Abstract
There is a growing interest in water reuse and in recovery of nutrients from wastewater. Because many advanced treatment processes are designed to remove organic matter, a better understanding of the composition of dissolved organic matter (DOM) in wastewater is needed. To that end, we assessed DOM in the Nine Springs Wastewater Treatment Plant in Madison, Wisconsin by UV–visible spectroscopy and Fourier transform-ion cyclotron resonance mass spectrometry. Samples were collected from the influent and effluent of two different secondary treatment processes and their respective secondary clarifiers, the UV disinfection unit, and an Ostara treatment system, which produces struvite via chemical precipitation. The optical properties reveal that DOM throughout the plant is relatively aliphatic and is low in molecular weight compared to DOM in freshwater systems. Furthermore, the DOM is rich in heteroatoms (e.g., N, S, P, and Cl) and its molecular formulas are present in the lipid-, protein-, carbohydrate-, and lignin-like regions of van Krevelen diagrams. Secondary treatment produces DOM that is more aromatic and more complex, as shown by the loss of highly saturated formulas and the increase in the number of CHO, CHON, and CHOP formulas. The two secondary treatment processes produce DOM with distinct molecular compositions, while the secondary clarifiers and UV disinfection unit result in minimal changes in DOM composition. The Ostara process decreases the molecular weight of DOM, but does not otherwise alter its composition. The optical properties agree with trends in the molecular composition of DOM within the main treatment train of the Nine Springs plant.
Keywords: Wastewater, Dissolved organic matter, High-resolution mass spectrometry, UV-visible spectroscopy, Secondary treatment, Struvite precipitation
1. Introduction
There is a growing interest in wastewater reuse as water scarcity, droughts, and population growth increase demand for freshwater resources (Hering et al., 2013). While the use of reclaimed water for irrigation or indirect potable reuse are relatively common applications, direct potable reuse is increasingly under consideration in waterstressed regions (Guest et al., 2009; Hering et al., 2013). Applications of wastewater reuse rely on removal of dissolved organic compounds using advanced treatment techniques, such as advanced oxidation processes or membrane-based processes. The removal of dissolved organic matter (DOM) by advanced processes depends on the type and amount of organic compounds present (Michael-Kordatou et al., 2015). Therefore, better understanding of the composition of DOM in treated wastewater effluent is needed.
Simultaneously, wastewater is increasingly recognized as a resource, rather than simply a waste product. Municipal waste-water contains thermal energy, organic compounds, and nutrients that can be recovered and used in other applications (Hering et al., 2013; van Loosdrecht and Brdjanovic, 2014). Energy recovery in the form of biogas produced from sludge is widely applied, while more novel applications involve the production of biopolymers and other products from the organic content in wastewater (Guest et al., 2009; van Loosdrecht and Brdjanovic, 2014). Phosphorus and nitrogen can be separated from wastewater and used as fertilizer (Guest et al., 2009). For example, phosphorus and ammonia can be recovered in the form of struvite (NH4MgPO4·6H2O) through chemical precipitation (Cornel and Schaum, 2009). These advanced wastewater treatment processes have the potential to further alter the composition of DOM in the final wastewater effluent.
The dissolved organic component of wastewater effluent consists of recalcitrant natural organic matter present in drinking water sources, soluble microbial products produced during biological wastewater treatment, trace concentrations of synthetic organic compounds produced during domestic or industrial use, and disinfection by-products produced during drinking or waste-water disinfection (Michael-Kordatou et al., 2015; Shon et al., 2006). The exact composition of DOM varies depending on the source of the wastewater and the wastewater treatment processes (Michael-Kordatou et al., 2015). While the general compound classes in treated effluent are known, less is known about how conventional and advanced treatment processes alter the molecular composition of DOM.
Ultrahigh-resolution mass spectrometry is uniquely suited to assess the molecular composition of DOM. Fourier transform-ion cyclotron resonance mass spectrometry (FT-ICR MS) is widely applied to determine molecular formulas in DOM from natural aquatic systems (Hertkorn et al., 2008; Koch et al., 2007), but has received little attention for the characterization of DOM in waste-water treatment systems. Previous studies of wastewater DOM by FT-ICR MS focused on formulas containing only C, H, O, and S and are limited to either single samples or samples collected during one treatment process (e.g., secondary treatment; Gonsior et al., 2011; Mesfioui et al., 2012; Shakeri Yekta et al., 2012, Tseng et al., 2013). Despite these limitations, the past studies indicate that DOM in wastewater treatment systems is highly complex, rich in S-containing formulas, and distinctive from DOM present in natural systems.
The aim of this study is to evaluate the composition of DOM throughout a municipal wastewater treatment plant by combining bulk measurements, such as UV–visible spectroscopy, with data derived from FT-ICR MS measurements. Samples were collected throughout the Nine Springs Wastewater Treatment Plant to evaluate how specific treatment processes alter the composition of DOM, including the presence of formulas containing S, N, P, and Cl. The processes of interest include two different secondary treatment processes, secondary clarification, UV disinfection, and struvite precipitation. Additionally, similarities between optical properties and individual molecular formulas are used to demonstrate that simple bulk measurements provide insight into how different wastewater treatment processes alter the composition of DOM.
2. Material and methods
2.1. Materials
Acetonitrile (HPLC grade), hydrochloric acid (certified ACS plus), and methanol (HPLC grade) were obtained from Fisher Scientific. Milli-Q water (18 MU cm) was generated by a Millipore A10 system.
2.2. Sample collection
The Nine Springs Wastewater Treatment Plant (~42 MGD) is an advanced secondary treatment facility operated by the Madison Metropolitan Sewerage District that serves 39 separate municipal entities in and around Madison, Wisconsin, USA. Raw wastewater first passes through primary treatment to remove solids (Fig. 1). The plant employs two different activated sludge treatment processes to achieve biological carbon and phosphorus removal and to nitrify ammonia to nitrate. Approximately 90% of the primary effluent undergoes treatment in a Modified University of Cape Town (UCT) process, which consists of an anaerobic stage, an anoxic stage, and an aerobic stage. The remaining primary effluent is treated using an A/O process, which is an activated sludge process with an anaerobic zone prior to the aeration basin. Although dedicated secondary clarifiers are not used for the UCT and A/O effluents, most of the A/O effluent is treated in certain clarifiers (referred to as “A/O secondary clarifier” in the text). Flow from all clarifiers is blended prior to disinfection by low pressure ultraviolet (UV) light. The plant also operates an Ostara treatment process to recover struvite. The influent to the Ostara unit consists of liquid filtrate produced by gravity belt thickening of sludge generated during secondary clarification and anaerobic digestion (Figure S1). The Ostara process involves the addition of magnesium chloride and sodium hydroxide to produce struvite via chemical precipitation.
Fig. 1.
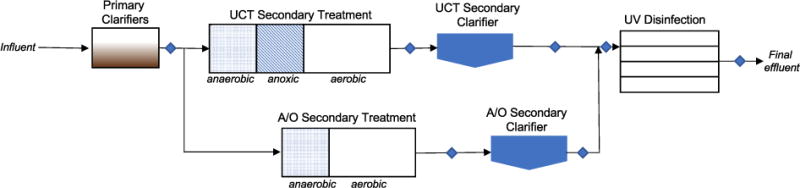
Schematic of liquids handling in the Nine Springs Wastewater Treatment Plant. Sampling locations are indicated by diamonds.
Nine samples (1–4 L) were collected in September 2016 (Fig. 1 and S1). Samples were sequentially vacuum filtered through 0.7 mm (Whatman GF/F, borosilicate glass) and 0.45 μm (MDI, nylon) membranes, then stored in the dark at 4°C in pre-combusted glass bottles.
2.3. Bulk analysis
Methods for quantifying dissolved organic carbon ([DOC]), pH, alkalinity, anions, and cations are described in Section S1. UV–visible spectra were collected using a Shimadzu UV-2401 PC in 1 cm quartz cuvettes. Measurements were collected at 1 nm increments against a Milli-Q water reference, and were corrected for blank and long wavelength (700–800 nm) absorption. SUVA254 is the ratio of the decadal absorbance at 254 nm to [DOC] (Weishaar et al., 2003). E2:E3 is the ratio of absorbance at 250 nm and 365 nm, while the spectral slope S275–295 is a measure of the slope of the absorbance spectrum over the wavelengths 275–295 nm (Fichot and Benner, 2012; Helms et al., 2008).
2.4. FT-ICR MS analysis
DOM was concentrated by solid phase extraction (SPE) as described previously (Dittmar et al., 2008). Approximately 500 mL of sample was acidified to pH 2 with 1 M HCl and passed through methanol-rinsed Agilent Bond Elut-PPL cartridges (500 mg, 6 mL). The cartridges were then rinsed with 0.01 M HCl and dried with air. DOM was eluted from the cartridges with methanol and eluents were stored in the dark at 4°C until aliquots were poured off for analysis. The extraction procedure retained approximately 60% of DOC, which is similar to previous studies that concentrated wastewater-derived samples via SPE (Gonsior et al., 2011; Phungsai et al., 2016; Tseng et al., 2013; Urai et al., 2014).
