Abstract
Fluid overload (FO) is commonly seen during hospitalization for allogeneic hematopoietic stem-cell transplantation (AHSCT). We hypothesized that FO is associated with transplant outcomes and evaluated this complication in two cohorts of patients and graded based on post-transplant weight gain, symptoms, and need for treatment, and was scored in real time by an independent team. The first cohort (study cohort) underwent haploidentical transplantation for hematologic malignancies (N=145) following a melphalan-based conditioning regimen. In univariate analysis, factors associated with Day 100 non-relapse mortality (NRM) were FO Grade ≥2 (HR=15, CI 4.2–55, p<0.001), creatinine >1 mg/dL (HR=4.7, CI 1.6–14, p=0.005), and age >55 years (HR=4.5, CI 1.5–13, p=0.008). In multivariate analysis, factors associated with Day 100 NRM were FO Grade ≥2 (HR=13.1, CI 3.4–50, p<0.001) and creatinine >1 mg/dL at transplant admission (HR=3.5, CI 1.1–11, p=0.03). These findings were verified in a separate cohort (validation cohort) of patients with acute myeloid leukemia/myelodysplastic syndrome who underwent HLA-matched transplant (N=449) with busulfan-based conditioning. In multivariate analysis, factors associated with Day 100 NRM were FO Grade ≥2 (HR=34, CI 7.2–158, p<0.001) and, in patients with FO Grade <2, advanced disease status (HR=5, CI 1.1–22, p=0.03). A higher NRM translated to significantly poorer 1-year overall survival rates for patients with FO ≥2 than for patients without FO, 70% vs. 42% (p<0.001) in the study cohort and 64% vs. 38% (p<0.001) in the validation cohort. In conclusion, FO Grade ≥2 is strongly associated with higher NRM and shorter survival and should be considered an important prognostic factor in transplantation.
INTRODUCTION
The Common Terminology Criteria for Adverse Events (CTCAE) is a set of standardized criteria for the classification of adverse effects of medications or interventions used during cancer therapy1–3. Many clinical trials, now extending beyond oncology, encode their observations according to the CTCAE system. Notwithstanding, the CTCAE does not encompass all potential adverse events and was not designed for high-dose chemotherapy regimens. Allogeneic hematopoietic cell transplantation (AHSCT) is a well-established treatment for a variety of malignant and non-malignant hematologic disorders4. Despite significant advances in supportive care, AHSCT remains a high-risk procedure with significant treatment-related complications that can lead to death. The risk of non-relapse mortality (NRM) after transplant, although significantly improved over time, is still approximately 10–15% at Day 100 (D100) and approximately 25% at 2 years post-transplant5,6.
In 1988, Bearman et al. proposed a toxicity scoring system for patients undergoing high-dose therapy with AHSCT7. This system was empirically developed and retrospectively tested in a group of 195 patients who received a myeloablative total body irradiation (TBI)–based conditioning regimen prior to either autologous or allogeneic transplantation. There are few reports in the literature regarding optimal scoring of AHSCT toxic effects, particularly in the context of non-TBI regimens or reduced-intensity conditioning regimens8. In addition, most regulatory agencies and the pharmaceutical industry require that toxic effects be scored using current versions of the CTCAE.
We identified a syndrome of “fluid overload” (FO), which happens early post-transplant in the absence of other known transplant complications, characterized by various degrees of weight gain, edema requiring fluid removal, with or without organ toxicity.
Since FO is not characterized in the CTCAE standardized criteria, we developed a proposed grading system for fluid toxicity based on degree of severity. The purposes of this study were to describe the prevalence and severity of FO, defined according to this grading system, in AHSCT recipients and to test the hypothesis that FO early in the transplant course has an impact on outcome, specifically NRM and survival. The frequency, severity, and outcomes following FO were evaluated in two separate contemporaneous cohorts of patients who underwent AHSCT at our institution between the years 2010 and 2015.
METHODS
Real-time AHSCT toxicity data collection
All patients undergoing transplantation in our department are prospectively registered in the institutional database, which is maintained by DMT. Toxicity data are entered systematically in the database, capturing event start and resolution dates, maximum grade observed, cause, and attribution by a group of data management professionals.
One of these data managers is assigned to each inpatient rounding team, their primary role being to identify and record these toxic effects. The data manager rounds at least twice a week with the transplant team recording potential toxic effects that may require further assessment and scoring and discussing with the rounding team the assignment of appropriate grade, cause, and attribution. The data captured are entered simultaneously in the patient record and the institutional database.
