Abstract
Objective
To further refine a measure of self-management, the Responsibility and Familiarity with Illness Survey (REFILS), and to determine if this score predicts medication adherence and thus fewer instances of allograft rejection among pediatric liver transplant recipients.
Study design
Participants were 400 liver transplant recipients and their parents recruited for the Medication Adherence in Children Who Had a Liver Transplant (MALT) study, from five United States pediatric transplant centers. The REFILS was administered to participants (ages 9–17) and their parents at enrollment (N=213 completed dyads). The REFILS scores, and a discrepancy score calculated between patient and parent report of the patient’s self-management, were used to predict Medication Level Variability Index (MLVI), a measure of medication adherence (higher MLVI = more variability in medication levels) and central pathologist-diagnosed rejection over a two-year follow-up.
Results
When patients reported greater self-management, their adherence was lower (higher MLVI, r = .26, P <.01). Discrepancies between patient and parent report (patients endorsing higher levels than parents) were associated with lower adherence (r = .20, P <.01). Greater patient reported self-management and higher discrepancy scores also predicted rejection.
Conclusions
We found that when patients endorse more responsibility for their care, clinical outcomes are worse, indicating that indiscriminate promotion of self-management by adolescents may not be advisable. A discrepancy between patient and parent perception of self-management emerged as a novel strategy to gauge the degree of risk involved in transitioning care responsibilities to the child.
Keywords: self-management, transition, adherence, pediatric transplant
Findings suggest that patients struggle during the “transition” to the adult health care system. Increased rates of non-adherence have been observed during transition, and the period has been shown to be associated with poorer clinical outcomes and increased mortality.1,2 It is therefore important to be able to assess self-management during transition.
It is largely unknown whether self-management skills are associated with clinical outcomes like medication adherence, although the tacit assumption is that they are. If some adolescents are not ready for transition, prematurely forcing self-management might lead to poorer, not better, outcomes. This seems to resonate with the state of affairs for pediatric self-management; there are many different approaches being implemented for its evaluation but a lack of data on how self-management translates into clinical outcomes.3
There are different approaches to the measurement of self-management acquisition. One is measuring allocation of responsibility, or how health care management tasks are divided between patients and their parents.4–8 Previous work in pediatric transplant has suggested that greater self-management, as measured by allocation of responsibility, is associated with poorer medication adherence among adolescent/young adult liver transplant recipients.4,9 A second approach is to calculate a numeric score or “level” of patient health care management skills.10–12 This level can be monitored over time to determine if self-management acquisition is increasing.
As patients transition from pediatrics, perhaps the most salient concern is medical instability related to non-adherence and faulty self-management acquisition. We have previously reported preliminary reliability and validity information for a checklist of skills, called the Responsibility and Familiarity with Illness Survey (REFILS).11 We have used this tool to track self-management when patients transition from pediatric to adult clinics.14 However, development of this measure, like others, has been limited by single-center data collection, a less than adequate sample size for demonstrating psychometric properties15, and a lack of robust correlation with medical outcomes.
This multisite, prospective cohort study aimed to further develop the REFILS through multi-site collection, analysis of technical adequacy and correlating it with medical outcomes. We furthermore evaluated whether REFILS score are associated with non-adherence to medications as well as with allograft rejection, in pediatric liver transplant recipients. Two different approaches to scoring the measure were employed: a cumulative score, and a “discrepancy” score, calculating the degree to which patients report greater self-management than concurrent parent report of their self-management.
Methods
Participants were enrolled in the Medication Adherence in Children Who Had a Liver Transplant (MALT) cohort.16 This multisite prospective trial recruited 400 children or adolescents ages 1–17 and their families from five pediatric liver transplant centers in the United States (Cincinnati Children’s Hospital Medical Center; Mattell Children’s Hospital, UCLA; Ann & Robert H. Lurie Children’s Hospital of Chicago; Children’s Hospital of Pittsburgh of UPMC; and Mount Sinai Medical Center, New York) and followed them each for 2 years.
