Key Clinical Message
Although the clinical significance of hypothyroidism in TAFRO syndrome is unknown, vascular endothelial growth factor (VEGF) levels decreased with improvements in the condition of our refractory TAFRO cases after thyroxine supplement therapy. Our results indicate that elevated VEGF levels are a potential factor in the pathogenesis and anasarca of TAFRO syndrome with hypothyroidism.
Keywords: Hypothyroidism, idiopathic multicentric Castleman disease, TAFRO syndrome, thyroid‐stimulating hormone, vascular endothelial growth factor
Introduction
Multicentric Castleman disease (MCD) is a rare disorder characterized by episodes of systemic inflammation, the reactive proliferation of morphologically benign lymphocytes, multicentric lymphadenopathy, polyclonal gammaglobulinemia, microcytic anemia, hypoalbuminemia, and elevated serum inflammatory proteins, such as C‐reactive protein (CRP), according to pathological hypercytokinemia 1. While human herpesvirus‐8 (HHV‐8) drives hypercytokinemia in a cohort of immunocompromised patients, the etiology of HHV‐8‐negative MCD is idiopathic MCD (iMCD) 2. TAFRO syndrome identifies a subset of iMCD patients with shared manifestations, including thrombocytopenia, anasarca, myelofibrosis, renal dysfunction, organomegaly, and typically normal immunoglobulin levels 3. Patients with TAFRO syndrome exhibit systemic inflammation, polyclonal lymphoproliferation, and a wide spectrum of symptoms caused by a cytokine storm often including interleukin (IL)‐6 and vascular endothelial growth factor (VEGF) 2, 4.
Vascular endothelial growth factor is an angiogenic and mitogenic substance that appears to be active in vascular endothelial cells and plays an important role in tumor growth and the metastatic process 5. VEGF is also a vascular permeability factor that induces a rapid and reversible increase in vascular permeability 5. The relationship between elevated VEGF levels and many pathological conditions, including POEMS syndrome, ovarian hyperstimulation syndrome, preeclampsia, and diabetes treated with troglitazone, has been demonstrated 6, 7, 8, 9. Moreover, a correlation has been reported between elevated serum VEGF and thyroid‐stimulating hormone (TSH) levels in patients with hypothyroidism 6. Although hypothyroidism is a common endocrine abnormality, its clinical significance in TAFRO syndrome remains unclear.
The etiology of TAFRO syndrome remains unknown, and a standard therapeutic strategy has not yet been established. Although all patients in previous case reports were treated with steroids and some improved with the addition of cyclosporine or tocilizumab with rituximab therapy, others relapsed or died. We herein report two cases of refractory TAFRO syndrome that were successfully treated with thyroxine supplements. Our cases indicate that even biochemical subclinical hypothyroidism causes severe clinical manifestations.
Case Report
Case 1
In March 2012, a 72‐year‐old female was referred to a local hospital with fever and dyspnea. She had been diagnosed with subclinical hypothyroidism 20 years previously and was given no treatment because she showed neither symptoms of hypothyroidism nor elevated TSH levels. Computed tomography (CT) findings revealed thyroid enlargement, mediastinal lymphadenopathy (<2 cm in diameter), bilateral pleural effusion, ascites, and splenomegaly (Fig. 1A and B). Mediastinal lymph node biopsy showed atrophic germinal centers, an expanded interfollicular zone, the proliferation of highly dense endothelial venules, and few plasma cells (Fig. 2A and B). Five days later, the patient was referred to our hospital due to the rapid deterioration of her general condition and the progression of thrombocytopenia and anemia.
Figure 1.
