ABSTRACT
The extracellular matrix protein biglycan (BGN) has oncogenic or tumor suppressive potential depending on the cellular origin. HER-2/neu overexpression in murine fibroblasts and human model systems is inversely correlated with BGN expression. Upon its restoration BGNhigh HER-2/neu+ fibroblasts were less tumorigenic in immune competent mice when compared to BGNlow/neg HER-2/neu+ cells, which was associated with enhanced immune cell responses and higher frequencies of immune effector cells in tumors and peripheral blood. The increased immunogenicity of BGNhigh HER-2/neu+ fibroblasts appears to be due to upregulated MHC class I surface antigens and reduced expression levels of transforming growth factor (TGF)-β isoforms and the TGF-β receptor 1 suggesting a link between BGN, TGF-β pathway and HER-2/neu-mediated downregulation of MHC class I antigens. Treatment of BGNlow/neg HER-2/neu+ cells with recombinant BGN or an inhibitor of TGF-β enhanced MHC class I surface antigens in BGNlow/neg HER-2/neu-overexpressing murine fibroblasts, which was mediated by a transcriptional upregulation of major MHC class I antigen processing components. Furthermore, BGN expression in HER-2/neu+ cells was accompanied by an increased expression of the proteoglycan decorin (DCN). Since recombinant DCN also elevated MHC class I surface expression in BGNlow/neg HER-2/neu+ cells, both proteoglycans might act synergistically. This was in accordance with in silico analyses of mRNA data obtained from The Cancer Genome Atlas (TCGA) dataset available for breast cancer (BC) patients. Thus, our data provide for the first time evidence that proteoglycan signatures are modulated by HER-2/neu and linked to MHC class I-mediated immune escape associated with an altered TGF-β pathway.
KEYWORDS: Proteoglycans, biglycan, HER-2/neu, MHC class I, tumor
Introduction
The biglycan (BGN) gene localized on chromosome X in humans and mice1 encodes an extracellular matrix (ECM) protein of the small leucine-rich proteoglycan (SLRP) family characterized by cysteine residues in the N-terminus and a protein core with side chains containing chondroitin and/or dermatan sulfate. It is expressed in most tissues in particular in the ECM of epithelial cells. Major functions of BGN include modulation of matrix assembly, cell migration, adhesion, bone mineralization, inflammation, cell growth, autophagy regulation as well as apoptosis.2 Consequently BGN is involved in several physiologic and pathophysiologic processes such as tumorigenesis.3 SLRPs including BGN interact with a number of receptors that regulate growth, motility and immune responses. Consequently, proteoglycans can induce a cross talk among various families of receptors and interact with natural receptor ligands.4 In their soluble form, proteoglycans can become stress signals and may e.g. act as ligands for toll-like receptors (TLR) thereby regulating innate immunity. Furthermore, BGN has been shown to enhance antigen-specific T cell activation thereby triggering autoimmune peri-myocarditis.5,6
The role of BGN in tumorigenesis is currently controversially discussed. In some tumor types, higher levels of BGN expression have been detected when compared to normal counterparts7-9, which was associated with enhanced migration and invasion in vitro and in vivo. Overexpression of BGN in e.g. colorectal cancer (CRC) cells possesses pro-angiogenic properties by binding to vascular endothelial growth factor (VEGF)-A leading to an activation of vascular endothelial growth factor receptor (VEGF-R) signaling and the extracellular signal-regulated kinase (ERK) pathway.4, 10 Furthermore, BGN can promote tumorigenesis via enhanced Wnt/β-catenin signaling.11 Thus, BGN plays an important role in cancer progression and metastasis.12
In contrast, in bladder cancer silencing of BGN resulted in enhanced tumor cell proliferation indicating that BGN acts as a growth suppressor in this disease.13 In vivo BGN expression might inhibit tumor growth of established tumors by creating a TLR2/4-mediated pro-inflammatory microenvironment.14 Decorin (DCN) is another member of the SLRP family and was first described in dcn/p53 double knock out mice, which developed tumors faster than wild type (wt) counterparts.3 BGN shares > 65% homology with DCN in mouse and human.15 Like BGN, DCN exert pro-angiogenic activities16. In addition, impaired DCN expression was found in many solid tumor entities including CRC17-20 and breast cancer (BC).21 Due to the inhibitory properties against receptor tyrosine kinases (RTK) and cancer growth pathways other SLRP members have been shown to have tumor suppressive effects in vivo and in vitro.16,22-25
These data were further confirmed using a comparative proteomic approach of Ki-ras-transformed mouse fibroblasts and respective controls: BGN expression was downregulated when compared to parental NIH3T3 cells or mock transfectants.26 Similar results were also obtained upon HER-2/neu transformation of fibroblasts.27 Restoration of BGN expression in HER-2/neu+ cells reduced their growth and migration capacity27 when compared to BGNlow/neg HER-2/neu+ cells, which was due to the enhanced expression and function of the cAMP response element binding protein (CREB).28 HER-2/neu amplification and/or overexpression were found in a variety of human epithelial tumors of distinct origin including breast and lung cancer, which was in the majority of studies associated with a more aggressive disease and poor patients' outcome.29 Poor survival patients' outcome was also correlated with an overexpression of the transforming growth factor (TGF)-β in breast cancer,30 which is required for the activation of the epidermal growth factor receptor (EGF-R).31 In in vitro models high levels of HER-2/neu expression in murine fibroblasts and human HER-2/neu-overexpressing tumor cells caused a downregulation of MHC class I surface expression leading to a reduced T cell recognition.32-35 This inverse expression of HER-2/neu and MHC class I surface antigens was caused by a transcriptional downregulation of major components of the MHC class I antigen processing machinery (APM) and controlled by activation of the mitogen-activated protein kinase (MAPK) pathway.32, 36, 37
Since a link between the lack of BGN expression and impaired MHC class I expression in HER-2/neu+ cells has not yet been described, the role of BGN in murine and human HER-2/neu-overexpressing cells on MHC class I APM components in vitro and/or in vivo was determined by overexpressing BGN in BGNlow/neg HER-2/neu+ cells or by their treatment with recombinant BGN.
Results
BGN-mediated induction of MHC class I expression in HER-2/neu overexpressing cells
In order to determine the effect of BGN on the MHC class I surface expression of HER-2/neu+ cells, both a BGN expression vector and a vector control were stably transfected into BGNlow/neg HER-2/neu+ cells followed by analyses of the BGN and MHC class I mRNA and/or protein expression. The BGNlow/neg HER-2/neu+ cells showed low transcription levels of MHC class I antigens (Fig. 1A), whereas BGN overexpression in HER-2/neu+ cells resulted in an upregulation of MHC class I heavy chain transcription (Fig. 1A) as well as MHC class I surface expression (Fig. 1B, C). In addition, treatment of BGNlow/neg HER-2/neu+ cells with exogenous recombinant BGN significantly increased MHC class I surface expression (Fig. 1D, S1A). Vice versa, silencing of BGN expression in BGNhigh NIH3T3 fibroblasts by shRNA resulted in a downregulation of MHC class I surface expression in these cells (Fig. 1E).
Figure 1.
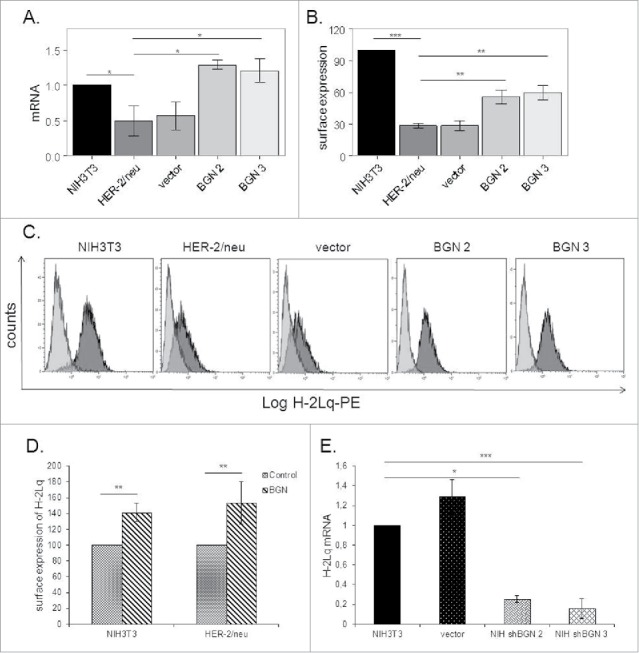
Induction of MHC class I expression upon BGN overexpression in BGNlow/neg HER-2/neu+ cells. A. mRNA expression levels of the MHC class I heavy chain in BGNlow/neg vs. BGNhigh HER-2/neu+ cells. Transcription of H-2Lq was analysed by qPCR as described in Materials and Methods. B. BGN-mediated regulation of MHC class I surface antigen expression MHC class I surface expression was assessed by flow cytometry as described in Materials and Methods. C. A representative histogram of MHC class I surface expression. D. Influence of recombinant BGN on MHC class I surface antigens. Cells were left untreated or treated with recombinant BGN (1 µg/mL) for 24 h before MHC class I expression was assessed by flow cytometry using an anti-H-2Lq mAb as described in Materials and Methods. E. Downregulation of MHC class I surface expression upon silencing of BGN. BGN expression was silenced in parental BGNhigh HER-2/neulow/neg NIH3T3 cells using shBGN and transfectants were analysed by qPCR for the expression of H-2Lq as described in Materials and Methods. The results were displayed as bar diagrams and represent the mean ± SE format of three independent experiments.