The study was approved by the respective Institutional Review Boards and involved parent/caregiver consent and child assent. At their enrollment visit, parents and patients were asked to complete a brief questionnaire assessing possible predictors of non-adherence (described below) as well as the REFILS, to capture self-management level and to examine its predictive validity. In the event that more than one parent attended the enrollment visit, families were asked to choose one respondent. In addition, patient medical variables and outcomes were followed for a 2-year period (ending in June 2015). Quarterly chart reviews were conducted during which time all tacrolimus values were obtained in order to characterize adherence (as described below). Data were sent via a secure web-based interface to a data-coordinating center (The EMMES Corporation, Rockville, Maryland).
The MALT study included a brief psychosocial assessment aimed at measuring self-management and possible predictors of nonadherence. In the present study, for further validation of the REFILS, measures assessing barriers to adherence17 were included.
The Responsibility and Familiarity with Illness Survey (REFILS)
Originally, the REFILS consisted of 22-items,11 drawing on the work of Vessey and Miola,18 but to decrease participant burden it was shortened to 13 items for this study based on factor analyses; there are companion patient and parent versions. Similar to other questionnaires that investigate responsibility for health care in children and adolescents7,8, the REFILS taps into 2 domains: perceived knowledge about the illness and responsibility for medical management. Patients and their parent are asked to choose from three options, “Never,” “Sometimes,” or “Always,” indicating how often the patient engages in the behavior listed, scored on a scale from 1–3 respectively. We calculated the total score from 13 items with possible scores ranging from 13 to 36. Higher scores therefore indicate greater self-management and lower scores may indicate that either parents or no one is overseeing the task. The REFILS was administered to dyads when the patient was age 9 and older (corresponding to just before “young teens” as per Centers for Disease Control definitions).19 Additionally, a “discrepancy” score was calculated; this score is the difference between patient and parent report of the patient’s self-management level. Higher discrepancy scores indicate not only greater disagreement between patients and parents, but also that patients rated their self-management higher than parents.
Adolescent Medication Barriers Scale (AMBS) & Parent Medication Barriers Scale (PMBS)
The AMBS/PMBS17 are scales designed to assess parent/patient perceived barriers to child medication adherence. These companion measures consist of 17 (AMBS) or 16-items (PMBS). Each item is rated on a 5-point Likert-like scale from ‘strongly disagree’ to ‘strongly agree.’ Reliability and validity have been established with Cronbach’s alphas of .86 and .87 respectively as well as factor analyses supporting the composition of items.17
Chart Review
The MLVI20 is defined as the degree of variation in blood levels of tacrolimus, the primary immunosuppressive medication used to prevent allograft rejection in liver transplant recipients. Measurement of trough blood levels of tacrolimus was standard practice in participating centers and was obtained approximately once every three months. The MLVI is calculated as the standard deviation of at least 3 consecutive tacrolimus trough blood levels for each patient. A higher MLVI denotes more fluctuation in levels. MLVI also was treated as a dichotomy (a predefined value greater than 2.5 units was considered to denote clinically significant nonadherence based on previous data). A higher MLVI was a significant predictor of future rejection in MALT16 and other cohorts.20,21
The primary clinical outcome measure in the MALT study was biopsy-defined rejection, as determined based on two independent readings in a central pathology laboratory; if the pathologists disagreed, the case was adjudicated by the senior study pathologist.16 For each participant, if there was at least 1 biopsy-proven episode of rejection during the study period, it was entered as a positive value (positive rejection). Thus, even if a participant had more than one rejection, it was counted as one event for the primary analysis (yes/no rejection occurring during the follow-up period, regardless of the number of rejection episodes).
Statistical Analyses
Reliability of the REFILS was examined using Cronbach alpha to measure internal consistency and Kappa coefficients and intra-class correlations were used to determine inter-rater reliability. For validation of the measure, factor analysis with orthogonal rotation (varimax) was utilized to identify factor loadings. Pearson correlation coefficients were used to test correlations between numeric assessment scores. Chi-square tests were used to test differences in distribution for categorical variables. Non-parametric Kruskal Wallis tests were used to determine the difference between two or more groups for continuous variables. In order to compare the distributions of the REFILS patient and parent reports, non-parametric Wilcoxon sign rank test was performed to compare the continuous total scores, and marginal homogeneity tests were implemented for nominal assessment outcomes with more than 2 categories. Statistical analyses were performed using the SAS System for Windows 9.3 (SAS Institute Inc, Cary, North Carolina).
