ABSTRACT
Myeloid-derived suppressor cells (MDSCs) are a heterogeneous group of myeloid cells that suppress T-cell activity in a tumor microenvironment. However, the suppressive function of MDSCs on B cells and its underlying mechanism remain unclear. Here, we show that in 4T1 breast cancer mice, a significantly increased number of MDSCs, in parallel with splenic B cells, are accumulated when compared to normal mice. In the presence of MDSCs, the surface molecules of B cells are remolded, with checkpoint-related molecules such as PD-1 and PD-L1 changing prominently. MDSCs also emerge as vital regulators in B-cell immune functions such as proliferation, apoptosis and the abilities to secrete antibodies and cytokines. Our study further identifies that MDSCs can transform normal B cells to a subtype of immuno- regulatory B cells (Bregs) which inhibit T-cell response. Furthermore, we identified a novel kind of Bregs with a specific phenotype PD-1−PD-L1+CD19+, which exert the greatest suppressive effects on T cells in comparison with the previously reported Bregs characterized as CD1d+CD5+CD19+, CD5+CD19+ and Interleukin (IL)-10-secreting B cells. Our results highlight that MDSCs regulate B-cell response and may serve as a therapeutic approach in anti-tumor treatment. Investigation of this new Breg subtype extends our understanding of regulation of T-cell response and sheds new light on anti-tumor immunity and immune therapy.
KEYWORDS: breast cancer, PD-1/PD-L1 axis, MDSC, Regulatory B cells, tumor immunity
Introduction
MDSCs are major regulators of immune response that dampen T-cell function in several types of cancers, including mammary, melanoma, lung and colon cancers.1 In mice, MDSCs are historically defined as Gr-1+CD11b+. As the Gr-1-positive population is composed of both monocytic and granulocytic cells, MDSCs are divided into two subsets based on the expression of Ly6C and Ly6G molecules: M-MDSCs (CD11b+Ly6C+Ly6G−) and G-MDSCs (CD11b+Ly6C−Ly6G+). MDSCs have been known for the ability to suppress T-cell response through the production of nitric oxide (NO), arginase-1 (Arg-1), and reactive oxygen species (ROS) , as well as through other untypical mechanisms.2 Recent researches have shown that MDSCs are associated with tumor progression, poorer clinical outcome, and decreased efficacy in immuno-therapeutic strategies. However, little is known about the effect of MDSCs on B-cell response, especially within the tumor micro-environment.
The B-cell repertoire performs potent roles in either promoting or inhibiting tumor development, which underscores the potential significance of B cells as a therapeutic target. On one hand, B cells can inhibit tumor development through the secretion of antibodies and cytokines, antigen presentation, and interacting with tumor tissue. On the other hand, B cells also act as inhibitory immune regulators by serving as Bregs,3 which interact with tumor tissues or certain types of immunocytes. Specific surface markers of Bregs haven't been clearly identified in mice or human. In mice, Bregs have been described as CD1dhiCD5+CD19+, or an IL-10-secreting B cell subtype.4 With no clear definition of Breg specific biomarkers, there is a great need for better understanding of the immuno-suppressive molecules in Bregs.
In this study, we explored the potent effects of MDSCs on B-cell response, shedding lights on the complex roles of MDSCs and B cells in tumor development. The goal of this study was to characterize the function of a novel subtype of immuno-suppressive B cells defined as PD-1−PD-L1+CD19+, which exerted the greater inhibitory effect on T-cell related immune response. Most importantly, an increasing proportion of PD-1−PD-L1+CD19+ B cells was observed in the peripheral blood of breast cancer patients, underscoring the clinical application of this Breg subset in breast cancer prognosis. Therefore, these findings demonstrated an important role of these newly identified immuno-regulatory B cells in tumor immunity.
2. Results
2.1. The concurrent increase of MDSCs and B cells in 4T1-bearing mice
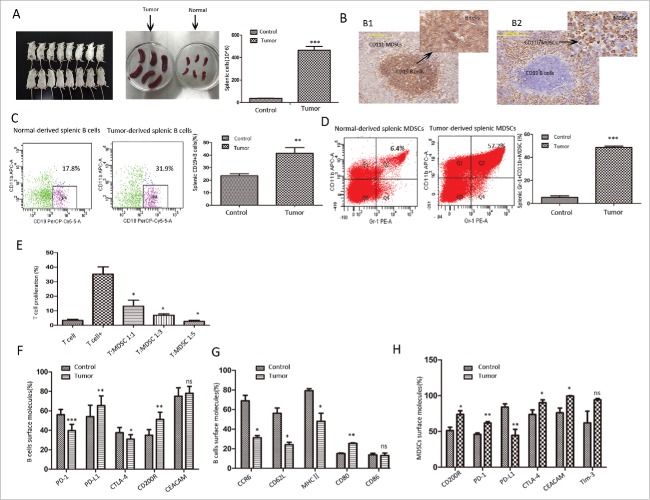
BALB/C mice were inoculated with 4T1 breast cancer cells subcutaneously, and were sacrificed at day 21. We first showed that the overall number of splenic cells in tumor-bearing mice increased by 12.5-fold (Fig. 1A). To gain insight into the anatomical localization of MDSCs and B cells, we evaluated the distribution of CD11b+ and CD19+ cells in the spleens of tumor-bearing mice using immunohistochemistry analysis. As shown in Fig. 1B, CD11b+ increased heavily and co-localized with marginal zone CD19+ B cells. The co-localization makes it possible for the two types of immunocytes interact with each other through cell-to-cell contact. Specifically, the percentage of B cells and MDSCs both presented a great increase (Fig. 1C and Fig. 1D). Tumor-derived MDSCs showed the capacity to inhibit T-cell proliferation in a dose-dependent manner (Fig. 1E), demonstrating the classic immuno-suppressive effect of MDSCs within the tumor micro-environment.
Figure 1.
The concurrent increases of MDSCs and B cells in breast cancer mice and surface molecules of splenic B cells and MDSCs in tumor or normal mice. Wide-type BALB/c mice were injected subcutaneously with 1 × 106 4T1 murine breast cancer cells, and were sacrificed at day 21 after tumor inoculation. (A) The brief descriptions of tumor-bearing status were shown in the left and middle panel. The overall splenic cells were analyzed from tumor-bearing and tumor-free mice (right panel). (B) Paraffin-embedded spleen tissues were collected from tumor-bearing mice to detect CD19+B cells (left panel) and CD11b+ MDSCs (right panel) by IHC staining (original magnification × 200 and × 400). (C) The percentage of B cells was detected using anti-CD19 Ab from tumor and normal splenic cells. (D) Splenic MDSCs were stained for CD11b and Gr-1, and detected by FC (right panel). The percentages are indicated in the representative dot plots (left two panels). (E) Isolated splenic MDSCs were assessed for their ability to suppress CD3+ T-cell proliferation at a 1:1, 3:1 or 5:1 of Gr-1+CD11b+cell: T-cell ratio. (F) FC analysis of immune checkpoint molecules including PD-1, PD-L1, CTLA-4, Tim-3, CD200R and CEACAM showing their expression frequencies in B cells. (G) Expression of the co-stimulatory molecules and activation markers including CD80, CD86, MHC II, CCR6 and CD62L on the surface of splenic CD19+ B cells from 4T1 tumor-bearing and control mice. (H) Immune checkpoint molecules on MDSCs were measured by FC from normal and tumor-bearing mice. The results are shown as the mean±SEM from five independent experiments with 8 mice per group per experiment. The t test or One-way ANOVA analysis were used for statistical analysis.* = P < 0.05, ** = P < 0.01, *** = P < 0.001, ns = not significant. FC, flow cytometry; MDSC, Myeloid-derived suppressor cells; IHC, immunohistochemistry.
2.2. The surface molecules of splenic B cells and MDSCs in tumor-bearing and normal mice
Immune checkpoint factors involved in undergo profound modifications in the tumor environment. Beyond their potent inhibitory effects on T cells, checkpoint factors also play a role in regulating B cell response.5,6 We analyzed checkpoint molecules PD-1, PD-L1, CTLA-4, CD200R, and CEACAM expressed on normal and tumor splenic B cells. As shown in Fig. 1F, a significant reduction in the expression levels of PD-1 and CTLA-4 was found in the tumor-bearing group. In contrast, we observed a significant increase in the surface expression levels of PD-L1 and CD200R, with no significant difference in CEACAM expression between the two groups.
Next, we assessed the expression of co-stimulatory molecules and activation markers on B cells, and found significant upregulation of the co-stimulatory molecule CD80. Surprisingly, CD86 showed no significant increase, while the levels of CCR6, CD62L and MHC II decreased in B cells from tumor-bearing mice (Fig. 1G).
