ABSTRACT
Macrophages exhibit phenotypic heterogeneity under both physiological and pathological conditions. Applications targeting M2-like tumor-associated macrophages (TAMs) improve outcome in solid tumors. Considerable differences are detected between leukemia-associated macrophages (LAMs) and TAMs. However, application to induce M1 characteristics in heterogeneous LAMs has not been established. Here we analyzed clinical relevance of macrophage phenotypes in human acute myeloid leukemia (AML), studied phenotypic evolution of bone marrow (BM) and spleen (SP) LAMs in mouse AML and T cell acute lymphoblastic leukemia (T-ALL) models, explored mechanism leading to different LAM phenotypes and tried to eliminate pro-leukemic effects by inducing M1 characteristics. The results showed that more M2-like LAMs but not total LAMs correlated with worse prognosis in AML patients. Heterogeneity of LAM activation in tissue-specific leukemic microenvironments was observed in both AML and ALL models, i.e. SP LAMs evolved with more M2 characteristics while BM LAMs with more M1 characteristics. Furthermore, IRF7 contributed to M1 characteristics through the activation of SAPK/JNK pathway. Moreover, targeting IRF7-SAPK/JNK pathway to induce M1 characteristics in LAMs contributed to prolonged survival in leukemia mice. Our study provides the potential target for macrophage based immuno-therapy strategy against leukemia.
KEYWORD: IRF7, Leukemia-associated macrophages, Phenotypic heterogeneity, Phenotype conversion, SAPK/JNK pathway
Introduction
Macrophages are essential cellular components of tissue microenvironments and take part in diverse physiological and pathological processes.1 Plasticity and diversity are two essential hallmarks of macrophage differentiation and activation.2 In different microenvironments or upon diverse stimuli, macrophages are polarized to heterogeneous activation phenotypes, especially under pathological conditions.3 Typically, two well-established phenotypes are classically activated (M1) and alternatively activated (M2) macrophages, which represent two extreme poles of phenotypes upon IFNγ/LPS or IL-4/IL-13 stimulation, respectively.4 M1-associated genes, such as IL-12 and iNOS, are up-regulated through JAK-STAT1 pathway,5 whereas M2-associated genes, such as Arg1 and Mrc1, are up-regulated through JAK–STAT6 pathway.6 Nevertheless, M2 macrophages, which are further divided into M2a to M2d subpopulations, nowadays refer to all non-M1 macrophages.7 A growing panel of phenotype-associated genes have been reported in macrophage subpopulations.2 However, in vivo macrophage activation phenotype model is scant due to complexity and inadequate understanding,8 even though macrophages are classified as M1/M2 or M1/M2-like.9 In fact, most macrophages are signal-dependently polarized to a continuum of states between M1 and M2 poles rather than either M1 or M2 phenotype in vivo.10
Tumor associated macrophages (TAMs) show distinctive transcriptional profiles and characteristics from either typical M1 or M2 macrophages though they are considered as M2 macrophages.11 Despite the fact that TAMs promote progression and metastasis in most tumors by diverse mechanisms,12 phenotypic heterogeneity of TAMs has been reported.13,14 Infiltrating TAMs are consist of iNOS+/MHCIIhi M1-like and CD163+ M2-like subsets in colorectal cancer or non-small cell lung cancer (NSCLC) patients.15,16 M2-like TAMs with superior proangiogenic activity are enriched in hypoxic areas whereas M1-like TAMs are in perivascular areas in vivo.17,18 Increased counts of M1-like TAMs in either islets or stroma significantly correlate with prolonged survival in NSCLC.19 In general, TAMs with M1-like phenotype have anti-tumor effects whereas those with M2-like phenotype have pro-tumor effects.20 Hence, approaches have been explored to enhance anti-tumor activity of TAMs by depleting M2-like TAMs or converting TAMs from M2-like to M1-like phenotype.21-23
In hematopoietic malignancies, TAM count in lymphoma sections is suggested for risk stratification.24,25 Anti-CD47 treatment has therapeutic effects in lymphoma and leukemia.26 Compares with solid tumors and lymphoma, leukemia has different clinical features. We previously reported that leukemia associated macrophages (LAMs) from bone marrow (BM), spleen (SP), liver and peritoneal cavity in Notch1-induced mouse T cell acute lymphoblastic leukemia (T-ALL) showed considerable diversities in function and gene expression profiles.27-29 Acute myeloid leukemia (AML) cells polarized macrophages towards a leukemia supporting state.30 However, depleting all LAMs by Clodronate treatment has little effect on survival of leukemia mice,27 suggesting that it's necessary to eliminate pro-leukemic LAM subsets by either selective depletion or phenotypic conversion. However, the distribution of phenotypically diverse LAMs and molecular mechanism how pro-leukemic LAMs evolve in complex in vivo leukemic microenvironments during the development of leukemia have not been established.
Here we analyzed clinical relevance of macrophages in human AML samples, studied the evolution and phenotype of BM and SP LAMs in mouse leukemia models, explored mechanism leading to diverse phenotypes of LAMs from different leukemic microenvironments and tried to eliminate pro-leukemic effects of LAM by targeting signal pathway. We demonstrated that more M2-like macrophages correlated with worse prognosis in AML. SP leukemic microenvironments were more potent to induce M2 characteristics than BM. Furthermore, IRF7 contributed to M1 characteristics in BM LAMs through the activation of SAPK/JNK pathway. Moreover, inducing LAMs with more M1 characteristics contributed to prolonged survival in leukemia mice.
Materials and methods
Mice
C57BL/6J and C57B6.SJL mice were provided by the Animal Center of the Institute of Hematology and Blood Diseases Hospital, CAMS & PUMC. 6- to 8-weeks old female mice were used and maintained in the specific pathogen-free certified animal facility. Mice were randomly assigned to experimental groups. The procedures for the animal experiments were approved by the Animal Care and Use Committee at the institution.
Coculture assay
Coculture assay was used to test the effects of LAMs on leukemia cells. Control macrophages or LAMs from BM or spleen (5 × 104 cells/well) were cocultured with GFP+ AML cells (2 × 105 cells/well) isolated from BM or spleen of leukemia mice on day 20 for 24, 48 or 72 hrs. Cells were resuspended and harvested in PBS. Total cell number each well was counted by a Neubauer hemocytometer, and percentage of GFP+ cells was detected by flow cytometry. Absolute number of GFP+ AML cells each well was determined. BrdU incorporation assay and apoptosis assay were used to study proliferation and apoptosis of AML cells. For BrdU incorporation assay, 1 mM BrdU was added 3 hrs prior to harvest of cells. Then cells were stained with PerCP-cy5.5-labeled CD45.1 followed by BD Pharmingen™ APC BrdU Flow Kit (BD, CA) according to manufacturer's instructions. BrdU+ cells in CD45.1+ AML cells were analyzed by flow cytometry. For Apoptosis assay, cells were stained with PerCP-cy5.5-labeled CD45.1 followed by Annexin V antibody (Biolegend) and PI (Sigma-Aldrich) according to the manufacturer's instructions.
RNA sequencing (RNA-seq) and data analysis
Control macrophages and LAMs on day 15 were sorted by flow cytometry from BM and spleen samples. RNA-seq was carried out by the Beijing Genomics Institute following standard protocols. The RNA-seq data of AML LAMs are available in the National Center for Biotechnology Information Gene Expression Omnibus database under accession number GSE72803. RNA-seq data of control macrophages and LAMs in mouse T-ALL model were from GSE63751.27 Different expression genes (DEGs) were filtrated by FC ≥ 1.4 and FDR < 0.01. The expression pattern among LAMs was analyzed by Hierarchical Cluster normalized to peritoneal macrophages (GSE37236).31 K-Mean cluster, Gene Ontology and Pathway were analyzed by MeV 4.8.1, FunNet and KEGG, respectively.
