Abstract
Chlamydia trachomatis urogenital serovars primarily replicate in epithelial cells lining the reproductive tract. Epithelial cells recognize Chlamydia through cell surface and cytosolic receptors, and/or endosomal innate receptors such as Toll-like receptors (TLRs). Activation of these receptors triggers both innate and adaptive immune mechanisms that are required for chlamydial clearance, but are also responsible for the immunopathology in the reproductive tract. We previously demonstrated that Chlamydia muridarum (Cm) induces IFN-β in oviduct epithelial cells (OE) in a TLR3-dependent manner, and that the synthesis of several cytokines and chemokines are diminished in Cm-challenged OE derived from TLR3-/- 129S1 mice. Furthermore, our in vitro studies showed that Cm replication in TLR3-/- OE is more efficient than in wild-type OE. Because TLR3 modulates the release inflammatory mediators involved in host defense during Cm infection, we hypothesized that TLR3 plays a protective role against Cm-induced genital tract pathology in congenic C57BL/6N mice. Using the Cm mouse model for human Chlamydia genital tract infections, we demonstrated that TLR3-/- mice had increased Cm shedding during early and mid-stage genital infection. In early stage infection, TLR3-/- mice showed a diminished synthesis of IFN-β, IL-1β, and IL-6, but enhanced production of IL-10, TNF-α, and IFN-γ. In mid-stage infection, TLR3-/- mice exhibited significantly enhanced lymphocytic endometritis and salpingitis than wild-type mice. These lymphocytes were predominantly scattered along the endometrial stroma and the associated smooth muscle, and the lamina propria supporting the oviducts. Surprisingly, our data show that CD4+ T-cells are significantly enhanced in the genital tract TLR3-/- mice during mid-stage Chlamydial infection. In late-stage infections, both mouse strains developed hydrosalpinx; however, the extent of hydrosalpinx was more severe in TLR3-/- mice. Together, these data suggest that TLR3 promotes the clearance of Cm during early and mid-stages of genital tract infection, and that loss of TLR3 is detrimental in the development hydrosalpinx.
Introduction
Chlamydia trachomatis (Ct), a Gram-negative obligate intracellular bacterium, is the leading cause of bacterial sexually transmitted diseases worldwide with an estimated incidence of 105.7 million infections in adults every year [1]. Although Ct infections in women are treatable with antibiotics, these infections can remain largely asymptomatic and go undetected in approximately 70–80% of the cases [2]. Chlamydia trachomatis infections in the reproductive tract of women can also lead to cervicitis and endometritis as well as to the development of serious complications, including pelvic inflammatory disease (PID), tubal scarring and infertility, fallopian tube blockage with serous fluid (hydrosalpinx), chronic pelvic pain, and ectopic pregnancy [2, 3]. Clearance of Chlamydia needs the coordinated action of both the innate immune response and host CD4+ T-cells, which together are essential for the optimal resolution of primary chlamydial genital infections in mice. [3–5]. However, Chlamydia infection causes the induction of a specific subset of innate inflammatory mediators and the recruitment of CD8+ T-cells into the female genital tract, and these factors are known to have a significant role in development of genital tract pathology [6–8]. The ultimate goal of the continued research on Ct pathogenesis is to identify immune mediators that generate long-term protective immune responses against Ct infections, and to ascertain immune targets that modulate the immune responses leading to upper reproductive tract pathology.
The murine model of Chlamydia muridarum genital tract infection recapitulates many aspects of the pathogenesis and immunity associated with Ct genital tract infections in women [9]. The initial immune response in the genital tracts of mice infected with C. muridarum is dominated by myeloid cell infiltrates, including neutrophils, which are predominantly recruited to the cervical epithelium [8, 10]. As infection progresses in mice, Chlamydia disseminates to epithelial surfaces lining the uterine horns and oviducts, which become infiltrated by CD4+ and CD8+ T-cells, plasma cells, and macrophages [9, 10]. Late stages of C. muridarum infection in mice can then lead to hydrosalpinx, fibrosis and/or infertility [4, 11, 12], which are also common post-infection sequelae in women. There are multiple inflammatory cells infiltrating the genital tract of mice throughout the course of Cm infection; however, none of them are the primary target of infection. Instead, epithelial cells are the primary targets as Chlamydia selectively replicates within the reproductive tract epithelium, and these cells are also critical on initiating and propagating the immune response during infection [13, 14].
As an obligate intracellular pathogen, Chlamydia species are known to interact with host-cell pattern recognition receptors (PRRs), including Toll-like receptors (TLRs) and a variety of other intracellular cytosolic receptors [13]. Activation of these receptors triggers the secretion of pro-inflammatory cytokines and chemokines, which in succession induces the recruitment of inflammatory cells. Chlamydial LPS and heat shock proteins (HSPs) are ligands for TLR4 [15–17], while chlamydial plasmid-regulated ligands and peptidoglycans are ligands for TLR2 in phagocytes [13, 18, 19]. Several other types of intracellular sensors have been shown to play role in the recognition of Chlamydia in phagocytes, including the intracellular nucleotide sensors cyclic GMP–AMP (cGAMP) synthase (cGAS), stimulator of interferon genes (STING), nucleotide-binding oligomerization domain-containing 1 (NOD1), and NLR family pyrin domain containing 3 (NLRP3) inflammasome. [13, 20, 21].
TLR3 is a receptor for double-stranded RNA (dsRNA) and is known to activate transcription of IFN-β via the adaptor protein Toll-IL-1 receptor (TIR) domain-containing adaptor molecule-1 (TICAM-1) [also called TIR-domain-containing adapter inducing IFN-β (TRIF)] [22, 23]. TLR3 has been identified as the major MyD88-independent PRR stimulated in the type-1 IFN responses to many different viral infections due to its intracellular localization [24]; however, its role in bacterial infection is poorly understood. Although a double-stranded RNA moiety for Chlamydia has not yet been identified as part of chlamydial structure, we previously showed that C. muridarum infected murine oviduct epithelial (OE) cells secrete IFN-β in a largely TLR3 dependent manner, and that OE cells deficient in TLR3 showed dramatic reductions in the synthesis of other inflammatory immune mediators in addition to IFN-β [25, 26]. Our previous data also showed IL-6 and CCL5 synthesis was diminished during early stages of Cm infection in the genital tract of TLR3-/- mice from a 129S1 genetic background [26]. Furthermore, C. muridarum replication in murine OE cells is more robust in the absence of TLR3, suggesting that activation of TLR3 negatively affects Chlamydia growth. Because TLR3 modulates the release of inflammatory mediators involved in host defense in vitro as well as during Cm genital tract infection in mice, we hypothesized that TLR3 plays a protective role against Cm-induced genital tract pathology in congenic C57BL/6N mice.
In this study, we further examine the role of TLR3 in the development of genital tract pathology during chlamydial infection. Using the Cm model of chlamydial genital tract infection, we infected wild-type and TLR3-/- mice, and compared the vaginal synthesis of cytokines and chemokines, Chlamydia burden, CD4+/CD8+ T-cell profiles, and genital tract pathology at different time points throughout infection. We found that TLR3 was critical in the synthesis of several intravaginal pro-inflammatory cytokines such as IFN-β, IL-1β, and IL-6, and had an altered synthesis of IL-10, TNF-α, and IFN-γ during early stages of Cm genital tract infection. TLR3 also played a role in host defense as TLR3-/- mice had significantly higher levels of genital tract chlamydial shedding than wild-type mice during the first three weeks of genital tract infection. Furthermore, TLR3-/- mice exhibited significantly higher lymphocytic endometritis and salpingitis than wild-type mice during mid-stages of genital tract infection. Interestingly, the presence of CD4+ T-cells were markedly enhanced in the genital tract of TLR3-/- mice at this stage of Chlamydia infection. Finally, TLR3-/- mice exhibited a significantly increased frequency in the development of hydrosalpinx than wild-type mice during late-stages of genital tract infection, suggesting that TLR3 had a role in preventing the development of hydrosalpinx in vivo.
Materials and methods
Ethics statement
C57BL/6J (control) and TLR3-deficient mice were purchased from The Jackson Laboratory (Bar Harbor, ME) at 6–8 weeks of age. All mice were provided food and water ad libitum, and kept on a standard 12-hour light/dark cycle. All mice were given at least a 1-week period where they were allowed to acclimate to their new environment. After acclimation, female C57BL/6NJ and TLR3-deficient mice were injected with 2.5 mg Depo-Provera after being briefly anesthetized with isoflurane prior to any experiment, and all mice were allowed to recover for no less than 1-week after the Depo-Provera treatment. To alleviate any possible distress, the mice were also briefly anesthetized with isoflurane prior to either intravaginal infection with C. muridarum, the insertion of calcium alginate swabs, or the insertion of aseptic vaginal sponges. All mice were monitored daily for lethargy, signs of vaginal bleeding, and/ or death. None of the mice exhibited morbidity during the course of these studies. All mice were euthanized by either exposure to isoflurane or inhalation of carbon dioxide, followed by exsanguination. The Indiana University Institutional Animal Care and Use Committee (IACUC) approved all experimental animal protocols. All care was used to ensure that steps were taken to ameliorate animal suffering in all work involved in the removal of genital-tract tissue.
Chlamydia stocks
Mycoplasma-free C. muridarum Nigg, previously known as C. trachomatis strain (MoPn), was grown and titrated in McCoy cells (ATCC) as described [27, 28]. Elementary bodies were harvested from infected cells, resuspended in SPG buffer (250mM sucrose, 10mM sodium phosphate, and 5mM L-glutamic acid, pH 7.2), and quantified on McCoy cells using methodology described previously [26, 27, 29].
Mice and infections
Wild-type control mice C57BL/6NJ [Stock No 005304] and TLR3-deficient mice B6N.129S1-Tlr3tm1Flv/J [Stock No 009675] were purchased from The Jackson Laboratory at 6–8 weeks of age. This TLR3 deficient mouse strain is a fully congenic on the C57BL/6N genetic background. Briefly, this particular strain of TLR3 deficient mice were the result of a backcross into C57BL/6J F1 hybrids that were self-crossed to generate F2 hybrids identified as homozygous with or without the TLR3 deletion. The homozygous mice are maintained at The Jackson Laboratory by breeding descendants of the original homozygous F2 hybrids. All mice were housed in Indiana University Purdue University-Indianapolis specific pathogen-free (SPF) facilities. Age-matched mice were used for the experiments in this study. The Indiana University Institutional Animal Care and Utilization Committee (IACUC) approved all experimental protocols.