Finally, to assess the immunological characteristics of MDSCs within the tumor environment, we tested the expression of checkpoint molecules in MDSCs from both control and tumor-bearing mice. The expression of CD200R, PD-1, CTLA-4 and CEACAM was significantly up-regulated in tumor-bearing mice, while the PD-L1 molecule displayed an opposite change (Fig. 1H). The Tim-3 molecules showed no significant changes. These data functionally validated that MDSCs might be influenced by tumor-related factors, undergoing specific modification of surface expression, and might utilize an unconventional mechanism to influence B-cell function.
2.3. Tumor-derived MDSCs regulate normal B cell function through a contact-dependent mechanism
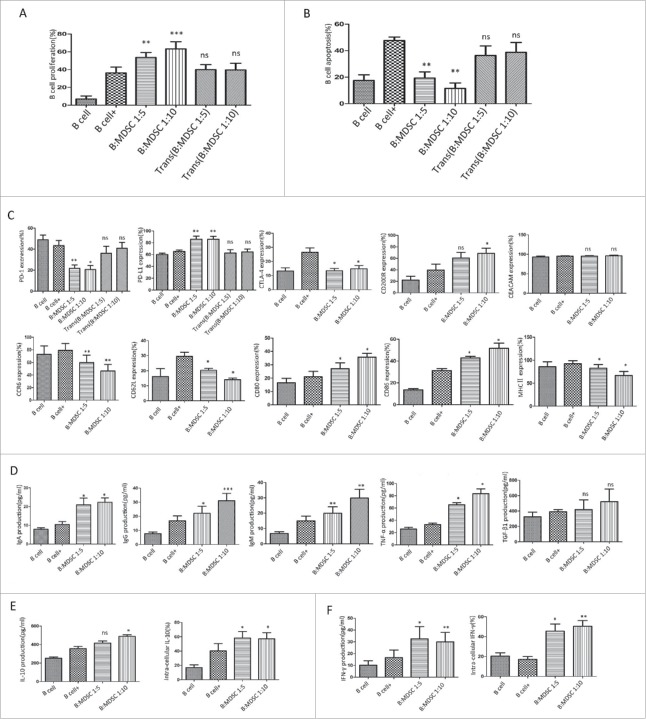
We systematically assessed the potential of MDSCs to affect B-cell responses. In our present work, MDSCs promoted the proliferation (Fig. 2A) and suppressed the apoptosis (Fig. 2B) of B cells, both in a dose-dependent manner with most pronounced results observed with the 1:5 and 1:10 ratios of co-culturing. We further analyzed whether MDSC-mediated B-cell regulations required cell-to-cell contact by using a Transwell co-culture system. We confirmed that the educational effect required a contact-dependent mechanism, since Transwell-separated B cells were not affected by MDSCs in terms of proliferation and apoptosis (P>0.05). The cultured supernatant derived from tumor-MDSCs was collected and co-cultured with normal B cells. As shown in Supplement Figure 1A-B, MDSC-conditioned medium showed no influence on B-cell proliferation and apoptosis, which further support the necessity of cell-to-cell contact in B-cell response.
Figure 2.
MDSCs regulate B-cell function through a contact-dependent mechanism. Purified splenic B cells were cultured alone or with MDSCs in the presence or absence of IL-4 (200 ng/ml) plus anti-CD40 antibody (10μg/ml) (A) The proliferation of CD19+ B cells was assessed by FC using BrdU labeling method. (B) CD19+B cell apoptosis was detected by FC using an Apoptosis Detection Kit. (C) The expression of immune checkpoint molecules (upper panel) and co-stimulatory or activation markers (bottom panel) on the surface of B cells were analyzed by FC. (D) The antibody subtypes (IgA, IgG, and IgM) and cytokine (TNF-α, TGFβ1) concentrations were determined from the culture supernatants after 48 h with specific ELISA Kit. ELASA data were collected from 5 independent experiments. (E) IL-10 and (F) IFN-γ production in the co-cultured groups were both detected by ELISA (left panels) and FC analysis (right panels). Data represent the mean±SEM of 5 independent experiments. * = P < 0.05, ** = P < 0.01, *** = P < 0.001, ns = not significant, as determined with One-way ANOVA analysis. FC, flow cytometry; BrdU, bromodeoxyuridine; ELISA, enzyme-linked immunosorbent assay; Ig, immunoglobulin; MDSCs, Myeloid-derived suppressor cells; IL, interleukin; IFN, interferon.
To further investigate any possible effects of MDSCs on B cells in vitro, we prospectively analyzed the modulation of immune checkpoint molecules and other surface factors on B cells as previously described. Under the treatment with MDSCs, significantly reduced levels of PD-1 and CTLA-4 were found in MDSC-co-cultured group. In contrast, we observed significantly increased levels of PD-L1 and CD200R, which showed similar changes in splenic B cells from tumor-bearing mice. MDSCs had no significant effect on CEACAM expression (Fig. 2C, upper panels). As for co-stimulatory molecules and activation markers on B cells, we found that CD80 and CD86 molecules were upregulated in MDSCs treatment, serving as a hallmark of B-cell activation. However, MHC II expression showed significant decrease, which coincided with modulation of CCR6 and CD62L (Fig. 2C, bottom panels). These changes suggest the possibility that the beneficial anti-tumor response of B cells was dampened by MDSCs.
Finally, we assessed whether MDSCs affect B cells' abilities to produce antibodies and cytokines. We tested immunoglobulin (Ig) A, IgG and IgM production in co-culture system by ELISA assay. As showed in Fig. 2D, stimulated B cells released increasing levels of IgA (10.49 pg/ml vs 20.91 pg/ml, P = 0.017 for 1:5 system, and 10.49 pg/ml vs 22.29 pg/ml, P = 0.013 for 1:10 system), IgG (16.79 pg/ml vs 22.19 pg/ml, P = 0.016 for 1:5 system, and 16.79 pg/ml vs 31.08 pg/ml, P = 0.0003 for 1:10 system), and IgM (14.92 pg/ml vs 19.96 pg/ml, P = 0.0076 for 1:5 system, and 14.92 pg/ml vs 29.83 pg/ml, P = 0.0021 for 1:10 system) in the presence of MDSCs. As for the cytokines, IL-10 (Fig. 2E, left panel), IFN-γ (Fig. 2F, left panel), and TNF-α (Fig. 2D) were upregulated in the MDSC-co-cultured groups, while no significant change was seen in TGF-β1 secretion (Fig. 2D). The production of IL-10 and IFN-γ by B cells was further tested by flow cytometry (FC) (Fig. 2E–F, right panels), with a higher percentage of IL-10+ (40.20% vs 58.18%, P = 0.04 for 1:5 group and 40.20% vs 57.25%, P = 0.02 for 1:10 group) and IFN-γ+ cells (17.10% vs 45.43%, P = 0.025 for 1:5 group and 17.10% vs 50.43%, P = 0.0095 for 1:10 group) detected in the CD19+ group in the presence of MDSCs.
2.4. The presence of MDSCs endowed B cells with suppressive functions
MDSCs are known to suppress T-cell response by inhibiting T-cell proliferation and cytotoxic activity, and by promoting Treg expansion to dampen the host immune responses against tumor.7 Based on the data above, we speculated that MDSCs may educate normal B cells into a unique subtype with immuno-suppressive properties on T-cell response. As described above, MDSCs were co-cultured with B cells for 24 or 48 hours, respectively. After inoculation, B cells were selected by FACS-sorting, and co-cultured with normal splenic T cells for 48 h with corresponding stimulus. We observed that after educated by MDSCs for 24 h or 48 h, isolated B cells were able to inhibit T-cell proliferation (Fig. 3A), promote the ability of IL-10 production (Fig. 3C, upper panel), and decrease the release of IFN-γ (Fig. 3C, bottom panel). However, B cells show no significant effect on T-cell apoptosis (Fig. 3B) or the induction of Tregs (CD4+CD25+CD127low) (Fig. 3D). In all comparative groups, T-cell response was not affect by B cells isolated from Transwell-incubated with MDSCs.
Figure 3.
