Abstract
Fibromyalgia syndrome (FMS) is a highly prevalent, chronic musculoskeletal condition characterized by widespread pain and evoked pain at tender points. This study evaluated various aspects of body awareness in a sample of 14 women with FMS and 13 healthy controls, such as plasticity of the body schema, body esteem, and interoceptive awareness. To this end, the Rubber Hand Illusion (RHI), the Body Esteem Scale (BES), and the Body Perception Questionnaire (BPQ) were used, respectively. Consistent with increased plasticity of the body schema, FMS patients scored higher, with large or very large effect sizes, across all three domains evaluated in the RHI paradigm, namely proprioceptive drift and perceived ownership and motor control over the rubber hand. Scores on all items addressed by the BES were consistently lower among FMS subjects (2.52, SEM .19 vs 3.89, SEM .16, respectively, p < .01, Cohen’s d = .38-.66). In the FMS sample, BES scores assigned to most painful regions also were lower than those assigned to the remaining body sites (1.58, SEM .19 vs 2.87, SEM .18, respectively, p < .01). Significantly higher scores (p < .01, Cohen’s d = .51-.87) were found in the FMS sample across awareness (3.57 SEM .15 vs 1.87 SEM .11), stress response (3.76 SEM .11 vs 1.78 SEM .11), autonomic nervous system reactivity (2.59 SEM .17 vs 1.35 SEM .07), and stress style 2 (2.73 SEM .27 vs 1.13 SEM .04) subscales of the BPQ. Intensity of ongoing clinical pain was found to be strongly correlated with interoceptive awareness (r = .75, p = .002). The results suggest a disturbed embodiment in FMS, characterized by instability of the body schema, negatively biased cognitions regarding one’s own body, and increased vigilance to internal bodily cues. These manifestations may be interpreted as related with the inability of incoming sensory inputs to adequately update negatively biased off-line somatorepresentations stored as long-term memory.
Introduction
Fibromyalgia syndrome (FMS) is a chronic musculoskeletal condition characterized by widespread pain and evoked pain at tender points [1–2]. Most patients with FMS also present with co-morbid anxiety and depression, and common findings also include fatigue and non-restorative sleep, memory and cognitive impairment, muscle stiffness, and gastrointestinal disorders. In addition, findings from several studies are consistent with central sensitization in FMS patients [3–5]. Up to 5.8% of the population of industrial countries may suffer from FMS [6–9].
The experience of pain is modulated on a cognitive level depending on attention, anticipation, emotion and memory of previous pain [10–12]. In addition, the ability to localize and confine a sensation to the body requires an intact body representation, and there is increasing evidence supporting the association of chronic pain with disturbed mental representations of the body [13–15]. Two concepts commonly referred to in the context of body awareness are body schema and body image, consistent with the notion that perception and action require different sensory signal processing as posed by the general functional hypothesis. Body schema is viewed as the dynamic, action-oriented implicit representation of one’s own body, which reflects the position and movement of the body in space, whereas body image refers to a cognitive and interpretative representation that integrates the conscious perceptual corporeal experiences and contributes to beliefs and attitudes towards the body [16–18]. The pain experience is an embodied one, inasmuch as the body is one’s way of experiencing the world and harbors the body schema which makes a person’s pain experience possible. Maintenance of the body schema and body image depends on multisensory bodily inputs, and there is growing evidence that both body schema and body image may be altered in patients suffering from chronic pain. In patients presenting with complex regional pain syndrome (CRPS), for example, mental representation of movement of the painful body part has been found to be slower [19–20], and visuospatial perception and laterality recognition may be impaired [21–22]. Patients with this condition also often present with altered motor performance, including limited range of motion, weakness or dystonia [23–26]. In addition, a number of studies in patients suffering from phantom pain or CRPS have reported distorted perceptions of the painful body parts [27–31]. In patients with FMS, poor balance and higher frequency of falls may be seen as indications of disturbed sensorimotor function and an altered body schema [32–33]. In addition, FMS patients perceive enlarged body size and shrinkage of the surrounding space during exacerbations of pain [34], suggesting a positive link between distortion of the body image and pain. Such relationship has been confirmed by direct correlations between poor body image and severity of the clinical status and ongoing pain [35].
On the other hand, interoception refers to the sense of the physiological condition of the body [36]. Interoceptive accuracy has been found to be positively linked with anxiety [37], while diminished interoception may be associated with depression and alexithymia [38–40]. Despite the fact that FMS is comorbid with anxiety and depression, the involvement of alterations of interoceptive awareness in this condition has not yet been established.
In order to gain insight into embodied pain in FMS, the current study explored various fundamental aspects of body awareness such as plasticity of the body schema, body image and interoceptive awareness. To this end, we administered the rubber hand illusion paradigm both to a sample of women with FMS and a group of healthy controls, and we used standard, self-administered questionnaires such as the Body Esteem Scale (BES) and the Body Perception Questionnaire (BPQ) to assess body image and interoceptive awareness, respectively.
Methods
Study participants and ethical statement
A convenience sample of fourteen right-handed women with a formal diagnosis of fibromyalgia syndrome was evaluated (average age 54.35 years, SEM 1.89), as well as 13 age-matched, healthy right-handed women which served as controls (average age 53.86 years, SEM 3.30). Patients were recruited February 2015 from a local support group through advertisements and informative presentations. No one participant had previously been subjected to the rubber had illusion (RHI, cf. below). The study protocol was approved by the Ethical Review Board of the University of the Basque Country and all participants provided written informed consent before participating.
Pain and clinical status
Pain, clinical status and body perception were assessed by using self-administered instruments. Subjects provided an overall measure of pain severity on a visual analog scale (VAS), and the Spanish version of the short form of the Brief Pain Inventory (BPI-SF) [41] was used to assess the impact of ongoing pain on daily function. The Spanish version of the Fibromyalgia Impact Questionnaire (FIQ) [42–43] was used to assess physical functioning, work status, depression, anxiety, sleep, pain, stiffness, and fatigue. Health-related quality of life was evaluated by using the SF-12 Health Survey, a multipurpose survey comprised by 12 questions selected from the SF-36 Health Survey [44] that provides scores in both mental and physical domains as Mental Component Summary and Physical Component Summary scales, respectively.
Rubber hand illusion
The RHI [5] is a well known somatosensory paradigm that can be induced when the experimental subject views a life-sized rubber hand being stroked by a paintbrush while his/her hand is hidden out of sight yet likewise stroked by another paintbrush. Simultaneous stroking of both the real and the rubber hand creates a sensory conflict between what one sees and feels, and the paradigm evaluates how the brain resolves such conflict by adapting the underlying body representation to embody the rubber hand. The RHI is thought to be based on visuotactile integration and can be used for assessing self-attribution and plasticity of the body schema [45–49]. Usual behavioral measures of the RHI include a drift of the perceived position of the subject’s hand toward the rubber hand, which is known as proprioceptive drift, as well as perceived appropriation (ownership) and motor control (agency) over the rubber hand.
In the present study, each participant sat at an office table and placed her right hand inside an opaque plexiglas box (Fig 1). A rubber hand had been placed palm down between the real hand and the body mid-line. The subject had direct visual access to the fake hand, whereas her real hand was kept hidden inside the box. Two round paint-brushes (diameter about 1 cm) [50] were used for brushing both the patient’s right hand and the rubber hand in a synchronous manner for 1 min, starting at the middle phalanx on each finger and ending at the fingernail. A questionnaire based on items from previously published studies [51–58] was administered immediately thereafter to assess the perceived location of the real and rubber hands (5 items), as well as perceived appropriation (ownership) and motor control (agency) over the rubber hand (7 and 6 items, respectively). Responses were measured on 5-point Likert scales (1—total disagreement, 5—total agreement).
Fig 1. Experimental setting for inducing the rubber hand illusion.
