ABSTRACT
Despite substantial clinical progress with targeted therapies, current antibody-based approaches have limited efficacy at controlling HER2/neu-positive breast cancers, especially in the absence of chemotherapies. Previously, we showed that the combination of IFNγ and anti-HER2/neu antibody synergistically reduces tumor growth in an in vivo implanted mammary tumor model. Here, we report a recombinant approach to produce an anti-HER2/neu scFv and IFNγ fusion protein using an engineered effector domain (EED) scaffold. The new molecule induces in vitro apoptosis in an IFNγ receptor-dependent manner. At a very low dose in the in vivo xenografted tumor models, the new EED-IFNγ fusion protein demonstrates superior activity over the anti-HER2/neu antibody and is even active on tumors that are resistant to anti-HER2/neu antibody therapy. Examination of tumor infiltrated macrophages and lymphocytes reveals that the fusion protein can induce changes in tumor microenvironment to support immune reactivity against tumors. Our studies have defined a targeted immunotherapy approach for the treatment of cancers.
KEYWORDS: Antibody, cancer, engineered effector domain (EED), HER2, IFNγ
Introduction
HER2/neu-targeted therapies originated in our laboratory after we described that the p185neu protein residing on the cell surface1 could serve as a downregulatable target for monoclonal anti-HER2/neu antibodies.2-4 Those studies demonstrated that the antibodies, upon disabling the Her2/neu kinase complex, induced a reversion of the malignant phenotype.3,5 It was also demonstrated that two antibodies binding to distinct ectodomain epitopes led to more complete phenotypic reversion and inhibition of tumor growth.
Similar antibodies have since been developed for human use. Two anti-HER2/neu antibodies (trastuzumab and pertuzumab) and an antibody-drug conjugate (trastuzumab emtansine) are used to treat HER2/neu positive breast cancer and stomach cancer.6-9 In addition, kinase inhibitors that limit HER2/neu tyrosine kinase activity have also been developed.10 HER2/neu-targeted therapies have greatly helped HER2/neu positive breast cancers in clinical treatments, improving overall survival even in patients with metastatic breast cancers.11
Despite substantial progress, current targeted therapies remain insufficient at controlling HER2/neu positive breast cancers. Trastuzumab as a single agent only demonstrates efficacy in about 30% of HER2/neu positive tumors,12 and currently is administrated in combination with chemotherapy. Most importantly, about 30% of patients develop resistance to trastuzumab and disease will recur, and almost all patients with advanced disease develop resistance to HER2 targeted therapies over time and succumb to the disease.13 Efforts have been made to understand the effect of immune modulation to aid targeted therapies.
It is reported that the clinical efficacy of anti-HER2/neu antibody is associated with the activation of both the adaptive and innate immune system.14,15 Stagg et al. suggested that mAb therapy requires type 1 and 2 IFNs, and found IFNγ induced CD8+ T cells were determinants for effective tumor inhibition.16 More recently, we have showed that a combination of IFNγ and anti-HER2/neu antibody synergistically reduces tumor growth in an in vivo implanted mammary tumor model.17
We have been focusing on improving the effectiveness of antibody effector functions, and report here a recombinant approach to produce an anti-HER2/neu scFv and IFNγ fusion protein. This fusion protein is an extension of previous structurally based studies using the Grababody scaffold,18 which is an scFv protein containing an engineered effector domain (EED). At a very low dose, the new fusion protein demonstrates superior activity over the anti-HER2/neu antibody. Furthermore, it is active even on tumors that are resistant to anti-HER2/neu antibody therapy. The IFNγ scFv–EED represents an approach to take advantage of the synergistic activity of IFNγ and the anti-HER2/neu antibody, while targeting the IFNγ activity precisely to the HER2-expression tumor cells.
Results
IFNγ scFv–EED retains the target-binding activity of the anti-HER2/neu antibody as well as the IFNγ activity
Previously, we reported that the anti-HER2/neu scFv–EED, namely 4D5scFvZZ, was able to bind the HER2/neu receptor proteins that were either immobilized on Biacore chips or expressed on the cell surface.18 One structural component in the scFv–EED is the EED feature, which was originally derived from the immunoglobulin Fc binding unit of the staphylococcal protein A (SPA), and is designed to capture circulating immunoglobulins to promote antibody effector functions.18
We recombinantly fused the human IFNγ to the C-terminus of the 4D5scFvZZ. The recombinant protein 4D5scFvZZ-IFNγ was expressed in bacteria and purified to confirm its binding activity for HER2/neu. A control construct without EED was also generated (4D5scFv-IFNγ). We performed FACS analysis on T6–17 cells, which are mouse fibroblasts overexpressing human HER2/neu receptor.18 As shown in Fig. 1A, both 4D5scFvZZ-IFNγ and 4D5scFv-IFNγ were able to bind T6–17 cells, indicating that both constructs were properly folded to obtain an active 4D5scFv binding unit.
Figure 1.
