Abstract
Protein-targeted therapies are expected to selectively kill tumor cells that express the targeted protein biomarker. Although a tumor mass may initially respond to targeted therapies based on expression of the targeted protein, all cells within a tumor may not express the targeted protein above a critical threshold level; therefore, those cells that do not express, or that downregulate expression of, the targeted protein may not be responsive to therapy. The ability to monitor the dynamic expression of these protein biomarkers throughout the course of therapy may allow for treatment to be personalized in real-time in response to the evolving nature of the tumor. This report demonstrates, by monitoring a single patient through multiple therapies, how targeted mass spectrometry is an effective, quantitative method that provides real-time analysis of multiple therapeutically associated targeted proteins that can be used to personalize a patient’s treatment strategy throughout the course of care.
Background
Patients with metastatic or locally advanced gastroesophageal cancer whose tumors overexpress the HER2 protein are eligible to receive the HER2-targeted therapy trastuzumab.1 The expectation for trastuzumab or any other biomarker-targeted treatment is that tumor cells expressing the targeted biomarker above a threshold will be selectively killed.2 Because all cells composing a tumor typically do not exhibit a homogeneous biomarker profile, those cells that do not express the biomarker of the targeted therapy will therefore remain unaffected. This “selective pressure” imposed on cancers by targeted therapies is considered to contribute to drug resistance.3 Although the term drug resistance is typically associated with active mechanisms pre-existing or reactive (ie, protein downregulation) within cells to withstand the toxicity of drugs, it does not represent the scenario of selective pressure for the subset of cells not expressing the target of treatment at the onset. Regardless, whether adaptive or selective, tumor molecular evolution over time as a resistance mechanism to therapy is now widely appreciated.4 Serial biopsy to ascertain whether resistance to targeted therapy is due to acquired downregulation of target expression may allow for an adjusted regimen of targeted therapies accordingly.
Standard diagnostic tests, such as immunohistochemistry (IHC), are unable to accurately measure changes in biomarker expression through multiple stages of treatment with adequate resolution; for example, IHC has a very narrow dynamic range at high protein expression levels, and cannot distinguish changes in expression levels within accepted scoring classifications (0 to ≥3). Targeted mass spectrometry is a novel method that provides absolute linear quantitation of protein expression over 5 orders of magnitude, and can be used to measure multiple specific protein biomarkers simultaneously throughout the course of care.5,6