BGN-mediated upregulation of MHC class I antigens due to transcriptional increase of APM components
To test whether the BGN-mediated increase of MHC class I surface antigens was due to a transcriptional upregulation of MHC class I APM components, mRNA expression and/or promoter activity of selected APM components were determined by qPCR and promoter reporter assays, respectively37. As shown in Fig. 2A and 2B, the transcript and protein expression levels of TAP1 and TAP2 were increased in BGN transfectants when compared to BGNlow/neg HER-2/neu+ cells, but to a different extent. This was further confirmed by enhanced TAP1 and TAP2 promoter activities in BGN transfectants using luciferase (luc) reporter assays (Fig. 2C), which was more pronounced for TAP2. In contrast, LMP2 transcription (Fig. 2A) and promoter activity (Fig. 2C) were reduced in BGN transfectants, while the expression of TPN (data not shown) and other APM components (Figure S2) were comparable in BGN transfectants and BGNlow/neg HER-2/neu+ cells.
Figure 2.
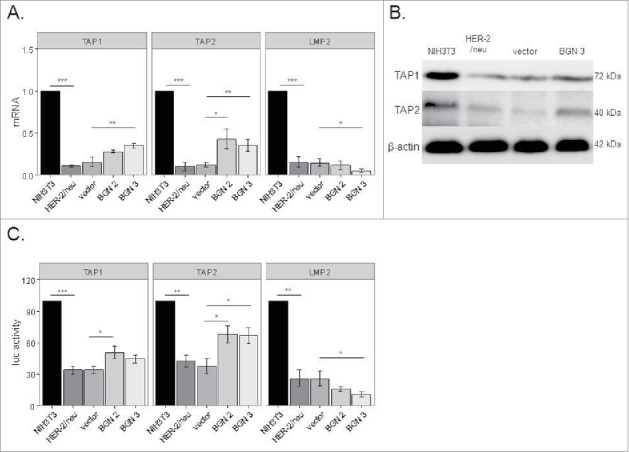
Transcriptional upregulation of MHC class I APM components by BGN overexpression in BGNlow/neg HER-2/neu+ cells. A. Relative mRNA expression levels of selected APM components in BGNlow/neg and BGNhigh HER-2/neu+ cells. The mRNA expression levels of APM components in BGNlow/neg vs. BGNhigh HER-2/neu+ cells were analysed by qPCR as described in Materials and Methods. The results represent the mean of 3 independent experiments. B. Western blot analysis. Protein expression was determined as described in Materials and Methods. A representative Western blot staining is presented. C. Transcriptional upregulation of APM components by BGN overexpression. The activity of selected APM promoters was determined in BGNlow/neg HER-2/neu+ cells, mock transfectants and two independent BGN transfectants (clones 2 and 3) as described in Materials and Methods. APM promoters were transiently co-transfected with the β-gal vector using lipofectamine in the respective cells 48 h prior to determination of the luc activity. The data were normalized to β-gal activity. The experiments were performed at least 3 times. Error bars in all panels indicate standard error. rel luc activity, relative luc activity.
Inverse correlation of BGN expression with HER-2/neu expression in human tumor cells
To confirm the inverse correlation of BGN and HER-/neu expressions in the murine model system, human HER-2/neu-overexpressing breast cancer cells were selected for further experiments. As expected, an inverse correlation of BGN and HER-2/neu expression was detected in wt HER-2/neu (HTB122 E2), but not in mut HER-2/neu (HTB122 E2A)-transfected HTB122 cells (Fig. 3). In addition, qPCR analysis of a large panel of melanoma cells with known HER-2/neu status demonstrated an inverse correlation of HER-2/neu and BGN expression levels (Figure S3).
Figure 3.
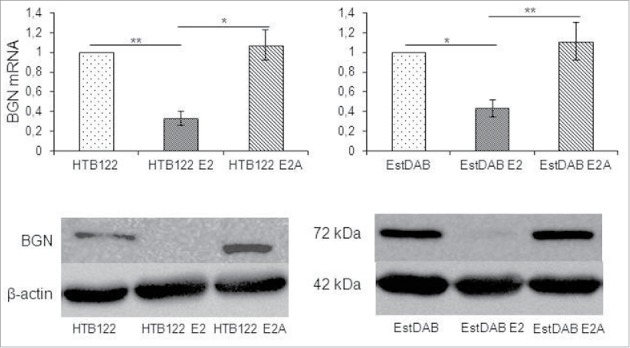
Downregulation of BGN expression in HER-2/neu overexpressing human tumor cells. A. Effect of HER-2/neu overexpression on BGN transcription in melanoma cells. Wt and mut HER-2neu-overexpressiong melanoma cells were subjected to qPCR as described in Materials and Methods. The qPCR data are expressed in bar charts relative to parental cells (set 1) and represent the mean ± SE format from 3 independent experiments. B. Effect of HER-2/neu overexpression on BGN protein expression in melanoma cells. For Western blot analysis 50 µg protein/cell line was separated by 10% SDS-PAGE and transferred onto a nitrocellulose membrane, before immunostaining with a BGN specific mAb was performed as described in Materials and Methods. Staining with a mAb directed against β-actin served as control.
Role of BGN expression in vivo
In order to assess whether BGN overexpression in BGNlow/neg HER-2/neu+ cells also affects the in vivo tumorgenicity, BGNlow/neg HER-2/neu+ cells, mock and BGN transfectants were injected into both immune competent (DBA-1) and immune deficient mice (Fox 1 nude), respectively, and tumor growth regarding incidence and tumor diameter was monitored over time. BGNhigh HER-2/neu+ cells exhibited a reduced frequency of tumor formation and tumor diameter in immune competent mice, which was associated with an increased survival of mice (Fig. 4A) when compared to BGNlow/neg HER-2/neu+ cells. Despite the tumor formation in immune deficient mice was an early event in mice injected with BGNlow/neg HER-2/neu+ cells compared to those injected with BGNhigh HER-2/neu+ cells, all mice injected with either BGNlow/neg or BGNhigh HER-2/neu+ cells, respectively, developed tumors at later stage without a significant difference in tumor diameter. The expression of HER-2/neu and BGN in tumors was determined by qPCR, western blot (Fig. 4B) and/or immunohistochemistry (IHC) demonstrating high levels of HER-2/neu expression in all tumor lesions analyzed independent of the presence of BGN, while BGN expression was only detected in the BGN-transfected HER-2/neu+ cells. Along with BGN expression, IHC analysis displayed also a higher frequency of CD3+ cells in BGNhigh HER-2/neu+ cells when compared to BGNlow/neg or BGNhigh HER-2/neu+ cells (Fig. 4C, 4D). Analysis of the immune cell infiltration in tumor tissues determined by qPCR revealed higher transcript levels of CD3 and CD8, but reduced CD4 mRNA levels in BGNhigh HER-2/neu+ cells, which were accompanied by high MHC class I heavy chain mRNA expression in these tumors (Fig. 4E). This was further supported by the fact that the mice bearing tumors induced by BGNhigh HER-2/neu+ cells showed an enhanced CD8+ T cell frequency and slight reduction of CD4+/CD25high cells in peripheral blood (Fig. 4F).
Figure 4.