Infections of mice were done as described in [18] with some minor modifications. Mice were treated with 2.5 mg of Depo-Provera (medroxyprogesterone acetate; Pfizer, New York, NY) in 0.1 ml saline to synchronize the estrous cycle and increase their susceptibility to a chlamydial infection, one week before vaginal infection with 105 IFU C. muridarum (approximately 100 times the ID50) in 10μl sucrose-phosphate-glutamic acid (SPG) buffer. The infection was monitored by swabbing the vaginal vault and cervix with a calcium alginate swab (Spectrum Medical Industries, Los Angeles, CA) and determining the number of inclusion forming units (IFU) recovered from the swabs by plating serial dilutions on McCoy cell monolayers as described [27, 28, 30]. Briefly, swabs were suspended in DMEM/ 10% FCS supplemented with vancomycin, gentamicin, and cycloheximide and then vortexed for 1 min in the presence of glass beads to release chlamydial organisms from the swab. Individual wells of McCoy cell monolayers in 96-well plates were inoculated with serial dilutions of each sample in a total volume of 200μl/well, followed by centrifugation at 1200 rpm (200 × g) in a table-top centrifuge for 1 hour. After centrifugation, the plates were then incubated at 37°C in a 5% CO2 humidified incubator for 2h before removing supernatants and replacing it with fresh medium. Plates were returned to the incubator for an additional 32h. Afterwards, the plates were removed from the incubator, supernatants removed, and the monolayers were washed with PBS before being fixed with methanol. Chlamydial inclusions were identified by the addition of anti-Chlamydia immune sera and anti-mouse immunoglobulin G (IgG) conjugated to fluorescein-isothiocyanate secondary antibody (ICN Immunobiologicals, Irvine, Calif.). The number of inclusion bodies within 20 fields was counted under a fluorescent microscope, and IFU/ml were calculated. Each mouse strain (wildtype and TLR3-deficient) were infected in groups of five, and each experiment was repeated at least three times.
Determination of cytokine production
Vaginal swab samples for measurement of genital secretions of innate-immune cytokines and chemokines were collected via the aseptic sponge technique [18]. Aseptic surgical sponges (ear wicks, 2 x 5 mm) (DeRoyal, Powell, TN) were inserted into the vaginas of anesthetized Chlamydia-infected and mock infected mice, each day during the first 21 days post-infection. Each sponge was retrieved 30 min later and individually collected in microfuge tubes for storage at -80°C. The sponges were then soaked in 300μl of sterile resuspension buffer (PBS supplemented with 0.5% BSA and 0.05% TWEEN 20) for one hour on ice, and filtered through a pre-blocked 0.2-μm cellulose acetate filter by centrifugation in a Spin-X microfuge tube (Fisher Scientific, Pittsburgh, PA) prior to analysis. The filtrates were frozen at -80°C and shipped on dry-ice to Aushon Biotechnology (Billerica, MA) for multiplex analyses. The analyses of cytokine and chemokine synthesis was performed by Aushon using their in-house Ciraplex™ multiplex assay. The samples were analyzed in triplicate, and the assays were conducted on all three replicates of mouse infections.
Tissue processing for isolation of lower genital tract and splenic T-cells
Genital tracts were excised from each group of wild-type and TLR3-deficient mice sacrificed at day 7 and day 21 post-infection. Each mouse was handled separately, and the lower portion of the genital tract of each mouse (defined as the area from cervix to the start of uterine the horn) was separated from the rest of the genital tract as previously described [31]. The lower genital tracts were minced in RPMI-1640 medium supplemented with 10% FBS (Sigma-Aldrich, St. Louis, MO) and filtered throw a 70μm nylon mesh cell strainer. The resulting slurry was then digested with freshly prepared collagenase I solution in RPMI-1640 supplemented with 10% FBS (1mg/ml; Sigma-Aldrich, St. Louis, MO) for 1h at 37 °C to obtain a single-cell suspension. The lymphocytes were isolated using Percoll™ gradient (density 1.129g/ml, GE Healthcare Bio-sciences AB, Sweden) as described in the protocol [32]. Spleens from individual mouse were minced and filtered through a 70μm nylon mesh cell strainer. Red blood cells (RBC) were lysed with 1x RBC lysis solution (eBioscience, SanDiego CA) for 5 min at room temperature. Total splenocytes were re-suspended in RPMI-1640 medium supplemented with 10% FBS after centrifugation.
Flow cytometry
Isolated murine genital tract and splenic T-cells were initially blocked for 20 min in 10% rabbit serum (Sigma-Aldrich, St. Louis, MO). Following blocking for non-specific binding, 1×106 cells were stained with anti-mouse CD3-PE-Cy7 (clone 17A2, BioLegend, San Diego CA), CD4-FITC (Ref# MCD0401, Invitrogen, Carlsbad CA) and CD8a-PE (Invitrogen) for 30 min at 4°C. Isotype control antibodies FITC Rat IgG2λ κ (clone RTK2758), PE Rat IgG2b κ (clone RTK4530), and PE-Cy7 Rat IgG2b κ (clone RTK4530) used in these experiments were obtained from BioLegend. Cell population analysis was conducted by LSR4 flow cytometer equipped with BD FACSDiva software (BD Biosciences, Franklin Lakes, NJ). A lymphocyte specific gating was set according to forward and side scatters profiles.
Histopathology
Genital tracts were extracted en bloc from mice sacrificed on day 7, 21, or 56 post-infection and then fixed in 4% paraformaldehyde for 48 hrs. Paraffin-embedded, 4-μm sections containing cervix and both uterine horns and oviducts were stained with hematoxylin and eosin (H&E). Genital tract sections (cervix, uterine body and horns, and oviducts) were independently examined and graded for the presence of acute inflammation (neutrophilic infiltrates), chronic inflammation (lymphocytic infiltrates), epithelial erosion and disruption, and luminal distension of uterine horns and oviducts using a previously described four-tiered semiquantitative scoring system [33, 34]. Inflammatory cell infiltrates or epithelial erosion and disruption were scored as follows: 0, normal; 1, occasional foci or minimal increase in the parameter; 2, scattered (2 to 4) aggregates or mild focal to multifocal increase in parameter; 3, numerous aggregates (< 4) or moderate multifocal to coalescing increase in parameter; 4, numerous, dense or confluent aggregates or severe diffuse or multifocal to coalescing increase in parameter. The uterine horn dilation and oviduct hydrosalpinges were assessed grossly and microscopically, and scoring of these parameters were performed using a semiquantitative scoring system described previously [34]. The dilation of the uterine horns and oviducts (hydrosalpinges) were scored as follows: 0, normal; 1, mild increase in dilatation of a single segment of these parameters; 2, one to three, unilateral or bilateral, dilated segments of these parameters; 3, more than three, unilateral or bilateral, dilated segments of these parameters; and 4, confluent pronounced or marked unilateral or bilateral dilatation of uterine horns and oviducts. All scoring was performed in a double-blinded fashion with regards to the treatment groups, and scores for each evaluated parameter were combined and averaged to provide one score per animal.
Immunohistochemistry (IHC)
Paraffin-embedded sections were processed at the UC Davis Center for Genomic Pathology Laboratory for immunohistochemical staining of CD4 and CD8 T-cells in the genital tracts of a subset uninfected and infected mice as previously described [35]. Sections of spleen were used as internal control. Rat monoclonal anti-mouse CD4 (clone RM4-5, BD Biosciences) and rat monoclonal anti-mouse CD8 (clone 53–6.7, Biolegend) were individually diluted in 0.5% bovine serum albumin (BSA) in PBS (pH 7.4) and incubated with tissue sections for 1 hr at room temperature. The slides were then rinsed twice with PBS buffer for 5 min each. Immunohistochemical reaction was performed by using a VECTASTAIN Elite ABC kit (Vector Laboratories, Burlingame, CA) with biotinylated anti-rat IgG (Vector Laboratories) following manufacturer’s protocol. After the slides were washed twice with PBS, sections were then incubated in 0.1% 3,3’-diaminobenzidine (DAB) solution (Dako, Carpinteria, CA) following the manufacturer’s directions. Paraffin-embedded sections from uninfected and infected mice (n = 3 per group) were processed at the California Animal Health and Food Safety Laboratory System for immunohistochemical detection of Chlamydia LPS antigen in genital tracts of these mice s previously described [36]. Sections of liver from a Chlamydia infected bird was used as an internal control for positive reaction.
Statistical analysis
Statistical comparison between the two experimental groups for the vaginal cytokines levels and the CD4—CD8 counts in tissues over the course of infection were made by a Student’s t-test with Welch correction. For analysis of histopathology scores, the Mann-Whitney rank sum test was performed to assess differences in inflammation between the two experimental groups on three separate time points of infection. A p value ≤ 0.05 was considered statistically significant. Analyses were performed using GraphPad Prism v7 statistical program (GraphPad Software, San Diego, CA).
Results
Chlamydia muridarum shedding is more robust in the genital tracts of TLR3-deficient C57BL/6 mice
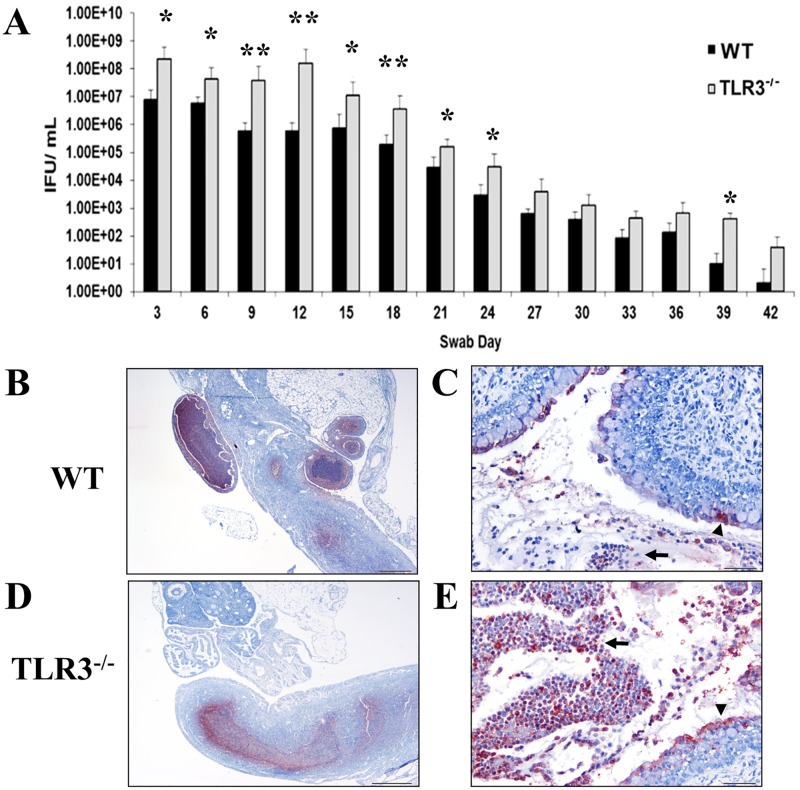
We previously showed that TLR3-/- mice bred in the 129S1 background exhibited significantly higher levels of chlamydial shedding from the genital tract when compared to wild-type mice within the first four weeks of infection [25, 26]. Additionally, our previous studies showed that Chlamydia replication in oviduct epithelial cells (OE) deficient in TLR3 is more efficient than the 129S1 wild-type OE cells. The data from these experiments also demonstrated that TLR3 had a biological impact on the innate immune response to Chlamydia infection in mice; however, the exact effect that TLR3 signaling had on the genital tract pathology of C. muridarum infection in mice was unclear. To determine the biological significance that TLR3 had on chlamydial shedding in mice bred in the C57BL/6N genetic background, we infected groups of five wild-type and five C57BL/6N TLR3-/- (referred as TLR3-/-) intravaginally with 105 IFU C. muridarum. As shown in Fig 1A, TLR3-/- mice bred in the C57BL/6 genetic background mice shed Chlamydia at significantly higher levels throughout the first 24 days of infection than did wild-type mice. Similar to our previous data using 129S1 mice, the chlamydial titers for both C57BL/6N strains were virtually the same after day 24 and for the remainder of infection, and the clearance kinetics were similar between both strains. To illustrate the localization of C. muridarum in the genital tract of mice, we performed immunohistochemistry in a subset of genital tracts of mice at day 7 of infection (Fig 1B–1F). Chlamydia antigen was detected in the cytoplasm of epithelial cells lining the oviducts as well as the endometrium of the uterine horns and cervical regions. There was also Chlamydia antigen commonly associated with degenerate and necrotic neutrophils present in the lumina of the genital tracts of wild-type and TLR3-/- mice (Fig 1B). Collectively, our data suggest that TLR3 controls C. muridarum replication during early and middle genital tract infection in both C57BL/6N and 129S1 mouse strains.