Samples were diluted in 10× in 1:1 acetonitrile:Milli-Q and analyzed with a 12T FT-ICR MS (Bruker solariX XR) coupled with an automated sample delivery system (TriVersa Nanomate). Samples were atomized with a gas pressure of 0.3 psi and analyzed using both positive and negative polarity electrospray ionization (ESI) under an applied voltage of ±1.4 kV. Ion accumulation time was adjusted to give an intensity of ~109 and 350 scans were collected per sample. Spectra were collected over 170–1200 m/z.
Peaks identified in mass spectra with 170–620 m/z and S/N > 3 were exported to R for processing. Prior to formula assignment, m/z values detected in negative mode were calibrated against commonly identified CHOS and CHO masses (186–504 m/z; Gonsior et al., 2011; Koch et al., 2005), while m/z values detected in positive mode were calibrated against commonly identified CHO and CHON masses (280–479 m/z; Koch et al., 2005). Negative mode m/z were converted to nominal masses by the addition of a proton, while positive mode m/z were converted to nominal masses by the subtraction of Na+ for CHO formulas and by the subtraction of either H+ or Na+ for CHON formulas (Koch et al., 2005). Potential formulas were composed of 12C1H16O13C0–1Cl0–1N0–1P0–1S0–1, had whole number double bond equivalent (DBE) values (0–40), 0 ≤ O ≤ C, 0 ≤ H 2C + 2, and obeyed the nitrogen rule. Formulas assignments were considered valid if the mass error was <0.2 ppm and if the associated m/z peak intensity was greater than 2% of the average of the 500 most intense matched peaks. DBE values were determined as: DBE = C - ½H + ½N + 1 (Stenson et al., 2003). Aromaticity index (AI) values were calculated as: AI = [1 + C – O -½(H + Cl)]/[C – O – S – N – P] (Koch and Dittmar 2006). The molecular lability boundary (MLBL) was calculated as the percentage of formulas with H:C ≥ 1.5 (D’Andrilli et al., 2015). Bray-Curtis dissimilarity was calculated with the R package pvclust. For comparison of molecular composition before and after wastewater treatment processes, the formula lists were examined for formulas that were present in only the influent or the effluent. For common formulas, their change in intensity was compared using a relative intensity index (Equation S1). Pearson correlations were calculated using the relative intensity of CHO and CHON formulas detected in all seven samples in the main treatment train (i.e., all samples except for the Ostara influent and effluent).
3. Results and discussion
3.1. Bulk and optical properties
Optical properties provide a simple way to assess the overall composition of DOM. SUVA254 is a measure of aromaticity of DOM (Maizel and Remucal, 2017; Weishaar et al., 2003) and ranges from 1.16 L mg-C−1 m−1 in the primary effluent to 2.22 L mg-C−1 m−1 in the A/O secondary clarifier effluent (Table 1). The SUVA254 values observed here are low relative to the typical values of 4–5 L mg-C−1 m−1 observed in terrestrially-dominated aquatic systems (Weishaar et al., 2003), but are similar to those of microbially-dominated aquatic systems (e.g., 1.3 L mg-C−1 m−1 in Lake Superior; Minor and Stephens, 2008). Likewise, the SUVA254 value for the Nine Springs primary effluent agrees with the previous report of 1.2 L mg-C−1 m−1 for primary effluent (Barber et al., 2001). SUVA254 values for secondary effluent are widely reported and range from 1.2 to 2.6 L mg-C−1 m−1 (Krasner et al., 2009; Sirivedhin and Gray, 2005), which agree with the two secondary clarifier effluents reported here. Overall, the SUVA254 values observed throughout the Nine Springs Plant indicate that the DOM is most similar to microbially-derived DOM and has low aromaticity.
Table 1.
Summary of bulk and optical properties of samples collected in the main treatment train of the Nine Springs Wastewater Treatment Plant.
| Sample | pH | [DOC] (mg-C L−1) | E2:E3 | S275–295 | SUVA254 (L mg-C−1 m−1) | Alkalinity (g-CaCO3 L−1) |
|---|---|---|---|---|---|---|
| Primary Effluent | 7.37 | 28.4 ± 0.5 | 6.64 ± 0.02 | 0.020 ± 0.000 | 1.16 ± 0.02 | 0.467 |
| A/O Effluent | 8.39 | 8.1 ± 0.2 | 5.24 ± 0.03 | 0.011 ± 0.000 | 2.17 ± 0.07 | 0.295 |
| A/O Secondary Clarifier | 7.83 | 7.9 ± 0.2 | 5.41 ± 0.02 | 0.011 ± 0.000 | 2.22 ± 0.06 | 0.292 |
| UCT Effluent | 8.09 | 9.0 ± 0.6 | 5.06 ± 0.01 | 0.012 ± 0.000 | 1.92 ± 0.14 | 0.313 |
| UCT Secondary Clarifier | 7.92 | 8.7 ± 0.3 | 5.46 ± 0.01 | 0.012 ± 0.000 | 2.03 ± 0.08 | 0.288 |
| UV Influent | 7.92 | 8.2 ± 0.4 | 5.45 ± 0.02 | 0.011 ± 0.000 | 2.13 ± 0.09 | 0.285 |
| UV Effluent | 7.95 | 8.5 ± 0.3 | 5.34 ± 0.03 | 0.011 ± 0.000 | 2.03 ± 0.08 | 0.281 |
E2:E3 and S275–295 describe the slope of the UV–vis spectrum of DOM and are inversely related to molecular weight (Fichot and Benner, 2012; Maizel and Remucal, 2017). E2:E3 within the conventional treatment train of the Nine Springs plant ranges from 5.06 in the UCT effluent to 6.64 in the primary effluent (Table 1). E2:E3 increases from 3.88 ± 0.05 to 5.19 ± 0.01 within the Ostara treatment unit. E2:E3 values are typically 3.5–5 in terrestrially-impacted aquatic systems (Peterson et al., 2012) and 7–10 in microbially-dominated freshwater or marine systems (Helms et al., 2008; Peterson et al., 2012). The E2:E3 values of the Nine Springs secondary effluents are within the range of values previously reported for secondary effluent (i.e., 4.6–7.0; Bodhipaksha et al., 2017; Mostafa and Rosario-Ortiz, 2013), while E2:E3 has not been previously reported for primary effluent. The E2:E3 values indicate that the molecular weight of most of the Nine Springs samples is similar to microbially-derived DOM, while the relatively high molecular weight of the Ostara influent is more similar to terrestrially-derived DOM. S275–295 has not been previously reported in wastewater samples, but behaves similarly to E2:E3 throughout the treatment plant (Table 1).
Changes in SUVA254, E2:E3, S275–295, and bulk chemical concentrations provide insight into changes in the composition of DOM as it passes through the treatment plant. For all parameters, the largest differences are observed between the primary effluent and both secondary effluents. DOC, alkalinity, E2:E3, and S275–295 decrease, while SUVA254 increases during secondary treatment (Table 1), indicating that biological treatment produces DOM that is slightly more aromatic and higher in molecular weight. These changes are accompanied by a decrease in chloride and sodium concentrations, and an increase in the concentration of nitrate (Tables S1–S2). The optical properties, DOC, alkalinity, anions, and cations are essentially constant in the remainder of the plant. For example, the secondary clarifiers result in a very small increase in both SUVA254 and E2:E3, while UV disinfection slightly decreases both parameters. In contrast, the large increase in E2:E3 within the Ostara treatment unit indicates that struvite precipitation decreases the molecular weight of the DOM.
The increase in SUVA254 and decrease in E2:E3 observed between the primary and secondary effluents agrees with the limited data available in previous studies. For example, SUVA254 is higher in secondary effluent compared to raw sewage or primary effluent (Barber et al., 2001; Park et al., 2010). Likewise, treatment plants with nitrification systems produce secondary effluent with higher SUVA254 values compared to plants with poor or no nitrification (Krasner et al., 2009). While E2:E3 has not been previously studied throughout a treatment plant, secondary treatment leads to a higher molecular weight distribution measured using sequential ultrafiltration (Esparza-Soto et al., 2006). This data agrees with the observation of a decrease in E2:E3 during secondary treatment. In contrast, UV disinfection was previously reported to result in a small increase in E2:E3 (Mostafa and Rosario-Ortiz, 2013); the opposite trend is observed in the Nine Springs treatment plant. The assessment of both SUVA254 and E2:E3 throughout a wastewater treatment plant provides evidence that secondary treatment results in DOM that is more aromatic and higher in molecular weight compared to primary effluent. Furthermore, the optical measurements suggest that other processes (e.g., secondary clarification or UV disinfection) result in subtle changes in DOM composition.
