ABSTRACT
Obinutuzumab (OBZ) shows stronger antibody-dependent cell cytotoxicity (ADCC) compared to rituximab and improved clinical activity for treating certain CD20+ neoplasia. However, the efficacy of monoclonal antibody (mAb) as a monotherapy is limited. Natural Killer (NK) cells are mediators of ADCC. Hematological cancer patients possess antitumor NK cells that are unable to control disease, possibly because they are dysfunctional. The immunomodulatory drug lenalidomide (LEN) could be a treatment to restore exhausted NK cell cytotoxic functions. The clinical trial GALEN is a Phase Ib/II study of OBZ combined with LEN for the treatment of relapsed/refractory follicular and aggressive (DLBCL and MCL) B-cell Lymphoma. During treatment, we analyzed specific aspects of NK cell biology. Treatment reversed the immature NK phenotype of patients and increased expression of NK activating receptors. Inhibitory receptors were either unchanged or decreased. There was a strong NK response at the end of the 1st cycle: NK number and intracellular granzyme B (GrzB) expression decreased, degranulation increased and NK responded better to allogeneic target challenge. Moreover, the interaction of NK cells with B cell targets, measured by trogocytosis, decreased during treatment. At the end of treatment, when target cells had been wiped out, the proportion of reactive NK cells (CD69+, CD45RARO+, CD107a+, CD19+) strongly decreased. Because all patients received LEN and OBZ, it was uncertain which drug was responsible of our observations, or even if a combination of both products was necessary for the described effects on this lymphocyte lineage.
KEYWORDS: diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL), lenalidomide, obinutuzumab, NK cell
Introduction
The anti-CD20 IgG1 monoclonal antibody (mAb) rituximab (RTX) has improved the treatment of B-cells lymphocytic leukemia (B-CLL) and B-cells non-Hodgkin lymphomas (B-NHL). Its success is related to its capacity to induce Fc- (antibody-dependent cell-mediated cytotoxicity (ADCC). One receptor for human IgG1 is FcγRIIIa (CD16 a), which is expressed on natural killer (NK) cells and macrophages. The influence of FcγRIIIa-158VF polymorphism on RTX clinical response strongly suggests that ADCC is critical.1 Based on these results, there has been an attempt to produce new anti-CD20 mAbs that exhibit higher affinity for FcγRIIIa either by Fc mutations or by glycoengeenering.2,3 This later strategy, leading to low fucose content of the N-glycan, is currently under clinical investigations in B-cell malignancies with the mAb obinutuzumab (OBZ; previously GA101, Roche, Genentech), which shows stronger ADCC in vitro and in a lymphoma xenograft mouse model compared to RTX4 and improved clinical activity for treating chronic lymphocytic leukemia (CLL).5 This clinical benefit has been observed in other B-cell malignancies.4,6,7 OBZ is approved for first-line CLL in association with chlorambucil and in combination with bendamustine for the treatment of patients with follicular lymphoma (FL) who relapse or are refractory to RTX-containing regimen.8
However, it is remarkable to note that the mAbs themselves have modest clinical activity. For example, RTX or OBZ when used as monotherapy in patients with relapsed follicular lymphoma have demonstrated short progression-free survival (PFS).8 These data indicate that there is a need to optimize their use in co-therapy. In this sense, hematological cancer patients possess antitumor NK cells that are unable to control disease.9,10 Blood-borne cancer cells use different mechanisms for immune escape,11,12 e.g. inducing NK cell dysfunction.13,14 In addition, NK cell differentiation may be inhibited by the presence of tumor cells e.g. acute myeloid leukemia (AML) cells infiltrating bone-marrow.15,16 Therefore, the failure of mAb as monotherapy could be related to impaired NK cell function and hence, there is a clinical interest to reactivate patient NK cells.17
Lenalidomide (LEN; Revlimid; Celgene) is an immune-modulatory drug that can activate NK cells.14,18–21 LEN treatment during and after stem cell transplantation (SCT) increases NK cell proliferation, enhances NKp44 expression on NK cells14 and increases circulating NK-cell numbers in leukemia patients.22,23 LEN increases co-stimulatory receptor expression on NK cells, such as CD16 and Lymphocytes Function-associated Antigen (LFA)14 and stabilizes NK cell:target cell immunological synapse.20,23,24 These effects lead to increased cytotoxic activity and increased proliferation of LEN-stimulated NK cells.14,19,20 LEN has similar effects in B-NHL patients restoring synapse formation, ADCC, and cytotoxic functions in NK cells.25,26 Of particular clinical importance, LEN allows NK cells to be activated by lower doses of RTX.20 Finally, it also favors target recognition by inducing expression of NKG2D and DNAM-1 ligands on malignant cells.27 LEN mechanism of action is thus predominantly immune-mediated, making LEN a suitable treatment to restore exhausted NK cell cytotoxic functions.