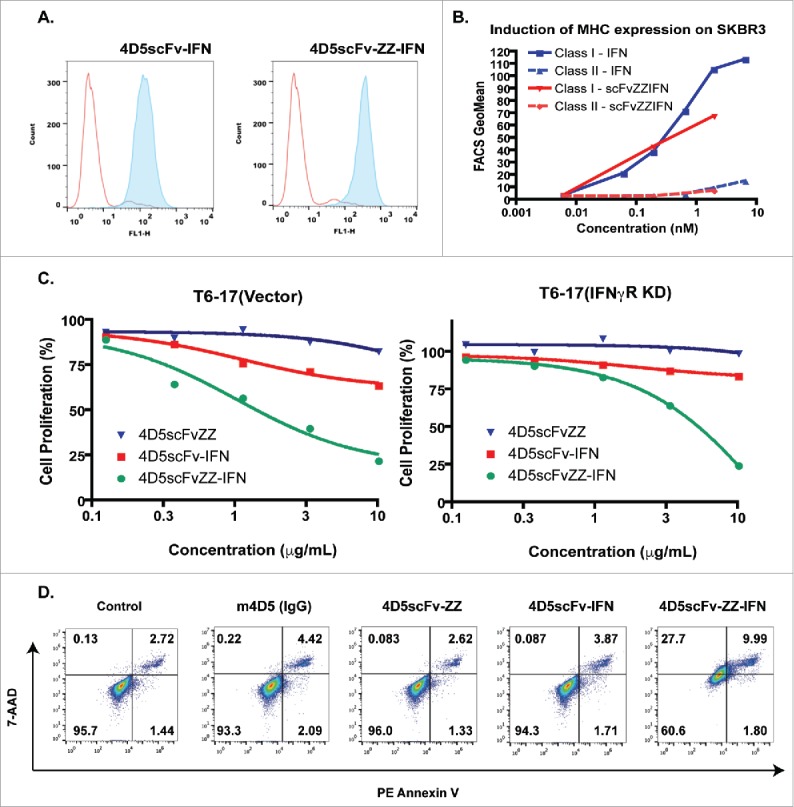
In vitro activity of 4D5scFvZZ-IFNγ. (A) Binding to the target. Both 4D5scFvZZ-IFNγ and 4D5scFv-IFNγ bind to cell surface p185her2/neu. T6–17 cells with the expression of p185her2/neu were prepared for Fluorescence-activated cell sorting (FACS). Histograms represent staining with 0.5 μg of 4D5scFv-IFNγ or 4D5scFvZZ-IFNγ, as indicated in the figure, followed by His-Probe antibody and Alexa488-conjugated goat anti-rabbit antibodies (filled peak). The control staining (unfilled peak) was obtained with only the His-Probe antibody and the secondary antibody. (B) Effect of 4D5scFvZZ-IFNγ on MHC expression. SKBR3 cells were incubated with IFNγ or 4D5scFvZZ-IFNγ for 24 h at different doses. The expression levels of both class I and class II MHC antigens was analyzed by FACS using monoclonal antibodies W6/32 and L243, respectively. (C) Proliferation by MTT assay. 2000 T6–17(Vector) or IFNγR knocked-down T6–17(IFNγ R KD) cells were plated in 96-well plates and incubated with different concentrations of proteins for 72 h. Cell viability was determined by MTT assay as described in materials and methods. (D) 4D5scFvZZ-IFNγ induced apoptosis/necrosis. HER2/neu expressing T6–17 cells were treated with control, the antibody 4D5, 4D5scFvZZ, 4D5scFv-IFNγ, and 4D5scFvZZ-IFNγ (10 ug/mL each), for 2 d, then stained with Annexin V/7-AAD staining kit for FACS analysis. The lower and upper right quadrants represent early and late apoptotic cells, respectively. Only the 4D5scFvZZ-IFNγ treatment induced apoptosis/necrosis significantly.
IFNγ is known to induce class I MHC antigen expression in breast and ovarian cancer cells.19 To verify that the IFNγ subunit in the fusion protein is active, we examined its activity on MHC expression in SKBR3, a human breast cancer cell line overexpressing HER2/neu. As shown in Fig. 1B, 4D5scFvZZ-IFNγ and free IFNγ were both able to induce the expression of class I MHC. Neither IFNγ nor 4D5scFvZZ-IFNγ had any effect on class II MHC antigen expression. Therefore, the engineered Fc domain fusion protein was confirmed to mediate defined IFNγ-related activities.
The EED contributes to the anti-proliferative activity of 4D5scFvZZ-IFNγ
Human HER2/neu expressing T6–17 cells were used to study the anti-proliferative activity of the IFNγ scFv–EED fusion protein. To confirm that the activity of the fusion protein is through IFNγ signaling in the transformed cells, we transfected shRNA to knock down the IFNγ receptor and established the T6–17(IFNγR KD) cell line. A control cell line, T6–17(vector) was generated with the empty shRNA vector. As shown in Fig. 1C, 4D5scFvZZ-IFNγ demonstrated dose-dependent activity to limit the proliferation of both cell lines, but it was more active in T6–17(vector) with the intact receptor. The calculated IC50 for 4D5scFvZZ-IFNγ to inhibit T6–17(vector) and T–17(IFNγR KD) was 2.46 μg/mL and 5.04 μg/mL, respectively. Since the IFNγ receptor expression in T6–17(IFNγR KD) cells is only diminished, but not totally eliminated,17 the two-fold change in IC50 suggests that the anti-proliferative activity of 4D5scFvZZ-IFNγ is influenced by the IFNγ receptor level on tumor cells.
We observed that 4D5scFvZZ-IFNγ caused ∼75% inhibition of cell proliferation at 10 μg/mL in both cell lines, an activity that was superior to that of either 4D5scFvZZ or 4D5scFv-IFNγ. In the proliferation assay, although the scFv–IFNγ fusion (4D5scFv-IFNγ) had better activity than the original 4D5scFvZZ species,18 the combination of the EED and IFNγ in one construct exhibited the highest inhibition activity.
To determine if the fusion protein caused apoptosis/necrosis in tumor cells, we performed 7-aminoactinomycin D (7-AAD) and PE-conjugated Annexin V staining, and analyzed the stained cells by FACS (Fig. 1D). We noticed a substantial reduction in the frequency of live cells (bottom left quadrant) after cells were treated for 2 d with 4D5scFvZZ-IFNγ (60.6% vs. 95.7% in control). Treatment by other agents, including the anti-HER2/neu antibody m4D5, failed to demonstrate a significant reduction in live cell population. Cell death induced by 4D5scFvZZ-IFNγ was determined to be primarily necrotic, as the staining of the entire cell population was shifted to upper right quadrant (Fig. 1D).
