Abstract
INTRODUCTION
Investigations of the independent associations of physical inactivity with cancer endpoints have been mounting in the epidemiological literature, in part due to the high prevalence of physical inactivity among cancer patients and to evidence that inactivity associates with carcinogenesis via pathways independent of obesity. Yet, physical inactivity is not currently recognized as a well-established risk or prognostic factor for lung cancer. As such, we examined the associations of lifetime physical inactivity with lung cancer risk and mortality in a hospital-based, case-control study.
PRESENTATION OF CASE
Materials and Methods
The analyses included data from 660 lung cancer patients and 1335 matched cancer-free controls. Multivariable logistic regression analyses were utilized to assess the association between lifetime physical inactivity and lung cancer risk, and Cox proportional hazards models were utilized to estimate the association between lifetime physical inactivity and mortality among lung cancer cases.
Results
We observed a significant positive association between lifetime physical inactivity and lung cancer risk: [Odds ratio (OR)=2.23, 95% confidence interval (CI): 1.77–2.81]; the association remained significant among never smokers (OR=3.00, 95% CI:1.33–6.78) and non-smokers (OR=2.33, 95% CI: 1.79–3.02). We also observed a significant positive association between lifetime physical inactivity and lung cancer mortality [Hazard ratio (HR)=1.40, 95% CI: 1.14–1.71]; the association remained significant in non-smokers (HR=1.51, 95% CI: 1.16–1.95).
DISCUSSION/CONCLUSION
These data add to the body of evidence suggesting that physical inactivity is an independent risk and prognostic factor for cancer. Additional research utilizing prospectively collected data is needed to substantiate the current findings.
Keywords: lung cancer epidemiology, lung cancer risk, lung cancer survival, lung cancer mortality, physical inactivity
INTRODUCTION
In 2018, lung cancer will account for over 234,000 new cancer diagnoses and 154,000 cancer deaths in the United States, making it the second most commonly diagnosed, and the most deadly, cancer in the U.S.[1] Active cigarette smoking is the most well-established behavioral risk factor for lung cancer, accounting for as much as 90% of newly diagnosed lung cancer cases [2]. Thus, the majority of efforts to prevent lung cancer and improve prognosis are focused on smoking cessation. However, given the high burden of lung cancer incidence and the poor survival outcomes, the identification of additional behavioral risk and prognostic factors for lung cancer could be of significant public health importance, especially among never-smokers. In fact, lung cancer among never smokers represents the seventh most common cancer globally [3], and lung cancer rates among female never-smokers are rising [4].
While a recently published meta-analysis of epidemiological evidence reported an inverse association between the highest level of recreational physical activity exposure and lung cancer risk [2], data representing the associations between physical activity and lung cancer endpoints among women, non-smokers, and among the individual subtypes of lung cancer are limited [2, 4]. Importantly, physical activity exposure is not currently recognized by the National Cancer Institute or the American Cancer Society as a well-established protective or prognostic factor for lung cancer [5, 6], and little is known about the independent association of physical inactivity with lung cancer risk and prognosis.
Despite national guidelines encouraging Americans to avoid physical inactivity [7], current reports suggest that 50 to 79% of Americans are insufficiently physically active during their leisure time [8]. As a result, researchers have called for more investigations of the associations between physical inactivity and cancer endpoints [9]. Yet, under the prevailing paradigm of epidemiological physical activity research, the “no-activity”, or the lowest activity level, is typically identified as the referent group. Thus, the independent associations of physical inactivity with cancer risk and survival often remain unreported. This may be an important public health oversight due to a recently published report suggesting that even the smallest amounts of physical activity associate with decreased mortality [10]. Therefore, investigations emphasizing physical inactivity as an independent exposure of interest may be especially impactful for disease sites in which physical activity exposure has not been identified as a well-established protective or prognostic factor. That is, a lack of a consistent association between incrementally higher quantities of physical activity with cancer endpoints should not preclude additional investigations of the independent associations of inactivity with cancer risk and mortality merely due to convention. Rather, given the high prevalence of physical inactivity at the population level and the hypothesis that the greatest protective benefits can be achieved by increasing activity levels among those at the low end of the activity continuum [11], physically inactive individuals could be a particularly important group to study from a public health perspective. Further, there is also a body of literature suggesting that self-reported physical inactivity is assessed with less exposure misclassification in comparison to self-reported incrementally higher levels of activity exposure [11, 12], and that inactivity may associate with cancer endpoints independently of obesity [13] [9].
Based on this collective knowledge, we sought to investigate the associations of lifetime recreational physical inactivity with lung cancer risk and mortality. We hypothesized that lung cancer patients would be more likely to report a history of lifetime physical inactivity in comparison to controls without cancer and that physically inactive patients would have poorer survival outcomes. To address additional gaps in the literature [2, 4], we also examined the associations of physical inactivity with lung cancer endpoints in subgroups based upon sex, smoking status, body mass index (BMI) and lung cancer histology.
MATERIALS AND METHODS
Study Population
The study population for the current analyses included individuals who received medical services at Roswell Park Cancer Institute (RPCI) between 1990 and 1998 who also agreed to complete a comprehensive epidemiological questionnaire and participate in the Patient Epidemiology Data System (PEDS). Lung cancer cases were identified from the RPCI Tumor Registry and Diagnostic Index and included 660 individuals diagnosed with primary, incident lung cancer. Controls were age-frequency matched to cases on five-year age strata and included 1,335 individuals identified from a pool of 10,642 potentially eligible controls. Control participants came to RPCI with a suspicion of cancer but were diagnosed with conditions that included non-malignant diseases of the circulatory system, genitourinary system, gastrointestinal system, respiratory system, or other conditions. The RPCI Institutional Review Board approved the conduct of the study and all participants provided written informed consent.
Epidemiological Questionnaire
The PEDS questionnaire, including a lifetime recreational physical activity assessment, was a self-administered epidemiological questionnaire offered to patients receiving medical service at RPCI. The PEDS questionnaire was offered to all new patients upon admission, regardless of diagnosis or reason for seeking care at RPCI. All questionnaires were completed within six months from date of diagnosis (median 21 days), with a 50% response rate among all admitted patients [14]. The detailed content and administration methods associated with the PEDS questionnaire have been previously described [15–17].
Physical Inactivity
The recreational physical activity section of the PEDS questionnaire was comprised of items assessing the age of onset of regular activity, the total years of regular activity, and the frequency of the activity (i.e., number of times per week or month). Recreational activity was defined as regularly exercising for health or pleasure in activities such as jogging, walking, or aerobics. We defined recreational physical inactivity in accordance with The 2008 Physical Activity Guidelines for Americans [7]. Thus, individuals reporting no regular, weekly, recreational physical activity throughout their adult lifetime (on average, less than one session per week or less than four sessions per month) were classified as physically inactive. Conversely, participants reporting, on average, at least one regular, weekly session of physical activity were classified as active. In exploratory analyses, we also examined physical inactivity during the time period spanning two decades prior to study enrollment, as this may be a more relevant exposure window relative to carcinogenesis.
Identification of Confounding Variables
We pre-specified age, sex, BMI, family history of lung cancer, and smoking (pack years) as important variables for adjustment in risk analyses. For survival analyses, we pre-specified age, sex, stage, grade, smoking (pack years), and BMI as important adjustment variables. We also examined the potential confounding effects of additional putative epidemiological risk and prognostic factors (i.e., education, race, treatment regimen, etc.) by applying the ten percent change-in-estimate method described by Maldonado et al.[18].
