ABSTRACT
Cytokine-induced killer (CIK) cells that are stimulated using mature dendritic cells (DCs), referred to as (DC-CIK cells) exhibit superior anti-tumor potency. Anti-programmed death-1 (PD-1) antibodies reinvigorate T cell-mediated antitumor immunity. This phase I study aimed to assess the safety and clinical activity of immunotherapy with PD-1 blockade (pembrolizumab)-activated autologous DC-CIK cells in patients with advanced solid tumors. Patients with selected types of advanced solid tumors received a single intravenous infusion of activated autologous DC-CIK cells weekly for the first month and every 2 weeks thereafter. The primary end points were safety and adverse event (AE) profiles. Antitumor responses, overall survival (OS), progression-free survival (PFS) and cytolytic activity were secondary end points. Treatment-related AEs occurred in 20/31 patients. Grade 3 or 4 toxicities, including fever and chills, were observed in two patients. All treatment-related AEs were reversible or controllable. The cytotoxicity of DC-CIK cells induced up-regulation of PD-L1 expression on autologous tumor cells. When activated using pembrolizumab ex vivo, DC-CIK cells exerted superior antitumor properties and elevated IFN-γ secretion. Objective responses (complete or partial responses) were observed in 7 of the 31patients.These responses were durable, with 6 of 7 responses lasting more than 5 months. The overall disease control rate in the patients was 64.5%. At the time of this report, the median OS and PFS were 270 and 162 days, respectively. In conclusions, treatment with pembrolizumab-activated autologous DC-CIK cells was safe and exerted encouraging antitumor activity in advanced solid tumors. A larger phase II trial is warranted.
KEYWORDS: advanced solid tumors, DC-CIK cell, PD-1 blockade, safety, clinical activity
Introduction
Our previous studies demonstrated that cytokine-induced killer (CIK) cell treatment have an adjuvant immunomodulatory effect by prolonging survival in several types of cancer patients who undergo curative treatment,1-4 yet it appears to be unsatisfactory in advanced malignancies. As an adoptive immunotherapy, CIK cells antitumor activity is restricted by immunosuppressive pathways in the tumor microenvironment.5 Thus, it would be of considerable interest to explore novel strategies, such as overcoming the potential immune barrier in vivo, to enhance how CIK cells respond to tumors and to thereby improve the therapeutic effects of CIK cells in patients with advanced malignancy.
Recently, many immune checkpoint inhibitors, especially those that blocked the PD-1/PD-L1 pathway, showed remarkable clinical success in a variety of cancers.6-9 However, most patients do not have a robust objective clinical response to these checkpoint blockades,10 indicating that we should pay attention to the immunocompetence of the patients when using these drugs that depend on pre-existing endogenous antitumor immunity.11 Indeed, a large fraction of cancer patients, especially those being treated with high-dose chemotherapies, which induce the collapse of the immune system, lack a detectable immune reaction to cancer.12
It is therefore logical to assume that combining immune checkpoint inhibitors and activated immune effector cells may result in potent antitumor effects in cancer patients, including immunocompromised patients. A recent series of studies showed that inhibitory receptors are also expressed on CIK cells and that blocking these receptors resulted in a remarkable increase in CIK-mediated cytotoxicity against tumor cells.13,14 These findings focused attention on enhancing the CIK cell-mediated antitumor response that is induced by PD-1/PD-L1 pathway inhibitors to develop a new immunotherapeutic strategy for advanced malignancies. In addition, based on what is known of CIK cell generation, we established a culture method to stimulate CIK cells using autologous dendritic cells (DCs), referred to as (DC-CIK cells). This modification of CIK cells resulted in significantly enhanced proliferation, antitumor activity and clinical effects.15 Given the promise of these findings, we performed a phase I clinical trial to evaluate the safety and antitumor activity of a low-dose of PD-1 blockade-activated autologous DC-CIK cells in patients with advanced solid tumors.
Results
Patient characteristics
A total of 37 patients with advanced solid tumors were enrolled into the protocol and treated with pembrolizumab-activated autologous DC-CIK cells. These patients included 10 with hepatocellular carcinoma (HCC), 8 with renal cell cancer (RCC), 6 with colorectal cancer (CRC), 5 with non–small-cell lung cancer (NSCLC), 3 with ovarian cancer, 2 with bladder cancer, 2 with breast cancer and one with nasopharyngeal carcinoma (NPC). Six patients did not continue treatment or undergo follow-up although they did receive activated autologous DC-CIK cell infusions, and the details of their clinical characteristics are presented in Supplementary Table S1. The detailed treatments administered to the patients are shown in Fig. 1. Finally, 31 patients underwent follow-up and subsequent clinical assessments. Of these 31 patients, 24 were male and 7 were female, and the median age was 52 years old (range, 31–71 years old). The median number of DC-CIK cell infusion cycles was 12 (range, 3–26) (Table 1). Seven patients received less than 8 cycles of treatment (range, 3–7) due to disease progression during the treatment period.
Figure 1.
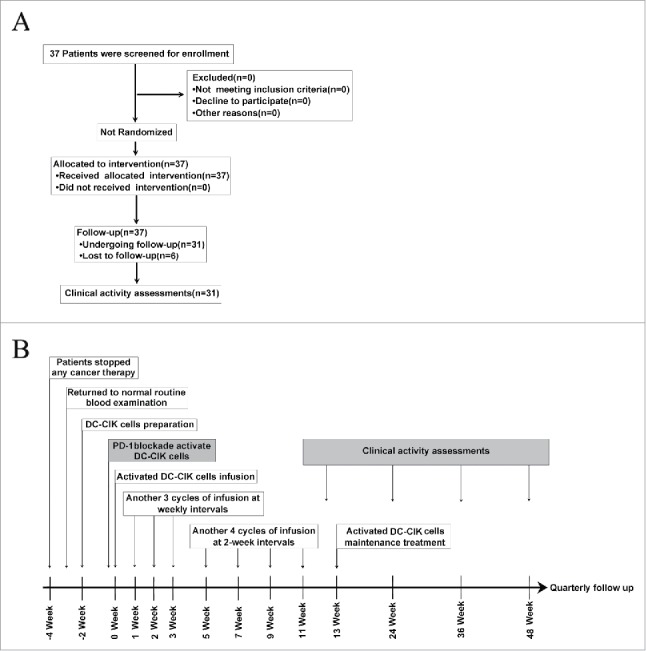
Clinical trial scheme and protocol. (A) Flow diagram illustrating the enrollment and assignment protocols and the analyses performed in the patients in this trial. In all, 37 eligible patients were allocated to receive the intervention treatment. However, 6 patients were lost during the treatment or follow-up period, and 31 patients were finally included for the clinical evaluation. (B) Overview of the clinical trial scheduling. Enrolled patients stopped cancer therapies so that they could generate activated DC-CIK Cells. Two weeks are required to manufacture DC-CIK cells. Anti-PD-1 antibodies were added, and the cells were then incubated for 30–40 min before the activated DC-CIK cells were infused. The patients received at least 8 cycles of infusions within 11 weeks. The response was assessed at 12, 24 and 36 weeks and quarterly thereafter. For eligible patients, maintenance infusions were given starting at 13 weeks.