With this view, the clinical trial GALEN is a Phase Ib/II study of OBZ combined with LEN for the treatment of relapsed/refractory follicular and aggressive B-cell lymphoma (diffuse large B-cell lymphoma (DLBCL) and mantle cell lymphoma (MCL) by the LYSA Lymphoma Study Association. The primary objective of the Phase IB part of the study was to determine the recommended dose (RD) of LEN when administered in association with OBZ. The primary objective of the Phase II part of the study was to assess the efficacy of the association of the recommended dose of LEN in combination with OBZ, as measured by the overall response rate (ORR) at the end of 6 cycles in these 2 different populations of lymphoma patients. We developed a pilot exploration of some specific aspects of NK cell biology. In this respect, we monitored the following time points: i) C1D1 predose; ii) C1D28 and iii) C6D28 (supplemental Fig. 1).
Results
Effect of treatment on lymphocyte populations
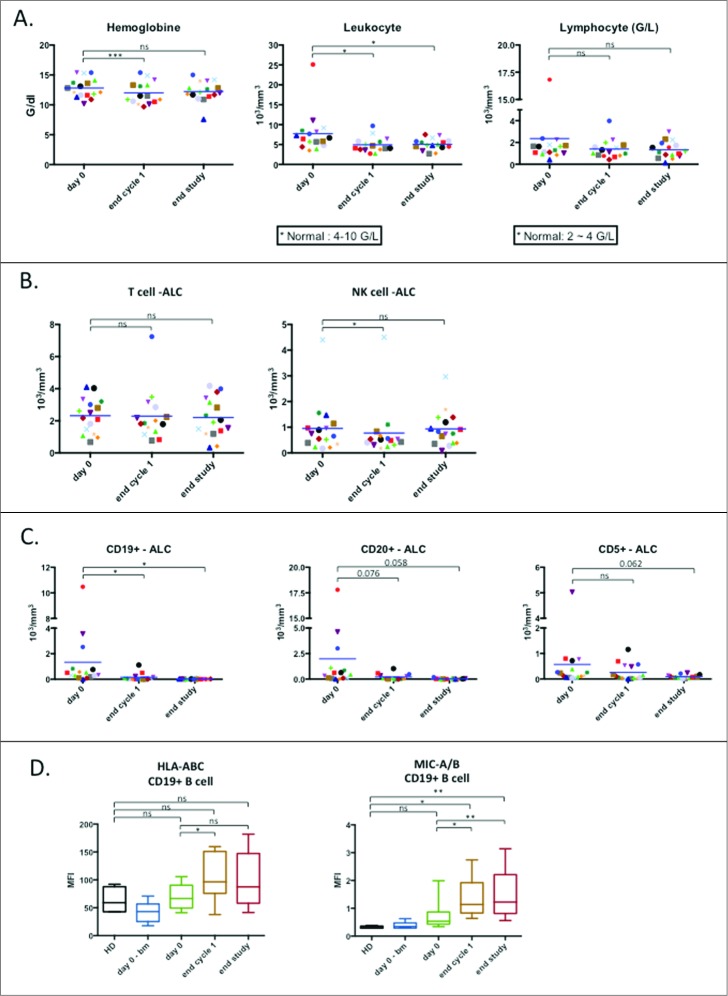
Patients were treated with a combination of LEN (orally administered) and 3 doses of OBZ in the first cycle and a single dose on the first day of the following for total of six consecutive treatment cycles (see supplemental Fig. 1 for treatment and sampling protocol). We did not observe differences in the NK cell parameters tested between the different lymphoma types in our pilot study, hence we analyzed them together (both FL and DLBCL patients). We observed a transient decrease in hemoglobin levels, a significant decrease in leucocytes and a trend towards a decrease in lymphocytes (Fig. 1A). T cell numbers were unchanged and there was a transient decrease in NK cells at the end of the first cycle (Fig. 1B). B cells (CD19+) decreased in numbers (Fig. 1C). The CD20+ population, which is the main target of OBZ, showed a tendency to decrease (Fig. 1C), similar to CD5+ cells (Fig. 1C). The remaining CD19+ cells showed increased expression of the major histocompatibility complex-I (MHC-I), as has been observed in other hematological neoplasias14; but also of the stress ligands MHC class I polypeptide-related sequence A (MICA) and MICB (Fig. 1D). The increased expression of MHC-I and MICA/B could have countervailing effects on NK cells because they are recognized by KIRs, inhibitory receptors, and NKG2D, activating receptor, respectively. Hence the final effect on NK cell recognition in remaining target cells is unclear.