3.2. Molecular composition of DOM
The molecular composition of the Nine Spring samples was determined using FT-ICR MS with both negative and positive electrospray ionization. Each sample produced 17,568–20,422 peaks in negative mode and 16,565–19,478 peaks in positive mode (Figures S2–S3; Tables S3–S4). The resulting mass spectra were analyzed to determine molecular formulas containing C, H, O, N, P, S, and Cl in negative mode, and for formulas containing C, H, O, and N in positive mode. Very few P, S, and Cl-containing formulas were determined using positive mode in preliminary data analysis and these heteroatoms were not included in the final analysis. In total, 2,106–3,013 unique molecular formulas were identified in each sample using negative mode (Table 2), and 815–1,949 formulas were identified in positive mode (Table S4). The number of assigned negative mode formulas is similar to past studies of wastewater effluent by FT-ICR MS (Gonsior et al., 2011; Tseng et al., 2013).
Table 2.
The total number of formulas identified by FT-ICR MS in negative mode for each sample, as well as the average (±the standard deviation) of the H:C, O:C, DBE, AI, mlbl, and mass accuracy of all assigned formulas.
| Sample | Total | H:Cavg | O:Cavg | DBEavg | AIavg | MLBL (%) | error (ppm) |
|---|---|---|---|---|---|---|---|
| Primary Effluent | 2227 | 1.48 ± 0.35 | 0.48 ± 0.18 | 5.36 ± 3.24 | 0.06 ± 0.35 | 49.1 | 0.01 ± 0.10 |
| A/O Effluent | 2995 | 1.30 ± 0.33 | 0.52 ± 0.18 | 7.24 ± 3.55 | 0.17 ± 0.31 | 28.9 | 0.00 ± 0.13 |
| A/O Secondary | 2754 | 1.24 ± 0.31 | 0.49 ± 0.16 | 8.23 ± 3.70 | 0.24 ± 0.23 | 22.7 | −0.01 ± 0.17 |
| UCT Effluent | 3013 | 1.33 ± 0.33 | 0.48 ± 0.17 | 7.07 ± 3.60 | 0.18 ± 0.26 | 31.9 | −0.01 ± 0.12 |
| UCT Secondary | 2668 | 1.32 ± 0.32 | 0.50 ± 0.17 | 7.19 ± 3.43 | 0.17 ± 0.33 | 30.4 | 0.00 ± 0.17 |
| UV Influent | 2892 | 1.29 ± 0.32 | 0.50 ± 0.17 | 7.70 ± 3.67 | 0.19 ± 0.32 | 27.9 | 0.00 ± 0.16 |
| UV Effluent | 2924 | 1.30 ± 0.32 | 0.49 ± 0.16 | 7.35 ± 3.37 | 0.19 ± 0.26 | 29.0 | 0.02 ± 0.35 |
| Ostara Influent | 2106 | 1.39 ± 0.33 | 0.45 ± 0.16 | 6.97 ± 3.82 | 0.15 ± 0.24 | 40.1 | 0.04 ± 0.37 |
| Ostara Effluent | 2475 | 1.39 ± 0.33 | 0.44 ± 0.15 | 6.99 ± 3.68 | 0.17 ± 0.23 | 39.5 | −0.05 ± 0.10 |
The resulting formulas for each sample are presented using van Krevelen diagrams in which the H:C ratio for each identified formula is plotted versus its O:C ratio (Figs. 2–3, S4–S21). van Krevelen diagrams are a useful way to visualize the large number of formulas in each sample because different regions correspond to different compound classes. For example, formulas with H:C values of 1.5–2.2 and O:C values 0–0.3 correspond to lipid-like compounds (Table S5; Hockaday et al., 2009; Sleighter and Hatcher, 2007). Although these class assignments are not definitive (i.e., a lignin-like formula is not necessarily derived from lignin), they provide insight into the general compound classes present in each sample. This section discusses the compound classes present in the Nine Springs samples, while Section 3.3 discusses the changes occurring in each unit process.
Fig. 2.
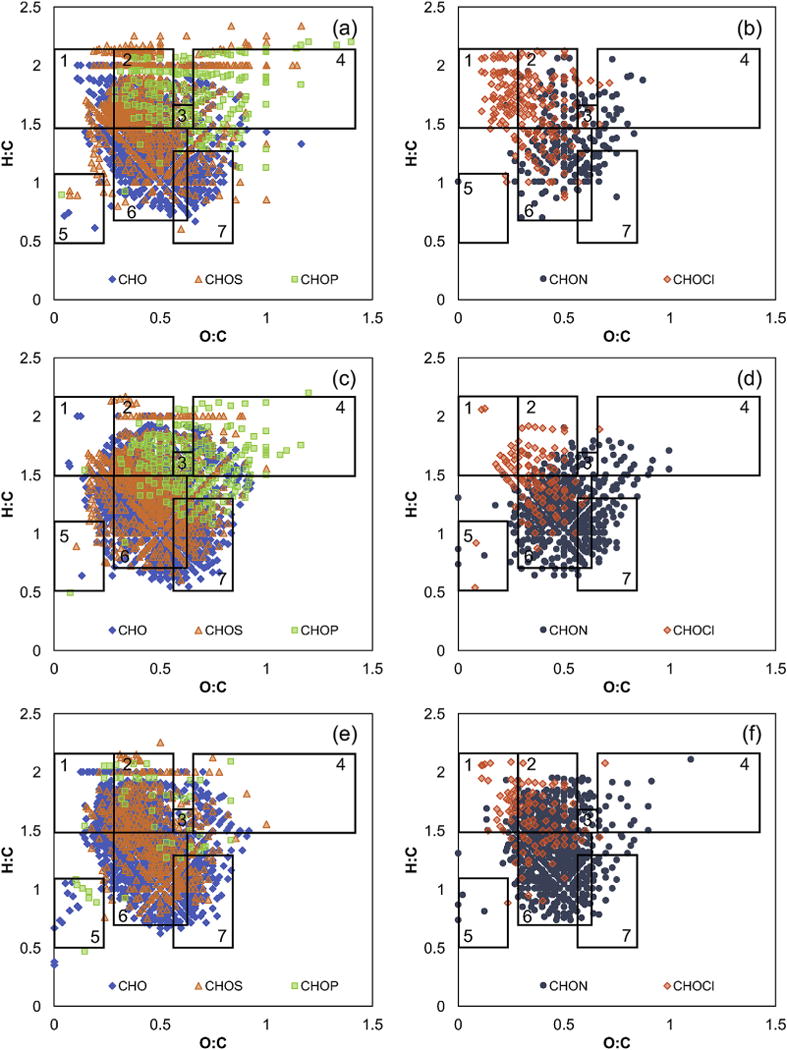
Representative van Krevelen diagrams showing all formula classes for (a, b) primary effluent, (c, d) final effluent, and (e, f) Ostara effluent samples determined using negative mode FT-ICR MS. The rectangles indicate regions associated with (1) lipids, (2) proteins, (3) aminosugars, (4) carbohydrates, (5) condensed hydrocarbons, (6) lignin, and (7) tannin.
Fig. 3.
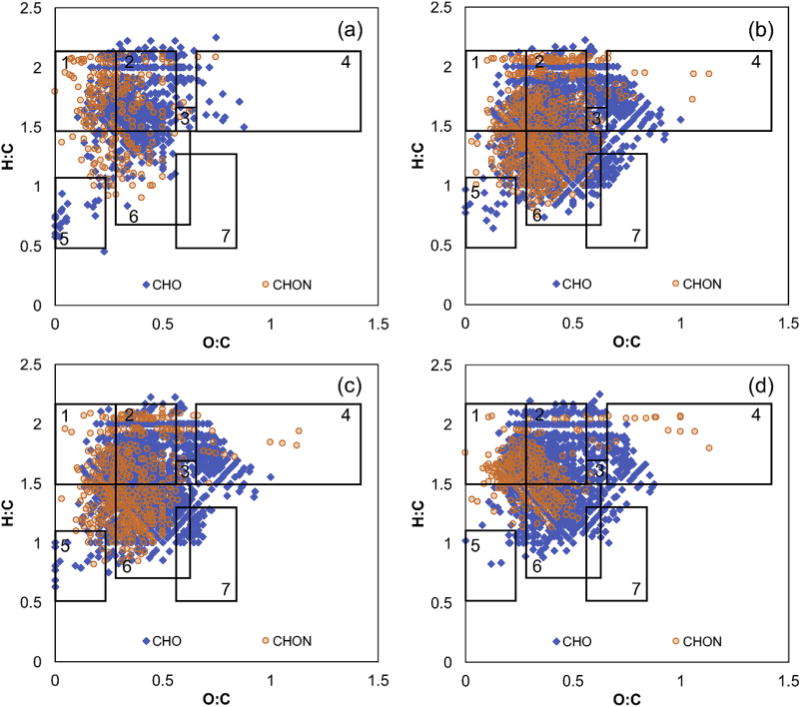
Representative van Krevelen diagrams showing all formula classes for (a) primary effluent, (b) UCT secondary clarifier effluent, (c) final effluent, and (d) Ostara effluent samples determined using positive mode FT-ICR MS. The rectangles indicate regions associated with (1) lipids, (2) proteins, (3) aminosugars, (4) carbohydrates, (5) condensed hydrocarbons, (6) lignin, and (7) tannin.