Finally, consistent with the primary MALT analyses, the sample was divided into pre-adolescent (1–12 years old, but in this study only patients age 9–12 completed the REFILS) and adolescent patients (13–17 years old) because of the known higher prevalence of non-adherence among adolescent transplant recipients,21 which coincides with shifts in self-management germane to the present analyses.
Results
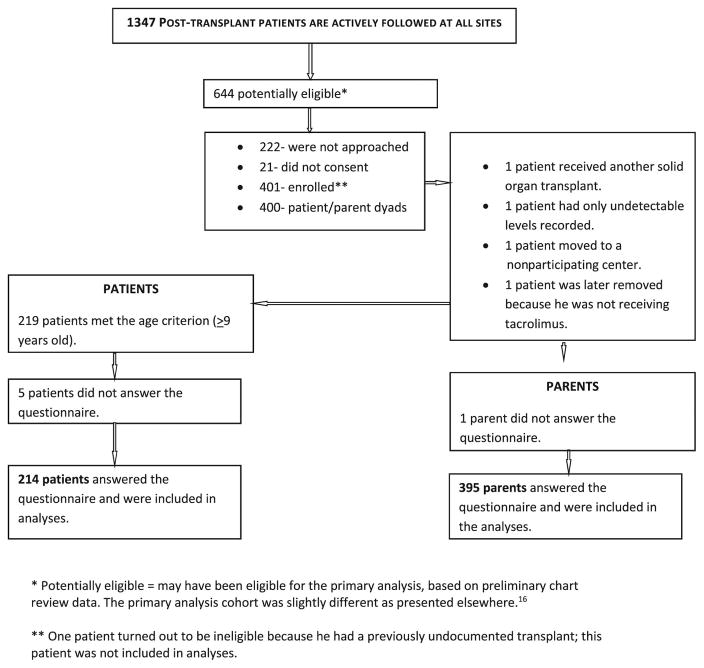
The MALT centers enrolled 400 patients; of these 395 (99%) parents and 214/219 patient participants age 9 and older (98%) completed the REFILS as shown in Figure 1 (available at www.jpeds.com). Table I (available at www.jpeds.com) depicts characteristics of the study sample. As shown, there were 213 patient-parent dyads with completed REFILS.
Figure I.
Consort Diagram
Table I.
Demographic Characteristics of Patient-Parent Dyads
| Participants with REFILS Patient and Parent Score | ||
|---|---|---|
| N | % | |
| Total | 213 | 100.0 |
| Age at Baseline | 106 | 49.8 |
| 9–12 years | ||
| ≥13 years | 107 | 50.2 |
| Gender of participant | 101 | 47.4 |
| Male | ||
| Female | 112 | 52.6 |
| Race of participant | 11 | 5.2 |
| Missing | ||
| Asian | 11 | 5.2 |
| Black or African American | 28 | 13.1 |
| White or Caucasian | 145 | 68.1 |
| Other | 18 | 8.5 |
| Ethnicity of participant | 4 | 1.9 |
| Missing | ||
| Hispanic or Latino | 51 | 23.9 |
| Not Hispanic or Latino | 158 | 74.2 |
| Type of insurance | 12 | 5.6 |
| Uninsured, Donation, or Other | ||
| Public insurance | 68 | 31.9 |
| Private insurance | 133 | 62.4 |
| Primary parent’s education level | 11 | 5.2 |
| Missing | ||
| Some high school or less | 24 | 11.3 |
| High school degree/GED | 53 | 24.9 |
| Vocational school or some college | 38 | 17.8 |
| College degree | 63 | 29.6 |
| Professional or graduate degree | 24 | 11.3 |
| Marital status of primary parent | 2 | 0.9 |
| Missing | ||
| Single, Divorced, or Widowed | 42 | 19.7 |
| Married | 169 | 79.3 |
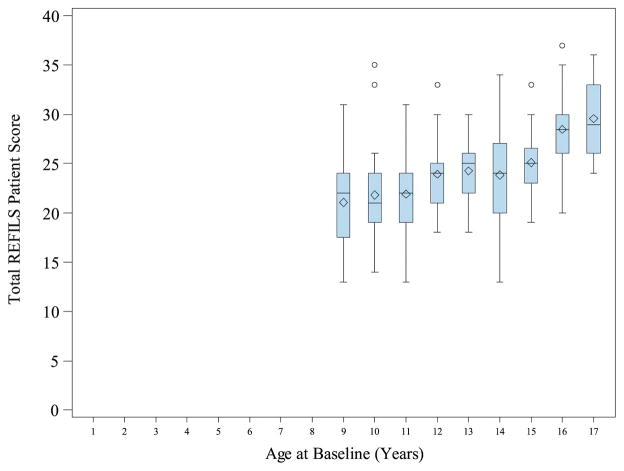