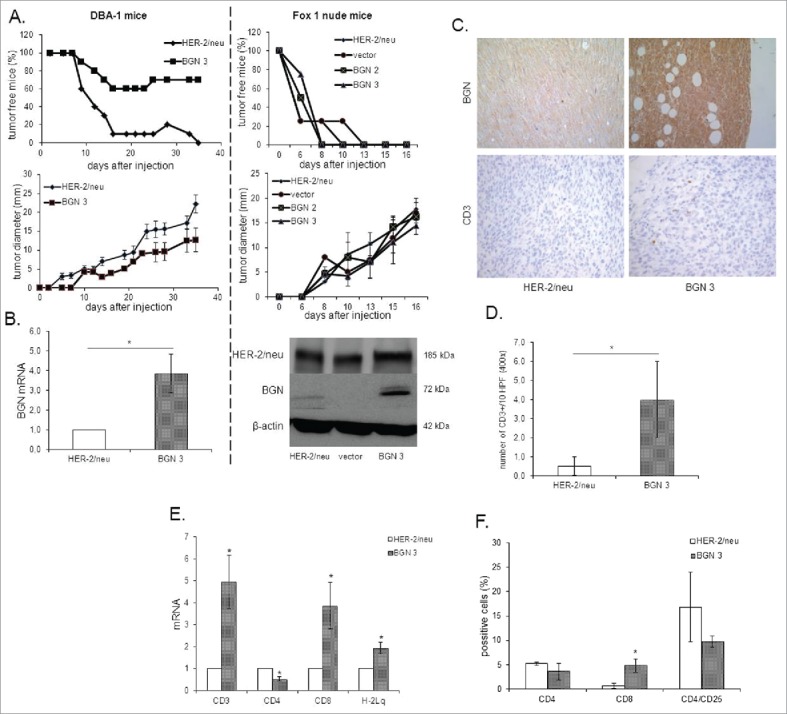
Reduced tumor growth of BGN transfectants in vivo in immune competent mice. A. Frequency and diameter of tumors 1 × 106 BGNlow/neg and BGNhigh HER-2/neu+ cells/mouse were subcutaneously injected as described in Material and Methods. Tumor growth was monitored overtime regarding frequency and diameter of the tumors. B. mRNA and/or protein expression of HER-2/neu and BGN in BGNlow/neg vs. BGNhigh HER-2/neu+ tumors. The mRNA and/or protein expression of HER-2/neu and BGN was determined in theBGNlow/neg and BGNhigh tumors as described in Materials and Methods using qPCRand Western blot analysis. C. Immunohistochemical staining of BGN and CD3 in BGNlow/neg and BGNhigh HER-2/neu+ cells. IHC of tumors was performed as described in Materials and Methods using anti- BGN, anti-HER-2/neu and anti-CD3 specific mAbs, respectively. D. Analysis of the number of CD3+ immune cells. The number of CD3+ immune cells was determined upon staining with an anti-CD3 mAb at 400 x magnification (HPF) considering preferentially areas with higher intra-tumoral immune cell density. E. Determination of the mRNA expression of immune markers and MHC class I expression in BGNlow/neg and BGNhigh HER-2/neu+ cells. MRNA expression of the different immune cell markers CD3, CD4, CD8, IL-2, FoxP3 and the MHC class I HC was determined by qPCR as described in Materials and Methods. The expression levels of these markers were compared in BGNlow/neg vs. BGNhigh HER-2/neu+ tumor lesions. F. Immune marker expression in peripheral blood of tumor bearing mice. The expression of levels of percentages of CD4, CD8 and CD4/CD25 were determined after injection of BGNlow vs. BGNhigh HER-2/neu+ tumor cells in the peripheral blood of mice using flow cytometry as described in Materials and Methods.
Correlation of BGN expression with TGF-β-mediated MHC class I downregulation
BGNlow/neg HER-2/neu+ cells expressed significantly higher mRNA levels of TGF-β1, TGF-β3 and TGF-βR1 than parental NIH3T3 cells and BGNhigh HER-2/neu+ cells suggesting that BGN overexpression inhibits the HER-2/neu-mediated TGF-β signaling (Fig. 5A). These in vitro data were further confirmed in vivo demonstrating a significant downregulation of molecules of the TGF-β pathway in the BGNhigh HER-2/neu+ tumor lesions (Fig. 5B).
Figure 5.
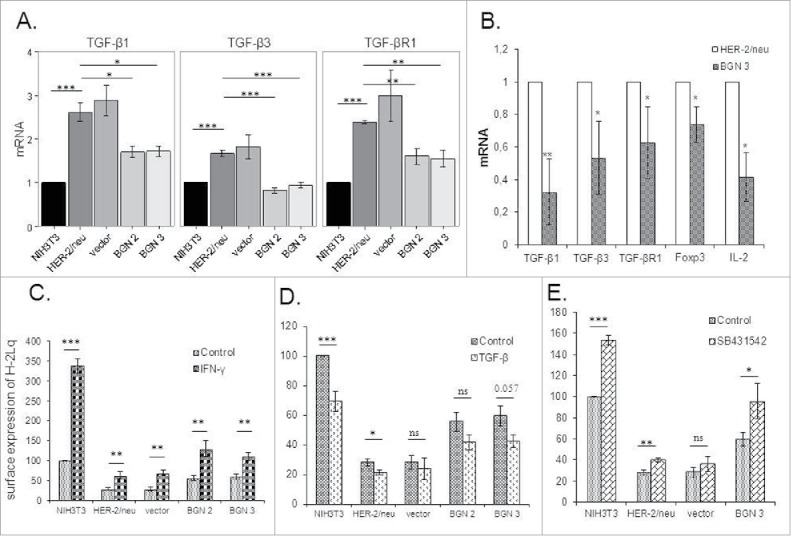
Changes of the TGF-β pathway and MHC class I surface antigens by treatment with TGF-β and IFN-γ. A. mRNA expression of TGF-β isoforms 1 (TGF-β1 and 3) and the TGF-βR1 in BGNlow/neg and BGNhigh HER-2/neu+ cells. The expression of components of the TGF-β pathway was analysed by qPCR as determined in Materials and Methods. The results represent the mean of three independent experiments and expressed relative to NIH3T3 cells (set 1). B. Analysis of TGF-β and Treg in BGNlow vs. BGNhigh HER-2/neu+ cells. The expression levels of TGF-β isoforms (TGF-β1 and 3) and receptor (TGF-βR1), were analysed in BGNhigh HER-2/neu+ tumor lesions in vivo using qPCR. C. Enhanced MHC class I surface expression upon IFN-γ treatment. Untreated and 20ng/ml IFN-γ-treated cells were subjected to flow cytometry as described in Materials and Methods. MFI was determined and expressed relative to NIH3T3 cells (100%). D. Effects of TGF-β on MHC class I surface expression. Untreated and 40ng/ml TGF-β-treated cells were subjected to flow cytometry as described in Materials and Methods. E. Influence of the TGF-β inhibitor on MHC class I surface expression. NIH3T3, BGNlow/neg and BGNhigh HER-2/neu+ cells were either left untreated or treated with 20 ng/ml TGF-β inhibitor (SB431542), before MHC class I surface expression was determined by flow cytometry. MFI of MHC class I surface expression of untreated and SB431542-treated BGNlow/neg and BGNhigh HER-2/neu+ cells was correlated to MFI of untreated NIH3T3 cells, which was set 100%. The experiments were at least performed three times and results represent the mean of these experiments.
In order to study a possible link between BGN and MHC class I expression with the TGF-β pathway BGNhigh cells were treated with recombinant TGF-β1, while BGNlow HER-2/neu+ cells were treated with the TGF-β1 inhibitor SB431542. IFN-γ treatment of the cells served as positive control. As expected IFN-γ significantly enhanced the expression of MHC class I surface antigens (Fig. 5C, S1B) in these cells, while it was downregulated in the presence of TGF-β1 (Fig. 5D) and slightly increased in the presence of SB431542 (Fig. 5E, S1C). The combination of TGF-β1 and IFN-γ treatment demonstrated that IFN-γ counteracted the TGF-β1-mediated inhibition of MHC class I surface antigens (Figure S4H) suggesting that IFN-γ overcomes the TGF-β-mediated downregulation of MHC class I surface expression. These results were further confirmed in human HER-2/neu model systems (Figure S4).
BGN-mediated upregulation of DCN expression
Since the SLRP member DCN has been shown to exhibit a tumor-suppressive activity,24, 25 it was analysed whether BGN overexpression influences DCN expression. As shown in Fig. 6A, BGNhigh HER-2/neu+ cells expressed higher levels of DCN at mRNA and protein levels than BGNlow HER-2/neu+ cells (Fig. 6A) suggesting a link between BGN and DCN expression in these cells. The BGN-induced DCN expression was also found in in vivo in BGNhigh HER-2/neu+ tumor lesions (Fig. 6C) and in the human melanoma model systems (Fig. 6B). Analogous to recombinant BGN, treatment of BGNlow/neg HER-2/neu+ cells with recombinant DCN enhanced MHC class I surface antigens (Fig. 6D, S1A).
Figure 6.