In vivo activity of 4D5scFvZZ-IFNγ
We next examined if 4D5scFvZZ-IFNγ could have better in vivo activity than the original 4D5scFvZZ species, which had been studied in the T6–17 tumor model.18 As shown in Fig 2A, 4D5scFvZZ-IFNγ had better activity than 4D5scFvZZ in limiting the growth of T6–17 tumors. In addition, 4D5scFvZZ-IFNγ was able to dose-dependently reduce the growth of implanted tumors (Fig. 2B). The humanized anti-HER2/neu bivalent antibody 4D5 (trastuzumab) was used in these experiments as a positive control. In both experiments, the activity of 4D5scFvZZ-IFNγ was superior to that seen with 4D5.
Figure 2.
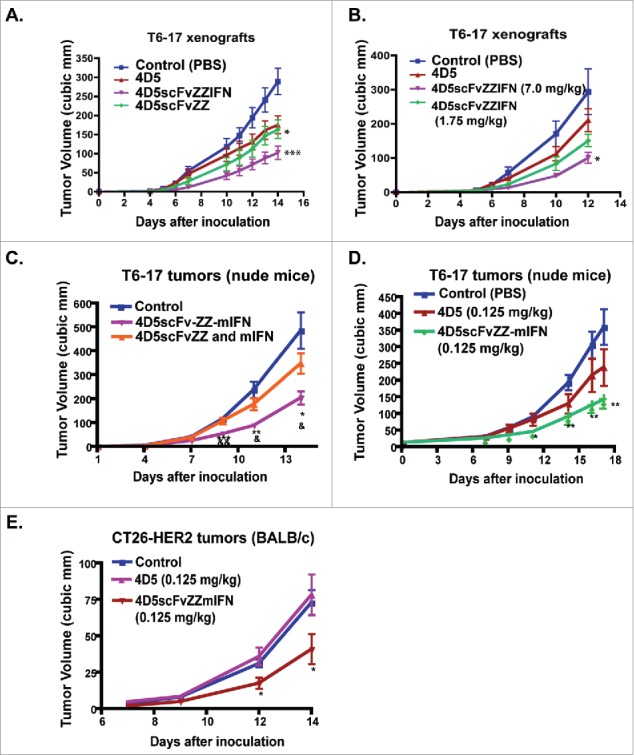
In vivo activity of scFv–EED–IFNγ on the growth of xenografted tumors. (A & B) In vivo activity of 4D5scFvZZ-IFNγ. (A) 4D5scFvZZ-IFNγ has better activity than 4D5scFvZZ. T6–17 tumor cells (5 × 105) were injected subcutaneously into both sides of the back of 6∼10-week old female athymic nude mice. Tumors were palpable 5 d after the inoculation of transformed T6–17 cells. Mice were treated with control (PBS), 4D5 mAb (1 mg/kg, twice; then 7 mg/kg twice, for a total of four treatments in 2 weeks), 4D5scFvZZI-IFNγ (7 mg/kg, five times per week), or 4D5scFvZZ (7 mg/kg, five times per week). Tumor growth in the 4D5scFvZZ-IFNγ group was highly suppressed compared with other groups. (B.) Dose-dependent activity of 4D5scFvZZ-IFNγ. Mice were treated with control (PBS), 4D5 mAb (1 mg/kg, twice per week), or 4D5scFvZZ-IFNγ (7 mg/kg or 1.75 mg/kg; five times per week). Tumor growth was dose-dependently suppressed by 4D5scFvZZ-IFNγ. (C) 4D5scFvZZ-mIFNγ has better activity than the combination of 4D5scFvZZ and free mouse IFNγ. Doses for each construct: 4D5scFvZZ-mIFNγ: 0.05 mg/kg; 4D5scFvZZ: 0.05 mg/kg; mIFNγ: 0.015 mg/kg. The dose of mIFNγ was adjusted to contain the equal molar amount of IFNγ as the 4D5scFvZZ-mIFNγ. (D) 4D5scFvZZ-mIFNγ has better activity than the HER2/neu antibody 4D5. For both C & D, T6–17 tumor cells (5 × 105) were injected subcutaneously into both flanks of the back of 6–10 weeks nude mice. Treatment started the next day after tumor implantation. (E) In vivo activity of 4D5scFvZZ-mIFNγ on 4D5 resistant tumors. CT26-HER2 tumor cells (1 × 106) were injected subcutaneously into both sides of the back of 6–10 weeks BALB/c female mice. Treatment started the next day after tumor implantation. The dosages of each treatment are indicated in the chart. Treatments were administrated five times per week via i.p.. Data represent mean + SEM t-test (two-sided) was performed to compare the difference in the tumor size for different treatment groups. *p < 0.05, **p < 0.01, ***p < 0.001, compared with control. All experimental groups in A–D that were compared for statistical difference had unequal variance, except the “4D5scFvZZ-mIFN” and “4D5scFvZZ and mIFN” comparison, which and Fig. 2E had equal variance.
The in vivo activity of 4D5scFvZZ-IFNγ is greatly improved by replacing human IFNγ with the mouse homolog
In the previous study, we have shown that the synergistic action of anti-HRE2 antibody and IFNγ is dependent on the IFNγ receptor on tumor cells (Fig. 1C). It has been reported that IFNγ is species specific, and human IFNγ activity is minimal in mouse cell lines.20 The first construct 4D5scFvZZ-IFNγ is designed for use in human and it contains the human IFNγ sequence. Both human IFN-Rα and IFN-Rβ would be required to render mouse cells to be fully responsive to human IFNγ.20
To better assess the in vivo activity in the mouse model, we constructed 4D5scFvZZ-mIFNγ that carries the mouse IFNγ sequence. Compared with the construct containing human IFNγ, 4D5scFvZZ-mIFNγ had far more potent activity in the T6–17 tumor model and we could use the protein at a much lower dosage. We found that the fusion protein was superior to the co-treatment of 4D5scFvZZ and free IFNγ(Fig. 2C). Furthermore, we compared side-by-side the activity of 4D5scFvZZ-mIFNγ and the 4D5 antibody, both at a low dose of 0.125 mg/kg and five times per week via i.p. injection. As shown in Fig. 2D, 4D5scFvZZ-mIFNγ had much better activity than 4D5 in limiting the growth of T6–17 tumors. In our experience with the T6–17 model, the activity we observed for the fusion protein at such a low dose is comparable to that of 4D5 at the much higher dose (5–10 mg/kg).