Statistical Analysis
Physical Inactivity and Lung Cancer Risk
In descriptive analyses, differences in demographic and risk factor characteristics between lung cancer cases and controls were evaluated with two-tailed t-tests and Pearson’s Chi-square. In risk analyses, we utilized age-adjusted and multivariable-adjusted binary logistic regression models to estimate the odds ratios (OR) and 95% confidence intervals (CI) representing the association between lifetime physical inactivity and lung cancer risk. We estimated associations of inactivity with lung cancer risk overall, and also by subgroups based upon sex, BMI status (normal weight versus overweight/obese), smoking status (never-smoker, former-smoker, current-smoker, non-smoker) and histological subtype (adenocarcinoma, squamous-cell carcinoma, small-cell carcinoma and ‘other histology,’ which consisted of all additional histological subtypes of lung cancer). In subgroup analyses by smoking status, the non-smoker group was defined as those participants who were not smoking at the time of study entry, and included never smokers and former smokers who had quit at least one year prior to study enrollment.
For all subgroup analyses, if we observed evidence that point estimates varied considerably across strata based upon sex, BMI, or smoking status, we evaluated the potential for statistical interaction via the inclusion of a physical inactivity cross-product term in multivariable models. Lastly, in exploratory analyses designed to examine the possibility of a synergistic effect of physical inactivity and smoking relative to lung cancer risk, we evaluated whether there was evidence of a positive, additive interaction among physically inactive smokers.
Physical Inactivity and Lung Cancer Mortality
The study population for the survival analyses included 579 lung cancer cases with available follow-up data. Follow-up data were obtained from the RPCI Tumor Registry update to the PEDS dataset and included information on vital status, date of last follow-up, date of death, and cause of death. Time to last follow-up was calculated in days from the date of diagnosis until the date of death or date of last follow-up, with the most recent Tumor Registry update occurring in February 2016. Lung cancer cases that died within 30 days of diagnosis were excluded from the survival analyses.
In descriptive analyses, differences in demographic and prognostic factors between physically inactive and physically active lung cancer patients were evaluated with two-tailed t-tests and Pearson’s Chi-square. To characterize the survival experience of physically inactive and physically active lung cancer patients, Kaplan-Meier plots were generated to compare the overall and disease-specific survival among lung cancer cases. Log rank tests were utilized to assess whether significant differences in survival were observed between physically inactive and physically active patients. Multivariable Cox proportional hazards models were also utilized to calculate hazards ratios (HRs) and 95% CIs representing the association of lifetime physical inactivity with all-cause and lung cancer-specific mortality while controlling for potential confounding variables. We assessed associations between lifetime physical inactivity and mortality in the overall study population and in subgroups based upon sex, BMI, smoking status, histological subtype, tumor stage (local/regional vs. distant disease) and type of treatment (surgery, chemotherapy and radiation therapy). For all subgroup analyses, if we observed evidence that hazard ratios varied considerably across strata, statistical interactions for physical inactivity with sex, BMI, smoking status, histological subtype and/or treatment type were assessed via the inclusion of a cross-product term in multivariable models. All statistical analyses for both risk and survival analyses were performed using SAS for Windows, version 9.4 and were considered significant at p<0.05.
RESULTS
Physical Inactivity and Lung Cancer Risk
The descriptive characteristics of the study population by case-control status are presented in Table 1. As expected, in comparison to controls, lung cancer cases were more likely to be smokers (p<0.001), had a significantly lower BMI (p<0.001), were more likely to be physically inactive (p<0.001), and were more likely to have a family history of cancer (p=0.002) (Table 1). We observed no significant differences in age distribution between cases and controls (Table 1).
Table 1.
Descriptive characteristics of the study population by case-control status (N=1995)1
| Characteristic | Cases (N = 660) N (SD or %) |
Controls (N = 1335) N (SD or %) |
p-value2 |
|---|---|---|---|
| Age (Continuous) | 63.42 (9.96) | 63.40 (10.20) | 0.969 |
| Sex | <0.001 | ||
| Male | 379 (57.42%) | 579 (43.40%) | |
| Female | 281 (42.58%) | 755 (56.60%) | |
| Family History of Lung Cancer | 0.002 | ||
| Yes | 87 (14.90%) | 120 (9.95%) | |
| No | 497 (85.10%) | 1086 (90.05%) | |
| BMI (kg/m2) | 25.63 (4.42) | 26.58 (5.16) | <0.001 |
| BMI Classification | 0.005 | ||
| Underweight | 16 (2.45%) | 18 (1.36%) | |
| Normal | 289 (44.33%) | 538 (40.79%) | |
| Overweight | 261 (40.03%) | 513 (38.89%) | |
| Obese | 86 (13.19%) | 250 (18.95%) | |
| Recreational Inactivity | <0.001 | ||
| Active | 209 (31.67%) | 674 (50.49%) | |
| Inactive | 451 (68.33%) | 661 (49.51%) | |
| Smoking (Pack Years) | <0.001 | ||
| 0 | 35 (5.34%) | 552 (41.47%) | |
| >0–31 | 23 (3.51% | 93 (6.99%) | |
| >31–46 | 30 (4.57%) | 38 (2.85%) | |
| >46–64 | 31 (4.73%) | 59 (4.43%) | |
| >64 | 537 (81.86%) | 589 (44.25%) | |
| Smoking Status (3 levels)3 | <0.001 | ||
| Never-smoker | 39 (5.91%) | 551 (41.27%) | |
| Former-smoker | 486 (73.64%) | 608 (45.54%) | |
| Current Smoker | 135 (20.45%) | 176 (13.18%) | |
| Smoking Status (2 levels)4 | <0.001 | ||
| Current Smoker | 135 (25.14%) | 176 (13.72%) | |
| Non-Smoker | 402 (74.86%) | 1107 (86.28%) |
Numbers may not sum to total due to missing data
p-value represents Chi square test for independence for categorical variables and t-test for continuous variables
Former smokers included all participants who reported quitting smoking prior to study enrollment; Current smokers were actively smoking at the time of study enrollment.
Current smokers were actively smoking at the time of study enrollment; non-smokers included only those who were never-smokers and those who had quit smoking at least one year prior to study enrollment.
Associations between lifetime physical inactivity and lung cancer risk are presented in Table 2. In multivariable-adjusted logistic regression models, we observed a significant positive association between lifetime physical inactivity and lung cancer risk in the overall study population (OR=2.23, 95% CI: 1.77– 2.81). When we examined the exposure window spanning only two decades prior to study entry, the observed association between physical inactivity and lung cancer risk was similar in magnitude and remained statistically significant (OR=1.98; 95% CI: 1.58–2.49).
Table 2.