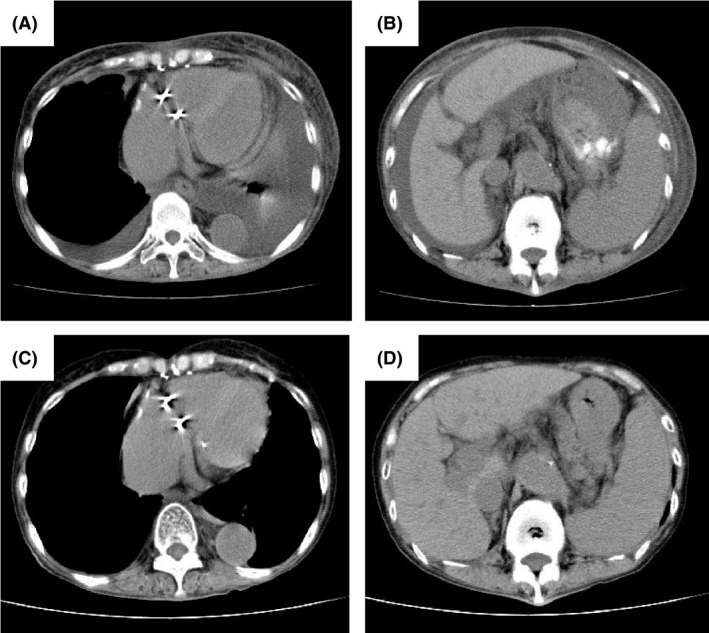
Computed tomography (CT) on admission and after treatment with thyroxine supplements in Case 1. (A, B) CT showing bilateral pleural effusion, ascites, and splenomegaly. (C, D) CT showing the disappearance of bilateral pleural effusion and ascites.
Figure 2.
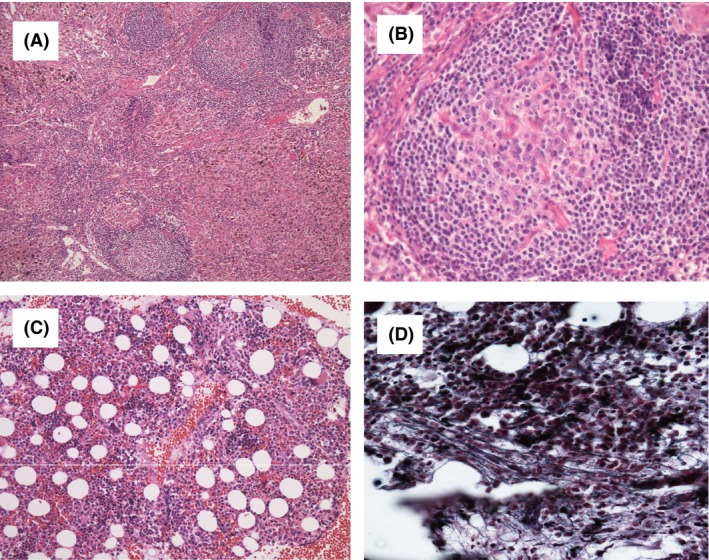
Histological findings of TAFRO syndrome lymph nodes (A, B) and bone marrow (C, D). (A) A biopsy specimen of a mildly enlarged lymph node shows atrophic centers and intact sinuses (Hematoxylin and Eosin staining, ×20). (B) The marked proliferation of high endothelial venules was observed in germinal centers and interfollicular zones (×40). (C) A biopsy specimen showing hypercellular marrow (×20). (D) Silver staining showing a very loose network of reticulin fibers (×200).