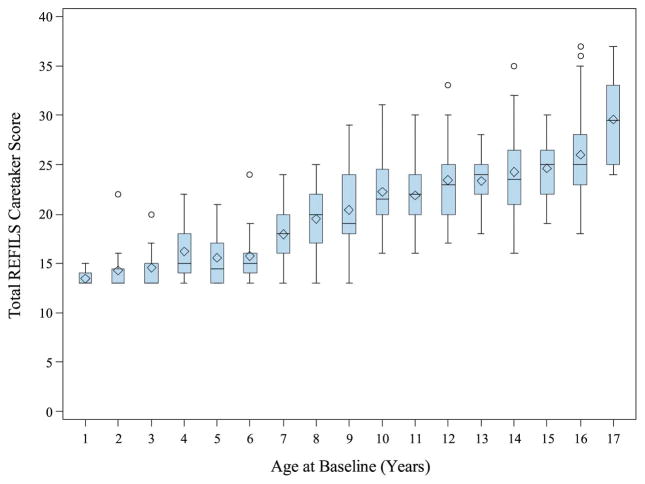
Reliability of the REFILS was examined using Cronbach alpha to measure internal consistency. For the patient version, Cronbach alpha was .80 and for the parent version, it was .88; both values are considered adequate. As would be expected, REFILS scores increased with patient age according to both patient and parent report. Figures 2 and 3 depict these findings.
Figure II.
Total REFILS Patient Score by Age at Baseline
Figure III.
Total REFILS Patient Score by Age at Baseline
Factor analysis on the 13 item REFILS measure was performed for both patient and parent versions to assess the dimensionality of the data. Two factors were retained in both analyses, with similar clustering of items, based on eigenvalues, proportion of common variance in extracted factors and inspection of scree plots. Each factor had acceptable reliability in both analyses with Cronbach alpha >0.74.
As a measure of convergent validity, REFILS scores were compared with AMBS and PMBS scores respectively. Patient score on the REFILS was significantly correlated with AMBS score, r = −.22, P < .01, meaning that greater self-management was associated with fewer reported barriers to medication adherence. However, there was not a significant correlation between parent REFILS score and PMBS score, r = .08, P = .11.
Patient and parent scores were correlated, r = .58, P < .01. Marginal homogeneity tests were conducted to compare the distribution of responses for each item as displayed in Table II. In addition, inter-rater reliability was calculated for each item; Kappa coefficients range from 0.17 to 0.42 which suggests a poor to moderate agreement between the patients and the caretaker.22 However, for total REFILS score, the intra-class correlation coefficient of 0.72 indicates a substantial agreement between the patient and the caretaker. The summation of the 13 items led to a better agreement than the individual items. Given the overall strong reliability of total score, it was used for further analyses.
Table II.