Quantitative reverse-transcription polymerase chain reaction (qRT-PCR)
Total RNA was isolated using a Qiagen RNeasy Mini Kit (Qiagen) or Trizol agent. cDNA was synthesized using SuperScript III (Invitrogen). Real-time PCR was performed using an ABI-Prism 7900 Sequence Detector (Applied Biosystems, CA). The expression level of target genes was analyzed by the relative quantity (RQ) value calculated using the ΔΔCt method [ΔΔCt = (CtTARGET – CtGAPDH)sample – (CtTARGET – CtGAPDH)calibrator]. For each gene, the RQ value of control group was designated as 1.000. All primer sequences are listed in Table S1.
Effects of LPS, IL-4 or polyIC on macrophage cell lines
RAW-V and RAW-IRF7 cells were cultured in 24-well plates with LPS (Sigma-Aldrich, St. Louis) or IL-4 (PeproTech, NJ) while those without stimulation were set as controls. To test dose-dependent effects, cells were treated with 10, 100, 1000 ng/ml LPS or 10, 20, 40 ng/ml IL-4 for 24 hrs, respectively. To test time-dependent effects, cells were treated with 100 ng/ml LPS or 20 ng/ml IL-4 for 2, 6 and 24 hrs, respectively. RAW-SC, RAW-IRF7sh1 and RAW-IRF7sh2 cells were treated with 50 ug/ml polyIC (Roche) for 24 hrs.
Effects of repolarized LAMs with more M1 or M2 characteristics on the progression of leukemia
The JNK inhibitor SP600125 (Medchem Express, NJ) was used to induce M2 characteristics by blocking SAPK/JNK pathway in vitro and in vivo. PolyIC (Roche) was used to induce M1 characteristics by promoting IRF7 expression in vivo. RAW-IRF7 cells were treated with 10 µM SP600125 for 24 hrs. AML mice were i.p. administrated with SP600125 (15 mg/kg) or polyIC (10 mg/kg) on day 13 and 14 before sacrificed on day 15 for verification of induction effects. PolyIC induced LAMs were cocultured with leukemia cells to test their effects on the proliferation of leukemia cells. The targeting efficiency of SP600125 was tested by RAW264.7 in vitro and peritoneal macrophages in vivo by Western blot. For survival analysis, AML mice were i.p. administrated with same dose of SP600125 or polyIC on day 5, 10, 13, 16, 19 and 22. Peripheral blood (PB) leukemia cells were monitored by flow cytometry and survival time was recorded.
Two-dimensional illustration of macrophage phenotypes
Two-dimensional illustration was established to describe activation phenotype of macrophages based on their expression of M1- and M2-associated genes. For each gene, the value of control macrophages from BM was designated 0, respectively. The M1 and M2 values of a specific group of macrophages were calculated as follow:
Statistical analysis
Results were shown as means (± s.d.). Sample size (n) represents biological replicates. Comparisons between two groups were analyzed by unpaired Student's t test, whereas comparisons among multiple groups were analyzed by one-way ANOVA. Survival time was compared by Kaplan–Meier analysis. Analysis was done using the SPSS16.0 software package (SPSS, IL) or GraphPad Prism 5.0 (GraphPad Software, CA). Statistical significance was accepted when the P values are less than 0.05.
Additional experimental procedures are provided in Supplementary Experimental Methods.
Results
High level expression of CD163 correlate with poor prognosis in human AML
CD68 and CD163 are typical markers for macrophages and M2 macrophages in human.15,16 To assess the potential clinical relevance of macrophages in human AML, an open clinical database, BloodSpot,32 was used and the expression of CD68 and CD163 were analyzed in AML patients. The expression of CD68 and CD163 showed no significant difference between AML patients and healthy donors (Fig S1A). Among certain AML subtypes with abnormal karyotype, the expression pattern of them is different (Fig S1B, normalized to healthy control). However, patients in high level CD163 group had a shorter lifetime than those in low level CD163 group whereas no difference was detected for CD68, when all AML cases were subdivided into two groups based on their level of CD68 or CD163, respectively (Fig. 1A and Fig. 1B). These results suggest that more M2 macrophages rather than total macrophages may be a worst prognostic factor for AML patients.
Figure 1.
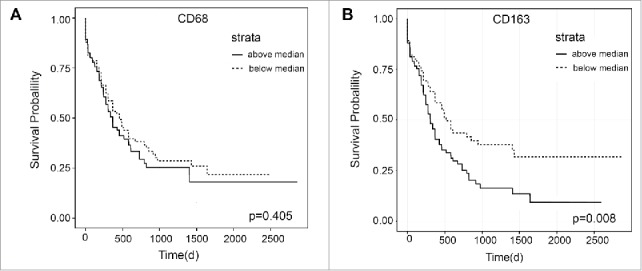
Expression of CD68 and CD163 in AML patients. The expression of CD68 and CD163 in AML patients was analyzed from open clinical database BloodSpot. The survival of AML patients were compared based on their expression of CD68 (A, n = 88 for high level group, n = 84 for low level group) or CD163 (B, n = 87 for high level group, n = 85 for low level group) by Kaplan–Meier analysis.
Activation phenotype of BM and SP LAMs in AML mice
To explore the mechanism, a non-irradiated MLL-AF9 induced mouse AML model with median survival time of 25 days after injection of AML cells was used.33 The experimental design is shown in Fig. 2A. Typical size of spleens as well as the phenotype and tissue infiltration of AML cells is shown in Fig S1C-S1E. The distribution of macrophages in BM and SP, the major hematopoietic sites in mice, was detected by flow cytometry (Fig. 2B for typical results). Macrophages in leukemic mice are defined as LAMs. Based on BM leukemia cells (Fig. 2C), we suggested that day 10, 15 and 20 were typical time points for early, middle and late stages of leukemia, respectively. With steady increase of leukemia cells, the percentage of LAMs in both BM and SP increased on day 10 and 15, then decreased on day 20 (Fig. 2C). The absolute number of BM LAMs increased on day 10, 15 but decreased on day 20, whereas that of SP LAMs increased on three time points due to splenomegaly (Fig. 2D).
Figure 2.
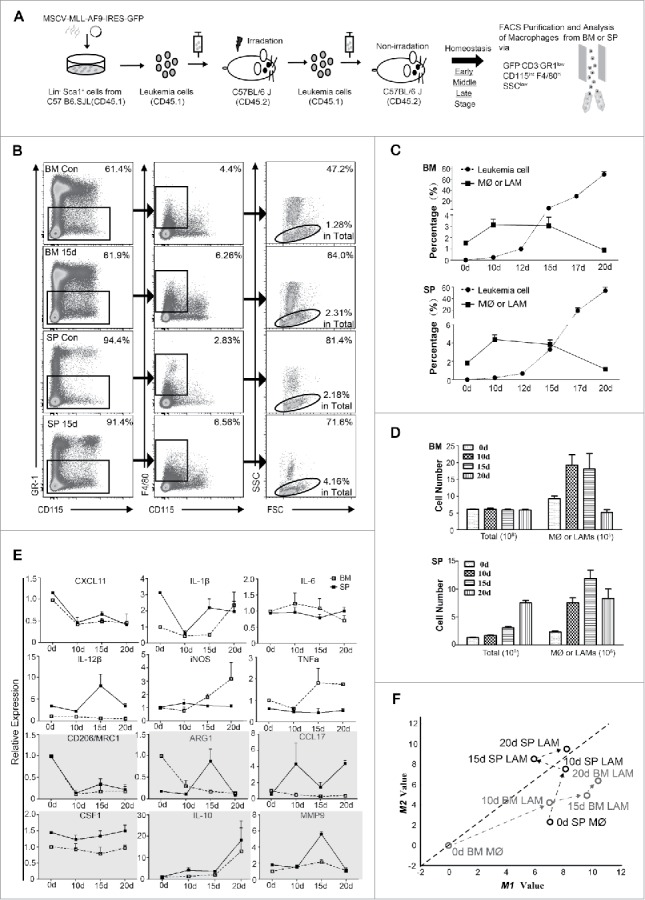
Distribution and phenotypic evolution of LAMs in BM and SP in MLL-AF9 induced mouse AML model. (A) Experimental design. (B) Macrophages from BM and SP were detected or sorted by flow cytometry as the Gr-1low F4/80+ CD115int SSClow subpopulation in GFP−CD3− population. Typical results on day 0 and day 15 are shown. (C) The dynamic distribution (in percentage) of macrophages and leukemia cells was analyzed by flow cytometry (n = 6 per group). (D) Cell counts of total cells and macrophages in BM and SP are plotted (n = 6 per group). (E) Mice were sacrificed at the indicated time points and macrophages were sorted. The expression of phenotype-associated genes was monitored during the development of AML by real-time PCR from three independent experiments. For each gene, the RQ value of control macrophage on day 0 was designated 1.000 (n = 4 per group). (F) Two-dimensional illustration of macrophage phenotype is shown. The evolution of LAMs is indicated by arrows. Values are given as means (±s.d.). Data are representative of at least three independent experiments.
