Abstract
Klebsiella pneumoniae (KP) is after Escherichia coli (EC) the most common gram-negative species causing invasive infections. Herein, we analyzed risk factors and prognosis in invasive infections caused by KP versus EC, in an area with low antimicrobial resistance. Moreover, we compared antimicrobial resistance and relative prevalence of KP and EC (KP/EC-ratio) in different European countries, using EARS-Net data. Adult patients admitted to Karolinska University Hospital 2006–2012 with invasive infection caused by KP (n = 599) were matched regarding sex and age with patients infected by EC. The medical records were retrospectively reviewed. Comorbidity was adjusted for with multivariable analysis. European data were retrieved from the EARS-Net database. No differences were observed in 7- and 30-day mortality between the groups. The 90-day mortality was significantly higher in the KP cohort (26% versus 17%, p<0.001), but not after adjusting for comorbidity. Malignancy was seen in 53% of the patients with KP versus 38% with EC, OR 1.86 (1.34–2.58). A significant increase in the rate of ESBL-production was observed in EC, but not in KP. The KP/EC-ratio remained stable. In contrast, European data showed increasing percentages of isolates non-susceptible to third-generation cephalosporins in EC and KP, and increasing KP/EC-ratio. Invasive infection caused by KP is a disease affecting patients with high comorbidity and associated with high 90-d mortality. The stable KP/EC-ratio and low occurrence of antimicrobial resistance in data from Karolinska University Hospital compared to aggregate data from 20 EARS-Net countries could be related to absence of clonal spread of multidrug-resistant KP.
Introduction
Klebsiella pneumoniae is second to Escherichia coli the most common gram-negative pathogen associated with a wide spectrum of infections, such as urinary tract infection (UTI), pneumonia, intra-abdominal infection, bloodstream infection (BSI), meningitis and pyogenic liver abscess (PLA) [1–4]. During the last decades the rates of extended-spectrum cephalosporin-resistant K. pneumoniae producing extended-spectrum β-lactamases (ESBL) have dramatically increased worldwide, and in most parts of the world K. pneumoniae is the pathogen mostly associated with dissemination of ESBLs and other horizontally transmissible resistance genes [5, 6].
Invasive infections caused by K. pneumoniae have been associated with comorbidities such as cancer, diabetes, and previous organ transplantation [7, 8]. A high case fatality rate has been reported, ranging between 18 and 49%, where more recent studies focus on infections caused by multi-drug resistant isolates [3, 7, 9–13]. A population-based study on bloodstream infection (BSI) caused by K. pneumoniae 2000–2007 in Canada, a setting with low prevalence of antimicrobial resistance, showed an increase of the burden of disease during the last decade and a case fatality rate of 19% [7]. In the same demographic area 2000–2006 the case fatality rate in BSI caused by E. coli was 11% [14]. Studies comparing community- and hospital-acquired BSI caused by K. pneumoniae demonstrate differences in risk factors and outcome [10, 15, 16], where neoplastic disease and antimicrobial resistance are common among patients with hospital-acquired infections. In a few studies BSI caused by K. pneumoniae or E. coli have been compared but there is still limited data on early and late mortality and differences in risk factors for acquisition between patient groups affected by the respective pathogens [11, 17].
The primary aim of this study was to compare risk factors for acquisition and mortality, using the endpoints 7-, 30-, and 90-day mortality, of invasive infection caused by K. pneumoniae compared to E. coli. A secondary aim was to use European EARS-Net data on invasive infections to compare temporal trends in rates of resistance in E. coli and K. pneumoniae and relative occurrence of the two species, pursuing the hypothesis that some resistant clones of K. pneumoniae may contribute to a species-shift, as measured by the ratio between K. pneumoniae and E. coli (KP/EC-ratio).
Methods
Study populations and bacterial isolates
Data from Karolinska University Hospital, Sweden
All adult (≥18-year-old) patients admitted to Karolinska University Hospital, Sweden, a tertiary medical center with 1700 beds, between 2006 and 2012, with growth of K. pneumoniae either in blood (n = 646), cerebrospinal fluid (CSF) (n = 4), or both (n = 2) were included in this retrospective cohort study (S1 Fig). These patients are referred to as the “extended K. pneumoniae cohort”. Isolates and patients were identified by searches in the clinical microbiology laboratory information system at the Karolinska University Laboratory. Species identification was done with the API 20E system (bioMérieux, Marcy l’Etoile, France) or VITEK2 (bioMérieux). Antimicrobial susceptibility testing was performed with the disk diffusion method on Isosensitest agar (Oxoid, Basingstoke, UK) and interpreted according to the guidelines of the Swedish Reference Group for Antibiotics (SRGA) [18]. Co-infection with E. coli was seen in 53/652 patients (8%). The 599 patients without co-infection with E. coli are referred to as the “K. pneumoniae cohort”. For each patient in the K. pneumoniae cohort a patient with invasive (BSI or meningitis) E. coli infection, matched 1:1 regarding sex, age and year of onset of infection, was selected at random from patients in the microbiological database. These 599 patients are referred to as the “E. coli cohort”. For all study subjects (n = 1,251) the medical records were reviewed by an infectious disease specialist regarding patient risk factors, hospital- versus community-acquired disease, source of infection, antimicrobial treatment, and mortality. Only the first episode of an infection during the study period was included in the analyses.
