpus callosum and amygdala have been observed in a large sample of contact (hockey and football players) relative to non-contact athletes (track, crew and Nordic skiing), with postseason WM changes associated with head impact measures (McAllister et al. 2014).
There are many potential mechanistic explanations for findings of reduced FA including alterations to axon membranes/organelles (Spain et al. 2010; Browne et al. 2011) as well as changes in intracellular/extracellular water fractions. Importantly, traditional DTI metrics do not specifically measure individual compartment water fractions (Zhang et al. 2012), rather characterizing the anisotropic motion of water (FA) or the total displacement of water molecules (MD) from a strictly Gaussian perspective. To overcome limitations associated with a purely Gaussian model of diffusion (i.e., DTI), the current study examined independent estimates of voxel-wise free water fractions (Viso), intracellular water fractions (Vic) and neurite dispersion (ODI) via a multishell acquisition sequence and a geometric modelling (Zhang et al. 2012). To our knowledge, the current study is the first to quantitatively examine both intracellular and free water estimates in any sample of mTBI patients.
Our first methodological goal was to establish the basic statistical relationships that exist between traditional (FA, AD, RD and MD) and geometric measures at the single-subject level (healthy tissue only). Results indicated a strong negative relationship between FA and estimates of neurite dispersion (ODI) in WM and GM for both the reduced and the full model. Thus, current results provide evidence of concurrent validity for geometric estimates of neurite dispersion properties relative to estimates obtained directly from anisotropic diffusion (Daducci et al. 2015; Sepehrband et al. 2015; Zhang et al. 2012). The negative direction of this relationship is purely conventional, as voxels with low neurite dispersion (e.g., as occurs in corpus callosum) are assigned low ODI values (e.g., 0.0–0.20) versus high estimates of anisotropic diffusion. Equally important, only moderate relationships existed between estimates of free/intracellular water fractions and traditional DTI metrics in both WM and GM. These findings suggest that geometric dMRI measures provide unique information about cellular processes relative to traditional DTI measures from a statistical perspective, a finding that was largely confirmed during group-wise comparisons.
Specifically, results from group-wise comparisons of ODI and FA metrics were largely overlapping, confirming results observed in our single-subject analyses. Thus, current results suggest that although ODI and FA are derived from very different types of dMRI models, they ultimately provide similar information about underlying microstructure properties. Similarly, there was spatial overlap for group differences in free water fractions and MD within the sagittal striatum, inferior putamen and hippocampus. However, unique findings of increased free water fractions (MMA > HC) were also observed within several WM tracts including the superior longitudinal fasciculus and anterior corona radiata. Finally, there was minimal spatial overlap between findings of increased intracellular water fractions in MMA within brainstem, cerebellum or striatal gray and white matter and traditional DTI metrics. To our knowledge, current results represent the first evidence that rmTBI may induce increased intracellular and free water fractions, which is critical for improved understanding of how edema may ultimately affect injury outcomes in rmTBI.
It is important to note that several of the dMRI abnormalities were located in tissue junctions that are highly susceptible to partial voluming effects. Computational models and classic studies from Holbourn suggest that differences in tissue rigidity and density render parenchymal interfaces (e.g., gray-white boundaries, gray-CSF boundaries) more susceptible to shear-strains during rapid rotational acceleration/deceleration (Gentry 1994; Holbourn 1943; Liu et al. 1999). Thus, similar to more severe trauma studies (Kim and Gean 2011; Liu et al. 1999), it is biologically plausible that rmTBI exerts a larger effect along tissue junctions as well as in deep gray and white matter. Alternatively, current findings could represent an artifact related to the increased head motion that was observed between MMA and HC. Head motion confounds dMRI results (Ling et al. 2012a; Storey et al. 2007), and is particularly problematic within regions that have high differences in basic signal-to-noise ratios as tends to occur at tissue junctions (Mayer et al. 2007). Several statistical measures (covariation and RESTORE analyses) specifically adopted to correct for motion artifacts did not have a large impact on our findings. However, we cannot fully rule out motion artifact as a contributory factor.