Age-adjusted and multivariable-adjusted odds ratios and 95% confidence intervals representing the association between lifetime recreational physical inactivity and lung cancer risk (N=1995)1
| Subgroup | Recreational (In)Activity | Case (N= 660) | Control (N=1335) | Age-Adjusted Model | Multivariable-Adjusted Model2 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |||||
| Overall Study Population | Active | 209 | 674 | Ref | -- | -- | Ref | -- | -- | |
| Inactive | 451 | 661 | 2.23 | 1.83 – 2.72 | <.001 | 2.23 | 1.77 – 2.81 | <.001 | ||
| Sex | Male | Active | 110 | 275 | Ref | -- | -- | Ref | -- | -- |
| Inactive | 269 | 304 | 2.32 | 1.75 – 3.06 | <.001 | 2.14 | 1.55 – 2.94 | <.001 | ||
| Female | Active | 99 | 398 | Ref | -- | -- | Ref | -- | -- | |
| Inactive | 182 | 357 | 2.01 | 1.51 – 2.67 | <.001 | 2.29 | 1.64 – 3.21 | <.001 | ||
| BMI Classification | Normal weight (BMI 18.5–24.99) | Active | 94 | 312 | Ref | -- | -- | Ref | -- | -- |
| Inactive | 211 | 244 | 2.92 | 2.17 – 3.94 | <.001 | 2.74 | 1.92 – 3.92 | <.001 | ||
| Overweight and Obese (BMI>25.0) | Active | 113 | 357 | Ref | -- | -- | Ref | -- | -- | |
| Inactive | 234 | 406 | 1.84 | 1.41 – 2.40 | <.001 | 1.84 | 1.36 – 2.49 | <.001 | ||
| Smoking Status3 | Non-smoker | Active | 136 | 587 | Ref | -- | -- | Ref | -- | -- |
| Inactive | 266 | 520 | 2.16 | 1.70 – 2.75 | <.001 | 2.33 | 1.79 – 3.02 | <.001 | ||
| Never-smoker | Active | 10 | 293 | Ref | -- | -- | Ref | -- | -- | |
| Inactive | 29 | 258 | 2.83 | 1.34 – 5.98 | .007 | 3.00 | 1.33 – 6.78 | 0.008 | ||
| Former-smoker | Active | 161 | 317 | Ref | -- | -- | Ref | -- | -- | |
| Inactive | 325 | 291 | 2.25 | 1.76 – 2.88 | <.001 | 2.39 | 1.82 – 3.13 | <.001 | ||
| Current smoker | Active | 38 | 64 | Ref | -- | -- | Ref | -- | -- | |
| Inactive | 97 | 112 | 1.48 | 0.90 – 2.42 | 0.124 | 1.43 | .829 – 2.47 | 0.198 | ||
| Lung Cancer Histological Subtype | Adenocarcinoma | Active | 61 | 674 | Ref | -- | -- | Ref | -- | -- |
| Inactive | 109 | 661 | 1.88 | 1.35 – 2.63 | <.001 | 1.87 | 1.30 – 2.69 | <.001 | ||
| Squamous-cell Carcinoma | Active | 44 | 674 | Ref | -- | -- | Ref | -- | -- | |
| Inactive | 148 | 661 | 3.29 | 2.31 – 4.70 | <.001 | 3.26 | 2.20 – 4.83 | <.001 | ||
| Small-cell Carcinoma | Active | 25 | 674 | Ref | -- | -- | Ref | -- | -- | |
| Inactive | 62 | 661 | 2.74 | 1.69 – 4.44 | <.001 | 2.82 | 1.59 – 4.99 | <.001 | ||
| Other4 | Active | 79 | 674 | Ref | -- | -- | Ref | -- | -- | |
| Inactive | 132 | 661 | 1.82 | 1.32 – 2.50 | <.001 | 1.77 | 1.26 – 2.47 | 0.001 | ||
Numbers may not sum to total due to missing data
Multivariable models adjusted for sex, smoking (pack years), BMI, family history of lung cancer, and age
Non-smokers include those who have never smoked and who had quit longer than one year prior to study enrollment; former smokers include any participant who had reported quitting smoking by the time of study enrollment; current smokers were actively smoking at the time of study enrollment
The ‘other’ histological subtype included all other lung cancer histological subtypes
We also observed positive associations between lifetime physical inactivity and lung cancer risk in every subgroup we examined. For example, in subgroup analyses by histological subtype, the association between lifetime physical inactivity and lung cancer remained significant for each of the four groups examined: adenocarcinoma (OR= 1.87, 95% CI: 1.30–2.69); squamous-cell carcinoma (OR= 3.26, 95% CI: 2.20–4.83); small-cell carcinoma (OR=2.82, 95% CI: 1.59–4.99); and ‘other’ histological subtypes (OR=1.77, 95% CI: 1.26–2.47) (Table 2). In subgroup analyses by BMI status, the association between lifetime physical inactivity and lung cancer risk persisted and remained significant in both normal-weight (OR= 2.74, 95% CI: 1.92–3.92) and overweight/obese participants (OR =1.84, 95% CI: 1.36–2.49) (Table 2) and although the observed associations are of greater magnitude among the normal-weight group, we observed no statistical evidence of effect modification by BMI classification (p-for-interaction=0.463).
When we evaluated associations of lifetime physical inactivity with lung cancer risk by smoking status, we observed increased risks of lung cancer in inactive never-smokers (OR=3.00, 95% CI: 1.33–6.78), former smokers (OR=2.39, 95% CI: 1.82–3.13), non-smokers (OR=2.33, 95% CI: 1.79–3.02), and current smokers (OR=1.43, 95% CI: 0.83–2.47), but the association was shy of significance among current smokers (Table 2). While the magnitudes of the observed associations are different across smoking status strata, we observed no statistical evidence of an inactivity-by-smoking status interaction via a cross product term (p-for-interaction = 0.322). However, in additional exploratory analyses, we observed evidence suggestive of a positive, additive interaction among physically inactive smokers. Specifically, utilizing fully adjusted multivariable models to assess associations, the relative excess risk due to interaction (RERI) was 4.05 (95% CI: −6.65–14.75) for current- versus never-smokers and 13.58 (95% CI: 1.21–25.95) for ever- versus never-smokers, but the CI contained the null for the former comparison.
Lastly, in subgroup analyses by sex, we observed significant positive associations between lifetime physical inactivity and lung cancer risk in women (OR=2.29, 95% CI: 1.64–3.21) and men (OR=2.14, 95% CI: 1.55–2.94) (Table 2). In additional exploratory analyses stratified by sex and smoking status, the association between lifetime physical inactivity and lung cancer risk persisted among female non-smokers (OR = 2.14, 95% CI: 1.44–3.19) and male non-smokers (OR=2.42, 95% CI: 1.71–3.44) (data not shown).
Physical Inactivity and Lung Cancer Mortality
Prognostic characteristics among lung cancer cases by inactivity status are presented in Table 3. Physically inactive cases were older (p=0.003) and were more likely to have received chemotherapy as part of their treatment (p=0.011) (Table 3). However, we observed no significant differences between physically inactive and physically active cases by surgery or radiation therapy, tumor stage, tumor grade, BMI, family history of lung cancer, smoking status or the number of pack years smoked (p>0.05). After 18 years of follow-up, there were 560 total deaths and 481 lung cancer-specific deaths among the 579 lung cancer cases included in the analysis. Collectively, median follow-up time for lung cancer cases was 475 days.
Table 3.