Definitions
In this study K. pneumoniae refers to K. pneumoniae sensu latu [19]. Infections were defined as hospital-acquired if the sample was obtained >48 h after admittance. Healthcare-associated community-onset infection was defined as either having been admitted to hospital, having had surgery, received outpatient hemodialysis, living at a long-term care facility, or attended daycare within the previous 30 days before the onset of infection [20, 21]. For comparison of antimicrobial resistance with EARS-Net data the same algorithm as for international data (i.e. one isolate per year and patient, and inclusion of all ages) was used. The infection was classified as polymicrobial if at least one additional species was recovered from blood specimens drawn within 24 h from the recovery of K. pneumoniae. Presence in one blood culture bottle was considered sufficient except for frequent skin contaminants that had to be present in at least two venipunctures [22]. Comorbidities were analyzed individually and as Charlson comorbidity index [23].Time to antimicrobial therapy was defined as time from arrival to hospital or, when onset at hospital, time from onset of symptoms until administration of adequate antimicrobial therapy. Adequate antimicrobial therapy was defined as treatment including at least one antibiotic susceptible to the present pathogen/pathogens. Data on combination versus single therapy was not evaluated.
Data from the European Antimicrobial Resistance Surveillance Network (EARS-Net)
The European Antimicrobial Resistance Surveillance Network (EARS-Net) is an international surveillance network that collects routine clinical antibiotic susceptibility data from all 28 European Union (EU) Member States and two European Economic Area (EEA) countries, Iceland and Norway. The network is coordinated by the European Centre for Disease Prevention and Control (ECDC). Only invasive isolates from blood or cerebrospinal fluid are included in the EARS-Net data. The AST results are ascertained according to agreed protocols [24, 25], and the general quality and comparability of the data are evaluated through an annual external quality assessment offered to the participating laboratories.
Data on invasive E. coli and K. pneumoniae isolates with AST information for third-generation cephalosporins reported for the period 2006–2012 were extracted from the database at ECDC. Resistance to third-generation cephalosporins was defined as resistance to at least one of the third-generation cephalosporins under surveillance by EARS-Net: ceftriaxone, ceftazidime or cefotaxime. Isolates were considered as non-susceptible for this study when tested and interpreted as intermediate (I) or resistant (R) in agreement with the clinical breakpoint criteria used by the local laboratory. To reduce sampling bias, countries either reporting few isolates (median value of <300 E. coli isolates per year), or lacking data for parts of the period, were excluded. Twenty countries were included in the analysis: Austria, Czech Republic, Germany, Denmark, Greece, Finland, France, Hungary, Ireland, Italy, Lithuania, Luxemburg, the Netherlands, Norway, Poland, Portugal, Spain, Sweden, Slovenia, and the United Kingdom.
Statistical analysis
Sample size was based on calculation of a 6% difference in mortality within 30 days (17 and 11% respectively for the K. pneumoniae and E. coli cohort) with a power of 0.8. Conditional logistic regression was used when comparing variables (clinical characteristics) between the K. pneumoniae cohort and the E. coli cohort. For categorical variables within the cohorts, Fisher´s exact test and Chi-square test were used. The Mann Whitney test was used to compare continuous variables. For all tests a two-sided p-value <0.05 was considered significant. A multivariable model was built based on stepwise forward and backward selection using the parameters with a p-value <0.2 in univariate analysis and comparing models with the likelihood ratio test (LRT). Of the strongly correlating risk factors only one was picked. Odds Ratio (OR) and 95% Confidence Intervals (CI) were calculated. Since hospital-acquired infection might correlate with other comorbidities we also performed a model without including the variables hospital-acquired, healthcare-associated community-onset and community-acquired infection. These results (data not shown) were similar to the results of our final model. To analyze data on mortality logistic regression was used, and similar multivariable models were built, restricted by the number of outcomes.
For international comparison, K. pneumoniae to E. coli ratios and percentages of K. pneumoniae and E. coli isolates non-susceptible to third-generation cephalosporins were calculated for each data source (Karolinska University Hospital and EARS-Net), country and year. The association between the national KP/EC ratio and the percentage third-generation cephalosporins non-susceptible isolates was assessed by Pearson correlation coefficient. The command ptrend in Stata was used for trend analysis of proportions. For EARS-Net data, a population-weighted EU/EEA mean resistance percentage was determined by applying population-based weights to each country’s data before calculating the arithmetic mean for all reporting countries. Country weights were used to adjust for imbalances in reporting propensity and population coverage, as the total number of reported isolates per country in most cases does not reflect the population size. The weight applied to each national data point represented the proportion of the country’s population out of the total population of all countries included in the calculation. Annual population data were retrieved from the Eurostat on-line database [26].
All statistical analyses were performed using Stata Statistical Software Release 12 (StataCorp., College Station, TX, USA).
Ethical considerations
Ethical approval for the study was obtained from the Karolinska Institutet Regional Ethics Committee of Stockholm (recordals 2009/1985-31/4, 2011/1377-32, and 2012/2159-32). The committee approved that no written or verbal consent had to be given by the study subjects, as the study only pertained to extracting limited clinical data from patient charts. Patient records were anonymized and de-identified prior to analysis.