Our final methodological aim investigated how different algorithms for registering individual subject dMRI data to a canonical template affected group-wise comparisons (Chung et al. 2008; Schwarz et al. 2014; Smith et al. 2006; Tustison et al. 2014). The pipelines examined included registration of individual participant’s dMRI (FA or ODI) data to their T1 image, followed by registration of the native T1 image to a T1 template using either linear or non-linear algorithms. In addition, the TBSS default pipeline, which includes a nonlinear algorithm, was also used to register each individual participant’s FA data to a FA template (Smith et al. 2006). Importantly, the general pattern of results (e.g., reduced FA and increased ODI) was consistent across all registration pipelines, indicating that current findings were generally reproducible regardless of the chosen pipeline. However, the non-linear registration pipeline was more sensitive (operationally defined as increased group-wise differences) relative to both the linear registration and TBSS pipelines.
Previous studies have also indicated the superiority of the T1 non-linear pipeline in terms of accuracy against gold-standard manual tracings (Klein et al. 2009) as well as standard TBSS analyses in other neurological populations (Schwarz et al. 2014). Moreover, utilization of the native T1 image also reduces biases recently associated with individual FA to template FA registration schemes (Tustison et al. 2014). Both the linear and the non-linear native T1 pipelines revealed significant findings at gray-white junctions, which were not present in TBSS analyses due to the “skeletonized” registration focus on the center of white matter tracts (Smith et al. 2006). As noted above, future studies will need to confirm whether gray-white junctions represent an important region of pathology for rmTBI by eliminating poor registration as a contributing factor to false positives.
There are several limitations that should be noted. First, a relatively small sample was studied in the current experiment, increasing the likelihood of sampling bias and under-powered results. Second, the current study has no metric for relating traditional DTI (e.g., FA or MD) or newer geometric dMRI measures of microstructure damage to actual cellular physiology (i.e., ground truth), with all conclusions based on statistical comparisons. Importantly, this caveat is true for all human imaging studies given orders of magnitude difference that exists for imaging tissue volume (typically 8 μl) versus the cellular milieu for a single axon (typically 8 × 10−9 μl). Finally, HC were also purposely selected to match MMA on general physical fitness levels while excluding for a history of participation in contact sports. While the selection of this cohort reduces the likelihood of group differences due to exercise confounds (Schlaffke et al. 2014), it may have inadvertently introduced other cardiovascular biases not present in the general population. Third, the majority of MMA data were collected prior to recruitment of HC to facilitate the individual case matching criteria. Although there were no differences across several QA metrics, we cannot rule out temporal sampling effects.
In conclusion, current findings highlight how novel dMRI metrics can be used to complement traditional DTI measurements. Current findings suggest that rmTBI largely results in a pattern of decreased anisotropic diffusion in conjunction with increased intracellular and free water fractions, highlighting the potential role of edema as a fundamental pathological process in rmTBI. Current findings suggest that pathology associated with rmTBI aggregates at tissue junctions where the accumulation of shear-stresses has been shown to be the greatest in computational and animal models. Although a ground-truth metric was not available, current results also suggest the potential improved sensitivity of non-linear registration pipelines that maximize the anatomical information available in T1-weighted images. However, all of these findings require replication in independent samples of patients at risk for long-term neurological damage due to repetitive head traumas.
Supplementary Material
Acknowledgments
We would like to thank the staff and mixed-martial artists at Jackson’s Mixed Martial Arts gym in Albuquerque, New Mexico for their help and participation in the current study.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s11682-016-9546-1) contains supplementary material, which is available to authorized users.
Compliance with ethical standards
Conflict of interest Andrew Mayer, Josef Ling, Andrew Dodd, Timothy Meier, Faith Hanlon and Stefan Klimaj declare that they have no conflicts of interest.
Informed consent All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, and the applicable revisions at the time of the investigation. Informed consent was obtained from all patients for being included in the study.