Table 1.
Patient Characteristics.
| Characteristic | No. of Patients | % |
|---|---|---|
| Gender | ||
| Male | 24 | 77.4 |
| Female | 7 | 22.6 |
| Age (years) | ||
| Median | 52 | |
| Range | 31-71 | |
| Tumor histology | ||
| HCC | 9 | 29.0 |
| RCC | 8 | 25.8 |
| CRC | 6 | 19.4 |
| NSCLC | 3 | 9.7 |
| Bladder cancer | 2 | 6.5 |
| Breast cancer | 1 | 3.2 |
| NPC | 1 | 3.2 |
| Ovarian cancer | 1 | 3.2 |
| ECOG PS | ||
| 0 | 22 | 71.0 |
| 1 | 9 | 29.0 |
| Prior therapies | ||
| Surgery | 22 | 68.2 |
| Chemotherapy* | 16 | 51.6 |
| Radiation therapy | 5 | 16.1 |
| Immunotherapy† | 6 | 16.1 |
| Treatment cycle | ||
| Median | 12 | |
| Range | 3-26 |
Abbreviations: HCC, hepatocellular carcinoma; RCC, renal cell carcinoma; CRC, colorectal cancer; NSCLC, non–small-cell lung cancer; NPC, nasopharyngeal carcinoma; ECOG, Eastern Cooperative Oncology Group; PS, performance status.
Includes molecularly targeted therapies.
Includes a single adjuvant cytokine-induced killer cell (CIK) immunotherapy.
Characteristics of cultured DC-CIK cells
The total number of pembrolizumab-activated DC-CIK cells at the time of infusion was an average of 8.8 × 109 cells (range, 5.5–11 × 109). The DC-CIK cells were primarily CD3+T cells (median, 95.8%; range, 72.3% to 99.4%) and comprised CD8+T cells (median, 65.1%; range, 42.0% to 82.3%), CD4+T cells (median, 28.1%; range, 15.7% to 43.3%), NK cells (CD3-CD56+, median, 2.9%; range, 0.5% to 24.2%), and NKT cells (CD3+CD56+, median, 17.8%; range, 9.5% to 40.0%). PD-1 was expressed on a median of 20.5% (range, 5.8% to 39.9%) of the infused DC-CIK cells, primarily on CD8+T cells (median, 15.0%; range, 4.4% to 30.2%). Although the DC-CIK cell phenotypes were predominantly effector and effector memory (CD8+CD45RO+CD62 L-; median, 49.5%; range, 27.2% to 79.0%), a median of 8.0% (range, 2.8% to 22.6%) of the cells were central memory cells (CD8+ CD45RO+CD62 L+). A substantial proportion of naïve T cells (CD8+CD45RA+ CD62 L+; median, 18.5%; range, 3.7% to 30.4%) were also detected in the final product (Fig. 2).
Figure 2.
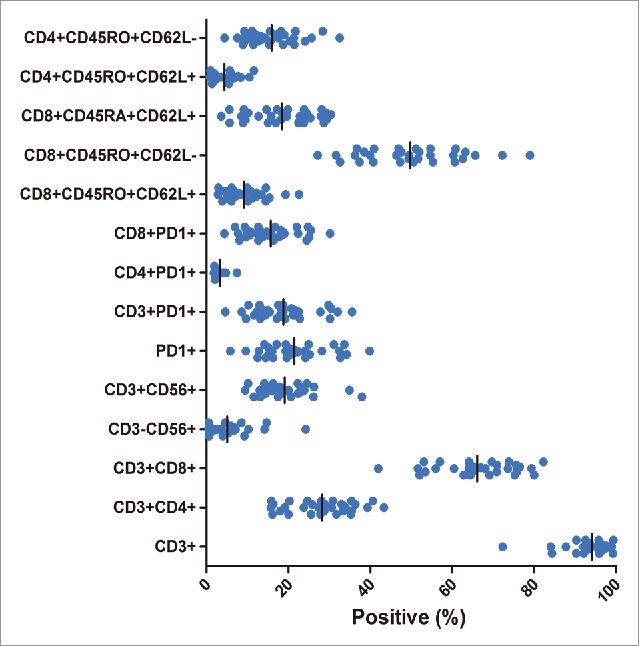
Phenotype of DC-CIK cells at the time of infusion. The cells were predominantly CD3+ and CD8+T cells. A substantial proportion of PD-1+ cells (a median of 20.5%) were also detected in the final product.
Treatment-related toxicities
The most common adverse events (AEs) arising from treatment with pembrolizumab-activated autologous DC-CIK cells during the study were fever, decreased albumin levels, increased serum AST and ALT levels, anemia, leukopenia, thrombocytopenia, chills, hypothyroidism and vitiligo (Table 2). All grades of treatment-related AEs occurred in 20 of 31 patients (64.5%), and most of them were grade 1 or 2 (18 of 20 patients, 90%). Grade 3 or 4 treatment-related AEs were observed in only two patients (6.4%). One patient with HCC exhibited a grade 3 fever during treatment, but objective antitumor regression (partial response) was observed in this patient after 8 cycles of infusions. A grade 3 chill was observed in another patient with bladder cancer, and this was resolved by treatment with promethazine hydrochloride. The most frequently observed AEs were fevers, which occurred in 9 of the 31 patients (29%). Almost all fevers rose no higher than 38°C and spontaneously resolved within 12 hours. No treatment-related serious AEs appeared in any of the patients, including those lost to follow-up (Supplementary Table S1). No infections, fatigue, nausea, vomiting, or allergic reactions were observed following infusion with pembrolizumab-activated autologous DC-CIK cells (Table 2). No treatment was discontinued in any patients because of treatment-related AEs.
Table 2.
Treatment-Related Adverse Events in patients in response to therapy (n = 31).
| No. (%) of Patients With Adverse Event |
|||
|---|---|---|---|
| Adverse event | All Grades | Grade 1 or 2 | Grade 3 or 4 |
| Overall incidence | 20 (64.5) | 18 (58.1) | 2 (6.4) |
| Fatigue | 0 (0) | 0 (0) | 0 (0) |
| Fever | 9 (29.0) | 8 (25.8) | 1 (3.2) |
| Chills | 3 (9.7) | 2 (6.4) | 1 (3.2) |
| Anemia | 4 (12.9) | 4 (12.9) | 0 (0) |
| Nausea | 0 (0) | 0 (0) | 0 (0) |
| Vomiting | 0 (0) | 0 (0) | 0 (0) |
| AST increased | 5 (16.1) | 5 (16.1) | 0 (0) |
| ALT increased | 5 (16.1) | 5 (16.1) | 0 (0) |
| Albumin decreased | 6 (19.4) | 6 (19.4) | 0 (0) |
| Leukopenia | 4 (12.9) | 4 (12.9) | 0 (0) |
| Thrombocytopenia | 4 (12.9) | 4 (12.9) | 0 (0) |
| Vitiligo | 1 (3.2) | 1 (3.2) | 0 (0) |
| Hypothyroidism | 3 (9.7) | 3 (9.7) | 0 (0) |
| Allergic reaction | 0 (0) | 0 (0) | 0 (0) |
Clinical activity
The objective response rate (RR) across the 31 enrolled patients was 7 of 31 (22.5%), including 2 (6.4%) with a complete response (CR) and 5 (16.1%) with a partial response (PR) (Fig. 3A and 3B; Table 3). The disease control rate (DCR) was 20 of 31 (64.5%) (Table 3). In the patients who had objective responses, six of seven cases experienced durable and evident responses (Fig. 3A).