Results
Risk factors for infection
Patient characteristics of the K. pneumoniae and the E. coli cohort are presented in Table 1 (multivariable analysis) and S1 Table (univariate analysis). The median age was 68 years and 348/599 patients (58%) were male. In multivariable analysis, hematological, colorectal and bile/liver/pancreatic malignancies, OR 1.70 (95% CI 1.07–2.70), 2.56 (95% CI 1.34–4.89) and 3.45 (95% CI 1.77–6.75) respectively, were more common in the K. pneumoniae cohort, as well as Chronic Obstructive Pulmonary Disease (COPD), OR 1.96 (95% CI 1.14–3.36), kidney disease, OR 1.90 (95% CI 1.28–2.82), peripheral vascular disease, OR 3.74 (95% CI 1.65–8.48) and bile disease, OR 3.10 (1.44–6.66). Urinary catheters/abnormalities (including urinary ileostomy, suprapubic catheter and indwelling urinary catheter) and central venous catheters or ports were associated with K. pneumoniae infection, OR 2.36 (95% CI 1.64–3.40) and 2.32 (95% CI 1.53–3.54) respectively. K. pneumoniae were significantly more often healthcare-associated community-onset infections, OR 3.06 (95% CI 2.03–4.62). An identified urinary tract source of infection was more frequent for E. coli. Invasive infection caused by K. pneumoniae was significantly more often polymicrobial, even when excluding cases with growth of both K. pneumoniae and E. coli, OR 1.42 (95% CI 1.00–2.00).
Table 1. Clinical characteristics of patients with invasive infection caused by K. pneumoniae versus E. coli, multivariable analysis.
| K. pneumoniae | E. coli | Adjusted odds | |
|---|---|---|---|
| n = 599 | n = 599 | ratio | |
| Patients factors | No (%) | No (%) | (95% CI) |
| K. pneumoniae vs | |||
| E. coli | |||
| Peripheral vascular disease | 30 (5) | 14 (2) | 3.74 (1.65–8.48) |
| COPD | 58 (10) | 37 (6) | 1.96 (1.14–3.36) |
| Kidney disease | 105 (18) | 69 (12) | 1.90 (1.28–2.82) |
| Bile disease | 36 (6) | 15 (3) | 3.10 (1.44–6.66) |
| Hematological malignancy | 112 (19) | 76 (13) | 1.70 (1.07–2.70) |
| Bile/liver/pancreas malignancy | 51 (9) | 22 (4) | 3.45 (1.77–6.75) |
| Colorectal malignancy | 42 (7) | 24 (4) | 2.56 (1.34–4.89) |
| Urinary catheter | 191 (32) | 111 (19) | 2.36 (1.64–3.40) |
| Central catheter | 190 (32) | 96 (16) | 2.32 (1.53–3.54) |
| Hospital-acquireda) | 178 (30) | 197 (33) | 0.53 (0.37–0.77) |
| Healthcare- associated community-onseta) | 163 (27) | 55 (9) | 3.06 (2.03–4.62) |
a) in relation to community-acquired infection
Additional factors (non-significant) included in the final multivariable analysis: arrhythmia, cerebrovascular disease with sequela, intestinal disease (ulcerative colitis, Crohn´s disease, op-ileostomy, intestinal co-infection), breast malignancy and melanoma
Mortality
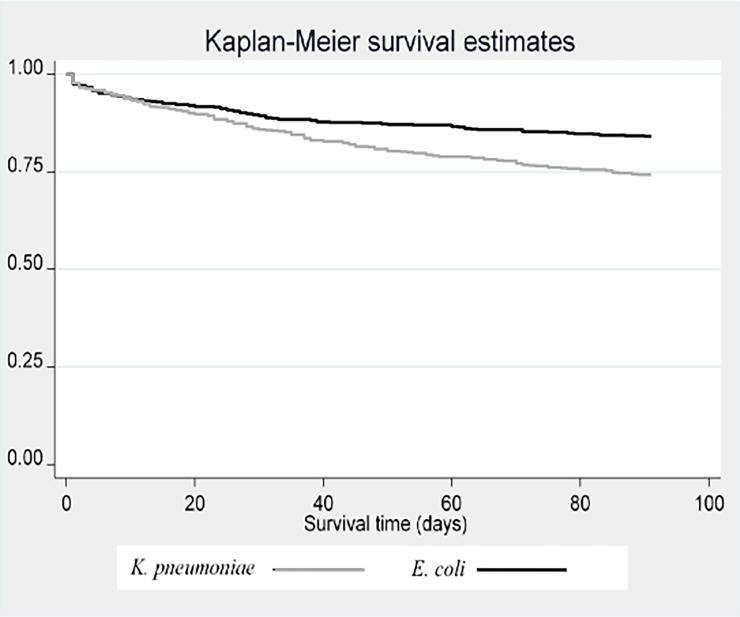
The 7-day–and 30-day mortalities were similar in the K. pneumoniae and E. coli cohorts, 34/599 (6%) versus 39/599 (7%), p = 0.55, and 87/599 (15%) versus 70/599 (12%), p = 0.15 respectively. The 90-day mortality was significantly higher in the K. pneumoniae 156/599 (26%) versus 101/599 (17%) in the E. coli cohort, p<0.001. As seen in the Kaplan-Meier survival analysis (Fig 1) mortality difference increased between K. pneumoniae and E. coli over time. However, there was no increased 90-day mortality in patients with K. pneumoniae OR 1.17 (95% CI 0.85–1.62), after adjustment for polymicrobial infections, site of acquisition (healthcare-acquired, community-acquired, or healthcare-associated community-acquired), source of infection and comorbidities (S2 Table).