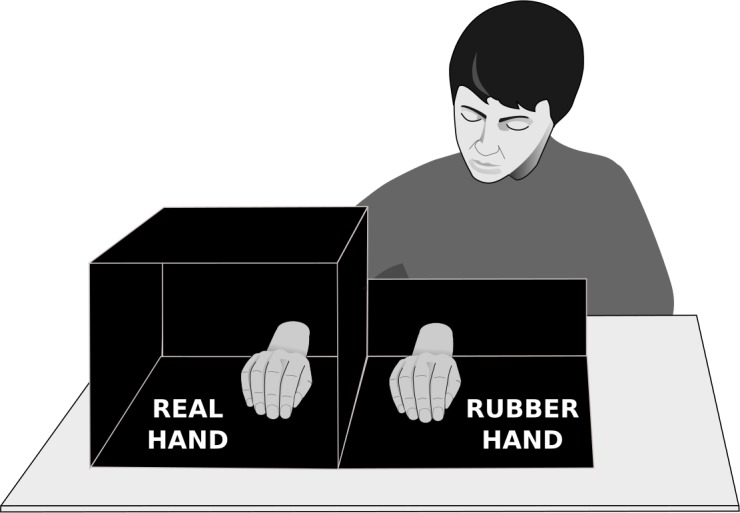
The subject was sitting at a table with eyes fixed on the rubber hand and her actual right hand placed inside an opaque box. The positions of the chair and the box both were adjusted so that the subject had the fake hand, but not the real one in sight. Small paint-brushes were used to synchronously stroke both the rubber hand and the subject’s hidden hand.
Body esteem and interoceptive awareness
Body esteem, an important dimension of self-esteem, was measured by using the Body Esteem Scale (BES) [59]. This instrument evaluates perception and self-evaluation of one’s body by measuring the feeling towards various body parts and functions on a 5-point Likert scale (1 labeled as very negative and 5 as very positive).
The Body Perception Questionnaire (BPQ) [60] was used as a measure of self-rated bodily awareness. This tool uses 5-point scoring scales (1 denoting no awareness at all, whereas 5 indicates permanent awareness) to measure body perception and interoceptive awareness on four subscales, including awareness (perception of bodily processes, e.g. swallowing), stress response (perception of bodily changes in stressful situations), autonomic nervous system reactivity (perception of one’s own autonomic nervous system reactions, e.g. beating of one's heart), and stress style (e.g. frustration or dizziness).
Data analysis and statistics
The SPSS® v. 22 statistical package (SPSS, Chicago, IL) was used for all statistical analyses. Normality of distribution was checked using the Shapiro-Wilk test, and the unpaired-sample two-tailed t-test or the Mann-Whitney-Wilcoxon U-test were used to investigate statistical significance of means differences between the two study groups.
Alpha values of .05 and .01 were used as criteria of statistical significance (indicated where appropriate). Primary data are presented as means and standard errors, and effects sizes are presented as Cohen’s d (95% confidence intervals). Likert scale data from individual questionnaire items were treated as ordinal data, whereas subscale scores were treated as interval data.
Results
Baseline clinical status of FMS patients
Subjects in the FMS sample scored an average of 88.38 (SEM 2.72) on the FIQ (data summarized in Table 1), which was slightly above the reported values of ca. 76 by studies in this geographic area [61–62]. Ongoing pain intensity was 8.89 (SEM .98) on the VAS, and scores on the Brief Pain Inventory averaged 7.39 (SEM .37), which can be considered as indicative of severe clinical pain with high interference with daily function. Differences on both pain measures relative to healthy controls were statistically significant. In addition, scores on the Physical- and Mental Component Summaries of the 12-Item Short-Form Health Survey (SF-12) both were significantly lower, i.e. they reflected an inferior clinical status, in the FMS sample (33.27, SEM 1.98 in the FMS sample, vs 23.10, SEM .82 in healthy controls).
Table 1. Ongoing pain and clinical status in the two study groups.
| FMS | Healthy Controls | |
|---|---|---|
| Fibromyalgia Impact Questionnaire (FIQ) | 88.38 (2.72) | – |
| Brief Pain Inventory–Short Form | 7.39 (.37) ** | .16 (.09) |
| Pain intensity (Visual Analog Scale) | 8.89 (.28) ** | .15 (.12) |
| SF12 –Physical Component Summary | 23.10 (.82) ** | 53.68 (1.23) |
| SF12 –Mental Component Summary | 33.27 (1.98) ** | 54.08 (2.09) |
Subjects in the FMS sample had lower health indicators in terms of physical and mental summary scales of the 12-Item Short-Form Health Survey (SF-12), and exhibited significantly higher levels of clinical pain intensity on the VAS. The Fibromyalgia Impact Questionnaire (FIQ) was administered only to FMS subjects. Data are presented as mean (SEM).
** p < .01 on the Student’s t test for independent samples.
Rubber hand illusion
FMS patients scored higher across all items of the assessment questionnaire (Table 2; raw data provided in S1 File). Observed effect sizes were large or very large across the vast majority of the items, with the sole exception of item 3 within the ownership domain (Cohen’s d < .5). Albeit high, effect sizes in the ownership domain were generally lower than in proprioceptive drift and agency domains, and differences between the two groups failed to attain statistical significance. Differences in scorings between groups were largest in the proprioceptive drift domain, in which statistically significant differences (Mann-Whitney-Wilcoxon U-test) and large effect sizes were found across all presented items (e.g. Cohen’s d value of 3.10 on the statement It seemed as if the rubber hand and my own hand were approaching).
Table 2. Assessment of the RHI in fibromyalgia and healthy controls.
| FMS | Healthy controls | Effect size | |
|---|---|---|---|
| Proprioceptive drift | |||
| It seemed like the rubber hand was where my own hand was | 4.07 (.22) * | 3.08 (.38) | .88 (.07–1.68) |
| I felt as if my own hand was drifting towards the rubber hand | 3.14 (.25) ** | 1.38 (.24) | 1.95 (1.01–2.88) |
| It seemed as if the touch I was feeling came from somewhere between my own hand and the rubber hand | 3.07 (.32) ** | 1.38 (.24) | 1.61 (.73–2.50) |
| It seemed (visually) as if the rubber hand was drifting towards my own hand | 3.21 (.28) ** | 1.23 (.12) | 2.46 (1.44–3.48) |
| It seemed as if the rubber hand and my own hand were approaching | 3.50 (.25) ** | 1.23 (.21) | 3.10 (1.96–4.24) |
| Ownership | |||
| It seemed like the rubber hand belonged to me | 3.92 (.26) | 3.15 (.37) | .60 (-.18–1.38) |
| It seemed like the rubber hand began to resemble my real hand | 3.50 (.32) | 2.76 (.37) | .57 (-.21–1.35) |
| It seemed like I was looking directly at my own hand, rather than at a rubber hand | 4.07 (.28) | 3.46 (.41) | .46 (-.31–1.24) |
| It seemed like the rubber hand was part of my body | 4.07 (.26) | 3.15 (.37) | .78 (-.01–1.58) |
| It seemed like the rubber hand was my hand | 3.85 (.27) | 3.15 (.38) | .57 (-.21–1.35) |
| The rubber hand began to resemble my own hand in terms of shape, skin tone, freckles or some other visual features | 3.50 (.27) | 2.53 (.44) | .72 (-.07–1.51) |
| It seemed as if I was feeling the touch of the paintbrush in the location where I saw the rubber hand touched | 4.21 (.21) | 3.15 (.50) | .75 (-.03–1.55) |
| Agency | |||
| It seemed like I was unable to move my own hand | 3.92 (.24) ** | 1.53 (.31) | 2.34 (1.34–3.34) |
| It seemed like I could not really tell where my hand was | 3.50 (.22) ** | 1.69 (.32) | 1.76 (.85–2.66) |
| It seemed like my own hand had disappeared | 3.78 (.26) ** | 2.00 (.39) | 1.47 (.60–2.33) |
| It seemed like my own hand was out of my control | 3.57 (.22) ** | 1.54 (.27) | 2.24 (1.26–3.23) |
| It seemed like I could move the rubber hand if I would like | 3.21 (.33) | 2.38 (.43) | .58 (-.19–1.37) |
| It seemed like I was in control of the rubber hand | 3.28 (.33) | 2.38 (.43) | .63 (-.15–1.42) |
FMS patients scored generally higher relative to healthy controls across all items in all three domains of the questionnaire. Data from 5-point Likert scales (1 –totally disagree, 5- totally agree) are presented as means (SEM; * p < .05, ** p < .01 on the Mann-Whitney-Wilcoxon U-test). Effect size is provided as Cohen’s d (95% CI).
Lower body esteem in FMS
The Body Esteem Scale (BES) was used here to evaluate body satisfaction. We found that the average global BES score was lower among FMS subjects (2.52, SEM .19) relative to healthy controls (3.89, SEM .16), the difference being statistically significant (p < .01 on the Student’s t-test for independent samples), i.e. patients reported less satisfaction with their bodies than did healthy individuals. Scores assigned by subjects from the FMS sample were consistently lower across all items within the BES, effect sizes being moderate to high with Cohen’s d values ranging between .38 and .66 (Table 3). Inter-group comparisons revealed statistically significant differences in BES scores on items addressing 14 out of 18 body regions across all four main body segments evaluated (head, superior and inferior limbs, and trunk). We also wished to ascertain whether body regions receiving lower scores corresponded to those primarily affected by ongoing clinical pain. To this end, we extracted the BES score assigned by each FMS patient to the body site regarded as most painful, and we then obtained the average of scores assigned to the remaining body regions addressed by the BES, which were either less painful or not painful at all. We found that average BES scores corresponding to most painful regions in the FMS sample were indeed lower (1.58, SEM .19) than those assigned to less painful or non-painful sites (2.87, SEM .18). Differences were statistically significant (p < .01 on the Student’s t-test for independent samples).