Figure 1.
Effect of treatment on lymphocyte populations. (A) Absolute values of hemoglobin (left), total leukocyte count (middle) and total lymphocyte count (right) are reported as before the first induction (day 0), after first round of treatment (end cycle 1) and after the final round of treatment (end study) respectively (n = 16). (B) Absolute count of T lymphocyte (CD3+CD56-) population and NK cell (CD3-CD56+) populations from total PBMC (n = 16). (C) Absolute count of lymphocyte populations carrying specific markers associated with B-cell lymphoma (n = 16). (D) Expression of HLA and the stress ligands MIC-A and MIC-B on CD19+ population in term of mean fluorescence intensity (HD: n = 4; patients: n = 10). Significance was determined by paired t-test between day 0 versus following time-points, and one-way ANOVA between HD and patients at every time-points with * p ≤ 0.05, ** p ≤ 0.01 and *** p ≤ 0.001.
Treatment induces maturation of the immature NK cell population
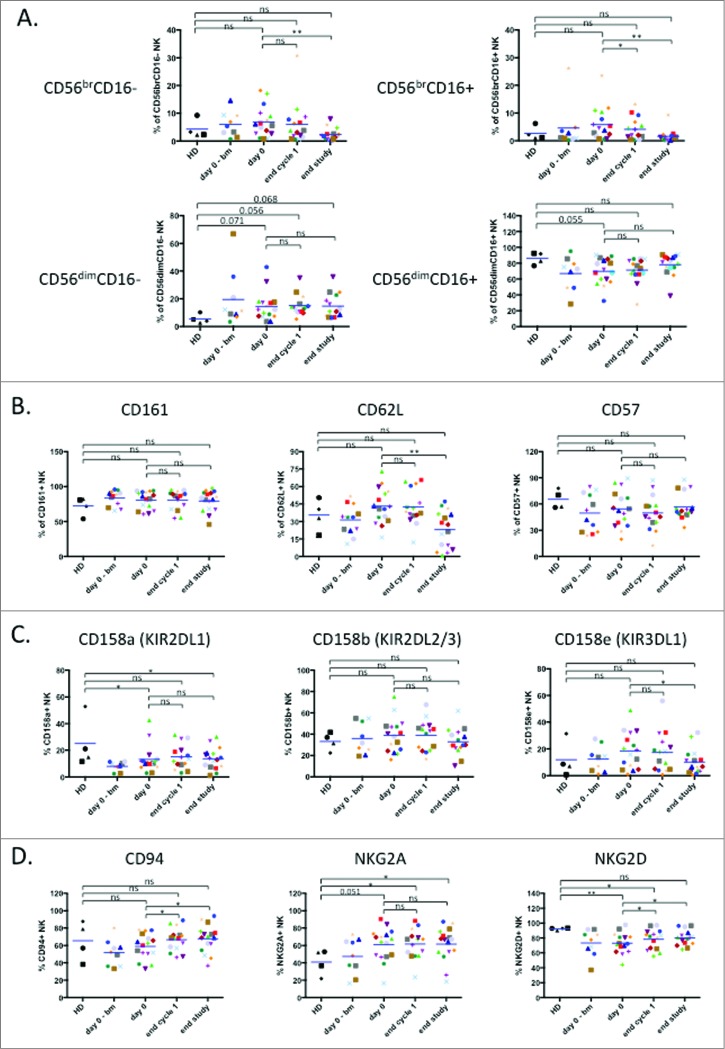
We next directly investigated the physiological status of NK cells during treatment. In the peripheral blood, human NK cells are mostly CD3−CD56dim cells with high cytotoxic activity, while CD3−CD56bright cells excel in cytokine production28. In vitro evidence indicates that CD56bright NK cells are precursors of CD56dim NK cells and this might also be the case in vivo29. In addition, combined analysis of CD56 and CD16 expression during NK cell development indicates that their profiles changes as follows: CD56brightCD16− → CD56brightCD16dim→ CD56dimCD16dim→ CD56dimCD16+. Additional markers can be used to identify specific subsets within these NK cell populations30,31. As previously described9,10, we observed a tendency to a higher proportion of immature NK cells in patients compare to HD, which correlated with a decrease in the full mature CD56dimCD16+ (Fig. 2A). At the end of treatment most patients lost the immature subsets and gained a NK distribution similar to healthy donors (Fig. 2A), i.e. with less immature cells.