Descriptive characteristics of the survival study population by lifetime recreational physical inactivity status (N=579)
| Characteristic | N1 | Median Length of Follow-up (Days) | Active (N = 186) N (SD or %) | Inactive (N = 393) N (SD or %) | p-value2 |
|---|---|---|---|---|---|
| Age (Continuous) | 579 | -- | 61.23 (10.63) | 64.46 (9.65) | 0.003 |
| Sex | 0.083 | ||||
| Male | 335 | 495 | 98 (52.69%) | 237 (60.31%) | |
| Female | 244 | 468 | 88 (47.31%) | 156 (39.69%) | |
| BMI (kg/m2) (Continuous) | 574 | -- | 25.63 (3.94) | 25.66 (4.71) | 0.926 |
| BMI (Categorical) | 0.453 | ||||
| Underweight | 12 | 546 | 2 (1.08%) | 10 (2.54%) | |
| Normal weight | 258 | 525 | 79 (42.47%) | 179 (45.55%) | |
| Overweight | 228 | 401 | 80 (43.01%) | 148 (37.66%) | |
| Obese | 76 | 544 | 23 (12.37%) | 53 (13.49%) | |
| Smoking (Pack Years) | 0.775 | ||||
| 0 | 30 | 383.5 | 8 (4.30%) | 22 (5.60%) | |
| >0–31 | 18 | 427.5 | 7 (3.76%) | 11 (2.80%) | |
| >31–46 | 27 | 402 | 11 (5.91%) | 16 (4.07%) | |
| >46–64 | 30 | 397 | 9 (4.84%) | 21 (5.34%) | |
| >64 | 474 | 523.5 | 151 (81.18%) | 323 (82.19%) | |
| Smoking Status (3 Level)3 | |||||
| Never Smoker | 30 | 383.5 | 8 (4.30%) | 22 (5.60%) | 0.396 |
| Former Smoker | 426 | 532 | 142 (76.34%) | 284 (72.26%) | |
| Current Smoker | 123 | 402 | 36 (19.35%) | 87 (22.14%) | |
| Smoking Status (2 Level)4 | 0.336 | ||||
| Current Smoker | 123 | 402 | 36 (19.35%) | 87 (22.14%) | |
| Non-Smoker | 347 | 546 | 118 (63.44%) | 229 (58.27%) | |
| Family History | 0.475 | ||||
| Yes | 76 | 421.5 | 22 (11.83%) | 54 (13.74%) | |
| No | 438 | 524 | 145 (77.96%) | 293 (74.55%) | |
| Stage5 | 0.793 | ||||
| Localized | 106 | 1787 | 30 (16.13%) | 76 (19.34%) | |
| Regional | 198 | 619 | 64 (34.41%) | 134 (34.10%) | |
| Distant | 247 | 307 | 83 (44.62%) | 164 (41.73%) | |
| Unknown | 20 | 553 | 7 (3.76%) | 13 (3.31%) | |
| Grade | 0.086 | ||||
| 1 | 19 | 2211 | 11 (5.91%) | 8 (2.04%) | |
| 2 | 89 | 610 | 28 (15.05%) | 61 (15.52%) | |
| 3 | 208 | 532.5 | 59 (31.72%) | 149 (37.91%) | |
| 4 | 131 | 394 | 38 (20.43%) | 93 (23.66%) | |
| Undetermined | 130 | 442.5 | 50 (26.88%) | 80 (20.36%) | |
| Chemotherapy | 0.011 | ||||
| Yes | 347 | 414 | 128 (68.82%) | 219 (55.73%) | |
| No | 228 | 679.5 | 57 (30.65%) | 171 (43.51%) | |
| Surgery | 0.060 | ||||
| Yes | 226 | 1068.5 | 83 (44.62%) | 143 (36.39%) | |
| No | 349 | 347 | 102 (54.84%) | 247 (62.85%) | |
| Radiation Therapy | 0.104 | ||||
| Yes | 363 | 439 | 108 (58.06%) | 255 (64.89%) | |
| No | 212 | 622.5 | 77 (41.40%) | 135 (34.35%) |
Numbers may not sum to total due to missing data
p-value represents Chi square test for independence for categorical variables and t-test for continuous variables
Former smokers included all participants who reported quitting smoking prior to study enrollment; Current smokers were actively smoking at the time of study enrollment.
Non-smokers included those who were never-smokers and those who had quit smoking at least one year prior to study enrollment.
Local stage indicates a tumor is confined to primary site; Regional stage indicates the tumor spread to regional lymph nodes; Distant stage indicates the tumor has metastasized; Unknown stage indicates the tumor was unable to be staged by a pathologist.
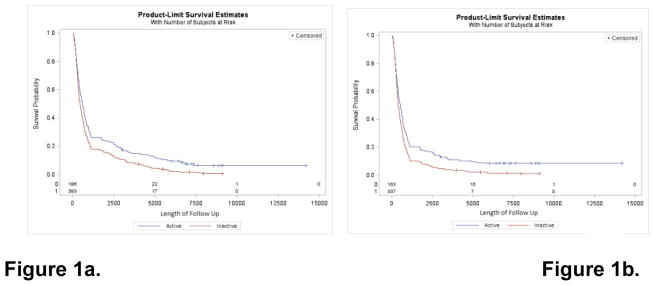
Kaplan Meier survival curves comparing the survival experience of physically inactive and physically active lung cancer cases are presented in Figures 1a and 1b. For overall survival, we observed significantly different survival probabilities between physically inactive and physically active cases (log rank p<0.001), with a median survival disadvantage of 170 days among physically inactive cases (Figure 1a). The significant survival disadvantage among physically inactive lung cancer cases persisted in analyses limited to lung-cancer-specific survival, with physically inactive cases experiencing a 133-day survival disadvantage in comparison to more physically active cases (log rank p<0.001) (Figure 1b).
Figure 1.
Kaplan-Meier survival plots depicting the (1a) overall survival and (1b) lung cancer-specific survival experience of lung cancer cases according to self-reported physical (in)activity status
Hazard ratios and 95% CIs representing the associations of lifetime physical inactivity with all-cause and lung cancer-specific mortality are presented in Table 4. In multivariable Cox regression models, we observed significant positive associations of lifetime physical inactivity with all-cause mortality (HR=1.31, 95% CI: 1.09–1.58) and lung cancer-specific mortality (HR=1.40, 95% CI: 1.14–1.71) in the overall study population. The association between lifetime physical inactivity and mortality remained significant in non-smokers for both all-cause mortality (HR=1.45, 95% CI: 1.14–1.84) and lung cancer-specific mortality (HR=1.51, 95% CI: 1.16–1.95) (Table 4).
Table 4.
Multivariable adjusted hazard ratios representing the associations of habitual recreational physical inactivity with all-cause and lung cancer-specific mortality (N=579).
| Subgroup | N1 | Median Length of Follow-Up (Days) | All-Cause Mortality | Lung Cancer-Specific Mortality | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Multivariable HR2 | 95% Confidence Interval | p-value | Multivariable HR2 | 95% Confidence Interval | p-value | |||||
| Overall Study Population | 579 | 476 | 1.31 | 1.09 | 1.58 | 0.004 | 1.40 | 1.14 | 1.71 | 0.001 |
| Sex | ||||||||||
| Male | 335 | 495 | 1.30 | 1.01 | 1.68 | 0.039 | 1.53 | 1.17 | 2.01 | 0.002 |
| Female | 244 | 468 | 1.35 | 1.02 | 1.79 | 0.039 | 1.32 | 0.97 | 1.80 | 0.081 |
| Smoking Status3 | ||||||||||
| Current Smoker | 123 | 402 | 1.1 | 0.73 | 1.65 | 0.649 | 1.15 | 0.74 | 1.79 | 0.538 |
| Former Smoker | 426 | 532 | 1.39 | 1.12 | 1.72 | 0.003 | 1.47 | 1.16 | 1.85 | 0.001 |
| Never Smoker | 30 | 384 | 0.97 | 0.38 | 2.52 | 0.957 | 0.54 | 0.16 | 1.81 | 0.318 |
| Non-Smoker | 347 | 446 | 1.45 | 1.14 | 1.84 | 0.002 | 1.51 | 1.16 | 1.95 | 0.002 |
| Histological Subtype | ||||||||||
| Adenocarcinoma | 148 | 427 | 1.17 | 0.81 | 1.67 | 0.407 | 1.24 | 0.85 | 1.81 | 0.273 |
| Squamous-cell Carcinoma | 170 | 626 | 1.14 | 0.77 | 1.68 | 0.523 | 1.24 | 0.80 | 1.93 | 0.332 |
| Small-cell Carcinoma | 81 | 395 | 0.70 | 0.40 | 1.25 | 0.231 | 0.87 | 0.49 | 1.56 | 0.655 |
| Other | 180 | 544 | 1.68 | 1.21 | 2.34 | 0.002 | 1.73 | 1.18 | 2.53 | 0.005 |
| BMI Classification | ||||||||||
| Normal weight | 304 | 445 | 1.21 | 0.91 | 1.62 | 0.193 | 1.10 | 0.81 | 1.49 | 0.539 |
| Overweight/Obese | 258 | 525 | 1.41 | 1.09 | 1.81 | 0.008 | 1.73 | 1.31 | 2.28 | <.001 |
Numbers may not sum to total due to missing data
Multivariable models adjusted for age, sex, tumor stage, tumor grade, smoking (pack years), and BMI
Current smokers were actively smoking at the time of study enrollment; non-smokers include those who had never smoked or who had quit longer than one year prior to study enrollment; former smokers include any participant who had reported quitting smoking by the time of study enrollment
The ‘other’ histological subtype included all other lung cancer histological subtypes
In sub-group analyses by sex, the significant positive association between lifetime physical inactivity and all-cause and lung cancer-specific mortality persisted among men: HR=1.30, (95%CI: 1.01–1.68) and HR=1.53, (95% CI: 1.17–2.01), respectively. Among women, we observed a significant association between lifetime physical inactivity and all-cause mortality (HR=1.35, 95% CI: 1.02–1.79), and the association for lung-cancer specific mortality was borderline significant (HR=1.32, 95% CI: 0.97–1.80) (Table 4). In further analyses by BMI status, the point estimates representing the association between lifetime physical inactivity and mortality were above null in both normal-weight and overweight/obese subgroups, but were only significant among overweight/obese subgroups for both all-cause and lung-cancer-specific mortality: HR=1.41, (95% CI:1.09–1.81) and HR= 1.73, (95% CI: 1.31–2.28), respectively (Table 4). In further analyses by histological subtype, we observed evidence of a positive association between lifetime physical inactivity and mortality for adenocarcinoma and squamous-cell carcinoma, but the association was only statistically significant in the ‘other’ subgroup: HR=1.73, (95% CI:1.18–2.53) (Table 4). In stratified analyses by disease stage, we observed a significant positive association of lifetime physical inactivity with local/regional disease (HR=1.73, 95% HR: 1.32–2.26), but the association did not reach statistical significance for distant disease (HR=1.13, 95% CI: 0.90–1.49). Lastly, we observed no considerable differences in hazard ratios between treatment groups in stratified analyses by chemotherapy, surgery, or radiation therapy status (data not shown).