Table 3. Scores from FMS patients and healthy controls on the Body Esteem Scale.
| FMS | Healthy Controls | Effect size | |
|---|---|---|---|
| Body scent | 3.21 (.23) | 3.69 (.39) | .38 (-1.18-.3) |
| Appetite | 3.64 (.17) * | 4.38 (.28) | .39 (-.04–1.54) |
| Nose | 3.42 (.29) | 3.61 (.24) | .38 (-.58-.96) |
| Physical stamina | 1.92 (.30) ** | 3.92 (.30) | .45 (.85–2.67) |
| Reflexes | 2.85 (.39) ** | 4.50 (.15) | .44 (.60–2.38) |
| Lips | 3.43 (.31) * | 4.31 (.21) | .40 (.09–1.70) |
| Muscular strength | 1.71 (.30) ** | 4.23 (.20) | .52 (1.58–3.68) |
| Waist | 2.21 (.24) ** | 3.61 (.31) | .42 (.52–2.24) |
| Energy level | 1.50 (.17) ** | 4.23 (.20) | .66 (2.63–5.29) |
| Thighs | 2.36 (.34) ** | 3.69 (.26) | .41 (.35–2.02) |
| Ears | 3.29 (.27) * | 4.15 (.22) | .47 (1.11–3.02) |
| Biceps | 2.38 (.24) ** | 3.77 (.28) | .44 (.58–2.35) |
| Chin | 3.07 (.22) ** | 4.08 (.21) | .42 (.42–2.10) |
| Body build | 2.42 (.32) ** | 4.30 (.17) | .46 (.99–2.86) |
| Physical coordination | 2.50 (.32) ** | 4.53 (.14) | .48 (1.19–3.13) |
| Buttocks | 2.62 (.35) * | 3.85 (.27) | .42 (.24–1.92) |
| Agility | 1.85 (.29) ** | 4.38 (.21) | .52 (1.60–3.72) |
| Width of shoulders | 2.93 (.32) * | 4.08 (.26) | .41 (.23–1.87) |
| Arms | 2.86 (.25) | 3.54 (.29) | .39 (-.11–1.47) |
| Chest or breasts | 2.50 (.27) ** | 3.77 (.23) | .42 (.50–2.21) |
| Appearance of eyes | 3.00 (.31) * | 3.92 (.23) | .40 (.08–1.70) |
| Cheeks /cheekbones | 3.29 (.24) | 3.84 (.25) | .39 (-.16–1.40) |
| Hips | 2.29 (.29) ** | 3.77 (.20) | .44 (.73–2.51) |
| Legs | 2.14 (.27) ** | 3.69 (.26) | .43 (.56–2.29) |
| Figure or physique | 2.84 (.27) ** | 3.84 (.24) | .45 (.85–2.67) |
| Sex drive | 2.42 (.37) ** | 3.92 (.21) | .42 (.47–2.18) |
| Feet | 2.62 (.33) | 3.54 (.31) | .40 (-.04–1.58) |
| Sex organs | 2.84 (.27) * | 3.84 (.24) | .41 (.22–1.89) |
| Appearance of stomach | 2.14 (.31) * | 3.31 (.29) | .41 (.23–1.88) |
| Health | 1.42 (.13) ** | 4.53 (.18) | .81 (3.63–6.88) |
| Sex activities | 2.14 (.43) ** | 3.76 (.28) | .41 (.37–2.04) |
| Body hair | 2.50 (.34) | 3.23 (.36) | .39 (-.22–1.34) |
| Physical condition | 1.50 (.22) ** | 4.15 (.24) | .56 (1.89–4.15) |
| Face | 3.00 (.26) ** | 4.08 (.18) | .42 (.46–2.16) |
| Weight | 2.21 (.35) * | 3.38 (.31) | .40 (.14–1.77) |
| General BES score | 2.52 (.17) ** | 3.89 (.16) | .48 (1.23–3.18) |
FMS patients assigned lower scores to all evaluated body features, relative to healthy controls. Differences were statistically significant across most items of the questionnaire, as well as in the general BES score (* p < .05, **p < .01 on the Mann-Whitney-Wilcoxon U-test). Data are presented as mean (SEM), and Cohen’s d (95% CI) is provided as a measure of effect size.
Increased interoceptive awareness in FMS
We used the Body Perception Questionnaire (BPQ) to assess awareness of one’s own body processes, stress responses, autonomic nervous system reactivity and stress style. We found higher scores in the FMS sample relative to healthy controls in all addressed subscales, with large effect sizes and statistically significant differences (Student’s t-test for independent samples) on all of them with the sole exception of the stress style 1 subscale (Table 4; scores across all measured 94 items are provided in S1 Table). In addition, we found a strong and significant (p = .002) correlation (two-tailed Pearson’s correlation r = .75) between intensity of ongoing pain reported on the VAS and the awareness subscale of the BPQ in the FMS sample.
Table 4. Scores from FMS subjects and healthy controls on the Body Perception Questionnaire.
| Domains | FMS | Healthy Controls | Effect size |
|---|---|---|---|
| Awareness | 3.57 (.15) ** | 1.87 (.11) | .61 (2.28–4.73) |
| Stress response | 3.76 (.11) ** | 1.78 (.11) | .87 (3.89–7.40) |
| Autonomic Nervous System Reactivity | 2.59 (.17) ** | 1.35 (.07) | .51 (1.49–3.56) |
| Stress style 1 | 3.25 (.20) | 2.55 (.38) | .40 (-.02–1.60) |
| Stress style 2 | 2.73 (.27) ** | 1.13 (.04) | .68 (2.76–5.49) |
Scores from FMS patients were higher across all subscales, with large effect sizes and statistically different differences on most subscales (** p < .01 on the Student’s t-test for independent samples). Effect size is provided as Cohen’s d (95% confidence interval).
Discussion
Increased plasticity of the body schema
Internal representations of one’s own body include the notions of shape and contours of the own body, the location of the body parts and the boundaries between them [17,63–64]. Such representations are subject to substantial plasticity, as exemplified by the experience of perceiving a body part as alien and, conversely, by conditions of embodiment of external objects as one's own.
The RHI is a misperception in which tactile sensations are referred to an alien limb. The illusion is commonly employed for assessing plasticity of the body schema [45–49]. In the current study, the RHI could consistently be induced in participants from the FMS group, whose scores were significantly higher relative to healthy controls in questions addressing the perceptions of motor control over the fake hand and proprioceptive drift. Scores regarding the sense of ownership of the alien hand also were all higher in the FMS group, showing large effect sizes on the vast majority of items. Albeit still large, effect sizes were less pronounced on the ownership subscale, where between-group contrasts failed to attain statistical significance. Although the RHI directly addresses the easiness to embody a foreign object, the outcome of this procedure is best conceptualized as a measure of plasticity of embodiment of one’s own limb [46], as the illusion can also be induced successfully in conditions involving the converse, i.e. disembodiment of body parts, as found in somatoparaphenia [48,65] or in psychiatric patients involving dissociative states [56]. Previous studies have found direct correlation between the intensity of the RHI and plasticity of body representations measured with the Trinity Assessment of Body Plasticity in healthy subjects [66]. Interestingly, previous reports also have shown enhanced RHI in patients with low back pain [67–68], although its intensity may be rather variable in subjects suffering from CRPS [69]. On the other hand, studies conducted in CRPS patients have reported manifestations of disembodiment of the painful limb such as neglect-like symptoms [70–73] and a range of feelings of disassociation and lack of ownership [29]. These findings, including the present ones in people with FMS, raise the possibility that instability of the body schema might play a role in the pathophysiology of chronic pain.
The intensity of the RHI is sensitive to detection of visuotactile mismatch or inconsistencies between incoming sensory inputs from the actual hand and expected attributes based on stored somatorepresentations, e.g. regarding texture or position [45,74]. Studies show that in individuals suffering from eating disorders, who indeed experience the RHI more strongly than healthy controls [75–76], negative cognitive bias toward one’s body may compromise adequate updating of offline body representations by incoming sensory input [77–78]. An analogous mechanism may potentially operate in FMS, since such negative bias has been reported by others (cf below) and also found here in terms of low body esteem.