Figure 2.
Treatment induces maturation of the immature NK cell population. (A) Analysis of NK cell subpopulations based on level of CD56 and CD16 expression that divides NK cells in 4 subpopulations: CD56brCD16− (top, left panel), CD56brCD16+ (top, right panel), CD56dimCD16− (bottom, left panel) and CD56dimCD16+ (bottom, right panel). (B) Assessment of NK cell maturation status determined by expression of the maturation markers CD161, CD62 L and CD57. (C) Expression of several KIR receptors on healthy donor and patient NK cells: CD158 a (KIR2DL1), CD158b (KIR2DL2/3) and CD158e (KIR3DL1). (D) Expression of the inhibitory heterodimer complex NKG2 A/CD94 and the activating receptor NKG2D. Patient: n = 16, healthy donor: n = 4. Significance was determined by paired t-test between day 0 versus following time-points, and one-way ANOVA between HD and patients at every time-points with * p ≤ 0.05, ** p ≤ 0.01 and *** p ≤ 0.001.
We next analyzed the maturation marker CD161-killer cell lectin-like receptor subfamily B, member 1 (KLRB1) that is expressed early in NK cell development and before CD5632. The expression of this marker did not change during treatment in the CD56+ NK compartment (Fig. 2B).
During in vivo maturation CD56bright cells become CD56dimCD62L+CD57− cells that produce perforin, while maintaining high IFN-γ production in response to cytokines28,33. On the other hand, CD56dimCD62L−CD57+ cells show low response to cytokines and higher cytotoxic capacity28,34. CD62L was slightly increased in patients and the treatment decreased the expression (Fig. 2B). In contrast CD57 was lower in patients and remained unchanged by the treatment (Fig. 2B). This suggests that at the end of treatment the NK cells show decreased expression of an immature marker, i.e. CD62L. When NK cells reach fully mature CD56dimCD16+ status, they gain full expression of killer inhibitory receptors (KIRs) receptors. KIR expression in patients before and after treatment was variable and expression of the 3 KIRs taken together was similar in patients and healthy donors (Fig. 2C).