Criteria for fluid overload and grading system
Our proposed grading system defines FO according to degree of severity, as follows: Grade 1: weight gain <10% from baseline, asymptomatic or mild edema, may require diuretic therapy or decrease in intravenous fluid replacement; Grade 2: symptomatic fluid retention, with or without weight gain ≥10% to <20% from baseline, requiring ongoing diuretic therapy; Grade 3: weight gain ≥20% from baseline, not responding to diuretic therapy, with possible renal, pulmonary, or cardiac dysfunction requiring further treatment; Grade 4: progressive dysfunction of more than one organ system or requiring intensive care.
FO was documented according to these criteria as it occurred at any time between the date of patient admission for transplantation and resolution of the FO or patient discharge following transplantation, whichever occurred first. The FO grade reported for each patient was the maximal grade observed between the date of admission and D30 post AHSCT in the absence of another obvious cause or syndrome.
Study cohorts
In this retrospective study, we evaluated the prevalence, severity, and prognostic value of fluid toxicity in two separate cohorts of AHCT recipients. The first cohort (referred to as “study cohort”) included all recipients of a transplant from an HLA haploidentical donor following a melphalan-fludarabine–based conditioning regimen treated at our institution between 2010 and 2015 for hematologic malignancies. These patients received cyclophosphamide, tacrolimus, and mycophenolate mofetil for graft vs. host disease (GVHD) prophylaxis. To validate our findings in this cohort, we evaluated the prevalence, severity, and prognostic value of FO in a second cohort (referred to as “validation cohort”), which included all recipients of a transplant from an HLA-matched related or unrelated donor following a busulfan-fludarabine–based conditioning regimen for the treatment of acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS) during the same period. This cohort received tacrolimus and “mini”-methotrexate for GVHD prophylaxis. This retrospective analysis was approved by the Institutional Review Board of The University of Texas MD Anderson Cancer Center under the number PA15-0644.
Statistical methods
The primary endpoints of the study were the prevalence and severity of FO and its impact on NRM. Secondary endpoints included the impact of FO on overall survival (OS) and progression of the underlying malignancy. FO was diagnosed between the day of admission for transplantation and D30 post AHSCT. To assess whether FO has an impact on survival, we evaluated outcomes in landmark analysis starting on D30 following transplant since all cases of FO were diagnosed at or before D30. The cumulative incidence of NRM starting on D30 was estimated considering progression of malignancy or relapse death as competing risks. Actuarial OS was estimated by the Kaplan-Meier method9. Predictors of D100 NRM were evaluated using competing risks regression for univariate and multivariate analyses. Interaction effects were evaluated and adjusted for as indicated. Predictors of NRM that were evaluated included grade ≥2 FO, diagnosis, age at AHSCT, disease status at AHSCT, conditioning regimen, comorbidity index at AHCT, baseline creatinine level (> vs. ≤1 mg/dL), baseline body weight (> vs. ≤80 kg), baseline diffusing capacity of the lungs for carbon dioxide (DLCO; >70 vs ≤70), and chest X-ray abnormality. Differences in the rate of progression of malignancy and OS according to FO grade were compared on univariate analysis using competing risks regression analysis and Cox proportional hazards regression, respectively10. Factors found to be significant on univariate analysis were considered in multivariate analysis. Backward elimination selection approach was used to identify significant predictors on multivariate analysis. The proportionality of hazards assumption was tested for all factors significant on univariate and multivariate analyses and was not found to be violated. Interaction effects were evaluated and adjusted for as indicated. Statistical significance was set at the 0.05 level. All p values were two-sided. Statistical analyses were primarily performed using STATA software.
RESULTS
Patient characteristics
Baseline characteristics of the study and validation cohorts are summarized in Table 1. Most of the 145 patients in the study cohort (94%) received a bone marrow graft, as previously described11. Other characteristics of this cohort are summarized in Table 2. The median age was 45 years (range, 19–69), 43% of patients were in first or second complete remission (CR1/2), and 58% were males. Median weight was 82 kg (range, 37–180 kg) at study entry before starting the preparative regimen. The median creatinine level was 0.75, and the creatinine was >1.0 mg/dL in 16% of the patients (range, 0.37–1.6). Median DLCO was 70, and ejection fraction (EF) was normal (>40%) in all except two patients.
Table 1.