4D5scFvZZ-mIFNγ is active on 4D5-resistant tumors
Previously, CT-26-HER2 was established by engineering the BALB/c syngeneic tumor line CT-26 to express human HER2/neu.21 CT-26-HER2 has been confirmed to be a resistance model for HER2/neu antibody therapies as it carries the oncogenic K-RasG12D mutation.22,23 To investigate if 4D5scFvZZ-mIFNγ mediates activity against this resistant tumor line, we compared it with 4D5 in BALB/c mice carrying implanted CT-26-HER2 tumors. As shown in Fig. 2E, while 4D5 treatment had no effect on the in vivo CT-26-HER2 tumors, 4D5scFvZZ-mIFNγ was able to significantly reduce the tumor growth.
The in vivo activity of 4D5scFvZZ-mIFNγ is less affected by the expression level of the IFNγ receptor in tumor cells
In the in vitro proliferation assay (Fig. 1C), the activity of the 4D5scFvZZ-mIFNγ was reduced when the IFNγ receptor levels in the transformed cell were diminished by shRNA. However, the in vivo activity of the fusion protein is less dependent on the IFNγ receptor status in the tumor cells. As shown in Fig. 3A and B, even in T6–17(IFNγR KD) tumors with diminished expression of the IFNγ receptor, 4D5scFvZZ-mIFNγ clearly showed good activity comparable to that in the control T6–17(vector) tumors. This study suggests that the slightly reduced anti-proliferation activity of 4D5scFvZZ-mIFNγ toward tumor cells with reduced IFNγ receptor levels is compensated in vivo by the host immune response that IFNγ can induce. The IFNγ receptor-independent in vivo activity of scFv–EED–IFN supports this new engineering strategy for patients with tumors with low IFNγ receptor expression.
Figure 3.
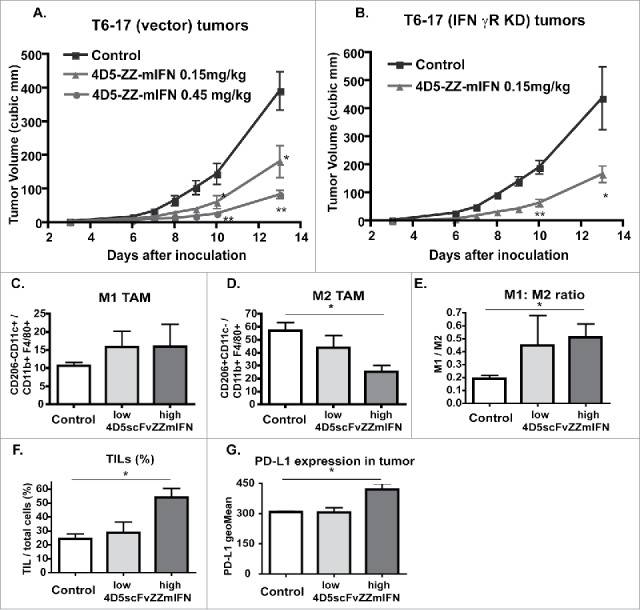
Effect of 4D5scFvZZ-mIFNγ on host immune cells. (A & B) In vivo activity of 4D5scFvZZ-mIFNγ is independent on the IFNγ receptor level. T6–17(Vector) or T6–17(IFNγR KD) tumor cells (5 × 105) were injected subcutaneously into the back of 6 ∼10 week old female nude mice. The next day, mice were treated with control or 4D5scFvZZ-mIFNγ, five times per week. t-test (two-sided) was performed to compare the difference in the tumor size of different groups. (A) At the end of the experiment, tumors in the low dose group were significantly smaller than those in the control group (*p < 0.05, equal variance). Tumors in the high dose group were very significantly smaller than those in the control group (**p < 0.01, unequal variance). (B) Tumors in the treatment group were significantly smaller than those in the control group (*p < 0.05, unequal variance). (C–G) At the end of the treatments, tumors from mice in Fig. 3A were collected and examined for macrophages (C D & E) and TILs (F). (G) Expression levels of PD-L1 in the tumors after treatments. Compared with the control group, tumors in the mice treated with high dose 4D5scFvZZ-mIFN had less M2 TAM, higher M1:M2 ratio, and more TILs (two tail t-test, equal variance, p < 0.05). In addition, there was a significant difference in the PD-L1 expression levels between treatment and control groups (one-way ANOVA, equal variance, p < 0.05). Data represent mean + SEM.
Previously, we had reported that, in the treatment of HER2 tumors in the syngeneic MMTVneu transgenic mice, the combination of IFNγ and anti-HER2/neu antibody induced the shift of tumor associated macrophages (TAMs) from M2 to M1 and reduced MDSC infiltration into tumors.17 Here, we want to find out whether the treatment with the new fusion protein also induced similar changes. The T6–17(vector) tumors at the end of treatments were isolated and analyzed for TAMs and tumor infiltrating lymphocytes (TIL) (Fig. 3). Treatment of 4D5scFvZZ-mIFNγ slightly increased M1 types of TAM and clearly reduced M2 type in a dose-dependent manner. As a result, the M1:M2 ratio was increased after the treatment. In addition, the treatment significantly increased CD45+ TILs in the high dose group. We also observed modestly increased PD-L1 levels in tumors treated with 4D5scFvZZ-mIFNγ, which is in consistent with the known mIFNγ effect on the expression of this checkpoint molecule (Fig. 3).