The CD94 glycoprotein heterodimerizes with the natural-killer group 2 (NKG2) receptors, which are type II transmembrane proteins. CD94/NKG2 A is an inhibitory receptor that recognizes HLA-E and it is the first inhibitory receptor expressed during NK cell maturation32,35. CD94 can also associate with the activating receptors NKG2C and E32,35. The activating receptor NKG2D represents an exception: it is a homodimer32,35. CD94 was lower in patients and increased on treatment (Fig. 2D). In contrast, NKG2A was higher in patients and was not modified by treatment (Fig. 2D). NKG2D, which was lower in patients, significantly increased after treatment (Fig. 2D). In summary, NK activating receptors tend to increase while inhibitory receptors are either unchanged or decreased.
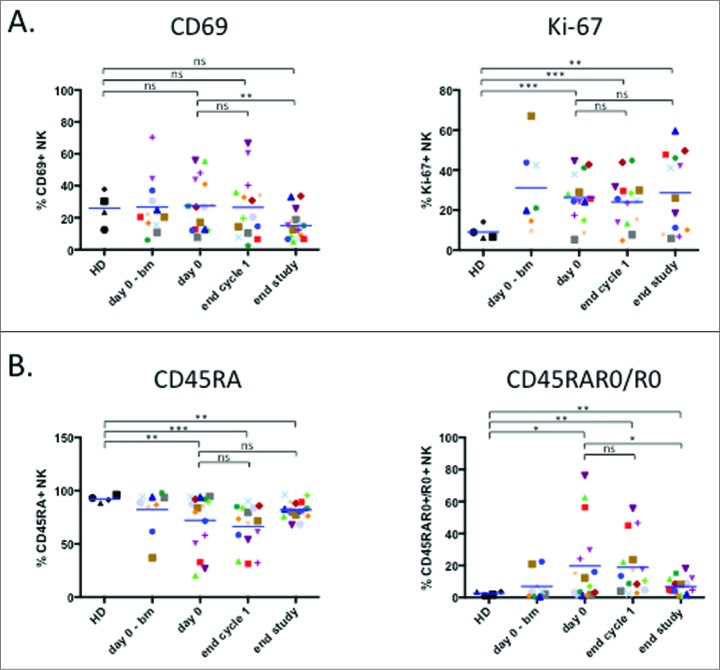
Treatment decreases the activated NK cell population
The proliferation marker Ki-67 is increased in NK cells from hematological cancer patients.9,10 Fig. 3A showed that the elevated values remained unchanged during treatment. In contrast, levels of the activation marker CD69, which were similar to healthy donors, decreased at the end of treatment (Fig. 3A).
Figure 3.
Treatment decreases the activated NK cell population. (A) Proliferation potency of NK cell presented as intracellular staining of the nuclear marker Ki-67 and NK activation status determined by expression of the activation marker CD69. (B) Analysis of NK populations based on differential expression of CD45 isoforms: CD45RA+ (left panel) and CD45RA+R0+ plus CD45R0+ (right panel). Patient: n = 16, healthy donor: n = 4. Significance was determined by paired t-test between day 0 versus following time-points, and one-way ANOVA between HD and patients at every time-points with * p ≤ 0.05, ** p ≤ 0.01 and *** p ≤ 0.001.