References
- Adluru G, Gur Y, Anderson JS, Richards LG, Adluru N, DiBella EV. Assessment of white matter microstructure in stroke patients using NODDI. Conf Proc IEEE Eng Med Biol Soc; 2014. pp. 742–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry–the methods. NeuroImage. 2000;11(6 Pt 1):805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Avants BB, Tustison N, Song G. Advanced normalization tools (ANTS) Insight Journal. 2009;2:1–35. [Google Scholar]
- Barrio JR, Small GW, Wong KP, Huang SC, Liu J, Merrill DA, et al. In vivo characterization of chronic traumatic encephalopathy using [F-18] FDDNP PET brain imaging. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(16):E2039–E2047. doi: 10.1073/pnas.1409952112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazarian JJ, Zhu T, Zhong J, Janigro D, Rozen E, Roberts A, et al. Persistent, long-term cerebral white matter changes after sports-related repetitive head impacts. PLoS ONE. 2014;9(4):e94734. doi: 10.1371/journal.pone.0094734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR in Biomedicine. 2002;15(7–8):435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Borich M, Makan N, Boyd L, Virji-Babul N. Combining whole-brain voxel-wise analysis with in vivo tractography of diffusion behavior after sports-related concussion in adolescents: a preliminary report. Journal of Neurotrauma. 2013;30(14):1243–1249. doi: 10.1089/neu.2012.2818. [DOI] [PubMed] [Google Scholar]
- Browne KD, Chen XH, Meaney DF, Smith DH. Mild traumatic brain injury and diffuse axonal injury in swine. Journal of Neurotrauma. 2011;28(9):1747–1755. doi: 10.1089/neu.2011.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang LC, Jones DK, Pierpaoli C. RESTORE: robust estimation of tensors by outlier rejection. Magnetic Resonance in Medicine. 2005;53(5):1088–1095. doi: 10.1002/mrm.20426. [DOI] [PubMed] [Google Scholar]
- Chung S, Pelletier D, Sdika M, Lu Y, Berman JI, Henry RG. Whole brain voxel-wise analysis of single-subject serial DTI by permutation testing. NeuroImage. 2008;39(4):1693–1705. doi: 10.1016/j.neuroimage.2007.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R, Glen D. Efficient, robust, nonlinear, and guaranteed positive definite diffusion tensor estimation. Proceedings of the International Society for Magnetic Resonance and Medicine, 14th Scientific Meeting; Seattle, WA. 2006. [Google Scholar]
- Daducci A, Canales-Rodriguez EJ, Zhang H, Dyrby TB, Alexander DC, Thiran JP. Accelerated Microstructure Imaging via Convex Optimization (AMICO) from diffusion MRI data. NeuroImage. 2015;105:32–44. doi: 10.1016/j.neuroimage.2014.10.026. [DOI] [PubMed] [Google Scholar]
- Davenport EM, Whitlow CT, Urban JE, Espeland MA, Jung Y, Rosenbaum DA, et al. Abnormal white matter integrity related to head impact exposure in a season of high school varsity football. Journal of Neurotrauma. 2014;31(19):1617–1624. doi: 10.1089/neu.2013.3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destrieux C, Fischl B, Dale A, Halgren E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. NeuroImage. 2010;53(1):1–15. doi: 10.1016/j.neuroimage.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didehbani N, Munro CC, Mansinghani S, Conover H, Hart J., Jr Depressive symptoms and concussions in aging retired NFL players. Archives of Clinical Neuropsychology. 2013;28(5):418–424. doi: 10.1093/arclin/act028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich O, Raya JG, Reeder SB, Reiser MF, Schoenberg SO. Measurement of signal-to-noise ratios in MR images: influence of multichannel coils, parallel imaging, and reconstruction filters. Journal of Magnetic Resonance Imaging. 2007;26(2):375–385. doi: 10.1002/jmri.20969. [DOI] [PubMed] [Google Scholar]
- Dodd AB, Epstein K, Ling JM, Mayer AR. Diffusion tensor imaging findings in semi-acute mild traumatic brain injury. Journal of Neurotrauma. 2014;31(14):1235–1248. doi: 10.1089/neu.2014.3337. [DOI] [PubMed] [Google Scholar]