Discussion
There is evidence that ADCC plays a role in the clinical activity of HER2-targeted antibody therapy. In a study of 18 HER2+ operable breast cancer cases, 15 patients (83%) showed a trastuzumab-induced ADCC activity.24 One patient with strong ADCC activity had complete pathologic response to the treatment, while four patients with intermediate induced ADCC activity had partial response. All three patients with no induced ADCC had significant tumor regression. In addition, objective response rate (ORR) and progression-free survival (PFS) after trastuzumab-based therapies are reported to be correlated with the 158 V/V genotype of the Fc receptor FcγRIIIa, which demonstrates a significantly higher trastuzumab-mediated ADCC than other genotypes.25-27
However, studies also show that ADCC is weakened over time in adjuvant and metastatic breast cancer patients as compared with healthy controls.28 This is in contrast to the trastuzumab-induced ADCC observed in the neoadjuvant setting, which only lasts about 5 week.24 It is speculated that long time trastuzumab treatment leads to an immune suppressive mechanism that inhibits ADCC. Therefore, an immunotherapy that limits the ADCC inhibition may enhance the clinical activity of trastuzumab.
For solid tumors, one immunosuppressive mechanism is the hypoxia-driven adenosine accumulation in the tumor microenvironment. Through the A2A adenosine receptor, adenosine can prevent T cells and NK cells from killing tumor cells. A2AR antagonists have been tested to prevent the inhibition of antitumor T cells and NK cells.29 Another approach is to change immune profiles of tumor microenvironment with immune-stimulatory cytokines. In a clinical trial with trastuzumab plus IL-12 in metastatic breast cancer patients, sustained production of IFNγ and other cytokines was associated with response to the treatment.30
Ever since the prototype of the anti-HER2/neu antibody was developed 30 y ago,3 we and others have tried to produce engineered peptides or proteins that might provide even better therapeutic activity.18,31,32 The IFNγ scFv–EED now represents the first engineered antibody-like protein that demonstrates superior in vivo activity. Endowed with multiple capabilities including disabling the receptor target, promoting the infiltration of T cells, and shifting TAMs to M1 type, this new species of mAb-like protein is active on trastuzumab- resistant tumors. The fusion protein approach is an improvement from our recent observation that IFNγ and anti-HER2/neu antibody synergistically inhibit in vivo tumor growth.17 By linking IFNγ recombinantly to scFv–EED, this new therapeutic protein is able to target the IFNγ effect toward the tumor and reduce the unwanted systemic activity of this cytokine.
Currently, immunocytokines in clinical development are mostly in the format of fusion protein with either antibodies or antibody fragments.33 While antibody-cytokine fusion proteins generally have longer in vivo half-lives due to binding to neonatal Fc receptors, their tissue penetration into solid tumor is less than optimal.34 In contrast, fusion proteins with smaller antibody fragments, such as scFv, tend to have better tumor penetration but shorter half-lives.35 In this study, we directly fused IFNγ to anti-HER2/neu scFv–EED. Since IgG binding domain was shown before to extend the plasma half-life of scFv fusion protein,36 this recombinant fusion protein is thought able to penetrate better into solid tumor with a prolonged in vivo half-life.
Bacterially expressed scFv–EED–IFNγ was used at a very low in vivo dose of 0.1–0.45 mg/kg. That is about 10–20-fold less than the usual dose for a recombinant protein in this type of experiment. The high potency of the molecule provides an exciting opportunity to dramatically reduce the medical cost for targeted cancer therapies. This is a very critical feature for this approach considering the problem and burden of the high cost of cancer drugs to the health care system.
In summary, the scFv–EED–IFNγ fusion protein represents a novel targeted immunotherapy. Its effects on TMAs repolarization and TILs infiltration indicate that this new type of molecules can fundamentally change the tumor microenvironment to support immune reactivity against tumors. We have started to generate similar species using scFvs targeting other oncogene encoded cancer-specific receptors. The 4D5scFvZZ-IFNγ fusion protein, once properly humanized, has the potential to be developed clinically as an immune-competent treatment of HER2/neu positive cancers.
Materials and methods
Cells and antibodies
T6–17, a gift from Dr JH Pierce, was derived from NIH3T3 by overexpressing the p185her2/neu receptor.37 CT26-HER2 was kindly provided by Drs Cristina Jaime-Ramirez and William Carson in OSUMC. The cell line was originally from Dr Sherie L. Morrison of UCLA. SKBR3 was obtained from the American Type Culture Collection. Authenticity of these cells was determined by confirming their known expression profiles for receptors using fluorescence-activated cell sorting (FACS) periodically. CT26-HER2 was cultured in RPMI 1640 media, plus L-glutamine with 10% FBS, 1% Antibiotic–Antimycotic (100X, ThermoFisher, Cat.# 15240062) and prophylactic Plasmocin (InvivoGen, Cat.# ant-mpp). All other cells were grown in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% heat inactivated fetal calf serum, L-glutamine (2mM), penicillin (100 U/mL) and streptomycin (100 mg/mL) at 37°C in a humidified 5% CO2 atmosphere. All cell lines are routinely checked to confirm the absence of mycoplasma. Both T6–17 and CT26HER2 were confirmed virus free by the IMPACT II test (IDEXX Bioresearch). Anti-class I and class II MHC antigens antibodies, FITC anti-human HLA-A,B,C antibody (clone W6/32, Cat.# 311403) and FITC anti-human HLA-DR antibody (clone L243, Cat.# 307603), respectively, were purchased from Biolegend (San Diego, CA).
Cell proliferation assay
To measure cell proliferation, we used the modified MTT assay as described previously.18,32 For T6–17 cells, exponentially growing cells were seeded at a density of 5,000 cells/well in 96-well plates. For each data point, five wells of cells were prepared for the same treatment to obtain the average value. The MTT assay was usually repeated at least once to confirm.