Biased body representation in FMS
Disturbances in body perception are increasingly recognized as accompanying chronic pain states. Phantom limb pain, which genuinely exemplifies distorted representations of one’s own body, is commonly accompanied by the perception of the affected body part as altered in size or consistency, or placed in unusual or non-anatomic positions [28,79–81]. In addition, decreased tactile acuity and inability to delineate the body outline both have been reported to coincide with the distribution of pain in individuals suffering from chronic low back pain [82]. Alterations of body perception in CRPS patients include faulty estimation of the size of the affected limb [27], spatial mislocalization [22,30], or decreased tactile acuity with a direct correlation between body perception disturbance and pain [31]. Indeed, the progression of body image distortions in CRPS patients parallels that of pain [83–84], raising the question of whether the altered somatorepresentations may contribute to generating or maintaining chronic pain. Interestingly, this notion appears to receive support from the observation that therapeutic approaches based on rehabilitation of tactile acuity can reduce pain in patients suffering from CRPS or chronic low back pain [85–87].
Body image distortions may be driven not only by sensory inputs but also by body dissatisfaction. For example, somatosensory disturbances including impaired tactile acuity are commonplace in anorexia nervosa, a condition in which body dissatisfaction is pivotal [88–90]. In individuals with FMS, a previous report has shown alteration of the perceived image of the whole body using the 10-item Body Image Scale [35]. Here, we found that participants in the FMS group scored significantly lower than healthy controls on the 35-item Body Esteem Scale, showing low levels of satisfaction regarding a variety of diverse body parts and functional features. Lower scores were found in the FMS patient sample across the vast majority of body regions addressed by the survey, and statistically significant differences were found in scores assigned to 14 out of 18 body regions addressed by the BES. Furthermore, we found that the body site regarded by each patient as the primary focus of ongoing clinical pain was consistently perceived as less satisfactory in terms of BES scores than the rest of evaluated body parts.
Low body esteem is strongly characterized by body dissatisfaction [91–92], which arises from discrepancy between current perceived-self and ideal body model [93–94]. FMS is a form of chronic widespread pain and therefore not circumscribed to a particular body region. Lower scores on the BES indicate distorted, overall negative body perceptions, a finding that is in general agreement with prior quantitative and qualitative studies in people with this condition [95–96]. Further, pain severity and negative body perceptions are reportedly directly correlated [35]. This generalized form of distorted body perception is based on a biased cognitive appraisal of one’s own body. Our current study design did not permit us to address whether body image distortion was primary or secondary to chronic pain. However, there are indications that at least some conditioning factors such as childhood trauma or physical or emotional abuse, which may be causal to low self-esteem [97–98], precede widespread pain in FMS. Studies have reported that a history of childhood trauma or abuse may be commonly found in FMS patients relative to the general population [99–102] and even a risk factor of FMS [101]. Moreover, evidence suggests that childhood trauma is related with pain severity in FMS [101,103]. Interestingly, high rates of child abuse and stressful life events have been reported in a variety of forms of chronic pain, including generalized pain [104–106], pelvic pain and vulvodynia [107–108], chronic musculoskeletal pain [109], headache [110], and irritable bowel syndrome and gastro-intestinal conditions [111–113]. Whether or not preceded by stressful life events or other pathogenic route, our present results support the existence of a link between pain and negatively biased cognitions of one’s own body in FMS, including low body esteem.
Increased interoceptive awareness
Pain is a salient experience that capitalizes attention and interferes with cognitive ad emotional processes [114]. Cognitive changes accompanying chronic pain often involve disproportionate attentional selection of pain and pain-associated information at the expense of the remaining sensory inputs [114–116]. Hypervigilance to pain is associated both with higher sensitivity to experimental pain [117–118] and greater clinical pain in FMS and other conditions [117,119]. Further, there is an emerging view that increased vigilance in people with FMS may not be circumscribed to painful inputs but rather represent a generalized, perceptual style of amplification of a variety of sensory information [120], including innocuous or auditory inputs [121]. In this perspective, in the current study we show that female patients with FMS exhibited increased interoceptive awareness relative to healthy individuals; supporting the notion that hypervigilance may not be limited to external sensory input but also involves an enhanced awareness of internal bodily cues. To our knowledge, only one prior recent study has addressed interoceptive awareness in a sample of FMS patients, by using The Multidimensional Assessment of Interoceptive Awareness questionnaire [122], which however failed to find significant differences with respect to their control sample. This apparent discordance may well be due to differences in sensitivity of the employed tools. In addition to higher scores on the BPQ, we found here that enhanced awareness of one’s own body processes as reflected by the scores on the awareness subscale was strongly associated with the intensity of ongoing clinical pain, a core clinical feature of FMS. Based on these findings, one may speculate that the coping style to pain in FMS patients involves perceptual amplification also including increased interoceptive awareness, or alternatively that perceptual amplification might lead to chronic pain in FMS.
Is the ability to update negatively biased somatorepresentations impaired in FMS?
Body-referenced spatial representations, as a part of the spatial experience, derive from both on-line afferent somatosensory inputs subserved by short-term memory and off-line representations or embodied information stored as long-term memory [78,123–124]. Studies in eating disorders suggest that if the mutual influence between somatosensory inputs and off-line body representations is disrupted, then the contents of the stored reference frame fail to be adequately updated and the individual becomes locked to negative representations of their bodies, a model termed as allocentric lock [125–127]. A role in this disturbance is thought to be played by the connection between the amygdala and the hippocampal complex, due to the ability of the former to enhance long-term consolidation of emotionally arousing perceptions [128]. Here, we found evidence of negatively biased body image in persons suffering from FMS, which was accompanied by instability and increased plasticity of the body schema. Importantly, such biased cognition prevailed in FMS patients despite an ongoing state of sensory hypervigilance, including increased awareness to internal bodily cues. We suggest that disturbed embodiment as observed here in FMS patients may share fundamental analogies with the allocentric lock framework, where inadequate updating of stored, negatively biased off-line somatorepresentations may play be pivotal. Consistent with this view, brain imaging studies in FMS patients have found axonal metabolic dysfunction in the left hippocampus, including decreased myoinositol/creatine ratios and lower levels of choline and N-acetyl aspartate [129]. In addition, diffusion tensor imaging data from FMS patients have revealed increased fractional anisotropy indicating tissue complexity and neuronal disorganization increase in amygdalae and hippocampi among other loci [130], although it remains to be established whether such changes are primary or secondary to FMS.
Limitations of the study
The asynchronous stroking condition in the RHI was omitted in the present study due to fatigue and difficulties maintaining mental focus throughout the procedure in the FMS population sample. However, this was unlikely to have a substantial impact on the interpretation of our findings, as these were based on comparisons between FMS subjects and age-matched, healthy individuals. On the other hand, primary data are presented as inter-group comparisons of individual items within questionnaires, rather than differences among multiple group means.
In summary, we present experimental evidence of disturbed embodiment in FMS that was characterized by instability of the body schema and negatively biased cognitions regarding one’s own body, in addition to increased vigilance to internal bodily cues. These pieces of evidence may be interpreted collectively in a framework centered upon the inability of incoming sensory inputs to adequately update negatively biased off-line somatorepresentations stored as long-term memory.