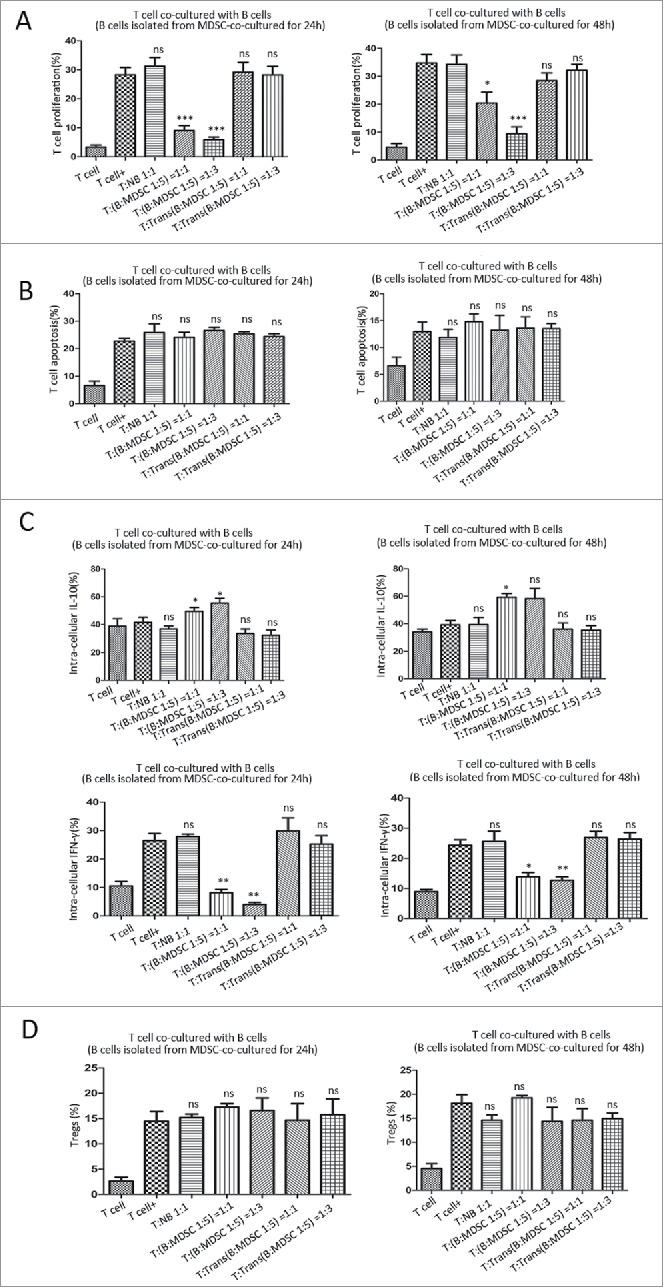
MDSCs educate B cells into regulatory B cells with immune suppressive effects on T-cell response. After co-cultured with MDSCs for 24 h or 48 h, B cells were isolated by FACS, and then co-incubated with normal splenic T cells with anti-CD3/CD28 dynabeads for 2 days. T cells alone with or without stimuli were used as control groups. (A) The proliferation of CD3+ T cells was assessed by FC using BrdU labeling method. (B) CD3+ T cell apoptosis was detected using an Apoptosis Detection Kit. (C) Cytokine concentrations were determined by FC to assess the T-cell intra-cellular secretion. T cells cultured for 2 days with or without B cells, were fixed, permeabilized and stained with PE-anti-IL-10 or FITC-anti-IFN-γ antibodies. (D) The percentage of Tregs was evaluated by FC analysis. Data represent the mean ± SEM of 5 independent experiments. * = P < 0.05, ** = P < 0.01, *** = P < 0.001, ns = not significant, as determined with One-way ANOVA analysis. BrdU, bromodeoxyuridine; MDSCs, myeloid-derived suppressor cells; Tregs, regulatory T cells; FC, flow cytometry; IL, interleukin; IFN, interferon
2.5. A unique PD-1−PD-L1+ CD19+ B cell subtype exert immuno-regulatory properties
2.5.1. PD-1−PD-L1+CD19+B cells were enriched in the spleen of 4T1-tumor-bearing mice, and in co-culture with MDSCs
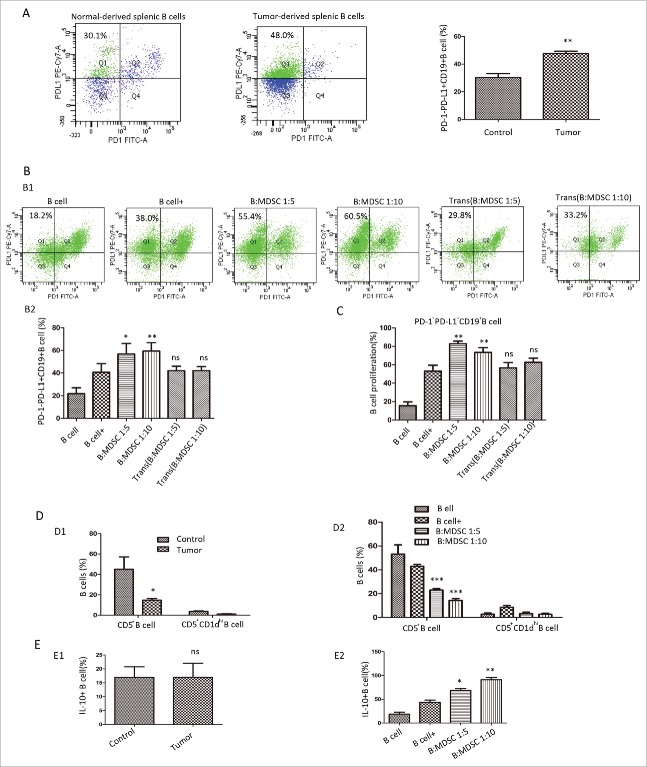
PD-1−PD-L1+B cells were expended in the spleen of tumor-bearing mice (30.33 ± 2.85% vs. 47.73 ± 1.64%, P = 0.019) when compared to normal mice (Fig. 4A). Likewise, in the co-cultured system, this B-cell subset showed significant increase (40.60% vs.56.78%, P = 0.0152 for B: MDSCs = 1:5 group, 40.60% vs.59.43%, P = 0.0023 for B: MDSCs = 1:10 group) in the presence of MDSCs (Fig. 4B). The B subset also exhibited greatest levels of induced proliferation (Fig. 4C). However, the Transwell-culture system (Fig. 4B–C) and MDSC-medicated medium (Supplement Figure 1C) couldn't recapitulate these effects.
Figure 4.
A typical PD-1−PD-L1+CD19+ B-cell subtype exert immuno-regulatory properties. (A) The left two panels show a representative dot plots of PD-1−PD-L1+ B cells gated on CD19+ cells from normal or tumor-bearing spleen. The right panel depicted values from 8 independent experiments. (B) Representative FC analysis showing the percentage of PD-1−PD-L1+CD19+ B cells at 48 h in the presence or absence of MDSCs in the culture system (B1). B2 shows summarized data from 5 independent experiments. (C) The percentage of PD-1−PD-L1+CD19+ B cells in BrdU-labeled B cells at 48 h with or without MDSCs from 8 independent experiments. (D) The percentage of CD5+ and CD5+CD1dhi B cells in tumor-bearing or tumor-free mice (D1) and (D2) in the MDSC-co-cultured system was evaluated by FC. (E) The intra-cellular IL-10 level of B cells was analyzed by FC in 4T1 or normal mice (E1) and (E2) in the MDSC-co-cultured group. * = P < 0.05, ** = P < 0.01, *** = P < 0.001, ns = not significant as determined with the t test. MDSC, myeloid-derived suppressor cells; FC, flow cytometry; IL, interleukin.
In mouse tumor models, no well-known surface markers of Bregs were clearly defined. It was previously demonstrated that CD5+CD1dhiCD19+ B cells,8 a well-characterized IL-10-producing Breg subset, owned the immuno-regulatory effect. Moreover, IL-10 production was regarded as a hallmark of B-cell immuno-suppressive effect. In autoimmune diseases, CD5+ B cells displayed some unique properties such as self-renewing and the potential to promote malignant transformation.9 Therefore, we compared PD-1−PD-L1+ B cells with CD5+ B cells, CD5+CD1dhi B cells and IL-10+ B cells for the aspects tested above. As shown in Fig. 4D, for CD5+B cells, the tumor-bearing group showed a lower percentage in splenic B cells. The portion of CD5+ B cells also decreased in the MDSC-co-cultured group. We further noticed that the percentage of CD5+CD1dhi B cells only represented a small portion of CD19+B cells, which experienced no significant difference in both systems respectively. No pronounced effect was observed in the percentage of IL-10-secreting B cells from tumor-bearing mice (Fig. 4E). However, the B-cell subset showed a significant increase in the presence of MDSCs. Our finding was in line with the studies10, 11 which indicated Bregs activity did not necessarily involve IL-10. Collectively, the specific B-cell subset PD-1−PD-L1+CD19+ B cells predominately increased in MDSC-co-cultured group. Since MDSC educated normal B cells into immuno-suppressive cells, we hypothesized that PD-1−PD-L1+CD19+ B cells might exert immuno-regulatory properties on T cells.