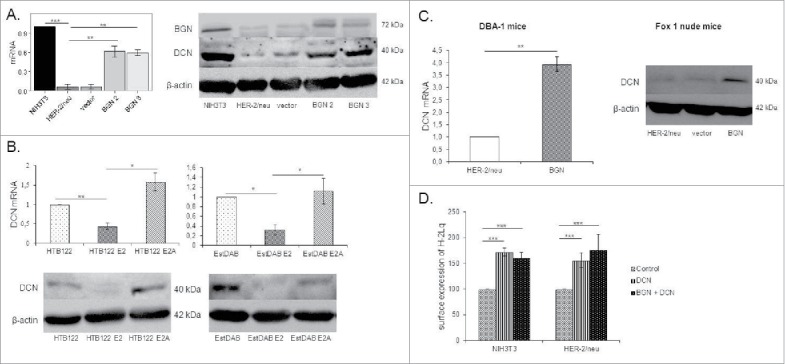
Induction of DCN expression in BGN transfectants. A. Determination of DCN expression in BGNhigh HER-2/neu+ cells. Relative mRNA and protein expression of DCN were determined by qPCR and Western blot, respectively. DCN mRNA expression was analyzed in the NIH3T3 BGNlow/neg and BGNhigh HER-2/neu+ cells and transcription was correlated to NIH3T3 cells (set 1). For protein expression, 50 µg protein/cell line was separated by 10% SDS-Page, transferred onto a nitrocellulose membrane, before immunostaining was performed with an anti-DCN-specific mAb as described in Materials and Methods. Staining of the Western blot with an anti-β-actin-specific mAb served as loading control. B. Downregulation of DCN expression by HER-2/neu overexpression in human tumor cells. The mRNA and protein expression of DCN was determined in human HER-2/neu model systems as described in Materials and Methods. C. Enhanced expression of DCN in BGNhigh HER-2/neu+ cells in vivo. DCN mRNA and protein expression was determined in BGNlow and BGNhigh HER-2/neu+ tumors as described in Materials and Methods. D. Increased MHC class I surface expression in the presence of DCN and/or BGN. Cells were treated with recombinant DCN (1.5 µg/mL) alone or in combination with recombinant (1 µg/mL) BGN for 23h, before MHC class I expression of untreated DCN and DCN/BGN-treated cells was determined by flow cytometry using an anti-H-2Lq mAb. MFI of untreated NIH3T3 cells and BGNlow HER-2/neu+ cells was set 100%.
Correlation of BGN and DCN expression with clinical parameters
In order to determine the clinical relevance of ERBB2, BGN, DCN, HLA-B, HLA-C, CD3, CD8, CD25, TGF-β1 and Foxp3 expression, in silico analysis of TCGA data from BC patients was performed by correlating the mRNA expression levels with the clinical outcome of BC patients. As shown in Figure S5, high BGN (nearly significant; p 0.061) and DCN (significant p 0.008) expression levels along with nearly significant expression levels of HLA-B, HLA-C, CD3 and CD8 had prognostic value and were considerably associated with an increased progression free survival (PFS) of patients. This was not the case for ERBB2, TGF-β1 and Foxp3, where their lower expression levels might support a higher patient's survival.
Discussion
HER-2/neu amplification and/or overexpression has been shown to be associated with altered growth properties and a reduced immunogenicity of tumors, which might be at least partially mediated by a HER-2/neu-induced downregulation of MHC class I surface expression due to transcriptional suppression of major APM components leading to escape from immune surveillance.39, 40 Furthermore, the tumor-induced modification of the tumor microenvironment (TME) is accompanied by a reduced activation, migration and cytotoxic activity of T cells, while the frequency of immune suppressive cells, e.g. Treg, M2 macrophages and myeloid suppressor cells, is increased.41 Thus, there is an urgent need to recover the immune escape phenotype of tumors to enhance the efficacy of T cell-based immunotherapies.42
The SLRP BGN has a broad range of functions. It links soluble matrix with innate immune responses via TLR 2 and 4 thereby inducing “danger” signals14, 43. Furthermore, BGN has been shown to promote angiogenesis via VEGF signaling44 and tumorigenesis via the wnt/β-actin pathway.11 In contrast, HER-2/neu-transformed cells with high angiogenic activity expressed decreased levels of BGN in a PKI/CREB-dependent manner.28 In the present study, the lack of BGN expression in HER-2/neu transformants was associated with reduced MHC class I surface expression, which could be reverted by BGN overexpression or by the addition of exogenous BGN. In vivo, BGN overexpression in HER-2/neu+ cells resulted in reduced tumorigenicity of these cells in immune competent mice when compared to BGNlow/neg HER-2/neu+ cells suggesting that BGN acts as a tumor suppressor and enhances immunogenicity. This might be explained by a stronger immune cell infiltration as shown by an increased frequency of CD3+ cells and higher mRNA expression levels of CD3 and CD8 in BGNhigh vs BGNlow/neg HER-2/neu+ tumors. Particularly the presence of T cells (CD3+) and T cell subpopulations (CD8+) are indicators for a better prognosis,45 strongly suggesting that the anti-tumoral immune responses could be exploited as a therapeutic option. It is noteworthy that CD4 transcription was low in BGNhigh HER-2/neu+ cells. These data suggest that the reduced frequency and size of BGNhigh HER-2/neu+ tumors might be due to an increased immunogenicity of these cells accompanied by a strong infiltration of effector T cells when compared to BGNlow/neg HER-2/neu+ tumors. Since CD4 transcription was reduced in BGNhigh HER-2/neu+ tumors, one might speculate that BGN restoration downregulates the frequency of immune suppressive CD4+ FoxP3+ Treg. In addition, overexpression of BGN in HER-2/neu+ cells was accompanied by an upregulation of DCN expression suggesting a link between BGN and DCN expression. Treatment of BGNlow/neg HER-2/neu+ cells with recombinant BGN or DCN resulted in an upregulation of MHC class I surface antigens due to increased expression of major APM components including TAP1 and TAP2 demonstrating that both SLRPs have an immune modulatory potential.
Neoplastic malignancies often overexpress TGF-β and its receptor.46 In a murine neu-driven BC model, TGF-β can accelerate metastasis formation possibly through the synergistic activation of PI3K/AKTand Ras/MAPK pathways with neu-dependent signaling.47 Furthermore, TGF-β signaling is activated in HER-2/neu-overexpressing BC cells,48 which is accompanied by increased tumor cell motility and metastatic progression. The crosstalk between HER2 and TGF-β not only alters intracellular signaling in cancer cells, but also influences components of the TME through the induction of several pro-invasive growth factors. In BGNlow/neg HER-2/neu+ cells high transcript levels of TGF-β and of the TGF-β receptor were detected, while BGN expression in HER-2/neu+ cells reduced their expression. This is in line with the regulation of BGN by the ALK5-Smad2/3 TGF-β1 signaling pathway49 and its function as a TGF-β repressor.50 Thus, BGN expression could be linked to changes in the TGF-β pathway known to negatively interfere with MHC class I surface expression51 and anti-tumoral immune responses.52 These data confirm the TGF-β-mediated escape from immune surveillance due to downregulation of MHC class I expression53 and an induction of the epithelial mesenchymal transition53 as demonstrated by increased SNAIL expression and activation of MMP954. So far, there exist no data on the effect of BGN in this context. Interestingly, TGF-β inhibition induced MHC class I expression in BGNlow/neg HER-2/neu+ cells. Such data highlight the important the role of microenvironmental TGF-β signaling on escape of tumor cells from immune surveillance leading to progression.
On the other hand, DCN has also been shown to block TGF-β transcription and protein expression in glioblastoma cells, which was accompanied by a strong inhibition of tumor formation in vivo.55 DCN-expressing glioblastoma showed an altered TME characterized by an increased frequency of infiltrating T and B cells. Furthermore, the DCN-induced inhibition of TGF-β was accompanied by significantly enhanced anti-glioblastoma immune responses in tumor necrosis factor-α-converting enzyme signaling.55 These data are in line with our BGNhigh HER-2/neu model demonstrating an increased DCN expression in these cells. Thus, a better understanding of the contextual networks of BGN and DCN in tumors is required to modulate immunogenicity by targeting the MHC class I surface expression.
TGF-β1 binds to its receptors (TGF-βRI and TGF-βR2) and induces the phosphorylation of the tumor necrosis factor-α-converting enzyme (TACE) (Fig. 7), which resulted in its translocation to the cell surface, where TACE induces integrins and cleaves the epidermal growth factor receptor (EGF-R) pro-ligands56. EGF-R ligands will initiate an autocrine and paracrine EGF-R signaling, which is amplified in HER-2/neu-overexpressing cells (BGNlow/neg HER-2/neu+ cells). In BGNhigh HER-2/neu+ cells, BGN and DCN bind to TGF-β1 and restrict HER-2/neu signaling, which might induce tumor suppression (Fig. 7). It can be suggested that TGF-β by signaling via the TGF-β receptor enhances the HER-2/neu-initiated signal transduction by increasing HER-2/neu ligand shedding, HER-2/neu-containing heterodimers, and their cross talk with integrins.57 In our study, an enhanced expression of TGF-β1 and its receptor TGF-βR1 was found in BGNlow HER-2/neu, which could be reverted to normal levels by BGN overexpression. Both BGN and DCN regulate the TGF-β availability in BGNhigh HER-2/neu cells. In BGNhigh HER-2/neu+ cells, the amount of BGN and DCN in the ECM increases and both proteoglycans bind to TGF-β and sequester it to the ECM. In this way both DCN and BGN translocate TGF-β from the membrane thereby reducing the binding to its receptor resulting in a decreased TGF-β signaling and restricting HER-2/neu-mediated carcinogenic effects.