This report describes a patient with a HER2-overexpressing metastatic esophagogastric cancer whose HER2 protein expression status, as measured by targeted mass spectrometry, evolved reciprocally with introduction, discontinuation, and reintroduction of anti-HER2 therapy, along with increased levels of HER3, suggesting mechanisms of trastuzumab resistance. This case study exemplifies the possibility for combining serial biopsies with quantitative measurements of protein expression by targeted mass spectrometry to deliver evolving personalized treatment plans to improve clinical outcomes.
Case Presentation
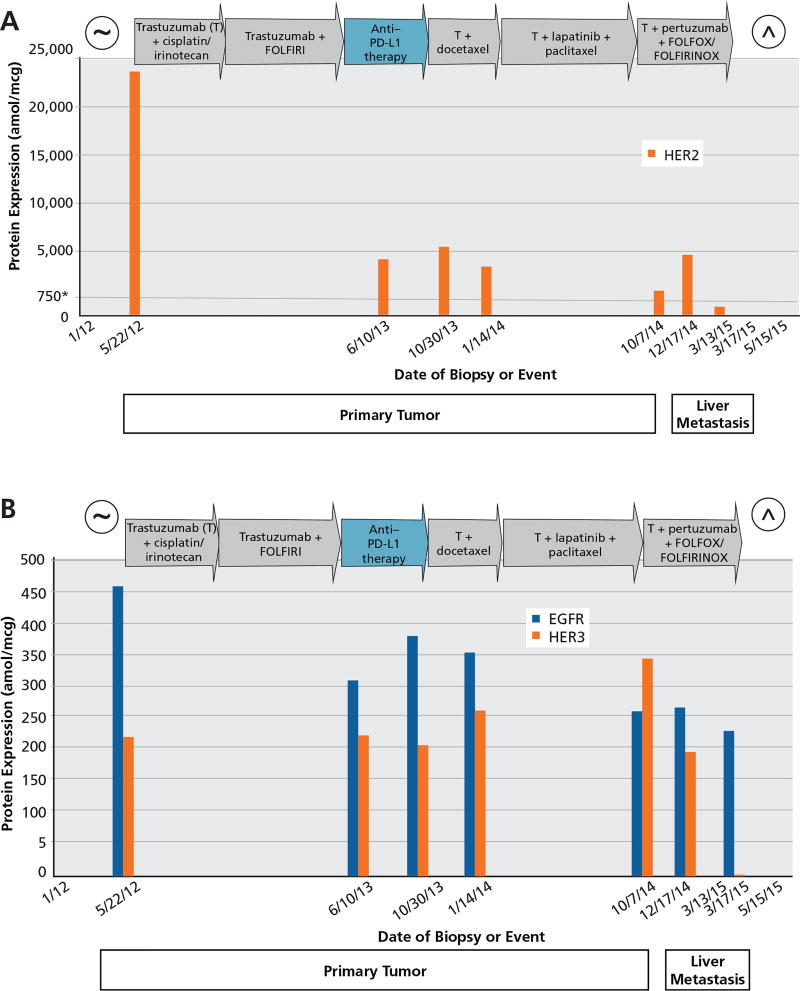
A 39-year-old man was diagnosed with stage IV HER2-amplified esophagogastric cancer in January 2012 after presenting with dysphagia and back pain demonstrating vertebral metastases. The patient had palliative radiation to the spine before commencing systemic therapy. The HER2 status of the patient’s tumor was initially scored as 3+ by IHC staining for HER2 protein expression and HER2 gene amplified by fluorescence in situ hybridization (FISH) ratio (10.15/2.15 mean copy number/cell = 4.72 ratio). By next-generation sequencing (NGS), this biopsy demonstrated 9 copies of HER2. The HER2, EGFR, and HER3 protein levels were also assessed by targeted mass spectrometry5; the HER2 protein expression level was found to be consistent with gene amplification,6,7 with a value of 24,671 attomoles per microgram (amol/mcg). We have previously reported that HER2 levels greater than 750 amol/mcg are consistent with current thresholds for clinical HER2 positivity,6 whereas more than 1,825 amol/mcg may actually better predict benefit of anti-HER2 therapy, and more than 2,383 amol/mcg may indicate an overall better prognosis (CY Ock, MD, unpublished data, 2016). Over the course of treatment, serial primary tumor biopsies5 were taken from the patient when opportune (usually at progression time points) and 2 later serial biopsies were taken of a liver metastasis, and multiple proteins, including HER2, EGFR, and HER3, were monitored using targeted mass spectrometry analysis (Figure 1). In addition, CT scans were taken throughout the course of therapy, showing changes in response to therapy (Figure 2).
Figure 1.
Temporal assessment of (A) HER2, and (B) EGFR and HER3 protein expression in a patient with HER2-positive esophagogastric cancer treated with anti-HER2 therapy–containing regimens. Seven biopsies were collected over the course of treatment (x axis) and tested for HER2, EGFR, and HER3 protein levels measured in attomoles per microgram (amol/mcg; y axis). Treatments and their reported durations are shown in arrows. Continual treatment with anti-HER2 therapies resulted in decreased HER2 protein levels, whereas non-HER2 therapy (anti–programmed death-ligand 1 [PD-L1]) resulted in an increase in HER2 protein levels. The first 5 biopsies were from the primary tumor, and the last 2 biopsies were from progressing liver metastatic lesions.
*A level of 750 amol/mcg of HER2 protein (gray horizontal line) correlates with HER2 gene amplification by FISH.6
~Date of diagnosis.
^Date of death.
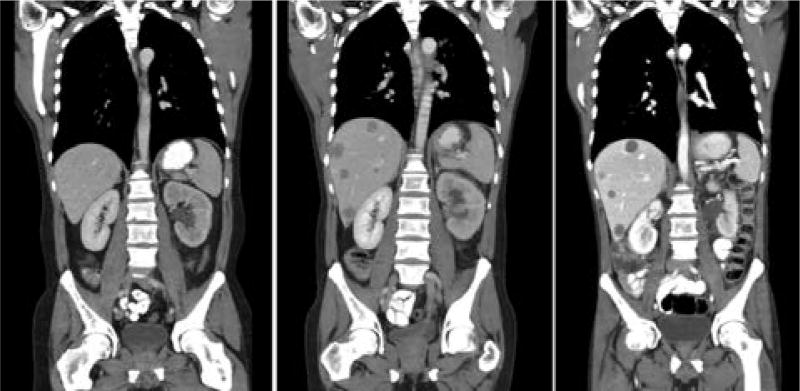
Figure 2.