Fig 1. The levels of Chlamydia muridarum shedding from genital tracts of TLR3-/- mice are significantly higher during the first 24 days of infection.
A) Genital tract infections were performed, swabs were collected every third day for 42 days, and C. muridarum titers were determined by infecting fresh McCoy cell monolayers (see Materials and methods). Representative data from an average of 5 mice are shown. IFU = inclusion forming units. * = p <0.05; ** = p <0.005. B-E) Immunostaining of Chlamydia muridarum in genital tracts to wild-type (WT) and TLR3-/- mice at day 7 post-challenge. Detection of Chlamydia antigen (red staining) within neutrophils (arrow) and epithelial cells (arrowhead) in oviducts (B), endometrium (C), and cervix from a representative WT mouse. Detection of Chlamydia antigen (red staining) associated with neutrophils (arrows) and epithelial cells (arrowhead) in endometrium and cervix (D and E), from a representative TLR3-/- mouse.
TLR3 deficiency in mice results in increased chronic endometritis and salpingitis during the middle stages of C. muridarum infection
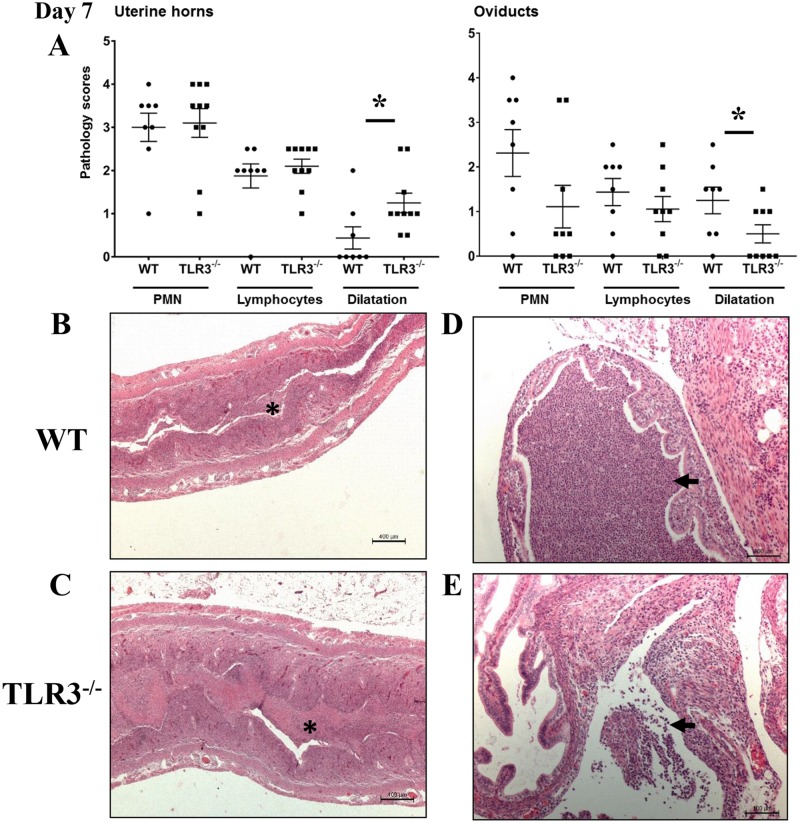
Because TLR3-/- mice exhibited increased genital tract shedding of Chlamydia throughout the first three weeks of infection, we next evaluated whether TLR3-/- mice also developed more severe pathology in the genital tracts during Chlamydia infection. Groups of 5 wild-type and TLR3-/- mice were intravaginally inoculated with C. muridarum, and histological lesions of their uterine horns and oviducts collected at days 7 and 21 of infection were scored as described in Materials and Methods. There were no significant differences in neutrophilic or lymphocytic infiltration in the uterine horns and oviducts between infected TLR3-/- and wild-type mice (Fig 2A). The uterine horn from both strains were moderately inflamed with multifocal to coalescing dense intraluminal aggregates of degenerate and necrotic neutrophils mixed with karyorrhectic nuclear debris and fibrin (Fig 2B and 2C). The endometrial epithelium was mildly disrupted at multiple regions of suppurative inflammation. The underlying endometrial stroma along the uterine horns was predominantly infiltrated with small numbers of lymphocytes (Fig 2B and 2C). The uterine horns from TLR3-/- mice were slightly distended, and this feature was more pronounced in TLR3-/- mice (Fig 2A). Although neutrophilic inflammation in oviducts and periovarian spaces of wild-type mice was not significantly different to the same areas in TLR3-/- mice, the extent and distribution of the neutrophilic inflammation in these regions were slightly enhanced in wild-type mice (Fig 2A). The oviducts, periovarian spaces, and ovarian bursa from wild-type mice were predominantly infiltrated with multifocal aggregates of neutrophils and small numbers of lymphocytes in a background karyorrhectic nuclear debris and eosinophilic proteinaceous fluid (Fig 2D). Conversely, the inflammatory cell infiltrates in oviducts, periovarian spaces, and ovarian bursa from TLR3-/- were composed by multifocal small aggregates of neutrophils, individual lymphocytes, and small amounts of eosinophilic proteinaceous fluid (Fig 2E). The oviduct epithelium from both group of mice were minimally eroded at sites of exudative inflammation. Oviductal dilation was mild and most commonly noted in wild-type mice (Fig 2A).
Fig 2. Acute inflammation in uterine horns and oviducts of wild-type (WT) and TLR3 deficient (TLR3-/-) mice on day 7 of Chlamydia infection.
A) The extent of histopathological changes of both uterine horns and oviducts were scored using a four-tiered semi-quantitative scoring system in a double-blind fashion. These results were representative of 8–10 mice per group from three independent experiments. Differences between groups for each parameter were determined by two-tailed Mann-Whitney test (* = p < 0.05). Only significant results are displayed in graphs. B-C) Histopathological evaluation of uterine horns from WT and TLR3-/- mice shows that PMNs were commonly infiltrating the lumina of the uterine horns (asterisk) in both mouse strains. Uterine horn dilatation was frequently noted in infected TLR3-/- mice (scale bar 100μm, 25x magnification). D-E) Histopathological evaluation of oviducts from WT and TLR3-/- mice on day 7 of infection revealed mild oviduct dilatation in WT mice, but minimal oviduct dilatation in TLR3-/- mice (scale bar 400μm, 100x magnification). There were PMN clusters in the lumina of oviducts (arrow). No significant differences of neutrophilic or lymphocytic infiltration was noted between the genital tracts of WT and TLR3-/- mice. Representative images for WT and TLR3-/- mice are shown. (• = WT mouse; ▪ = TLR3-/- mouse).
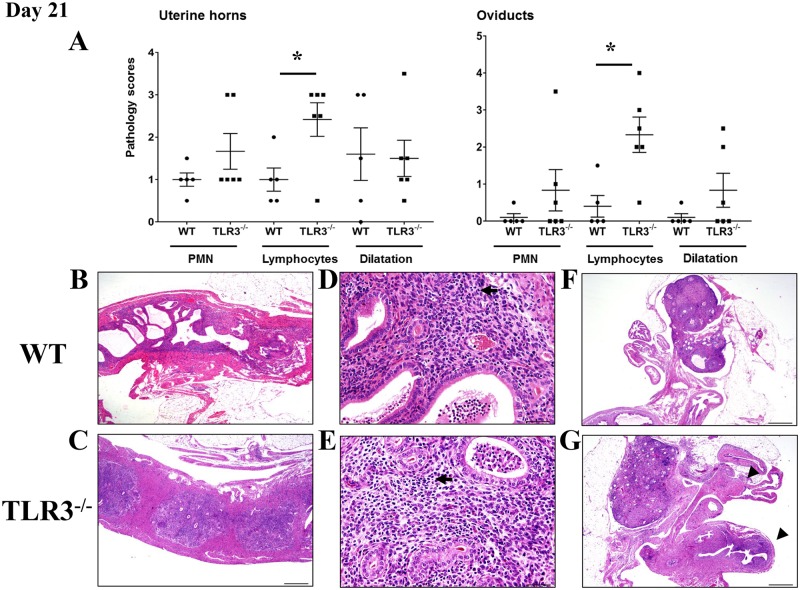
The inflammatory cell infiltrate in the genital tract of wild-type and TLR3-/- mice at middle stages (day 21 of infection) of C. muridarum infection was predominantly composed of lymphocytes when compared with mock-inoculated controls. As shown in Fig 3, the degree of lymphocytic inflammation in the genital tract of TLR3-/- mice was significantly different to the inflammation noted in the genital tract of wild-type mice. Scattered throughout the endometrial and oviductal lamina propria and ovarian bursa of TLR3-/- mice were small to moderate numbers of lymphocytes with variable amounts of intraluminal proteinaceous fluid and clusters of degenerate neutrophils (Fig 3A and 3D–3E). In contrast, the lymphocytic inflammation in the endometrium and oviducts of wild-type mice was minimal (Fig 3A and 3A–3C). The uterine horns and oviducts from both mouse strains were from minimally to mildly expanded in size (Fig 3A). Together, these findings suggest that TLR3 signaling plays a role in the recruitment of lymphocytes to the endometrium and oviducts during middle stages of C. muridarum infection in mice.
Fig 3. Lymphocytic endometritis and salpingitis is enhanced in the absence of TLR3 in mice during middle stage of C. muridarum infection.
A) The extent of histopathological changes of uterine horns and oviducts were scored using a four-tiered semi-quantitative scoring system as described in Materials and Methods. These results were representative of 5–6 mice per group from two independent experiments. Differences between groups for each parameter were determined by two-tailed Mann-Whitney test (* = p < 0.05). Only significant results are displayed in graphs. B-E) Histopathological evaluation of uterine horns from WT and TLR3-/- mice showed that lymphocytes (arrows) were commonly infiltrating the endometrial stroma at day 21 of C. muridarum infection. Reproductive tracts of TLR3-/- mice exhibited significantly higher lymphocytic inflammation than WT mice (A asterisk; B-E 20–400x magnification). F-G) Histopathological evaluation of oviducts from WT and TLR3-/- mice showed that lymphocytic (arrowhead) inflammation was commonly found within the oviducts of TLR3-/- mice (G 400x magnification). No significant differences in PMN infiltration was noted between the genital tracts of WT and TLR3-/- mice. Representative images for WT and TLR3-/- mice are shown. (• = WT mouse; ▪ = TLR3-/- mouse).