- Eierud C, Craddock RC, Fletcher S, Aulakh M, King-Casas B, Kuehl D, LaConte SM. Neuroimaging after mild traumatic brain injury: review and meta-analysis. Neuroimage Clinical. 2014;4:283–294. doi: 10.1016/j.nicl.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson CJ. An effect size primer: a guide for clinicians and researchers. Professional Psychology Research and Practice. 2009;40(5):532. [Google Scholar]
- Gentry LR. Imaging of closed head injury. Radiology. 1994;191(1):1–17. doi: 10.1148/radiology.191.1.8134551. [DOI] [PubMed] [Google Scholar]
- Grossman EJ, Ge Y, Jensen JH, Babb JS, Miles L, Reaume J, et al. Thalamus and cognitive impairment in mild traumatic brain injury: a diffusional kurtosis imaging study. Journal of Neurotrauma. 2012;29(13):2318–2327. doi: 10.1089/neu.2011.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman EJ, Jensen JH, Babb JS, Chen Q, Tabesh A, Fieremans E, et al. Cognitive impairment in mild traumatic brain injury: a longitudinal diffusional kurtosis and perfusion imaging study. AJNR - American Journal of Neuroradiology. 2013;34(5):951–953. doi: 10.3174/ajnr.A3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harel O. The estimation of R 2 and adjusted R 2 in incomplete data sets using multiple imputation. Journal of Applied Statistics. 2009;36(10):1109–1118. [Google Scholar]
- Hart J, Jr, Kraut MA, Womack KB, Strain J, Didehbani N, Bartz E, et al. Neuroimaging of cognitive dysfunction and depression in aging retired national football league players: a cross-sectional study. JAMA Neurology. 2013;70(3):326–335. doi: 10.1001/2013.jamaneurol.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry LC, Tremblay S, Boulanger Y, Ellemberg D, Lassonde M. Neurometabolic changes in the acute phase after sports concussions correlate with symptom severity. Journal of Neurotrauma. 2010;27(1):65–76. doi: 10.1089/neu.2009.0962. [DOI] [PubMed] [Google Scholar]
- Holbourn AHS. Mechanics of head injuries. The Lancet. 1943;242(6267):438–441. [Google Scholar]
- Hulkower MB, Poliak DB, Rosenbaum SB, Zimmerman ME, Lipton ML. A decade of DTI in traumatic brain injury: 10 years and 100 articles later. AJNR - American Journal of Neuroradiology. 2013;34(11):2064–2074. doi: 10.3174/ajnr.A3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansons KM, Alexander DC. Persistent angular structure: new insights from diffusion magnetic resonance imaging data. Inverse Problems. 2003;19(5):1031. doi: 10.1007/978-3-540-45087-0_56. [DOI] [PubMed] [Google Scholar]
- Jensen JH, Helpern JA. MRI quantification of non-gaussian water diffusion by kurtosis analysis. NMR in Biomedicine. 2010;23(7):698–710. doi: 10.1002/nbm.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen JH, Helpern JA, Ramani A, Lu H, Kaczynski K. Diffusional kurtosis imaging: the quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magnetic Resonance in Medicine. 2005;53(6):1432–1440. doi: 10.1002/mrm.20508. [DOI] [PubMed] [Google Scholar]
- Jones DK, Horsfield MA, Simmons A. Optimal strategies for measuring diffusion in anisotropic systems by magnetic resonance imaging. Magnetic Resonance in Medicine. 1999;42(3):515–525. [PubMed] [Google Scholar]
- Jones DK, Griffin LD, Alexander DC, Catani M, Horsfield MA, Howard R, Williams SC. Spatial normalization and averaging of diffusion tensor MRI data sets. NeuroImage. 2002;17(2):592–617. [PubMed] [Google Scholar]
- Kim JJ, Gean AD. Imaging for the diagnosis and management of traumatic brain injury. Neurotherapeutics. 2011;8(1):39–53. doi: 10.1007/s13311-010-0003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A, Andersson J, Ardekani BA, Ashburner J, Avants B, Chiang MC, et al. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. NeuroImage. 2009;46(3):786–802. doi: 10.1016/j.neuroimage.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koay CG, Yeh P-H, Ollinger JM, Irfanoglu MO, Pierpaoli C, Basser PJ, et al. Tract Orientation and Angular Dispersion Deviation Indicator (TOADDI): a framework for single-subject analysis in diffusion tensor imaging. 