Fig 1. Kaplan-Meier survival curve K. pneumoniae versus E. coli cohort during 90 days.
Log-rank test p<0.001.
Predictors for mortality were evaluated in the extended K. pneumoniae cohort (n = 652). Associated factors significant in multivariable analysis are presented in Table 2, and univariate analyses are shown in S3 Table. High age, lung malignancy and infections emanating from the lungs were factors associated both with early (within 7 days) and late (up to 90 days) mortality. For mortality within 30 and 90 days host factors were of major importance. Patients with Charlson index >5, compared to patients with Charlson index ≤5, had an adjusted OR of 7.17 (95% CI 2.69–19.14) and 11.82 (95% CI 5.32–26.24) (not shown in table) respectively for fatal outcome within 30 and 90 days respectively. Within 30 and 90 days, mortality was also higher among patients with a hospital-acquired or a healthcare-associated infection, OR 1.99 (95% CI 1.21–3.28) and 1.69 (95% CI 1.02–2.79) respectively compared to community-acquired infection. Polymicrobial infection was a poor prognostic factor for mortality within 7 days, OR 3.07 (95% CI 1.51–6.27) (Table 2). Infections emanating from the lungs were associated with higher mortality than infections with other origins and were also more common among patients at the ICU.
Table 2. Associated factors for mortality in the extended K. pneumoniae cohort, factors significant in multivariable analysis.
| Mortality within | Mortality within | Mortality within | |
|---|---|---|---|
| 7d | 30 d | 90 d | |
| Associated factors* | (n = 43) | (n = 101) | (n = 176) |
| Adjusted OR | Adjusted OR | Adjusted OR | |
| Age | 1.03 (1.00–1.05) | 1.02 (1.00–1.04) | 1.03 (1.01–1.04) |
| Polymicrobial infection | 3.07 (1.51–6.27) | 2.20 (1.32–3.68) | |
| Kidney disease | 2.33 (1.41–3.84) | ||
| CNS disease | 3.12 (1.73–5.62) | 2.09 (1.26–3.45) | |
| Lung malignancy | 13.45 (3.94–45.90) | 13.20 (4.11–42.38) | 20.77 (6.01–71.73) |
| Urogenital, GI, bile/liver/pancreas malignancy | 2.07 (1.10–3.91) | 3.07 (1.87–5.05) | |
| Hematological malignancy | 3.13 (1.47–6.63) | 2.50 (1.33–4.72) | |
| Other malignancy** | 6.18 (1.87–20.41) | 6.34 (2.54–15.85) | 3.77 (1.63–8.74) |
| Hospital-acquired | 1.99 (1.21–3.28) | ||
| Healthcare-associated community-onset | 1.69 (1.02–2.79) | ||
| Source of infection | |||
| Respiratory tract | 3.62 (1.01–13.04) | 3.79 (1.32–10.87) | 3.74 (1.44–9.68) |
| Bile/liver, GI | 1.92 (1.00–4.16) | 1.91 (1.15–3.15) | |
| Unknown | 2.09 (1.05–4.16) |
*Compared to variable being absent except for hospital-acquired and healthcare-associated community-onset where compared to community-acquired infection and for source of infection where compared to urinary tract.
**Breast, miscellaneous, melanoma
Variables included in models: 7-d mortality: age, polymicrobial infection, malignancies, onset of disease and source of infection. 30-d and 90-d mortality: age, polymicrobial infection, malignancies, onset of disease, source of infection, CNS disease, cardiovascular disease, lung disease and kidney disease.
The time to adequate antimicrobial treatment was almost equal in the two cohorts. Within 2 hours 210 (35%) versus 194 (32%) (p = 0.33) patients in the K. pneumoniae- and E. coli cohorts, within 4 hours 356 (59%) versus 373 (62%) (p = 0.31), and finally within 24 hours 525 (89%) versus 541 (91%) patients received adequate antimicrobial treatment. Out of the 68 patients in the K. pneumoniae cohort receiving adequate antibiotics after more than 24 h, or not receiving antibiotics at all, 20 (29%) died within 90 days (p = 0.47 compared to those receiving antibiotics within 24 hours).
Bacterial characteristics and antimicrobial resistance
The isolates from Karolinska University Hospital showed low levels of resistance (S4 Table) in the K. pneumoniae cohort (n = 599). Only nine isolates (2%) were extended-spectrum beta-lactamase (ESBL)-producers, including one strain that also produced VIM-metallo-beta-lactamase (detected in a patient who had been hospitalized abroad). The most common resistance was against trimethoprim-sulfamethoxazole (n = 62, 10%) and ciprofloxacin (n = 47, 8%). A total of 106 isolates (18%) exhibited resistance to at least one antimicrobial group while only 15 (3%) showed multidrug-resistance, i.e. were resistant to ≥ three classes of antimicrobials. Among the E. coli isolates the susceptibility pattern was different showing resistance in 29% (n = 172) against trimethoprim-sulfamethoxazole, 17% (n = 103) against ciprofloxacin, and 6% (n = 36) were ESBL-producers, all p<0.001 compared to K. pneumoniae.