Figure 3.
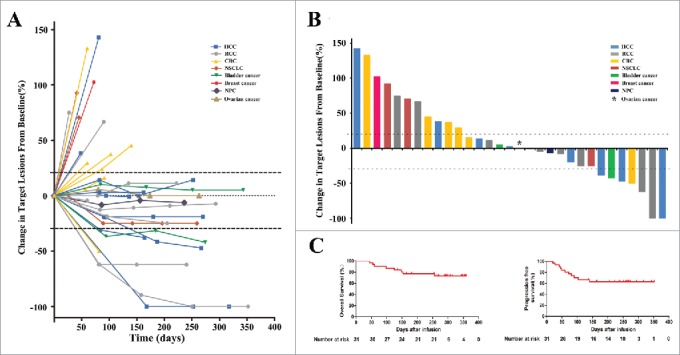
Clinical outcomes in 31 patients who were treated with PD-1 blockade-activated DC-CIK cells. (A) Spider plots showing the time and duration of clinical responses and changes in the tumor burden from baseline in patients who received pembrolizumab-activated autologous DC-CIK cell infusions. The thresholds for an objective response (-30%) and progressive disease (+20%) are marked by horizontal dashed lines, respectively, according to RECIST (version 1.1). (B) A waterfall plot of all patients showing the maximum reduction achieved in the target lesions from baseline as the sum of the longest diameter; +20% and −30% are marked by horizontal dashed lines. (C) Overall survival and progression-free survival were estimated using the Kaplan-Meier method for the 31 included patients. Censoring events are indicated by vertical tick marks.
Table 3.
Clinical effect of PD-1 blockade activated DC-CIK cells treatment.
| Response |
No. (%) of Patients |
|||||||
|---|---|---|---|---|---|---|---|---|
| Tumor histology | No. of Patients | CR | PR | SD | PD | RR | DCR | |
| HCC | 9 | 1 | 2 | 4 | 2 | 3(33.3) | 7 (77.8) | |
| RCC | 8 | 1 | 1 | 4 | 2 | 2(25.0) | 6(75.0) | |
| CRC | 6 | 0 | 1 | 1 | 4 | 1(16.7) | 2(33.3) | |
| NSCLC | 3 | 0 | 0 | 1 | 2 | 0(0) | 1(33.3) | |
| Bladder cancer | 2 | 0 | 1 | 1 | 0 | 1(50.0) | 2(100) | |
| Breast cancer | 1 | 0 | 0 | 0 | 1 | 0(0) | 0(0) | |
| NPC | 1 | 0 | 0 | 1 | 0 | 0(0) | 1(100) | |
| Ovarian cancer | 1 | 0 | 0 | 1 | 0 | 0(0) | 1(100) | |
| All | 31 | 2 | 5 | 13 | 11 | 7(22.5) | 20(64.5) | |
Abbreviations: CR, complete response; PR, partial response; SD, stable disease; PD, disease progression; RR, response rate (ie, CR and PR);DCR, disease control rate (ie, CR, PR, and SD).
In patients with HCC, one achieved a CR, two experienced a PR, and four exhibited stable disease. The objective RR and DCR were 33.3% and 77.8%, respectively. The patient who experienced a CR was a 61-year-old male with recurrent multiple intrahepatic metastases after transarterial chemoembolization (TACE) and treatment with radiofrequency ablation (RFA). After 14 cycles of pembrolizumab-activated autologous DC-CIK cell infusions, the multiple intrahepatic lesions disappeared, and the level of tumor marker alpha fetoprotein (AFP) decreased to a normal range (Fig. 4A). This patient experienced PFS for 310+ days with further maintenance treatments. The two partial responders also achieved an antitumor response for over 150 days after they received 8+ cycles of activated autologous DC-CIK cell infusions.
Figure 4.
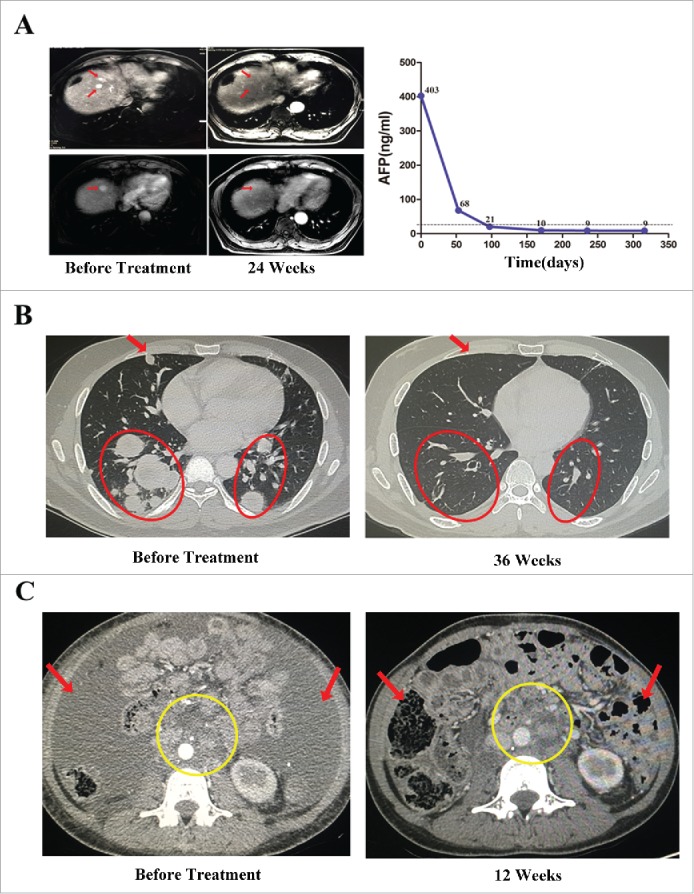
Activity levels of PD-1 blockade-activated DC-CIK cells in three patients with advanced hepatocellular carcinoma (HCC) and renal cell carcinoma (RCC). (A) A 61-year-old male with recurrent HCC experienced a complete response (CR) after receiving 14 cycles of pembrolizumab-activated autologous DC-CIK cell infusion. Although multiple intrahepatic metastases were observed on a baseline MRI (left), these lesions were no longer apparent at 24 weeks after the start of treatment (middle). The arrows indicate the regression of the lesions. The expression of the tumor marker alpha fetoprotein (AFP) had also decreased to a normal range after treatment (right). (B) A 34-year-old male with advance RCC achieved a CR after he was treated with pembrolizumab-activated autologous DC-CIK cells. Chest CT scans showing extensive bilateral pulmonary metastasis (arrow and circles) at baseline (left). This was followed by complete regression at 36 weeks after the start of the treatment (right). (C) Partial regression of metastatic RCC in a 31-year-old female within 12 weeks of the initiation of activated autologous DC-CIK cell infusions. The arrows show regression of ascites, and the yellow circle indicates regression of the lymph node metastases.