CT analysis over treatment course before and after the reintroduction of anti-HER2 therapy–containing regimens. CT scans were taken over the course of treatment with anti-HER2 and anti– programmed death-ligand 1 (PD-L1) therapies. Multiple new liver lesions developed when anti-HER2 therapies were discontinued. Liver metastases regressed in number (smaller lesions were no longer visible), whereas larger lesions remain unchanged to slightly decreased in size) after anti-HER2 therapy was reintroduced.
Given that the tumor was HER2-positive as determined by standard diagnostic methods, the patient initially received a regimen containing anti-HER2 therapy (cisplatin/irinotecan plus trastuzumab) for 4.5 months under the care of a community oncologist. Although the tumor and tumor markers responded positively to treatment (his CA 19-9 level decreased from 3,663 to 114 U/mL), because of toxicity concerns, the patient presented to the University of Chicago for another opinion on therapy, and the patient’s chemotherapy regimen backbone was changed to FOLFIRI (folinic acid, fluorouracil, irinotecan hydrochloride) plus trastuzumab. The patient had stable disease for an additional 13.5 months. Ultimately the patient demonstrated signs of progression with worsening dysphagia and increasing tumor markers (CA 19-9, 471 U/mL). By the end of this 17-month period from diagnosis, the HER2 protein level in the primary tumor had decreased by 78% (from 24,671 to 5,350 amol/ mcg), suggesting a contributing mechanism of evasion by downregulation and/or selection of cells with lower expression levels of the HER2 receptor (Figure 1A). Interestingly, NGS revealed an increase in HER2 gene copy from 9 at diagnosis to 20.
The patient was then enrolled in a clinical trial of anti–programmed cell death-1 (PD-1) therapy. After 4 doses (over 8 weeks), innumerable new liver metastases developed, and a new biopsy of the primary tumor showed the HER2 protein status had increased by 26% to 6,750 amol/mcg, suggesting continued dependence on the HER2 pathway (Figure 1A). At this time, HER3 protein levels remained relatively stable (Figure 1B). Given these results, reinstatement of anti-HER2 therapy in the form of docetaxel plus trastuzumab was given for 10 cycles with the rationale of changing to new cytotoxic therapy but maintaining pressure on the HER2 pathway given an apparent persistent dependency, with consequent stabilization of disease. After 10 cycles of docetaxel plus trastuzumab, the patient experienced bleeding from the primary tumor, leading to treatment with radiation, and then experienced subsequent embolization of the left gastric artery and tumor neovascularity caused by refractory bleeding despite radiation. Biopsy of the primary tumor at this time (after docetaxel plus trastuzumab) revealed a 30% reduction (4,725 amol/mcg) of expressed HER2 protein (Figure 1A). The EGFR and HER3 protein levels were now 358 and 266 amol/mcg, respectively (Figure 1B). After bleeding was controlled, treatment was initiated with paclitaxel, trastuzumab, and lapatinib, a dual HER2/EGFR tyrosine kinase inhibitor.8–10 This regimen led to continued tumor reduction observed on serial scans over approximately 6 months (May 9, 2014, and July 11, 2014, scans), with ultimate progression in the liver lesions by October 3, 2014. A repeat biopsy on October 7, 2014, of the primary tumor demonstrated further decrease in the expression status of HER2 protein (2,140 amol/mcg) and showed decreased EGFR protein (265 amol/mcg) from before introduction of lapatinib; the HER3 protein level had in contrast increased 30.8% from the last reading (from 266 amol/ mcg up to 348 amol/mcg).