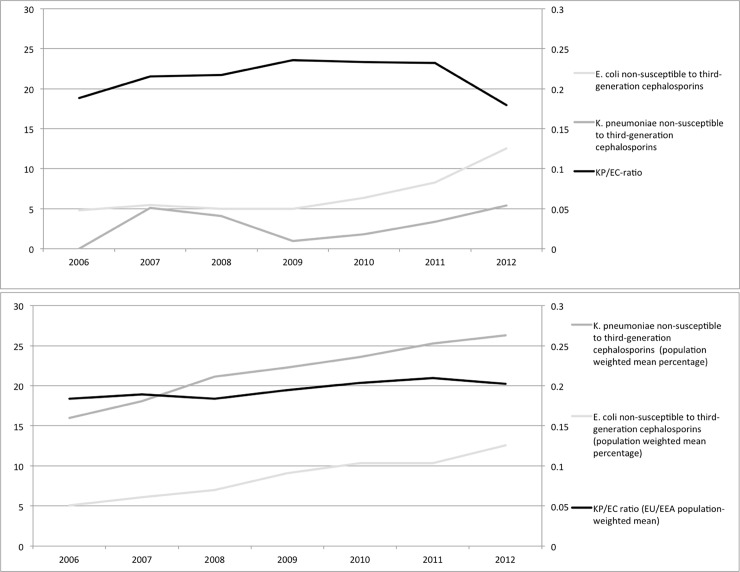
In total (children and adults, reporting 1 patient/year) there were an increasing number of K. pneumoniae invasive infections from 2006 (70 patients) to 2012 (111 patients) (data not shown in table). A similar increase was observed of invasive E. coli infections from 356 in 2006 to 557 in 2012, so the proportion of K. pneumoniae to E. coli (KP/EC-ratio) ranged between 0.18–0.23 from year to year, but showed no changing trend during the period (p = 0.52). During this period the frequency of obtained blood cultures at the hospital has increased in total, but the ratio of positive cultures has remained unchanged (unpublished laboratory statistics, Karolinska University Hospital). Among the K. pneumoniae isolates there was no increase in the proportion of ESBL-producers during the period. In contrast, for E. coli ESBL-producing isolates increased significantly, from 2% 2006 to 11% 2012 (p<0.001). The proportion of non-susceptibility among third-generation cephalosporins (Fig 2A) in data from Karolinska University Hospital increased from 5 to 13% among the E. coli isolates and varied between 0 and 5% among the K. pneumoniae isolates.
Fig 2. Rates of invasive isolates non-susceptible to third-generation cephalosporins among K. pneumoniae and E. coli 2006–2012, and K. pneumoniae / E. coli (KP/EC) ratio.
A) Karolinska University Hospital. B) Twenty countries within EU/EEA reporting to EARS-Net. Population-weighted data.
European data
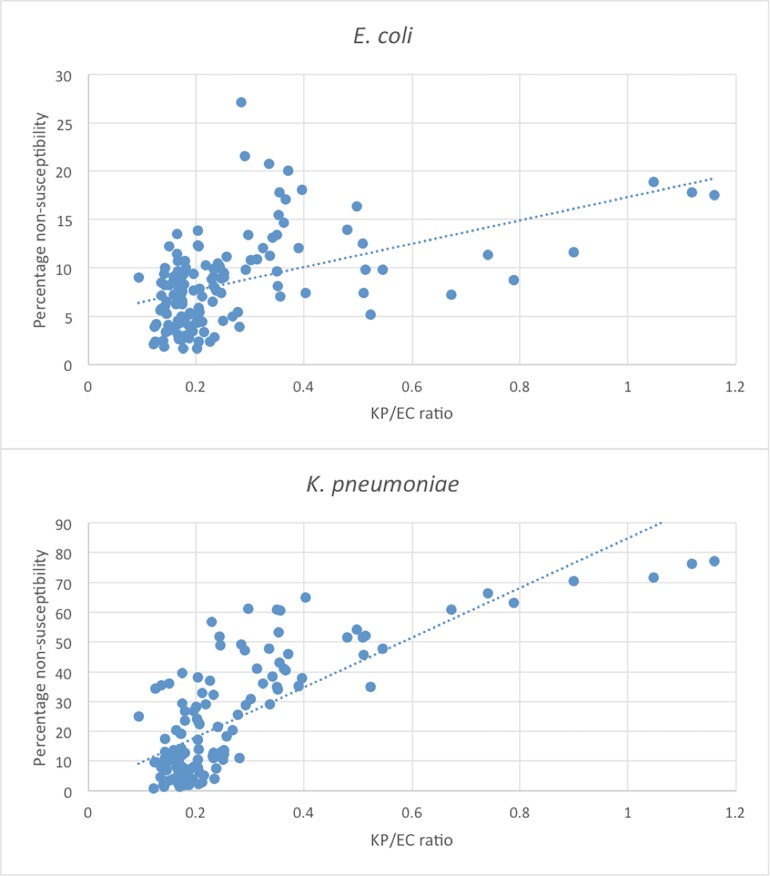
The EU/EEA population-weighted proportion of isolates non-susceptible to third-generation cephalosporins increased over time, both among K. pneumoniae (from 16% to 26% in the 20 countries) and in E. coli (from 5% to 13%). A moderate correlation between an increase in KP/EC-ratio and non-susceptibility to third-generation cephalosporin was observed for E. coli (r: 0.48 p<0.001) and the correlation was even more pronounced for non-susceptible K. pneumoniae (r: 0.77 p<0.001) (Fig 3). Also, the increasing third-generation cephalosporin-non-susceptibility in E. coli and K. pneumoniae paralleled a shift in the KP/EC-ratio 2006–2012 (p<0.001). In the Stockholm area, an increase in third-generation cephalosporin-resistant E. coli was observed, but contrary to the EARS-Net data, no change was observed in the KP/EC-ratio (p = 0.90) (Fig 2).