To dynamically monitor the activation status of LAMs, BM and SP LAMs at different stages were sorted with over 95% purity (Fig S1F). Since no single gene, even iNOS or Arg1, can accurately define activation phenotype of macrophages2, 12 phenotype-associated genes (6 for M1 and 6 for M2) were included (Fig. 2E). Their expression varied between BM and SP LAMs. To give an intuitive view of phenotypic evolution, a two-dimensional illustration of macrophage phenotypes was developed. The M1 and M2 values of macrophage populations were determined by the expression of above genes. To simplify the model, each gene was assumed to have equal weight. It was interesting that SP LAMs moved to M2 area while BM LAMs remained in M1 area during the development of AML (Fig. 2F). These results suggest that SP LAMs have more M2 characteristics whereas BM LAMs have more M1 characteristics.
SP LAMs had more M2 functional characteristics than BM LAMs
To further investigate their functional characteristics, LAMs were sorted from BM or SP at middle stage and control macrophages from BM, SP or peritoneal cavity (PC) were sorted on day 0.
Coculture experiments demonstrated that SP LAMs had stronger growth promoting effects on AML cells than BM LAMs (Fig. 3A). Both SP and BM LAMs did not induce apoptosis of AML cells (Fig. 3B). However, more BrdU+ AML cells (absolute count) were detected when AML cells were cocultured with SP LAMs (Fig. 3C). These results indicate that SP LAMs are more effective to promote proliferation of AML cells than BM LAMs. Meanwhile, more apoptotic SP LAMs were detected in coculture systems than BM LAMs at 48 hrs and 72 hrs (Fig S2A and S2B).
Figure 3.
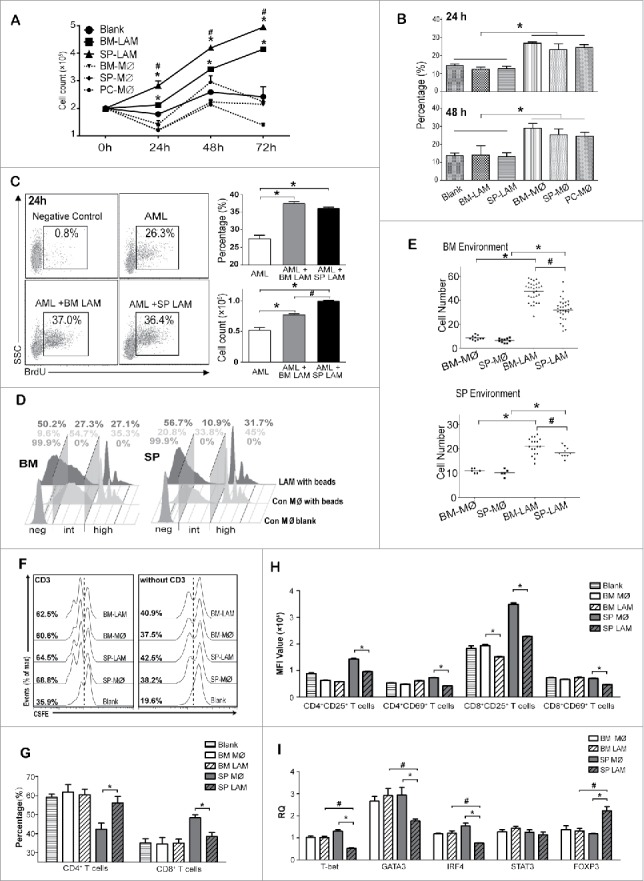
Functional characteristics of LAMs from AML model. (A-E) Control macrophages (day 0) were sorted from BM, SP and PC while LAMs (day 15) were sorted from BM and SP. (A-C) 2 × 105 leukemia cells were cocultured without or with 5 × 104 LAMs/control macrophages. GFP+ leukemia cells (A) and apoptotic rates of CD45.1+ leukemia cells (B) are plotted. (C) BrdU was added 3 hrs before harvest. Positive rates (upper) and absolute cell counts (lower) of BrdU+ leukemia cells are shown. (D) Control macrophages and LAMs were cultured with FITC labeled 2 µm latex beads for 15 mins and phagocytic activity was studied by flow cytometry. (E) 1 × 105 LAMs or control macrophages were seeded in the upper compartment of 8 μm transwell plate and 3 × 106 normal BM or SP cells were placed in the lower compartment for 24 hrs. After stained with crystal violet, macrophage counts from at least five random fields were obtained. Cells migrated into BM (upper) or SP (lower) compartment are plotted. (F-I) T cells from normal SP were labeled with CSFE and cocultured without or with control macrophages (day 0) or LAMs (day 15) in low serum medium for 72 hrs. (F) CFSE-labeled T cells with (left) or without (right) 5ug/ml coated CD3 were detected by flow cytometry. (G) CD4+ and CD8+ T cells in coculture system with CD3 were analyzed. (H) Expression of CD25 and CD69 in CD4+ or CD8+ T cells were detected by flow cytometry and shown in mean fluorescence intensities (MFI). (I) Expression of T cell differentiation-related genes in cocultured CD4+ T cells were detected by real time PCR. For each gene, the RQ value of CD4+ T cells without macrophage or LAM was designated 1.000. Values are given as means (±s.d.). * P<0.05, vs. respective control; # P<0.05, SP LAMs vs. BM LAMs (Student's t-test or ANOVA). Data are representative of at least three independent experiments.
Phagocytic potential of LAMs was studied. Compared with control, increase in negative population while decrease in intermediate and high positive populations were observed in both BM or SP LAMs (Fig. 3D).
Transwell experiments indicated that increased migration potential was detected in BM and SP LAMs. The ability of LAMs recruiting AML cells had no significant difference among different groups (Fig S2C). However, SP LAMs were significantly lower than BM LAMs (Fig. 3E and Fig S2D).
M2 macrophages have immune suppressing effects34. The effects of LAMs on T cell activation were studied. LAMs had little effect on total T cell proliferation (Fig. 3F). However, more CD4+ but less CD8+ T cells were detected in the presence of SP LAMs and CD3 (Fig. 3G and Fig S2E). Furthermore, SP LAMs down-regulated the expression of CD25 and CD69, which are up-regulated in activated T cells, in both CD4+ and CD8+ populations whereas BM LAMs down-regulated CD25 expression in CD8+ cells (Fig. 3H and Fig S2F). Collectively, LAMs, especially from SP, suppress T cell activation. Moreover, SP LAMs suppressed T-bet, GATA3 and IRF4 expression but enhanced FOXP3 expression in CD4+ T cells whereas BM LAMs had little effect (Fig. 3I). In addition, CD4+ T cells cocultured with SP LAMs had lower level T-bet, GATA3 and IRF4, but higher level Foxp3 than those cocultured with BM LAMs (Fig. 3I). These results suggest that SP LAMs are more potent to induce suppressive immune microenvironment than BM LAMs.