Reports suggest that trastuzumab does not effectively disrupt the formation of ligand-induced HER2–HER3 heterodimers.11 Overexpression of HER3 and generation of high levels of ligand-stimulated HER2–HER3 heterodimers may also contribute to trastuzumab resistance.11 Pertuzumab is an anti-HER2 antibody that binds near the heterodimer interface of HER2,12 thus limiting the ability for other HER family members (like HER3) to bind HER2. In preclinical studies, HER3 protein expression has been shown to be a predictive biomarker of pertuzumab efficacy by interrupting HER2/HER3 signaling.13,14 The CLEOPATRA study of first-line therapy for patients with HER2-positive metastatic breast cancer demonstrated significantly improved overall survival (OS) with the combination of trastuzumab and pertuzumab (with docetaxel) versus trastuzumab alone (with docetaxel).15 Therefore, with tumor markers and CT scans showing progressive disease after 6 months of paclitaxel plus trastuzumab and lapatinib, and with an increase in the expression of HER3 protein, treatment was amended to trastuzumab plus pertuzumab with a FOLFOX-chemotherapy backbone. The patient demonstrated some clinical improvement with dysphagia, anorexia, and fatigue, but objectively showed progressive disease after 4 doses on December 5, 2014, by CT with a slight progression in tumor markers; a new biopsy of a liver lesion on December 17, 2014, showed HER2 protein expression at 5,850 amol/mcg (no prior liver lesion to compare, but 273% higher than the primary tumor before the introduction of pertuzumab), whereas HER3 protein expression was now low at 198 amol/ mcg (43% lower than the primary tumor before the addition of pertuzumab therapy). Again, interestingly, by NGS, the HER2 gene copy number was now increased to 41 copies. At this time, treatment was amended to FOLFIRINOX/trastuzumab/pertuzumab (a total of 5 doses from January 19, 2015, to March 13, 2015) with a partial response in the liver, improved clinical symptoms of dysphagia and abdominal pain, and dramatically decreased tumor markers (CA 19-9, from 25,000 to 5,300 U/mL). Unfortunately, during a planned and necessary chemotherapy break from March 13 to April 24, 2015, due to grade 2 fatigue and diarrhea and grade 3 thrombocytopenia, there was rapid disease progression on CT and tumor markers along with significant clinical deterioration. A seventh biopsy (liver) was taken on March 17, 2015, just before the chemotherapy break, with the HER2 protein status showing a dramatic decline of 91% (531 amol/mcg) from the previous reading, with HER3 protein also dramatically decreased (not detected) and EGFR protein at 232 amol/mcg. The patient was enrolled in hospice and died on May 15, 2015, 41 months after diagnosis of stage IV disease.
Discussion
The addition of biomarker-targeted therapies to standard chemotoxic regimens improves outcomes in select patient groups.16–18 Studies, including the ToGA trial19 and others,1 demonstrate that addition of trastuzumab to the standard chemotherapy regimen results in an OS benefit in metastatic gastroesophageal cancer. However, tumors ultimately evolve mechanisms of resistance and this first-line therapy fails.
A critical need remains for both the development of more effective agents and the identification of predictive molecular markers to select which patients might benefit most from specific targeted therapies.20 From the perspective of the mechanism of action of a targeted therapy, as cells overexpressing the target of a therapy are eliminated, profiling the remaining tumor cells to determine mechanisms of resistance can lead to adjusted targeted therapy management. It is unclear whether a generalized continuation of anti-HER2 therapy is warranted for gastroesophageal cancer that is initially HER2-positive. The TyTAN (Tykerb with Taxol in Asian HER2-Positive Gastric Cancer) study was a second-line trial of paclitaxel with or without lapatinib in patients with HER2-positive disease that failed to respond to first-line therapy, which showed overall negative results; it did, however, demonstrate a survival benefit in highly expressing patients.10 Recently, a trial of T-DM1 (ado-trastuzumab emtansine) versus docetaxel in the second-line treatment of patients with HER2-positive gastroesophageal cancer also showed negative results.21 HER2 status evaluation after progression on first-line trastuzumab-based therapy was not required in either trial, and it is possible that conversion to HER2-negative (downregulation or selection of HER2-negative clones) occurred in a subset of patients, which likely affected trial outcomes.