A physical examination revealed a temperature of 38.6°C, blood pressure of 104/64 mmHg, heart rate of 100 bpm, and SpO2 of 94%. Generalized edema was present. Her laboratory data showed microcytic anemia (hemoglobin, 9.6 mg/dL) and severe thrombocytopenia (platelets, 3.4 × 109/L) as well as elevated C‐reactive protein (CRP) (7.45 mg/dL, normal range: <0.3 mg/dL), alkaline phosphatase (ALP) (480 IU/L, normal range: 104–338 IU/L), and soluble interleukin‐2 receptor (sIL2R) (1300 U/mL, normal range: 145–519 U/mL) levels. Her serum IgG level was 1200 mg/dL (normal range: 870–1700 mg/dL) and monoclonal bands were not observed in immunofixation tests. Hypoalbuminemia, renal dysfunction with a creatinine level of 2.32 mg/dL (normal range: 0.4–0.8 mg/dL), and proteinuria were detected. Her thyroid function test revealed subclinical hypothyroidism, such as a slightly elevated TSH level (8.82 μIU/mL, normal range: 0.43–4.82 μIU/mL), while free T3 (FT3) (3.35 pg/mL, normal range: 2.39–3.86 pg/mL) and FT4 (1.10 ng/dL, normal range: 0.87–1.72 ng/dL) were both within normal ranges. Antithyroid peroxidase antibodies and antithyroglobulin antibodies were positive. Serum rheumatoid factor and antibodies including antinuclear, anti‐SS‐A, anti‐SS‐B, anti‐DNA, anti‐Sm, and anti‐RNP antibodies, the perinuclear antineutrophil cytoplasmic antibody, and cytoplasmic antineutrophil cytoplasmic antibody were all negative. Serum antibodies against hepatitis B and C viruses, HIV, human T‐lymphotropic virus‐1, and HHV‐8 were all negative. Her serum IL‐6 and VEGF levels were 21.2 pg/mL (normal range: <4.0 pg/mL) and 130 pg/mL (normal range: <38.3 pg/mL), respectively. Bone marrow aspiration revealed hypercellular marrow with myelofibrosis and an increased number of megakaryocytes without pathological cells or the dysplastic changes of hemophagocytosis (Fig. 2C and D). As her clinical features were considered to be compatible with TAFRO syndrome, steroid pulse therapy was initiated at 1 g/day of methylprednisolone for 3 days. Ten days later, fever, edema, pleural effusion, ascites, anemia, and renal dysfunction gradually improved with decreases in serum IL‐6 and VEFG levels (7.6 and 12 pg/mL, respectively). Her TSH level was slightly elevated (6.42 μIU/mL) and FT4 was within normal ranges (1.13 ng/dL, respectively). The dose of steroids administered was tapered to 5 mg/day of prednisolone. However, anemia, thrombocytopenia, and pleural effusion appeared 14 months after disease onset with an elevated VEGF level (173 pg/mL) and TSH level (7.21 μIU/mL). The administration of cyclosporine was initiated at 150 mg/day as second‐line therapy in combination with prednisolone. Fourteen days later, pleural effusion, anemia, and thrombocytopenia improved with decreases in serum VEGF levels (10 pg/mL). Her TSH level was slightly elevated (6.32 μIU/mL) and FT4 was within normal ranges (1.60 ng/dL).
Thirty‐six months after disease onset, severe relapse occurred. Her serum IL‐6 and VEGF levels were 15.0 and 186 pg/mL, respectively. Her TSH level was slightly elevated (8.40 μIU/mL) and FT4 was within normal ranges (1.30 ng/dL). Steroid pulse therapy was reinitiated at 1 g/day of methylprednisolone for 3 days, but was not effective. Therefore, the patient was administered weekly injections of the anti‐IL‐6 receptor antibody (tocilizumab 8 mg/kg) in combination with steroids. Despite anti‐IL‐6 therapy with steroids, renal dysfunction, anasarca, and thrombocytopenia persisted for 2 weeks. After informed consent was obtained for off‐label use, she received rituximab (375 mg/m2, day 1) plus cyclophosphamide (750 mg/m2, day 2), doxorubicin (50 mg/m2, day 2), vincristine (1.4 mg/m2, day 2), and prednisolone (50 mg/m2, days 2–6) (R‐CHOP) chemotherapy, and her CT findings markedly improved after one course. Anemia and renal dysfunction improved after four courses of R‐CHOP chemotherapy, whereas anasarca persisted.
Forty‐two months after disease onset, another severe relapse occurred. Her serum IL‐6 and VEGF levels increased (16.5 and 213 pg/mL, respectively). Although her FT4 level was within the normal range (1.04 ng/dL), her TSH level was elevated (20.6 μIU/mL). She was treated with R‐CHOP chemotherapy and thyroid hormone replacement therapy. Levothyroxine sodium was administered at a dose of 25 μg/day and then gradually increased to 100 μg/day. Renal dysfunction and anasarca gradually improved with a decrease in the level of VEGF (10.2 pg/mL) and the normalization of her TSH level (2.79 μIU/mL) (Fig. 1D and E). After four courses of R‐CHOP chemotherapy, the patient's condition improved and has remained stable for 4 months with no recurrence with the administration of levothyroxine sodium (100 μg/day).