Taken together, our results suggest that SP LAMs have more M2-like functional characteristics than BM LAMs.
Gene expression profiles of SP LAMs and BM LAMs in different leukemia models
To further explore the mechanism, RNA-seq was performed on control macrophages, LAMs from middle stage of T-ALL (day 21, GSE63751) and AML (day 15, GSE72803) mice.
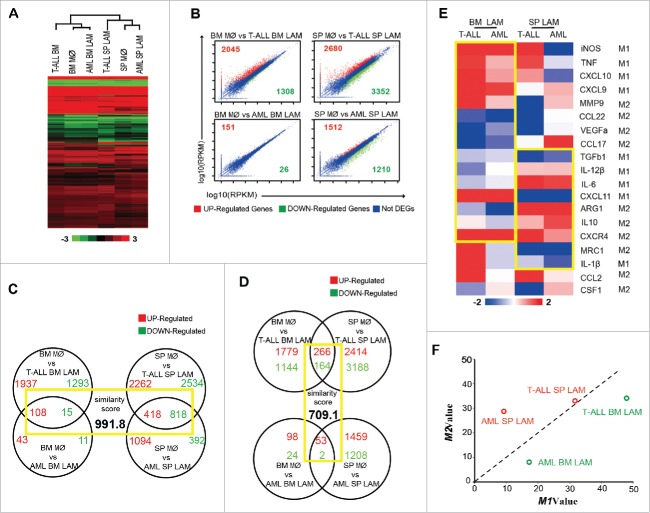
Hierarchical Cluster analysis was performed after normalized to data from peritoneal macrophages (GSE37236), showing that macrophages from the same origin (BM or SP) clustered and shared more similarities, suggesting that LAMs from a specific organ in different types of leukemia might have common features (Fig. 4A). To further understand the roles of leukemia microenvironment on gene expression in LAMs, DEGs were analyzed by the strategy that BM LAMs (no matter AML or T-ALL) were normalized to BM control macrophages while SP LAMs were normalized to SP control macrophages, respectively. Counts of DEGs in four LAMs samples are plotted in Fig. 4B. Total DEGs each sample were undergone GO and KEGG analysis. Top GO and KEGG annotations of four LAM samples are shown in Fig S3 and S4, respectively. It's interesting that two kinds of BM LAMs shared 15 GO annotations, such as regulation of leukocyte activation, positive regulation of immune system process and immune system process, etc. whereas two kinds of SP LAMs shared 2 GO annotations, i.e. binding and cellular process. Furthermore, KEGG results showed that Metabolic pathway was enriched in LAMs from T-ALL BM and SP as well as AML BM, MAPK signaling pathway was enriched in LAMs from AML BM and SP, and Cell cycle was enriched in LAMs from T-ALL BM and SP. To verify that LAMs from organ-specific leukemic microenvironment have similar gene expression signatures, an ordered list algorithm35 was used to quantify inter-microenvironmental or inter-leukemia model similarity. Higher scores, which are determined by gene ranks that are comparable and statistically significant, denote increased degree of similarity. It's worth noting that the similarity score in LAM samples with same microenvironment (991.8) (Fig. 4C) was higher than that with same type of leukemia (709.1) (Fig. 4D). Moreover, the expression of 19 genes, covering M1/M2 phenotype-associated genes reported in the literature, in different LAMs is shown in Fig. 4E. Similar pattern in expression was observed in 15 genes between BM LAM samples and in 9 genes between SP samples from two leukemia models. Most importantly, when the above 19 genes were taken into consideration, BM LAMs from both models were located in the M1 area whereas SP LAMs were located in the M2 area (Fig. 4F). These results suggest that SP LAMs have more M2 characteristics whereas BM LAMs have more M1 characteristics in leukemia models.
Figure 4.
Gene expression profiles of LAMs from AML and T-ALL models. Mice were sacrificed on day 0, day 15 in AML model and day 21 in T-ALL model. Macrophages were sorted by flow cytometry and RNAseq was performed. (A) Heat map shows gene expression profiles of LAMs from BM or SP of AML and T-ALL mice, which are normalized to PC macrophages (GSE37236). Hierarchical Cluster (HCL) was performed. (B) Comparison of gene expression between control macrophages and LAMs. FC≥1.4, FDR<0.01. (C-D) The Venn diagram illustrates number of overlapped genes (red for up-regulated and green for down-regulated) in LAMs. Overlaps of DEGs are highlighted and similarity score is provided. (E) Heat map shows the expression of phenotype-associated genes from RNA-seq data. (F) Two-dimensional illustration of macrophage phenotype is shown.
Molecular basis for different phenotypes of LAMs
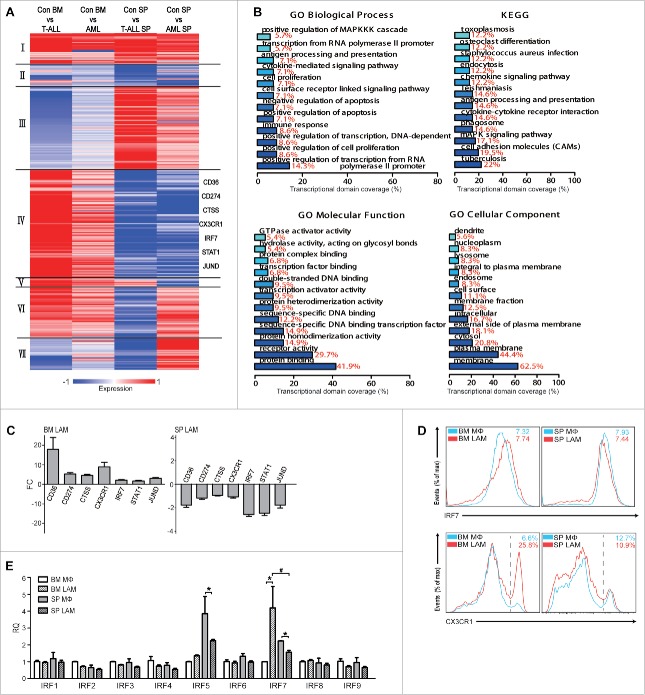
To study the molecular basis for different phenotypes of LAMs, DEGs in four LAM samples were screened and 303 genes were selected (Table S2). These genes were further clustered (K-Mean) into different categories based on their expression patterns among LAM samples (Fig. 5A). As expected, genes in categories I and II (27 and 20), identical pattern among LAMs, were less than those in categories III and IV (75 and 99), different patterns between BM and SP LAMs. These results further confirm that BM and SP LAMs modified by organ-specific microenvironments show more diversities than identities. Furthermore, the genes in categories IV were studied by GO and KEGG analysis. Enrichment of positive regulation of MAPKKK cascade, cytokine-mediated signaling pathway, transcription factor binding and receptor activity, etc. was found in GO annotations, and enrichment of cytokine-cytokine signaling pathway and MAPK signaling pathway was detected in KEGG pathways (Fig. 5B). Moreover, six genes (CD36, CD274, CTSS, CX3XR1, IRF7, STAT1 and JUND) in categories IV were validated by qRT-PCR (Fig. 5C) and two of them (IRF7 and CX3CR1) were further validated by flow cytometry (Fig. 5D) in AML model. IRF7 expression in LAMs from T-ALL model was also validated by qRT-PCR (Fig S5A). Since IRF7 belongs to the IRF family, the expression of all family members was also studied by qRT-PCR. Concomitant increase in BM LAMs but decrease in SP LAMs was only detected in IRF7 though decrease in SP LAMs was also observed in IRF5. In addition, BM LAMs expressed higher level IRF7 than SP LAMs (Fig. 5E). Together, these results indicate that the expression of IRF7 is reversely modulated in BM and SP LAMs, which may be a possible reason for their different phenotypes. Furthermore, MAPK pathway may also be involved.