In patients with RCC, the objective RR and DCR were 25% and 75%, respectively, including one CR, one PR, and four stable diseases. The patient who achieved a CR was a 34-year-old male with extensive bilateral pulmonary metastasis and continuous progressive disease after surgery and treatment with axitinib. At 36 weeks after initiation of the treatment, his metastatic disease had completely regressed (Fig. 4B). This patient continues to receive maintenance treatments and experience a CR. The other partial responder was a 31-year-old female with massive ascites and multiple retroperitoneal lymph node metastases after palliative surgery. Within 12 weeks of the initiation of pembrolizumab-activated autologous DC-CIK cell infusions, these ascites almost disappeared and the lymph node metastatic lesions were reduced (Fig. 4C). However, she requested that the treatments be discontinued after 16 cycles because of anemia, increased serum AST and ALT levels, and hypothyroidism. Interestingly, this patient continues to experience PR (more than 240 days) as of a recent follow-up.
In patients with other solid tumors, including CRC, NSCLC, bladder cancer, breast cancer, NPC and ovarian cancer, only one patient with CRC and one patient with bladder cancer achieved a PR. The objective RR and DCR were 14% and 50%, respectively.
A total of 11 patients experienced disease progression, eight of whom died as a result of their cancer progression. The median overall survival (OS) and progression-free survival (PFS) thus far were 270 and 162 days, respectively (Fig. 3C).
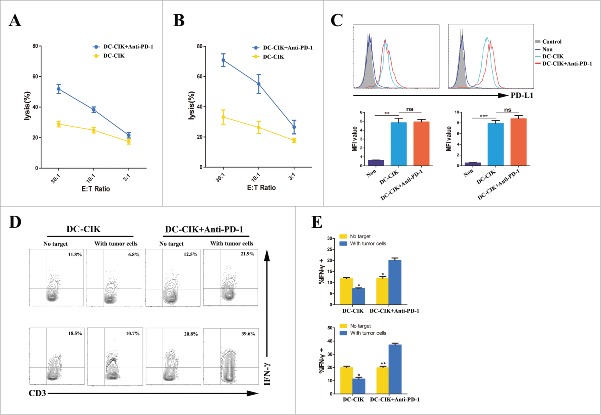
Cytotoxicity of DC-CIK cells and changes in PD-L1 expression on tumor cells in vitro
To confirm the antitumor response achieved by activated DC-CIK cells,the cytotoxicity of DC-CIK cells was tested ex vivo against autologous tumor cells that were obtained from two RCC patients who received palliative resection followed by pembrolizumab -activated autologous DC-CIK cell infusions. These two patients subsequently achieved PR (patient 3435) and stable disease (patient 5942). Cytolytic activity of DC-CIK cells was observed to response to their respective autologous tumor cells. However, higher levels of cytotoxicity were observed in the DC-CIK cells activated by pembrolizumab than non-activated DC-CIK cells (Fig. 5A and 5B). We hypothesize that up-regulation of PD-L1 on tumor cells may affect the cytolytic activity of DC-CIK cells that express PD-1 by an adaptive immune resistance mechanism.16 To address this possibility, PD-L1 expression on the tumor cells was analyzed before and after the cells was co-cultured with autologous DC-CIK cells. As expected, PD-L1 expression was rapidly induced on tumor cells by tumor-reactive DC-CIK cells, which secrete IFN-γ (Fig. 5C). Because the PD-1/PD-L1 axis was negatively regulated, the level of IFN-γ secretion from non-activated DC-CIK cells was lower after co-culturing with autologous tumor cells. However, the activity status of the PD-1 blockade-activated DC-CIK cells was improved when co-culturing with tumor cells (Fig. 5D and 5E).
Figure 5.
Cytotoxicity of DC-CIK cells that were derived from patients with advanced RCC. (A and B) The cytolytic activity of non-activated DC-CIK cells or activated DC-CIK cells in response to their respective autologous tumor cells, which were obtained from two RCC patients (patient 5942 (A) and patient 3435 (B)). E:T Ratio, effector cell to target cell ratio. (C) Changes in PD-L1 expression on tumor cells were analyzed using flow cytometry in the presence of non-activated DC-CIK cells or activated DC-CIK cells, as indicated, in patient 5942 (left) and patient 3435 (right). The corresponding mean fluorescence intensity (MFI) of three experiments is shown below the flow cytometry histogram plot. (D) Flow cytometric analysis of the expression of IFN-γ in non-activated DC-CIK cells or activated DC-CIK cells after the cells were co-cultured in medium or their respective autologous tumor cells. The samples were gated using CD3+ cells. Upper panels: patient 5942, lower panels: patient 3435. (E) The percentage of IFN-γ positive cells in three experiments is shown as a bar graph for patient 5942 (upper) and patient 3435 (lower). The data are presented as the mean ± SD. (*) P<0.05. (**) P<0.01, (***) P<0.001. ns, no significance.
Discussion
While a recent series of clinical trials have shown that an antitumor response is induced by PD-1/PD-L1 blockade therapy or adoptive cell therapy (ACT) in patients with various types of advanced cancer,7,17,18 this exploratory study is the first to combine ACT (DC-CIK cells) with PD-1/PD-L1 blockade in patients with advanced solid tumors. Strikingly, the results show that this new strategy exhibits a promising antitumor effect and a satisfactory clinical response, with 7 of 31 patients achieved objective tumor regression. Indeed, inhibiting the PD-1/PD-L1 checkpoint released the brake on active T cells and restored immune responsiveness, resulting in the elimination of the tumor.19 Interestingly, the DC-CIK cells we used in this study contained a substantial proportion of PD-1+ T cells (Fig. 2), suggesting that a PD-1 blockade might significantly increase the cytotoxic potency of DC-CIK cells. Consistent with these results, recent preclinical and clinical data have also demonstrated that specifically blocking PD-1 immunosuppression significantly increases the antitumor efficacy of adoptive T-cell immunotherapy performed with chimeric antigen receptor (CAR) T cells.20,21
In this study, modified CIK cells (DC-CIK cells) were administered to patients. DC-CIK cells have been demonstrated to exhibit superior antitumor potency, increased proliferative capacity, higher CD3+CD8+ ratios, and increased IFN-γ secretion.15,22 Moreover, this study shows that a substantial proportion of DC-CIK cells express PD-1, suggesting that PD-1 blockade has a strong potential for activating DC-CIK cells. Additionally, and just as important, PD-1 expression, which is significantly up-regulated when T cells recognize tumor targets and are consequently activated, is increasingly recognized as a component of the repertoire of clonally expanded tumor-reactive cells.23,24 Based on this hypothesis, we sought to determine whether a higher level of antitumor activity is exhibited by DC-CIK cells in the combined immunotherapeutic strategy. Nevertheless, much less is known about checkpoint inhibitors' in vivo pharmacokinetics, and anti–PD-1 mAb, which is unlikely to solely act on T cells, may interact with various host components (e.g., myeloid cells or macrophages) in the tumor bed.25 We therefore activated DC-CIK cells using pembrolizumab in vitro prior to infusion, and inferred that the anti-PD-1 antibodies (at a lower dose) could conjugate directly with DC-CIK cells and that this interaction would be more thorough and precise in the absence of complex interfering factors.