2.5.2. Splenic PD-1−PD-L1+CD19+ Bregs exert immuno-regulatory properties
To demonstrate the regulatory properties of the specific B-cell subset, a co-culture system was set up as previously described. Briefly, stimulated CD19+, CD19+PD-1−, CD19+PD-L1+, CD19+PD-1−PD-L1+, CD19+PD-1+PD-L1+, CD19+PD-1−PD-L1−, CD19+PD-1+PD-L1− and CD19+CD5+ B cells from tumor-bearing mice were incubated with autologous splenic T cells upon CD3/CD28 stimulus for 48h. As shown in Fig. 5A, when B cells from tumor-bearing mice were added to the system, the proliferation of T cells decreased at the ratio of 1:1. The PD-1−PD-L1+ B cell subset exerted the greatest inhibitory significance on T-cell proliferation. In addition, this specific B-cell subset was able to promote the apoptosis of T cells (Fig. 5B). T-cell abilities in secreting cytokines IL-10, IFN-γ, IL-2 and TGF-β1 were measured by FC. In a cell-to-cell co-culture system, a higher percentage of IL-10 secretion and lower IFN-γ were seen when T cells were co-cultured with PD-1−PD-L1+ B-cell subset (41.57% vs 66.73%, P = 0.0045 for IL-10; 24.33% vs 5.1%, P = 0.0014 for IFN-γ). However, these T cells harbored no significant levels of IL-2 and TGF-β1 (Fig. 5D). No significant difference was found between the groups in the induction of Tregs (Fig. 5C).
Figure 5.
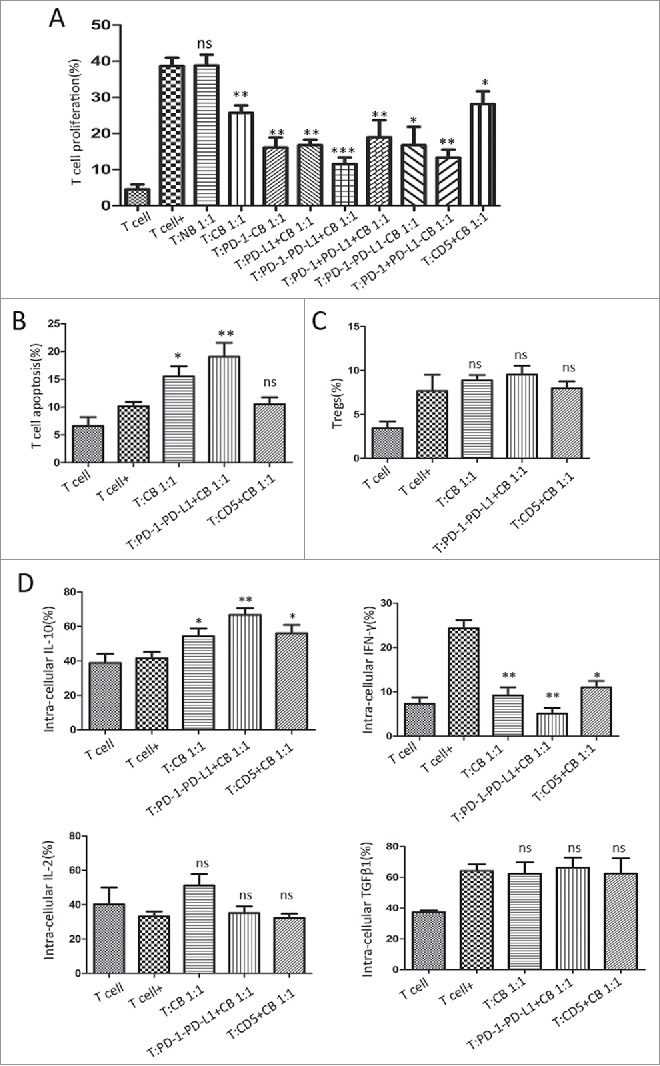
PD-1−PD-L1+CD19+ B cells display immuno-regulatory properties on activated T cells. PD-1−PD-L1+CD19+ B cells were isolated from the splenic cells of tumor-bearing mice. T cells were cultured for 2 days with or without B cells for the subsequent analyses. (A) Regulation of CD3+ T-cell proliferation by different B cell subsets is shown in relative to T cells cultured in the absence of B cells from 8 independent experiments. (B) The apoptosis of CD3+ T cell was detected by FC at 48 h following incubation with splenic CD19+ cells, PD-1−PD-L1+CD19+cells or CD5+B cells from tumor-bearing mice. (C) The percentage of Tregs (CD4+CD25+CD125low) with or without B cell incubation was evaluated by FC analysis. (D) Cytokine concentrations (IL-10, IFN-γ, TGFβ1, and IL-2) were determined by FC to analyze T-cell intra-cellular production. Data were collected from 5 independent experiments. Data represent the mean ± SEM of 5 independent experiments. * = P < 0.05; ** = P < 0.01; *** = P < 0.001, ns = not significant as determined with t test or One-way ANOVA. BrdU, bromodeoxyuridine; MDSCs, myeloid-derived suppressor cells; Tregs, regulatory T cells; FC, flow cytometry; TNF, tumor necrosis factor; TGF, transforming growth factor; IL, interleukin; IFN, interferon.
Subsequently, we investigated the suppressive efficacy of PD-1−PD-L1+B cells in comparison with CD5+ B cells. As shown in Fig. 5, We found PD-1−PD-L1+ B cells exerted the greater suppressive functions on T-cell proliferation (38.66% vs 11.48%, P<0.0001 for PD-1−PD-L1+B cells system; and 38.66% vs 28.1%, P = 0.03 for CD5+B cells system). No significant effect of CD5+B cells was seen (P = 0.57) with regard to T-cell apoptosis. As for IL-10 and IFN-γ production, CD5+B cells showed similar trends but with less significance compared to PD-1−PD-L1+ B cells. PD-1−PD-L1+ and CD5+B cells both exert no significant impacts on T-cell secreting IL-2 and TGF-β1. These results collectively indicated that PD-1−PD-L1+CD19+ Bregs suppress T-cell functions in the co-cultured system, which exert predominate efficacy when compared to other Breg subsets.
2.6. The distribution of PD-1−PD-L1+ Bregs in BM, peripheral blood, spleen and LN
As shown above, PD-1−PD-L1+ Bregs exerted potent immuno-regulation within the tumor environment, which might serve as a target in immunotherapy. We next determined the distribution of PD-1−PD-L1+ Bregs in BM, peripheral blood, spleen and tumor-draining LNs (TDLNs) in mice. As shown in Fig. 6A, PD-1−PD-L1+ Bregs were upregulated in BM (15.98 ± 1.51% vs. 23.30 ± 0.57%, P = 0.03), spleen (30.33 ± 2.85% vs. 47.73 ± 1.64%, P = 0.019) and peripheral blood (36.57 ± 1.63% vs. 47.73 ± 1.64%, P = 0.0009) in tumor-bearing mice. In the LNs, this B-cell subset showed significant expansion in TDLNs in comparison to normal LNs (38.98 ± 2.46% vs. 30.00 ± 2.03%, P = 0.03), whereas reduced levels in the primary tumor tissue were observed (38.98 ± 2.46% vs 12.43 ± 1.90, P = 0.0005). Since LNs play a central role in the development of adaptive immunity and particularly the generation of antigen-specific B cell responses, changes in the percentage of PD-1−PD-L1+ B cells might be involved in the process of tumor metastasis. Most importantly, the percentage of MDSCs presented a similar increase in TDLNs as well as tumor bed (Fig. 6B). The similar modulation of PD-1−PD-L1+ B cells and MDSCs may suggest potential mechanisms on the inter-connections, which needed further investigations.
Figure 6.