Patient and treatment characteristics
| Characteristic | Study Cohort | Validation Cohort |
|---|---|---|
|
| ||
| N | 145 | 449 |
|
| ||
| Age, years, median (range) | 45 (19–69) | 58 (18–77) |
|
| ||
| Age >55 years | 40 (28%) | 260 (62%) |
|
| ||
| Sex, F/M | 61/84 (42%/58%) | 174/275 (39%/61%) |
|
| ||
| Diagnosis | ||
| AML/MDS | 85 (59%) | 449 (100%) |
| ALL | 20 (14%) | |
| CML/MPD | 15 (10%) | |
| NHL/HD/MM/CLL | 23 (16%) | |
| Other | 2 (1%) | |
|
| ||
| Months from diagnosis to AHCT, median (range) | 15 months (2–254) | 7 months (2–504) |
|
| ||
| Disease status at AHCT | ||
| CR1/CR2 | 62 (43%) | 191 (42%) |
| Other | 83 (57%) | 258 (57%) |
|
| ||
| Donor type | ||
| 8/8 MRD | 190 (42%) | |
| 8/8 MUD | 259 (58%) | |
| >7/8 Mismatched related | 145 (100%) | |
|
| ||
| Weight, Kg, pre AHCT, median (range) | 82 (37–180) | 81 (47–170) |
|
| ||
| Stem cell source | ||
| Bone marrow | 137 (94%) | 143 (32%) |
| Peripheral blood | 8 (6%) | 306 (68%) |
|
| ||
| DLCO, median (range) | 70 (33–116) | 71 (38–132) |
|
| ||
| Ejection fraction, median (range) | 57 (42–74) | 57 (32–87) |
|
| ||
| HCT comorbidity index | ||
| Median (range) | 1 (0–13) | 2 (0–10) |
| >3 | 24 (17%) | 135 (30%) |
|
| ||
| Creatinine, pre-transplant, mg/dL | ||
| Median (range) | 0.75 (0.4–1.6) | 0.8 (0.4–1.8) |
| >1 | 22 (15%) | 86 (19%) |
|
| ||
| Conditioning regimen | ||
| Melphalan-based | 145 (100%) | 0 |
| Busulfan-based | 0 | 449 (100%) |
|
| ||
| GVHD prophylaxis | ||
| Tacrolimus +/− other | 1 (1%) | 416 (93%) |
| Post-transplant cyclophosphamide | 143 (99%) | 31 (7%) |
| Other | 1 | 2 |
|
| ||
| Year of AHCT | ||
| 2010–2012 | 70 (48%) | 255 (57%) |
| 2013–2015 | 75 (52%) | 194 (43%) |
|
| ||
| FO grade | ||
| <2 | 115 (79%) | 422 (94%) |
| ≥2 | 30 (21%) | 27 (6%) |
F, female; M, male; AML, acute myeloid leukemia; MDS, myelodysplastic syndromes; CML, chronic myeloid leukemia; MPD, myeloproliferative disease; MM – multiple myeloma; NHL – non-Hodgkin’s lymphoma; HD – Hodgkin’s disease; CLL, chronic lymphocytic leukemia; AHCT, allogeneic hematopoietic stem cell transplantation; CR1, first complete remission; CR2, second complete remission; MRD, matched related donor; MUD, matched unrelated donor; DLCO, diffusing capacity of the lungs for carbon dioxide; GVHD, graft-versus-host disease; FO, fluid overload.
Table 2.
Univariate and multivariate analyses of predictors of Day 100 non-relapse mortality in the study cohort
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR | p | HR 95%CI | p | |
| FO, grade | ||||
| <2 | Reference | Reference | ||
| ≥2 | 15 | <0.001 | 13 3.4–50 | <0.001 |
| Age, years | ||||
| ≤55 | Reference | |||
| >55 | 4.5 | 0.008 | NS | |
| Diagnosis | ||||
| AML/MDS | Reference | |||
| ALL | 1.1 | 0.9 | ||
| CML/MPD | 1.3 | 0.7 | ||
| NHL/HD/MM/CLL | 0.5 | 0.5 | ||
| Disease status at AHCT | ||||
| CR1/CR2 | Reference | |||
| Not CR1/CR2 | 1.2 | 0.8 | ||
| Conditioning regimen | ||||
| Thiotepa | Reference | |||
| TBI | 1.1 | 0.9 | ||
| Other | 1.3 | 0.6 | ||
| HCT comorbidity index | ||||
| ≤2 | Reference | |||
| >2 | 3.1 | 0.04 | NS | |
| Baseline creatinine, mg/dL | ||||
| ≤1 | Reference | Reference | ||
| >1 | 4.7 | 0.005 | 3.5 | 0.03 |
| Baseline weight, kg | ||||
| ≤80 | Reference | |||
| >80 | 0.8 | 0.65 | ||
| Baseline DLCO | ||||
| ≤70 | Reference | |||
| >70 | 0.85 | 0.8 | ||
HR, hazard ratio; FO, fluid overload; AML, acute myeloid leukemia; MDS, myelodysplastic syndromes; CML, chronic myeloid leukemia; MPD, myeloproliferative disease; NHL, non-Hodgkin lymphoma; HD, Hodgkin disease; MM, multiple myeloma; CLL, chronic lymphocytic leukemia; AHCT, allogeneic hematopoietic cell transplant; CR1, first complete remission; CR2, second complete remission; TBI, total body irradiation; DLCO, diffusing capacity of the lungs for carbon dioxide.