TLR3 modulates cytokine production in the genital tract of C57BL/6N during the acute phase of Chlamydial infection
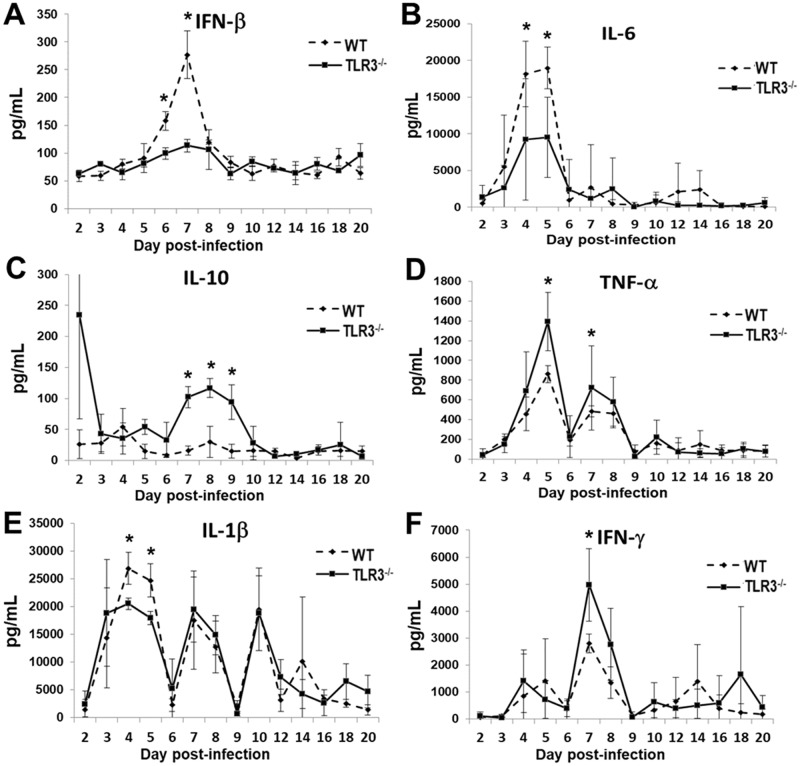
Our previous findings have demonstrated that C. muridarum infection in OE cells induced the synthesis of IFN-β in a TLR3 dependent manner, and the synthesis of other inflammatory mediators, such as IL-6, CCL5, and CXCL10 were substantially diminished in OE cells isolated from TLR3 deficient mice derived from 129S1 genetic background [25, 26]. Moreover, the synthesis of IFN-β, IL-6, and CCL5 were diminished in the vaginal secretions of 129S1 TLR3-/- mice infected with C. muridarum [25, 26]. These data suggest that oviductal epithelial cells play an important role in the synthesis of inflammatory mediators in the upper genital tract of mice during C. muridarum infection. To determine the significance that TLR3 has on the secretion of innate-immune cytokines and chemokines in the genital tract of C57BL/6N mice, groups of 5 wild-type and TLR3-/- mice were intravaginally inoculated with 105 IFU C. muridarum one week after treatment with Depo-Provera. As shown in Fig 4A and 4B, levels of IFN-β and IL-6 were significantly lower in TLR3-/- mice, at day 7 and day 4–5 respectively (Fig 4A and 4B), corroborating with our earlier studies demonstrating that the 129S1-bred TLR3-deficient mice and TLR3-deficient OE cells were defective in the Chlamydia-induced synthesis of these cytokines (21, 23). IL-1β synthesized during early Chlamydia infection was also significantly diminished in TLR3-deficient mice between days 4 and 5 (Fig 4E), but was virtually identical to wild-type mice after day 5 of infection. In contrast, the TLR3-/- mice secreted higher levels of IL-10, TNF-α and IFN-γ than wild-type mice (Fig 4C, 4D and 4F) at day 7 of infection, suggesting that TLR3 deficiency did not result in a global downregulation in synthesis of cytokines during early stages of chlamydial infection. Genital tract secretion levels of CXCL1, IL-2, and IL-4 were also measured; however, we found that the expression levels for these inflammatory mediators were virtually identical between the wild-type and TLR3-/- mice (data not shown). Collectively, these in vivo data suggest an important role for TLR3 in modulating the synthesis of cytokines in the genital tract of mice during the early stages of C. muridarum infection.
Fig 4. TLR3 modulates cytokine production in the murine genital tract during the acute phase of Chlamydia infection.
Genital tract secretions were obtained during the first 20 days post-infection, and analyzed by multiplex cytokine assay for: (A) IFN-β, (B) IL-6, (C) IL-10, (D) TNF-α, (E) IL-1β, and (F) IFN-γ synthesis. Data are from a representative experiment where n = 5 mice. * = p <0.05; ** = p <0.005.
CD4+ T-cells recruitment is significantly altered in the genital tracts of TLR3-/- mice during mid-Chlamydial infection
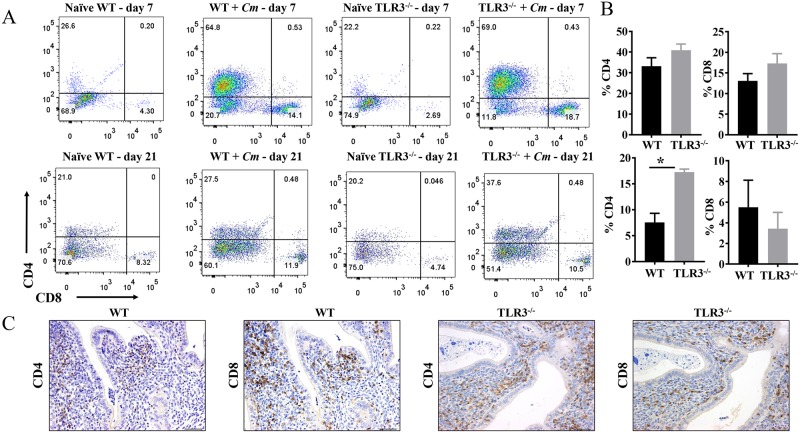
In the experimental murine model for human intravaginal Chlamydial infections, CD4+ T-cells are required and sufficient to clear C. muridarum primary genital tract infections [5, 37–39]. Conversely, Chlamydia-specific CD8+ T-cell responses have been associated with scaring and immunopathology of upper genital tract infections in mice [40, 41]. We reported that TLR3-deficient OE cells and TLR3-/- mice were defective in the Chlamydia-induced synthesis of several chemokines, such as CCL5, CXCL10, CXCL11, and CXCL16, that can act as chemo-attractants of T-cells in vivo [26, 42, 43]. Because the experiments above showed that the endometrial and oviductal stroma of TLR3-/- C57BL/6 mice were significantly infiltrated with lymphocytes during middle stages of C. muridarum genital tract infections, we next evaluated the contribution of TLR3 on CD4+ and CD8+ T-cells in the genital tract of C57BL/6N mice. Groups of five wild-type and five TLR3-/- mice were infected intravaginally with 105 IFU C. muridarum before being sacrificed at either day 7 or day 21 of infection, and their lower genital tracts processed for flow cytometry as described in Materials and Methods. As shown in Fig 5, there were no significant differences in CD4+ and CD8+ T responses in the genital tracts between wild-type and TLR3-/- mice during the early (acute) phase of C. muridarum infection (Fig 5A and 5B; Day 7). By day 7 post-infection, the CD4+ T-cell populations in the wild-type and TLR3-deficient mice were 64.8% and 69%, respectively, which represented an increased frequency of CD4+ T-cells in the genital tract of wild-type and TLR3-/- mice was 38.2% and 46.8% over the mock controls. There were 14.1% and 18.7% CD8+ T-cells in the wild-type and TLR3-deficient mice, respectively, at day 7 post-infection, which indicated a 9.8% and 16.01% increase over mock controls. Compared to the total percentage of CD4+ T-cells present in the genital tract tissue at day 7, there was an approximate 3 to 4-fold reduction in the total frequency of CD8+ T-cells in the genital tracts of the wild-type and TLR3-/- mice in the representative example. However, by day 21 post-infection, the frequency of CD4+ T-cells in the genital tract of wild-type and TLR3-/- mice was significantly different (Fig 5B). As shown, the frequency of CD4+ T-cells in the genital tract of wild-type and TLR3-/- mice was 6.5% and 17.4%, respectively. There were no significant differences on CD8+ T-cell recruitment in the genital tracts of wild-type and TLR3-/- mice (3.58% vs 5.76%; representative example) during the middle stages of C. muridarum infection. Collectively, these results suggest that TLR3 may be involved in modulating the recruitment of CD4 T-cells required to clear C. muridarum from the genital tracts of C57BL/6N mice during middle stages of infection.
Fig 5. CD4+ and CD8+ T-cells recruitment into genital tracts of infected wild-type and TLR3-/- mice.
A) Groups of C57BL/6 and TLR3-/- mice were infected intravaginally with 1×105 C. muridarum. At day 7 and day 21 post-challenge, reproductive tracts from each group of mice were harvested, and lymphocytes were isolated as described in Materials and Methods. FACS plots showing percentage of CD4+ and CD8+ cells within the CD3+ T-cell population (gated on CD3+CD4+ or CD3+CD8+) in the genital tract of a mouse from each group. FACS data showing representative results from each group of mice at days 7 and 21 of chlamydial infection. B) The percentages of CD4 and CD8 T-cells from each group were summarized in the graphs. Data shown are representative results from two independent experiments with three to five mice per time point. Bar graphs show mean number ± SEM. * indicate P values of <0.05. C) Immunohistochemical staining of lymphocytes with antibodies to CD4 or CD8 in the genital tract of an infected mouse at day 21 of infection. Brown stain indicates CD4 or CD8 positive cells. Original magnification ×200.
To illustrate the distribution of CD4 and CD8 T-cells in the genital tract of wild-type and TLR3-/- mice at day 21 of C. muridarum infection, representative cross-sections of the genital tracts of these mice were stained by immunohistochemistry using anti-CD4 and anti-CD8 antibodies. CD4+ and CD8+ T-cell were frequently detected along the endometrial stroma and myometrium of uterine horns (Fig 5C) and occasionally infiltrating mucosal folds and smooth muscle of oviducts of wild-type and TLR3-/- mice.