The formulas containing only C, H, and O are the most abundant formula class, comprising 38.2–43.3% of all identified formulas in negative mode (Table S3) and 53.3–83.3% of all identified formulas in positive mode (Table S4). The dominance of CHO formulas agrees with one previous study of secondary and secondary clarifier effluents using negative ESI FT-ICR MS, which found that 52.7–61.1% of the formulas contained only C, H, and O when CHOS were considered for formula assignments (Tseng et al., 2013). In contrast, two previous FT-ICR MS studies that were limited to CHOS formula assignments found that primary effluent and secondary effluent were dominated by CHOS formulas, with 27–34% of formulas containing only C, H, and O (Gonsior et al., 2011; Tseng et al., 2013). The other sample types included here (e.g., UV disinfection and struvite precipitation) have not been previously analyzed by high-resolution mass spectrometry.
The CHO formulas are found in a wide range of compound classes depending on the sample type. For example, the primary effluent and Ostara effluent contain many formulas in the lipid, protein, and lignin-like regions based on the negative mode formula assignments (Fig. 2). The secondary effluents, secondary clarifier effluents, and final effluent also have many protein- and lignin-like formulas, but have fewer lipid-like and more tannin-like formulas compared to primary effluent (Fig. 2b and S5–S10). The dominant compound classes for CHO formulas determined using positive mode ESI are similar (i.e., primarily lipid-, protein-, and lignin-like formulas; Fig. 3), but many of the low H:C and high O:C formulas present in the negative mode samples are absent (Figure S22). A similar trend was observed when Suwannee River fulvic acid was analyzed by both negative and positive mode ESI FT-ICR MS due to preferential ionization of certain compounds depending on the polarity (Hertkorn et al., 2008). Thus, even though there are hundreds to thousands of formulas detected in these samples, it is important to note that only a subset of the compounds present in DOM are amenable to the selected ionization modes. Despite this caveat, the CHO compound classes detected throughout the Nine Springs treatment plant agree with the general classification of the types of compounds expected in municipal wastewater treatment systems. For example, effluent organic matter is generally considered to contain recalcitrant organic matter from drinking water sources (i.e., lignin-like compounds), synthetic organic compounds produced during domestic use and disinfection, and soluble microbial products from biological processes (e.g., proteins, carbohydrates, and lipids; Shon et al., 2006). In addition, primary effluent contains a mixture of biodegradable and recalcitrant organic matter derived from human and industrial waste (Park et al., 2010). In contrast, terrestrially-derived aquatic DOM is generally comprised primarily of lignin- and tannin-like compounds, with nearly all formulas with H:C < 1.5 (Hertkorn et al., 2008; Maizel and Remucal, 2017).
Molecular formulas containing a single sulfur atom are common throughout the Nine Springs plant and comprise 17.7–31.6% of all assigned formulas (Table S3). Wastewater effluent is typically elevated in sulfur content relative to natural DOM due to the presence of detergents, surfactants, amino acids, and pharmaceuticals (Greenwood et al., 2012). Past FT-ICR MS studies of wastewater have focused on CHOS formulas because they are highly abundant and ionize well in negative mode, as observed here. These studies report that 73% of primary effluent, 40.6–42.2% of secondary effluent, and 8–18% of advanced effluent formulas contain S, all of which are higher than the abundance of S-containing formulas in natural DOM samples (Gonsior et al., 2011; Mesfioui et al., 2012; Tseng et al., 2013). All of the Nine Springs samples have CHOS formulas within the protein and lignin-like regions, while the primary effluent also contains lipid- and carbohydrate-like CHOS formulas (Fig. 2 and S4–S12). Compared to CHO formulas, there are numerous CHOS formulas with H:C ≥ 2.0. The location of the CHOS formulas observed here agrees with past studies (Gonsior et al., 2011; Mesfioui et al., 2012; Tseng et al., 2013). The most intense CHOS formulas detected here have been previously identified as linear alkyl benzene sulfonates and their metabolites (Gonsior et al., 2011; Tseng et al., 2013). The absence of numerous CHOS formulas in positive mode has been reported previously and is likely due to the limited ability of sulfonates to undergo positive ionization (Gonsior et al., 2011).
N-containing formulas comprise 9.5–25.6% of negative mode formulas and 16.7–46.7% of positive mode formulas in the Nine Spring plant (Tables S3–S4). N-containing compounds derived from proteins and amino sugars should be common in wastewater based on measurements using elemental analysis, Fourier transform infrared spectroscopy, and pyrolysis gas chromatography-mass spectrometry (Sirivedhin and Gray, 2005). Despite the expected abundance of CHON compounds, nitrogen-containing formulas have received minimal attention in past FT-ICR MS studies of wastewater and have been limited to negative mode ESI. For example, CHON formulas made up 9–19% of formulas detected by FT-ICR MS in tertiary effluent (Mesfioui et al., 2012). In contrast, N-containing formulas could not be assigned to any peaks observed using negative ESI FT-ICR MS in primary or secondary effluent (Tseng et al., 2013). While compounds with amine functional groups should ionize well in positive mode (Arnold et al., 2014), the interpretation of positive mode ESI spectra is complicated by the presence of sodium adducts (Koch et al., 2005); this challenge may explain why positive mode ESI data of wastewater-related samples has not been previously reported. To address this issue, both [M+H]+ and [M+Na]+ ions were considered for the assignment of CHON formulas (Koch et al., 2005) and only formulas that were part of a +CH2 or (+CH4 – O) homologous series were considered (Koch et al., 2007; Kujawinski and Behn, 2006). In this data set, 66.2–96.7% of the assigned CHON formulas were present [M+H]+ as ions and 1.7–10.9% of the CHON formulas were found as both [M+H]+ and [M+Na]+ ions; duplicate formula assignments are not included in the reported number of assigned formulas (Table S4).
The CHON formulas fall within the expected regions of the van Krevelen diagram. The negative mode CHON formulas are generally protein- and lignin-like, while the secondary effluents, secondary clarifier effluents, and UV effluents contain additional carbohydrate-like formulas that are absent in the primary effluent (Fig. 2 and S4–S12). The positive mode CHON formulas are also present in the protein-like region but are shifted towards lower O:C values, with fewer lignin-like and many more lipid-like formulas (Fig. 3 and S13–S21). Similarly, negative mode CHON formulas in the protein-like (i.e., aliphatic) and lignin-like regions were found in tertiary wastewater effluent (Mesfioui et al., 2012) and DOM derived from the sludge of continuously-stirred tank biogas reactors (Shakeri Yekta et al., 2012), which also contains protein-derived compounds of human and microbial origin. Although positive mode CHON formulas have not been previously reported in wastewater, they have been identified in septic-impacted groundwater using FT-ICR MS (Arnold et al., 2014). The CHON formulas in the septic-impacted samples were found in the lipid- and protein-like regions, in agreement with the Nine Springs samples. Unlike the data presented here, the septic-impacted groundwater had numerous condensed aromatic CHON formulas and fewer lignin-like formulas.
The presence of P-containing formulas is highly variable in the Nine Springs plant, ranging from 19 formulas in the Ostara influent to 240 formulas in the UV disinfection influent (0.9–8.3% of all formulas; Table S3). The CHOP formulas are in the protein, amino sugar and carbohydrate regions (Fig. 2 and S4–S12), and are typically highly saturated (i.e., high H:C) and highly oxygenated (i.e., high O:C) compared to the CHON and CHOS formulas. The secondary clarifier and UV effluents also contain lignin- and tannin-like CHOP formulas. The Ostara influent and effluent have very few CHOP formulas, likely because the preceding treatment steps are designed to convert P into an inorganic form (Figure S1; Cornel and Schaum, 2009). Very few CHOP formulas are present at S/N > 3 in the UCT secondary effluent, which is unexpected because the primary effluent and the UCT secondary clarifier effluent have ~200 CHOP formulas. Many of the expected formulas are found at lower S/N, but not shown here due to the conservative formula assignment criteria. Organophosphorus formulas are rarely reported in FT-ICR MS studies of natural aquatic DOM because of the low abundance of P compounds and the challenge of resolving low mass differences between CHOP and CHO formulas (Reemtsma, 2009). Although CHOP formulas have not been previously reported for wastewater-derived samples, organic phosphorus compounds are present in municipal wastewater (Cornel and Schaum, 2009; Gu et al., 2011; Qin et al., 2015) and the types of formulas presented here are similar to the lipid- and carbohydrate-like CHOP formulas in Dismal Swamp water (Sleighter and Hatcher, 2008). These formulas are likely derived from phospholipids and phosphorylated carbohydrates, and are indicative of autochthonous primary production (Sleighter and Hatcher, 2008).