As shown in previous clinical trials, PD-1 signal-blocking therapies are associated with immune-related AEs, including serious AEs.17,26 However, these toxicities appear to be less frequent and milder in patients who are treated with pembrolizumab-activated autologous DC-CIK cells. Most of the events observed in this study, including fever, chills, hepatic impairment, anemia, leukopenia, thrombocytopenia, and hypothyroidism, were expected, and all were controllable. Drug-related grade 3 or 4 toxic effects occurred in only 6% of the patients, and there were no treatment-related deaths, pneumonitis, or inflammatory colitis, suggesting that this therapy can be conducted in an outpatient setting with minimal supportive care. The safety and tolerance of patients to our treatment may result from the administration of autologous DC-CIK cells and the low dose of PD-1-blocking mAbs we used. Indeed, autologous CIK/DC-CIK cells can home preferentially to tumor sites and do not cause more normal tissue impairment, and treatment with these cells (range, 6–15 × 109) was safe and well tolerated in cancer patients.4,27-30 These results reassure us that inducing a blockade of PD-1 does not significantly increase the autoimmunity-related cytotoxicity of DC-CIK cells. Second, after considering immune-related AEs, which are inherent to the use of immune checkpoint inhibitors, we conducted a dose-escalation trial of pembrolizumab to select the optimal dose for activating DC-CIK cells. As shown in Supplementary Figure S1, pembrolizumab that was administered at a dose of 2 µg per million cells produced a superior increase in DC-CIK-mediated killing against tumor targets. This dose (10–20 mg per cycle of infusion) of anti-PD-1 antibodies is far less compared to what was used in other studies, and have little effect on the disease control.7,26,31 Therefore, we consider that it is the infusion of the ex vivo pre-activated DC-CIK cells that resulted in the satisfactory clinical response with less toxicity in the patients.
The efficacy of ACT requires transferred T cells to persist in vivo, to home to tumor sites and to present a durable antitumor response. DC-CIK may represent an ideal cell population for adoptive immunotherapy. DC-CIK cells have been confirmed as a heterogeneous cell population, including CD3+ CD56+ (NKT cells), CD3+CD56− (T cells), and CD3−CD56+ (NK cells), which could identify the target cells not only through the TCR and MHC, but also could through the non-MHC restriction such as NK cell activated receptor.32 They also express CD4, CD8, TCRαβ, CD45RA, CD45RO, CD127, CCR7, CD27, CD28 and CD62 L.33-35 The CD8+CD45RO+CD62 L- effector memory cell subset could deliver potent cytotoxicity for the immediate destruction of tumor cells. The less potent CD8+ CD45RO+CD62 L+ central memory cell and CD8+CD45RA+ CD62 L+ naïve cell subset in the DC-CIK cell population could proliferate and persist in vivo for a longer duration and therefore provide a persistent source of cells with long-term anti-tumor effective.34,35 The antitumor mechanisms of DC-CIK cells include effector cell-target cell contacts through binding of the surface adhesion molecules, cell signaling pathway involving the binding and activity of NK-cell receptors to their ligands, and induction of tumor cell apoptosis by Fas ligand via the Fas signaling pathway.33 However, whether there are specific antitumor immune responses during the DC-CIK cells infusion remain unclear. The potential mechanism of the memory cell subset in the cultured DC-CIK cells could be correlated with the culture conditions or a probable unidentified antitumor specific immune response against the tumor-associated antigens. And our frequent infusions of PD-1 blockade-activated DC-CIK cells (1–2 weeks interval between cycles) may simultaneously enhance the immune response. The further precise mechanism of how memory cell phenotype arising will be investigated in the future work.
It is important to consider factors that might improve the antitumor effect because not all patients were responsive to the treatment in this study. The accumulated literature shows that immunotherapies and anti-PD-1 antibodies exert suboptimal effects in patients with a non-inflamed tumor microenvironment or a compromised immune system.36,37 This data may support the notion that patients with immunogenic tumors (e.g., HCC and RCC) are more susceptible to ACT, especially those that attempt to block immune checkpoints. Another important factor to predict outcomes may be intratumoral PD-L1 expression.8,38 However, PD-L1 expression was not detected on the tumor cells before treatment with activated autologous DC-CIK cells for patients' benefit in this phase I clinical trial. Instead, we collected two RCC specimens from patients who received palliative resection in our hospital to investigate the potential predictive factor. In the two RCC patients who achieved favorable clinical outcomes after activated DC-CIK cell therapy, PD-L1 expression could not be detected on the tumor cells before the treatment, whereas it was significantly up-regulated in co-cultures of tumor and autologous DC-CIK cells. (Fig. 5C). These results highlight the linkage between PD-L1 expression and DC-CIK cells, which is thought to be that cytotoxic DC-CIK cells release cytokines such as INF-γ to driving the up-regulation of PD-L1 that leads to a negative feedback regulation. Indeed, it is increasingly being recognized that PD-L1 expression can be adaptively induced by immune responses, especially IFN-γ within the tumor microenvironment.39 These suggest that PD-L1 expression may dynamically change during treatment with PD-1 blockade-activated DC-CIK cells. Thus, the dynamic expression of PD-L1 on the tumor surface but not PD-L1 expression should be considered a predictive biomarker of responsiveness to treatment with immunotherapies, PD-1 blockade therapy, or PD-1 blockade-activated DC-CIK cell treatment, though more adequately powered samples are needed.
In conclusion, we provide the first data showing that pembrolizumab-activated autologous DC-CIK cells have a promising safety profile and demonstrate an encouraging clinical response in patients with advanced solid tumors. Therefore, this study promotes the development of a powerful treatment option for patients with advanced cancers. A randomized, phase II trial based on these findings is being planned in patients with advanced RCC and HCC.