Figure 7.
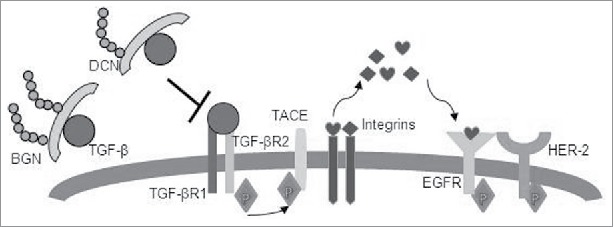
Schematic representation of BGN- and DCN-mediated inhibition of the TGF-β pathway and restriction of HER-2/neu signaling. TGF-β1 binds to its receptors (TGF-βRI and TGF-βR2) and induces phosphorylation of TACE, resulting in its translocation to the cell surface, where TACE induces integrins and cleaves EGFR pro-ligands. EGFR ligands will initiate autocrine and paracrine EGFR signaling, which is amplified in HER2-overexpressing cells (BGNlow/neg HER-2/neu+ cells). In BGNhigh HER-2/neu+ cells, BGN and DCN bind to TGF-β1 and restricts HER-2/neu signaling, thus allowing tumor suppression to occur.
In cancer, contradicting data exist regarding the clinical significance of BGN expression. In some tumors an increased BGN expression was linked to poor prognosis,58 whereas in others its overexpression was associated with inhibition of cancer cell growth and a good prognosis.10,59 The expression of BGN was increased in liver, ovarian, endometrial, pancreatic and gastric cancer60 suggesting an important role of BGN in the pathogenesis of these malignancies. In contrast, several other studies demonstrated an anti-tumoral activity of BGN associated with anti-proliferative capacity. Similar results were obtained in our HER-2/neu model system suggesting that BGN has tumor suppressive activity, which might be associated with an increased immunogenicity. In silico analysis of TCGA data from BC patients demonstrated a prognostic value of BGN and DCN, which is in line with our in vitro results. Based on these reports BGN displays very contradicting roles, which might depend on the cellular context. Thus further in vitro and in vivo studies are required to elucidate the precise underlying molecular mechanisms of BGN in the tumor development and progression.
Material and methods
Cell culture and treatment
Murine NIH3T3 fibroblasts were purchased from ATCC, while the HER-2/neu-overexpressing NIH3T3 cells (termed BGNlow/neg HER-2/neu+) were kindly provided by H. Bernhard (University Hospital of the Technical University, Munich, Germany) and have been described elsewhere.32 BGN-overexpressing HER-2/neu+ cells (HER-2/neu+ BGN+ Clone 2 and HER-2/neu+ BGN+ Clone 3, termed BGNhigh HER-2/neu+) were cultured in Eagle's modified essential medium (EMEM; Lonza) supplemented with 10% (v/v) heat-inactivated fetal calf serum (FCS), 2 mmol/L L-glutamine, 100 U/mL penicillin, and 100 mg/mL streptomycin (PAA). Human breast cancer cells, HTB122 transfected with wild type (wt) HER-2/neu (HTB122 E2) and mutant (mut) HER-2/neu (signal transduction deficient; HTB122 E2A) were employed and have been previously described.35
All experiments were carried out during the logarithmic growth phase of the cells. For IFN-γ stimulation, cells were treated for 24 to 48 h at 37°C with 20 – 40 ng/mL murine or human recombinant IFN-γ (Roche Diagnostics), respectively. In addition, cells were treated with 20 ng/mL human and 50 ng/mL murine TGF-β1 for 24 h, respectively, 1.0 µg/mL recombinant BGN (R&D Systems), 1.5 µg/mL recombinant DCN (R&D Systems) and 20 ng/mL TGF-β1 inhibitor (SB431542).
RNA extraction and real-time quantitative PCR
Total cellular RNA was isolated using the NucleoSpin RNA II kit (Macherey-Nagel). An equal amount of total RNA (2 μg) was reverse transcribed into cDNA using the Revert H Minus First Strand cDNA synthesis kit (Fermentas) and oligo(dT)18 primer according to manufacturer's instructions. Real-time PCR was performed as previously described37 using gene-specific and control primers (Table S1). Comparative quantification of gene expression was performed as previously described.61 The experiments were independently performed three times with two technical replicates.
Western blot analyses
For Western blot analysis 5 × 106 cells were harvested as previously described26, proteins were solubilized according to Laemmli62. 50 µg protein/lane were separated in 10% SDS-PAGE gels29, transferred onto nitrocellulose membranes (Schleicher & Schuell) and stained with Ponceau S as previously described.61 Membranes were incubated over night at 4°C with primary monoclonal antibodies (mAb) directed against BGN (Proteintech), DCN (Sigma-Aldrich), TAP1 (Santa Cruz Biotechnology Inc.), TAP2 (kindly provided by K. Früh (Howard Johnsson, La Jolla, CA)), β-actin (Sigma-Aldrich) and GAPDH (Cell Signaling Technology) followed by incubation for 1h with horseradish peroxidase-linked secondary antibody and developed using the ECL method. Chemiluminescence signals were visualized with the Lumi-Light Western Blotting Substrate (Roche Diagnostics) and recorded with a LAS3000 system (Fuji). For quantification of the protein expression, the respective area of the signal was integrated using an AIDA image analyser (Raytest) and subsequently normalized to β-actin or GAPDH.
Flow cytometry
The mAbs used for flow cytometry were the phycoerythrin (PE)-labeled anti-H-2Ld/q (Cedarlane Laboratories LTD) and the respective PE-labeled isotype mouse immunoglobulin (Beckman Coulter). Whereas, human cells were stained with a FITC-labeled HLA class I-specific mAb (Beckman Coulter) using a FITC-labeled lgG2a mAb (Beckman Coulter) as control. Flow cytometric analysis was performed as recently described.26 Briefly, 5 × 105 cells were incubated with the appropriate amount of antibodies at 4°C for 30 minutes, before the stained cells were measured on a FACScan unit (Becton Dickinson) and subsequently analyzed with the CellQuest software (Becton Dickinson). The data are represented as mean specific fluorescence intensity (MFI) from three independent experiments.
Promoter assay
TAP1/LMP2, TAP2, and TPN promoter sequences were amplified from genomic DNA and then cloned into the pGl3 luciferase (luc) vector (Promega) as recently described.32 For transient transfections, 1 × 105 cells were incubated overnight in 100 µL OptiMEM (Invitrogen), followed by transfection with 0.3 µg promoter construct and 0.016 µg β-galactosidase (β-gal) using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. Forty-eight hours after transfection, the luc activity was determined with the luc substrate (Promega) using a luminometer and normalized to the transfection efficiency determined by β-gal enzyme activity.26
In vivo tumor formation
All animal experiments described were approved by the Regional Council of Halle (Germany). The animals were maintained in accordance with the Guides for the Care and Use of Laboratory Animals. Adult (2-3-month old, 20 ± 4g body weight, male and female), specific pathogen-free in-bred and immune competent DBA/10IaHsd mice (Harlan Laboratories) and immune deficient mice (Fox 1 nude) were used for analysis of tumor formation. These mice were randomly split into three groups, with 10 mice in groups I and II [group I: HER-2/neu+ cells; group II: BGN-transfected HER-2/neu+ cells (Bgn clone 3) and 5 mice in group III (mock-transfected HER-2/neu+ cells), and 1 × 106 cells in 200 µL PBS/mouse were subcutaneously injected into the left lateral abdominal wall. The right lateral abdominal wall was used for sham injections with PBS. Tumor diameter was monitored three times a week by caliper measurements of the greatest longitudinal diameter.