Supporting information
(CSV)
(DOCX)
Acknowledgments
We are deeply indebted to AVAFAS (Asociación Vasca de Fibromialgia y Astenia Crónica) for participating in this study. Supported by the Basque Government (Euskal unibertsitate-sistemako ikerketa-taldeen jarduerak bultzatzeko diru-laguntzak, GIC15/25) and the University of the Basque Country UPV/EHU (PPG17/06).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the Basque Government (Euskal unibertsitate-sistemako ikerketa-taldeen jarduerak bultzatzeko diru-laguntzak, GIC15/25) and the University of the Basque Country UPV/EHU (PPG17/06). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33(2):160–72. [DOI] [PubMed] [Google Scholar]
- 2.Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, Mease P, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken). 2010;62(5):600–10. [DOI] [PubMed] [Google Scholar]
- 3.Desmeules JA, Cedraschi C, Rapiti E, Baumgartner E, Finckh A, Cohen P, et al. Neurophysiologic evidence for a central sensitization in patients with fibromyalgia. Arthritis Rheum. 2003;48(5):1420–29. doi: 10.1002/art.10893 [DOI] [PubMed] [Google Scholar]
- 4.Arendt-Nielsen L, Graven-Nielsen T. Central sensitization in fibromyalgia and other musculoskeletal disorders. Curr Pain Headache Rep. 2003;7(5):355–61. [DOI] [PubMed] [Google Scholar]
- 5.Sörensen J, Graven-Nielsen T, Henriksson KG, Bengtsson M, Arendt-Nielsen L. Hyperexcitability in fibromyalgia. J Rheumatol. 1998;25(1):152–5. [PubMed] [Google Scholar]
- 6.Wolfe F, Brähler E, Hinz A, Häuser W. Fibromyalgia prevalence, somatic symptom reporting, and the dimensionality of polysymptomatic distress: results from a survey of the general population. Arthritis Care Res (Hoboken). 2013;65(5):777–85. [DOI] [PubMed] [Google Scholar]
- 7.Branco JC, Bannwarth B, Failde I, Abello Carbonell J, Blotman F, Spaeth M, et al. Prevalence of fibromyalgia: a survey in five European countries. Semin Arthritis Rheum. 2010;39(6):448–53. doi: 10.1016/j.semarthrit.2008.12.003 [DOI] [PubMed] [Google Scholar]
- 8.Ablin JN, Oren A, Cohen S, Aloush V, Buskila D, Elkayam O, et al. Prevalence of fibromyalgia in the Israeli population: a population-based study to estimate the prevalence of fibromyalgia in the Israeli population using the London Fibromyalgia Epidemiology Study Screening Questionnaire (LFESSQ). Clin Exp Rheumatol. 2012;30(6 Suppl 74):39–43. [PubMed] [Google Scholar]
- 9.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. ; National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26–35. doi: 10.1002/art.23176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9(4):463–84. doi: 10.1016/j.ejpain.2004.11.001 [DOI] [PubMed] [Google Scholar]
- 11.Borsook D, Becerra L. Pain imaging: future applications to integrative clinical and basic neurobiology. Adv Drug Deliv Rev. 2003;28;55(8):967–86. [DOI] [PubMed] [Google Scholar]
- 12.Treede RD. Assessment of pain as an emotion in animals and in humans. Exp Neurol. 2006;197(1):1–3. doi: 10.1016/j.expneurol.2005.10.009 [DOI] [PubMed] [Google Scholar]
- 13.Moseley GL. Why do people with complex regional pain syndrome take longer to recognize their affected hand? Neurology. 2004;62(12):2182–6. [DOI] [PubMed] [Google Scholar]
- 14.Moseley GL, Gallace A, Spence C. Bodily illusions in health and disease: physiological and clinical perspectives and the concept of a cortical 'body matrix'. Neurosci Biobehav Rev. 2012;36(1):34–46. doi: 10.1016/j.neubiorev.2011.03.013 [DOI] [PubMed] [Google Scholar]
- 15.Tsay A, Allen TJ, Proske U, Giummarra MJ. Sensing the body in chronic pain: a review of psychophysical studies implicating altered body representation. Neurosci Biobehav Rev. 2015;52:221–32. doi: 10.1016/j.neubiorev.2015.03.004 [DOI] [PubMed] [Google Scholar]
- 16.Coslett HB, Saffran EM, Schwoebel J. Knowledge of the human body: a distinct semantic domain. Neurology. 2002;13;59(3):357–63. [DOI] [PubMed] [Google Scholar]
- 17.Schwoebel J, Coslett HB. Evidence for multiple, distinct representations of the human body. J Cogn Neurosci. 2000;(4):543–53. [DOI] [PubMed] [Google Scholar]
- 18.Dijkerman HC, de Haan EH. Somatosensory processes subserving perception and action. Behav Brain Sci. 2007;30(2):189–239. doi: 10.1017/S0140525X07001392 [DOI] [PubMed] [Google Scholar]
- 19.Schwoebel J, Friedman R, Duda N, Coslett HB. Pain and the body schema: evidence for peripheral effects on mental representations of movement. Brain. 2001;124(Pt 10):2098–104. [DOI] [PubMed] [Google Scholar]
- 20.Schwoebel J, Coslett HB, Bradt J, Friedman R, Dileo C. Pain and the body schema: effects of pain severity on mental representations of movement. Neurology. 2002;59(5):775–7. [DOI] [PubMed] [Google Scholar]
- 21.Reinersmann A, Haarmeyer GS, Blankenburg M, Frettlöh J, Krumova EK, Ocklenburg S, et al. Left is where the L is right. Significantly delayed reaction time in limb laterality recognition in both CRPS and phantom limb pain patients. Neurosci Lett. 2010;486(3):240–5. doi: 10.1016/j.neulet.2010.09.062 [DOI] [PubMed] [Google Scholar]
- 22.Reinersmann A, Landwehrt J, Krumova EK, Ocklenburg S, Güntürkün O, Maier C. Impaired spatial body representation in complex regional pain syndrome type 1 (CRPS I). Pain. 2012;153(11):2174–81. doi: 10.1016/j.pain.2012.05.025 [DOI] [PubMed] [Google Scholar]
- 23.Schwartzman RJ, Kerrigan J. The movement disorder of reflex sympathetic dystrophy. Neurology. 1990;40(1):57–61 [DOI] [PubMed] [Google Scholar]
- 24.Harden RN, Bruehl S, Stanton-Hicks M, Wilson PR. Proposed new diagnostic criteria for complex regional pain syndrome. Pain Med. 2007;8(4):326–31. doi: 10.1111/j.1526-4637.2006.00169.x [DOI] [PubMed] [Google Scholar]
- 25.Galer BS, Butler S, Jensen MP. Case reports and hypothesis: a neglect-like syndrome may be responsible for the motor disturbance in reflex sympathetic dystrophy (Complex Regional Pain Syndrome-1). J Pain Symptom Manage. 1995;10(5):385–91. [DOI] [PubMed] [Google Scholar]
- 26.Maihöfner C, Baron R, DeCol R, Binder A, Birklein F, et al. The motor system shows adaptive changes in complex regional pain syndrome. Brain. 2007;130(10):2671–87. [DOI] [PubMed] [Google Scholar]
- 27.Moseley GL. Distorted body image in complex regional pain syndrome. Neurology. 2005;65(5):773 doi: 10.1212/01.wnl.0000174515.07205.11 [DOI] [PubMed] [Google Scholar]
- 28.Lotze L. Moseley GL. Role of distorted body image. Curr Rheumatol Rep. 2007;9(6):488–96. [DOI] [PubMed] [Google Scholar]
- 29.Lewis JS, Kersten P, McCabe CS, McPherson KM, Blake DR. Body perception disturbance: a contribution to pain in complex regional pain syndrome (CRPS). Pain. 2007;133(1–3):111–9. doi: 10.1016/j.pain.2007.03.013 [DOI] [PubMed] [Google Scholar]
- 30.Lewis JS, Kersten P, McPherson KM, Taylor GJ, Harris N, McCabe CS, Blake DR. Wherever is my arm? Impaired upper limb position accuracy in complex regional pain syndrome. Pain. 2010;149(3):463–9. doi: 10.1016/j.pain.2010.02.007 [DOI] [PubMed] [Google Scholar]
- 31.Lewis JS, Schweinhardt P. Perceptions of the painful body: the relationship between body perception disturbance, pain and tactile discrimination in complex regional pain syndrome. Eur J Pain. 2012;16(9):1320–30. doi: 10.1002/j.1532-2149.2012.00120.x [DOI] [PubMed] [Google Scholar]