Case 2
A previously healthy 67‐year‐old female was referred to our hospital in August 2017 with fever (between 37 and 38°C) for 14 days and dyspnea. Generalized edema was present. CT findings revealed cervical lymphadenopathy (<2 cm in diameter), bilateral pleural effusion, and ascites (Fig. 3A and B). Within 2 h, severe dyspnea with 90% SpO2 in 10 L of oxygen developed, and the patient had to be intubated and ventilated. She showed microcytic anemia (hemoglobin, 7.4 mg/dL) and thrombocytopenia (platelets, 2.3 × 109/L) as well as elevated CRP (25.5 mg/dL), ALP (650 IU/L), and sIL2R (1100 U/mL) levels. Her serum IgG level was 1120 mg/dL with no monoclonal bands and renal dysfunction was noted with a creatinine level of 3.46 mg/dL. Her thyroid function test revealed subclinical hypothyroidism, including a slightly elevated TSH level (8.40 μIU/mL), with decreased FT3 (0.35 pg/mL) and FT4 (0.60 ng/dL) levels. Antithyroid peroxidase antibodies and antithyroglobulin antibodies were positive. Serum antibodies against HHV‐8 were negative. Although anti‐SS‐A and Anti‐SS‐B antibodies were positive, she showed no symptoms. Her serum IL‐6 and VEGF levels were 6.5 and 246 pg/mL, respectively. Cervical lymph node biopsy showed atrophic germinal centers, an expanded interfollicular zone, the proliferation of highly dense endothelial venules, and few plasma cells (Fig. 4A and B). Bone marrow biopsy revealed hypercellular marrow with an increased number of megakaryocytes and myelofibrosis (Fig. 4C). TAFRO syndrome was suspected based on her clinical features. Steroid pulse therapy was initiated at 1 g/day of methylprednisolone for 3 days, but was not effective. Fourteen days after disease onset, the administration of cyclosporine was initiated at 150 mg/day as second‐line therapy. However, anasarca and hypotension (systolic blood pressure of 80 mmHg and diastolic blood pressure of 50 mmHg) deteriorated 30 days after disease onset. Levothyroxine sodium was initially administered at a dose of 25 μg/day and was gradually increased to 150 μg/day. Anemia, thrombocytopenia, renal dysfunction, anasarca, and hypotension gradually improved with a decrease in the level of VEGF (22 pg/mL) and the normalization of her TSH level (2.79 μIU/mL) (Fig. 3C and D). Three months after disease onset, the patient's condition improved and has remained stable with no recurrence with the administration of cyclosporine with levothyroxine sodium (150 μg/day).
Figure 3.
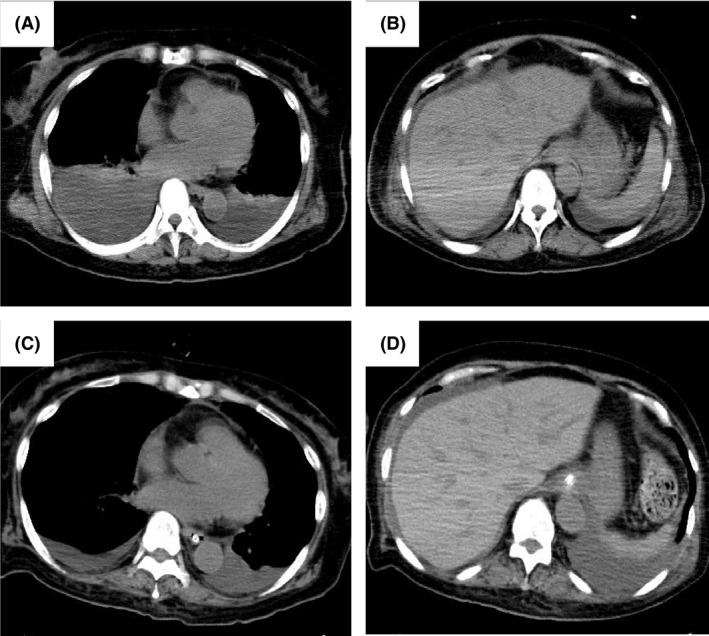
Computed tomography (CT) on admission and after treatment with thyroxine supplements in Case 2. (A, B) CT showing bilateral pleural effusion and ascites. (C, D) CT showing decreased bilateral pleural effusion and ascites.