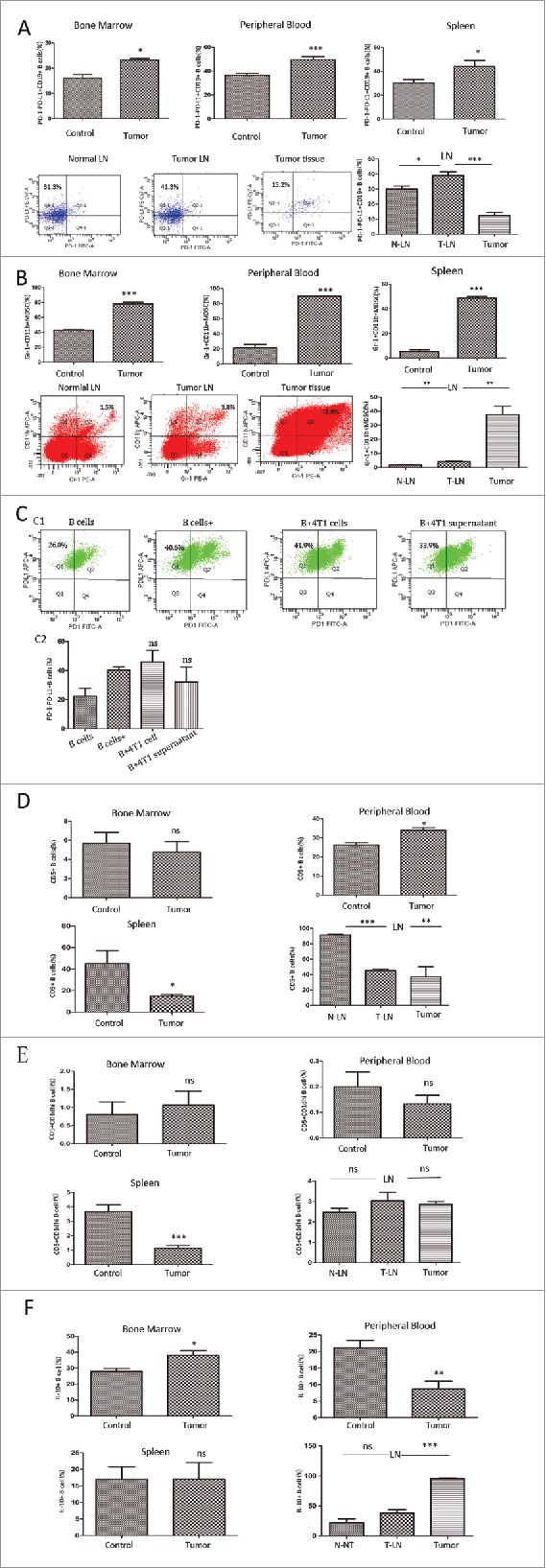
The distribution of different B cell subsets in BM, peripheral blood, spleen and TDLNs. (A) PD-1−PD-L1+CD19+ B cells was expanded in the BM, peripheral blood, spleen, LNs and primary tumor tissue in tumor-bearing or normal mice . Representative dot plots of FC in LNs were shown. (B) The percentage of Gr-1+CD11b+MDSCs and representative dot plots in tumor-bearing or tumor-free mice from 5 independent experiments with triplicate samples was shown. (C) Normal splenic B cells were cultured with 4T1 cells or 4T1-supernatant, and the percentage of PD-1−PD-L1+CD19+ B cells was detected by FC. (D) The percentage of CD5+CD19+ B cells (E) CD5+CD1dhi CD19+B cells and (F) intra-cellular staining was with PE-anti-IL-10 antibody to detect IL-10+ B cells determined by FC. Data show mean±SEM of 7 independent experiments. * = P < 0.05, ** = P < 0.01, *** = P < 0.001, ns = not significant as determined by t test. BM, bone marrow; LN, lymph node; TDLN, tumor-draining lymph node; MDSC, Myeloid-derived suppressor cells.
In order to analysis the influence of tumor cells or tumor-culture supernatant exerted on normal B cells, we built co-cultured systems containing B cells with 4T1 cells or tumor supernatant, respectively. As shown in Fig. 6C, the percentage of PD-1−PD-L1+ B cells showed no significant difference in the groups (P = 0.56 for 4T1 cells group, and P = 0.35 for supernatant group).
Next, we evaluated the distributions of other Breg subsets. As seen in Fig. 6D, the percentage of CD5+ immuno-suppressive B cells decreased in TDLNs and spleen but, increased in peripheral blood, with no significant changes in the BM of tumor-bearing mice. For CD5+CD1dhi B cells, only trend of decrease was seen in tumor-bearing mice, while no significant difference was found in other comparative groups (Fig. 6E). Previous studies identified IL-10 as a key mediator of Bregs function in cancer.12 We further analyzed the intra-cellular IL-10 of B cells by FC (Fig. 6F), and found no changes in the frequency of IL-10-producing B cells in the TDLN compared to normal LNs. The IL-10+B cells elevated in the BM of tumor-bearing mice, showed no significant changes in splenic B cells, but increased in the peripheral blood, which deserves further investigation. In comparison, the accumulation of specific PD-1−PD-L1+ B cells might act as a more vital biomarker for cancer prognosis.
2.7. PD-1−PD-L1+CD19+ Bregs were expanded in breast cancer patients
Subsequently, we investigated the role of PD-1−PD-L1+ B cells in breast cancer patients and healthy controls. Elevated numbers of CD19+B cells and CD45+CD33+CD13+CD14−CD15− MDSCs were both seen in the peripheral blood of breast tumor patients (Fig. 7A, 7B). Most importantly, as shown in Fig. 7C, the percentage of PD-1−PD-L1+CD19+ cells (18.13% ± 1.15%, n = 35, P<0.0001) in the peripheral blood of breast tumor patients was also significantly up-regulated compared to healthy participants (8.50% ± 0.47%, n = 20). Since most patients suffer from Stage I (24/35) tumors, we did not find any correlations of the Breg subset with histopathological characteristics and tumor stages (Fig. 7E).
Figure 7.
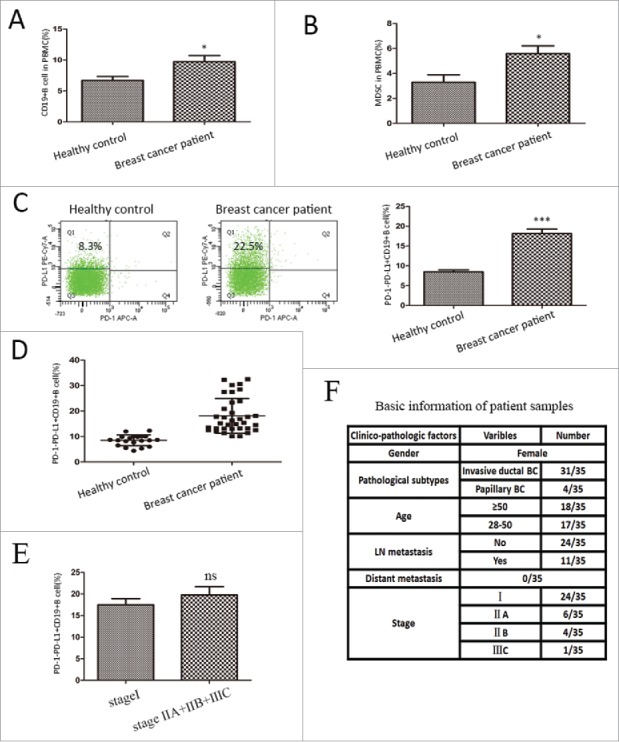
PD-1−PD-L1+CD19+ Bregs were expanded in the peripheral blood of breast cancer patients. The percentages of indicated cell types were determined in 35 breast cancer patients and 20 healthy female controls. The results are shown as the mean±SEM. of the percentage of (A) CD19+ B cells, and (B) CD45+CD33+CD13+CD14−CD15− MDSCs in the periphery blood. (C) PD-1−PD-L1+ Bregs were expanded in the peripheral blood of breast cancer patients when compared with healthy controls. Representative dot plots were shown in the left panel. (D) A scatter plot for the percentage of PD-1−PD-L1+CD19+ B cells in two groups. (E) The correlation of the PD-1−PD-L1+ CD19+ cells with tumor stages. (F) The histopathological characteristics and other basic information of the patients involved. The analysis was determined by unpaired t test. * = P <0.05, *** = P < 0.001, ns = not significant. Bregs, regulatory B cells; MDSC, Myeloid-derived suppressor cells.
3. Discussion
MDSCs are well-recognized to suppress T-cell response in various pathological conditions including cancer,13 inflammation14 and autoimmune diseases.15 However, little research focused on the potential influence of MDSCs on B cells, especially within the tumor microenvironment. Our current study has opened a new avenue to study MDSC-mediated regulations of B cells and provided new insights into the possible manipulation by B cell-mediated suppression of T-cell response. Here, we have shown that a unique Breg subset termed PD-1−PD-L1+CD19+ cells exerted great inhibitory effect on T-cell immunity. Most importantly, this B cell subtype is dramatically up-regulated in the peripheral blood of breast cancer patients. It underscored the potential of this B cell subset to serve as a clinical biomarker for the pathological characteristics of tumors.
In tumor-bearing mice, MDSCs increased heavily and co-located with marginal zone B cells, which was in line with the study by Xu et al.13 It provided the possibility of cell-to-cell contact between the immunocytes. Unlike their effects on T cells, MDSCs promote proliferation and inhibit apoptosis of B cells and this requires direct cell-to-cell contacts. However, the underlying mechanisms of MDSCs' function remained unclear. A recent study suggested that in mice with fibrosarcoma, MDSCs could boost the proliferation and differentiation of B cells into IgA-producing plasma cells in a TNFR2-dependent manner.13 MDSCs were required for in vivo priming and expansion of antigen-specific B cells,16 consistent with our findings here. Current studies17-20 focused on the regulation of MDSCs in other pathological conditions beyond tumor. The complexity of tumor and other pathological conditions may play a critical role to be addressed in future studies.