Comparison of Patient and Parent Report on the REFILS
| Item | Patient REFILS | Parent REFILS | P value | ||||
|---|---|---|---|---|---|---|---|
| Never | Sometimes | Always | Never | Sometimes | Always | ||
| Understands key aspects of liver disease | 33 (15.8%) | 66 (31.6%) | 110 (52.6%) | 18 (8.6%) | 63 (30.1%) | 128 (61.2%) | <.01 |
| Discusses management plan w/team | 66 (31.3%) | 101 (47.6%) | 45 (21.2%) | 58 (27.4%) | 95 (44.8%) | 59 (27.8%) | 0.17 |
| Self-manages liver regimen | 109 (52.1%) | 80 (38.3%) | 20 (9.6%) | 116 (55.5%) | 74 (35.4%) | 19 (9.1%) | 0.72 |
| Knows name/dose of medications | 13 (6.2%) | 50 (23.7%) | 148 (70.1%) | 7 (3.3%) | 68 (32.2%) | 136 (64.5%) | 0.03 |
| Keeps track of medications | 42 (19.8%) | 68 (32.1%) | 102 (48.1%) | 64 (30.2%) | 55 (25.9%) | 93 (43.9%) | <.01 |
| Correctly takes medications | 19 (9.0%) | 28 (13.2%) | 165 (77.8%) | 7 (3.3%) | 40 (18.9%) | 165 (77.8%) | <.01 |
| Calls pharmacy for refills | 168 (79.3%) | 22 (10.4%) | 22 (10.4%) | 193 (91.0%) | 11 (5.2%) | 8 (3.8%) | <.01 |
| Knows different types of providers | 77 (36.3%) | 82 (38.7%) | 53 (25.0%) | 62 (29.3%) | 97 (45.7%) | 53 (25.0%) | 0.15 |
| Knows date of next appointment | 50 (23.7%) | 102 (48.3%) | 59 (28.0%) | 54 (25.6%) | 82 (38.9%) | 75 (35.5%) | 0.06 |
| Makes Appointments | 193 (91.5%) | 11 (5.2%) | 7 (3.3%) | 200 (94.8%) | 6 (2.8%) | 5 (2.4%) | 0.21 |
| Know insurance details | 140 (66.4%) | 51 (24.2%) | 20 (9.5%) | 157 (74.4%) | 33 (15.6%) | 21 (10.0%) | 0.02 |
| Understands insurance plan | 143 (68.4%) | 44 (21.1%) | 22 (10.5%) | 162 (77.5%) | 29 (13.9%) | 18 (8.6%) | 0.06 |
| Keeps health care Records | 173 (81.6%) | 22 (10.4%) | 17 (8.0%) | 190 (89.6%) | 15 (7.1%) | 7 (3.3%) | 0.01 |
In order to capture differences between patient and parent reporting which could suggest gaps in management, a difference score was calculated by subtracting each patient score from parent scores. On average, the difference between respondents was 0.3 (SD=4.2). We also calculated difference scores for pre-adolescent (0.1, SD=4.0) and adolescent patients (0.5, SD=4.4).
Kruskal-Wallis tests were conducted to determine if there were any demographic differences in scores or possible psychosocial correlates. There were no differences in scores by patient sex (χ2 =.26, P = .61 for patient report; χ2 =2.74, P =.10 for parent report), ethnicity (χ2 =3.82, P = .05 for patient score, χ2 =.05, P =.84 for parent score), parent marital/partner status (χ2 =3.01, P = .08 for patient score; χ2 =.11, P =.74 for parent score) or insurance type (χ2 =2.37, P = .31 for patient score; χ2 =5.09, P =.08 for parent score). However, there were significant associations between REFILS scores and parent education level based on both patient (χ2 =4.94, P = .03) and parent report (χ2 =4.72, P = .03).
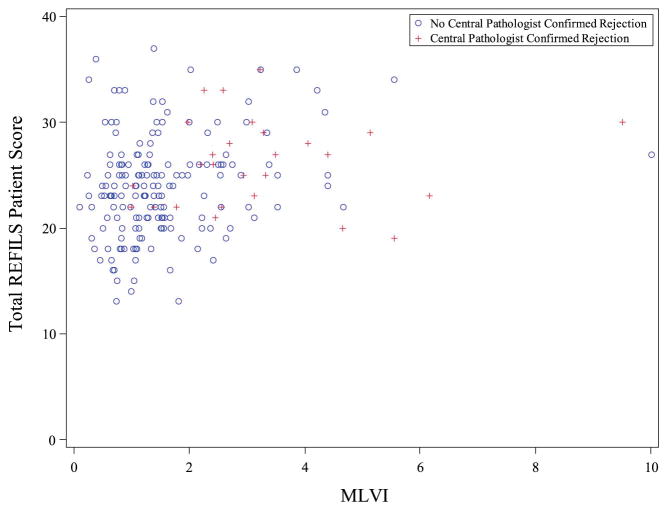
The ability of the REFILS to predict MLVI first was examined using correlational analyses. Overall, patient score was positively correlated with MLVI, r = .26, P <.01; please see Figure 4 (available at www.jpeds.com) for a scatterplot of this finding with patients who experienced rejection highlighted. Interestingly, the magnitude of this correlation was smaller when looking at adolescents alone, r = .20, P = 05. Parent score was not correlated with MLVI (all patients, r = −.01, P = .85, adolescents, r = −.01, P = .92).