Figure 4.
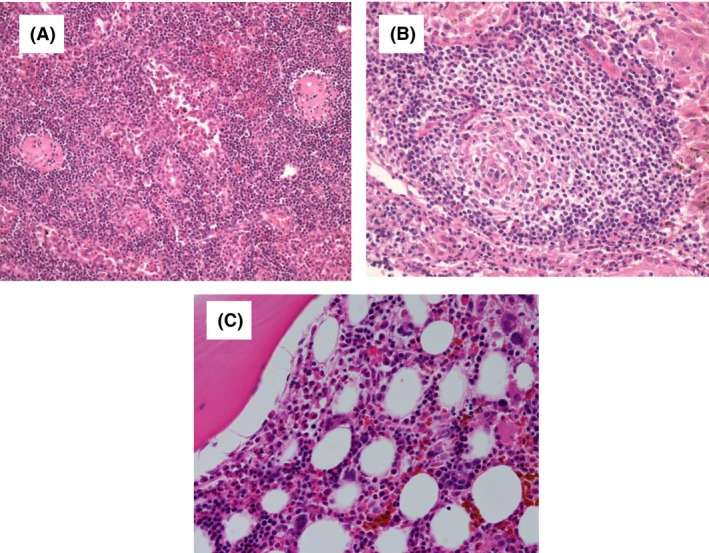
Histological findings of TAFRO syndrome lymph nodes (A, B) and bone marrow (C). (A) A biopsy specimen of a mildly enlarged lymph node shows atrophic centers and intact sinuses (Hematoxylin and Eosin staining, ×20). (B) The marked proliferation of high endothelial venules was observed in germinal centers and interfollicular zones (×40). (C) A biopsy specimen showing hypercellular marrow with fibrosis (×20).
Discussion
TAFRO syndrome identifies a subset of iMCD patients with heterogeneous clinical features, a characteristic lymph node histopathology, and often deadly multiple organ dysfunctions. Fajgenbaum et al. proposed that hypercytokinemia in iMCD is driven by one or more of the following mechanisms: autoimmune mechanisms, a germline genetic mutation in a gene involved in innate immunity, the paracrine secretion of cytokines such as IL‐6, VEGF, IL‐1, and tumor necrosis factor α, or a non‐HHV‐8 virus 2, 10, 11.
Although standard protocols have yet to be established, three treatment strategies have been employed 4: anti‐inflammatory and immunosuppressive therapies, the cytotoxic elimination of cells responsible for hypercytokinemia, and the blockade of IL‐6 signaling with monoclonal antibodies. Corticosteroids, cyclosporin, rituximab, cytotoxic lymphoma‐based chemotherapies (e.g., cyclophosphamide, doxorubicin, vincristine, and prednisone), and treatments directly targeting IL‐6 (e.g., tocilizumab and siltuximab) induce responses in a large number of iMCD cases; however, relapse is common, and these treatments are not effective in all patients 12, 13. The pathogenesis of TAFRO syndrome needs to be elucidated in more detail in order to provide additional candidate‐targeted therapies.
In patients with TAFRO syndrome, the positivity of autoantibodies is a characteristic clinical finding 14. Yu et al. 15 reported that 39% of iMCD patients had a history of autoimmune diseases, which were typically stable at the time of diagnosis. Furthermore, treatments resulted in the improvement or resolution of iMCD and the signs and symptoms of autoimmune connective tissue diseases. Based on the overlap between iMCD and autoimmune diseases, autoimmunity may be responsible for initiating or perpetuating the cytokine storm in iMCD via an autoantibody antigenic stimulation. Thus, hypothyroidism with the presence of autoantibodies suggests that autoimmunity is a pathological cause of TAFRO syndrome 16, 17.
Vascular endothelial growth factor induces a rapid and reversible increase in vascular permeability, and these functions may induce the development of clinical manifestations, such as ascites, pleural effusion, peripheral edema, and organomegaly 18. VEGF is expressed in a number of normal adult tissues, including the kidney, lung, uterus, ovary, brain, heart, skin, pituitary gland, and macrophages 19. It has been demonstrated in vitro that VEGF is produced by thyroid follicular epithelial cells in response to the stimulation of the TSH receptor 20, 21. Moreover, Sorvillo et al. 22 showed that TSH in vivo may regulate VEGF production from extrathyroidal tissues.