Our investigations further suggested decreased percentage of immune checkpoint molecules PD-1, together with increased PD-L1 expression were observed in the presence of MDSCs. Previous studies have identified PD-L1 as a critical mediator of Bregs. Guan and colleagues6 reported that PD-L1 contributed to the immunosuppressive role of CD19+CD24+CD38+ Bregs in invasive breast cancer patients. PD-L1 expression on B cells can result in the inhibition of T-cell proliferation21 and T-cell-dependent immunogenic chemotherapy.22 By contrast, PD-1hi represents a pro-tumorigenic B-cell subset in hepatocellular carcinoma,23 therefore PD-1/PD-L1 axis24, 25 may be predominantly involved in B-cell suppressive mechanism. Here, the modulation of PD-1/PD-L1 expression might contribute to the B-cell suppressive properties, which was confirmed by our data showing the consequent regulation of. Our results also confirmed B-cell activation was constrained in MDSC groups, for the decreasing expressions of CCR6, CD62L and MHCII. CCR6 is restricted to functionally mature B cells capable of responding to antigen challenge26 and promoting migration events.27 As for CD62L, it acts as a homing receptor on B cells and is induced on activated B cells.28 MHC II is expressed on professional APCs including B cells and supports CD4+ T-cell activation by displaying antigens.29 These modifications were in line with the study by Olkhanud et al., which identified a designated Bregs with surface markers PD-L1hiCD86hi CD80hi CD62Llow to facilitate lung metastasis.30 Xu et al. 13 concluded similar trends in terms of CD80 and CD86 expression. The evidence may suggest the positive anti-tumor response of B cells is dampened with the presence of MDSCs.
Results from our study showed that MDSC promoted B-cell capabilities of secreting antibodies, suggesting a potential stimulatory effect of B cells differentiating into plasma cells. The results coincided with the report that an IgA+ B-cell subpopulation was immunosuppressive via multiple tumor-promotion mechanisms,31 one of which is to up-regulate PD-L1 expression thus inducing T-cell exhaustion. As for cytokines production, the elevated secretion of IL-1032, IFN-γ 33 and TNF-α34 serves as immunosuppressive effect and gives rise to progressive tumors. Interestingly, Dai et al. confirmed a positive correlation of PD-L1 with IFN-γ in gastric cancer tissues,35 consistent with the evidence that inductions of PD-L1, IDO, and Tregs are driven by IFN-γ and CD8+ T cells in melanoma model.36
All the aforementioned studies reveal that MDSC dampen the positive effect of B cells in vitro, which educate normal B cells into a subset of Bregs that inhibit T-cell response. Our studies provide the first evidence that a specific subset of PD-1−PD-L1+ B cells exert the greatest suppressive effect on T-cell response when compared with specific inhibitory Breg subsets. Evidence for Bregs' inhibitory effect on T cells has been well established.22,37 However, no exact surface markers of Bregs have been recognized. In previous studies, CD5+CD1dhi B cells8, 9 were the well-characterized Breg cells. We therefore compared the suppressive capacity between PD-1−PD-L1+ and CD5+CD1dhi B cells. In fact, CD5+CD1dhi B cell subset accounts for a small portion of B cells (less than 5%) in mice spleen (Fig. 4D). Additionally, in MDSC-co-cultured group, CD5+CD1dhi B-cell group did not change significantly. In contrast, CD5+ B cells suffer a great decrease in tumor-bearing spleen and in MDSC-co-cultured system. Therefore, we compared the immune response of PD-1−PD-L1+ B cells with CD5+ B cells. As shown in Fig. 5, PD-1−PD-L1+ B cell subset owned the higher suppressive capability on T-cell proliferation, promoted apoptosis, and affected T-cell cytokines production. We provided convincing evidence for a distinct subset of immuno-suppressive B cells, thus opening a new avenue to study B cell-mediated suppression and providing new insights into the possible manipulation through specific B-cell surface markers. The precise phenotype of Bregs needs to be further characterized in other types of cancers.
Next, we tested the percentage of PD-1−PD-L1+ Bregs in the BM, spleen, peripheral blood, TDLN and primary tumor site. In the study of Ruddell et al., TDLN demonstrated an increased B-cell accumulation, which accelerates melanoma metastasis.38 B-cell deficient μ MT mice failed to develop LN lymphangiogenesis39 and presented reduced tumor growth.40 The accumulation of PD-1−PD-L1+B cells and MDSCs in TDLN when compared to negative ones (Fig. 6A–B) provided the possibility that MDSCs might be recruited to TDLNs by tumor-related factors, and thereby, educate normal B cells into PD-1−PD-L1+ Bregs. The enhanced accumulation of this Bregs in TDLN might be crucial for tumor dissemination, for which the mechanisms remain largely unexplored. However, 4T1 tumor cells were previously reported to convert normal B cells into TGFβ-producing Bregs, therefore induced Tregs and facilitated lung metastasize.30 In our study, the percentage of PD-1−PD-L1+ Bregs showed no significant difference in co-culturing with 4T1 cells and tumor supernatant (Fig. 6C). These data suggest that the transformation of PD-1−PD-L1+ B cells may be not involved in 4T1-related molecules in a direct manner. However, our current data do not explore whether and how MDSCs influence the accumulation of PD-1−PD-L1+ Bregs in TDLNs. We are trying to evaluate whether there is similar phenomenon in breast cancer patients, and the exact regulatory process, which might shed light on enhanced therapeutic efficacy of strategies targeting MDSCs and Bregs.
We further observed that this newly identified PD-1−PD-L1+CD19+ cell subset was elevated in patients with pre-treated breast cancer (Fig. 7C–D), however, the correlations with tumor stage and early recurrence of patients cannot be analyzed due to the limited patient samples and confined clinical stages. Here, we demonstrated for the first time that elevated level of circulating PD-1− PD-L1+ B cell frequency in breast cancer patients, both for invasive ductal and papillary subtypes. These results corresponded well to our findings in murine models. Recently, immunotherapy has been demonstrated as a new and attractive approach, underscoring the emergence of immune checkpoint blockade with monoclonal antibodies as a successful treatment for cancer patients. The modulation of PD-1 and PD-L1 on B cells might identify promising targets for immunotherapy. Its predictive role in tumor stage prognosis and the connections with clinical-pathological molecules exerted promising prospect for anti-tumor treatment. Additionally, the dynamic changes in peripheral Bregs might be more important in cancer progression and help physicians adjust treatments,41 which need to be further elaborated. The surface biomarkers of Bregs in peripheral blood provided an opportunity for us to predict tumor generation, development and progression.
4. Materials and methods
Mice
Female BALB/C mice aged 6–8 weeks were purchased from Weitonglihua Corporation. All of the mice were maintained in a pathogen-free barrier facility at Tianjin Medical University Cancer Institute and Hospital. The animal experiments were carried out with the approval of the Institutional Laboratory Animal Care and Use Committee.
Cell lines
4T1 mammary cells are of BALB/C origin and were acquired from the American Type Culture Collection (ATCC). All of the cells were cultured in Roswell Park Memorial Institute (RPMI) -1640 medium that was supplemented with 10% fetal bovine serum (FBS) as described.42 Cells were suspended in phosphate-buffered saline (PBS) at the concentration of 5 × 106/ml and injected subcutaneously (1 × 106cells in 200μL medium/mouse) into recipient mice.
Cell isolation of murine splenic cells
Twenty-one days after inoculation, splenic lymphocytes were isolated using Red Blood Cell Lysis Buffer (Beyotime Biotechnology) following standard procedures. B cells or T cells were purified using the mouse B-cell or T-cell Isolation Kit (Miltenyi Biotec), respectively, and negatively selected according to manufacturer's instructions. The purity of B or T cells was always above 93%. MDSCs were purified by using Gr-1 micro-beads for positive selection (Miltenyi Biotec). The purity of MDSC cells was always above 95%. Purified B cells and MDSCs were cultured in RPMI 1640 medium containing 10 % FBS.
Specific phenotypes of B cell were sorted from tumor-bearing mice using the FACS-ARIA II sorter (BD Bioscience). Cell populations were defined as CD19+PD-1−; CD19+PD-L1+; CD19+PD-1−PD-L1+; CD19+PD-1+PD-L1+; CD19+PD-1−PD-L1− ; CD19+PD-1+PD-L1−; CD19+CD5+CD1d+ and CD19+CD5+. The purity of magnetic sort was above 97%. Purified cells were cultured in RPMI 1640 medium containing 10 % FBS.
In vitro culture of purified splenic cells (co-cultured experiments)
Co-cultured tumor-derived MDSCs with normal B cells Purified B cells (1 × 106 cells/mL) from naïve mice were co-cultured with tumor-bearing MDSCs in 6-well flat-bottom plates in the presence or absence of Recombinant Murine IL-4 (200 ng/ml, Pepotech) plus anti-mouse CD40 (10 μg/ml, Biolegend) at 37˚C in a 5% CO2 incubator, and the ratio of purified B cells to MDSCs was 1:5 and 1:10. Culture of B cells alone with or without stimulus was used as control groups. After 48 hours, harvested cells were examined using FC for proliferation and apoptosis analysis, and B-cell surface molecules detection. Culture supernatants were harvested to detect the levels of cytokines by ELISA assays.