DISCUSSION
In this epidemiological investigation, we observed significant positive associations between lifetime recreational physical inactivity and lung cancer risk and mortality. Our analyses provided consistent evidence of a positive, significant association of lifetime physical inactivity with lung cancer risk in men and women, normal-weight and overweight/obese subgroups, and among each of the lung cancer histological subgroups examined herein. We also observed consistent evidence of an association between lifetime physical inactivity and overall survival and lung-cancer specific survival in the total population of lung cancer cases. To our knowledge, no previous studies have systematically examined lifetime physical inactivity as an independent exposure of interest relative to both lung cancer risk and mortality. However, the most recent meta-analysis summarizing the associations of incrementally higher levels of physical activity exposure with lung cancer risk from 28 epidemiological studies reported a 24% decreased risk of lung cancer among the most physically active individuals, but the protective effects of physical activity were not seen among never smokers [2]. Herein, we observed a significant positive association between lifetime physical inactivity and lung cancer risk among never smokers and non-smokers who had quit at least one year prior to study enrollment, but the association was only borderline significant among current smokers. We also observed a significant positive association between lifetime physical inactivity and lung cancer risk among female non-smokers, a population in which lung cancer incidence has been increasing.
Only four previous studies have examined associations of varying quantities of physical activity exposure with lung cancer risk among never-smokers, and the evidence has been inconclusive [2], suggesting that previously reported associations between physical activity and lung cancer risk in smokers may be the result of confounding by smoking. That is, individuals who participate in regular, weekly recreational physical activity may be less likely to be heavy smokers, while regular smokers may be more likely to be physically inactive because of a clustering of lifestyle patterns or because of impaired lung function associated with their smoking behavior [2, 19]. Importantly, in the current analyses, we uncovered two sources of evidence suggesting that the observed association between lifetime physical inactivity and lung cancer risk cannot be entirely explained due to confounding by smoking. First, we observed a significant association between lifetime physical inactivity and lung cancer risk in never smokers. Second, we observed evidence of a positive additive interaction among physically inactive smokers, suggesting a potential synergistic effect between smoking and physical inactivity. Thus, not only did our data suggest that lifetime physical inactivity is associated with an increased risk of lung cancer among never smokers; lifetime physical inactivity also contributed to considerably elevated risks in current and ever-smokers.
Similarly, it is commonly reported that observed associations between physical activity (or inactivity) and cancer endpoints are likely confounded by overweight and/or obesity. However, recently emerging epidemiological evidence suggests that the associations between physical activity (or inactivity) and cancer endpoints are, at least in part, independent of obesity [9, 13, 20, 21]. Our findings are consistent with these data in that obesity is not an established risk factor for lung cancer, and we observed evidence of a robust association between lifetime physical inactivity and lung cancer risk which was consistently seen among both normal-weight and overweight/obese participants.
In addition to our findings of a positive association between lifetime physical inactivity and lung cancer risk, we also observed significant positive associations between pre-diagnostic lifetime physical inactivity and all-cause mortality and lung cancer-specific mortality in the overall study population. These findings are consistent with one additional study that reported a positive association between post-diagnostic self-reported lifetime physical inactivity and lung cancer mortality (HR=1.29, 95% CI: 1.003–1.669, p=0.047) [22]. Four additional studies have evaluated the associations between incrementally higher amounts of pre-diagnostic physical activity exposure and lung cancer outcomes, and each were suggestive of an inverse association between physical activity and mortality [4, 23–25]. Alfano et al. reported a significant inverse association between the highest level of physical activity and lung cancer mortality among women, but not among men [23]. An additional study observed a significant inverse association between the highest level of physical activity and lung cancer mortality when men and women were considered jointly (0.84; 0.77–0.92; p-trend <0.001) [24]. A third study of men demonstrated that cardiorespiratory fitness was inversely associated with lung cancer mortality, but only among current and former smokers [25]. Most recently, Wang and colleagues reported a significant trend between increasing levels of physical activity and decreased lung cancer mortality in post-menopausal women for low activity (HR= 0.80, 95% CI: 0.69–0.92), medium activity (HR=0.68, 95% CI: 0.59–0.80), and high activity (HR=0.78, 95% CI: 0.66–0.93) [4].
The biologic pathways specifically linking increased levels of physical activity with decreased risk of lung cancer have been previously summarized [2] and may involve increases in pulmonary function, which likely reduces the duration of exposure to carcinogenic agents in the lungs [2]. Further, regular physical activity has been shown to attenuate the decline in forced expiratory volume (FEV) and forced vital capacity (FVC), which are commonly seen in current and former smokers [26]. Additional mechanisms accounting for an association between recreational physical inactivity and lung cancer risk may include, but are not limited to, chronic inflammation, impaired immune surveillance and responsiveness, increased free-radical production, and impaired DNA repair capacity [2, 27, 28].
An important strength of the current analysis is that we were able to examine the potential confounding role of a number of important epidemiological risk and prognostic factors that may be associated with lung cancer risk and survival, as well as other factors that tend to parallel physical activity (or inactivity) in lifestyle patterns. Further, our use of two inactivity exposures (i.e., self-reported inactivity spanning all of the years of adulthood prior to study enrollment and self-reported inactivity spanning two decades prior to study enrollment), decreases the likelihood that the observations reported herein were due to a reverse causation bias. That is, in order to be categorized as physically inactive in any of the analyses, respondents had to report never engaging in any regular, weekly, recreational physical activity throughout adulthood and/or throughout the two decades prior to study enrollment. Additional strengths of our survival analyses include the long duration of follow-up and our ability to examine the association of lifetime physical inactivity with lung cancer-specific mortality while adjusting for important prognostic variables.