Materials and Methods
Patients
Patients were required to have a documented diagnosis of advanced HCC, RCC, CRC, NSCLC, bladder cancer, breast cancer, NPC, or ovarian cancer and to have experienced disease progression after at least one previous course of tumor-appropriate treatment for advanced or metastatic disease. Other inclusion criteria included stopping any cancer therapy before enrollment, an age of 18 to 75 years, a life expectancy of greater than 12 weeks, an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, adequate organ function, and lesions that could be evaluated using the Response Evaluation Criteria in Solid Tumors (RECIST) guidelines. The following exclusion criteria were applied: previous treatment with anti-CTLA-4 or anti-PD-1/PD-L1 therapy, any form of primary immunodeficiency or history of autoimmune disease, ongoing systemic infections and concurrent systemic steroid therapy, and recruitment into other clinical trial. All participating patients provided informed consent.
Study design and procedures
This single-center, open-label, phase I trial was approved by the Review Board and Clinical Research Ethics Committee of Sun Yat-sen University Cancer Center (SYSUCC) and is registered at www.clinicaltrial.gov. (NCT 02886897). All methods and procedures associated with this study were conducted in accordance with the Good Clinical Practice guidelines and accorded ethically with the principles of the Declaration of Helsinki and local laws. All authors had access to the study data and reviewed and approved the final manuscript.
The enrolled patients received intravenous infusions of pembrolizumab-activated autologous DC-CIK cells at the Biotherapy Center of SYSUCC. All participants received at least 8 cycles of infusions (4 cycles at weekly intervals followed by 4 cycles every 2 weeks) or received cycles until they experienced disease progression or unacceptable adverse effects or withdrew consent. Patients with disease progression were taken off of the study, and they received multidisciplinary synthetic therapy or were recruited into other clinical trials according to the recommendation by physicians.If the patients had an objective response or stable disease after treatment, they were viewed as eligible to receive additional cycles of maintenance treatment every 2 weeks. The design and procedures used in the clinical trial are shown in Fig. 1.
Outcome measures
The primary objective was to evaluate the safety and adverse-event profiles of intravenous infusions of pembrolizumab-activated autologous DC-CIK cells in the patients. Secondary objectives included assessments of clinical responses, OS, PFS, and cytolytic activity. Safety evaluations primarily consisted of clinical and laboratory abnormalities that were monitored throughout the study up until two weeks after the last infusion of activated autologous DC-CIK cells. AEs were evaluated using the National Cancer Institute Common Toxicity Criteria version 4.0. Treatment-related AEs were monitored during the treatment and observation periods, and the highest observed grade was recorded for each patient. In each patient, lesions were evaluated using computed tomography (CT) or magnetic resonance imaging (MRI) every 12 weeks for up to 1 year, until disease progression occurred or until the patient was lost to follow-up. The overall responses were assessed by RECIST version 1.1.
Generation of DC-CIK cells and activation by Anti-PD-1 antibodies
Autologous DC-CIK cells were generated according to our previous report.15 Briefly, when a routine blood examination revealed a return to normal conditions, 50–60 ml of heparinized peripheral blood was obtained from each patient over a 2-week period. Peripheral blood mononuclear cells (PBMCs) were separated using Ficoll-Hypaque gradient centrifugation. The cells were cultured in X-VIVO 15 containing 2% autologous serum and allowed to adhere for 1 h. The suspended cells were then collected and induced to become CIK cells using 1000 U/ml rhIFN-γ for the first 24 h followed by stimulation with 100 ng/ml OKT-3, 1000 U/ml rhIL-2 and 100 U/ml IL-1α. The adherent cells were cultured using DC medium (X-VIVO 15 serum-free medium supplemented with 1000 U/ml GM-CSF and 30 ng/ml IL-4). On the sixth day, another 10 ng/ml of TNFα was added to the DCs to induce maturation. On the next day, the CIK cells were mixed with DCs (DC-DIK cells) at a ratio of 20:1 and cultured in fresh medium containing 1000 U/ml rhIL-2 for another 7 days. At 14 days, the DC-CIK cells were harvested, and their number, viability, and phenotype and whether they were contaminated were analyzed. Before the DC-CIK cells were transferred to patients, they were incubated with pembrolizumab (2 µg/106cells) for 30–40 min in a 37℃ thermostat to allow the antibodies to bind to the DC-CIK cells. Pembrolizumab (Merck & Co., Inc.) is a humanized IgG4 anti-PD-1 monoclonal antibody that binds to PD-1 to prevent it from engaging with PD-L1 or PD-L2. It thereby enhances the antitumor activity of T cells. All of the drugs used in this trial were identically packaged.
Immunophenotyping
The phenotypes of the DC-CIK cells were characterized using flow cytometry with anti-CD3, -CD4, -CD8, -CD56, -PD-1, -CD62 L, -CD45RA, and -CD45RO monoclonal antibodies, all which were obtained from BD Biosciences (San Diego, CA, USA). The number of labeled cells was measured using an FC500 flow cytometer, and the obtained data were analyzed using CXP software (Beckman Coulter).
Cytotoxicity assays
The cytotoxic specificity of the DC-CIK cells obtained from two RCC patients was analyzed using an impedance-based tumor cell killing assay (xCELLigence) as previously described.40,41 The effector cells in these tests were DC-CIK cells that were blocked with or without anti-PD-1 antibodies (pembrolizumab), and the target cells were autologous tumor cells that were separated from surgically resected tissues obtained from the patients and cultured in RPMI-1640 medium containing 10% fetal bovine serum and 1% penicillin-streptomycin. Cytotoxicity was quantified after the effector and target cells were co-incubated for 24 h. To evaluate the reactivity between the DC-CIK cells and autologous tumor cells and the checkpoint ligands expressed by tumor cells before and after co-incubation, we measured the expression of IFN-γ in DC-CIK cells and PD-L1 on tumor cells using flow cytometry with anti-IFN-γ and PD-L1 antibodies (BD Biosciences), respectively.
Statistical analysis
All statistical analyses were conducted using SPSS 20.0 or GraphPad Prism 5 software. Descriptive statistics were used to summarize the patient characteristics, treatment-related AEs, overall responses and DC-CIK cell phenotypes. The Mann–Whitney U-test was used to compare continuous variables, and Fisher's exact test was used for categorical variables between groups. Survival rates were calculated using the Kaplan–Meier method. Overall survival (OS) was defined from the date of first treatment to the date of death resulting from any cause or the date of last follow-up. Progression-free survival (PFS) was calculated from the time of first treatment to the time of first disease progression or last follow-up. A difference of p <0.05 was considered statistically significant in all the analyses.