Figure 5.
Screening for key molecules governing differential evolution of LAMs in BM and SP. (A) K-mean clustering of 303 DEGs in BM and SP LAMs from leukemic microenvironment in T-ALL and AML mice. (B) GO annotation and KEGG pathway analysis were performed on DEGs, which were up-regulated in BM LAMs but down-regulated in SP LAMs. Each bar represents a significant annotation (P<0.05). The x axis shows the transcriptional domain coverage. (C) The genes up-regulated in BM LAMs but down-regulated in SP LAMs were validated by real time PCR. Fold change of those was analyzed against control macrophages from BM (left) or SP (right), respectively. (D) Expression of IRF7 and CX3CR1 in BM and SP LAMs from AML mice was detected by flow cytometry. The means of MFI ( × 104) or positive rates (in percentage) are indicated. (E) Expression of IRF family genes were detected by real-time PCR. The RQ value of control macrophages from BM was designated as 1.000. Data represent means (±s.d.). * P<0.05, vs. respective control; # P<0.05, SP LAMs vs. BM LAMs (Student's t-test). Data are representative of at least three independent experiments.
IRF7 promoted M1 characteristics and activation of SAPK/JNK pathway in macrophages
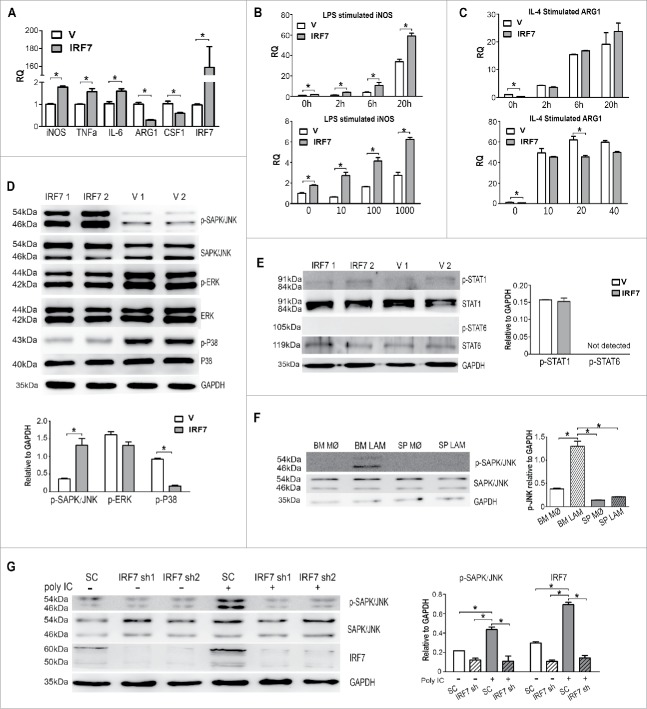
To investigate the roles of IRF7 on macrophages, RAW264.7 cells were infected with blank MSCV-GFP retrovirus or retrovirus carrying IRF7 (Fig S5B). After cell sorting, stably transfected cell lines, verified by RT-PCR (Fig. 6A) and Western blot (Fig S5C), were named RAW-V, RAW-IRF7, respectively. RAW-IRF7 expressed higher level of M1-associated genes (iNOS, TNFa and IL-6) but lower level of M2-realated genes (ARG1 and CFS1) than RAW-V (Fig. 6A). Furthermore, LPS stimulated the expression of iNOS, the classical M1 marker, while IL-4 stimulated the expression of ARG1, the classical M2 marker, in RAW-V and RAW-IRF7 in both time-and dose-dependent manners (Fig. 6B, 6C). Notably, only upon LPS stimulation, the increase in RAW-IRF7 was more dramatic than in RAW-V.
Figure 6.
IRF7 contribute to M1-like phenotypes through the activation of SAPK/JNK pathway. RAW264.7 cells were infected with blank retrovirus (RAW-V) or retrovirus encoding IRF7 (RAW-IRF7). (A) Expression of M1/M2 phenotype-associated genes and IRF7 in RAW-V and RAW-IRF7 cells were detected by real-time PCR. The RQ value of RAW-V was designated as 1.000. (B) Time-dependent (100 ng/ml) and dose-dependent (6 hrs) effects of LPS on iNOS expression were analyzed. (C) Time-dependent (20 ng/ml) and dose-dependent (6 hrs) effects of IL-4 on ARG1 expression were studied. The RQ value of RAW-V without stimulation was designated as 1.000. (D) Phosphorylation of SAPK/JNK, ERK1/2 and p38 was detected by Western blot. (E) Phosphorylation of STAT1 and STAT6 was detected by Western blot. (F) Phosphorylation of SAPK/JNK in LAMs (day 15) and control macrophages was detected. (G) Phosphorylation of JNK in RAW-SC, RAW-IRF7sh1 and RAW-IRF7sh2 cells with or without 50ug/ml polyIC stimulation was detected. Results were quantified by grey value analysis (Image J). Data represent means (±s.d.). * P<0.05, vs. respective control (Student's t-test). Data are representative of at least three independent experiments.
To figure out the involvement of MAPK pathways, which are strongly suggested by GO and KEGG analysis, Western blot was carried out. Higher level phosphorylation of SAPK/JNK but lower level phosphorylation of P38 was observed in RAW-IRF7 than RAW-V (Fig. 6D). However, no significant activation of STAT1 or STAT6, important for macrophage polarization,36 was detected (Fig. 6E). Higher level phosphorylation of SAPK/JNK was also detected in freshly isolated BM LAMs than SP LAMs (Fig. 6F).
To further verify high level IRF7 resulted in the activation of SAPK/JNK pathway, IRF7 knockdown cell lines, RAW-IRF7sh1 and RAW-IRF7sh2, were established and verified by RT-PCR (Fig S5D) and Western blot (Fig. 6G). PolyIC modulated the expression of IRFs, especially up-regulated the expression of IRF3 and IRF7.37 PolyIC induced up-regulated expression of IRF7 in RAW-SC and activation of SAPK/JNK, which were blocked by knockdown of IRF7 in RAW-IRF7sh1 and RAW-IRF7sh2 cells (Fig. 6G). A specific SAPK/JNK pathway inhibitor, SP600125, was also used to elucidate the involvement of SAPK/JNK pathway in the regulation of macrophage phenotype by IRF7. SP600125 effectively lowered down the phosphorylation level of SAPK/JNK at the dosage of 10 µM in vitro (Fig S5E) and at the dosage of 15mg/kg in vivo (Fig S5F). SP600125 had no effect on the expression of IRF7 in vitro and in vivo (Fig S5G). It significantly down-regulated M1-associated genes but up-regulated M2-associated genes in RAW-IRF7 (Fig S5H).
These results suggest that IRF7 promotes M1 characteristics in LAMs through the activation of SAPK/JNK pathway.
Induction of M1 characteristics in LAMs contributed to prolonged survival in leukemia mice
To study the effects of LAMs with more M1 or M2 characteristics on the progression of leukemia in vivo, two induction models were designed as illustrated in Fig. 7A. Administration of SP600125 significantly decreased the expression of all six M1-associated genes while increased the expression of M2-associated genes including CD206, CCL17 and CSF1 in BM LAMs (Fig. 7B). Up-regulation of M2-associated genes including ARG1, CCL17 and IL-10 was also detected in SP LAMs (Fig S5I). Hence, SP600125 induces M2 characteristics in LAMs (Fig. 7C). On the contrary, administration of polyIC significantly increased the expression of M1-associated genes including CXCL11, IL-6, IL-12β, iNOS and TNFα while decreased the expression of M2-associated genes in SP LAMs (Fig. 7D). Down-regulation of M2-associated genes including ARG1, IL-10 and up-regulated of M1-associated genes including IL-1β and iNOS were also detected in BM LAMs (Fig S5J). Hence, polyIC induces M1 characteristics in LAMs (Fig. 7E). Furthermore, polyIC-induced LAMs from both BM (Fig. 7F) and SP (Fig. 7G) had eliminated growth promoting effects by in vitro coculture experiments.