Flow cytometry assays (Fluorescence-activated cell sorting, FACS)
For the determination of cell surface expression of MHC receptors in response to IFNγ, cells were incubated with appropriate FITC labeled primary antibodies for 1 h on ice. After wash and collection, the cell pellets were resuspended in FACS buffer. 10,000 cells were run for each sample on a FACSCalibur™ flow cytometer and analyzed using the CellQuest software.
For apoptosis assay, T6–17 cells were treated for 2 d with 10 ug/mL of control antibody 9BJ5, 4D5, 4D5scFvZZ, 4D5scFv-IFNγ and 4D5scFvZZ-IFNγ, respectively. Cells were harvested and washed with phosphate-buffered saline (PBS), and then stained with PE-Annexin V and 7-AAD according to the manufacturer's instructions (eBioscience, San Diego, CA). Stained cells were analyzed with Accuri C6 flow cytometer (BD Biosciences) and the acquired FACS data were analyzed with FlowJo software (Tree Star, Ashland, OR).
For TILs, tumors were collected for single cell suspensions at 1 d after final treatment. Tumor tissues were cut and digested with Collagenase D (Roche, 1 mg/mL) and DNAse (Sigma, 1 mg/mL) in 2% FBS RPMI for 30 min. Then cells were filtered through a cell strainer (Falcon). Cell surface antigens were stained with anti-CD45, F4/80, CD11b, CD11c, CD206 and PD-L1 antibodies (Biolegend). Cells were analyzed with FACS LSR (BD Biosciences) and FACS data were analyzed with FlowJo software (Tree Star, Ashland, OR).
IFNγR knock down by shRNA
TRC mouse lentiviral shRNA clones targeting IFNγR1 and the control pLKO.1 were obtained from The Open Biosystems Expression Arrest™TRC Library (Thermo Scientific). Lentiviruses were produced by VairaSafe™ Lentivirus expression system (Cell Biolabs inc., San Diego, CA) as manufacturer's instructions. T6–17 cells were transfected with those lentiviruses and selected with 1 μg/mL puromycin and analyzed by FACS with anti-IFNγR antibody (Biolegend).
In vivo tumor studies
All mouse procedures were performed according to the guidelines and protocols approved by the IACUC of University of Pennsylvania. In general, we used four mice for each treatment group, and we routinely implanted two tumors on each mouse. Mice were randomly grouped and treated with various reagents as described in the text. Control mice were treated with PBS. Tumor size was measured with a Vernier caliper, and tumor volume was calculated by the formula: 3.14* length * width * height/6.
Statistical analysis
For our routine tumor study, the minimal number of tumors in each group is 6. By having at least six tumors in each group, the probability is 87% that the study will detect a treatment difference at a two-sided 0.05 significance level, if the true difference between treatments is two times the standard deviation. In our experience, this will allow us to detect a 40% reduction in the average tumor size of the treatment group as compared with the control group. All data expressed as mean ± s.e.m. Student's t-test and one-way analysis of variance (ANOVA) were used to compare two groups (GraphPad Prism 6, La Jolla, CA, USA). p-value < 0.05 was considered statistically significant. F-test was performed to check for variance.
Funding Statement
This work was supported by grants from the Breast Cancer Research Foundation and the National Institutes of Health to M.I.G. (R01CA089481, R01CA149425), grants from NIH to A.T. (R21EB018863, R21CA187657), and the DOD IDEA grant to S.S. (W81XWH-15–1–0362).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
Flow Cytometry was performed at the Abramson Cancer Center Flow Cytometry and Cell Sorting Shared Resource, a member of Path BioResource, in the Perelman School of Medicine of the University of Pennsylvania.
References
- 1.Schechter AL, Stern DF, Vaidyanathan L, Decker SJ, Drebin JA, Greene MI, Weinberg RA. The neu oncogene: an erb-B-related gene encoding a 185,000-Mr tumour antigen. Nature 1984; 312(5994):513-6; PMID:6095109; https://doi.org/ 10.1038/312513a0 [DOI] [PubMed] [Google Scholar]
- 2.Drebin JA, Stern DF, Link VC, Weinberg RA, Greene MI. Monoclonal antibodies identify a cell-surface antigen associated with an activated cellular oncogene. Nature 1984; 312(5994):545-8; PMID:6504162; https://doi.org/ 10.1038/312545a0 [DOI] [PubMed] [Google Scholar]
- 3.Drebin JA, Link VC, Stern DF, Weinberg RA, Greene MI. Down-modulation of an oncogene protein product and reversion of the transformed phenotype by monoclonal antibodies. Cell 1985; 41(3):697-706; PMID:2860972; https://doi.org/ 10.1016/S0092-8674(85)80050-7 [DOI] [PubMed] [Google Scholar]
- 4.Drebin JA, Link VC, Greene MI. Monoclonal antibodies reactive with distinct domains of the neu oncogene-encoded p185 molecule exert synergistic anti-tumor effects in vivo. Oncogene 1988; 2(3):273-77; PMID:2451200 [PubMed] [Google Scholar]
- 5.Drebin JA, Link VC, Weinberg RA, Greene MI. Inhibition of tumor growth by a monoclonal antibody reactive with an oncogene-encoded tumor antigen. Proc Natl Acad Sci U S A 1986; 83(23):9129-33; PMID:3466178; https://doi.org/ 10.1073/pnas.83.23.9129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nielsen DL, Kumler I, Palshof JA, Andersson M. Efficacy of HER2-targeted therapy in metastatic breast cancer. Monoclonal antibodies and tyrosine kinase inhibitors. Breast 2013; 22(1):1-12; PMID:23084121; https://doi.org/ 10.1016/j.breast.2012.09.008 [DOI] [PubMed] [Google Scholar]