2015 doi: 10.1016/j.neuroimage.2015.11.046. arXiv preprint, 1510.02934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerte IK, Kaufmann D, Hartl E, Bouix S, Pasternak O, Kubicki M, et al. A prospective study of physician-observed concussion during a varsity university hockey season: white matter integrity in ice hockey players. Part 3 of 4. Neurosurgical Focus. 2012;33(6):E3–E7. doi: 10.3171/2012.10.FOCUS12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling J, Merideth F, Caprihan A, Pena A, Teshiba T, Mayer AR. Head injury or head motion? Assessment and quantification of motion artifacts in diffusion tensor imaging studies. Human Brain Mapping. 2012a;33(1):50–62. doi: 10.1002/hbm.21192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling JM, Pena A, Yeo RA, Merideth FL, Klimaj S, Gasparovic C, Mayer AR. Biomarkers of increased diffusion anisotropy in semi-acute mild traumatic brain injury: a longitudinal perspective. Brain. 2012b;135(Pt 4):1281–1292. doi: 10.1093/brain/aws073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton ML, Kim N, Zimmerman ME, Kim M, Stewart WF, Branch CA, Lipton RB. Soccer heading is associated with white matter microstructural and cognitive abnormalities. Radiology. 2013;268(3):850–857. doi: 10.1148/radiol.13130545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu AY, Maldjian JA, Bagley LJ, Sinson GP, Grossman RI. Traumatic brain injury: diffusion-weighted MR imaging findings. AJNR - American Journal of Neuroradiology. 1999;20(9):1636–1641. [PMC free article] [PubMed] [Google Scholar]
- Mayer AR, Franco AR, Ling J, Canive JM. Assessment and quantification of head motion in neuropsychiatric functional imaging research as applied to schizophrenia. Journal of the International Neuropsychological Society. 2007;13(5):839–845. doi: 10.1017/S1355617707071081. [DOI] [PubMed] [Google Scholar]
- Mayer AR, Ling JM, Dodd AB, Gasparovic C, Klimaj SD, Meier TB. A longitudinal assessment of structural and chemical alterations in mixed martial arts fighters. Journal of Neurotrauma. 2015;32(22):1759–1767. doi: 10.1089/neu.2014.3833. [DOI] [PubMed] [Google Scholar]
- McAllister TW, Ford JC, Flashman LA, Maerlender A, Greenwald RM, Beckwith JG, et al. Effect of head impacts on diffusivity measures in a cohort of collegiate contact sport athletes. Neurology. 2014;82(1):63–69. doi: 10.1212/01.wnl.0000438220.16190.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee AC, Stein TD, Nowinski CJ, Stern RA, Daneshvar DH, Alvarez VE, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013;136(Pt 1):43–64. doi: 10.1093/brain/aws307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Oishi K, Jiang H, Jiang L, Li X, Akhter K, et al. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. NeuroImage. 2008;40(2):570–582. doi: 10.1016/j.neuroimage.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugavel M, Cubon V, Putukian M, Echemendia R, Cabrera J, Osherson D, Dettwiler A. A longitudinal diffusion tensor imaging study assessing white matter fiber tracts after sports-related concussion. Journal of Neurotrauma. 2014;31(22):1860–1871. doi: 10.1089/neu.2014.3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omalu BI, DeKosky ST, Minster RL, Kamboh MI, Hamilton RL, Wecht CH. Chronic traumatic encephalopathy in a national football league player. Neurosurgery. 2005;57(1):128–134. doi: 10.1227/01.neu.0000163407.92769.ed. [DOI] [PubMed] [Google Scholar]
- Pasternak O, Koerte IK, Bouix S, Fredman E, Sasaki T, Mayinger M, et al. Hockey concussion education project, part 2. Microstructural white matter alterations in acutely concussed ice hockey players: a longitudinal free-water MRI study. Journal of Neurosurgery. 2014;120(4):873–881. doi: 10.3171/2013.12.JNS132090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaffke L, Lissek S, Lenz M, Brune M, Juckel G, Hinrichs T, et al. Sports and brain morphology - a voxel-based morphometry study with endurance athletes and martial artists. Neuroscience. 2014;259:35–42. doi: 10.1016/j.neuroscience.2013.11.046. [DOI] [PubMed] [Google Scholar]