TLR3 deficiency results in more pronounced hydrosalpinx in the upper genital tracts of mice at day 56 post-intravaginal inoculation with C. muridarum
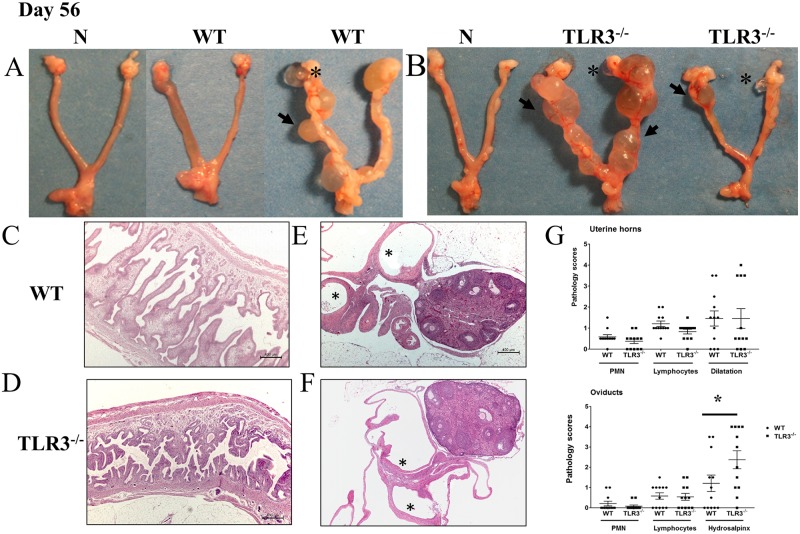
In the murine model of C. muridarum genital tract infections, the development of hydrosalpinx is used as one of the surrogate markers of chronic sequelae of infection and infertility [44]. Because TLR3-/- mice exhibited more obvious lymphocytic endometritis and salpingitis during middle stage of chlamydial infection, we further evaluated whether TLR3-/- mice also developed more severe hydrosalpinx in the upper genital tract. Groups of wild-type and TLR3-/- mice were intravaginally inoculated with C. muridarum, and histological lesions in the lower and upper genital tract at day 56 of infection were scored as described in methods. There were no obvious differences in inflammatory cellular infiltrates in the uterine horns and oviducts of wild-type and TLR3-/- mice at late stages of C. muridarum infection (Fig 6G). The endometrial and oviduct lamina propria (and associated smooth muscle) contain small numbers of lymphocytes and occasional neutrophils, with minimal erosion of the endometrial epithelial cells, an absence of intraluminal inflammatory cells, and variable degrees of unilateral and/or bilateral uterine horn dilation (Fig 6C, 6D and 6G). The periovarian space and oviducts from TLR3-/- mice were mildly to moderately dilated (Fig 6A and 6B) with variable small amounts of intraluminal acellular, fibrillar mucinous fluid (hydrosalpinx). These oviductal changes were either bilateral or unilateral and more severe in oviducts of TLR3-/- mice than oviducts of wild-type mice (Fig 6G). Dilated segments of the oviduct (e.g. ampulla, infundibulum and/or isthmus) were lined by an attenuated, intact surface epithelium. Additionally, the degree of periovarian dilation was commonly noted in oviducts with hydrosalpinx. Thus, these findings showed that TLR3 deficiency can lead to more pronounced chronic sequelae, such as hydrosalpinx, during late stages of C. muridarum genital tract infections in mice.
Fig 6. Hydrosalpinx is more commonly detected in TLR3-/-mice.
A-B) Representative genital tracts removed from groups of wild-type mice (left) and TLR3-/- mice (right) at day 56 of infection. Grossly, uterine horns were regionally or diffusely dilated in a subset of WT and TLR3-/- mice. Oviducts and ovarian bursa were mildly to severely dilated (hydrosalpinx/ hydrobursitis) in subset of animals. C-D) Evaluation of uterine horns from WT and TLR3-/- mice showed similar inflammatory changes characterized by small numbers of lymphocytic infiltration of the endometrial stroma with occasional neutrophils scattered throughout the uterine horn lumina. Uterine horns were partially filled with proteinaceous fluid with focal to multifocal endometrial cystic dilatation of glands that in some cases compressed and effaced the normal uterine wall architecture. E-F) Oviducts and ovarian bursa showed similarly mild inflammatory cell infiltrates in the oviducts, but significantly higher frequency and severity of hydrosalpinx in oviducts of TLR3-/- mice (25x magnification, asterisks = hydrosalpinx). G) The extent of histopathological changes of both uterine horns and oviducts were scored as described in Materials and Methods. These results were representative of 12 mice per group from three independent experiments. Differences between groups for each parameter were determined by two-tailed Mann-Whitney test (* = p < 0.05). Only significant results are displayed in graphs.
Discussion
The pathogenesis of genital tract Chlamydia infections involves an interplay between chlamydial PAMPs and various host PRRs. This interaction during infection results in the release of various inflammatory mediators and chemokines that induce an influx of neutrophils into the genital tract, activate nearby macrophage and antigen presenting cells, and ultimately dictate the ensuing acquired immune response [3]. Toll-like receptors via MyD88 play an important role in host defense against chlamydial urogenital infections in mice by both mediating the recognition and early inflammatory response, and by steering the adaptive immunity toward a Th1 cell-mediated immune response [45, 46]. In addition, MyD88 independent pathways were also shown to be involved in modulating the inflammatory response during C. muridarum genital tract infections [25, 26, 47]. Our previous studies demonstrated that TLR3 signaling during C. muridarum infection plays a role in host defense and in the secretion of a variety of inflammatory mediators (including IFN-β) into the genital tracts of mice [25]. In this study, we expanded our investigations on the contribution of TLR3 in host defense and the immunopathology during C. muridarum infection in a congenic C57BL/6N mouse strain.
Our findings showed that TLR3 deficiency in congenic C57BL/6N and 129S1 mice [26] leads to a significantly increased shedding of C. muridarum during early and middle stage of genital tract infections. This enhanced efficiency of Chlamydia shedding was hypothesized to be attributed to the increased replication of C. muridarum in epithelial cells lining the genital tracts of TLR3-/- mice. Our previous in vitro data support this hypothesis as C. muridarum growth in oviduct epithelial cells lacking TLR3 was more efficient when compared to wild-type OE cells, suggesting that stimulation of TLR3 by C. muridarum attenuates Chlamydia growth in these epithelial cells [26]. It is not yet clear whether the reduced replication of C. muridarum in wild-type OE cells are due to the direct interaction of Chlamydia factors with TLR3 in genital tract epithelial cells, or to a possible effect elicited by specific inflammatory mediators secreted during Chlamydia infection on the infected genital tract epithelial cells. Our in vitro data initially supported this later mechanism as IFN-β has an inhibitory effect on Chlamydia growth in oviduct epithelial cells [26]. However, our in vivo data showed that IFN-β likely modulates Chlamydia growth during early infection as IFN-β synthesis induced via TLR3 was simply elevated between day 6 and 8 of genital tract infections in wild-type mice. These findings suggest that additional host factors may modulate C. muridarum growth and survival in the genital tract of mice. For instance, it is possible that Chlamydia shedding is controlled by inducible nitric oxide synthase (iNOS) dependent mechanisms in the genital tract of wild-type mice. Investigations into the impact of iNOS dependent mechanisms on Chlamydia replication have shown that IFN-γ and T cell-epithelial cell contact results in upregulated iNOS genes and nitric oxide production, and these factors have been shown to be sufficient in controlling C. muridarum replication in genital tract epithelial cells [48, 49]. In support of this mechanism as a way to restrict chlamydial replication in these cells, other more recent studies have demonstrated Chlamydia replication in the genital tracts of mice is also modulated by iNOS independent mechanisms requiring T-cell degranulation [50, 51]. In addition, different lines of evidence have proposed that the interaction of Chlamydia with host cytoskeletal proteins plays a role in the release of infectious elementary bodies (EBs) from epithelial cells in vitro and in vivo [52–54]. Therefore, it will be of interest to determine whether TLR3-/- genital tract epithelial cells have a different profile in cytoskeletal assembly and junctional proteins during C. muridarum infection, which may lead to an increased release of infectious EBs.
In view of the Chlamydia shedding findings above, we anticipated at the start of our investigation that TLR3 would protect against genital tract immunopathology. However, our study revealed the TLR3-/- mice display comparable recruitment of inflammatory cells and/or disruption of the epithelium lining the reproductive tract (data not shown) with control mice during early genital tract infection. The enhanced dilation of the uterine horns in TLR3-/- mice or the oviducts in wild-type mice during early stages of C. muridarum infection may be mediated by presence of neutrophilic-rich exudate and inflammatory mediators secreted as a result of Chlamydia replication at sites of genital tract infections [55, 56]. For instance, the recruitment of neutrophils into uterine horns and oviducts of infected mice is likely mediated by a variety of chemokines and/or proinflammatory cytokines, such as CXCL1, CXCL2, CXCL5, TNF-α, and IL-1β, which have shown to be produced during active C. muridarum infection in the genital tract of mice [27, 55–57]. We then showed that TLR3 deficiency resulted in the aberrant synthesis of several innate-immune factors including IFN-β, IL-1β, TNF-α, IFN-γ, IL-6, and IL-10 during the early stage of C. muridarum genital tract infection. TLR3 deficiency in mice led to increased synthesis of TNF-α, IFN-γ, and IL-10; however, IFN-β, IL-1β, and IL-6 were secreted into the genital tracts of wild-type mice at significantly higher levels than in TLR3-/- mice during early infection. The impact that this differential expression of inflammatory mediators has on the overall outcomes of Chlamydia infection in TLR3-/- mice is uncertain. However, it is possible that the increased secretion of TNF-α and IFN-γ in the genital tract of C. muridarum infected TLR3-/- mice is in response to the relatively higher levels of Chlamydia replication during the first week of C. muridarum infection. In fact, our results on TNF-α synthesis were consistent with previous results showing that its synthesis is upregulated during the first week of C. muridarum infection in rodents [33, 58]. This may indicate that increased TNF-α levels enhances host defense in the C57BL6/N strain. Similarly, infectivity studies in IFN-γ-deficient mice from C57BL6 and 129S1 backgrounds [59, 60] demonstrated that IFN-γ played a major role in controlling C. muridarum genital tract infections in mice, and that CD4+ T-cells secreting gamma interferon (IFN-γ) were required for clearance of C. muridarum in vivo [5, 39, 61, 62].
The biological significance of the elevated synthesis of IL-10 in TLR3-/- mice during early C. muridarum genital tract infections is unclear. IL-10 has been identified as a key player in the establishment and perpetuation of viral persistence, is known to dysregulate type-I interferon production, and promotes a suppressive environment that diminishes the antiviral response [63–65]. Also, there are numerous studies in Chlamydia addressing the hypothesis that changes in the regulation of this critical anti-inflammatory cytokine is associated with increased chronic disease complications and persistence in several different species of Chlamydia [66, 67]. Based on these findings regarding IL-10’s purported role in suppressing IFN-β, we can extrapolate a similar role for IL-10 in the suppression of IFN-β during Chlamydia infection in TLR3-deficient mice. Our data in Fig 4C shows that IL-10 was synthesized at significantly higher levels in the TLR3 deficient mice, but that it coincided at a time where there was peak IFN-β synthesis levels in the wild-type mice (Day 7, Fig 4A). Although it is unlikely that the increased IL-10 synthesis in TLR3-deficient mice impacts the C. muridarum-induced IFN-β synthesized early during infection (that we have shown to be mostly TLR3 dependent in OE cells), its synthesis more likely suppresses IFN-β secreted from pathways other than TLR3, which are mostly IFN-β amplification pathways that are active in the cell late during infection [68]. The proposed IL-10 mediated attenuation of IFN-β synthesis during TLR3 deficiency likely promotes the increased C. muridarum replication that we have observed in the TLR3-deficient mice and in our in vitro studies [26]. This hypothesis is supported in other studies where it was demonstrated that IL-10 synthesis was markedly upregulated in the lower genital tracts of Cm infected wild type mice, which resulted in a decreased Th1 response and increased chlamydial replication in the lower genital tract when compared to IL-10-/- mice [67].