- 7.Pazo Cid RA, Anton A. Advanced HER2-positive gastric cancer: current and future targeted therapies. Critical Rev Oncol/Hematol 2013; 85(3):350-62; PMID:23021388; https://doi.org/ 10.1016/j.critrevonc.2012.08.008 [DOI] [PubMed] [Google Scholar]
- 8.Dent S, Oyan B, Honig A, Mano M, Howell S. HER2-targeted therapy in breast cancer: a systematic review of neoadjuvant trials. Cancer Treatment Rev 2013; 39(6):622-31; PMID:23434074; https://doi.org/ 10.1016/j.ctrv.2013.01.002 [DOI] [PubMed] [Google Scholar]
- 9.Brown-Glaberman U, Dayao Z, Royce M. HER2-targeted therapy for early-stage breast cancer: a comprehensive review. Oncology (Williston Park) 2014; 28(4):281-9; PMID:24839797 [PubMed] [Google Scholar]
- 10.Scaltriti M, Chandarlapaty S, Prudkin L, Aura C, Jimenez J, Angelini PD, Sanchez G, Guzman M, Parra JL, Ellis C et al.. Clinical benefit of lapatinib-based therapy in patients with human epidermal growth factor receptor 2-positive breast tumors coexpressing the truncated p95HER2 receptor. Clin Cancer Res 2010; 16(9):2688-95; PMID:20406840; https://doi.org/ 10.1158/1078-0432.CCR-09-3407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chia SK, Speers CH, D'Yachkova Y, Kang A, Malfair-Taylor S, Barnett J, Coldman A, Gelmon KA, O'Reilly S E, Olivotto IA. The impact of new chemotherapeutic and hormone agents on survival in a population-based cohort of women with metastatic breast cancer. Cancer 2007; 110(5):973-9; PMID:17647245; https://doi.org/ 10.1002/cncr.22867 [DOI] [PubMed] [Google Scholar]
- 12.Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, Slamon DJ, Murphy M, Novotny WF, Burchmore M et al.. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol 2002; 20(3):719-26; PMID:11821453; https://doi.org/ 10.1200/JCO.2002.20.3.719 [DOI] [PubMed] [Google Scholar]
- 13.Merry CR, McMahon S, Forrest ME, Bartels CF, Saiakhova A, Bartel CA, Scacheri PC, Thompson CL, Jackson MW, Harris LN et al.. Transcriptome-wide identification of mRNAs and lincRNAs associated with trastuzumab-resistance in HER2-positive breast cancer. Oncotarget 2016; 7(33):53230-44; PMID:27449296; https://doi.org/ 10.18632/oncotarget.10637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park S, Jiang Z, Mortenson ED, Deng L, Radkevich-Brown O, Yang X, Sattar H, Wang Y, Brown NK, Greene M et al.. The therapeutic effect of anti-HER2/neu antibody depends on both innate and adaptive immunity. Cancer Cell 2010; 18(2):160-70; PMID:20708157; https://doi.org/ 10.1016/j.ccr.2010.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bianchini G, Gianni L. The immune system and response to HER2-targeted treatment in breast cancer. Lancet Oncol 2014; 15(2):e58-68; PMID:24480556; https://doi.org/ 10.1016/S1470-2045(13)70477-7 [DOI] [PubMed] [Google Scholar]
- 16.Stagg J, Andre F, Loi S. Immunomodulation via chemotherapy and targeted therapy: a new paradigm in breast cancer therapy? Breast Care (Basel) 2012; 7(4):267-72; PMID:23904828; https://doi.org/ 10.1159/000342166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagai Y, Tsuchiya H, Runkle EA, Young PD, Ji MQ, Norton L, Drebin JA, Zhang H, Greene MI. Disabling of the erbB pathway followed by IFN-gamma modifies phenotype and enhances genotoxic eradication of breast tumors. Cell Rep 2015; 12(12):2049-59; PMID:26365188; https://doi.org/ 10.1016/j.celrep.2015.08.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cai Z, Fu T, Nagai Y, Lam L, Yee M, Zhu Z, Zhang H. scFv-based “Grababody” as a general strategy to improve recruitment of immune effector cells to antibody-targeted tumors. Cancer Res 2013; 73(8):2619-27; PMID:23396586; https://doi.org/ 10.1158/0008-5472.CAN-12-3920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boyer CM, Dawson DV, Neal SE, Winchell LF, Leslie DS, Ring D, Bast RC Jr.. Differential induction by interferons of major histocompatibility complex-encoded and non-major histocompatibility complex-encoded antigens in human breast and ovarian carcinoma cell lines. Cancer Res 1989; 49(11):2928-34; PMID:2497969 [PubMed] [Google Scholar]
- 20.Hemmi S, Bohni R, Stark G, Di Marco F, Aguet M. A novel member of the interferon receptor family complements functionality of the murine interferon gamma receptor in human cells. Cell 1994; 76(5):803-10; PMID:8124717; https://doi.org/ 10.1016/0092-8674(94)90355-7 [DOI] [PubMed] [Google Scholar]