Figure IV.
Scatterplot Depiction of Patient REFILS Score and MLVI Correlations
We then examined whether discrepancy scores were associated with MLVI. For patients of all ages, when patients endorsed higher levels of self-management than parent, it was associated with a higher MLVI, r = .20, P <.01. For adolescent patients, this was the case as well, r = .23, P = .02.
Finally, Kruskal-Wallis tests were conducted to determine if REFILS scores were predictive of future adherence when measured categorically (MLVI > 2.5) or rejection, as diagnosed by a central pathologist. Table III displays these findings. As shown, patient REFILS score and REFILS discrepancy score predicted non-adherence and future rejection episodes. Patient REFILS score significantly differed between those who did, 26.1 (SD=4.2) and did not experience rejection, 23.7 (SD=4.8). Similarly, discrepancies between patient and parent report were larger for those who experienced rejection, 1.6 (SD=4.2) versus .01 (SD=4.2).
Table III.
Prediction of Categorical Outcomes, MLVI & Rejection
| Categorical Outcomes | Patient REFILS | Parent REFILS | Difference Score | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Mean (SD) | χ2 P value | Mean (SD) | χ2 P value | Mean (SD) | χ2 P value | |
|
| ||||||
| MLVI > 2.5 | 13.86 | .04 | 6.46 | |||
| Yes | 26.6 (4.5) | < .01 | 20.0 (5.8) | .84 | 1.8 (4.0) | .01 |
| No | 23.5 (4.7) | 20.1 (5.4) | 0.0 (4.2) | |||
|
| ||||||
| Rejection | 6.54 | 2.51 | 4.07 | |||
| Yes | 26.1 (4.2) | .01 | 21.2 (5.5) | .11 | 1.6 (4.2) | .04 |
| No | 23.7 (4.8) | 20.0 (5.4) | 0.1 (4.2) | |||
Discussion
This prospective multisite study evaluated the psychometric properties of a transition measure and also its ability to predict robust outcomes. Our most important finding is that negative outcomes were more likely to occur if patients reported that they are “in charge.” A higher score, which denotes a higher level of (self-reported) management, was significantly and consistently correlated with worse adherence and organ rejection. Such findings are consistent with a prior report that greater self-management is associated with poorer medication adherence among young adult liver transplant recipients.9
We found that the REFILS questionnaire is quite feasible to administer, it can measure self-management level reliably and validity was demonstrated through convergence with a barriers to adherence measure as well as its ability to predict future outcomes. Additional validation studies are needed to explore the utility of possible subscales of the REFILS in the prediction of outcomes. The REFILS requires no interpretation beyond summing scores and comparing them which may be preferable to reviewing how tasks are allocated in detail (e.g., comparing who patients versus parents say is covering each task).
It appears that measuring the level of discrepancy of self-management skills between child/adolescent and parent might be an innovative, nuanced approach for understanding the transition process. We found that greater patient report scores compared with parent report scores of self-management correlates with poor clinical outcomes (non-adherence and more rejections).
Our study had several strengths including the prospective, multi-site design and clearly defined outcome measures thought to be the “gold standard”. However, based on the study design, self-report measures were only administered at baseline and therefore we are unable to gauge how changes in self-management impact clinical outcomes. Another limitation to consider is that the significant correlations we detected were largely small effects, likely reflecting the large sample size. Finally, the population studied was very specific (pediatric liver transplant recipients), which allowed us to look at these robust outcomes. Although it is possible that our results are not generalizable to other diseases, this study certainly sheds light on a construct that was never before examined prospectively in any other population. Therefore our results are of interest to more than transplant program clinicians.