In this case report, dramatic changes in HER2 protein levels and an increase of resistance markers (eg, HER3) were observed over time and as therapies were changed; however, it should be noted that HER2 levels in every biopsy were well above the levels associated with clinical overexpression6 until the final liver biopsy, suggesting continued dependency on this pathway throughout this case. NGS of both tumor and circulating tumor DNA fragments on 315- and 60-gene panels, respectively, did not show serial changes over time (genomic events identified at each time point included ERBB2 amplification, FBXW7 R465H, TP53 E286K, and EPHA3 R381H). It is interesting to note that with each serial biopsy after anti-HER2 therapy, HER2 expression levels decreased by mass spectrometry, whereas HER2 gene copy number increased by NGS. This may reflect the conflict of the cancer cell to have continued dependence of HER2 (selecting higher gene copies over time) but at the same time attempting to downregulate HER2 expression to evade anti-HER2 therapies. This concept requires further study to validate these observations and elucidate these biologic mechanisms of growth and resistance. Moreover, targeted mass spectrometry offered a unique advantage over traditional diagnostic methods, such as IHC, in that the dynamic range at high protein levels had the capability to discern the changes observed. Using standard IHC protocols for HER2 analysis, all of the serial biopsies had a consistent scoring of 3+ without resolution to detect serial changes.7
As seen with this patient and in the report of another patient with gastric cancer,22 reintroduction of an anti-HER2 therapy can be effective after initial progression on first-line anti-HER2 therapy. This case is the first to demonstrate an absolute measurable decrease in the level of HER2 protein expressed, a reversal when anti-HER2 therapies were discontinued, and then a decrease once again on reintroduction of anti-HER2 therapies. Furthermore, it is interesting to note that the patient described here likely benefited from treatment with multiple anti-HER2 therapies (trastuzumab, pertuzumab, and lapatinib) to address known mechanisms of resistance using ”vertical” (lapatinib-HER2) and ”horizontal” (pertuzumab-HER3 and lapatinib-EGFR) inhibitory strategies. Monitoring the expression levels of HER2 protein and others, such as HER3 and EGFR, over the course of anti-HER2 targeted therapy by using targeted mass spectrometry allowed implementation of a personalized treatment strategy as tumor biology evolved. This patient, surviving 41 months after an initial stage IV diagnosis, far surpassed the median OS rate of 13.8 months observed in patients who received only trastuzumab plus chemotherapy in one line of therapy.19 As such, further studies are warranted, including those using novel clinical trial designs testing such serial molecular profiling and targeted ”biologics beyond progression.”4
Conclusions
This case displays the integration of targeted therapies into cancer treatment regimens based on serial profiling results that demonstrated profound tumor molecular evolution. Potential mechanisms of resistance that were observed over the course of therapy included downregulation of HER2 expression and increase in co-stimulatory receptors, including HER3. There has thus far been no method that allows real-time monitoring of the expression level of these targets in tumor cells in a high-resolution and quantitative manner while the patient is receiving one or more targeted therapies. Protein expression analysis by targeted mass spectrometry offers a reliable and scalable method to monitor and react to changes in the status of multiple (currently 94) therapeutic protein targets serially, and merits further investigation.
Acknowledgments
This work was supported by NIH K23 award (CA178203-01A1), UCCCC (University of Chicago Comprehensive Cancer Center) Award in Precision Oncology- CCSG (Cancer Center Support Grant) (P30 CA014599), OncoPlex Diagnostics Collaborative Research Agreement, LLK (Live Like Katie) Foundation Award, and the Sal Ferrara II Fund for PANGEA (to D.V.T.C).
This research was performed under approved Institutional Review Board protocols at the University of Chicago.
Footnotes
Drs. Sellappan, Blackler, Liao, Thyparambil, Cecchi, and Hembrough have disclosed that they are employed by and hold equity interest in NantOmics, LL. Dr. Catenacci has disclosed that he is on the advisory board for Genentech, Inc. The remaining author have disclosed that they have no financial interests, arrangements, affiliations, or commercial interests with the manufacturers of any products discussed in this article or their competitors.
References
- 1.Herceptin [prescribing information] South San Francisco, CA: Genentech, Inc.; 2016. [Google Scholar]
- 2.Barrajon-Catalan E, Menendez-Gutierrez MP, Falco A, et al. Selective death of human breast cancer cells by lytic immunoliposomes: correlation with their HER2 expression level. Cancer Lett. 2010;290:192–203. doi: 10.1016/j.canlet.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 3.National Cancer Institute. [Accessed April 13, 2016];Provocative Questions: Identifying Perplexing Problems to Drive Progress Against Cancer. Available at: http://provocativequestions.nci.nih.gov/archived-rfas-and-pqs/rfa-archive-2012/mainquestions_listview?mqCategory=Group+D.