- Schwarz CG, Reid RI, Gunter JL, Senjem ML, Przybelski SA, Zuk SM, et al. Improved DTI registration allows voxel-based analysis that outperforms tract-based spatial statistics. NeuroImage. 2014;94:65–78. doi: 10.1016/j.neuroimage.2014.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepehrband F, Clark KA, Ullmann JF, Kurniawan ND, Leanage G, Reutens DC, Yang Z. Brain tissue compartment density estimated using diffusion-weighted MRI yields tissue parameters consistent with histology. Human Brain Mapping. 2015;36(9):3687–3702. doi: 10.1002/hbm.22872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenton ME, Hamoda HM, Schneiderman JS, Bouix S, Pasternak O, Rathi Y, et al. A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging and Behavior. 2012;6(2):137–192. doi: 10.1007/s11682-012-9156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin W, Mahmoud SY, Sakaie K, Banks SJ, Lowe MJ, Phillips M, et al. Diffusion measures indicate fight exposure-related damage to cerebral white matter in boxers and mixed martial arts fighters. AJNR - American Journal of Neuroradiology. 2014;35(2):285–290. doi: 10.3174/ajnr.A3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skare S, Hedehus M, Moseley ME, Li TQ. Condition number as a measure of noise performance of diffusion tensor data acquisition schemes with MRI. Journal of Magnetic Resonance. 2000;147(2):340–352. doi: 10.1006/jmre.2000.2209. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. NeuroImage. 2003;20(3):1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Spain A, Daumas S, Lifshitz J, Rhodes J, Andrews PJ, Horsburgh K, Fowler JH. Mild fluid percussion injury in mice produces evolving selective axonal pathology and cognitive deficits relevant to human brain injury. Journal of Neurotrauma. 2010;27(8):1429–1438. doi: 10.1089/neu.2010.1288. [DOI] [PubMed] [Google Scholar]
- Storey P, Frigo FJ, Hinks RS, Mock BJ, Collick BD, Baker N, et al. Partial k-space reconstruction in single-shot diffusion-weighted echo-planar imaging. Magnetic Resonance in Medicine. 2007;57(3):614–619. doi: 10.1002/mrm.21132. [DOI] [PubMed] [Google Scholar]
- Tustison NJ, Avants BB, Cook PA, Kim J, Whyte J, Gee JC, Stone JR. Logical circularity in voxel-based analysis: normalization strategy may induce statistical bias. Human Brain Mapping. 2014;35(3):745–759. doi: 10.1002/hbm.22211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virji-Babul N, Borich MR, Makan N, Moore T, Frew K, Emery CA, Boyd LA. Diffusion tensor imaging of sports-related concussion in adolescents. Pediatric Neurology. 2013;48(1):24–29. doi: 10.1016/j.pediatrneurol.2012.09.005. [DOI] [PubMed] [Google Scholar]
- Wedeen VJ, Hagmann P, Tseng WY, Reese TG, Weisskoff RM. Mapping complex tissue architecture with diffusion spectrum magnetic resonance imaging. Magnetic Resonance in Medicine. 2005;54(6):1377–1386. doi: 10.1002/mrm.20642. [DOI] [PubMed] [Google Scholar]
- Wu EX, Cheung MM. MR diffusion kurtosis imaging for neural tissue characterization. NMR in Biomedicine. 2010;23(7):836–848. doi: 10.1002/nbm.1506. [DOI] [PubMed] [Google Scholar]
- Zhang L, Heier LA, Zimmerman RD, Jordan B, Ulug AM. Diffusion anisotropy changes in the brains of professional boxers. AJNR - American Journal of Neuroradiology. 2006;27(9):2000–2004. [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Johnson B, Pennell D, Ray W, Sebastianelli W, Slobounov S. Are functional deficits in concussed individuals consistent with white matter structural alterations: combined FMRI & DTI study. Experimental Brain Research. 2010;204(1):57–70. doi: 10.1007/s00221-010-2294-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DC. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. NeuroImage. 2012;61(4):1000–1016. doi: 10.1016/j.neuroimage.2012.03.072. [DOI] [PubMed] [Google Scholar]
- Zhu DC, Covassin T, Nogle S, Doyle S, Russell D, Pearson RL, et al. A potential biomarker in sports-related concussion: brain functional connectivity alteration of the default-mode network measured with longitudinal resting-state fMRI over thirty days. Journal of Neurotrauma. 2015;32(5):327–341. doi: 10.1089/neu.2014.3413. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.