The validation cohort comprised 449 patients with AML/MDS who received an HLA-matched transplant from a matched related donor (MRD, n=190) or a matched unrelated donor (MUD, n=259) as previously described12. The median patient age was 58 years (range, 18–77), 42% were in CR1/2 at transplant, and 68% received a peripheral blood graft. Median weight was 81 kg (range, 47–170). Median creatinine level at transplant was 0.8 mg/dL (range, 0.38–1.8), and the creatinine was >1 mg/dL in 19%. Median DLCO was 71 and EF was normal (>40%) in all except two patients (Table 1).
Prevalence and severity of fluid overload
FO was diagnosed in 96 of the 145 patients (66.2%) in the study cohort and in 192 of the 449 (42.7%) patients in the validation cohort. The median time from admission to FO diagnosis was 2 days (range, 0–52) in the study cohort and 6 days (0–30) in the validation cohort. The prevalence of maximal grade ≥2 FO occurring between the date of admission for transplantation and D30 post AHCT was 21% (95% confidence interval [CI] 15–28%) in the study cohort and 6% (95% CI 4–9%) in the validation cohort. In the study cohort, maximal FO was grade 1 in 45% of the patients, grade 2 in 14% of the patients, grade 3 in 6% of the patients, and grade 4 in 1% of the patients. In the validation cohort, maximal FO was grade 1 in 37%, grade 2 in 5% and grade 3 in 1%. No grade 4 FO was observed in this cohort. Notably, baseline left ventricular ejection fraction was not associated with maximal grade ≥2 FO in the study or validation cohort; median left ventricular ejection fraction was 57% in patients with or without ≥2 FO in both cohorts.
Impact of fluid overload on transplant outcome
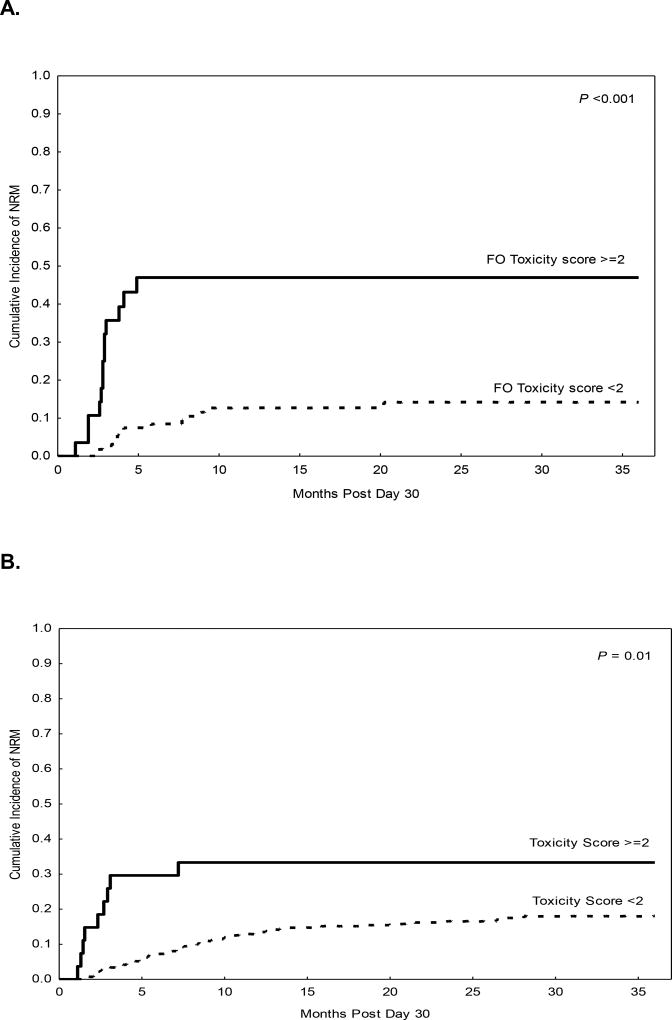
The median post-transplant follow-up interval in surviving patients was 18 months (range, 1–56) in the study cohort and 23 months (range, 2–62) in the validation cohort. Ninety-seven percent of patients, both in the study cohort (n=140/145) and in the validation (n=440/449) cohort, were alive and free of disease progression on D30 after transplant and were eligible for D100 NRM assessment. This included 28 of the 30 patients in the study cohort and all of the 27 patients in the validation cohort in whom grade ≥2 FO was diagnosed. In landmark analysis starting on D30, the overall cumulative incidence of D100 NRM was 9% in the study cohort (95% CI 6–16%) and 5% (95% CI 3–7%) in the validation cohort. On univariate analysis, Grade ≥2 FO was associated with significantly higher D100 NRM both in the study cohort (Table 2) and in the validation cohort (Table 3). Grade 0 and 1 FO were associated with comparably low D100 NRM in the study cohort (p=0.1) and the validation cohort (p=0.4). In the study cohort (Figure 1A), the cumulative incidence of D100 NRM was 36% in patients with Grade ≥2 FO and 3% in patients with Grade <2 FO (hazard ratio [HR]=15, p <0.001).