Organochlorine formulas comprise 2.8–8.0% of all identified formulas in the Nine Springs samples, with up to 227 unique formulas in a single sample (Table S3). The CHOCl formulas were verified by the presence of the 37Cl isotope and are generally in the lipid-, protein-, and lignin-like regions (Fig. 2 and Figures S4–S12). These compounds may derive from chlorination of drinking water or from pharmaceuticals present in raw wastewater (Heberer, 2002; Loraine and Pettigrove, 2006). Cl-containing compounds (e.g., chlorobutanoic acid and diclofenac) were detected in wastewater effluent and are markers of anthropogenic wastewater effluent (Heberer, 2002; Sirivedhin and Gray, 2005). Previous FT-ICR MS studies of wastewater-related samples did not consider the presence of organochlorine formulas.
3.3. Effect of processes on molecular composition
FT-ICR MS analysis of the Nine Springs samples reveals that the DOM is elevated in heteroatoms (i.e., N, S, P, and Cl) and that the formulas are generally lipid-, protein-, carbohydrate-, and lignin-like. However, this analysis does not provide information about how the specific processes within the wastewater treatment plant alter the composition of the DOM. The variability amongst the nine samples was first assessed using Bray-Curtis dissimilarity analysis of all formulas detected in negative mode (Fig. 4). Of the samples in the conventional treatment train, the largest difference is observed between the primary effluent and the two secondary effluents. Interestingly, the A/O and UCT secondary effluents do not cluster together, suggesting that the secondary treatment processes produce DOM with differing composition. Furthermore, the respective secondary clarifier effluents are distinct from the secondary effluents, suggesting that the physical settling process further alters the composition of DOM. The Ostara influent and effluent are different in composition from the other samples, which is expected because the DOM in these samples originates from dewatered sludge (Figure S1).
Fig. 4.
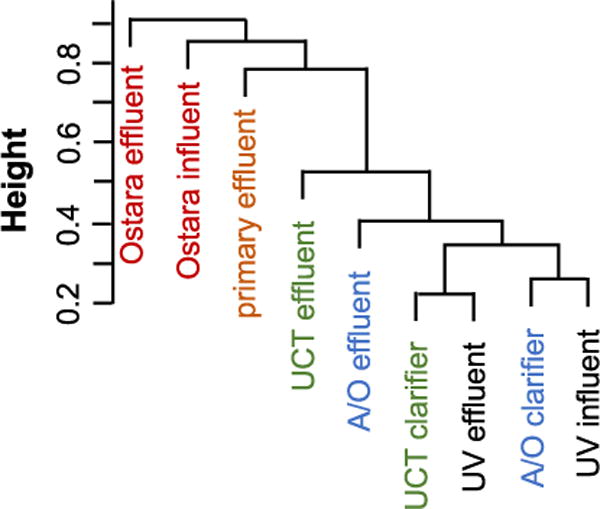
Bray-Curtis dissimilarity analysis of all assigned negative mode DOM formulas throughout the Nine Springs treatment plant.
The molecular composition of DOM was compared to determine which types of formulas are produced or consumed by each treatment process. Samples collected before and after a treatment process were first assessed for the presence of formulas that were unique to the influent or effluent. Next, formulas found in both the influent and effluent were compared using the relative intensity index (Equation S1) to determine which formulas were relatively enriched or depleted during the treatment process. While Bray-Curtis analysis indicates which samples are different from each other based on the presence or absence of individual formulas, this analysis provides information about how the DOM composition changes throughout the treatment plant.
3.3.1. Effect of secondary treatment
The largest change in optical properties and in molecular composition during a single treatment process is observed before and after the secondary treatment processes (Fig. 4). The increase in SUVA254 and decrease in E2:E3 and S275–295 during secondary treatment suggests that DOM becomes more aromatic and higher in molecular weight during biological processing, likely due to preferential removal of less aromatic, lower molecular weight DOM (Table 1). Similarly, the decrease in H:Cavg and increase in DBEavg and AIavg during secondary treatment also suggest that the DOM becomes more aromatic (Table 2). The decrease in MLBL suggests that the DOM becomes less bioavailable after secondary treatment (Table 2 and S4). No change is observed in O:Cavg across the treatment plant, which indicates that the effluent DOM is not more oxygenated than influent DOM. The H:Cavg and O:Cavg reported here agree with previously published values of 1.23 and 0.47 for CHO formulas in secondary effluent, respectively (Gonsior et al., 2011).
The change in DOM composition during A/O secondary treatment was further assessed by comparing the change in bulk metrics (i.e., H:C, O:C, and DBE) calculated for individual formula types and by inspecting changes in van Krevelen diagrams. The number of formulas containing C, H, O, N, and/or P increases during A/O secondary treatment (Tables S6–S7). The number of CHOS formulas is roughly constant during A/O secondary treatment, while the number of unique CHOCl formulas decreases from 104 to 55. For all formula types in both positive and negative mode, there is a large decrease in H:C, a large increase in DBE, and a smaller increase in O:C during secondary treatment (Tables S6–S7). For example, the DBE of unique CHO formulas is 5.21 in primary effluent and 9.36 in A/O secondary effluent. These changes are clearly observed in van Krevelen diagrams (Fig. 5 and S24–S26). Overall, many of the highly saturated formulas (i.e., H:C ≥ 2) are absent after A/O secondary treatment and the organic matter becomes more aromatic, in agreement with the increase in SUVA254. Furthermore, the increase in CHO, CHON, and CHOP formulas suggests that the organic matter becomes more complex during biological processing.
Fig. 5.
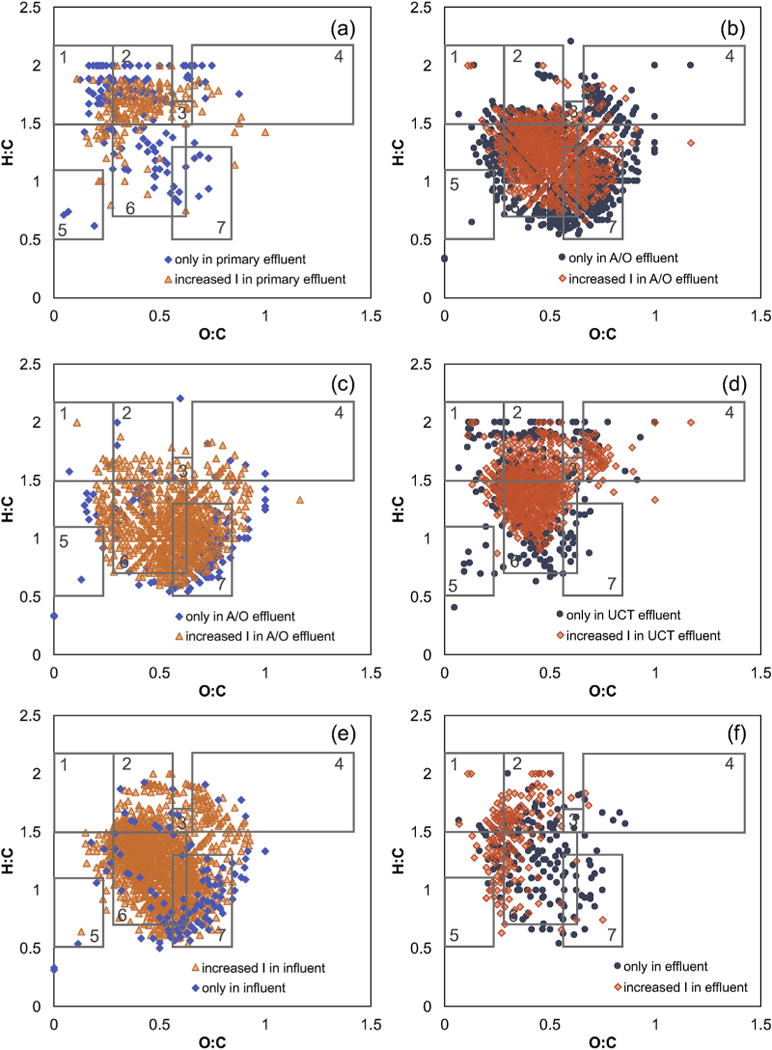
Representative van Krevelen diagrams showing negative mode CHO formulas that were unique to or had increased relative intensity (a) in primary effluent compared to A/O secondary effluent, (b) in A/O secondary effluent compared to primary effluent, (c) in A/O secondary effluent compared to UCT secondary effluent, (d) in UCT secondary effluent compared to A/O secondary effluent, and (e) before and (f) after UV disinfection.