Although the clinical significance of hypothyroidism in TAFRO syndrome is unknown, VEGF levels decreased with improvements in the condition of our refractory TAFRO cases after thyroid hormone replacement therapy. A possible explanation for this is that VEGF levels may be increased by the prolonged stimulation of TSH. Secreted VEGF may then stimulate VEGF receptors on endothelial cells, leading to an increase in vascular permeability and the development of TAFRO syndrome.
Our results indicate that even biochemical subclinical hypothyroidism causes severe clinical manifestations. They also prompted us to speculate that elevated VEGF levels are a potential factor in the pathogenesis and symptomatology of TAFRO syndrome with subclinical hypothyroidism. The etiology, pathology, and strategies for the optimal management of this syndrome remain largely unknown. Therefore, further studies to clarify its prognosis, pathophysiology, and appropriate treatments are needed.
Conflicts of Interest
None declared.
Authorship
SO: was involved in patients care and writing the manuscript. KO: has reviewed and arranged the pathology images. MN: was responsible for the concept of this case report. None: performed statistical analysis and obtained funding.
Clinical Case Reports 2018; 6(4): 644–650
References
- 1. Iwaki, N. , Fajgenbaum D. C., Nabel C. S., Gion Y., Kondo E., Kawano M., et al. 2016. Clinicopathologic analysis of TAFRO syndrome demonstrates a distinct subtype of HHV‐8‐negative multicentric Castleman disease. Am. J. Hematol. 91:220–226. [DOI] [PubMed] [Google Scholar]
- 2. Liu, A. Y. , Nabel C. S., Finkelman B. S., Ruth J. R., Kurzrock R., van Rhee F., et al. 2016. Idiopathic multicentric Castleman's disease: a systematic literature review. Lancet Haematol. 3:e163–e175. [DOI] [PubMed] [Google Scholar]
- 3. Kawabata, H. , Takai K., Kojima M., Nakamura N., Aoki S., Nakamura S., et al. 2013. Castleman‐Kojima disease (TAFRO syndrome): a novel systemic inflammatory disease characterized by a constellation of symptoms, namely, thrombocytopenia, ascites (anasarca), microcytic anemia, myelofibrosis, renal dysfunction, and organomegaly : a status report and summary of Fukushima (6 June, 2012) and Nagoya meetings (22 September, 2012). J. Clin. Exp. Hematop. 53:57–61. [DOI] [PubMed] [Google Scholar]
- 4. Fajgenbaum, D. C. , van Rhee F., and Nabel C. S.. 2014. HHV‐8‐negative, idiopathic multicentric Castleman disease: novel insights into biology, pathogenesis, and therapy. Blood 123:2924–2933. [DOI] [PubMed] [Google Scholar]
- 5. Ferrara, N. , Carver‐Moore K., Chen H., Dowd M., Lu L., O'Shea K. S., et al. 1996. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 380:439–442. [DOI] [PubMed] [Google Scholar]
- 6. Dun, X. Y. , Zhou F., Xi H., Yuan Z. G., and Hou J.. 2009. Thyroid function and its clinical significance in POEMS syndrome. Leuk. Lymphoma 50:2013–2016. [DOI] [PubMed] [Google Scholar]
- 7. McClure, N. , Healy D. L., Rogers P. A., Sullivan J., Beaton L., Haning R. V. Jr, et al. 1994. Vascular endothelial growth factor as capillary permeability agent in ovarian hyperstimulation syndrome. Lancet 344:235–236. [DOI] [PubMed] [Google Scholar]
- 8. Baker, P. N. , Krasnow J., Roberts J. M., and Yeo K. T.. 1995. Elevated serum levels of vascular endothelial growth factor in patients with preeclampsia. Obstet. Gynecol. 86:815–821. [DOI] [PubMed] [Google Scholar]