Table 3.
Univariate and multivariate analyses of predictors of Day 100 non-relapse mortality in the validation cohort
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR | p | HR 95%CI | p | |
| FO, grade | ||||
| <2 | Reference | |||
| ≥2 | 10 | <0.001 | 34 7.2–158 | <0.001 |
| Age, years | ||||
| ≤55 | Reference | |||
| >55 | 1.7 | 0.3 | ||
| Donor type | ||||
| MRD | Reference | |||
| MUD | 1.6 | 0.3 | ||
| Disease status at AHCT | ||||
| CR1/CR2 | Reference | |||
| Not CR1/CR2 | 3.5 | 0.02 | 5 | 0.03 |
| HCT comorbidity index | ||||
| ≤2 | Reference | |||
| >2 | 1.5 | 0.3 | ||
| ≤3 | Reference | |||
| >3 | 2.4 | 0.04 | NS | |
| Baseline creatinine, mg/dL | ||||
| ≤1 | ||||
| >1 | 2.2 | 0.09 | ||
| Baseline weight, kg | ||||
| ≤80 | ||||
| >80 | 1.3 | 0.5 | ||
| Baseline DLCO | ||||
| ≤70 | ||||
| >70 | 0.8 | 0.7 |
HR, hazard ratio; FO, fluid overload; MRD, matched related donor; MUD, matched unrelated donor; AHCT, allogeneic hematopoietic cell transplantation; CR1, first complete remission; CR2, second complete remission; DLCO, diffusing capacity of the lungs for carbon dioxide.
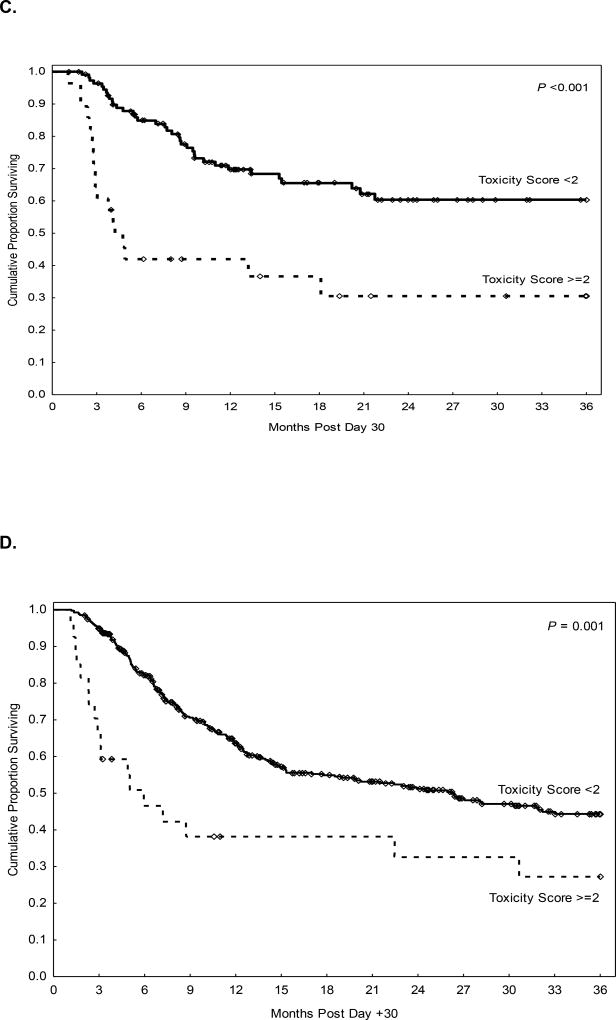
Figure 1.