UCT secondary treatment also increases the complexity and aromaticity of DOM, as shown by the increase in unique CHON and CHOS formulas, the decrease in H:C, and increase in DBE (Tables S8–S9; Figures S27–S29). However, a direct comparison of the secondary effluents demonstrates that the A/O and UCT processes produce DOM with differing compositions. There are more common CHON and CHOS formulas in the two secondary effluents compared to unique formulas (Tables S10–S11; Figures S30–S32). Interestingly, there are more unique CHOCl formulas in UCT secondary effluent compared to both primary effluent (Table S8) and A/O secondary effluent (Table S10), suggesting that the UCT process may favor the formation of novel chlorinated compounds. It is not possible to compare the effect of the UCT process on organophosphorus compounds due to S/N limitations in that sample (Table S3), as described above. Despite the differences in the number of unique formulas in the secondary effluents, the change in molecular composition during the two secondary treatment processes is consistent for all formula classes. Specifically, the UCT process produces DOM that has higher H:C, slightly lower O:C, and much lower DBE compared to DOM produced by the A/O process (Fig. 5; Tables S10–S11). In other words, the UCT secondary treatment process does not alter DOM as significantly as the A/O process, as indicated by Bray-Curtis dissimilarity analysis (Fig. 4). The lower aromaticity of UCT secondary effluent compared to A/O secondary effluent agrees with the lower SUVA254 and bulk DBEavg (Table 2 and S4). However, the H:Cavg of the two samples calculated using all formulas is nearly identical (Table S4) and the higher H:Cavg of formulas that appear in the UCT secondary effluent is only evident when comparing formulas that are unique to or enriched in the secondary effluent (Tables S10–S11).
The optical properties and FT-ICR MS data demonstrate that secondary treatment produces DOM that is higher in molecular weight, more aromatic, less bioavailable, and more complex. Furthermore, the effluent produced by two different secondary treatment processes is distinct; the A/O effluent is more aromatic than the UCT effluent. A similar increase in molecular complexity was observed in two parallel secondary treatment systems that treated the same primary effluent (Tseng et al., 2013). However, the previous study found that the two secondary treatment processes (i.e., activated sludge and integrated fixed film activated sludge) produced DOM that was nearly identical (Tseng et al., 2013). Similarly, the DOM in activated sludge and trickling filter secondary effluents was indistinguishable (Gonsior et al., 2011). The mechanism that results in less DOM transformation in the UCT secondary treatment process is unclear. The UCT treatment unit has a longer hydraulic residence time than the A/O unit (i.e., 14 vs. 11 h), so the difference may be attributable to the anoxic tank that is included to achieve partial denitrification (Fig. 1).
3.3.2. Effect of secondary clarification
Secondary clarification is a physical treatment process that separates suspended biomass from the treated wastewater. This process results in subtle, but detectable, changes in the optical properties and molecular composition of DOM. Both secondary clarifiers result in a small increase in both SUVA254 and E2:E3 (Table 1), suggesting that the aromaticity of DOM increases while its molecular weight decreases. This result is unexpected because lower molecular weight DOM tends to be less aromatic (Maizel and Remucal, 2017). Bray-Curtis analysis demonstrates that the molecular composition of the secondary clarifier effluents is distinct from their respective secondary effluents (Fig. 4). However, the bulk properties calculated from all identified formulas do not provide insight into how secondary clarification alters the composition of DOM (Table 2 and S4). Specifically, the changes in H:Cavg, O:Cavg, DBEavg, AIavg, and MLBL are small and inconsistent when the type of clarifier (i.e., A/O or UCT) or FT-ICR MS polarity are compared.
The change in molecular composition of DOM during secondary clarification is best visualized when individual formula types are plotted in van Krevelen diagrams (Figures S33–S38). For CHON and CHOS formulas, the cluster of points on the van Krevelen diagram tightens as many of the extreme formulas (e.g., high H:C and O:C) are lost or decrease in intensity in both clarifiers. Most of the formulas with higher relative intensity after the secondary clarifiers are protein-, lignin-, and tannin-like, as the few lipid- and carbohydrate-like formulas are preferentially removed. Additionally, CHOS formulas with H:C > 2 are absent after secondary clarification. These changes are not consistently observed when bulk parameters are calculated for each formula class (Tables S12–S15). For example, O:C decreases and DBE increases for CHON, CHOS, and CHOP negative mode formulas in the A/O secondary clarifier, but the trends in the UCT secondary clarifier are dependent upon the formula type (e.g., DBE increases for CHO formulas, but decreases for CHON formulas). The lack of clear trends in bulk metrics may be attributable the fact that many formulas present before and after the clarifiers fall in the same van Krevelen space or because changes in extreme formulas cancel each other out when the data are averaged (e.g., loss of low O:C and high O:C formulas does not change O:Cavg). This analysis suggests that multiple methods should be used to visualize FT-ICR MS data (e.g., Bray-Curtis analysis and van Krevelen diagrams) and that bulk metrics (e.g., H:Cavg, O:Cavg, and DBEavg) may not detect subtle changes in DOM composition during treatment processes.
3.3.3. Effect of UV disinfection
The blended secondary clarifier effluent is exposed to 254 nm light for <5 min during UV disinfection, resulting in a small decrease in SUVA254 (Table 1). A decrease in aromaticity was previously observed when DOM was photobleached using wavelengths within the solar spectrum for long irradiation periods (Sharpless et al., 2014). The slight decrease in E2:E3 from 5.45 to 5.34 (Table 1) is unexpected and does not agree with photo-bleaching studies using simulated sunlight or UV irradiation (Mostafa and Rosario-Ortiz, 2013; Sharpless et al., 2014). However, the change in E2:E3 observed here and the one previously reported wastewater sample is small (i.e., <0.2; Mostafa and Rosario-Ortiz, 2013) and there is no change in S275–295, which does not indicate a major shift in molecular weight in either study.
UV disinfection produces small changes in the molecular composition of DOM. The only detectable change in bulk FT-ICR MS metrics is a decrease in DBEavg from 7.70 to 7.35, calculated using all negative mode formulas (Table 2). The opposite trend is observed in DBEavg calculated using all positive mode formulas, and there is no clear shift in H:Cavg, O:Cavg, AIavg, or MLBL in either polarity (Table 2 and S4). Examination of the different formula classes demonstrate that most formulas are common in the UV influent and effluent (i.e., there are fewer unique formulas; Tables S16–S17). However, many formulas are more intense before UV irradiation, implying that photolysis decreases the relative intensity of some compounds. There is a clear shift toward higher H:C, lower O:C, and lower DBE in unique CHO formulas (Table S16; Fig. 5), in agreement with the observed decrease in SUVA254. While the average O:C and DBE values of the CHOS and CHON formulas also decrease during photolysis (Tables S16–S17), the formulas occupy the same regions of the van Krevelen diagrams before and after UV disinfection (Figures S40–S41). Few CHOP and CHOCl formulas are present in these samples. Therefore, the effect of UV disinfection on molecular composition is not consistent among formula types and primarily results in changes in CHO formulas, in contrast with the consistent changes observed during other wastewater treatment processes.
3.3.4. Effect of struvite precipitation
The Ostara treatment process utilizes magnesium chloride and sodium hydroxide to precipitate struvite. The molecular composition of DOM in the Ostara influent and effluent is unique compared the other Nine Springs samples because the DOM is derived from dewatering sludge (Figure S1). The Ostara process is also unique because it results in no detectable change in the molecular composition of DOM. Although the increase in E2:E3 from 3.88 to 5.19 within the Ostara treatment unit suggests that the molecular weight of DOM decreases during the chemical precipitation process, there is no corresponding shift in molecular composition determined using FT-ICR MS. For example, there is no change in H:Cavg, O:Cavg, DBEavg, AIavg, or MLBL before or after the Ostara process calculated using either all negative or positive mode formulas, or when formula types are assessed individually (Table 2, S4, S18, and S19). While there are numerous unique formulas in the Ostara influent and effluent, the formulas generally occupy the same regions of the van Krevelen diagram (Figures S42–S44).