Co-cultured T cells with different B cell subtypes FACS-sorted B-cell subsets (CD19+PD-1−; CD19+PD-L1+; CD19+PD-1− PD-L1+; CD19+PD-1−PD-L1+; CD19+PD-1+PD-L1+; CD19+PD-1−PD-L1− ; CD19+CD5+) from tumor-bearing mice, or B cells isolated from MDSC-cultured group after 24 or 48 hours respectively, thereafter, cultured with purified normal-derived splenic T-cells (5 × 105 cells/mL) at a ratio of 1:1 in the presence of anti-CD3/CD28 Dynabeads (15 μg/mL, Gibco), respectively. T cells with and without stimulus was used as control group. After 48 hours, cultured cells were harvested and analyzed by FC for T-cell related response.
Co-cultured B cells with 4T1 cells or 4T1-supernatant Purified B cells (1 × 106 cells/mL) from naïve mice were co-cultured with 4T1 cells (1 × 105 cells/mL) or 4T1 supernatant with B-cell stimuli. B cells alone with or without stimulus were used as control groups. After 48 hours, harvested cells were examined using FC.
Flow cytometry
Single-cell suspensions were prepared directly from spleens and post-cultured cells were stained with the following directly conjugated mouse specific monoclonal antibodies that were purchased from Biolegend, including PE-Gr-1, APC-CD11b, APC-CCR6, APC/Cy7-CD62L, FITC-PD-1, PE/Cy7-PD-L1, PE-MHC II, FITC-CD200R, APC-CTLA-4, PE/Cy7-Tim-3, PE-CECAM, APC-CD80, FITC-CD86 and PerCP-CD19 from BD Pharmingen. The appropriate isotype controls were used as negative controls (All from Biolegend). Cells were incubated with antibodies for 30 min at 4 ℃ in the dark. Then, the cells were washed twice with PBS. All samples were analyzed using a FACS in 3 replicates, and at least 20,000 events were acquired for each analysis.
For intra-cellular staining, cells were stimulated for 4 h with PMA-ionomycin-monensin [PMA 50 ng/mL, ionomycin 1 μL/mL (Sigma-Aldrich) and monensin (GolgiStop, 4 μL for 6 mL of culture)]. Cells were fixed with Fixation/Permeabilization solution (BD Pharmingen) and incubated with intra-cellular staining antibodies FITC-Tumor necrosis factor (TNF)-α, PE-IL-10, FITC-Interferon(IFN)-γ, FITC-IL-2, PE-Transforming growth factor (TGF)-β1 for 30 min. Labeled cells were re-suspended in 0.2 mL cell staining buffer, and analyzed with FC.
Immunohistochemical interpretations (IHC)
Formalin-fixed paraffin-embedded tissue samples were available for spleen tissues from tumor-bearing mice. The tissue sections were stained using anti-mouse antibody to CD19 (Abbiotec) for B cells and CD11b (Abcam) for MDSCs analysis. In brief, the sections were stained with an anti-CD19 (1:100) or anti-CD11b antibody (1:200) and incubated overnight at 4 ℃. Sites of binding were detected using the Envision technique with DAB (3-3′ diaminobenzidine), according to the manufacturer's instruction.
Cells proliferation and apoptosis assays
Cell proliferation was determined by bromodeoxyuridine (BrdU) labeling method using a BrdU Cell Proliferation Assay Kit (BD) by standard procedures. We stained targeted cells by specific biomarkers (B cells: PerCP-CD19; T cells: APC-CD3). Proliferation of the effectors was determined by comparing the amounts of incorporated BrdU in experimental samples to control samples. To detect cells apoptosis, we stained target cells by the specific biomarkers, provided in an Apoptosis Detection Kit (BD) and detected by FC.
ELISA and cytokine detection
The supernatants of co-culture group were collected and stored at -20˚C. ELISA kits were used to determine the levels of cytokines IL-10, IFN-γ, TNF-α, TGF-β1 (Dakewe Biotech Company), and antibodies IgA, IgG and IgM (Bethyl Laboratories, Inc), according to the manufacturer's instructions.
Patients and peripheral blood cells collection
A total of 55 participants including 35 breast cancer, and 20 healthy individuals were enrolled in this study. All patients are female. The patients aged 28–85 years, with a mean of 50 years. The age range of healthy individuals was from 35 to 71 years, with a mean of 58 years. None of the breast cancer patients had received any kind of chemical or radiation therapy before surgery. Clinical characteristics of the study cohort are summarized in Fig. 7F. This study was approved by the State Food and Drug Administration of China (2006L01023) and by the Ethical Committee of Cancer Hospital of Tianjin Medical University, China, according to the guidelines of the Declaration of Helsinki. Informed consent was obtained from all subjects before their entry into the study.
Peripheral blood mononuclear cells (1 × 105) were suspended in PBS containing 20% human serum and incubated for 30 min with appropriate antibodies: anti-human FITC-CD19, APC-PD-1 and PE/Cy7-PD-L1 (Biolegend). Cells stained with isotype control antibodies served as the negative control. Experiment was performed in accordance with the guidelines outlined aforementioned.
Statistical analysis
Statistical analysis was performed with Prism v5.0 (GraphPad Software). The values are expressed as the mean ± SEM, and the presented data are representative of at least three independent experiments. Depending on the distribution of the dataset, comparisons between groups were made using a parametric ANOVA test or a t test. P <0.05 were considered statistically significant.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding details
This work was supported by the National Key Technology R&D Program under Grant number 2015BAI12B12, the Natural Science Foundation of China under Grant Number 81472471;81272221 and the Science & Technology Development Fund of Tianjin Education Commission for Higher Education under Grant Number 2017KJ197.
References
- 1.Pyzer AR, Cole L, Rosenblatt J, Avigan DE. Myeloid-derived suppressor cells as effectors of immune suppression in cancer. Int J Cancer Journal international du cancer. 2016;139:1915–26. doi: 10.1002/ijc.30232. [DOI] [PubMed] [Google Scholar]
- 2.Peranzoni E, Zilio S, Marigo I, Dolcetti L, Zanovello P, Mandruzzato S, Bronte V. Myeloid-derived suppressor cell heterogeneity and subset definition. Curr Opin Immunol. 2010;22:238–44. doi: 10.1016/j.coi.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 3.Mauri C, Menon M. The expanding family of regulatory B cells. Int Immunol. 2015;27:479–86. doi: 10.1093/intimm/dxv038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wortel CM, Heidt S. Regulatory B cells: Phenotype, function and role in transplantation. Transpl Immunol. 2017;41:1–9. doi: 10.1016/j.trim.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Ren Z, Peng H, Fu YX. PD-1 Shapes B Cells as Evildoers in the Tumor Microenvironment. Cancer discovery. 2016;6:477–8. doi: 10.1158/2159-8290.CD-16-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guan H, Wan Y, Lan J, Wang Q, Wang Z, Li Y, Zheng J, Zhang X, Wang Z, Shen Y, et al.. PD-L1 is a critical mediator of regulatory B cells and T cells in invasive breast cancer. Sci Rep. 2016;6:35651. doi: 10.1038/srep35651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gantt S, Gervassi A, Jaspan H, Horton H. The role of myeloid-derived suppressor cells in immune ontogeny. Front Immunol. 2014;5:387 eCollection 2014. doi: 10.3389/fimmu.2014.00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hong C, Gao XM. Purification and immunophenotypic characterization of murine B10 B cells. Methods in molecular biology. 2014;1190:35–44. doi: 10.1007/978-1-4939-1161-5_3. [DOI] [PubMed] [Google Scholar]
- 9.Zhang C, Xin H, Zhang W, Yazaki PJ, Zhang Z, Le K Li W, Lee H, Kwak L, Forman S, et al.. CD5 Binds to Interleukin-6 and Induces a Feed-Forward Loop with the Transcription Factor STAT3 in B Cells to Promote Cancer. Immunity. 2016;44:913–23. doi: 10.1016/j.immuni.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swartz MA. Immunomodulatory roles of lymphatic vessels in cancer progression. Cancer Immunol Res. 2014;2:701–7. doi: 10.1158/2326-6066.CIR-14-0115. [DOI] [PubMed] [Google Scholar]