Conversely, as previously described [20] the potential measurement error associated with self-reported physical inactivity data categorized dichotomously is an important limitation of the current work. However, there is a body of literature suggesting that simplified activity questionnaires, including binary categorization of physical inactivity behavior, is a valid method for identifying the most physically inactive individuals in a population [29–35]. However, we also recognize that the validity of simplified questionnaires may be optimized for measurement of “current” activity rather than self-reported lifetime activity. However, the self-reported lifetime physical inactivity prevalence reported herein among cases (68%) and controls (50%) was well within the range of other self-reported population estimates suggesting that 50–79% of Americans are insufficiently active [8].
We also recognize that a referent group broadly defined as active could result in misclassification among individuals with lower, compared to higher, activity levels and that our approach also precludes the ability to examine a dose-response association between lung cancer endpoints and physical activity exposure [20]. However, reliance upon binary classification of physically inactive versus active participants likely underestimates the true ORs and HRs associated with inactivity because the referent group is not restricted to the most active participants. Importantly, the aim of the current analyses was to study the most physically inactive segment of the population by examining lifetime physical inactivity as the independent exposure of interest. Further, given that controls were also seen at a hospital and diagnosed with non-neoplastic conditions that could affect activity level, or the recall of activity, in a manner similar to cases, the potential for differential exposure misclassification is likely reduced. Therefore, if a biased measure of association were to occur, it most likely would be conservative and non-differential according to case-control status, resulting in an underestimation of the associations of lifetime physical inactivity with lung cancer risk. Similarly, although not likely a concern in the current survival analyses, non-differential misclassification according to vital status would also result in attenuation of observed hazard ratios estimating the association between lifetime physical inactivity and lung cancer mortality.
The current findings are also limited because we did not consider physical inactivity in other behavioral domains (i.e., occupational, household, etc.); nor can we account for explicitly sedentary behavior (i.e., hours of sitting) in these analyses, a distinct behavioral construct which is likely associated with cancer endpoints via similar physiological pathways as recreational physical inactivity. Survival analyses were also limited by the use of pre-diagnostic self-reported physical inactivity levels, which prevented us from assessing changes in physical activity that may have occurred after diagnosis. While data in the existing literature depicting the association between post-diagnostic physical activity and lung cancer survival are scant, there are data to suggest that post-diagnosis physical activity interventions designed for lung cancer patients and survivors are feasible, and may be associated with improved quality of life [36–42].
Furthermore, a limited number of events among never-smokers may have impaired our ability to detect an association between lifetime physical inactivity and lung cancer mortality among never-smokers. To combat this limitation, we adopted a similar approach as described in previously published reports and combined never-smokers and former smokers into a “non-smoking” group [2, 43, 44]. However, we limited former smokers to only those who had quit at least one year prior to study enrollment. While there are no previously published reports describing the association between lifetime physical inactivity and lung cancer risk in never-smokers, four previous studies of physical activity and lung cancer risk have yielded inconclusive results [2]. Thus, additional research of the associations between (in)activity and lung cancer endpoints among larger populations of never smokers will be an important area of focus to help rule out the confounding effects of cigarette smoking [2].
Our findings may also be limited by low response rates and the recruitment methods inherent in this hospital-based case-control study. For example, the identification of participants with non-neoplastic diagnoses as controls could have resulted in a higher prevalence of unhealthier, physically inactive individuals in the control group than are present in the overall population. Importantly, if control participants reported a higher prevalence of recreational physical inactivity than the general population, our observed risk estimates would be attenuated toward the null. Furthermore, we cannot account for the potential effects of a survivor bias, nor can we account for any temporal changes in the staging, classification and/or the treatment of lung cancer since the time of data collection.
Lastly, the potential for residual confounding and other non-causal explanations for the statistically significant observed associations between lifetime physical inactivity and lung cancer risk and mortality cannot be ruled out [2]. For example, smoking status and pack-years were based on self-report data and no biomarkers of tobacco use were available in the current analysis. Furthermore, other unmeasured factors that may be associated with both physical inactivity and smoking could also be a source of residual confounding. Healthier lifestyles associated with regular recreational physical activity may also be associated with healthier diets, including increased fruit and vegetable consumption, and it has been well-established that diets high in fruits and vegetables are inversely associated with lung cancer risk [45, 46]. Further, it is possible that more active individuals have increased exposure to vitamin D [47, 48], and vitamin D directly correlates with FEV in chronic obstructive pulmonary disorder (COPD) patients [49, 50] and is also directly associated with improved survival in early-stage lung cancer patients [51].
CONCLUSION
Our primary findings suggest that pre-diagnostic lifetime recreational physical inactivity is significantly and positively associated with lung cancer risk and mortality in the overall study population. Given that lung cancer is one of the most common and deadliest cancers in the United States, and given the persistence of recreational physical inactivity at the population level, replicating these findings in further investigations could be of significant public health importance. If the observed associations of lifetime recreational physical inactivity with lung cancer risk and mortality are corroborated in future reports, then additional intervention-based research should aim to address whether the associations of physical inactivity with lung cancer endpoints reflects a causal pattern, and if so, to further characterize the dose of physical activity necessary to improve lung cancer outcomes and decrease lung cancer risk.
CLINICAL PRACTICE POINTS
Mounting evidence has demonstrated the safety, efficacy and feasibility of exercise interventions designed to improve the quality of life and attenuate treatment-related toxicities among cancer patients. Recreational physical activity, including targeted exercise interventions designed for lung cancer patients and survivors, has been shown to reduce sleep disturbance, fatigue, depression, and anxiety, while improving health-related fitness and physical function. Importantly, initiating exercise rehabilitation prior to treatment can decrease the length of hospitalization and post-surgery treatment complications among lung cancer patients. The results of the current study, in combination with mounting evidence demonstrating the safety and efficacy of exercise for lung cancer patients throughout the cancer care continuum [36–42], suggest that physical activity counseling is an important aspect of lung cancer care, even among patients with metastatic disease. Clinical teams should be encouraged to develop services and community collaborations which promote and incorporate physical activity as part of a multi-disciplinary cancer care program. Further, well-planned randomized clinical exercise trials designed to examine the effects of supervised exercise on lung cancer clinical outcomes are also warranted.
Acknowledgments
K.B. Moysich was supported by New York State Department of Health (NYS DOH C019286)
Epidemiological and follow-up data were provided, in part, by Roswell Park Comprehensive Cancer Center shared resources, which are funded by the National Cancer Institute Cancer Center Support Grant (NIH P30 CA16056)
Abbreviations Used
- OR
odds ratio
- HR
hazard ratio
- CI
confidence intervals
- RPCI
Roswell Park Cancer Institute
- PEDS
Patient Epidemiology Data System
- BMI
body mass index
- RERI
Relative Excess Risk due to Interaction
- FEV
Forced Expiratory Volume
- FVC
Forced Vital Capacity
Footnotes
Conflicts of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Cancer Society. Cancer Facts & Figures 2017. Atlanta: American Cancer Society; 2017. [cited 2018]. Available from: http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-047079.pdf. [Google Scholar]
- 2.Brenner DR, Yannitsos DH, Farris MS, Johansson M, Friedenreich CM. Leisure-time physical activity and lung cancer risk: A systematic review and meta-analysis. Lung cancer (Amsterdam, Netherlands) 2016;95:17–27. doi: 10.1016/j.lungcan.2016.01.021. Epub 2016/04/05. [DOI] [PubMed] [Google Scholar]
- 3.Sun S, Schiller JH, Gazdar AF. Lung cancer in never smokers--a different disease. Nature reviews Cancer. 2007;7(10):778–90. doi: 10.1038/nrc2190. Epub 2007/09/21. [DOI] [PubMed] [Google Scholar]
- 4.Wang A, Qin F, Hedlin H, Desai M, Chlebowski R, Gomez S, et al. Physical activity and sedentary behavior in relation to lung cancer incidence and mortality in older women: The Women’s Health Initiative. International journal of cancer Journal international du cancer. 2016 doi: 10.1002/ijc.30281. Epub 2016/07/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.ACS. Lung Cancer Prevention and Early Detection. [updated 2015 February 62015 June 25]. Available from: http://www.cancer.org/cancer/lungcancer-non-smallcell/moreinformation/lungcancerpreventionandearlydetection/lung-cancer-prevention-and-early-detection-risk-factors.