Figure 7.
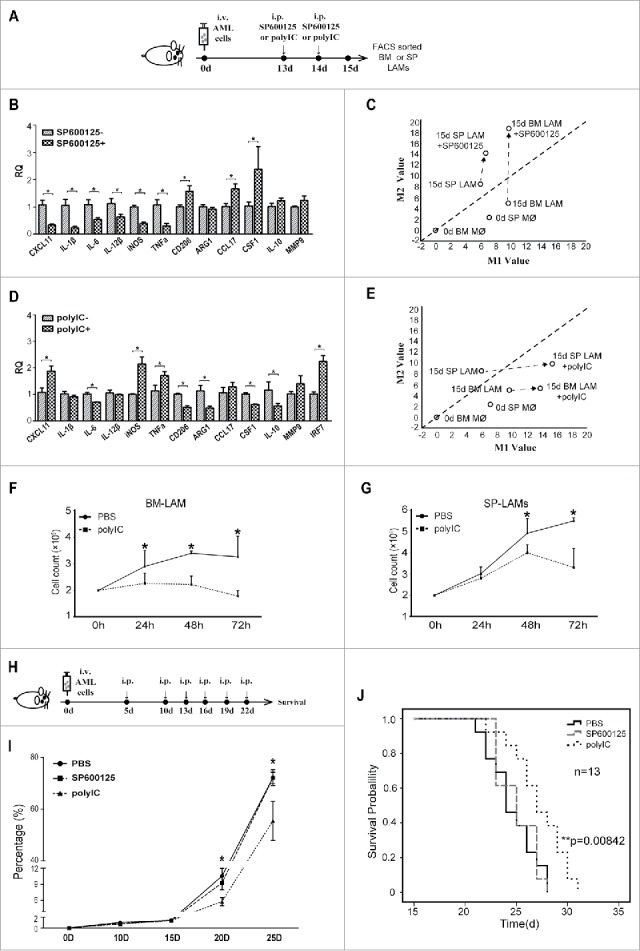
Effects of induced LAMs on the survival of AML mice. (A) Experimental design to test in vivo effects of SP600125(15 mg/kg) or polyIC (10mg/kg) on phenotypes of LAMs. (B) Effects of SP600125 on the expression of phenotype-associated genes in BM LAMs were detected by real-time PCR. The RQ value of BM LAMs sorted on day 15 without SP600125 was designated as 1.000. (C) Two-dimensional illustration of macrophage phenotype is shown. Induction of phenotype shift is indicated by arrows. (D) Effects of polyIC on the expression of phenotype-associated genes in SP LAMs were detected by real-time PCR. The RQ value of SP LAMs sorted on day 15 without PolyIC was designated as 1.000. (E) Two-dimensional illustration of macrophage phenotype is shown. Induction of phenotype shift is indicated by arrows. (F-G) Leukemic mice were treated with polyIC or PBS on day 13 and 14, then LAMs were sorted on day 15. 2 × 105 leukemia cells were cocultured with 5 × 104 BM LAMs (F) or SP LAMs (G). Counts of GFP+ leukemia cells are plotted. (H) Experimental design to study in vivo effects of induced LAMs on survival of AML mice. (I) Leukemia cells (in percentage) in peripheral blood of AML mice with induced M1-like or M2-like LAMs were monitored by flow cytometry. (J) Survival of AML mice upon induction by SP600125 and polyIC. Values are given as means (±s.d.). * P<0.05, ** P<0.01, vs. respective control (Student's t-test; Kaplan–Meier analysis for survival curve). Data are representative of at least three independent experiments.
Experiments were designed to explore in vivo effects of induced LAMs on the survival of leukemia mice (Fig 7H). Significantly less PB leukemia cells were detected at middle or late stages in polyIC-treated mice than those in control or SP600125-treated mice (Fig. 7I). Most importantly, polyIC-treated leukemia mice had significantly prolonged survival time (Fig. 7J). These results indicate that inducing LAMs with more M1 characteristics contribute to prolonged survival in leukemia mice and suggest that IRF7-JNK/SAPK pathway may be a potential target for macrophage-based strategy against AML.
Discussion
The functional phenotype of macrophages is controlled by specific microenvironments under both physiological and pathological conditions.38 Though M1/M2 criteria is widely accepted, most macrophages in pathological microenvironments are more often regarded as M1-like or M2-like based on their function and expression of phenotype-associated genes.39 TAMs are generally regarded as M2-like macrophages. However, TAMs are heterogeneous and those with M1 characteristics are detected in tumors.15-17 Furthermore, TAMs with more M2 characteristics have pro-tumor effects whereas TAMs with more M1 characteristics have anti-tumor effects.20 In leukemia patients, more M2-like LAMs rather than total LAMs correlate with worse prognosis, which suggest that M2-like LAMs have pro-leukemia effects.
Targeting M2-like LAMs should be an immuno-therapy strategy against leukemia. However, the evolution of LAMs and the distribution of heterogeneous LAMs have not been established. We studied LAMs from BM and SP, the two major hematopoietic tissues, in mouse AML and ALL models. As concomitant high level expression of iNOS and Arg1, the classical M1- and M2-associated genes, is detected in macrophages,28 not a single phenotype-associated gene can define the phenotype of macrophages. Here we developed a two-dimensional method based on the expression of a panel of phenotype-associated genes in two categories, and visualized the phenotypic evolution of LAM in different leukemic microenvironments and in response to inductions. An interesting observation from this approach is that polarization of LAMs is tissue-dependent, i.e. SP LAMs have more M2 characteristics whereas BM LAMs have more M1 characteristics. Functional study further confirmed that SP LAMs have more M2 characteristics than BM LAMs. It was reported that spleen microenvironment mediated immune tolerance to tumor antigens,40 spleen leukemic microenvironment promoted malignant phenotype of T-ALL cells and induced M2-polarization of macrophages in vitro and in vivo.27,41 As a consequence, SP LAMs form sterile inflammatory leukemic microenvironments may contribute to malignant evolution of leukemia cells.
Selective activation of intrinsic signal pathway contributes to divers macrophage phenotypes upon activation.39 IRF7 was selected as the potential candidate by screening of DEGs with special attention to those up-regulated in BM LAMs but down-regulated in SP LAMs. Several IRF members including IRF3,42 IRF443 and IRF544 have been reported to mediate macrophage activation. Most recently, IRF7 was reported to participate in LPS stimulated M1-like microglial polarization switch through an activation of STAT1 pathway.45 We found that IRF7-SAPK/JNK pathway rather than STAT1 or STAT6 pathway was crucial for the polarization of BM LAMs with more M1 characteristics. Sterile inflammation might account for this phenomenon since BM macrophages are less easily exposed to microbial stimuli versus SP counterparts. How BM leukemic microenvironment without microbial stimuli promotes the expression of IRF7 in LAMs has not been established.
Two directions of approaches targeting M2-like TAMs have been explored.46 Depletion of M2-like TAMs by CSR-1R antibody or small molecules significantly delays various tumor growth in murine models and patients.21,47 Repolarization of TAMs with more M1 characteristics and less M2 characteristics by antibody against phosphatidylserine, agonist antibody against CD40, trabectedin or blocking NF-κB pathway promote their anti-tumor effects in solid tumors.22,48-50 Here we repolarized LAMs to obtain more M1 characteristics by targeting the IRF7-JNK pathway. PolyIC treatment induced both BM and SP LAMs with more M1 characteristics and prolonged the survival of leukemia mice. PolyIC had no significant effect on the proliferation and apoptosis of leukemia cells (data not shown). Though polyIC may also affect other immune cell subsets, our results suggest that repolarized LAMs should contribute to prolonged survival of leukemia mice.