Immunohistochemistry staining and analysis
For staining with anti-HER-2/neu, anti-BGN and anti-CD3 antibodies 5 µm tissue sections of the tumors were deparaffinized with xylol and transferred via alcohol into aqua dest (Elix 5 Filter System, Merck-Millipore). Antigen decloaking for mAb CD3 was performed by steaming the slides with a preheated T-EDTA buffer (ZUC029-500, 1:10 dissolved, Zytomed Systems) at pH 6.0 at 98°C for 30 minutes in an oven (Braun, type 3216). No antigen decloaking was required for staining with BGN and HER-2/neu mAbs. The slides were blocked for 7-10 minutes with 3% H2O2. Following a rinsing step and application of washing buffer (ZUC202-2500, 1:20 solution, Zytochem Plus HRP Kit / Plus Polymer System, Zytomed), the primary mAbs were added dropwise on the tissue area. For BGN the primary mAb 16409-1-AP (Proteintech) was incubated at 1:50 for 60 minutes at room temperature (RT). The HER-2/neu staining was performed as previously described.63 For CD3 the primary mAb SP7 (RM-9107-S, Thermo-Fisher) was incubated at 1:200 for 60 minutes at RT. After vacuuming and washing off the primary Ab, the slides were incubated with a HRP-polymer secondary antibody (POLHRP-100, Zytochem Plus HRP Polymer System Mouse/Rabbit, Zytomed) for 15 (BGN, HER-2/neu) and for 30 minutes (CD3), respectively, at room temperature. After a washing step, the epitopes were visualized with DAB (10 minutes of DAB Substrate Kit, Zytomed), followed by a counterstain with hemalaun (Dr. K. Hollborn & Sons) for 30 seconds, then transferred into xylol and slip covered (Eukitt, ORSAtec). Negative controls were obtained by omitting the primary antibody. Microscopic analysis of the staining was independently performed by two pathologists (CW, DB). The staining intensity of BGN and HER-2/neu expression was scored as absent, weak, moderate or strong. The distribution of intra-tumoral CD3+ T cells was scored as homogenous or non-homogenous, while their density was scored as number of CD3+ cells per 10 high power fields (HPF, 400x).
Blood preparation and analysis
Between day 36 - 41 tumor bearing mice were anesthetised with 2.5% (v/v) isofluran and blood was collected by cardiac puncture into heparin containing tubes. Following lysis of erythrocytes in erythrocyte lysis buffer (c-c-pro GmbH, Germany), the cells were incubated with the rat anti-mouse CD16/32 (Beckman Coulter, Brea, CA, USA) to block non-specific antibody binding. Anti-CD4 PeCy7 (eBioscience/ThermoScientific), anti-CD8α FITC (Beckman Coulter) and anti-CD25 eFluor450 (eBioscience/ThermoScientific) were used. Before acquisition on a Navios flow cytometer (Beckman Coulter), the cells were stained with propidium iodide to exclude dead cells. Analysis was performed using the Kaluza software package (Beckman Coulter).
In silico analysis
The R2 web tool (http://r2.amc.nl) was used to predict the association of ERBB2, BGN, DCN, HLA-B, HLA-C, CD3, CD8, CD25, TGF-β1 and Foxp3 expression with survival of patients with breast cancer. R2 calculates the optimal cut-off in the expression level for each gene and are divided into two groups. The statistical differences in the gene expression values between the patient groups with ‘High’ and ‘Low’ mRNA expressions were evaluated by ANOVA tests implemented in the R2 web tool. The p-values were corrected for multiple testing according to the false discovery rate. All of the cut-off expression levels and their resulting groups are analyzed according to the patients' survival. The cut-off level is reported and was used to generate the Kaplan-Meier curves, which allowed to discriminate patients into ‘good’ and ‘bad’ prognosis cohorts59. Kaplan scan analysis was performed to estimate the overall survival according to the breast cancer microarray dataset called ‘Mixed Tumor Breast’ that included 104 breast cancer and 17 normal breast biopsies with different clinical characteristics.
Statistical analysis
Microsoft Excel version 2010, R (programming language), GenStat 15th Edition were used for student's t-test and one-way ANOVA. A p-value of < 0.05 was considered as significant result (*, p < 0.05; **, p < 0.01; ***, p < 0.001).
Supplementary Material
Funding Statement
Dr. Mildred Scheel Stiftung für Krebsforschung 10.13039/501100005973 (111105); Wilhelm Sander Stiftung (2014.003.1)
Disclose of interest
The authors have no conflict of interest.
Acknowledgments
We would like to thank Sylvi Magdeburg for excellent secretarial help and Jana Beer (Histology Laboratory of the Institute of Pathology) for her comprehensive technical assistance in IHC analysis.
The project was sponsored by a grant of the Wilhelm Sander Stiftung (BS 2014.003.1) as well as by a Roux grant of the Medical Faculty of the Martin Luther University in Halle, Germany.
Financial support
Roux grant of the Medical Faculty of the Martin Luther University in Halle, Germany and a grant from the Sander Stiftung (BS, 2014.003.1)
References
- 1.Geerkens C, Vetter U, Just W, Fedarko NS, Fisher LW, Young MF, Termine JD, Robey PG, Wöhrle D, Vogel W. The X-chromosomal human biglycan gene BGN is subject to X inactivation but is transcribed like an X-Y homologous gene. Hum Genet 1995;96:44-52. doi: 10.1007/BF00214185. PMID:7607653 [DOI] [PubMed] [Google Scholar]
- 2.Schaefer L, Tredup C, Gubbiotti MA, Iozzo RV. Proteoglycan neofunctions: regulation of inflammation and autophagy in cancer biology. FEBS J. 2017;284:10-26. doi: 10.1111/febs.13963. PMID:27860287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iozzo RV, Karamanos N. Proteoglycans in health and disease: emerging concepts and future directions. The FEBS J. 2010;277:3863. doi: 10.1111/j.1742-4658.2010.07796.x. PMID:20812984 [DOI] [PubMed] [Google Scholar]
- 4.Xing X, Gu X, Ma T, Ye H. Biglycan up-regulated vascular endothelial growth factor (VEGF) expression and promoted angiogenesis in colon cancer. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine. 2015;36:1773-80. doi: 10.1007/s13277-014-2779-y. PMID:25371074 [DOI] [PubMed] [Google Scholar]
- 5.Frey H, Schroeder N, Manon-Jensen T, Iozzo RV, Schaefer L. Biological interplay between proteoglycans and their innate immune receptors in inflammation. FEBS J. 2013;280:2165-79. doi: 10.1111/febs.12145. PMID:23350913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Popovic ZV, Wang S, Papatriantafyllou M, Kaya Z, Porubsky S, Meisner M, Bonrouhi M, Burgdorf S, Young MF, Schaefer L, et al.. The proteoglycan biglycan enhances antigen-specific T cell activation potentially via MyD88 and TRIF pathways and triggers autoimmune perimyocarditis. J Immunol. 2011;187:6217-26. doi: 10.4049/jimmunol.1003478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu L, Duan YT, Li JF, Su LP, Yan M, Zhu ZG, Liu BY, Yang QM. Biglycan enhances gastric cancer invasion by activating FAK signaling pathway. Oncotarget. 2014;5:1885-96. doi: 10.18632/oncotarget.1871. PMID:24681892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Y, Li W, Li X, Tai Y, Lu Q, Yang N, Jiang J. Expression and significance of biglycan in endometrial cancer. Archives of gynecology and obstetrics. 2014;289:649-55. doi: 10.1007/s00404-013-3017-3. PMID:24013431 [DOI] [PubMed] [Google Scholar]
- 9.Zhu YH, Yang F, Zhang SS, Zeng TT, Xie X, Guan XY. High expression of biglycan is associated with poor prognosis in patients with esophageal squamous cell carcinoma. Int J Clin Exp Pathol. 2013;6:2497-505. PMID:24228112 [PMC free article] [PubMed] [Google Scholar]
- 10.Berendsen AD, Pinnow EL, Maeda A, Brown AC, McCartney-Francis N, Kram V, Owens RT, Robey PG, Holmbeck K, de Castro LF, et al.. Biglycan modulates angiogenesis and bone formation during fracture healing. Matrix biology: journal of the International Society for Matrix Biology. 2014;35:223-31. doi: 10.1016/j.matbio.2013.12.004. PMID:24373744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berendsen AD, Fisher LW, Kilts TM, Owens RT, Robey PG, Gutkind JS, Young MF. Modulation of canonical Wnt signaling by the extracellular matrix component biglycan. Proc Natl Acad Sci U S A. 2011;108:17022-7. doi: 10.1073/pnas.1110629108. PMID:21969569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun H, Wang X, Zhang Y, Che X, Liu Z, Zhang L, Qiu C, Lv Q, Jiang J. Biglycan enhances the ability of migration and invasion in endometrial cancer. Arch Gynecol Obstet. 2016;293:429-38. doi: 10.1007/s00404-015-3844-5. PMID:26275380 [DOI] [PubMed] [Google Scholar]