The antitumor NK cell population is easily recognized by expression of CD45RO (CD45RO cells) in general together with CD45RA (CD45RARO cells). Patients show high levels of these cells, leading to a decrease in the CD45RA+RO− population (CD45RA cells).9,10 Patients in our cohort clearly showed this phenotype (Fig. 3B). At the end of treatment this phenotype tended to converge versus a healthy donor phenotype with increase in CD45RA cells and a decrease in CD45RO+ cells. Taken together these data suggest that elimination of target cells decreases NK cell activation status.
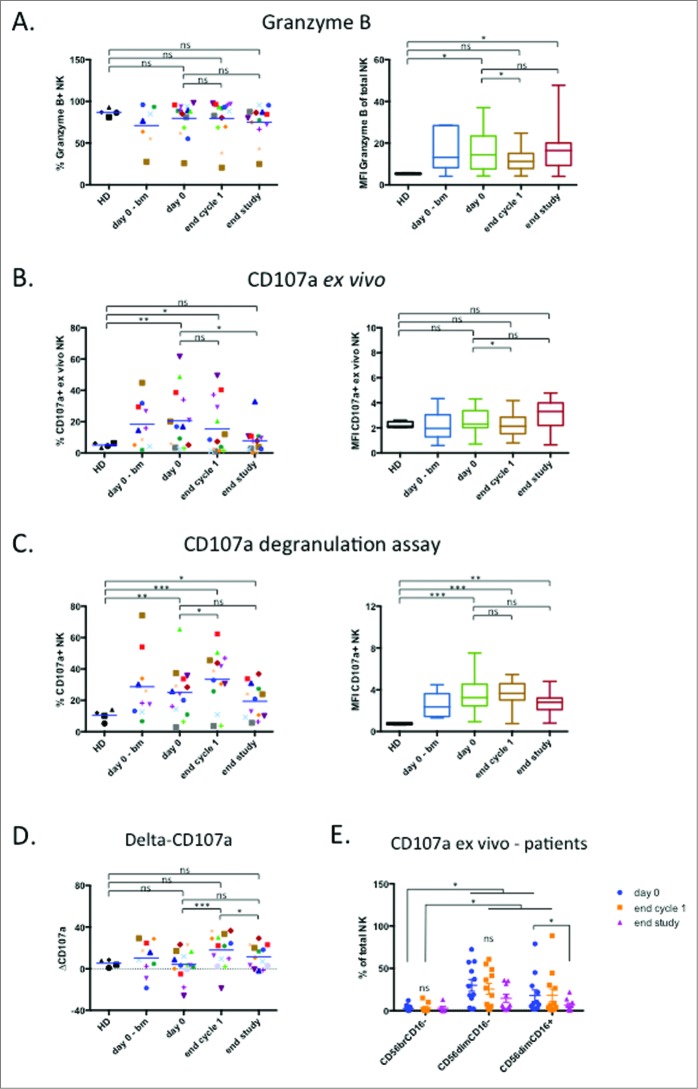
Treatment modulates NK cell cytotoxic activity
If NK cell target cells were disappearing, we also expect to find a decrease in NK degranulation because cytotoxicity is probably the main antitumor function of these lymphocytes in hematological neoplasias.9,10 The proportion of granzyme+ cells was similar to healthy donors and slightly decreased after treatment (Fig. 4A). The amount of granzyme, as measured by the median fluorescence intensity (MFI) values, was higher in patients, underwent a significant reduction at the end of cycle 1, and recovered to normal values at the end of treatment (Fig. 4A). In agreement with our previous results,9,10 we observed that more NK cells were degranulating in patients, measured by CD107a expression in the plasma membrane (Fig. 4B). At the end of treatment the proportion of degranulating cells had significantly decreased (Fig. 4B). NK cells degranulated at similar levels at the beginning and at the end of the treatment against the allogeneic non-Hodgkin B lymphoma cell line Daudi (Fig. 4C and D). Interestingly, after the first cycle NK cells were more active against the allogeneic targets (Fig. 4C and D). This suggests that the presence of targets cells and the treatment activate NK cells in vivo. CD56dim cells were responsible for CD107a expression ex vivo, whereas CD56bright cells lacked expression of this marker (Fig. 4E). CD56dimCD16− cells are considered immature cells and precursors of CD56dimCD16+ cells Unexpectedly, CD56dimCD16− cells expressed higher CD107a levels than CD56dimCD16+ cells.30,31 This is probably related to CD16 downregulation after NK cell activation36 and also explains the relative high CD56dimCD16− cell numbers in patients (Fig. 2A).