Fig 3.
The percentages of E. coli (3A) and K. pneumoniae (3B) isolates non-susceptible against third-generation cephalosporins plotted against the ratios of K. pneumoniae/E. coli (KP/EC ratio) among 20 European countries 2006–2012. Each dot (n = 140) represents one country a certain year.
Discussion
Invasive infection caused by K. pneumoniae is a disease with high 90-days mortality. There is a high level of comorbidity among the affected patients. Due to low resistance rates in K. pneumoniae in Sweden we could reduce the risk of bias that is often observed in other studies in assessment of mortality. In concordance with previous studies there are differences in patient populations affected by invasive infection caused by K. pneumoniae versus E. coli with a higher level of comorbidity in patients infected with K. pneumoniae. Patients with an impaired host defense are at greater risk of developing invasive infection caused by K. pneumoniae compared to E. coli, in this study 163/599 patients (27%) had a healthcare-associated community-onset infection in the K. pneumoniae cohort compared to only 55/599 (9%) in the E. coli cohort. In settings with a high prevalence of third-generation cephalosporin resistance among K. pneumoniae, the knowledge about risk factors for infections caused by this pathogen may have implications for selection of empiric treatment.
The K. pneumoniae isolates were in the Swedish setting more susceptible to antimicrobials than the E. coli isolates. This contrasts with most European countries where K. pneumoniae is associated with higher resistance levels compared to E. coli. The few countries reporting higher third-generation non-susceptibility in E. coli compared to K. pneumoniae all had low resistance frequencies, just as the cohort from Karolinska University Hospital [27]. As scatterplots in Fig 3A and 3B graphically show, there is a correlation between the ratio of E. coli/K. pneumoniae and the percentage of E. coli and K. pneumoniae isolates non-susceptible to third-generation cephalosporins. Among the isolates from the Swedish cohort, we could not see the same trend over time. There are reasons to believe that the ecological niches and resistance rates could be influenced by circulating epidemic clones [28–30]. The K. pneumoniae-isolates from patients admitted to Karolinska University Hospital Solna 2007–2009 (n = 139) were in a previous study subjected to molecular analysis [31]. In that study, there was a high level of diversity among sequence types, and internationally reported epidemic high-risk clones were not detected. The low antimicrobial resistance among K. pneumoniae isolates in the present study supports the previous results that there is no indication of circulating high-risk clones in the Stockholm area, which could offer an explanation to the relatively low proportion of K. pneumoniae in blood cultures. The 30-d mortality of 16% noted in our study is compared to other studies low, but comparison is not easily made between e.g. 30-d mortality and in-hospital mortality, and between settings of high-and low prevalence of ESBL-producers. Since most patients affected by invasive infection caused by K. pneumoniae have a known malignancy, studies investigating if invasive infection caused by K. pneumoniae should be regarded as a predictor for an underlying undiagnosed malignancy, would be of interest.
Due to the retrospective design of our study we could not control for all possible variables and data from the medical records can be incomplete. We lacked the possibility to retrieve severity score indexes (i.e. disease severity on onset of illness), a factor influencing both time to antimicrobial treatment and mortality. However, the study is large, containing nearly 600 patients in each group. As the E. coli cohort was selected to match the K. pneumoniae cohort in sex and age, results from this study are not generalizable to all E. coli invasive infections. Data from EARS-Net are much less detailed compared to the Karolinska University Hospital data, but the similar sampling framework (restricting data to invasive isolates and using the same algorithm for avoiding isolate duplication) allowed for a crude overall comparison without any patient-specific stratifications. It should however be noted that the pooled European data are based on various national surveillance systems with significant differences in population coverage and BSI case ascertainment of patients with BSIs. In countries where patients with community-acquired infections less frequently are sampled compared to those with healthcare-associated and hospital-acquired infections, the KP/EC-ratio might be overestimated. We believe that excluding the countries reporting <300 isolates of E. coli per year minimized this potential bias.