- 6.NCI. Lung Cancer Prevention-for health professionals (PDQ®) [updated 2015 February 62015 June 25]. Available from: http://www.cancer.gov/types/lung/hp/lung-prevention-pdq#link/_172_toc.
- 7.USDHHS. 2008 Physical Activity Guidelines for Americans. Washington, D.C: Office of Disease Prevention and Health Promotion; 2008. [Google Scholar]
- 8.USDHHS. Prevalence and Trends Data: Nationwide Physical Activity - 2013 Office of Surveillance, Epidemiology and Laboratory Services. US Department of Health and Human Services, Centers for Disease Control and Prevention; 2014. [cited 2014 November 1, 2014] [Google Scholar]
- 9.Sanchis-Gomar F, Lucia A, Yvert T, Ruiz-Casado A, Pareja-Galeano H, Santos-Lozano A, et al. Physical inactivity and low fitness deserve more attention to alter cancer risk and prognosis. Cancer prevention research (Philadelphia, Pa) 2015;8(2):105–10. doi: 10.1158/1940-6207.capr-14-0320. Epub 2014/11/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.LaMonte MJ, Buchner DM, Rillamas-Sun E, Di C, Evenson KR, Bellettiere J, et al. Accelerometer-Measured Physical Activity and Mortality in Women Aged 63 to 99. Journal of the American Geriatrics Society. 2017 doi: 10.1111/jgs.15201. Epub 2017/11/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bull FC, Armstrong TP, Dixon T, Ham S, Neiman A, Pratt M. WHO, editor. Comparitive Quantification of Health Risks: Global and Regional Burden of Disease Attributable to selected Major Risk Factors. Vol. 1. WHO; 2004. Physical Inactivity; pp. 729–881. [Google Scholar]
- 12.Celis-Morales CA, Perez-Bravo F, Ibanez L, Salas C, Bailey ME, Gill JM. Objective vs. self-reported physical activity and sedentary time: effects of measurement method on relationships with risk biomarkers. PloS one. 2012;7(5):e36345. doi: 10.1371/journal.pone.0036345. Epub 2012/05/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Byers T. Physical activity and gastric cancer: so what? An epidemiologist’s confession. Cancer prevention research (Philadelphia, Pa) 2014;7(1):9–11. doi: 10.1158/1940-6207.capr-13-0400. Epub 2013/12/19. [DOI] [PubMed] [Google Scholar]
- 14.Mowls DS, Brame LS, Martinez SA, Beebe LA. Lifestyle behaviors among US cancer survivors. Journal of cancer survivorship: research and practice. 2016 doi: 10.1007/s11764-016-0515-x. Epub 2016/01/29. [DOI] [PubMed] [Google Scholar]
- 15.Friel G, Liu CS, Kolomeyevskaya NV, Hampras SS, Kruszka B, Schmitt K, et al. Aspirin and Acetaminophen Use and the Risk of Cervical Cancer. Journal of lower genital tract disease. 2015;19(3):189–93. doi: 10.1097/lgt.0000000000000104. Epub 2015/04/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kolomeyevskaya NV, Szender JB, Zirpoli G, Minlikeeva A, Friel G, Cannioto RA, et al. Oral Contraceptive Use and Reproductive Characteristics Affect Survival in Patients With Epithelial Ovarian Cancer: A Cohort Study. International journal of gynecological cancer: official journal of the International Gynecological Cancer Society. 2015;25(9):1587–92. doi: 10.1097/igc.0000000000000540. Epub 2015/08/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCann SE, Moysich KB, Mettlin C. Intakes of selected nutrients and food groups and risk of ovarian cancer. Nutrition and cancer. 2001;39(1):19–28. doi: 10.1207/S15327914nc391_3. Epub 2001/10/09. [DOI] [PubMed] [Google Scholar]
- 18.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. American journal of epidemiology. 1993;138(11):923–36. doi: 10.1093/oxfordjournals.aje.a116813. Epub 1993/12/01. [DOI] [PubMed] [Google Scholar]
- 19.Brenner DR. Cancer incidence due to excess body weight and leisure-time physical inactivity in Canada: implications for prevention. Preventive medicine. 2014;66:131–9. doi: 10.1016/j.ypmed.2014.06.018. Epub 2014/06/27. [DOI] [PubMed] [Google Scholar]
- 20.Cannioto R, LaMonte MJ, Risch HA, Hong CC, Sucheston-Campbell LE, Eng KH, et al. Chronic Recreational Physical Inactivity and Epithelial Ovarian Cancer Risk: Evidence from the Ovarian Cancer Association Consortium. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2016 doi: 10.1158/1055-9965.epi-15-1330. Epub 2016/05/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cannioto RA, LaMonte MJ, Kelemen LE, Risch HA, Eng KH, Minlikeeva AN, et al. Recreational physical inactivity and mortality in women with invasive epithelial ovarian cancer: evidence from the Ovarian Cancer Association Consortium. British journal of cancer. 2016;115(1):95–101. doi: 10.1038/bjc.2016.153. Epub 2016/06/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sloan JA, Cheville AL, Liu H, Novotny PJ, Wampfler JA, Garces YI, et al. Impact of self-reported physical activity and health promotion behaviors on lung cancer survivorship. Health and quality of life outcomes. 2016;14:66. doi: 10.1186/s12955-016-0461-3. Epub 2016/05/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alfano CM, Klesges RC, Murray DM, Bowen DJ, McTiernan A, Vander Weg MW, et al. Physical activity in relation to all-site and lung cancer incidence and mortality in current and former smokers. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2004;13(12):2233–41. Epub 2004/12/16. [PubMed] [Google Scholar]
- 24.Arem H, Moore SC, Park Y, Ballard-Barbash R, Hollenbeck A, Leitzmann M, et al. Physical activity and cancer-specific mortality in the NIH-AARP Diet and Health Study cohort. International journal of cancer Journal international du cancer. 2014;135(2):423–31. doi: 10.1002/ijc.28659. Epub 2013/12/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sui X, Lee DC, Matthews CE, Adams SA, Hebert JR, Church TS, et al. Influence of cardiorespiratory fitness on lung cancer mortality. Medicine and science in sports and exercise. 2010;42(5):872–8. doi: 10.1249/MSS.0b013e3181c47b65. Epub 2009/12/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garcia-Aymerich J, Lange P, Benet M, Schnohr P, Anto JM. Regular physical activity modifies smoking-related lung function decline and reduces risk of chronic obstructive pulmonary disease: a population-based cohort study. American journal of respiratory and critical care medicine. 2007;175(5):458–63. doi: 10.1164/rccm.200607-896OC. Epub 2006/12/13. [DOI] [PubMed] [Google Scholar]
- 27.McTiernan A. Mechanisms linking physical activity with cancer. Nature reviews Cancer. 2008;8(3):205–11. doi: 10.1038/nrc2325. Epub 2008/02/01. [DOI] [PubMed] [Google Scholar]
- 28.Walsh NP, Gleeson M, Shephard RJ, Gleeson M, Woods JA, Bishop NC, et al. Position statement. Part one: Immune function and exercise. Exercise immunology review. 2011;17:6–63. Epub 2011/03/31. [PubMed] [Google Scholar]
- 29.Li S, Carlson E, Holm K. Validation of a single-item measure of usual physical activity. Perceptual and motor skills. 2000;91(2):593–602. doi: 10.2466/pms.2000.91.2.593. Epub 2000/11/07. [DOI] [PubMed] [Google Scholar]