In summary, more M2-like LAMs correlated with worse prognosis in AML patients. SP LAMs evolved with more M2 characteristics while BM LAMs with more M1 characteristics during the development of leukemia. Targeting IRF7-SAPK/JNK pathway, which accounted for different activation of LAMs, to induce M1 characteristics contributed to prolonged survival in leukemia mice. Our findings provide clues for new strategy against leukemia. However, before being clinically translated, fundamental research on important aspects should be done. A set of markers for high purity human macrophages from BM and SP should be determined since the scavenger receptor CD68 is also expressed in monocytes and CD163 is considered as an M2 markers though they are well accepted markers for human macrophages. The panel of human M1 and M2-associated genes as well as phenotype definition of human macrophages and LAMs (molecular mapping at single cell resolution if possible) need to be established. Moreover, whether activation of IRF7-SAPK/JNK pathway resulted in more M1 characteristics in human macrophages and LAMs should be verified. Nevertheless, repolarization of LAMs should be a prospective application for macrophage based immuno-therapy strategy against leukemia.
Supplementary Material
Funding Statement
This work was supported by grants (81370634, 81570153 and 81770183) from the National Natural Science Foundation of China (NSFC); programs (2016-I2M-2-006 and 2017-I2M-1-015) from CAMS Initiative for Innovative Medicine; and grant (17JCZDJC35000) from the Tianjin Natural Science Foundation. Z.GG. was a recipient of the New Century Excellent Talents in University (NCET-08-0329).
Data and materials availability
All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Author contributions
XY designed and performed the experiments, analyzed data and wrote the manuscript. WF, LW and RW helped with all experiments and assisted with the manuscript. FY and SC helped with the mouse experiments and flow cytometry. YR helped with ultrastructure analysis. TC assisted with experimental design and provided critical revisions of the manuscript. GZ conceived the study, designed the experiments, interpreted the results, wrote the paper and oversaw the research project. All authors approved all versions including the final version and are responsible for the accuracy and integrity of all aspects of the manuscript.
References
- 1.Sieweke MH. Waddington's Valleys and Captain Cook's Islands. Cell Stem Cell. 2015;16(1):7–8. doi: 10.1016/j.stem.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 2.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11(10):889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 3.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32(5):593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 4.Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol. 2011;11(11):750–761. doi: 10.1038/nri3088. [DOI] [PubMed] [Google Scholar]
- 5.Darnell JJ, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264(5164):1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 6.Brombacher F, Arendse B, Peterson R, Holscher A, Holscher C. Analyzing classical and alternative macrophage activation in macrophage/neutrophil-specific IL-4 receptor-alpha-deficient mice. Methods Mol Biol. 2009;531:225–252. doi: 10.1007/978-1-59745-396-7_15. [DOI] [PubMed] [Google Scholar]
- 7.Hambardzumyan D, Gutmann DH, Kettenmann H. The role of microglia and macrophages in glioma maintenance and progression. Nat Neurosci. 2016;19(1):20–27. doi: 10.1038/nn.4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies LC, Taylor PR. Tissue-resident macrophages: then and now. Immunology. 2015;144(4):541–548. doi: 10.1111/imm.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ginhoux F, Schultze JL, Murray PJ, Ochando J, Biswas SK. New insights into the multidimensional concept of macrophage ontogeny, activation and function. Nat Immunol. 2015;17(1):34–40. doi: 10.1038/ni.3324. [DOI] [PubMed] [Google Scholar]
- 10.Natoli G, Monticelli S. Macrophage Activation: Glancing into Diversity. Immunity. 2014;40(2):175–177. doi: 10.1016/j.immuni.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 11.Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol. 2006;177(10):7303–7311. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- 12.Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496(7446):445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Komohara Y, Jinushi M, Takeya M. Clinical significance of macrophage heterogeneity in human malignant tumors. Cancer Sci. 2014;105(1):1–8. doi: 10.1111/cas.12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laoui D, Movahedi K, Van Overmeire E, Van den Bossche J, Schouppe E, Mommer C, Nikolaou A, Morias Y, De Baetselier P, Van Ginderachter JA. Tumor-associated macrophages in breast cancer: distinct subsets, distinct functions. Int J Dev Biol. 2011;55(7–9):861–867. doi: 10.1387/ijdb.113371dl. [DOI] [PubMed] [Google Scholar]
- 15.Edin S, Wikberg ML, Dahlin AM, Rutegard J, Oberg A, Oldenborg PA, Palmqvist R. The distribution of macrophages with a M1 or M2 phenotype in relation to prognosis and the molecular characteristics of colorectal cancer. Plos One. 2012;7(10):e47045. doi: 10.1371/journal.pone.0047045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma J, Liu L, Che G, Yu N, Dai F, You Z. The M1 form of tumor-associated macrophages in non-small cell lung cancer is positively associated with survival time. Bmc Cancer. 2010;10:112. doi: 10.1186/1471-2407-10-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Movahedi K, Laoui D, Gysemans C, Baeten M, Stange G, Van den Bossche J, Mack M, Pipeleers D, In'T VP, et al.. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 2010;70(14):5728–5739. doi: 10.1158/0008-5472.CAN-09-4672. [DOI] [PubMed] [Google Scholar]
- 18.Goswami S, Sahai E, Wyckoff JB, Cammer M, Cox D, Pixley FJ, Stanley ER, Segall JE, Condeelis JS. Macrophages promote the invasion of breast carcinoma cells via a colony-stimulating factor-1/epidermal growth factor paracrine loop. Cancer Res. 2005;65(12):5278–5283. doi: 10.1158/0008-5472.CAN-04-1853. [DOI] [PubMed] [Google Scholar]
- 19.Ohri CM, Shikotra A, Green RH, Waller DA, Bradding P. Macrophages within NSCLC tumour islets are predominantly of a cytotoxic M1 phenotype associated with extended survival. Eur Respir J. 2009;33(1):118–126. doi: 10.1183/09031936.00065708. [DOI] [PubMed] [Google Scholar]
- 20.Chanmee T, Ontong P, Konno K, Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers (Basel). 2014;6(3):1670–1690. doi: 10.3390/cancers6031670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ries CH, Cannarile MA, Hoves S, Benz J, Wartha K, Runza V, Rey-Giraud F, Pradel LP, Feuerhake F, Klaman I, Jones T, et al.. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell. 2014;25(6):846–859. doi: 10.1016/j.ccr.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 22.Yin Y, Huang X, Lynn KD, Thorpe PE. Phosphatidylserine-targeting antibody induces M1 macrophage polarization and promotes myeloid-derived suppressor cell differentiation. Cancer Immunol Res. 2013;1(4):256–268. doi: 10.1158/2326-6066.CIR-13-0073. [DOI] [PubMed] [Google Scholar]
- 23.Nakanishi Y, Nakatsuji M, Seno H, Ishizu S, Akitake-Kawano R, Kanda K, Ueo T, Komekado H, Kawada M, Minami M, et al.. COX-2 inhibition alters the phenotype of tumor-associated macrophages from M2 to M1 in ApcMin/+ mouse polyps. Carcinogenesis. 2011;32(9):1333–1339. doi: 10.1093/carcin/bgr128. [DOI] [PubMed] [Google Scholar]