Landmark analysis at Day 30 post AHCT in the study cohort (haploidentical transplant) and validation cohort (HLA-matched transplant). A, B. Non-relapse mortality according to FOR grade in the study (A) and validation (B) cohorts; C, D. Overall survival according to FOR grade in the study (C) and validation (D) cohorts.
These rates were similar in the validation cohort (Figure 1B, 30% vs. 3%, HR=10, p<0.001). Notably, Grade ≥2 FO was associated with significantly higher D100 NRM in patients with MRD (p<0.001) or MUD (p=0.004). Additional significant predictors of D100 NRM in the study cohort included age greater than 55 years (HR=4.5, p=0.008), creatinine level at AHCT >1 mg/dL (HR=4.7, p=0.005), and HSCT-comorbidity index >2 (HR=3.1, p=0.04). In the validation cohort, advanced disease at the time of AHSCT (HR=3.5, p=0.02) and HSCT-comorbidity index >3 (HR=2.4, p=0.04) were associated with significantly higher D100 NRM. Notably, the 1-year rate of progression did not differ in patients with or without Grade ≥2 FO in either the study cohort (HR=0.8, p=0.6) or the validation cohort (HR=0.96, p=0.9).
Multivariate analysis adjusting for potential confounding factors revealed Grade ≥2 FO to be not only an independent predictor of D100 NRM in the study cohort (Table 2; HR=13, p<0.001) and the validation cohort (Table 3; HR=63, p<0.001) but also the strongest factor associated with NRM in these patients. The higher NRM rate for patients with >2 FO translated into poorer OS (Table 4) for both the study and validation cohorts (Figure 1C, 1D). Causes of D100 NRM according to FO grade are presented in Table 5.
Table 4.
Survival outcomes after Day 30 post AHCT according to FO grade in the study and validation cohorts
| Study Cohort | Validation Cohort | |||||
|---|---|---|---|---|---|---|
| FO grade | FO grade | |||||
| Outcome | ≥2 % (95% CI) |
<2 % (95% CI) |
p value |
≥2 % (95% CI) |
<2 % (95% CI) |
p value |
| Day 100 NRM | 36% (22–59) | 3% (1–8) | <0.01 | 31% (17–53) | 3% (2–6) | <0.001 |
| 1-year NRM | 47% (32–70) | 13% (8–21) | <0.01 | 33% (19–57) | 13% (10–17) | 0.01 |
| Day 100 OS | 61% (40–76) | 96% (90–99) | <0.01 | 59% (39–75) | 94% (91–96) | <0.001 |
| 1-year OS | 42% (23–59) | 70% (59–78) | <0.01 | 38% (20–56) | 64% (58–68) | <0.001 |
FO, fluid overload; CI, confidence interval; NRM, non-relapse mortality; OS, overall survival.
Table 5.
Causes of Day 100 NRM according to FO grade
| Study Cohort | Validation Cohort | |||
|---|---|---|---|---|
| FO grade | ≥2 (n=28) | <2 (n=112) | ≥2 (n=26) | <2 (n=412) |
| D100 NRM cases | 10 (%) | 3 (%) | 8 (%) | 14 (%) |
| Cause of NRM | ||||
| Graft failure | 1 (10) | 1 (33) | 1 (13) | 1 (7) |
| Infection | 3 (30) | 2 (67) | 2 (25) | 3 (21) |
| Acute GVHD | 1 (10) | 0 | 3 (38) | 5 (36) |
| Organ failure | 2 (20) | 0 | 0 | 2 (14) |
| Hemorrhage | 1 (10) | 0 | 1 (13) | 0 |
| ARDS | 2 (20) | 0 | 0 | 0 |
| Pneumonia | 1 (13) | 1 (7) | ||
| Secondary malignancy | 0 | 1 (7) | ||
| Other | 0 | 1 (7) | ||
NRM, non-relapse mortality; FOR, fluid overload/retention; GVHD, graft-versus-host disease; ARDS, adult respiratory distress syndrome.