- 32.Jones KD, Horak FB, Winters-Stone K, Irvine JM, Bennett RM. Fibromyalgia is associated with impaired balance and falls. J Clin Rheumatol. 2009;15(1):16–21 doi: 10.1097/RHU.0b013e318190f991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meireles SA, Antero DC, Kulczycki MM, Skare TL. Prevalence of falls in fibromyalgia patients. Acta Ortop Bras. 2014;22(3):163–6. doi: 10.1590/1413-78522014220300386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valenzuela-Moguillansky C. Pain and Body Awareness. An Exploration of the Bodily Experience of Persons Suffering from Fibromyalgia. Constructivist Foundations. 2013;8. [Google Scholar]
- 35.Akkaya N, Akkaya S, Atalay NS, Balci CS, Sahin F. Relationship between the body image and level of pain, functional status, severity of depression, and quality of life in patients with fibromyalgia syndrome. Clin Rheumatol. 2012;31(6):983–8. doi: 10.1007/s10067-012-1965-9 [DOI] [PubMed] [Google Scholar]
- 36.Craig AD. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol. 2003;13(4):500–5. [DOI] [PubMed] [Google Scholar]
- 37.Dunn BD, Stefanovitch I, Evans D, Oliver C, Hawkins A, Dalgleish T. Can you feel the beat? Interoceptive awareness is an interactive function of anxiety- and depression-specific symptom dimensions. Behav Res Ther. 2010;48(11):1133–8. doi: 10.1016/j.brat.2010.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pollatos O, Traut-Mattausch E, Schandry R. Differential effects of anxiety and depression on interoceptive accuracy. Depress Anxiety. 2009;26(2):167–73. doi: 10.1002/da.20504 [DOI] [PubMed] [Google Scholar]
- 39.Herbert BM, Herbert C, Pollatos O. On the relationship between interoceptive awareness and alexithymia: is interoceptive awareness related to emotional awareness? J Pers. 2011;79(5):1149–75. doi: 10.1111/j.1467-6494.2011.00717.x [DOI] [PubMed] [Google Scholar]
- 40.Terhaar J, Viola FC, Bär KJ, Debener S. Heartbeat evoked potentials mirror altered body perception in depressed patients. Clin Neurophysiol. 2012;123(10):1950–7. doi: 10.1016/j.clinph.2012.02.086 [DOI] [PubMed] [Google Scholar]
- 41.de Andrés Ares J, Cruces Prado LM, Canos Verdecho MA, Penide Villanueva L, Del Valle Hoyos M, Herdman M, et al. Validation of the Short Form of the Brief Pain Inventory (BPI-SF) in Spanish Patients with Non-Cancer-Related Pain. Pain Pract. 2015;15(7):643–53. doi: 10.1111/papr.12219 [DOI] [PubMed] [Google Scholar]
- 42.Burckhardt CS, Clark, Bennett RM. The fibromyalgia Impact questionnaire: development and validation. J Rheumatol. 1991;18(5):728–33. [PubMed] [Google Scholar]
- 43.Monterde S, Salvat I, Montull S, Fernández-Ballart J. Validación de la versión española del Fibromyalgia Impact Questionnaire. Rev. Esp. Reumatol. 2004;31:507–513. [Google Scholar]
- 44.Ware JE, Kosinski M, Keller SD. A 12-item short-form health survey: Construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–33. [DOI] [PubMed] [Google Scholar]
- 45.Tsakiris M, Haggard P. The rubber hand illusion revisited: visuotactile integration and self-attribution. J Exp Psychol Hum Percept Perform. 2005;31(1):80–91. doi: 10.1037/0096-1523.31.1.80 [DOI] [PubMed] [Google Scholar]
- 46.Kammers MP, de Vignemont F, Verhagen L, Dijkerman HC. The rubber hand illusion in action. Neuropsychologia. 2009;47(1):204–11. doi: 10.1016/j.neuropsychologia.2008.07.028 [DOI] [PubMed] [Google Scholar]
- 47.Rohde M, Di Luca M, Ernst MO. The Rubber Hand Illusion: feeling of ownership and proprioceptive drift do not go hand in hand. PLoS One. 2011;6(6):e21659 doi: 10.1371/journal.pone.0021659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Stralen HE, van Zandvoort MJ, Kappelle LJ, Dijkerman HC. The Rubber Hand Illusion in a patient with hand disownership. Perception. 2013;42(9):991–3. doi: 10.1068/p7583 [DOI] [PubMed] [Google Scholar]
- 49.Romano D, Sedda A, Brugger P, Bottini G. Body ownership: When feeling and knowing diverge. Conscious Cogn. 2015;34:140–8. doi: 10.1016/j.concog.2015.04.008 [DOI] [PubMed] [Google Scholar]
- 50.Zopf R, Savage G, Williams MA. Crossmodal congruency measures of lateral distance effects on the rubber hand illusion. Neuropsych 2010;48(3):713–25. [DOI] [PubMed] [Google Scholar]
- 51.Botvinick M. Cohen J. Rubber hands “feel” touch that eyes see. Nature 1998; 391;756 doi: 10.1038/35784 [DOI] [PubMed] [Google Scholar]
- 52.Preston C. The role of distance from the body and distance from the real hand in ownership and disownership during the rubber hand illusion. Acta Psychol. 2013; 142:177–83. [DOI] [PubMed] [Google Scholar]
- 53.Kállai J, Hegedüs G, Feldmann A, Rózsa S, Darnai G, Herold R, et al. Temperament and psychopathological syndromes specific susceptibility for rubber hand illusion. Psychiatry Res. 2015;229:410–19. doi: 10.1016/j.psychres.2015.05.109 [DOI] [PubMed] [Google Scholar]
- 54.Romano D, Caffa E, Hernández-Arieta A, Brugger P, Maravita A. The robot hand illusion: Inducing proprioceptive drift through visuo-motor congruency. Neuropsychologia. 2015;70:414–20. doi: 10.1016/j.neuropsychologia.2014.10.033 [DOI] [PubMed] [Google Scholar]
- 55.Kalckert A, Ehrsson HH. Moving a rubber hand that feels like your own: a dissociation of ownership and agency. Front Hum Neurosci. 2012;6(40):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peled A, Ritsner M, Hirschmann S, Geva BA, Modai I. Touch feel illusion in Schizophrenic Patients. Biol Psychiatry. 2000;48:1105–08. [DOI] [PubMed] [Google Scholar]
- 57.Bertamini M, Berselli N, Bode N, Lawson R, Wong TL. The rubber hand illusion in a mirror. Conscious Cogn. 2011;20:1108–19. doi: 10.1016/j.concog.2011.04.006 [DOI] [PubMed] [Google Scholar]
- 58.Guterstam A, Petkova IV, Ehrsson HH. The Illusion of Owning a Third Arm. PLoS One. 2011;6(2):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Franzoi SL, Shields SA. The Body-Esteem Scale: Multidimensional structure and sex differences in a college population. Journal of Personality Assessment. 1984;48:173–78. doi: 10.1207/s15327752jpa4802_12 [DOI] [PubMed] [Google Scholar]
- 60.Porges SW. Body perception questionnaire Laboratory of Developmental Assessment, University of Maryland; 1993. [Google Scholar]
- 61.Martín J, Torre F, Aguirre U, González N, Padierna A, Matellanes B, et al. Evaluation of the interdisciplinary PSYMEPHY treatment on patients with fibromyalgia: a randomized control trial. Pain Med. 2014;15(4):682–91. doi: 10.1111/pme.12375 [DOI] [PubMed] [Google Scholar]
- 62.Martín J, Torre F, Padierna A, Aguirre U, González N, Matellanes B, et al. Interdisciplinary treatment of patients with fibromyalgia: improvement of their health-related quality of life. Pain Pract. 2014;14(8):721–31. doi: 10.1111/papr.12134 [DOI] [PubMed] [Google Scholar]
- 63.de Vignemont F, Ehrsson HH, Haggard P. Bodily illusions modulate tactile perception. Curr Biol. 2005;15(14):1286–90. doi: 10.1016/j.cub.2005.06.067 [DOI] [PubMed] [Google Scholar]
- 64.Tsakiris M. My body in the brain: a neurocognitive model of body-ownership. Neuropsychologia. 2010;48(3):703–12. doi: 10.1016/j.neuropsychologia.2009.09.034 [DOI] [PubMed] [Google Scholar]
- 65.Vallar G, Ronchi R. Somatoparaphrenia: a body delusion. A review of the neuropsychological literature. Exp. Brain Res. 2009;192(3):533–51. doi: 10.1007/s00221-008-1562-y [DOI] [PubMed] [Google Scholar]
- 66.MacLachlan M, Desmond D, Horgan O. Psychological correlates of illusory body experiences. J Rehabil Res Dev. 2003;40(1):59–65. [DOI] [PubMed] [Google Scholar]
- 67.Osborn M, Smith JA. Living with a body separate from the self. The experience of the body in chronic benign low back pain: an interpretative phenomenological analysis. Scand J Caring Sci. 2006;20(2):216–22. doi: 10.1111/j.1471-6712.2006.00399.x [DOI] [PubMed] [Google Scholar]