- 13.Niedworok C, Rock K, Kretschmer I, Freudenberger T, Nagy N, Szarvas T, Vom Dorp F, Reis H, Rübben H, Fischer JW. Inhibitory role of the small leucine-rich proteoglycan biglycan in bladder cancer. PloS One. 2013;8:e80084. doi: 10.1371/journal.pone.0080084. PMID:24223213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schaefer L, Babelova A, Kiss E, Hausser HJ, Baliova M, Krzyzankova M, Marsche G, Young MF, Mihalik D, Götte M, et al.. The matrix component biglycan is proinflammatory and signals through Toll-like receptors 4 and 2 in macrophages. J Clin Invest. 2005;115:2223-33. doi: 10.1172/JCI23755. PMID:16025156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iozzo RV, Schaefer L. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix biology: journal of the International Society for Matrix Biology. 2015;42:11-55. doi: 10.1016/j.matbio.2015.02.003. PMID:25701227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neill T, Schaefer L, Iozzo RV. Decorin: a guardian from the matrix. Am J Pathol. 2012;181:380-7. doi: 10.1016/j.ajpath.2012.04.029. PMID:22735579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bostrom P, Sainio A, Kakko T, Savontaus M, Soderstrom M, Jarvelainen H. Localization of decorin gene expression in normal human breast tissue and in benign and malignant tumors of the human breast. Histochem Cell Biol. 2013;139:161-71. doi: 10.1007/s00418-012-1026-0. PMID:23007289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nyman MC, Sainio AO, Pennanen MM, Lund RJ, Vuorikoski S, Sundstrom JT, Järveläinen HT. Decorin in Human Colon Cancer: Localization In Vivo and Effect on Cancer Cell Behavior In Vitro. The journal of histochemistry and cytochemistry: official journal of the Histochemistry Society. 2015;63:710-20. doi: 10.1369/0022155415590830. PMID:26001829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santra M, Skorski T, Calabretta B, Lattime EC, Iozzo RV. De novo decorin gene expression suppresses the malignant phenotype in human colon cancer cells. Proc Natl Acad Sci U S A. 1995;92:7016-20. doi: 10.1073/pnas.92.15.7016. PMID:7624361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suhovskih AV, Aidagulova SV, Kashuba VI, Grigorieva EV. Proteoglycans as potential microenvironmental biomarkers for colon cancer. Cell Tissue Res. 2015;361:833-44. doi: 10.1007/s00441-015-2141-8. PMID:25715761 [DOI] [PubMed] [Google Scholar]
- 21.Sainio A, Nyman M, Lund R, Vuorikoski S, Bostrom P, Laato M, Boström PJ, Järveläinen H. Lack of decorin expression by human bladder cancer cells offers new tools in the therapy of urothelial malignancies. PloS One. 2013;8:e76190. doi: 10.1371/journal.pone.0076190. PMID:24146840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Biglari A, Bataille D, Naumann U, Weller M, Zirger J, Castro MG, Lowenstein PR. Effects of ectopic decorin in modulating intracranial glioma progression in vivo, in a rat syngeneic model. Cancer Gene Ther. 2004;11:721-32. doi: 10.1038/sj.cgt.7700783. PMID:15475879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farace C, Oliver JA, Melguizo C, Alvarez P, Bandiera P, Rama AR, Malaguarnera G, Ortiz R, Madeddu R, Prados J. Microenvironmental Modulation of Decorin and Lumican in Temozolomide-Resistant Glioblastoma and Neuroblastoma Cancer Stem-Like Cells. PloS one. 2015;10:e0134111. doi: 10.1371/journal.pone.0134111. PMID:26230845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neill T, Schaefer L, Iozzo RV. Oncosuppressive functions of decorin. Mol Cell Oncol. 2015;2:e975645. doi: 10.4161/23723556.2014.975645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neill T, Schaefer L, Iozzo RV. Decoding the Matrix: Instructive Roles of Proteoglycan Receptors. Biochemistry. 2015;54:4583-98. doi: 10.1021/acs.biochem.5b00653. PMID:26177309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Recktenwald CV, Mendler S, Lichtenfels R, Kellner R, Seliger B. Influence of Ki-ras-driven oncogenic transformation on the protein network of murine fibroblasts. Proteomics. 2007;7:385-98. doi: 10.1002/pmic.200600506. PMID:17211828 [DOI] [PubMed] [Google Scholar]
- 27.Recktenwald CV, Leisz S, Steven A, Mimura K, Muller A, Wulfanger J, Kiessling R, Seliger B. HER-2/neu-mediated down-regulation of biglycan associated with altered growth properties. J Biol Chem. 2012;287:24320-9. doi: 10.1074/jbc.M111.334425. PMID:22582394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steven A, Leisz S, Massa C, Iezzi M, Lattanzio R, Lamolinara A, Bukur J, Müller A, Hiebl B, Holzhausen HJ, et al.. HER-2/neu mediates oncogenic transformation via altered CREB expression and function. Mol Cancer Res: MCR. 2013;11:1462-77. doi: 10.1158/1541-7786.MCR-13-0125. PMID:24025972 [DOI] [PubMed] [Google Scholar]
- 29.Gutierrez C, Schiff R. HER2: biology, detection, and clinical implications. Archives of pathology & laboratory medicine. 2011;135:55-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu C, Wang Z, Cui R, He H, Lin X, Sheng Y, Zhang H. Co-expression of parathyroid hormone related protein and TGF-beta in breast cancer predicts poor survival outcome. BMC cancer. 2015;15:925. doi: 10.1186/s12885-015-1873-x. PMID:26597083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Samarakoon R, Dobberfuhl AD, Cooley C, Overstreet JM, Patel S, Goldschmeding R, et al.. Induction of renal fibrotic genes by TGF-beta1 requires EGFR activation, p53 and reactive oxygen species. Cell Signal. 2013;25: 2198-209. doi: 10.1016/j.cellsig.2013.07.007. PMID:23872073 [DOI] [PubMed] [Google Scholar]
- 32.Herrmann F, Lehr HA, Drexler I, Sutter G, Hengstler J, Wollscheid U, Seliger B. HER-2/neu-mediated regulation of components of the MHC class I antigen-processing pathway. Cancer Res. 2004;64:215-20. doi: 10.1158/0008-5472.CAN-2522-2. PMID:14729627 [DOI] [PubMed] [Google Scholar]
- 33.Maruyama T, Mimura K, Sato E, Watanabe M, Mizukami Y, Kawaguchi Y, Ando T, Kinouchi H, Fujii H, Kono K. Inverse correlation of HER2 with MHC class I expression on oesophageal squamous cell carcinoma. Br J Cancer. 2010;103:552-9. doi: 10.1038/sj.bjc.6605772. PMID:20628381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Milano F, Guarriera M, Rygiel AM, Krishnadath KK. Trastuzumab mediated T-cell response against HER-2/neu overexpressing esophageal adenocarcinoma depends on intact antigen processing machinery. PloS One. 2010;5:e12424. doi: 10.1371/journal.pone.0012424. PMID:20865050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mimura K, Ando T, Poschke I, Mougiakakos D, Johansson CC, Ichikawa J, Okita R, Nishimura MI, Handke D, Krug N, et al.. T cell recognition of HLA-A2 restricted tumor antigens is impaired by the oncogene HER2. Int J Cancer. 2011;128:390-401. doi: 10.1002/ijc.25613. PMID:20715101 [DOI] [PubMed] [Google Scholar]
- 36.Mimura K, Shiraishi K, Mueller A, Izawa S, Kua LF, So J, Yong WP, Fujii H, Seliger B, Kiessling R, et al.. The MAPK pathway is a predominant regulator of HLA-A expression in esophageal and gastric cancer. J Immunol. 2013;191:6261-72. doi: 10.4049/jimmunol.1301597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bukur J, Herrmann F, Handke D, Recktenwald C, Seliger B. Identification of E2F1 as an important transcription factor for the regulation of tapasin expression. J Biol Chem. 2010;285:30419-26. doi: 10.1074/jbc.M109.094284. PMID:20663889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodriguez GM, Bobbala D, Serrano D, Mayhue M, Champagne A, Saucier C, Steimle V, Kufer TA, Menendez A, Ramanathan S, et al.. NLRC5 elicits antitumor immunity by enhancing processing and presentation of tumor antigens to CD8(+) T lymphocytes. Oncoimmunology. 2016;5:e1151593. doi: 10.1080/2162402X.2016.1151593. PMID:27471621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clifton GT, Peoples GE. Overcoming cancer immune tolerance and escape. Clinical cancer research: an official journal of the American Association for Cancer Research. 2009;15:749-51. doi: 10.1158/1078-0432.CCR-08-2805. PMID:19188142 [DOI] [PubMed] [Google Scholar]