In summary, invasive infections caused by K. pneumoniae affect patients with high comorbidity. The disease is associated with high long-term mortality, even in a low-prevalence setting of ESBL-producers. Specific risk factors for invasive infection caused by K. pneumoniae compared to E. coli are healthcare-associated community-onset infection, malignancies, peripheral vascular disease, COPD, kidney disease, bile disease, and indwelling catheters. In settings with high resistance among K. pneumoniae this information could be taken into consideration when choosing empiric antibiotic treatment in severely ill patients. The increasing KP/EC-ratio observed in European data was not demonstrated in the Karolinska University Hospital data, and could be due to absence of epidemic K. pneumoniae clones, as the latter could explain both high prevalence of resistance and a higher overall burden of K. pneumoniae BSI.
Supporting information
(TIFF)
(PDF)
(PDF)
(PDF)
No of isolates with non-susceptibility (intermediate and resistant) to antimicrobials.
(PDF)
Acknowledgments
We would like to acknowledge all EARS-Net national representatives and laboratories for providing the European data included in this study. In particular, we thank Lise-Lotte Diaz Högberg at the European Centre for Disease Prevention and Control, Stockholm, for access to and assistance in analysis of EARS-Net data.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The study was supported by a grant from the European Society for Clinical Microbiology and Infectious diseases (ESCMID).
References
- 1.Podschun R, Ullmann U. Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev. 1998;11(4):589–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang JH, Liu YC, Lee SS, Yen MY, Chen YS, Wann SR, et al. Primary liver abscess due to Klebsiella pneumoniae in Taiwan. Clin Infect Dis. 1998;26(6):1434–8. [DOI] [PubMed] [Google Scholar]
- 3.Yinnon AM, Butnaru A, Raveh D, Jerassy Z, Rudensky B. Klebsiella bacteraemia: community versus nosocomial infection. QJM: monthly journal of the Association of Physicians. 1996;89(12):933–41. [DOI] [PubMed] [Google Scholar]
- 4.Anderson DJ, Moehring RW, Sloane R, Schmader KE, Weber DJ, Fowler VG Jr., et al. Bloodstream infections in community hospitals in the 21st century: a multicenter cohort study. PLoS One. 2014;9(3):e91713 doi: 10.1371/journal.pone.0091713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Queenan AM, Bush K. Carbapenemases: the versatile beta-lactamases. Clin Microbiol Rev. 2007;20(3):440–58, table of contents. doi: 10.1128/CMR.00001-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iredell J, Brown J, Tagg K. Antibiotic resistance in Enterobacteriaceae: mechanisms and clinical implications. BMJ (Clinical research ed). 2016;352:h6420. [DOI] [PubMed] [Google Scholar]
- 7.Meatherall BL, Gregson D, Ross T, Pitout JD, Laupland KB. Incidence, risk factors, and outcomes of Klebsiella pneumoniae bacteremia. Am J Med. 2009;122(9):866–73. doi: 10.1016/j.amjmed.2009.03.034 [DOI] [PubMed] [Google Scholar]
- 8.Wang LS, Lee FY, Cheng DL, Liu CY, Hinthorn DR, Jost PM. Klebsiella pneumoniae bacteremia: analysis of 100 episodes. Journal of the Formosan Medical Association = Taiwan yi zhi. 1990;89(9):756–63. [PubMed] [Google Scholar]
- 9.Jung Y, Lee MJ, Sin HY, Kim NH, Hwang JH, Park J, et al. Differences in characteristics between healthcare-associated and community-acquired infection in community-onset Klebsiella pneumoniae bloodstream infection in Korea. BMC infectious diseases. 2012;12:239 doi: 10.1186/1471-2334-12-239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsay RW, Siu LK, Fung CP, Chang FY. Characteristics of bacteremia between community-acquired and nosocomial Klebsiella pneumoniae infection: risk factor for mortality and the impact of capsular serotypes as a herald for community-acquired infection. Archives of internal medicine. 2002;162(9):1021–7. [DOI] [PubMed] [Google Scholar]
- 11.Thom KA, Schweizer ML, Osih RB, McGregor JC, Furuno JP, Perencevich EN, et al. Impact of empiric antimicrobial therapy on outcomes in patients with Escherichia coli and Klebsiella pneumoniae bacteremia: a cohort study. BMC infectious diseases. 2008;8:116 doi: 10.1186/1471-2334-8-116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marra AR, Wey SB, Castelo A, Gales AC, Cal RG, Filho JR, et al. Nosocomial bloodstream infections caused by Klebsiella pneumoniae: impact of extended-spectrum beta-lactamase (ESBL) production on clinical outcome in a hospital with high ESBL prevalence. BMC infectious diseases. 2006;6:24 doi: 10.1186/1471-2334-6-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tuon FF, Kruger M, Terreri M, Penteado-Filho SR, Gortz L. Klebsiella ESBL bacteremia-mortality and risk factors. The Brazilian journal of infectious diseases: an official publication of the Brazilian Society of Infectious Diseases. 2011;15(6):594–8. [DOI] [PubMed] [Google Scholar]
- 14.Laupland KB, Gregson DB, Church DL, Ross T, Pitout JD. Incidence, risk factors and outcomes of Escherichia coli bloodstream infections in a large Canadian region. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2008;14(11):1041–7. [DOI] [PubMed] [Google Scholar]