We also demonstrate that the TLR3-deficient mice show more signs of chronic disease complications such as lymphocytic endometritis and hydrosalpinx. Because IL-10 dysregulation is also possibly associated with outcomes of infection that are indicative of chronic sequalae of chlamydial disease [66], it is possible that the TLR3 signaling pathway either directly or indirectly regulates IL-10 synthesis as a way to limit genital tract pathology in the wild-type mice, and that putative regulation is absent in the TLR3-deficient mice. The proper balance between type-1 and type-2 IFN is critical in achieving the optimal immune response to Chlamydia infection, and dysregulation of that balance can have significant effect on outcomes of chlamydial disease [69]. Because our data show that IFN-β synthesis is diminished while IFN-γ synthesis is increased in the TLR3-deficient mice, we propose that this relative imbalance towards type-2 IFN in the genital tracts of infected mice may be one of the major reason IL-10 is more abundant compared to the wild-type controls. However, further study is needed to address the interplay between TLR3 signaling and IL-10 synthesis in order to better understand how dysregulation of IL-10 synthesis during Chlamydia infection of TLR3-deficient mice leads to more substantial genital tract sequalae.
Our in vivo findings have shown that C. muridarum infected TLR3-/- mice generated in both the 129S1 and C57BL/6N backgrounds are defective in the synthesis of IFN-β on day 6 and 8 of infection, respectively. This data also support our previous findings of severely diminished IFN-β synthesis in TLR3-/- OE cells [25, 26], which appeared to be a major contributor in the production of IFN-β in vitro [25]. Together, these findings suggest that C. muridarum, by a yet unidentified ligand, induces the expression of interferon regulatory factor 3 (IRF3) and IFN-β in a TRIF-dependent manner in oviduct epithelial cells during the acute phase of C. muridarum genital tract infection. Interestingly, other investigators have shown that IFN-β synthesis was downregulated during the first days of C. muridarum infections in IRF3-/- mice when compared to wild-type mice, but the differential expression in IFN-β returned to normal levels after the first week of C. muridarum infection [70]. In addition, our previous in vitro studies showed that Chlamydia replication was more robust in the TLR3-/- OE cells when compared to wild-type, and that pretreating the TLR3-/- OE cells with IFN-β prior to infection significantly reduced chlamydial replication in these cells [26]. Our findings are suggestive that IFN-β has a detrimental role on C. muridarum replication during early genital tract of mice.
Similar to other investigators, IL-1β synthesis is markedly upregulated in the lower tract of C57BL6 mice during early C. muridarum infection, where the neutrophils and macrophages are thought to be the primary sources of this cytokine during infection [57]. The role and source of IL-6 synthesized during chlamydial genital tract immunopathology in mice is somewhat lesser defined and variable [71, 72]. IL-6 synthesis is upregulated in the genital tract of C57BL6 mice during the first week of C muridarum infection, and this process was initially shown to be independent of TLR4 and TLR3 [18, 26]. Furthermore, TLR2 has been proposed to induce the synthesis of IL-6 in vitro, as Chlamydia stimulates the production of IL-6 in murine oviduct epithelial cells and peritoneal macrophages in a TLR2 dependent manner [18, 73]. In a seemingly contrasting finding, our results here suggest that TLR3 signaling modulates the release of this pro-inflammatory cytokine during the first week of Chlamydia infection in mice, suggesting instead that its synthesis is TLR3 dependent. However, results of our earlier in vitro investigations into this phenomenon show that Chlamydia-induced TLR3-dependent IFN-β upregulates TLR2 gene expression, protein synthesis, and protein function in murine OE cells [26]. This interplay between TLRs 2 and 3 is representative of a possible regulation of TLR2-dependent immune responses by TLR3, and that this mechanism of regulation may be important to help control outcomes of Chlamydia infection in mice; particularly early- and mid-infection. It is likely that the TLR3 induced augmentation of TLR2 activity is absent early times during infection during TLR3 deficiency, and is what contributes to an aberrant immune response to C. muridarum in the TLR3 deficient mice.
Our findings showed that the endometrium and oviducts of TLR3-/- mice exhibited significantly higher lymphocytic inflammation when compared to wild-type mice at day 21 post-infection. Furthermore, CD4+ T-cells in part contributes to this differential recruitment of lymphocytes in the genital tract of TLR3-/- mice. A possible interpretation for this enhanced recruitment of CD4+ T-cells could be attributed to the increased burden of Chlamydia in the genital tract of TLR3-/- mice, as CD4+ T-cells are essential in controlling Chlamydia burden from the genital tract mice [5, 39]. In addition, the kinetics of CD4+ T-cells in the genital tract of TLR3-/- mice during the early and middle stages of infection is similar to what is observed during C. muridarum infections in BALB/c and C57BL/6 mice showing that Th1 T-cells remain high after 3 weeks post-infection [10, 74, 75]. The mechanism how TLR3 signaling recruits CD4+ T-cells during C. muridarum genital tract infection is currently unknown. Our studies studying the kinetics of lymphocytes during C. muridarum infection did not detect differences in total lymphocyte counts from peripheral blood and numbers of CD4+ T-cell in the spleen between TLR3-/- and wild-type mice at day 7 and 21 of C. muridarum infection (data not shown). Thus, it is possible that TLR3-/- mice displays an increased chlamydial-antigen-specific T-cell response derived from regional lymph nodes at later time points of genital tract infections. This finding is consistent with data from IRF3-/- mice showing an enhanced proliferation chlamydial-antigen-specific T-cells derived from the iliac lymph node at day 14 and 21 of C. muridarum infection [70]. Alternatively, it is possible that C. muridarum infection in the genital tract of TLR3-/- mice induces a more robust local expansion of chlamydial-antigen-specific T-cells when compared to wild-type mice. For instance, this enhanced CD4+ T-cells in the genital tract of TLR3-/- mice may represent local expansion of tissue resident T-cells during C. muridarum infection in the genital tract of mice [76]. Further experimentation is required to determine the source of this enhanced proliferation of CD4+ T-cells in TLR3-/- mice, and the chemokine/cytokine profile associated with this phenotype.
Our data showed that the incidence of oviduct hydrosalpinx is increased in the genital tracts of TLR3-/- mice. Both the host’s innate and adaptive immunity have been purported to play a significant role in the development of upper genital tract pathology [77, 78]. In addition, the development of hydrosalpinx vary among different mouse strains from different genetic backgrounds and is independent to the duration and levels of Chlamydia shedding [79]. Previous studies have demonstrated that C57BL/6 exhibits the shortest course of Chlamydia shedding and markedly reduces development of hydrosalpinx at day 42 of chlamydial infection [33]. Similar to our experiments, no inflammatory infiltrates or fibrosis in the oviducts were detected in any of the groups of mice infected with C. muridarum at day 56 post-challenge. The exact mechanism on how TLR3 contributes to hydrosalpinx in mice remains enigmatic, but this lesion may have developed as a result of the chronic salpingitis from earlier time points of C. muridarum infection.
Our novel observations showing that TLR3 is stimulated during infection to exacerbate reproductive tract pathology in mice infected with C. muridarum, presents an interesting mechanism of bacterial-induced pathogenesis that may not be readily explained based upon our current understanding of TLR3 biology. Double-stranded RNA and possibly stathmin (a cellular protein that is a ubiquitously expressed regulator of microtubule formation [80]), are the only known ligands for TLR3. Our findings that TLR3 has such a prominent role in the pathogenesis of a bacterial pathogen not known to be associated with either ligand is both significant and enigmatic. One possible scenario that can provide some insight to this proposed TLR3-dependent mechanism of Chlamydia induced immunopathogenesis in mice intimates the possibility of a novel TLR3 PAMP (either bacterial or cellular) associated with Chlamydia infection. In this scenario, we propose that there is possibly a structural component of Chlamydia that is not likely a double-stranded RNA, since there is no known double-stranded RNA species associated with the chlamydial structure. We would hypothesize that this novel TLR3 PAMP associated Chlamydia could possibly be a chlamydial protein that has a similar α-helical structure of stathmin, and can potentially be identified via fractionation methodology [81].
Another possibility is that Chlamydia infection of reproductive tract epithelium somehow induces a cellular response resulting in the syntheses of cellular double-stranded RNAs, which could then bind-to and stimulate TLR3-dependent immune responses. The involvement of micro-RNAs (miRNAs) that are induced in response to bacterial infection has been investigated in gastric cancers during chronic Helicobacter pylori infection [82], and bacterial infections of the Arabidopsis plants [83]. In this scenario, we hypothesize the possible contributions of novel miRNAs that are endogenous to the epithelial tissue lining the reproductive tract, that are potentially induced during Chlamydia infection of these cells. In either scenario, the identification of the TLR3 PAMP that is presented during Chlamydia infection would represent a significant contribution to our understanding of TLR3 biology.
Overall, our results showed that TLR3 plays a role in host defense during the early and middle time points of infection in congenic C57BL/6N mice by modulating the production of proinflammatory cytokine at earlier time points, and by regulating the proliferation of CD4+ T-cells in the genital tract of mice at middle time points of infection. In addition, our data demonstrated the absence of TLR3 in the genital tract led to more severe symptoms of chronic inflammation and hydrosalpinx. Therefore, these findings suggest that TLR3 has a role in protection from uterine horn and oviduct pathology at middle and late time points of infection in mice. Greater understanding of the mechanism how TLR3 signaling modulates the development of upper genital tract pathology in mice may allow us to identify novel targets to reduce the risk of chronic sequelae caused by Chlamydia genital tract infections.