- 68.Nishigami T, Mibu A, Osumi M, Son K, Yamamoto S, Kajiwara S, et al. Are tactile acuity and clinical symptoms related to differences in perceived body image in patients with chronic nonspecific lower back pain? Man Ther. 2015;20(1):63–7. doi: 10.1016/j.math.2014.06.010 [DOI] [PubMed] [Google Scholar]
- 69.Reinersmann A, Landwehrt J, Krumova EK, Peterburs J, Ocklenburg S, Güntürkün O, et al. The rubber hand illusion in complex regional pain syndrome: preserved ability to integrate a rubber hand indicates intact multisensory integration. Pain. 2013;154(9):1519–27. doi: 10.1016/j.pain.2013.03.039 [DOI] [PubMed] [Google Scholar]
- 70.Förderreuther S, Sailer U, Straube A. Impaired self-perception of the hand in complex regional pain syndrome (CRPS). Pain. 2004;110(3):756–61. doi: 10.1016/j.pain.2004.05.019 [DOI] [PubMed] [Google Scholar]
- 71.Moseley GL, Gallace A, Spence C. Space-based, but not arm-based, shift in tactile processing in complex regional pain syndrome and its relationship to cooling of the affected limb. Brain. 2009;132(Pt 11):3142–51. doi: 10.1093/brain/awp224 [DOI] [PubMed] [Google Scholar]
- 72.Bultitude JH, Walker I, Spence C. Space-based bias of covert visual attention in complex regional pain syndrome. Brain. 2017;140(9):2306–2321. doi: 10.1093/brain/awx152 [DOI] [PubMed] [Google Scholar]
- 73.Filbrich L, Alamia A, Verfaille C, Berquin A, Barbier O, Libouton X, Fraselle V, Mouraux D, Legrain V. Biased visuospatial perception in complex regional pain syndrome. Sci Rep. 2017;7(1):9712 doi: 10.1038/s41598-017-10077-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Haans A, Ijsselsteijn WA, de Kort YA. The effect of similarities in skin texture and hand shape on perceived ownership of a fake limb. Body Image. 2008;5(4):389–94. doi: 10.1016/j.bodyim.2008.04.003 [DOI] [PubMed] [Google Scholar]
- 75.Eshkevari E, Rieger E, Longo MR, Haggard P, Treasure J. Increased plasticity of the bodily self in eating disorders. Psychol Med. 2012;42(4):819–28. doi: 10.1017/S0033291711002091 [DOI] [PubMed] [Google Scholar]
- 76.Mussap AJ, Salton N. A 'rubber-hand' illusion reveals a relationship between perceptual body image and unhealthy body change. J Health Psychol. 2006;11(4):627–39. doi: 10.1177/1359105306065022 [DOI] [PubMed] [Google Scholar]
- 77.Smeets MA, Ingleby JD, Hoek HW, Panhuysen GE. Body size perception in anorexia nervosa: a signal detection approach. J Psychosom Res. 1999;46(5):465–77. [DOI] [PubMed] [Google Scholar]
- 78.Tsakiris M, Fotopoulou A. Is my body the sum of online and offline body-representations? Conscious Cogn. 2008;17(4):1317–23. doi: 10.1016/j.concog.2008.06.012 [DOI] [PubMed] [Google Scholar]
- 79.Flor H, Nikolajsen L, Jensen TS. Phantom limb pain: a case of maladaptive CNS plasticity? Nat Rev Neurosci. 2006;7:873–81. doi: 10.1038/nrn1991 [DOI] [PubMed] [Google Scholar]
- 80.Giummarra MJ, Gibson SJ, Georgiou-Karistianis N, Bradshaw JL. Central mechanisms in phantom limb perception: the past, present and future. Brain Res Rev. 2007;54:219–31. [DOI] [PubMed] [Google Scholar]
- 81.Ramachandran VS, Altschuler EL. The use of visual feedback, in particular mirror visual feedback, in restoring brain function. Brain. 2009;132:1693–1710. doi: 10.1093/brain/awp135 [DOI] [PubMed] [Google Scholar]
- 82.Moseley GL. I can't find it! Distorted body image and tactile dysfunction in patients with chronic back pain. Pain. 2008;140(1):239–43. doi: 10.1016/j.pain.2008.08.001 [DOI] [PubMed] [Google Scholar]
- 83.Osumi M, Okuno H, Nishigami T, Ueta K, Morioka S. Tactile localization training for pain, sensory disturbance, and distorted body image: a case study of complex regional pain syndrome. Neurocase. 2015;21(5):628–34. doi: 10.1080/13554794.2014.961482 [DOI] [PubMed] [Google Scholar]
- 84.Bultitude JH, Rafal RD. Derangement of body representation in complex regional pain syndrome: report of a case treated with mirror and prisms. Exp Brain Res. 2010;204:409–18. doi: 10.1007/s00221-009-2107-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ryan C, Harland N, Drew BT, Martin D. Tactile acuity training for patients with chronic low back pain: a pilot randomised controlled trial. BMC Musculoskelet Disord. 2014; 26:15–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Moseley GL, Wiech K. The effect of tactile discrimination training is enhanced when patients watch the reflected image of their unaffected limb during training. Pain. 2009;144(3):314–9. doi: 10.1016/j.pain.2009.04.030 [DOI] [PubMed] [Google Scholar]
- 87.Trapp W, Weinberger M, Erk S, Fuchs B, Mueller M, Gallhofer B, et al. A brief intervention utilising visual feedback reduces pain and enhances tactile acuity in CLBP patients. J Back Musculoskelet Rehabil. 2015;28(4):651–60. doi: 10.3233/BMR-140561 [DOI] [PubMed] [Google Scholar]
- 88.Gaudio S, Brooks SJ, Riva G. Nonvisual multisensory impairment of body perception in anorexia nervosa: a systematic review of neuropsychological studies. PLoS One. 2014;9(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Keizer A, Smeets MA, Dijkerman HC, van den Hout M, Klugkist I, van Elburg A, et al. Tactile body image disturbance in anorexia nervosa. Psychiatry Res. 2011;190(1):115–20. doi: 10.1016/j.psychres.2011.04.031 [DOI] [PubMed] [Google Scholar]
- 90.Keizer A, Smeets MA, Dijkerman HC, van Elburg A, Postma A. Aberrant somatosensory perception in Anorexia Nervosa. Psychiatry Res. 2012;200(2–3):530–7. doi: 10.1016/j.psychres.2012.05.001 [DOI] [PubMed] [Google Scholar]
- 91.Tiggermann M. Body dissatisfaction and adolescent self-esteem: prospective findings. Body Image. 2005; 2(2):129–35. doi: 10.1016/j.bodyim.2005.03.006 [DOI] [PubMed] [Google Scholar]
- 92.Van den Berg PA, Mond J, Eisenberg M, Ackard D, Neumark-Sztainer D. The link between body dissatisfaction and self-esteem in adolescents: similarities across gender, age, weight status, race/ethnicity, and socioeconomic status. J Adolesc Health. 2010;47(3):290–6. doi: 10.1016/j.jadohealth.2010.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Strauman TJ, Vookles J, Berenstein V, Chaiken S, Higgins ET. Self-discrepancies and vulnerability to body dissatisfaction and disordered eating. J Pers Soc Psychol. 1991;61(6):946–56. [DOI] [PubMed] [Google Scholar]
- 94.Vartanian LR. Self-discrepancy theory and body image. Body Image and Human Appearance 2012;2:711–17. [Google Scholar]
- 95.Boyington JE, Schoster B, Callahan LF. Comparisons of Body Image Perceptions of a Sample of Black and White Women with Rheumatoid Arthritis and Fibromyalgia in the US. Open Rheumatol J. 2015;31(9):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Horwitz EB, Theorell T, Anderberg UM. Fibromyalgia patients' own experiences of video self-interpretation: a phenomenological-hermeneutic study. Scand J Caring Sci. 2003;17(3):257–64. [DOI] [PubMed] [Google Scholar]
- 97.Eubanks JR, Kenkel MY, Gardner RM. Body-size perception, body-esteem, and parenting history in college women reporting a history of child abuse. Percept Mot Skills. 2006;102(2):485–97. doi: 10.2466/pms.102.2.485-497 [DOI] [PubMed] [Google Scholar]
- 98.Wenninger K, Heiman JR. Relating body image to psychological and sexual functioning in child sexual abuse survivors. J Trauma Stress. 1998;11(3):543–62. doi: 10.1023/A:1024408830159 [DOI] [PubMed] [Google Scholar]
- 99.Goldberg RT, Pachas WN, Keith D. Relationship between traumatic events in childhood and chronic pain. Disabil Rehabil. 1999;21(1):23–30. [DOI] [PubMed] [Google Scholar]