Supplementary Material
Funding Statement
National Natural Science Foundation for Young Scholar of China ID: 81402560; Funding Source: Guangdong Province Science and Technology Plan Project ID: 2013B021800063; Funding Source: National Natural Science Foundation of China (NSFC) ID: 81472387; Funding Source: National Natural Science Foundation of China (NSFC) ID: 81572865
Abbreviations
- AFP
alpha fetoprotein
- AE
adverse event
- CAR
chimeric antigen receptor
- CIK
cytokine-induced killer
- CR
complete response
- CT
computed tomography
- CRC
colorectal cancer
- DC
dendritic cells
- DC-CIK
CIK cells are stimulated using mature DCs
- DCR
disease control rate
- ECOG
Eastern Cooperative Oncology Group
- HCC
hepatocellular carcinoma
- MRI
magnetic resonance imaging
- NSCLC
non–small-cell lung cancer
- NPC
nasopharyngeal carcinoma
- OS
overall survival
- PD-1
programmed death-1
- PFS
progression-free survival
- PR
partial response
- RR
response rate
- RFA
radiofrequency ablation
- RCC
renal cell cancer
- RECIST
Response Evaluation Criteria in Solid Tumors
- TACE
transarterial chemoembolization
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by a grant from the National Natural Science Foundation for Young Scholar of China (81402560),the National Natural Science Foundation of China (81572865 and 81472387), and the Guangdong Province Science and Technology Plan Project (2013B021800063).
Authenticity of this article
Our study has been validated by uploading the key raw data onto the Research Data Deposit public platform (www.researchdata.org.cn) at Sun Yat-sen University Cancer Center (approval number: RDDA2017000235).
References
- 1.Pan K, Guan XX, Li YQ, Zhao JJ, Li JJ, Qiu HJ, Weng DS, Wang QJ, Liu Q, Huang LX, et al.. Clinical activity of adjuvant cytokine-induced killer cell immunotherapy in patients with post-mastectomy triple-negative breast cancer. Clin Cancer Res. 2014;20:3003–11. doi: 10.1158/1078-0432.CCR-14-0082. PMID:24668644. [DOI] [PubMed] [Google Scholar]
- 2.Pan QZ, Tang Y, Wang QJ, Li YQ, Zhang L, Li XD, Zhao JJ, Weng DS, Liu Q, Huang LX, et al.. Adjuvant cellular immunotherapy in patients with resected primary non-small cell lung cancer. Oncoimmunology. 2015;4:e1038017. doi: 10.1080/2162402X.2015.1038017. PMID:26405607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li JJ, Gu MF, Pan K, Liu LZ, Zhang H, Shen WX, Xia JC. Autologous Cytokine-induced Killer Cell Transfusion in Combination With Gemcitabine Plus Cisplatin Regimen Chemotherapy for Metastatic Nasopharyngeal Carcinoma. J Immunother. 2012;35:189–95. doi: 10.1097/CJI.0b013e318241d9de. PMID:22306907. [DOI] [PubMed] [Google Scholar]
- 4.Xu L, Wang J, Kim Y, Shuang ZY, Zhang YJ, Lao XM, Li YQ, Chen MS, Pawlik TM, Xia JC, et al.. A randomized controlled trial on patients with or without adjuvant autologous cytokine-induced killer cells after curative resection for hepatocellular carcinoma. Oncoimmunology. 2015;5:e1083671. doi: 10.1080/2162402X.2015.1083671. PMID:27141337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi H, Qi X, Ma B, Cao Y, Wang L, Sun L, Niu H. The status, limitation and improvement of adoptive cellular immunotherapy in advanced urologic malignancies. Chin J Cancer Res. 2015;27:128–37. PMID:25937774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, et al.. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–44. doi: 10.1056/NEJMoa1305133. PMID:23724846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, Stankevich E, Pons A, Salay TM, McMiller TL, et al.. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–75. doi: 10.1200/JCO.2009.26.7609. PMID:20516446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, et al.. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–28. doi: 10.1056/NEJMoa1501824. PMID:25891174. [DOI] [PubMed] [Google Scholar]
- 9.El-Khoueiry AB, Melero I, Crocenzi TS. Phase I/II safety and antitumor activity of nivolumab in patients with advanced hepatocellular carcinoma (HCC): CA209–040. J Clin Oncol. 2015;33:LBA101. doi: 10.1200/jco.2015.33.18_suppl.lba101. [DOI] [Google Scholar]
- 10.Pauken KE, Wherry EJ. Overcoming T cell exhaustion in infection and cancer. Trends Immunol. 2015;36:265–76. doi: 10.1016/j.it.2015.02.008. PMID:25797516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, et al.. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–71. doi: 10.1038/nature13954. PMID:25428505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teng MW, Ngiow SF, Ribas A, Smyth MJ. Classifying Cancers Based on T-cell Infiltration and PD-L1. Cancer Res. 2015;75:2139–45. doi: 10.1158/0008-5472.CAN-15-0255. PMID:25977340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dai C, Lin F, Geng R, Ge X, Tang W, Chang J, Wu Z, Liu X, Lin Y, Zhang Z, et al.. Implication of combined PD-L1/PD-1 blockade with cytokine-induced killer cells as a synergistic immunotherapy for gastrointestinal cancer. Oncotarget. 2016;7:10332–44. doi: 10.18632/oncotarget.7243. PMID:26871284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poh SL, Linn YC. Immune checkpoint inhibitors enhance cytotoxicity of cytokine-induced killer cells against human myeloid leukaemic blasts. Cancer Immunol Immunother. 2016;65:525–36. doi: 10.1007/s00262-016-1815-8. PMID:26961084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang QJ, Wang H, Pan K, Li YQ, Huang LX, Chen SP, He J, Ke ML, Zhao JJ, Li JJ, et al.. Comparative study on anti-tumor immune response of autologous cytokine-induced killer (CIK) cells, dendritic cells-CIK (DC-CIK), and semi-allogeneic DC-CIK. Chin J Cancer. 2010;29:641–48. doi: 10.5732/cjc.009.10772. PMID:20591215. [DOI] [PubMed] [Google Scholar]
- 16.Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT, Gajewski TF. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med. 2013;5:200ra116. doi: 10.1126/scitranslmed.3006504. PMID:23986400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamanishi J, Mandai M, Ikeda T, Minami M, Kawaguchi A, Murayama T, Kanai M, Mori Y, Matsumoto S, Chikuma S, et al.. Safety and Antitumor Activity of Anti-PD-1 Antibody, Nivolumab, in Patients With Platinum-Resistant Ovarian Cancer. J Clin Oncol. 2015;33:4015–22. doi: 10.1200/JCO.2015.62.3397. PMID:26351349. [DOI] [PubMed] [Google Scholar]