An important message in our findings, consistent with clinical practice, is that transition of responsibilities from the parent/caregiver(s) to the adolescent may in fact not always be indicated or advisable. Relatedly, when a team member offers education about transition, this might be either helpful or misguided. It may be critical to consider the timing of such education, vis-à-vis a specific patient’s abilities at that stage. Therefore, process measurement methods which rely only on adolescent reports12 seem incomplete for two reasons. First, we found that the adolescent’s self-report may not be a good representation of reality (and in our case, was often discrepant with the parents’ view), and second, a program that provides education about self-care might actually be harming patients if this is done indiscriminately (and discourages parent involvement). It is probably prudent to discourage rather than encourage adolescents from assuming self-care in some cases. An important future direction is to determine with empirical support how to best time shifts to self-management for individual patients.
Acknowledgments
Supported by the National Institutes of Health (R01DK080740 to E.S.).
Abbreviations
- REFILS
Responsibility and Familiarity with Illness Survey
- MLVI
Medication Level Variability Index
- MALT
Medication Adherence in Children Who Had a Liver Transplant
- AMBS
Adolescent Medication Barriers Scale
- PMBS
Parent Medication Barriers Scale
- CDI
Children’s Depression Inventory ©
- CES-D
The Center for Epidemiologic Studies Depression Scale
- IES
Impact of Events Scale
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kahana SY, Frazier TW, Drotar D. Preliminary quantitative investigation of predictors of treatment non-adherence in pediatric transplantation: A brief report. Pediatr Transplant. 2008;12:656–660. doi: 10.1111/j.1399-3046.2007.00864.x. [DOI] [PubMed] [Google Scholar]
- 2.Watson AR. Non-compliance and transfer from paediatric to adult transplant unit. Pediatr Nephrol. 2000;14:469–472. doi: 10.1007/s004670050794. [DOI] [PubMed] [Google Scholar]
- 3.Porter M, Larsson S, Lee TH. Standardizing patient outcomes measurement. NEJM. 2016;374:504–506. doi: 10.1056/NEJMp1511701. [DOI] [PubMed] [Google Scholar]
- 4.Bilhartz JL, Lopez MJ, Magee JC, Shieck VL, Eder SJ, Fredericks EM. Assessing allocation of responsibility for health management in pediatric liver transplant recipients. Pediatr Transplant. 2015;19:538–546. doi: 10.1111/petr.12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilleland J, Amaral S, Mee L, Blount R. Getting ready to leave: transition readiness in adolescent kidney transplant recipients. J Pediatr Psychol. 2012;37:85–96. doi: 10.1093/jpepsy/jsr049. [DOI] [PubMed] [Google Scholar]
- 6.Pai AL, Gray E, Kurivial K, Ross J, Schoborg D, Goebel J. The Allocation of Treatment Responsibility scale: a novel tool for assessing patient and parent management of pediatric medical treatment regimens. Pediatr Transplant. 2010;14:993–999. doi: 10.1111/j.1399-3046.2010.01391.x. [DOI] [PubMed] [Google Scholar]
- 7.McQuaid EL, Koel SJ, Klein RB, Fritz GK. Medication adherence in pediatric asthma: Reasoning, Responsibility and Behavior. J Pediatr Psychol. 2003;28:323–333. doi: 10.1093/jpepsy/jsg022. [DOI] [PubMed] [Google Scholar]
- 8.Anderson BJ, Auslander WF, Jung KC, Miller JP, Santiago JV. Assessing family sharing of diabetes responsibilities. J Pediatr Psychol. 1990;15:477–492. doi: 10.1093/jpepsy/15.4.477. [DOI] [PubMed] [Google Scholar]