- 21.Jaime-Ramirez AC, Mundy-Bosse BL, Kondadasula S, Jones NB, Roda JM, Mani A, Parihar R, Karpa V, Papenfuss TL, LaPerle KM et al.. IL-12 enhances the antitumor actions of trastuzumab via NK cell IFN-gamma production. J Immunol 2011; 186(6):3401-9; PMID:21321106; https://doi.org/ 10.4049/jimmunol.1000328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castle JC, Loewer M, Boegel S, de Graaf J, Bender C, Tadmor AD, Boisguerin V, Bukur T, Sorn P, Paret C et al.. Immunomic, genomic and transcriptomic characterization of CT26 colorectal carcinoma. BMC Genomics 2014; 15:190; PMID:24621249; https://doi.org/ 10.1186/1471-2164-15-190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang B, Halder SK, Zhang S, Datta PK. Targeting transforming growth factor-beta signaling in liver metastasis of colon cancer. Cancer Letters 2009; 277(1):114-20; PMID:19147275; https://doi.org/ 10.1016/j.canlet.2008.11.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Varchetta S, Gibelli N, Oliviero B, Nardini E, Gennari R, Gatti G, Silva LS, Villani L, Tagliabue E, Menard S et al.. Elements related to heterogeneity of antibody-dependent cell cytotoxicity in patients under trastuzumab therapy for primary operable breast cancer overexpressing Her2. Cancer Res 2007; 67(24):11991-9; PMID:18089830; https://doi.org/ 10.1158/0008-5472.CAN-07-2068 [DOI] [PubMed] [Google Scholar]
- 25.Musolino A, Naldi N, Bortesi B, Pezzuolo D, Capelletti M, Missale G, Laccabue D, Zerbini A, Camisa R, Bisagni G et al.. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J Clin Oncol 2008; 26(11):1789-96; PMID:18347005; https://doi.org/ 10.1200/JCO.2007.14.8957 [DOI] [PubMed] [Google Scholar]
- 26.Musolino A, Naldi N, Dieci MV, Zanoni D, Rimanti A, Boggiani D, Sgargi P, Generali DG, Piacentini F, Ambroggi M et al.. Immunoglobulin G fragment C receptor polymorphisms and efficacy of preoperative chemotherapy plus trastuzumab and lapatinib in HER2-positive breast cancer. Pharmacogenomics J 2016; 16(5):472-7; PMID:27378608; https://doi.org/ 10.1038/tpj.2016.51 [DOI] [PubMed] [Google Scholar]
- 27.Gavin PG, Song N, Kim SR, Lipchik C, Johnson NL, Bandos H, Finnigan M, Rastogi P, Fehrenbacher L, Mamounas EP et al.. Association of polymorphisms in FCGR2A and FCGR3A with degree of Trastuzumab benefit in the adjuvant treatment of ERBB2/HER2-positive breast cancer: analysis of the NSABP B-31 trial. JAMA Oncol 2017;3(3):335-41; PMID:27812689; https://doi.org/ 10.1001/jamaoncol.2016.4884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petricevic B, Laengle J, Singer J, Sachet M, Fazekas J, Steger G, Bartsch R, Jensen-Jarolim E, Bergmann M. Trastuzumab mediates antibody-dependent cell-mediated cytotoxicity and phagocytosis to the same extent in both adjuvant and metastatic HER2/neu breast cancer patients. J Transl Med 2013; 11:307; PMID:24330813; https://doi.org/ 10.1186/1479-5876-11-307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hatfield SM, Sitkovsky M. A2A adenosine receptor antagonists to weaken the hypoxia-HIF-1alpha driven immunosuppression and improve immunotherapies of cancer. Curr Opin Pharmacol 2016; 29:90-6; PMID:27429212; https://doi.org/ 10.1016/j.coph.2016.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parihar R, Nadella P, Lewis A, Jensen R, De Hoff C, Dierksheide JE, VanBuskirk AM, Magro CM, Young DC, Shapiro CL et al.. A phase I study of interleukin 12 with trastuzumab in patients with human epidermal growth factor receptor-2-overexpressing malignancies: analysis of sustained interferon gamma production in a subset of patients. Clin Cancer Res 2004; 10(15):5027-37; PMID:15297404; https://doi.org/ 10.1158/1078-0432.CCR-04-0265 [DOI] [PubMed] [Google Scholar]
- 31.Park BW, Zhang HT, Wu C, Berezov A, Zhang X, Dua R, Wang Q, Kao G, O'Rourke DM, Greene MI et al.. Rationally designed anti-HER2/neu peptide mimetic disables P185HER2/neu tyrosine kinases in vitro and in vivo. Nat Biotechnol 2000; 18(2):194-8; PMID:10657127; https://doi.org/ 10.1038/72651 [DOI] [PubMed] [Google Scholar]
- 32.Masuda K, Richter M, Song X, Berezov A, Masuda K, Murali R, Greene MI, Zhang H. AHNP-streptavidin: a tetrameric bacterially produced antibody surrogate fusion protein against p185her2/neu. Oncogene 2006; 25(59):7740-6; PMID:16785990; https://doi.org/ 10.1038/sj.onc.1209745 [DOI] [PubMed] [Google Scholar]
- 33.List T, Neri D. Immunocytokines: a review of molecules in clinical development for cancer therapy. Clin Pharmacol 2013; 5:29-45; PMID:23990735; https://doi.org/ 10.2147/CPAA.S49231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jain RK, Baxter LT. Mechanisms of heterogeneous distribution of monoclonal antibodies and other macromolecules in tumors: significance of elevated interstitial pressure. Cancer Res 1988; 48(24 Pt 1):7022-32; PMID:3191477 [PubMed] [Google Scholar]
- 35.Yokota T, Milenic DE, Whitlow M, Schlom J. Rapid tumor penetration of a single-chain Fv and comparison with other immunoglobulin forms. Cancer Res 1992; 52(12):3402-8; PMID:1596900 [PubMed] [Google Scholar]
- 36.Hutt M, Farber-Schwarz A, Unverdorben F, Richter F, Kontermann RE. Plasma half-life extension of small recombinant antibodies by fusion to immunoglobulin-binding domains. J Biol Chem 2012; 287(7):4462-9; PMID:22147690; https://doi.org/ 10.1074/jbc.M111.311522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Di Fiore PP, Pierce JH, Kraus MH, Segatto O, King CR, Aaronson SA. erbB-2 is a potent oncogene when overexpressed in NIH/3T3 cells. Science 1987; 237(4811):178-82; PMID:2885917; https://doi.org/ 10.1126/science.2885917 [DOI] [PubMed] [Google Scholar]