- 18.Bollard CM, Gottschalk S, Torrano V, Diouf O, Ku S, Hazrat Y, Carrum G, Ramos C, Fayad L, Shpall EJ, et al.. Sustained complete responses in patients with lymphoma receiving autologous cytotoxic T lymphocytes targeting Epstein-Barr virus latent membrane proteins. J Clin Oncol. 2014;32:798–808. doi: 10.1200/JCO.2013.51.5304. PMID:24344220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, Chen L, Pardoll DM, Topalian SL, Anders RA. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20:5064–74. doi: 10.1158/1078-0432.CCR-13-3271. PMID:24714771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.John LB, Devaud C, Duong CP, Yong CS, Beavis PA, Haynes NM, Chow MT, Smyth MJ, Kershaw MH, Darcy PK. Anti-PD-1 antibody therapy potently enhances the eradication of established tumors by gene-modified T cells. Clin Cancer Res. 2013;19:5636–46. doi: 10.1158/1078-0432.CCR-13-0458. PMID:23873688. [DOI] [PubMed] [Google Scholar]
- 21.Chong EA, Melenhorst JJ, Lacey SF, Ambrose DE, Gonzalez V, Levine BL, June CH, Schuster SJ. PD-1 blockade modulates chimeric antigen receptor (CAR)-modified T cells: refueling the CAR. Blood. 2017;129:1039–41. doi: 10.1182/blood-2016-09-738245. PMID:28031179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hongeng S, Petvises S, Worapongpaiboon S, Rerkamnuaychoke B, Pakakasama S, Jootar S. Generation of CD3+ CD56+ cytokine-induced killer cells and their in vitro cytotoxicity against pediatric cancer cells. Int J Hematol. 2003;77:175–79. doi: 10.1007/BF02983217. PMID:12627854. [DOI] [PubMed] [Google Scholar]
- 23.Gros A, Robbins PF, Yao X, Li YF, Turcotte S, Tran E, Wunderlich JR, Mixon A, Farid S, Dudley ME, et al.. PD-1 identifies the patient-specific CD8(+) tumor-reactive repertoire infiltrating human tumors. J Clin Invest. 2014;124:2246–59. doi: 10.1172/JCI73639. PMID:24667641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gros A, Parkhurst MR, Tran E, Pasetto A, Robbins PF, Ilyas S, Prickett TD, Gartner JJ, Crystal JS, Roberts IM, et al.. Prospective identification of neoantigen-specific lymphocytes in the peripheral blood of melanoma patients. Nat Med. 2016;22:433–8. doi: 10.1038/nm.4051. PMID:26901407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arlauckas SP, Garris CS, Kohler RH, Kitaoka M, Cuccarese MF, Yang KS, Miller MA, Carlson JC, Freeman GJ, Anthony RM, et al.. In vivo imaging reveals a tumor-associated macrophage-mediated resistance pathway in anti-PD-1 therapy. Sci Transl Med. 2017;9:pii: eaal3604. doi: 10.1126/scitranslmed.aal3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al.. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. PMID:22658127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee JH, Lee JH, Lim YS, Yeon JE, Song TJ, Yu SJ, Gwak GY, Kim KM, Kim YJ, Lee JW, et al.. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology. 2015;148:1383–91. doi: 10.1053/j.gastro.2015.02.055. PMID:25747273. [DOI] [PubMed] [Google Scholar]
- 28.Liu L, Zhang W, Qi X, Li H, Yu J, Wei S, Hao X, Ren X. Randomized study of autologous cytokine-induced killer cell immunotherapy in metastatic renal carcinoma. Clin Cancer Res. 2012;18:1751–59. doi: 10.1158/1078-0432.CCR-11-2442. PMID:22275504. [DOI] [PubMed] [Google Scholar]
- 29.Nishimura R, Baker J, Beilhack A, Zeiser R, Olson JA, Sega EI, Karimi M, Negrin RS. In vivo trafficking and survival of cytokine-induced killer cells resulting in minimal GVHD with retention of antitumor activity. Blood. 2008;112:2563–74. doi: 10.1182/blood-2007-06-092817. PMID:18565854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang N, Qiao G, Wang X, Morse MA, Gwin WR, Zhou L, Song Y, Zhao Y, Chen F, Zhou X, et al.. Dendritic Cell/Cytokine-Induced Killer Cell Immunotherapy Combined with S-1 in Patients with Advanced Pancreatic Cancer: A Prospective Study. Clin Cancer Res. 2017;23:5066–73. doi: 10.1158/1078-0432.CCR-17-0492. PMID:28611200. [DOI] [PubMed] [Google Scholar]
- 31.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al.. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65. doi: 10.1056/NEJMoa1200694. PMID:22658128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pievani A, Borleri G, Pende D, Moretta L, Rambaldi A, Golay J, Introna M. Dual-functional capability of CD3+CD56+ CIK cells, a T-cell subset that acquires NK function and retains TCR-mediated specific cytotoxicity. Blood. 2011;118:3301–10. doi: 10.1182/blood-2011-02-336321. PMID:21821703. [DOI] [PubMed] [Google Scholar]
- 33.Guo Y, Han W. Cytokine-induced killer (CIK) cells: from basic research to clinical translation. Chin J Cancer. 2015;34:99–107. doi: 10.1186/s40880-015-0002-1. PMID:25962508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Linn YC, Lau SK, Liu BH, Ng LH, Yong HX, Hui KM. Characterization of the recognition and functional heterogeneity exhibited by cytokine-induced killer cell subsets against acute myeloid leukaemia target cell. Immunology. 2009;126:423–35. doi: 10.1111/j.1365-2567.2008.02910.x. PMID:18778291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu J, Wang L, Wang Y, Zhang W, Cao Y. Phenotypic characterization and anticancer capacity of CD8+ cytokine-induced killer cells after antigen-induced expansion. PLoS One. 2017;12:e0175704. doi: 10.1371/journal.pone.0175704. PMID:28426690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gajewski TF, Louahed J, Brichard VG. Gene signature in melanoma associated with clinical activity: a potential clue to unlock cancer immunotherapy. Cancer J. 2010;16:399–403. doi: 10.1097/PPO.0b013e3181eacbd8. PMID:20693853. [DOI] [PubMed] [Google Scholar]
- 37.Ji RR, Chasalow SD, Wang L, Hamid O, Schmidt H, Cogswell J, Alaparthy S, Berman D, Jure-Kunkel M, Siemers NO, et al.. An immune-active tumor microenvironment favors clinical response to ipilimumab. Cancer Immunol Immunother. 2012;61:1019–31. doi: 10.1007/s00262-011-1172-6. PMID:22146893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL, et al.. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4:127ra137. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64. doi: 10.1038/nrc3239. PMID:22437870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davenport AJ, Jenkins MR, Cross RS, Yong CS, Prince HM, Ritchie DS, Trapani JA, Kershaw MH, Darcy PK, Neeson PJ. CAR-T Cells Inflict Sequential Killing of Multiple Tumor Target Cells. Cancer Immunol Res. 2015;3:483–94. doi: 10.1158/2326-6066.CIR-15-0048. PMID:25711536. [DOI] [PubMed] [Google Scholar]
- 41.Hegde M, Mukherjee M, Grada Z, Pignata A, Landi D, Navai SA, Wakefield A, Fousek K, Bielamowicz K, Chow KK, et al.. Tandem CAR T cells targeting HER2 and IL13Ralpha2 mitigate tumor antigen escape. J Clin Invest. 2016;126:3036–52. doi: 10.1172/JCI83416. PMID:27427982. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.