Figure 4.
Treatment modulates NK cell cytotoxic activity. (A) Percentage of GrzB+ NK cells (left panel) and the MFI of GrzB+ population (right panel). (B) Ex vivo degranulation of NK cells determined by CD107 a staining (left panel: % of CD107 a+ NK cells; right panel: MFI of CD107 a+ NK cells. (C) Degranulation of NK cells upon overnight incubation with Daudi target cells at [E:T] = 1:10 determined by % of CD107 a+ cell (left panel) and MFI of CD107 a+ NK (right panel). (D) The increase in CD107 a in in vitro assays (ΔCD107 a) was calculated as the difference between the % of CD107 a+ in degranulation assay versus the % of CD107 a+ NK cell detected ex vivo for each data point. (E) Percentage of the different NK cell subsets that expressed CD107 a ex vivo. Patient: n = 16, healthy donor: n = 4. Significance was determined by paired t-test between day 0 versus following time-points, and one-way ANOVA between HD and patients at every time-points with * p ≤ 0.05, ** p ≤ 0.01 and *** p ≤ 0.001.
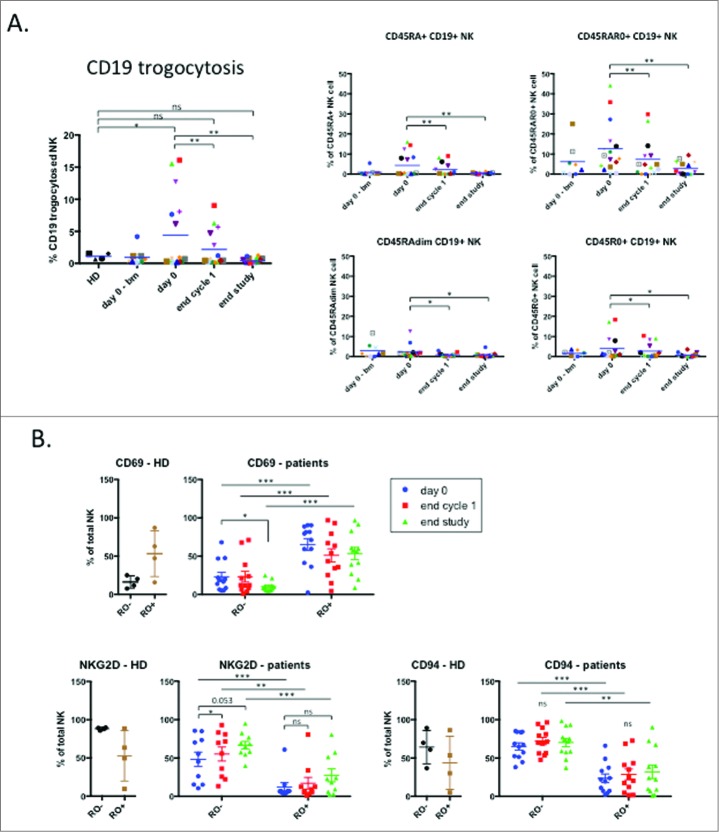
Treatment decreases the trogocytosis of tumor-associated markers by NK cells
These results suggested that NK cells were actively killing their targets during treatment and the absence of such targets at the end of treatment generated resting NK cells. To test this hypothesis, we investigated the proportion of NK cells that have killed CD19+ targets at the different time-points. We took advantage of the fact that NK cells gained target cell antigens, e.g. CD19, by trogocytosis.9,10 As expected, the percentage of CD19+ NK cells was higher in patients than in healthy donors and decreased with treatment (Fig. 5A). The CD45RARO NK subset expressed the highest level of CD19 and treatment successfully decreased CD19 expression (Fig. 5A). Other subsets also decreased CD19 expression (Fig. 5A). These results suggest that efficient treatments leading to elimination of NK cell target cells reduce NK: target cell interaction and lead to lack of target antigens on NK cell surface.