- 15.Tsai SS, Huang JC, Chen ST, Sun JH, Wang CC, Lin SF, et al. Characteristics of Klebsiella pneumoniae bacteremia in community-acquired and nosocomial infections in diabetic patients. Chang Gung medical journal. 2010;33(5):532–9. [PubMed] [Google Scholar]
- 16.Kang CI, Kim SH, Bang JW, Kim HB, Kim NJ, Kim EC, et al. Community-acquired versus nosocomial Klebsiella pneumoniae bacteremia: clinical features, treatment outcomes, and clinical implication of antimicrobial resistance. Journal of Korean medical science. 2006;21(5):816–22. doi: 10.3346/jkms.2006.21.5.816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hansen DS, Gottschau A, Kolmos HJ. Epidemiology of Klebsiella bacteraemia: a case control study using Escherichia coli bacteraemia as control. The Journal of hospital infection. 1998;38(2):119–32. [DOI] [PubMed] [Google Scholar]
- 18.Olsson-Liljequist B, Larsson P, Walder M, Miorner H. Antimicrobial susceptibility testing in Sweden. III. Methodology for susceptibility testing. Scandinavian journal of infectious diseases Supplementum. 1997;105:13–23. [PubMed] [Google Scholar]
- 19.Brisse S, Passet V, Grimont PA. Description of Klebsiella quasipneumoniae sp. nov., a novel species isolated from human infections, with two subspecies Klebsiella quasipneumoniae subsp. quasipneumoniae subsp. nov. and Klebsiella quasipneumoniae subsp. similipneumoniae subsp. nov., and demonstration that K. singaporensis is a junior heterotypic synonym of K. variicola. International journal of systematic and evolutionary microbiology. 2014. [DOI] [PubMed] [Google Scholar]
- 20.Kanoksil M, Jatapai A, Peacock SJ, Limmathurotsakul D. Epidemiology, microbiology and mortality associated with community-acquired bacteremia in northeast Thailand: a multicenter surveillance study. PLoS One. 2013;8(1):e54714 doi: 10.1371/journal.pone.0054714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moon HW, Ko YJ, Park S, Hur M, Yun YM. Analysis of community- and hospital-acquired bacteraemia during a recent 5-year period. J Med Microbiol. 2014;63(Pt 3):421–6. doi: 10.1099/jmm.0.069054-0 [DOI] [PubMed] [Google Scholar]
- 22.Kjellander C, Bjorkholm M, Cherif H, Kalin M, Giske CG. Hematological: Low all-cause mortality and low occurrence of antimicrobial resistance in hematological patients with bacteremia receiving no antibacterial prophylaxis: a single-center study. Eur J Haematol. 2012;88(5):422–30. doi: 10.1111/j.1600-0609.2012.01768.x [DOI] [PubMed] [Google Scholar]
- 23.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. [DOI] [PubMed] [Google Scholar]
- 24.ECDC. European Center for Disease Control and Prevention. EARS-Net Reporting Protocol. July 2015. Available from http://ecdc.europa.eu/en/activities/surveillance/EARS-Net/Documents/2015-EARS-Net-reporting-protocol.pdf 2015.
- 25.EUCAST. European Committee on Antimicrobial Susceptibility Testing. EUCAST guidelines for detection of resistance mechanisms and specific resistances of clinical and/or epidemiological importance. Version 1.0, December 2013. Available from http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Resistance_mechanisms/EUCAST_detection_of_resistance_mechanisms_v1.0_20131211.pdf 2013.
- 26.THe European Commission database on statistics. Available at: http://ec.europa.eu/eurostat/data/database
- 27.European Centre for Disease Prevention and Control. Antimicrobial resistance surveillance in Europe 2014 Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net). Stockholm:ECDC; 2015. [Google Scholar]
- 28.Rodrigues C, Machado E, Ramos H, Peixe L, Novais A. Expansion of ESBL-producing Klebsiella pneumoniae in hospitalized patients: a successful story of international clones (ST15, ST147, ST336) and epidemic plasmids (IncR, IncFIIK). International journal of medical microbiology: IJMM. 2014;304(8):1100–8. doi: 10.1016/j.ijmm.2014.08.003 [DOI] [PubMed] [Google Scholar]
- 29.Breurec S, Guessennd N, Timinouni M, Le TA, Cao V, Ngandjio A, et al. Klebsiella pneumoniae resistant to third-generation cephalosporins in five African and two Vietnamese major towns: multiclonal population structure with two major international clonal groups, CG15 and CG258. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2013;19(4):349–55. [DOI] [PubMed] [Google Scholar]
- 30.Woodford N, Turton JF, Livermore DM. Multiresistant Gram-negative bacteria: the role of high-risk clones in the dissemination of antibiotic resistance. FEMS microbiology reviews. 2011;35(5):736–55. doi: 10.1111/j.1574-6976.2011.00268.x [DOI] [PubMed] [Google Scholar]
- 31.Maatallah M, Vading M, Kabir MH, Bakhrouf A, Kalin M, Naucler P, et al. Klebsiella variicola is a frequent cause of bloodstream infection in the stockholm area, and associated with higher mortality compared to K. pneumoniae. PLoS One. 2014;9(11):e113539 Epub 2014/11/27. doi: 10.1371/journal.pone.0113539 ; PubMed Central PMCID: PMCPMC4245126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIFF)
(PDF)
(PDF)
(PDF)
No of isolates with non-susceptibility (intermediate and resistant) to antimicrobials.
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.