3.4. Relationship between DOM composition and optical properties
The optical properties generally followed the same trends as the FT-ICR MS-determined molecular composition of DOM within the main treatment train of the Nine Springs plant. For example, the increase in SUVA254 observed during the secondary treatment processes was accompanied by a decrease in H:Cavg (Tables 1 and 2). To investigate whether the same trend was observed in individual formulas, Pearson correlation coefficients were calculated for correlations between the relative intensity of each formula and the SUVA254, E2:E3, or S275–295 value determined in all seven samples in the main treatment train. Of these 769 CHO or CHON formulas, 690, 692, and 701 formulas had correlations with r ≤ −0.5 or ≥0.5 with SUVA254, E2:E3, and S275–295, respectively. Lignin- and tannin-like formulas with low H:C values correlated positively with SUVA254 and negatively with E2:E3 and S275–295 (Fig. 6 and S45). These formulas may be derived from terrestrial sources and are likely aromatic in nature, so it is unsurprising that they correlate with SUVA254, a measure of aromaticity (Weishaar et al., 2003). In contrast, protein- and carbohydrate-like formulas correlated negatively with SUVA254 and positively with E2:E3 and S275–295. Compounds in these classes may be derived from autochthonous (i.e., microbial) sources, which generally produce DOM that is low in molecular weight (i.e., with high E2:E3 and S275–295 values; Maizel and Remucal, 2017). Similar correlations between SUVA254 and the relative intensity of formulas detected by FT-ICR MS have been previously reported for DOM from natural systems (Kellerman et al., 2015), but this is the first report of this correlation in wastewater-derived samples. Correlations between E2:E3 or S275–295 and FT-ICR MS formula intensity have not been previously reported.
Fig. 6.
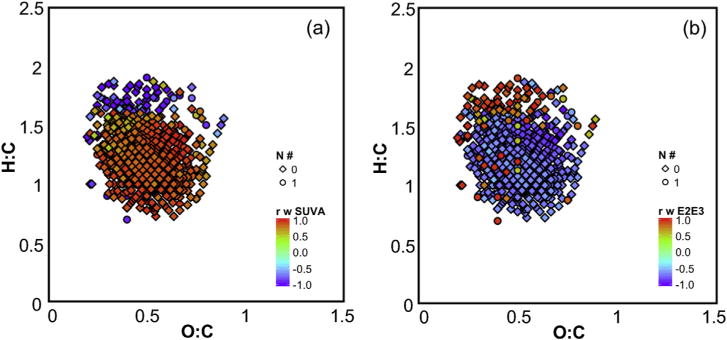
Pearson correlation coefficients calculated between the relative intensity of common FT-ICR MS CHO and CHON formulas and (a) SUVA254 or (b) E2:E3. The analysis was conducted using negative mode formulas in all samples excepting the Ostara influent and effluent. Only formulas with r ≤ −0.5 or ≥ 0.5 are shown.
4. Conclusions
This study combines optical property measurements with high-resolution mass spectrometry to assess how secondary treatment processes, secondary clarification, UV disinfection, and struvite precipitation alter the composition of DOM in a municipal wastewater treatment plant. Compared to DOM from natural aquatic systems, wastewater DOM is low in molecular weight and highly saturated. The molecular formulas determined using both positive and negative mode ESI FT-ICR MS are rich in heteroatoms and are generally present in the lipid-, protein-, carbohydrate-, and lignin-like regions of van Krevelen diagrams, which agrees with the types of compounds expected in wastewater. This study provides insight into the composition of DOM that must be removed during advanced treatment processes (e.g., membrane filtration, activated carbon absorption, or advanced oxidation processes) in water reuse applications. For example, the low aromaticity of the final effluent suggests that the DOM may not be as efficiently removed by activated carbon and that it will screen less light during UV-based advanced oxidation processes compared to DOM from natural systems.
The largest changes in DOM optical properties and composition are induced by secondary treatment, which results in the loss of highly saturated formulas and produces DOM that is more aromatic, more complex, and less bioavailable. The two secondary treatment processes produce DOM with distinct molecular composition, with the UCT process producing DOM that is less aromatic and less oxidized compared to DOM produced by the A/O process. Secondary clarification and UV disinfection result in subtle, but detectable, changes in DOM composition. The Ostara process decreases the molecular weight of DOM, but does not otherwise alter its composition. The optical properties generally follow the same trends as the molecular composition of DOM within the main treatment train of the Nine Springs plant, and formulas with low H:C values correlated positively with SUVA254 and negatively with E2:E3 and S275–295. The agreement between optical properties and FT-ICR MS data demonstrate that simple measurements may be appropriate for assessing bulk changes in DOM composition during wastewater treatment.
Supplementary Material
Acknowledgments
We thank Dr. Matt Seib (Madison Metropolitan Sewerage District) for assistance with collecting samples and for his insight into the treatment processes at the Nine Springs Wastewater Treatment Plant. Funding was provided by the University of Wisconsin-Madison Graduate School.
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.watres.2017.05.055.
References
- Arnold WA, Longnecker K, Kroeger KD, Kujawinski E. Molecular signature of organic nitrogen in septic-impacted groundwater. Environ Sci Process Impacts. 2014;16:2400–2407. doi: 10.1039/c4em00289j. [DOI] [PubMed] [Google Scholar]
- Barber LB, Leenheer JA, Noyes TI, Stiles EA. Nature and transformation of dissolved organic matter in treatment wetlands. Environ Sci Technol. 2001;35(24):4805–4816. doi: 10.1021/es010518i. [DOI] [PubMed] [Google Scholar]
- Bodhipaksha LC, Sharpless CM, Chin YP, MacKay AA. Role of effluent organic matter in the photochemical degradation of compounds of wastewater origin. Water Res. 2017;110:170–179. doi: 10.1016/j.watres.2016.12.016. [DOI] [PubMed] [Google Scholar]
- Cornel P, Schaum C. Phosphorus recovery from wastewater: needs, technologies and costs. Water Sci Technol. 2009;59(6):1069. doi: 10.2166/wst.2009.045. [DOI] [PubMed] [Google Scholar]
- D’Andrilli J, Cooper WT, Foreman CM, Marshall AG. An ultrahigh-resolution mass spectrometry index to estimate natural organic matter lability. Rapid Commun Mass Spectrom. 2015;29(24):2385–2401. doi: 10.1002/rcm.7400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmar T, Koch B, Hertkorn N, Kattner G. A simple and efficient method for the solid-phase extraction of dissolved organic matter (SPE-DOM) from seawater. Limnol Oceanogr Methods. 2008;6:230–235. [Google Scholar]
- Esparza-Soto M, Fox P, Westerhoff P. Transformation of molecular weight distributions of dissolved organic carbon and UV-absorbing compounds at full-scale wastewater-treatment plants. Water Environ Res. 2006;78(3):253–262. doi: 10.2175/106143005x90083. [DOI] [PubMed] [Google Scholar]
- Fichot CG, Benner R. The spectral slope coefficient of chromophoric dissolved organic matter (S275–295) as a tracer of terrigenous dissolved organic carbon in river-influenced ocean margins. Limnol Oceanogr. 2012;57(5):1453–1466. [Google Scholar]
- Gonsior M, Zwartjes M, Cooper WJ, Song W, Ishida KP, Tseng LY, Jeung MK, Rosso D, Hertkorn N, Schmitt-Kopplin P. Molecular characterization of effluent organic matter identified by ultrahigh resolution mass spectrometry. Water Res. 2011;45(9):2943–2953. doi: 10.1016/j.watres.2011.03.016. [DOI] [PubMed] [Google Scholar]
- Greenwood PF, Berwick LJ, Croué JP. Molecular characterisation of the dissolved organic matter of wastewater effluents by MSSV pyrolysis GC–MS and search for source markers. Chemosphere. 2012;87:504–512. doi: 10.1016/j.chemosphere.2011.12.051. [DOI] [PubMed] [Google Scholar]
- Gu AZ, Liu L, Neethling JB, Stensel HD, Murthy S. Treatability and fate of various phosphorus fractions in different wastewater treatment processes. Water Sci Technol. 2011;63(4):804. doi: 10.2166/wst.2011.312. [DOI] [PubMed] [Google Scholar]
- Guest JS, Skerlos SJ, Barnard JL, Beck MB, Daigger GT, Hilger H, Jackson SJ, Karvazy K, Kelly L, Macpherson L, Mihelcic JR, Pramanik A, Raskin L, Van Loosdrecht MCM, Yeh D, Love NG. A new planning and design paradigm to achieve sustainable resource recovery from wastewater. Environ Sci Technol. 2009;43(16):6126–6130. doi: 10.1021/es9010515. [DOI] [PubMed] [Google Scholar]