- 9.Fredericks EM, Dore-Stites D, Well A, Magee JC, Freed GL, Shieck V, et al. Assessment of transition readiness skills and adherence in pediatric liver transplant recipients. Pediatr Transplant. 2010;14:944–953. doi: 10.1111/j.1399-3046.2010.01349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferris M, Cohen S, Haberman C, Javalkar K, Massengill S, Mahan JD, et al. Self-Management and Transition Readiness Assessment: Development, Reliability, and Factor Structure of the STAR x Questionnaire. J Pediatr Nurs. 2015;30:691–699. doi: 10.1016/j.pedn.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 11.Annunziato RA, Parkar S, Dugan CA, Barsade S, Arnon R, Miloh T, et al. Deficits in health care management skills among adolescent and young adult liver transplant recipients transitioning to adult care settings. J Pediatr Psychol. 2011;36:155–159. doi: 10.1093/jpepsy/jsp110. [DOI] [PubMed] [Google Scholar]
- 12.Sawicki GS, Lukens-Bull K, Yin X, Demars N, Huang IC, Livingood W, et al. Measuring the transition readiness of youth with special healthcare needs: validation of the TRAQ—Transition Readiness Assessment Questionnaire. J Pediatr Psychol. 2009;36:160–171. doi: 10.1093/jpepsy/jsp128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sawicki GS, Garvey KC, Toomey SL, Williams KA, Chen Y, Hargraves JL, et al. Development and Validation of the Adolescent Assessment of Preparation for Transition: A Novel Patient Experience Measure. J Adolesc Health. 2015;57:282–287. doi: 10.1016/j.jadohealth.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Annunziato RA, Baisley MC, Arrato N, Barton C, Henderling F, Arnon R, et al. Strangers headed to a strange land? Utilization of a transition coordinator to improve transfer from pediatric to adult service. J Pediatr. 2013;163:1628–33. doi: 10.1016/j.jpeds.2013.07.031. [DOI] [PubMed] [Google Scholar]
- 15.Annunziato RA, Kim S-K. Assessment in Transition: Options, Challenges and Future Directions. Pediatr Transplant. 2015;19:446–448. doi: 10.1111/petr.12521. [DOI] [PubMed] [Google Scholar]
- 16.Shemesh E, Bucuvalas JC, Anand R, Mazariegos GV, Alonso EM, Venick RS, et al. The Medication Level Variability Index (MLVI) Predicts Poor Liver Transplant Outcomes. A Prospective Multi-Site Study. Am J Transplant. doi: 10.1111/ajt.14276. in press, on-line release. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simons LE, Blount RL. Identifying barriers to medication adherence in adolescent transplant. doi: 10.1093/jpepsy/jsm030. [DOI] [PubMed] [Google Scholar]
- 18.Vessey JA, Miola ES. Teaching adolescents self-advocacy skills. Pediatr Nurs. 1996;23:53–56. [PubMed] [Google Scholar]
- 19. [Accessed April 26, 2016]; http://www.cdc.gov/ncbddd/childdevelopment/positiveparenting/index.html.
- 20.Supelana C, Annunziato R, Vaidya S, Anand R, Vaidya S, Chuang K, et al. Medication Level Variability Index predicts rejection, possibly due to nonadherence, in adult liver transplant recipients. Liver Transpl. 2014;20:1168–1177. doi: 10.1002/lt.23930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shemesh E, Shneider BL, Savitzky JK, Arnott L, Gondolesi GE, Krieger NR, et al. Medication adherence in pediatric and adolescent liver transplant recipients. Pediatrics. 2004;113:825–832. doi: 10.1542/peds.113.4.825. [DOI] [PubMed] [Google Scholar]
- 22.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977:159–74. [PubMed] [Google Scholar]
- 23.American Academy of Pediatrics, American Academy of Family Physicians, & American College of Physicians-American Society of Internal Medicine. A consensus statement on health care transitions for young adults with special health care needs. Pediatrics. 2002;110:1304–1306. [PubMed] [Google Scholar]
- 24.Eccles JS, Midgley C, Wigfield A, Buchanan CM, Reuman D, Flanagan C, et al. Development during adolescence: The impact of stage-environment fit on young adolescents’ experiences in schools and in families. Am Psychol. 1993;48:90. doi: 10.1037//0003-066x.48.2.90. [DOI] [PubMed] [Google Scholar]
- 25.Sawyer SM, Aroni RA. Self-management in adolescents with chronic illness. What does it mean and how can it be achieved. Med J Aust. 2005;183:405. doi: 10.5694/j.1326-5377.2005.tb07103.x. [DOI] [PubMed] [Google Scholar]