- 30.Milton K, Bull FC, Bauman A. Reliability and validity testing of a single-item physical activity measure. British journal of sports medicine. 2011;45(3):203–8. doi: 10.1136/bjsm.2009.068395. Epub 2010/05/21. [DOI] [PubMed] [Google Scholar]
- 31.Rose S, Elley CR, Lawton BA, Dowell AC. A single question reliably identifies physically inactive women in primary care. The New Zealand Medical Journal. 2008;121(1268) [PubMed] [Google Scholar]
- 32.Schechtman KB, Barzilai B, Rost K, Fisher EB., Jr Measuring physical activity with a single question. American journal of public health. 1991;81(6):771–3. doi: 10.2105/ajph.81.6.771. Epub 1991/06/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siconolfi SF, Lasater TM, Snow RC, Carleton RA. Self-reported physical activity compared with maximal oxygen uptake. American journal of epidemiology. 1985;122(1):101–5. doi: 10.1093/oxfordjournals.aje.a114068. Epub 1985/07/01. [DOI] [PubMed] [Google Scholar]
- 34.Smith BJ, Marshall AL, Huang N. Screening for physical activity in family practice: evaluation of two brief assessment tools. American journal of preventive medicine. 2005;29(4):256–64. doi: 10.1016/j.amepre.2005.07.005. Epub 2005/10/26. [DOI] [PubMed] [Google Scholar]
- 35.Weiss TW, Slater CH, Green LW, Kennedy VC, Albright DL, Wun CC. The validity of single-item, self-assessment questions as measures of adult physical activity. Journal of clinical epidemiology. 1990;43(11):1123–9. doi: 10.1016/0895-4356(90)90013-f. Epub 1990/01/01. [DOI] [PubMed] [Google Scholar]
- 36.Andersen AH, Vinther A, Poulsen LL, Mellemgaard A. A modified exercise protocol may promote continuance of exercise after the intervention in lung cancer patients--a pragmatic uncontrolled trial. Supportive care in cancer: official journal of the Multinational Association of Supportive Care in Cancer. 2013;21(8):2247–53. doi: 10.1007/s00520-013-1781-z. Epub 2013/03/20. [DOI] [PubMed] [Google Scholar]
- 37.Chen HM, Wu YC, Tsai CM, Tzeng JI, Lin CC. Relationships of Circadian Rhythms and Physical Activity With Objective Sleep Parameters in Lung Cancer Patients. Cancer nursing. 2015;38(3):215–23. doi: 10.1097/ncc.0000000000000163. Epub 2014/06/20. [DOI] [PubMed] [Google Scholar]
- 38.Dhillon HM, van der Ploeg HP, Bell ML, Boyer M, Clarke S, Vardy J. The impact of physical activity on fatigue and quality of life in lung cancer patients: a randomised controlled trial protocol. BMC cancer. 2012;12:572. doi: 10.1186/1471-2407-12-572. Epub 2012/12/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Henke CC, Cabri J, Fricke L, Pankow W, Kandilakis G, Feyer PC, et al. Strength and endurance training in the treatment of lung cancer patients in stages IIIA/IIIB/IV. Supportive care in cancer: official journal of the Multinational Association of Supportive Care in Cancer. 2014;22(1):95–101. doi: 10.1007/s00520-013-1925-1. Epub 2013/09/03. [DOI] [PubMed] [Google Scholar]
- 40.Jensen W, Oechsle K, Baumann HJ, Mehnert A, Klose H, Bloch W, et al. Effects of exercise training programs on physical performance and quality of life in patients with metastatic lung cancer undergoing palliative chemotherapy--a study protocol. Contemporary clinical trials. 2014;37(1):120–8. doi: 10.1016/j.cct.2013.11.013. Epub 2013/12/10. [DOI] [PubMed] [Google Scholar]
- 41.Missel M, Pedersen JH, Hendriksen C, Tewes M, Adamsen L. Exercise intervention for patients diagnosed with operable non-small cell lung cancer: a qualitative longitudinal feasibility study. Supportive care in cancer: official journal of the Multinational Association of Supportive Care in Cancer. 2015;23(8):2311–8. doi: 10.1007/s00520-014-2579-3. Epub 2015/01/13. [DOI] [PubMed] [Google Scholar]
- 42.Wiskemann J, Hummler S, Diepold C, Keil M, Abel U, Steindorf K, et al. POSITIVE study: physical exercise program in non-operable lung cancer patients undergoing palliative treatment. BMC cancer. 2016;16(1):499. doi: 10.1186/s12885-016-2561-1. Epub 2016/07/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sprague BL, Trentham-Dietz A, Klein BE, Klein R, Cruickshanks KJ, Lee KE, et al. Physical activity, white blood cell count, and lung cancer risk in a prospective cohort study. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2008;17(10):2714–22. doi: 10.1158/1055-9965.epi-08-0042. Epub 2008/10/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yun YH, Lim MK, Won YJ, Park SM, Chang YJ, Oh SW, et al. Dietary preference, physical activity, and cancer risk in men: national health insurance corporation study. BMC cancer. 2008;8:366. doi: 10.1186/1471-2407-8-366. Epub 2008/12/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y, Li F, Wang Z, Qiu T, Shen Y, Wang M. Fruit and vegetable consumption and risk of lung cancer: a dose-response meta-analysis of prospective cohort studies. Lung cancer (Amsterdam, Netherlands) 2015;88(2):124–30. doi: 10.1016/j.lungcan.2015.02.015. Epub 2015/03/10. [DOI] [PubMed] [Google Scholar]
- 46.Kubik A, Zatloukal P, Tomasek L, Pauk N, Havel L, Dolezal J, et al. Interactions between smoking and other exposures associated with lung cancer risk in women: diet and physical activity. Neoplasma. 2007;54(1):83–8. Epub 2007/01/06. [PubMed] [Google Scholar]
- 47.CHOMISTEK AK, CHIUVE SE, JENSEN MK, COOK NR, RIMM EB. Vigorous Physical Activity, Mediating Biomarkers, and Risk of Myocardial Infarction. Medicine & Science in Sports & Exercise. 2011;43(10):1884–90. doi: 10.1249/MSS.0b013e31821b4d0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Giovannucci E, Liu Y, Rimm EB, Hollis BW, Fuchs CS, Stampfer MJ, et al. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. Journal of the National Cancer Institute. 2006;98(7):451–9. doi: 10.1093/jnci/djj101. Epub 2006/04/06. [DOI] [PubMed] [Google Scholar]
- 49.Monadi M, Heidari B, Asgharpour M, Firouzjahi A, Monadi M, Ghazi Mirsaied MA. Relationship between serum vitamin D and forced expiratory volume in patients with chronic obstructive pulmonary disease (COPD) Caspian journal of internal medicine. 2012;3(3):451–5. Epub 2012/07/01. [PMC free article] [PubMed] [Google Scholar]
- 50.Janssens W, Bouillon R, Claes B, Carremans C, Lehouck A, Buysschaert I, et al. Vitamin D deficiency is highly prevalent in COPD and correlates with variants in the vitamin D-binding gene. Thorax. 2010;65(3):215–20. doi: 10.1136/thx.2009.120659. Epub 2009/12/10. [DOI] [PubMed] [Google Scholar]
- 51.Zhou W, Suk R, Liu G, Park S, Neuberg DS, Wain JC, et al. Vitamin D is associated with improved survival in early-stage non-small cell lung cancer patients. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2005;14(10):2303–9. doi: 10.1158/1055-9965.epi-05-0335. Epub 2005/10/11. [DOI] [PubMed] [Google Scholar]