DISCUSSION
Although survival of patients receiving AHSCT has progressively improved, primarily because of improved supportive care reducing NRM5, fluid overload has not been recognized and categorized as a significant toxic effect of this procedure. We developed a grading system for this complication that takes into account the percentage of weight gain since admission, development of symptoms, and need for intervention. Using this grading system and two cohorts of AHSCT patients, we showed that significant FO (Grade ≥2) is a frequent occurrence, found in up to 20% of patients receiving AHSCT. We also found that FO is consistently associated with a significantly higher NRM rate and poorer survival than observed in transplanted patients who do not experience FO, supporting our hypothesis. In fact, our data show that Grade ≥2 FO diagnosed by D30 post AHSCT accounted for the majority of D100 and 1-yr NRM and was the strongest predictor of NRM in transplant patients. Among patients who did not experience FO, D100 NRM was only 3% and 1-year NRM 13% in both our study cohort (HLA haploidentical donor) and validation cohort (HLA-matched donor). Supporting the validity of the proposed grading system was our finding that Grade ≥2 FO was associated with comparable D100 NRM in the study (36%) and validation (30%) cohorts. This relationship was maintained at 1 year post-transplant. The proposed grading system, if validated in independent patient populations, could provide a useful tool to identify patients at high risk of NRM as early as D30 post AHSCT.
It is important to mention that FO occurs in the first 7 days from admission when patients receive intravenous hydration and conditioning chemotherapy. This clearly differentiates this syndrome from alternative diagnoses (like renal failure, heart failure and liver failure) as all patients were eligible for transplantation and had good organ function before admission for transplantation with no significant differences between those who developed this syndrome and who did not. However, it is possible that some patients may be more predispose to retain fluids and have capillary leak during this time. The excess fluid may accumulate in the extracellular compartments of the body, and when severe, may evolve to a capillary leak syndrome, which is poorly responsive to treatment with diuretics. This interstitial edema may mediate toxic effects in multiple organs. The capillary vascular endothelium may play an important role in the balance between extracellular and intravascular fluids,13 which could make FO part of a spectrum of endothelial damage disorders that includes sinusoidal obstructive syndrome, thrombotic microangiopathy, and idiopathic pneumonia syndrome.
Many of the life-threatening complications frequently associated with high-dose chemotherapy and AHSCT are caused by endothelial damage. This damage may result either from direct effects of high-dose chemotherapy on the vascular endothelium or from host-pathogen interactions from peri-transplant infections and the inflammatory cytokine milieu. Most patients require continued fluid replacement to maintain adequate hydration and facilitate clearance of the chemotherapy and the products of cellular destruction14. However, the literature regarding optimal fluid management in the setting of high-dose chemotherapy and HSCT is sparse. Furthermore, fluid retention may occur as a toxic effect of treatment. Our data suggest that at least 20% of allograft recipients receiving a myeloablative conditioning regimen will have an increase of at least 10–20% in baseline body weight requiring diuretic therapy and that these patients have a significant higher risk of non-relapse–related death. Remarkably, fluid toxicity appears to have the greatest impact on NRM among all known factors, highlighting the importance of recognizing and minimizing FOR early in the post-AHSCT course. Early recognition of this potential complication may allow more effective management and predictive factors for FO should be further explored. Moreover, biomarkers may provide insight into novel treatments or preventive measures, could potentially be explored in the future.
The CTCAE version 4 does not include fluid overload as a specific toxic effect. A simple and clear classification as proposed here may help earlier recognition and aggressive management to avoid morbidity and mortality associated with excessive fluid retention as well as facilitate further study of endothelial damage which may be present in these patients.
The major strengths of this study is the prospective real time collection of toxicity data, and the validation of our findings on the association between FO and survival in an independent cohort of patients. Remarkably, the rate of mortality associated with FO was consistent between the study and validation cohorts. Although we did not evaluate the causes of excessive fluid retention in the early post-transplant period, we recognize that other complications that occur after ASCT, like VOD, heart and renal failure occur much later in the course of treatment and unlikely to overlap with this early fluid toxicity described in this report as maximal Grade ≥2 FO was diagnosed early after admission for transplantation, and only two cases of VOD were diagnosed among patients with maximal Grade ≥2 FO, one in the study and one in the validation cohorts. On the other hand, baseline left ventricular ejection fraction was comparable in patients with and without maximal Grade ≥2 FO. Elevated baseline creatinine was associated with higher NRM in the study cohort, however, Grade ≥2 FO remained an independent significant predictor of NRM on multivariate analysis after adjusting for baseline creatinine.
In summary, the fluid overload should be considered an important adverse event post AHSCT. Grade 2 or greater FO was strongly associated with NRM and poorer survival in our two cohorts of AHSCT recipients. Caution is warranted to prevent excessive hydration and weight gain in the early post-transplant period. FOR should be considered an important peri-transplant prognostic factor for NRM that could potentially be modified if our understanding of its pathophysiology were improved. The grading criteria we propose can be easily implemented to capture this toxicity and form the basis for assessment of measures to prevent or treat this complication.
KEY POINTS.