- 24.Steidl C, Lee T, Shah SP, Farinha P, Han G, Nayar T, Delaney A, Jones SJ, Iqbal J, Weisenburger DD, et al.. Tumor-Associated Macrophages and Survival in Classic Hodgkin's Lymphoma. New Engl J Med. 2010;362(10):875–885. doi: 10.1056/NEJMoa0905680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Audrito V, Serra S, Brusa D, Mazzola F, Arruga F, Vaisitti T, Coscia M, Maffei R, Rossi D, Wang T, et al.. Extracellular nicotinamide phosphoribosyltransferase (NAMPT) promotes M2 macrophage polarization in chronic lymphocytic leukemia. Blood. 2015;125(1):111–123. doi: 10.1182/blood-2014-07-589069. [DOI] [PubMed] [Google Scholar]
- 26.Majeti R, Chao MP, Alizadeh AA, Pang WW, Jaiswal S, Gibbs KD, van Rooijen N, Weissman IL. CD47 Is an Adverse Prognostic Factor and Therapeutic Antibody Target on Human Acute Myeloid Leukemia Stem Cells. Cell. 2009;138(2):286–299. doi: 10.1016/j.cell.2009.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen SY, Yang X, Feng WL, Liao JF, Wang LN, Feng L, Lin YM, Ren Q, Zheng GG. Organ-specific microenvironment modifies diverse functional and phenotypic characteristics of leukemia-associated macrophages in mouse T cell acute lymphoblastic leukemia. J Immunol. 2015;194(6):2919–2929. doi: 10.4049/jimmunol.1400451. [DOI] [PubMed] [Google Scholar]
- 28.Chen S, Yang X, Feng W, Yang F, Wang R, Chen C, Wang L, Lin Y, Ren Q, Zheng G. Characterization of peritoneal leukemia-associated macrophages in Notch1-induced mouse T cell acute lymphoblastic leukemia. Mol Immunol. 2017;81:35–41. doi: 10.1016/j.molimm.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 29.Yang X, Feng W, Wang R, Yang F, Wang L, Chen S, Chen C, Ren Q, Zheng G. Hepatic leukemia-associated macrophages exhibit a pro-inflammatory phenotype in Notch1-induced acute T cell leukemia. Immunobiology. 2018;223(1):73–80. doi: 10.1016/j.imbio.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 30.Al-Matary YS, Botezatu L, Opalka B, Hones JM, Lams RF, Thivakaran A, Schutte J, Koster R, Lennartz K, Schroeder T, et al.. Acute myeloid leukemia cells polarize macrophages towards a leukemia supporting state in a Growth factor independence 1 dependent manner. Haematologica. 2016;101(10):1216–1227. doi: 10.3324/haematol.2016.143180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Behrendt R, Schumann T, Gerbaulet A, Nguyen LA, Schubert N, Alexopoulou D, Berka U, Lienenklaus S, Peschke K, Gibbert K, et al.. Mouse SAMHD1 has antiretroviral activity and suppresses a spontaneous cell-intrinsic antiviral response. Cell Rep. 2013;4(4):689–696. doi: 10.1016/j.celrep.2013.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bagger FO, Sasivarevic D, Sohi SH, Laursen LG, Pundhir S, Sonderby CK, Winther O, Rapin N, Porse BT. BloodSpot: a database of gene expression profiles and transcriptional programs for healthy and malignant haematopoiesis. Nucleic Acids Res. 2016;44(D1):D917–D924. doi: 10.1093/nar/gkv1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng H, Hao S, Liu Y, Pang Y, Ma S, Dong F, Xu J, Zheng G, Li S, Yuan W, Cheng T. Leukemic marrow infiltration reveals a novel role for Egr3 as a potent inhibitor of normal hematopoietic stem cell proliferation. Blood. 2015;126(11):1302–1313. doi: 10.1182/blood-2015-01-623645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sica A, Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J Clin Invest. 2007;117(5):1155–1166. doi: 10.1172/JCI31422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ingersoll MA, Spanbroek R, Lottaz C, Gautier EL, Frankenberger M, Hoffmann R, Lang R, Haniffa M, Collin M, Tacke F, et al.. Comparison of gene expression profiles between human and mouse monocyte subsets. Blood. 2010;115(3):e10–e19. doi: 10.1182/blood-2009-07-235028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol. 2011;11(11):750–761. doi: 10.1038/nri3088. [DOI] [PubMed] [Google Scholar]
- 37.Bauer CMT, DeWitte-Orr SJ, Hornby KR, Zavitz CCJ, Lichty BD, Stämpfli MR, Mossman KL. Cigarette Smoke Suppresses Type I Interferon-Mediated Antiviral Immunity in Lung Fibroblast and Epithelial Cells. Journal of Interferon & Cytokine Research. 2008;28(3):167–179. doi: 10.1089/jir.2007.0054. [DOI] [PubMed] [Google Scholar]
- 38.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122(3):787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11(11):723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ugel S, Peranzoni E, Desantis G, Chioda M, Walter S, Weinschenk T, Ochando JC, Cabrelle A, Mandruzzato S, Bronte V. Immune tolerance to tumor antigens occurs in a specialized environment of the spleen. Cell Rep. 2012;2(3):628–639. doi: 10.1016/j.celrep.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 41.Ma S, Shi Y, Pang Y, Dong F, Cheng H, Hao S, Xu J, Zhu X, Yuan W, Cheng T, Zheng G. Notch1-induced T cell leukemia can be potentiated by microenvironmental cues in the spleen. J Hematol Oncol. 2014;7(1):71. doi: 10.1186/s13045-014-0071-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Biswas SK, Gangi L, Paul S, Schioppa T, Saccani A, Sironi M, Bottazzi B, Doni A, Vincenzo B, Pasqualini F, et al.. A distinct and unique transcriptional program expressed by tumor-associated macrophages (defective NF-kappaB and enhanced IRF-3/STAT1 activation). Blood. 2006;107(5):2112–2122. doi: 10.1182/blood-2005-01-0428. [DOI] [PubMed] [Google Scholar]
- 43.Satoh T, Takeuchi O, Vandenbon A, Yasuda K, Tanaka Y, Kumagai Y, Miyake T, Matsushita K, Okazaki T, Saitoh T, et al.. The Jmjd3-Irf4 axis regulates M2 macrophage polarization and host responses against helminth infection. Nat Immunol. 2010;11(10):936–944. doi: 10.1038/ni.1920. [DOI] [PubMed] [Google Scholar]
- 44.Takaoka A, Yanai H, Kondo S, Duncan G, Negishi H, Mizutani T, Kano S, Honda K, Ohba Y, Mak TW, et al.. Integral role of IRF-5 in the gene induction programme activated by Toll-like receptors. Nature. 2005;434(7030):243–249. doi: 10.1038/nature03308. [DOI] [PubMed] [Google Scholar]
- 45.Tanaka T, Murakami K, Bando Y, Yoshida S. Interferon regulatory factor 7 participates in the M1-like microglial polarization switch. Glia. 2015;63(4):595–610. doi: 10.1002/glia.22770. [DOI] [PubMed] [Google Scholar]
- 46.Belgiovine C, D'Incalci M, Allavena P, Frapolli R. Tumor-associated macrophages and anti-tumor therapies: complex links. Cell Mol Life Sci. 2016;73(13):2411–2424. doi: 10.1007/s00018-016-2166-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Strachan DC, Ruffell B, Oei Y, Bissell MJ, Coussens LM, Pryer N, Daniel D. CSF1R inhibition delays cervical and mammary tumor growth in murine models by attenuating the turnover of tumor-associated macrophages and enhancing infiltration by CD8+ T cells. Oncoimmunology. 2013;2(12):e26968. doi: 10.4161/onci.26968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hagemann T, Lawrence T, McNeish I, Charles KA, Kulbe H, Thompson RG, Robinson SC, Balkwill FR. “Re-educating” tumor-associated macrophages by targeting NF-kappaB. J Exp Med. 2008;205(6):1261–1268. doi: 10.1084/jem.20080108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, Huhn RD, Song W, Li D, Sharp LL, et al.. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331(6024):1612–1616. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Germano G, Frapolli R, Belgiovine C, Anselmo A, Pesce S, Liguori M, Erba E, Uboldi S, Zucchetti M, Pasqualini F, et al.. Role of macrophage targeting in the antitumor activity of trabectedin. Cancer Cell. 2013;23(2):249–262. doi: 10.1016/j.ccr.2013.01.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.