- Heberer T. Tracking persistent pharmaceutical residues from municipal sewage to drinking water. J Hydrol. 2002;266(3):175–189. [PubMed] [Google Scholar]
- Helms JR, Stubbins A, Ritchie JD, Minor EC, Kieber DJ, Mopper K. Absorption spectral slopes and slope ratios as indicators of molecular weight, source, and photobleaching of chromophoric dissolved organic matter. Limnol Oceanogr. 2008;53(3):955–969. [Google Scholar]
- Hering JG, Waite TD, Luthy RG, Drewes JE, Sedlak DL. A changing framework for urban water systems. Environ Sci Technol. 2013;47(19):10721–10726. doi: 10.1021/es4007096. [DOI] [PubMed] [Google Scholar]
- Hertkorn N, Frommberger M, Witt M, Koch BP, Schmitt-Kopplin P, Perdue EM. Natural organic matter and the event horizon of mass spectrometry. Anal Chem. 2008;80(23):8908–8919. doi: 10.1021/ac800464g. [DOI] [PubMed] [Google Scholar]
- Hockaday WC, Purcell JM, Marshall AG. Electrospray and photoionization mass spectrometry for the characterization of organic matter in natural waters: a qualitative assessment. Limnol Oceanogr. 2009;7:81–95. [Google Scholar]
- Kellerman AM, Kothawala DN, Dittmar T, Tranvik LJ. Persistence of dissolved organic matter in lakes related to its molecular characteristics. Nat Geosci. 2015;8(6):454–457. [Google Scholar]
- Koch BP, Dittmar T. From mass to structure: an aromaticity index for high-resolution mass data of natural organic matter. Rapid Commun Mass Spectrom. 2006;20(5):926–932. [Google Scholar]
- Koch BP, Dittmar T, Witt M, Kattner G. Fundamentals of molecular formula assignment to ultrahigh resolution mass data of natural organic matter. Anal Chem. 2007;79(4):1758–1763. doi: 10.1021/ac061949s. [DOI] [PubMed] [Google Scholar]
- Koch BP, Witt M, Engbrodt R, Dittmar T, Kattner G. Molecular formulae of marine and terrigenous dissolved organic matter detected by electrospray ionization Fourier transform-ion cyclotron resonance mass spectrometry. Geochim Cosmochim Ac. 2005;69(13):3299–3308. [Google Scholar]
- Krasner SW, Westerhoff P, Chen B, Rittmann BE, Nam SN, Amy G. Impact of wastewater treatment processes on organic carbon, organic nitrogen, and DBP precursors in effluent organic matter. Environ Sci Technol. 2009;43(8):2911–2918. doi: 10.1021/es802443t. [DOI] [PubMed] [Google Scholar]
- Kujawinski EB, Behn MD. Automated analysis of electrospray ionization Fourier transform ion cyclotron resonance mass spectra of natural organic matter. Anal Chem. 2006;78(13):4363–4373. doi: 10.1021/ac0600306. [DOI] [PubMed] [Google Scholar]
- Loraine GA, Pettigrove ME. Seasonal variations in concentrations of pharmaceuticals and personal care products in drinking water and reclaimed wastewater in Southern California. Environ Sci Technol. 2006;40(3):687–695. doi: 10.1021/es051380x. [DOI] [PubMed] [Google Scholar]
- Maizel AC, Remucal CK. Molecular composition and photochemical reactivity of size-fractionated dissolved organic matter. Environ Sci Technol. 2017;51(4):2113–2123. doi: 10.1021/acs.est.6b05140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesfioui R, Love NG, Bronk DA, Mulholland MR, Hatcher PG. Reactivity and chemical characterization of effluent organic nitrogen from wastewater treatment plants determined by Fourier transform ion cyclotron resonance mass spectrometry. Water Res. 2012;46(3):622–634. doi: 10.1016/j.watres.2011.11.022. [DOI] [PubMed] [Google Scholar]
- Michael-Kordatou I, Michael C, Duan X, He X. Dissolved effluent organic matter: characteristics and potential implications in wastewater treatment and reuse applications. Water Res. 2015;77:213–248. doi: 10.1016/j.watres.2015.03.011. [DOI] [PubMed] [Google Scholar]
- Minor E, Stephens B. Dissolved organic matter characteristics within the Lake Superior watershed. Org Geochem. 2008;39:1489–1501. [Google Scholar]
- Mostafa S, Rosario-Ortiz FL. Singlet oxygen formation from wastewater organic matter. Environ Sci Technol. 2013;47(15):8179–8186. doi: 10.1021/es401814s. [DOI] [PubMed] [Google Scholar]
- Park MH, Lee TH, Lee BM, Hur J, Park DH. Spectroscopic and chromatographic characterization of wastewater organic matter from a biological treatment plant. Sensors. 2010;10(1):254–265. doi: 10.3390/s100100254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BM, McNally AM, Cory RM, Thoemke JD, Cotner JB, McNeill K. Spatial and temporal distribution of singlet oxygen in Lake Superior. Environ Sci Technol. 2012;46(13):7222–7229. doi: 10.1021/es301105e. [DOI] [PubMed] [Google Scholar]
- Phungsai P, Kurisu F, Kasuga I, Furumai H. Molecular characterization of low molecular weight dissolved organic matter in water reclamation processes using Orbitrap mass spectrometry. Water Res. 2016;100:526–536. doi: 10.1016/j.watres.2016.05.047. [DOI] [PubMed] [Google Scholar]
- Qin C, Liu H, Liu L, Smith S, Sedlak DL, Gu AZ. Bioavailability and characterization of dissolved organic nitrogen and dissolved organic phosphorus in wastewater effluents. Sci Total Environ. 2015;511:47–53. doi: 10.1016/j.scitotenv.2014.11.005. [DOI] [PubMed] [Google Scholar]
- Reemtsma T. Determination of molecular formulas of natural organic matter molecules by (ultra-) high-resolution mass spectrometry. J Chromatogr A. 2009;1216(18):3687–3701. doi: 10.1016/j.chroma.2009.02.033. [DOI] [PubMed] [Google Scholar]
- Shakeri Yekta S, Gonsior M, Schmitt-Kopplin P, Svensson BH. Characterization of dissolved organic matter in full scale continuous stirred tank biogas reactors using ultrahigh resolution mass spectrometry: a qualitative overview. Environ Sci Technol. 2012;46(22):12711–12719. doi: 10.1021/es3024447. [DOI] [PubMed] [Google Scholar]
- Sharpless CM, Aeschbacher M, Page SE, Wenk J, Sander M, McNeill K. Photooxidation-induced changes in optical, electrochemical, and photochemical properties of humic substances. Environ Sci Technol. 2014;48(5):2688–2696. doi: 10.1021/es403925g. [DOI] [PubMed] [Google Scholar]
- Shon HK, Vigneswaran S, Snyder SA. Effluent organic matter in wastewater: constituents, effects, and treatment. Crit Rev Environ Sci Technol. 2006;36(4):327–374. [Google Scholar]
- Sirivedhin T, Gray KA. Part I. Identifying anthropogenic markers in surface waters influenced by treated effluents: a tool in potable water reuse. Water Res. 2005;39:1154–1164. doi: 10.1016/j.watres.2004.11.032. [DOI] [PubMed] [Google Scholar]
- Sleighter RL, Hatcher PG. The application of electrospray ionization coupled to ultrahigh resolution mass spectrometry for the molecular characterization of natural organic matter. J Mass Spectrom. 2007;42(5):559–574. doi: 10.1002/jms.1221. [DOI] [PubMed] [Google Scholar]
- Sleighter RL, Hatcher PG. Molecular characterization of dissolved organic matter (DOM) along a river to ocean transect of the lower Chesapeake Bay by ultrahigh resolution electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry. Mar Chem. 2008;110(3–4):140–152. [Google Scholar]
- Stenson A, Marshall A, Cooper W. Exact masses and chemical formulas of individual Suwannee River fulvic acids from ultrahigh resolution electrospray ionization Fourier transform ion cyclotron resonance mass spectra. Anal Chem. 2003;75(6):1275–1284. doi: 10.1021/ac026106p. [DOI] [PubMed] [Google Scholar]
- Tseng LY, Gonsior M, Schmitt-Kopplin P, Cooper WJ, Pitt P, Rosso D. Molecular characteristics and differences of effluent organic matter from parallel activated sludge and integrated fixed-film activated sludge processes. Environ Sci Technol. 2013;47:10277–10284. doi: 10.1021/es4002482. [DOI] [PubMed] [Google Scholar]
- Urai M, Kasuga I, Kurisu F, Furumai H. Molecular characterization of dissolved organic matter in various urban water resources using Orbitrap Fourier transform mass spectrometry. Water Sci Technol Water Suppl. 2014;14(4):547. [Google Scholar]
- van Loosdrecht M, Brdjanovic D. Anticipating the next century of wastewater treatment. Science. 2014;344(6191):1452–1453. doi: 10.1126/science.1255183. [DOI] [PubMed] [Google Scholar]
- Weishaar JL, Aiken GR, Bergamaschi BA, Fram MS, Fujii R, Mopper K. Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon. Environ Sci Technol. 2003;37(20):4702–4708. doi: 10.1021/es030360x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