- 4.Catenacci DV. Next-generation clinical trials: novel strategies to address the challenge of tumor molecular heterogeneity. Mol Oncol. 2015;9:967–996. doi: 10.1016/j.molonc.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hembrough T, Thyparambil S, Liao WL, et al. Application of selected reaction monitoring for multiplex quantification of clinically validated biomarkers in formalin-fixed, paraffin-embedded tumor tissue. J Mol Diagn. 2013;15:454–465. doi: 10.1016/j.jmoldx.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Catenacci DV, Liao WL, Zhao L, et al. Mass-spectrometry-based quantitation of Her2 in gastroesophageal tumor tissue: comparison to IHC and FISH [published online ahead of print November 18, 2015] Gastric Cancer. doi: 10.1007/s10120-015-0566-0. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nuciforo P, Thyparambil S, Aura C, et al. High HER2 protein levels correlate with increased survival in breast cancer patients treated with anti-HER2 therapy. Mol Oncol. 2016;10:138–147. doi: 10.1016/j.molonc.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scaltriti M, Verma C, Guzman M, et al. Lapatinib, a HER2 tyrosine kinase inhibitor, induces stabilization and accumulation of HER2 and potentiates trastuzumab-dependent cell cytotoxicity. Oncogene. 2009;28:803–814. doi: 10.1038/onc.2008.432. [DOI] [PubMed] [Google Scholar]
- 9.Medina PJ, Goodin S. Lapatinib: a dual inhibitor of human epidermal growth factor receptor tyrosine kinases. Clin Ther. 2008;30:1426–1447. doi: 10.1016/j.clinthera.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 10.Satoh T, Xu RH, Chung HC, et al. Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: TyTAN--a randomized, phase III study. J Clin Oncol. 2014;32:2039–2049. doi: 10.1200/JCO.2013.53.6136. [DOI] [PubMed] [Google Scholar]
- 11.Gajria D, Chandarlapaty S. HER2-amplified breast cancer: mechanisms of trastuzumab resistance and novel targeted therapies. Expert Rev Anticancer Ther. 2011;11:263–275. doi: 10.1586/era.10.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuentes G, Scaltriti M, Baselga J, Verma CS. Synergy between trastuzumab and pertuzumab for human epidermal growth factor 2 (Her2) from colocalization: an in silico based mechanism. Breast Cancer Res. 2011;13:R54. doi: 10.1186/bcr2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas G, Chardes T, Gaborit N, et al. HER3 as biomarker and therapeutic target in pancreatic cancer: new insights in pertuzumab therapy in preclinical models. Oncotarget. 2014;5:7138–7148. doi: 10.18632/oncotarget.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee-Hoeflich ST, Crocker L, Yao E, et al. A central role for HER3 in HER2-amplified breast cancer: implications for targeted therapy. Cancer Res. 2008;68:5878–5887. doi: 10.1158/0008-5472.CAN-08-0380. [DOI] [PubMed] [Google Scholar]
- 15.Swain SM, Kim SB, Cortes J, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013;14:461–471. doi: 10.1016/S1470-2045(13)70130-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swain SM, Baselga J, Kim SB, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372:724–734. doi: 10.1056/NEJMoa1413513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pegram MD, Lipton A, Hayes DF, et al. Phase II study of receptor-enhanced chemosensitivity using recombinant humanized anti-p185HER2/neu monoclonal antibody plus cisplatin in patients with HER2/neu-overexpressing metastatic breast cancer refractory to chemotherapy treatment. J Clin Oncol. 1998;16:2659–2671. doi: 10.1200/JCO.1998.16.8.2659. [DOI] [PubMed] [Google Scholar]
- 18.Lordick F, Kang YK, Salman P, et al. Clinical outcome according to tumor HER2 status and EGFR expression in advanced gastric cancer patients from the EXPAND study [abstract] J Clin Oncol. 2013;31(Suppl) Abstract 4021. [Google Scholar]
- 19.Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 20.Qiu MZ, Xu RH. The progress of targeted therapy in advanced gastric cancer. Biomark Res. 2013;1:32. doi: 10.1186/2050-7771-1-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.GEN News Highlights. [Accessed April 13, 2016];Roche’s Kadcyla Fails Phase II/III Trial for Gastric Cancer. Available at: http://www.genengnews.com/gen-news-highlights/roche-s-kadcyla-fails-phase-ii-iii-trial-for-gastric-cancer/81251888/
- 22.Dubreuil O, Zaanan A, Pellerin O, et al. Trastuzumab re-introduction with FOLFIRI for treatment of HER2 overexpression-advanced gastric adenocarcinoma following failure of other trastuzumab-based chemotherapy regimens. Case Reports in Clinical Medicine. 2015;4:131–136. [Google Scholar]