- 40.Spranger S. Mechanisms of tumor escape in the context of the T-cell-inflamed and the non-T-cell-inflamed tumor microenvironment. Int Immunol. 2016;28:383-91. doi: 10.1093/intimm/dxw014. PMID:26989092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arina A, Corrales L, Bronte V. Enhancing T cell therapy by overcoming the immunosuppressive tumor microenvironment. Semin Immunol. 2016;28:54-63. doi: 10.1016/j.smim.2016.01.002. PMID:26872631 [DOI] [PubMed] [Google Scholar]
- 42.Garrido F, Aptsiauri N, Doorduijn EM, Garcia Lora AM, van Hall T. The urgent need to recover MHC class I in cancers for effective immunotherapy. Curr Opin Immunol. 2016;39:44-51. doi: 10.1016/j.coi.2015.12.007. PMID:26796069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moreth K, Frey H, Hubo M, Zeng-Brouwers J, Nastase MV, Hsieh LT, Haceni R, Pfeilschifter J, Iozzo RV, Schaefer L. Biglycan-triggered TLR-2- and TLR-4-signaling exacerbates the pathophysiology of ischemic acute kidney injury. Matrix biology: journal of the International Society for Matrix Biology. 2014;35:143-51. doi: 10.1016/j.matbio.2014.01.010. PMID:24480070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hu L, Zang MD, Wang HX, Li JF, Su LP, Yan M, Li C, Yang QM, Liu BY, Zhu ZG. Biglycan stimulates VEGF expression in endothelial cells by activating the TLR signaling pathway. Mol Oncol. 2016;10:1473-84. doi: 10.1016/j.molonc.2016.08.002. PMID:27590684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Darb-Esfahani S, Kunze CA, Kulbe H, Sehouli J, Wienert S, Lindner J, Budczies J, Bockmayr M, Dietel M, Denkert C, et al.. Prognostic impact of programmed cell death-1 (PD-1) and PD-ligand 1 (PD-L1) expression in cancer cells and tumor-infiltrating lymphocytes in ovarian high grade serous carcinoma. Oncotarget. 2016;7(2): 1486-1499. doi: 10.18632/oncotarget.6429. PMID:26625204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pickup M, Novitskiy S, Moses HL. The roles of TGFbeta in the tumour microenvironment. Nat Rev Cancer. 2013;13:788-99. doi: 10.1038/nrc3603. PMID:24132110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bandyopadhyay A, Lopez-Casillas F, Malik SN, Montiel JL, Mendoza V, Yang J, Sun LZ. Antitumor activity of a recombinant soluble betaglycan in human breast cancer xenograft. Cancer Res. 2002;62:4690-5. PMID:12183427 [PubMed] [Google Scholar]
- 48.Wang SE, Xiang B, Guix M, Olivares MG, Parker J, Chung CH, Pandiella A, Arteaga CL. Transforming growth factor beta engages TACE and ErbB3 to activate phosphatidylinositol-3 kinase/Akt in ErbB2-overexpressing breast cancer and desensitizes cells to trastuzumab. Mol Cell Biol. 2008;28:5605-20. doi: 10.1128/MCB.00787-08. PMID:18625725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hara T, Yoshida E, Shinkai Y, Yamamoto C, Fujiwara Y, Kumagai Y, et al.. Biglycan Intensifies ALK5-Smad2/3 Signaling by TGF-beta1 and Downregulates Syndecan-4 in Cultured Vascular Endothelial Cells. J Cell Biochem. 2016;118:1087-96. doi: 10.1002/jcb.25721. PMID:26990420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Groth S, Schulze M, Kalthoff H, Fandrich F, Ungefroren H. Adhesion and Rac1-dependent regulation of biglycan gene expression by transforming growth factor-beta. Evidence for oxidative signaling through NADPH oxidase. J Biol Chem. 2005;280:33190-9. doi: 10.1074/jbc.M504249200. PMID:16051607 [DOI] [PubMed] [Google Scholar]
- 51.Nagaraju K, Raben N, Loeffler L, Parker T, Rochon PJ, Lee E, Danning C, Wada R, Thompson C, Bahtiyar G, et al.. Conditional up-regulation of MHC class I in skeletal muscle leads to self-sustaining autoimmune myositis and myositis-specific autoantibodies. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:9209-14. doi: 10.1073/pnas.97.16.9209. PMID:10922072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hsiao YW, Liao KW, Chung TF, Liu CH, Hsu CD, Chu RM. Interactions of host IL-6 and IFN-gamma and cancer-derived TGF-beta1 on MHC molecule expression during tumor spontaneous regression. Cancer Immunol Immunother: CII. 2008;57:1091-104. doi: 10.1007/s00262-007-0446-5. PMID:18259750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen XH, Liu ZC, Zhang G, Wei W, Wang XX, Wang H, et al.. TGF-beta and EGF induced HLA-I downregulation is associated with epithelial-mesenchymal transition (EMT) through upregulation of snail in prostate cancer cells. Mol Immunol. 2015;65:34-42. doi: 10.1016/j.molimm.2014.12.017. PMID:25618241 [DOI] [PubMed] [Google Scholar]
- 54.Fu YF, Gui R, Liu J. HER-2-induced PI3K signaling pathway was involved in the pathogenesis of gastric cancer. Cancer Gene Therapy. 2015;22:145-53. doi: 10.1038/cgt.2014.80. PMID:25613482 [DOI] [PubMed] [Google Scholar]
- 55.Stander M, Naumann U, Dumitrescu L, Heneka M, Loschmann P, Gulbins E, Dichgans J, Weller M. Decorin gene transfer-mediated suppression of TGF-beta synthesis abrogates experimental malignant glioma growth in vivo. Gene Therapy. 1998;5:1187-94. doi: 10.1038/sj.gt.3300709. PMID:9930319 [DOI] [PubMed] [Google Scholar]
- 56.Henderson MA, Danks JA, Slavin JL, Byrnes GB, Choong PF, Spillane JB, Hopper JL, Martin TJ. Parathyroid hormone-related protein localization in breast cancers predict improved prognosis. Cancer Res. 2006;66(4):2250-6. doi: 10.1158/0008-5472.CAN-05-2814. PMID:16489028 [DOI] [PubMed] [Google Scholar]
- 57.Wang SE, Xiang B, Guix M, Olivares MG, Parker J, Chung CH, Pandiella A, Arteaga CL. Transforming growth factor β engages TACE and ErbB3 to activate PI3K/Akt in erbB2-overexpressing breast cancer and desensitizes cells to trastuzumab. Mol Cell Biol. 2008;28:5605-20. doi: 10.1128/MCB.00787-08. PMID:18625725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gu X, Ma Y, Xiao J, Zheng H, Song C, Gong Y, Xing X. Up-regulated biglycan expression correlates with the malignancy in human colorectal cancers. Clin Exp Med. 2012;12:195-9. doi: 10.1007/s10238-011-0155-4. PMID:21879307 [DOI] [PubMed] [Google Scholar]
- 59.Weber CK, Sommer G, Michl P, Fensterer H, Weimer M, Gansauge F, Leder G, Adler G, Gress TM. Biglycan is overexpressed in pancreatic cancer and induces G1-arrest in pancreatic cancer cell lines. Gastroenterology. 2001;121:657-67. doi: 10.1053/gast.2001.27222. PMID:11522750 [DOI] [PubMed] [Google Scholar]
- 60.Nishino R, Honda M, Yamashita T, Takatori H, Minato H, Zen Y, Sasaki M, Takamura H, Horimoto K, Ohta T, et al.. Identification of novel candidate tumour marker genes for intrahepatic cholangiocarcinoma. J Hepatol. 2008;49:207-16. doi: 10.1016/j.jhep.2008.03.025. PMID:18490072 [DOI] [PubMed] [Google Scholar]
- 61.Wulfaenger J, Niedling S, Riemann D, Seliger B. Aminopeptidase N (APN)/CD13-dependent CXCR4 downregulation is associated with diminished cell migration, proliferation and invasion. Mol Membr Biol. 2008;25:72-82. doi: 10.1080/09687680701551855. PMID:18097955 [DOI] [PubMed] [Google Scholar]
- 62.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970;227:680-5. doi: 10.1038/227680a0. PMID:5432063 [DOI] [PubMed] [Google Scholar]
- 63.Steven A, Leisz S, Sychra K, Hiebl B, Wickenhauser C, Mougiakakos D, Kiessling R, Denkert C, Seliger B. Hypoxia-mediated alterations and their role in the HER-2/neuregulated CREB status and localization. Oncotarget. 2016;7:52061-84. doi: 10.18632/oncotarget.10474. PMID:27409833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cimmino F, Pezone L, Avitabile M, Acierno G, Andolfo I, Capasso M, Iolascon A. Inhibition of hypoxia inducible factors combined with all-trans retinoic acid treatment enhances glial transdifferentiation of neuroblastoma cells. Sci Rep. 2015;5:11158. doi: 10.1038/srep11158. PMID:26057707 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