- 9. Emoto, M. , Anno T., Sato Y., Tanabe K., Okuya S., Tanizawa Y., et al. 2001. Troglitazone treatment increases plasma vascular endothelial growth factor in diabetic patients and its mRNA in 3T3‐L1 adipocytes. Diabetes 50:1166–1170. [DOI] [PubMed] [Google Scholar]
- 10. Fajgenbaum, D. C. , Uldrick T. S., Bagg A., Frank D., Wu D., Srkalovic G., et al. 2017. International, evidence‐based consensus diagnostic criteria for HHV‐8‐negative/idiopathic multicentric Castleman disease. Blood 129:1646–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gherardi, R. K. , Bélec L., Fromont G., Divine M., Malapert D., Gaulard P., et al. 1994. Elevated levels of interleukin‐1 beta (IL‐1 beta) and IL‐6 in serum and increased production of IL‐1 beta mRNA in lymph nodes of patients with polyneuropathy, organomegaly, endocrinopathy, M protein, and skin changes (POEMS) syndrome. Blood 83:2587–2593. [PubMed] [Google Scholar]
- 12. Muskardin, T. W. , Peterson B. A., and Molitor J. A.. 2012. Castleman disease and associated autoimmune disease. Curr. Opin. Rheumatol. 24:76–83. [DOI] [PubMed] [Google Scholar]
- 13. Williams, S. C. 2013. First IL‐6‐blocking drug nears approval for rare blood disorder. Nat. Med. 19:1193. [DOI] [PubMed] [Google Scholar]
- 14. Masaki, Y. , Kawabata H., Takai K., Kojima M., Tsukamoto N., Ishigaki Y., et al. 2016. Proposed diagnostic criteria, disease severity classification and treatment strategy for TAFRO syndrome, 2015 version. Int. J. Hematol. 103:686–692. [DOI] [PubMed] [Google Scholar]
- 15. Yu, L. , Tu M., Cortes J., Xu‐Monette Z. Y., Miranda R. N., Zhang J., et al. 2017. Clinical and pathological characteristics of HIV‐ and HHV‐8‐negative Castleman disease. Blood 129:1658–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ozawa, T. , Kosugi S., Kito M., Onishi M., Kida T., Nakata S., et al. 2014. Efficacy of rituximab for TAFRO syndrome, a variant type of multicentric Castleman's disease. Rinsho. Ketsueki. 55:350–355. [PubMed] [Google Scholar]
- 17. Edahiro, Y. , Ichikawa K., Sunami Y., Koike M., and Komatsu N.. 2015. Autoimmune hemolytic anemia in a patient with TAFRO syndrome. Rinsho. Ketsueki. 56:2346–2350. [DOI] [PubMed] [Google Scholar]
- 18. Arimura, K. , Hashiguchi T., and Watanabe O.. 2008. Crow‐Fukase syndrome and VEGF. Brain Nerve. 60:611–619. [PubMed] [Google Scholar]
- 19. Ferrara, N. , Houck K., Jakeman L., and Leung D. W.. 1992. Molecular and biological properties of the vascular endothelial growth factor family of proteins. Endocr. Rev. 13:18–32. [DOI] [PubMed] [Google Scholar]
- 20. Sato, K. , Yamazaki K., Shizume K., Kanaji Y., Obara T., Ohsumi K., et al. 1995. Stimulation by thyroid‐stimulating hormone and Grave's immunoglobulin G of vascular endothelial growth factor mRNA expression in human thyroid follicles in vitro and flt mRNA expression in the rat thyroid in vivo. J. Clin. Invest. 96:1295–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Soh, E. Y. , Duh Q. Y., Sobhi S. A., Young D. M., Epstein H. D., Wong M. G., et al. 1997. Vascular endothelial growth factor expression is higher in differentiated thyroid cancer than in normal or benign thyroid. J. Clin. Endocrinol. Metab. 82:3741–3747. [DOI] [PubMed] [Google Scholar]
- 22. Sorvillo, F. , Mazziotti G., Carbone A., Piscopo M., Rotondi M., Cioffi M., et al. 2003. Recombinant human thyrotropin reduces serum vascular endothelial growth factor levels in patients monitored for thyroid carcinoma even in the absence of thyroid tissue. J. Clin. Endocrinol. Metab. 88:4818–4822. [DOI] [PubMed] [Google Scholar]