- 100.Imbierowicz K, Egle UT. Childhood adversities in patients with fibromyalgia and somatoform pain disorder. Eur J Pain. 2003;7(2):113–9. doi: 10.1016/S1090-3801(02)00072-1 [DOI] [PubMed] [Google Scholar]
- 101.Walker EA, Keegan D, Gardner G, Sullivan M, Bernstein D, Katon WJ. Psychosocial factors in fibromyalgia compared with rheumatoid arthritis: II. Sexual, physical, and emotional abuse and neglect. Psychosom Med. 1997;59(6):572–7. [DOI] [PubMed] [Google Scholar]
- 102.Alexander RW, Bradley LA, Alarcón GS, Triana-Alexander M, Aaron LA, Alberts KR, et al. Sexual and physical abuse in women with fibromyalgia: association with outpatient health care utilization and pain medication usage. Arthritis Care Res. 1998;11(2):102–15. [DOI] [PubMed] [Google Scholar]
- 103.McBeth J, Macfarlane GJ, Benjamin S, Morris S, Silman AJ. The association between tender points, psychological distress, and adverse childhood experiences: a community-based study. Arthritis Rheum. 1999;42(7):1397–404. doi: 10.1002/1529-0131(199907)42:7<1397::AID-ANR13>3.0.CO;2-7 [DOI] [PubMed] [Google Scholar]
- 104.Finestone HM, Stenn P, Davies F, Stalker C, Fry R, Koumanis J. Chronic pain and health care utilization in women with a history of childhood sexual abuse. Child Abuse Negl. 2000;24(4):547–56. [DOI] [PubMed] [Google Scholar]
- 105.Green CR, Flowe-Valencia H, Rosenblum L, Tait AR. The role of childhood and adulthood abuse among women presenting for chronic pain management. Clin J Pain. 2001;17(4):359–64. [DOI] [PubMed] [Google Scholar]
- 106.Kendall-Tackett KA, Marshall R, Ness KE. Chronic pain syndromes and violence against women. Women and Therapy. 2003;26:45–56. [Google Scholar]
- 107.Harlow BL, Stewart EG. Adult-onset vulvodynia in relation to childhood violence victimization. Am J Epidemiol. 2005;161(9):871–80. doi: 10.1093/aje/kwi108 [DOI] [PubMed] [Google Scholar]
- 108.Lampe A, Doering S, Rumpold G, Sölder E, Krismer M, Kantner-Rumplmair W, et al. Chronic pain syndromes and their relation to childhood abuse and stressful life events. J Psychosom Res. 2003;54(4):361–7. [DOI] [PubMed] [Google Scholar]
- 109.Kopec JA, Sayre EC. Traumatic experiences in childhood and the risk of arthritis: a prospective cohort study. Can J Public Health. 2004;95(5):361–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Golding JM, Wilsnack SC, Cooper ML. Sexual assault history and social support: six general population studies. J Trauma Stress. 2002;15(3):187–97. doi: 10.1023/A:1015247110020 [DOI] [PubMed] [Google Scholar]
- 111.Drossman DA, Leserman J, Li Z, Keefe F, Hu YJ, Toomey TC. Effects of coping on health outcome among women with gastrointestinal disorders. Psychosom Med. 2000;62(3):309–17. [DOI] [PubMed] [Google Scholar]
- 112.Leserman J, Drossman DA, Li Z, Toomey TC, Nachman G, Glogau L. Sexual and physical abuse history in gastroenterology practice: how types of abuse impact health status. Psychosom Med. 1996;58(1):4–15. [DOI] [PubMed] [Google Scholar]
- 113.Talley NJ, Fett SL, Zinsmeister AR. Self-reported abuse and gastrointestinal disease in outpatients: association with irritable bowel-type symptoms. Am J Gastroenterol. 1995;90(3):366–71. [PubMed] [Google Scholar]
- 114.Eccleston C, Crombez G. Pain demands attention: A cognitive-affective model of the interruptive function of pain. Psychol Bull. 1999;125:356–66. [DOI] [PubMed] [Google Scholar]
- 115.Van Damme S, Legrain V, Vogt J, Crombez G. Keeping pain in mind: A motivational account of attention to pain. Neurosci Biobehav Rev. 2010;34:204–13. doi: 10.1016/j.neubiorev.2009.01.005 [DOI] [PubMed] [Google Scholar]
- 116.Legrain V, Van Damme S, Eccleston C, Davis KD, Seminowicz DA, Crombez G. A neurocognitive model of attention to pain: Behavioural and neuroimaging evidence. Pain. 2009;144:230–32. doi: 10.1016/j.pain.2009.03.020 [DOI] [PubMed] [Google Scholar]
- 117.Herbert MS, Goodin BR, Pero ST, Schmidt JK, Sotolongo A, Bulls HW, et al. Pain hypervigilance is associated with greater clinical pain severity and enhanced experimental pain sensitivity among adults with symptomatic knee osteoarthritis. Ann Behav Med. 2014;48(1):50–60. doi: 10.1007/s12160-013-9563-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Baum C, Huber C, Schneider R, Lautenbacher S. Prediction of experimental pain sensitivity by attention to pain-related stimuli in healthy individuals. Percept Mot Skills. 2011;112(3):926–46. doi: 10.2466/02.09.22.PMS.112.3.926-946 [DOI] [PubMed] [Google Scholar]
- 119.Crombez G, Eccleston C, Van den Broeck A, Goubert L, Van Houdenhove B. Hypervigilance to pain in fibromyalgia: the mediating role of pain intensity and catastrophic thinking about pain. Clin J Pain. 2004;20(2):98–102. [DOI] [PubMed] [Google Scholar]
- 120.McDermid AJ, Rollman GB, McCain GA. Generalized hypervigilance in fibromyalgia: evidence of perceptual amplification. Pain. 1996;66(2–3):133–44. [DOI] [PubMed] [Google Scholar]
- 121.Hollins M, Walters S. Experimental hypervigilance changes the intensity/unpleasantness ratio of pressure sensations: evidence for the generalized hypervigilance hypothesis. Exp Brain Res. 2016;234(6):1377–84. doi: 10.1007/s00221-015-4541-0 [DOI] [PubMed] [Google Scholar]
- 122.Borg C, Emond FC, Colson D, Laurent B, Michael GA. Attentional focus on subjective interoceptive experience in patients with fibromyalgia. Brain Cogn. 2015;101:35–43. doi: 10.1016/j.bandc.2015.10.002 [DOI] [PubMed] [Google Scholar]
- 123.Burgess N. Spatial memory: how egocentric and allocentric combine. Trends Cogn Sci. 2006;10(12):551–7. doi: 10.1016/j.tics.2006.10.005 [DOI] [PubMed] [Google Scholar]
- 124.Giudice NA, Klatzky RL, Bennett CR, Loomis JM. Perception of 3-D location based on vision, touch, and extended touch. Exp Brain Res. 2013;224(1):141–53. doi: 10.1007/s00221-012-3295-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Riva G, Gaudio S. Allocentric lock in anorexia nervosa: new evidences from neuroimaging studies. Med Hypotheses. 2012;79(1):113–7. doi: 10.1016/j.mehy.2012.03.036 [DOI] [PubMed] [Google Scholar]
- 126.Riva G, Gaudio S. Locked to a wrong body: Eating disorders as the outcome of a primary disturbance in multisensory body integration. Conscious Cogn. 2017; pii: S1053-8100(17)30209-X. [DOI] [PubMed] [Google Scholar]
- 127.Serino S, Dakanalis A, Gaudio S, Carrà G, Cipresso P, Clerici M, et al. Out of body, out of space: Impaired reference frame processing in eating disorders. Psychiatry Res. 2015;15;230(2):732–4. doi: 10.1016/j.psychres.2015.10.025 [DOI] [PubMed] [Google Scholar]
- 128.Mather M. Emotional Arousal and Memory Binding: An Object-Based Framework. Perspect Psychol Sci. 2007;2(1):33–52. doi: 10.1111/j.1745-6916.2007.00028.x [DOI] [PubMed] [Google Scholar]
- 129.Fayed N, Garcia-Campayo J, Magallón R, Andrés-Bergareche H, Luciano JV, Andres E, et al. Localized 1H-NMR spectroscopy in patients with fibromyalgia: a controlled study of changes in cerebral glutamate/glutamine, inositol, choline, and N-acetylaspartate. Arthritis Res Ther. 2010;12(4):R134 doi: 10.1186/ar3072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lutz J, Jäger L, de Quervain D, Krauseneck T, Padberg F, Wichnalek M, et al. White and gray matter abnormalities in the brain of patients with fibromyalgia: a diffusion-tensor and volumetric imaging study. Arthritis Rheum. 2008;58(12):3960–9. doi: 10.1002/art.24070 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(CSV)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.