- 11.Ganti SN, Albershardt TC, Iritani BM, Ruddell A. Regulatory B cells preferentially accumulate in tumor-draining lymph nodes and promote tumor growth. Scientific reports. 2015;5:12255. doi: 10.1038/srep12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balkwill F, Montfort A, Capasso M. B regulatory cells in cancer. Trends Immunol. 2013;34:169–73. doi: 10.1016/j.it.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Xu X, Meng Q, Erben U, Wang P, Glauben R, Kuhl AA, Wu H, Ma CW, Hu M, Wang Y, et al.. Myeloid-derived suppressor cells promote B-cell production of IgA in a TNFR2-dependent manner. Cell Mol Immunol. 2017;14:597–606. doi: 10.1038/cmi.2015.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao LY, Chung JS, Teshima T, Feigenbaum L, Cruz PD Jr., Jacobe HT, Chong BF, Ariizumi K. Myeloid-Derived Suppressor Cells in Psoriasis Are an Expanded Population Exhibiting Diverse T-Cell-Suppressor Mechanisms. Journal of investigative dermatology. 2016;136:1801–10. doi: 10.1016/j.jid.2016.02.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crook KR, Jin M, Weeks MF, Rampersad RR, Baldi RM, Glekas AS, Shen Y, Esserman DA, Little P, Schwartz TA, et al.. Myeloid-derived suppressor cells regulate T cell and B cell responses during autoimmune disease. Journal of leukocyte biology. 2015;97:573–82. doi: 10.1189/jlb.4A0314-139R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jordan MB, Mills DM, Kappler J, Marrack P, Cambier JC. Promotion of B cell immune responses via an alum-induced myeloid cell population. Science. 2004;304:1808–10. doi: 10.1126/science.1089926. [DOI] [PubMed] [Google Scholar]
- 17.Green KA, Cook WJ, Green WR. Myeloid-derived suppressor cells in murine retrovirus-induced AIDS inhibit T- and B-cell responses in vitro that are used to define the immunodeficiency. J Virol. 2013;87:2058–71. doi: 10.1128/JVI.01547-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Connor MA, Fu WW, Green KA, Green WR. Subpopulations of M-MDSCs from mice infected by an immunodeficiency-causing retrovirus and their differential suppression of T- vs B-cell responses. Virology. 2015;485:263–73. doi: 10.1016/j.virol.2015.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, Tu Z, Qian S, Fung JJ, Markowitz SD, Kusner LL, Kaminski HJ, Lu L, Lin F. Myeloid-derived suppressor cells as a potential therapy for experimental autoimmune myasthenia gravis. J Immunol. 2014;193:2127–34. doi: 10.4049/jimmunol.1400857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kennedy DE, Knight KL. Inhibition of B Lymphopoiesis by Adipocytes and IL-1-Producing Myeloid-Derived Suppressor Cells. J Immunol. 2015;195:2666–74. doi: 10.4049/jimmunol.1500957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang Y, Jiang Q, Ou Y, Zhang F, Qing K, Sun Y, Lu W, Zhu H, Gong F, Lei P, et al.. BIP induces mice CD19(hi) regulatory B cells producing IL-10 and highly expressing PD-L1, FasL. Mol Immunol. 2016;69:44–51. doi: 10.1016/j.molimm.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 22.Shalapour S, Font-Burgada J, Di Caro G, Zhong Z, Sanchez-Lopez E, Dhar D, Willimsky G, Ammirante M, Strasner A, Hansel DE, et al.. Immunosuppressive plasma cells impede T-cell-dependent immunogenic chemotherapy. Nature. 2015;521:94–8. doi: 10.1038/nature14395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiao X, Lao XM, Chen MM, Liu RX, Wei Y, Ouyang FZ, Chen DP, Zhao XY, Zhao Q, Li XF, et al.. PD-1hi Identifies a Novel Regulatory B-cell Population in Human Hepatoma That Promotes Disease Progression. Cancer Discov. 2016;6:546–59. doi: 10.1158/2159-8290.CD-15-1408. [DOI] [PubMed] [Google Scholar]
- 24.Santarpia M, Karachaliou N. Tumor immune microenvironment characterization and response to anti-PD-1 therapy. Cancer Biol Med. 2015;12:74–8. doi: 10.7497/j.issn.2095-3941.2015.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou W. Highlights of Miami winter symposium 2015: into the era of immunotherapy. Cancer Biol Med. 2015;12:68–9. doi: 10.7497/j.issn.2095-3941.2015.0008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams IR. CCR6 and CCL20: partners in intestinal immunity and lymphorganogenesis. Ann N Y Acad Sci. 2006 Aug;1072:52–61. doi: 10.1196/annals.1326.036. [DOI] [PubMed] [Google Scholar]
- 27.Reboldi A, Arnon TI, Rodda LB, Atakilit A, Sheppard D, Cyster JG. IgA production requires B cell interaction with subepithelial dendritic cells in Peyer's patches. Science. 2016;352:aaf4822. doi: 10.1126/science.aaf4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morva A, Lemoine S, Achour A, Pers JO, Youinou P, Jamin C. Maturation and function of human dendritic cells are regulated by B lymphocytes. Blood. 2012;119:106–14. doi: 10.1182/blood-2011-06-360768. [DOI] [PubMed] [Google Scholar]
- 29.Adler LN, Jiang W, Bhamidipati K, Millican M, Macaubas C, Hung SC, Mellins ED. The Other Function: Class II-Restricted Antigen Presentation by B Cells. Front Immunol. 2017;8:319. doi: 10.3389/fimmu.2017.00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olkhanud PB, Damdinsuren B, Bodogai M, Gress RE, Sen R, Wejksza K, Malchinkhuu E, Wersto RP, Biragyn A. Tumor-evoked regulatory B cells promote breast cancer metastasis by converting resting CD4(+) T cells to T-regulatory cells. Cancer Res. 2011;71:3505–15. doi: 10.1158/0008-5472.CAN-10-4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang JZ, Zhang YH, Guo XH, Zhang HY, Zhang Y. The double-edge role of B cells in mediating antitumor T-cell immunity: Pharmacological strategies for cancer immunotherapy. Int immunopharmacology. 2016;36:73–85. doi: 10.1016/j.intimp.2016.04.018. [DOI] [PubMed] [Google Scholar]
- 32.Liu J, Wang H, Yu Q, Zheng S, Jiang Y, Liu Y, Yuan G, Qiu L. Aberrant frequency of IL-10-producing B cells and its association with Treg and MDSC cells in Non Small Cell Lung Carcinoma patients. Human Immunol. 2016;77:84–9. doi: 10.1016/j.humimm.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 33.Mandai M, Hamanishi J, Abiko K, Matsumura N, Baba T, Konishi I. Dual Faces of IFNgamma in Cancer Progression: A Role of PD-L1 Induction in the Determination of Pro- and Antitumor Immunity. Clin Cancer Res : an official journal of the American Association for Cancer Research. 2016;22:2329–34. doi: 10.1158/1078-0432.CCR-16-0224. [DOI] [PubMed] [Google Scholar]
- 34.Schioppa T, Moore R, Thompson RG, Rosser EC, Kulbe H, Nedospasov S, Mauri C, Coussens LM, Balkwill FR. B regulatory cells and the tumor-promoting actions of TNF-alpha during squamous carcinogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:10662–7. doi: 10.1073/pnas.1100994108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dai C, Geng R, Wang C, Wong A, Qing M, Hu J, Sun Y, Lo AW, Li J. Concordance of immune checkpoints within tumor immune contexture and their prognostic significance in gastric cancer. Mol Oncol. 2016;10:1551–8. doi: 10.1016/j.molonc.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT, Gajewski TF. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med. 2013;5:200ra116. doi: 10.1126/scitranslmed.3006504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nouel A, Pochard P, Simon Q, Segalen I, Le Meur Y Pers JO, Hillion S. B-Cells induce regulatory T cells through TGF-beta/IDO production in A CTLA-4 dependent manner. Journal of autoimmunity. 2015;59:53–60. doi: 10.1016/j.jaut.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 38.Ruddell A, Harrell MI, Furuya M, Kirschbaum SB, Iritani BM. B lymphocytes promote lymphogenous metastasis of lymphoma and melanoma. Neoplasia. 2011;13:748–57. doi: 10.1593/neo.11756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harrell MI, Iritani BM, Ruddell A. Tumor-induced sentinel lymph node lymphangiogenesis and increased lymph flow precede melanoma metastasis. The American journal of pathology. 2007;170:774–86. doi: 10.2353/ajpath.2007.060761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shah S, Divekar AA, Hilchey SP, Cho HM, Newman CL, Shin SU, Nechustan H, Challita-Eid PM, Segal BM, Yi KH, et al.. Increased rejection of primary tumors in mice lacking B cells: inhibition of anti-tumor CTL and TH1 cytokine responses by B cells. International journal of cancer Journal international du cancer. 2005;117:574–86.doi: 10.1002/ijc.21177. [DOI] [PubMed] [Google Scholar]
- 41.Li W, Song D, Li H, Liang L, Zhao N, Liu T. Reduction in Peripheral CD19+CD24hCD27+ B Cell Frequency Predicts Favourable Clinical Course in XELOX-Treated Patients with Advanced Gastric Cancer. Cell Physiol Biochem: international journal of experimental cellular physiology, biochemistry, and pharmacology. 2017;41:2045–52. doi: 10.1159/000475435. [DOI] [PubMed] [Google Scholar]
- 42.Kim EJ, Hong JE, Eom SJ, Lee JY, Park JH. Oral administration of benzyl-isothiocyanate inhibits solid tumor growth and lung metastasis of 4T1 murine mammary carcinoma cells in BALB/c mice. Breast Cancer Res Treat. 2011;130:61–71. doi: 10.1007/s10549-010-1299-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.