-
-
We describe a new grading system for fluid toxicity in patients receiving allogeneic stem cell transplantation
-
-
Patients who experienced weight gain ≥10% (Grade 2) early during hospitalization experienced higher NRM and worse survival
-
-
Fluid toxicity had the highest impact on NRM of all known causes
-
-
Further studies are needed to better asses causes and prevent this complication
Acknowledgments
The authors thank Ruby Delgado, Victoria Francis, Maria Guillermo-Pacheco, Monique Kendrick, Johnny Nguyen, and Sofia Qureshi for their dedication to capturing real-time toxicity data; the faculty of the Department of Stem Cell Transplantation and Cellular Therapy and the APNs, PharmDs, and inpatient nurses for their support in collecting the data. We would also like to thank Kathryn Hale and Sergio Giralt, MD, for editorial advice.
Footnotes
Conflict of interest: The authors have no competing financial interests to disclose for this work.
This paper was presented in part at the American Society of Hematology Meeting, Orlando, FL, 2015.
Authorship Contribution: G.R. collected data and wrote the manuscript; R.S. analyzed data, interpreted the results, reviewed and approved the manuscript; J.C, C.L. contributed with data collection, reviewed and approved the manuscript; A.M.A., B.O, P.K., C.M.H., I.F.K., E.J.S, U.R.P., R.E.C. contributed with treatment of patients, reviewed and approved the manuscript; S.O.C. contributed with study design, data collection and interpretation, and manuscript writing.
References
- 1.Trotti A, Colevas AD, Setser A, Basch E. Patient-reported outcomes and the evolution of adverse event reporting in oncology. J Clin Oncol. 2007;25(32):5121–5127. doi: 10.1200/JCO.2007.12.4784. [DOI] [PubMed] [Google Scholar]
- 2.Common Terminology Criteria for Adverse Events (CTCAE) USDEPARTMENT OF HEALTH AND HUMAN SERVICES. National Institutes of Health National Cancer Institute; 2009. [Google Scholar]
- 3.CTEP. NCI Guidance on CTC Terminology Applications. National Cancer Institute; [Google Scholar]
- 4.Wieduwilt MaG, S. Clinical Hematopoietic Cell transplantation in American Society of Hematology Self Assessment Program. Blood [Google Scholar]
- 5.Gooley TA, Chien JW, Pergam SA, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363(22):2091–2101. doi: 10.1056/NEJMoa1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horan JT, Logan BR, Agovi-Johnson MA, et al. Reducing the risk for transplantation-related mortality after allogeneic hematopoietic cell transplantation: how much progress has been made? J Clin Oncol. 2011;29(7):805–813. doi: 10.1200/JCO.2010.32.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bearman SI, Appelbaum FR, Buckner CD, et al. Regimen-related toxicity in patients undergoing bone marrow transplantation. J Clin Oncol. 1988;6(10):1562–1568. doi: 10.1200/JCO.1988.6.10.1562. [DOI] [PubMed] [Google Scholar]
- 8.Chou A, Guo M, Tay J, et al. Serial assessment of toxicity after hematopoietic SCT can discern kinetics of transplant-related organ injury and patterns of recovery. Bone Marrow Transplant. 2012;47(10):1375–1376. doi: 10.1038/bmt.2012.27. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan EL, Meier P. Nonparametric Estimation from Incomplete Observations. Journal of the American Statistical Association. 1958;53(282):457–481. [Google Scholar]
- 10.Cox D. Regression Models and Life-Tables. Journal of the Royal Statistical Society. 1972;34(1972):187–220. [Google Scholar]
- 11.Gaballa S, Ge I, El Fakih R, et al. Results of a 2-arm, phase 2 clinical trial using post-transplantation cyclophosphamide for the prevention of graft-versus-host disease in haploidentical donor and mismatched unrelated donor hematopoietic stem cell transplantation. Cancer. 2016;122(21):3316–3326. doi: 10.1002/cncr.30180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alatrash G, de Lima M, Hamerschlak N, et al. Myeloablative reduced-toxicity i.v. busulfan-fludarabine and allogeneic hematopoietic stem cell transplant for patients with acute myeloid leukemia or myelodysplastic syndrome in the sixth through eighth decades of life. Biol Blood Marrow Transplant. 2011;17(10):1490–1496. doi: 10.1016/j.bbmt.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Connor ME, Prowle JR. Fluid Overload. Crit Care Clin. 2015;31(4):803–821. doi: 10.1016/j.ccc.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 14.Rimkus C. Acute complications of stem cell transplant. Semin Oncol Nurs. 2009;25(2):129–138. doi: 10.1016/j.soncn.2009.03.007. [DOI] [PubMed] [Google Scholar]