Acknowledgments
We thank Dr. Keith Condon for excellent histological preparation of the mouse genital tract tissue. We also acknowledge the thoughtful manuscript critiques provided by Drs. David Nelson and Matthew Muramatsu.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by National Institutes of Health Grants 1K22AI072072 and 1R01AI104944 (W.A.D.). S.E.C. was supported by the National Institute of Health's, NRSA T32 AI 060519, Immunology and Infectious Disease Program at IUSM. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Organization WH. Global incidence and prevalence of selected curable sexually transmitted infections–2008. In: Research DoRHa, editor.: World Health Organization; 2012; 2012. p. 20. [Google Scholar]
- 2.Brunham RC, Rey-Ladino J. Immunology of Chlamydia infection: implications for a Chlamydia trachomatis vaccine. Nature reviews Immunology. 2005;5(2):149–61. doi: 10.1038/nri1551 . [DOI] [PubMed] [Google Scholar]
- 3.Rey-Ladino J, Ross AG, Cripps AW. Immunity, immunopathology, and human vaccine development against sexually transmitted Chlamydia trachomatis. Hum Vaccin Immunother. 2014;10(9):2664–73. doi: 10.4161/hv.29683 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morrison RP, Caldwell HD. Immunity to murine chlamydial genital infection. Infection and immunity. 2002;70(6):2741–51. doi: 10.1128/IAI.70.6.2741-2751.2002 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morrison RP, Feilzer K, Tumas DB. Gene knockout mice establish a primary protective role for major histocompatibility complex class II-restricted responses in Chlamydia trachomatis genital tract infection. Infection and immunity. 1995;63(12):4661–8. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manam S, Nicholson BJ, Murthy AK. OT-1 mice display minimal upper genital tract pathology following primary intravaginal Chlamydia muridarum infection. Pathogens and disease. 2013;67(3):221–4. doi: 10.1111/2049-632X.12032 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murthy AK, Li W, Chaganty BK, Kamalakaran S, Guentzel MN, Seshu J, et al. Tumor necrosis factor alpha production from CD8+ T cells mediates oviduct pathological sequelae following primary genital Chlamydia muridarum infection. Infection and immunity. 2011;79(7):2928–35. doi: 10.1128/IAI.05022-11 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Redgrove KA, McLaughlin EA. The Role of the Immune Response in Chlamydia trachomatis Infection of the Male Genital Tract: A Double-Edged Sword. Front Immunol. 2014;5:534 doi: 10.3389/fimmu.2014.00534 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Clercq E, Kalmar I, Vanrompay D. Animal models for studying female genital tract infection with Chlamydia trachomatis. Infection and immunity. 2013;81(9):3060–7. doi: 10.1128/IAI.00357-13 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morrison SG, Morrison RP. In situ analysis of the evolution of the primary immune response in murine Chlamydia trachomatis genital tract infection. Infection and immunity. 2000;68(5):2870–9. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de la Maza LM, Pal S, Khamesipour A, Peterson EM. Intravaginal inoculation of mice with the Chlamydia trachomatis mouse pneumonitis biovar results in infertility. Infection and immunity. 1994;62(5):2094–7. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shah AA, Schripsema JH, Imtiaz MT, Sigar IM, Kasimos J, Matos PG, et al. Histopathologic changes related to fibrotic oviduct occlusion after genital tract infection of mice with Chlamydia muridarum. Sex Transm Dis. 2005;32(1):49–56. Epub 2004/12/23. . [DOI] [PubMed] [Google Scholar]
- 13.Elwell C, Mirrashidi K, Engel J. Chlamydia cell biology and pathogenesis. Nat Rev Microbiol. 2016;14(6):385–400. doi: 10.1038/nrmicro.2016.30 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rasmussen SJ, Eckmann L, Quayle AJ, Shen L, Zhang YX, Anderson DJ, et al. Secretion of proinflammatory cytokines by epithelial cells in response to Chlamydia infection suggests a central role for epithelial cells in chlamydial pathogenesis. The Journal of clinical investigation. 1997;99(1):77–87. doi: 10.1172/JCI119136 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prebeck S, Brade H, Kirschning CJ, da Costa CP, Durr S, Wagner H, et al. The Gram-negative bacterium Chlamydia trachomatis L2 stimulates tumor necrosis factor secretion by innate immune cells independently of its endotoxin. Microbes Infect. 2003;5(6):463–70. . [DOI] [PubMed] [Google Scholar]
- 16.Bulut Y, Faure E, Thomas L, Karahashi H, Michelsen KS, Equils O, et al. Chlamydial heat shock protein 60 activates macrophages and endothelial cells through Toll-like receptor 4 and MD2 in a MyD88-dependent pathway. J Immunol. 2002;168(3):1435–40. . [DOI] [PubMed] [Google Scholar]
- 17.Kol A, Bourcier T, Lichtman AH, Libby P. Chlamydial and human heat shock protein 60s activate human vascular endothelium, smooth muscle cells, and macrophages. The Journal of clinical investigation. 1999;103(4):571–7. doi: 10.1172/JCI5310 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Darville T, O’Neill JM, Andrews CW Jr., Nagarajan UM, Stahl L, Ojcius DM. Toll-like receptor-2, but not Toll-like receptor-4, is essential for development of oviduct pathology in chlamydial genital tract infection. J Immunol. 2003;171(11):6187–97. . [DOI] [PubMed] [Google Scholar]
- 19.O’Connell CM, Ionova IA, Quayle AJ, Visintin A, Ingalls RR. Localization of TLR2 and MyD88 to Chlamydia trachomatis inclusions. Evidence for signaling by intracellular TLR2 during infection with an obligate intracellular pathogen. The Journal of biological chemistry. 2006;281(3):1652–9. doi: 10.1074/jbc.M510182200 . [DOI] [PubMed] [Google Scholar]
- 20.Abdul-Sater AA, Koo E, Hacker G, Ojcius DM. Inflammasome-dependent caspase-1 activation in cervical epithelial cells stimulates growth of the intracellular pathogen Chlamydia trachomatis. The Journal of biological chemistry. 2009;284(39):26789–96. doi: 10.1074/jbc.M109.026823 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Welter-Stahl L, Ojcius DM, Viala J, Girardin S, Liu W, Delarbre C, et al. Stimulation of the cytosolic receptor for peptidoglycan, Nod1, by infection with Chlamydia trachomatis or Chlamydia muridarum. Cell Microbiol. 2006;8(6):1047–57. doi: 10.1111/j.1462-5822.2006.00686.x . [DOI] [PubMed] [Google Scholar]
- 22.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413(6857):732–8. doi: 10.1038/35099560 . [DOI] [PubMed] [Google Scholar]
- 23.Matsumoto M, Kikkawa S, Kohase M, Miyake K, Seya T. Establishment of a monoclonal antibody against human Toll-like receptor 3 that blocks double-stranded RNA-mediated signaling. Biochemical and biophysical research communications. 2002;293(5):1364–9. doi: 10.1016/S0006-291X(02)00380-7 . [DOI] [PubMed] [Google Scholar]
- 24.Perales-Linares R, Navas-Martin S. Toll-like receptor 3 in viral pathogenesis: friend or foe? Immunology. 2013;140(2):153–67. doi: 10.1111/imm.12143 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Derbigny WA, Johnson RM, Toomey KS, Ofner S, Jayarapu K. The Chlamydia muridarum-induced IFN-beta response is TLR3-dependent in murine oviduct epithelial cells. J Immunol. 2010;185(11):6689–97. Epub 2010/10/27. doi: 10.4049/jimmunol.1001548 . [DOI] [PubMed] [Google Scholar]
- 26.Derbigny WA, Shobe LR, Kamran JC, Toomey KS, Ofner S. Identifying a role for Toll-like receptor 3 in the innate immune response to Chlamydia muridarum infection in murine oviduct epithelial cells. Infection and immunity. 2012;80(1):254–65. Epub 2011/10/19. doi: 10.1128/IAI.05549-11 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson RM. Murine oviduct epithelial cell cytokine responses to Chlamydia muridarum infection include interleukin-12-p70 secretion. Infection and immunity. 2004;72(7):3951–60. doi: 10.1128/IAI.72.7.3951-3960.2004 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schachter J. Chlamydiae (Psittacosis-lymphogranuloma venereum-trachoma group). 3rd ed. ed: American Society for Microbiology, Washington, D.C.; 1980. [Google Scholar]
- 29.Caldwell HD, Kromhout J, Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infection and immunity. 1981;31(3):1161–76. Epub 1981/03/01. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelly KA, Robinson EA, Rank RG. Initial route of antigen administration alters the T-cell cytokine profile produced in response to the mouse pneumonitis biovar of Chlamydia trachomatis following genital infection. Infection and immunity. 1996;64(12):4976–83. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prantner D, Darville T, Nagarajan UM. Stimulator of IFN gene is critical for induction of IFN-beta during Chlamydia muridarum infection. J Immunol. 2010;184(5):2551–60. Epub 2010/01/29. doi: 10.4049/jimmunol.0903704 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang J, Kelly KA. Isolation of lymphocytes from mouse genital tract mucosa. J Vis Exp. 2012; (67):e4391 doi: 10.3791/4391 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Darville T, Andrews CW Jr., Laffoon KK, Shymasani W, Kishen LR, Rank RG. Mouse strain-dependent variation in the course and outcome of chlamydial genital tract infection is associated with differences in host response. Infection and immunity. 1997;65(8):3065–73. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang Y, Zhang Q, Yang Z, Conrad T, Liu Y, Zhong G. Plasmid-Encoded Pgp5 Is a Significant Contributor to Chlamydia muridarum Induction of Hydrosalpinx. PLoS One. 2015;10(4):e0124840 Epub 2015/04/29. doi: 10.1371/journal.pone.0124840 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mori H, Soonsawad P, Schuetter L, Chen Q, Hubbard NE, Cardiff RD, et al. Introduction of Zinc-salt Fixation for Effective Detection of Immune Cell-related Markers by Immunohistochemistry. Toxicologic pathology. 2015;43(6):883–9. doi: 10.1177/0192623315587593 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giannitti F, Anderson M, Miller M, Rowe J, Sverlow K, Vasquez M, et al. Chlamydia pecorum: fetal and placental lesions in sporadic caprine abortion. J Vet Diagn Invest. 2016;28(2):184–9. doi: 10.1177/1040638715625729 . [DOI] [PubMed] [Google Scholar]
- 37.Rank RG, Ramsey KH, Pack EA, Williams DM. Effect of gamma interferon on resolution of murine chlamydial genital infection. Infection and immunity. 1992;60(10):4427–9. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rank RG, Soderberg LS, Barron AL. Chronic chlamydial genital infection in congenitally athymic nude mice. Infection and immunity. 1985;48(3):847–9. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Su H, Caldwell HD. CD4+ T cells play a significant role in adoptive immunity to Chlamydia trachomatis infection of the mouse genital tract. Infection and immunity. 1995;63(9):3302–8. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson RM, Kerr MS, Slaven JE. An atypical CD8 T-cell response to Chlamydia muridarum genital tract infections includes T cells that produce interleukin-13. Immunology. 2014;142(2):248–57. doi: 10.1111/imm.12248 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vlcek KR, Li W, Manam S, Zanotti B, Nicholson BJ, Ramsey KH, et al. The contribution of Chlamydia-specific CD8(+) T cells to upper genital tract pathology. Immunol Cell Biol. 2016;94(2):208–12. doi: 10.1038/icb.2015.74 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barnes PJ. The cytokine network in asthma and chronic obstructive pulmonary disease. The Journal of clinical investigation. 2008;118(11):3546–56. doi: 10.1172/JCI36130 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Medoff BD, Thomas SY, Banerji A, Wain JC, Zhang H, Lilly CM, et al. Pathogenic T-cell recruitment into the airway in human disease. Annals of the New York Academy of Sciences. 2005;1062:220–41. doi: 10.1196/annals.1358.026 . [DOI] [PubMed] [Google Scholar]
- 44.Carey AJ, Cunningham KA, Hafner LM, Timms P, Beagley KW. Effects of inoculating dose on the kinetics of Chlamydia muridarum genital infection in female mice. Immunol Cell Biol. 2009;87(4):337–43. doi: 10.1038/icb.2009.3 . [DOI] [PubMed] [Google Scholar]
- 45.Chen L, Lei L, Chang X, Li Z, Lu C, Zhang X, et al. Mice deficient in MyD88 Develop a Th2-dominant response and severe pathology in the upper genital tract following Chlamydia muridarum infection. J Immunol. 2010;184(5):2602–10. doi: 10.4049/jimmunol.0901593 . [DOI] [PubMed] [Google Scholar]
- 46.Nagarajan UM, Ojcius DM, Stahl L, Rank RG, Darville T. Chlamydia trachomatis induces expression of IFN-gamma-inducible protein 10 and IFN-beta independent of TLR2 and TLR4, but largely dependent on MyD88. J Immunol. 2005;175(1):450–60. . [DOI] [PubMed] [Google Scholar]
- 47.Nagarajan UM, Sikes J, Prantner D, Andrews CW Jr., Frazer L, Goodwin A, et al. MyD88 deficiency leads to decreased NK cell gamma interferon production and T cell recruitment during Chlamydia muridarum genital tract infection, but a predominant Th1 response and enhanced monocytic inflammation are associated with infection resolution. Infection and immunity. 2011;79(1):486–98. Epub 2010/11/17. doi: 10.1128/IAI.00843-10 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Igietseme JU. Molecular mechanism of T-cell control of Chlamydia in mice: role of nitric oxide in vivo. Immunology. 1996;88(1):1–5. . [PMC free article] [PubMed] [Google Scholar]
- 49.Igietseme JU, Uriri IM, Hawkins R, Rank RG. Integrin-mediated epithelial-T cell interaction enhances nitric oxide production and increased intracellular inhibition of Chlamydia. J Leukoc Biol. 1996;59(5):656–62. . [DOI] [PubMed] [Google Scholar]
- 50.Johnson RM, Kerr MS, Slaven JE. Plac8-dependent and inducible NO synthase-dependent mechanisms clear Chlamydia muridarum infections from the genital tract. J Immunol. 2012;188(4):1896–904. Epub 2012/01/13. doi: 10.4049/jimmunol.1102764 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johnson RM, Kerr MS, Slaven JE. Perforin is detrimental to controlling [corrected] C. muridarum replication in vitro, but not in vivo. PLoS One. 2013;8(5):e63340 doi: 10.1371/journal.pone.0063340 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chin E, Kirker K, Zuck M, James G, Hybiske K. Actin recruitment to the Chlamydia inclusion is spatiotemporally regulated by a mechanism that requires host and bacterial factors. PLoS One. 2012;7(10):e46949 doi: 10.1371/journal.pone.0046949 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Slade JA, Hall JV, Kintner J, Phillips-Campbell R, Schoborg RV. Host Nectin-1 Promotes Chlamydial Infection in the Female Mouse Genital Tract, but Is Not Required for Infection in a Novel Male Murine Rectal Infection Model. PLoS One. 2016;11(8):e0160511 doi: 10.1371/journal.pone.0160511 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang C, Starr T, Song L, Carlson JH, Sturdevant GL, Beare PA, et al. Chlamydial Lytic Exit from Host Cells Is Plasmid Regulated. MBio. 2015;6(6):e01648–15. doi: 10.1128/mBio.01648-15 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Darville T, Andrews CW Jr., Sikes JD, Fraley PL, Rank RG. Early local cytokine profiles in strains of mice with different outcomes from chlamydial genital tract infection. Infection and immunity. 2001;69(6):3556–61. doi: 10.1128/IAI.69.6.3556-3561.2001 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee HY, Schripsema JH, Sigar IM, Murray CM, Lacy SR, Ramsey KH. A link between neutrophils and chronic disease manifestations of Chlamydia muridarum urogenital infection of mice. FEMS Immunol Med Microbiol. 2010;59(1):108–16. doi: 10.1111/j.1574-695X.2010.00668.x . [DOI] [PubMed] [Google Scholar]
- 57.Prantner D, Darville T, Sikes JD, Andrews CW Jr., Brade H, Rank RG, et al. Critical role for interleukin-1beta (IL-1beta) during Chlamydia muridarum genital infection and bacterial replication-independent secretion of IL-1beta in mouse macrophages. Infection and immunity. 2009;77(12):5334–46. Epub 2009/10/07. doi: 10.1128/IAI.00883-09 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Darville T, Andrews CW Jr., Rank RG. Does inhibition of tumor necrosis factor alpha affect chlamydial genital tract infection in mice and guinea pigs? Infection and immunity. 2000;68(9):5299–305. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cotter TW, Ramsey KH, Miranpuri GS, Poulsen CE, Byrne GI. Dissemination of Chlamydia trachomatis chronic genital tract infection in gamma interferon gene knockout mice. Infection and immunity. 1997;65(6):2145–52. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ito JI, Lyons JM. Role of gamma interferon in controlling murine chlamydial genital tract infection. Infection and immunity. 1999;67(10):5518–21. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Igietseme JU, Ramsey KH, Magee DM, Williams DM, Kincy TJ, Rank RG. Resolution of murine chlamydial genital infection by the adoptive transfer of a biovar-specific, Th1 lymphocyte clone. Reg Immunol. 1993;5(6):317–24. . [PubMed] [Google Scholar]
- 62.Perry LL, Feilzer K, Caldwell HD. Immunity to Chlamydia trachomatis is mediated by T helper 1 cells through IFN-gamma-dependent and -independent pathways. J Immunol. 1997;158(7):3344–52. . [PubMed] [Google Scholar]
- 63.Taylor DR, Shi ST, Romano PR, Barber GN, Lai MM. Inhibition of the interferon-inducible protein kinase PKR by HCV E2 protein. Science. 1999;285(5424):107–10. Epub 1999/07/03. . [DOI] [PubMed] [Google Scholar]
- 64.Wilson EB, Brooks DG. The role of IL-10 in regulating immunity to persistent viral infections. Curr Top Microbiol Immunol. 2011;350:39–65. doi: 10.1007/82_2010_96 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zuniga EI, Liou LY, Mack L, Mendoza M, Oldstone MB. Persistent virus infection inhibits type I interferon production by plasmacytoid dendritic cells to facilitate opportunistic infections. Cell host & microbe. 2008;4(4):374–86. doi: 10.1016/j.chom.2008.08.016 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hakimi H, Zare-Bidaki M, Zainodini N, Assar S, Arababadi MK. Significant roles played by IL-10 in Chlamydia infections. Inflammation. 2014;37(3):818–23. Epub 2014/01/09. doi: 10.1007/s10753-013-9801-1 . [DOI] [PubMed] [Google Scholar]
- 67.Marks E, Tam MA, Lycke NY. The female lower genital tract is a privileged compartment with IL-10 producing dendritic cells and poor Th1 immunity following Chlamydia trachomatis infection. PLoS Pathog. 2010;6(11):e1001179 doi: 10.1371/journal.ppat.1001179 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hosey KL, Hu S, Derbigny WA. Role of STAT1 in Chlamydia-Induced Type-1 Interferon Production in Oviduct Epithelial Cells. J Interferon Cytokine Res. 2015;35(11):901–16. doi: 10.1089/jir.2015.0013 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Darville T, Hiltke TJ. Pathogenesis of genital tract disease due to Chlamydia trachomatis. J Infect Dis. 2010;201 Suppl 2:S114–25. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Prantner D, Sikes JD, Hennings L, Savenka AV, Basnakian AG, Nagarajan UM. Interferon regulatory transcription factor 3 protects mice from uterine horn pathology during Chlamydia muridarum genital infection. Infection and immunity. 2011;79(10):3922–33. Epub 2011/07/27. doi: 10.1128/IAI.00140-11 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cunningham K, Stansfield SH, Patel P, Menon S, Kienzle V, Allan JA, et al. The IL-6 response to Chlamydia from primary reproductive epithelial cells is highly variable and may be involved in differential susceptibility to the immunopathological consequences of chlamydial infection. BMC immunology. 2013;14:50 doi: 10.1186/1471-2172-14-50 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sun X, Tian Q, Wang L, Xue M, Zhong G. IL-6-mediated signaling pathways limit Chlamydia muridarum infection and exacerbate its pathogenicity in the mouse genital tract. Microbes Infect. 2017;19(11):536–45. doi: 10.1016/j.micinf.2017.08.007 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Derbigny WA, Kerr MS, Johnson RM. Pattern recognition molecules activated by Chlamydia muridarum infection of cloned murine oviduct epithelial cell lines. J Immunol. 2005;175(9):6065–75. Epub 2005/10/21. . [DOI] [PubMed] [Google Scholar]
- 74.Darville T, Andrews CW Jr., Sikes JD, Fraley PL, Braswell L, Rank RG. Mouse strain-dependent chemokine regulation of the genital tract T helper cell type 1 immune response. Infection and immunity. 2001;69(12):7419–24. doi: 10.1128/IAI.69.12.7419-7424.2001 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kelly KA, Rank RG. Identification of homing receptors that mediate the recruitment of CD4 T cells to the genital tract following intravaginal infection with Chlamydia trachomatis. Infection and immunity. 1997;65(12):5198–208. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Johnson RM, Brunham RC. Tissue-Resident T Cells as the Central Paradigm of Chlamydia Immunity. Infection and immunity. 2016;84(4):868–73. doi: 10.1128/IAI.01378-15 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Morrison SG, Su H, Caldwell HD, Morrison RP. Immunity to murine Chlamydia trachomatis genital tract reinfection involves B cells and CD4(+) T cells but not CD8(+) T cells. Infection and immunity. 2000;68(12):6979–87. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tang L, Zhang H, Lei L, Gong S, Zhou Z, Baseman J, et al. Oviduct infection and hydrosalpinx in DBA1/j mice is induced by intracervical but not intravaginal inoculation with Chlamydia muridarum. PLoS One. 2013;8(8):e71649 doi: 10.1371/journal.pone.0071649 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen J, Zhang H, Zhou Z, Yang Z, Ding Y, Zhou Z, et al. Chlamydial induction of hydrosalpinx in 11 strains of mice reveals multiple host mechanisms for preventing upper genital tract pathology. PLoS One. 2014;9(4):e95076 doi: 10.1371/journal.pone.0095076 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bsibsi M, Bajramovic JJ, Vogt MH, van Duijvenvoorden E, Baghat A, Persoon-Deen C, et al. The microtubule regulator stathmin is an endogenous protein agonist for TLR3. J Immunol. 2010;184(12):6929–37. Epub 2010/05/21. doi: 10.4049/jimmunol.0902419 . [DOI] [PubMed] [Google Scholar]
- 81.Adachi J, Kumar C, Zhang Y, Olsen JV, Mann M. The human urinary proteome contains more than 1500 proteins, including a large proportion of membrane proteins. Genome biology. 2006;7(9):R80 Epub 2006/09/05. doi: 10.1186/gb-2006-7-9-r80 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Belair C, Darfeuille F, Staedel C. Helicobacter pylori and gastric cancer: possible role of microRNAs in this intimate relationship. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2009;15(9):806–12. Epub 2009/08/26. doi: 10.1111/j.1469-0691.2009.02960.x . [DOI] [PubMed] [Google Scholar]
- 83.Jin H. Endogenous small RNAs and antibacterial immunity in plants. FEBS letters. 2008;582(18):2679–84. Epub 2008/07/16. doi: 10.1016/j.febslet.2008.06.053 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.