Figure 5.
Trogocytosis of tumor associated marker on NK cell population. (A) Trogocytosis of CD19 tumor marker on the total NK population (left panel) or on different NK cell subpopulations regarding expression of CD45 isoforms: CD45RA+, CD45RAR0+, CD45RAdim and CD45R0+. (B) The percentage of NK cells expressing CD69, NKG2D and CD94 was analyzed in the CD45RO− and CD45RO+ populations. Patient: n = 16, healthy donor: n = 4. Significance was determined by paired t-test between day 0 versus following time-points, and one-way ANOVA between HD and patients at every time-points with * p ≤ 0.05, ** p ≤ 0.01 and *** p ≤ 0.001.
We next investigated which populations were responsible for other variations in NK cell markers and focused on three of them that changed after treatment. CD69 expression was higher in patients and decreased after treatment (Fig. 3A). CD45RO+ cells showed higher CD69 levels, and treatment did not decrease it. CD45RO− cells showed lower levels that decreased with treatment (Fig. 5B). Therefore the decrease in CD69 expression is linked to both the decrease in the number of CD45RO+ cells, which expressed higher CD69 levels and the decrease of CD69 in CD45RO− cells.
NKG2D expression was lower in patients and increased after treatment (Fig. 2D). NKG2D was lower in CD45RO+ cells and treatment increased expression in both CD45RO− and CD45RO+ (Fig. 5B). Hence the increase in NKG2D levels is due to the decrease in CD45RO+ cell numbers and the increase in NKG2D expression by all NK cells.
CD94 expression was not modified in patients but increased during treatment (Fig. 2D). CD45RO+ cells expressed less CD94 and both CD45RO+ and RO− non-significantly increased CD94 expression during treatment (Fig. 5B). The increase of CD94 on the whole NK cell population is thus related to both, the decrease in CD45RO+ cells and the general increase of CD94.
Treatment does not exhaust NK cells
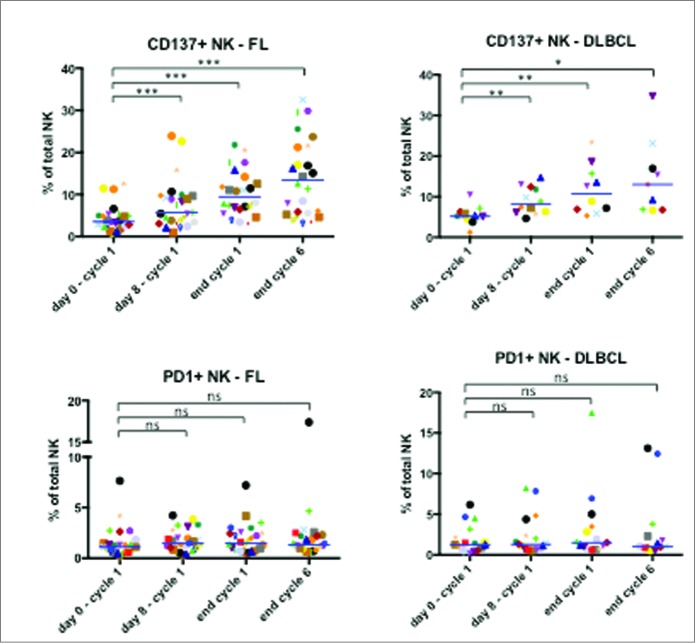
Finally, we investigated the effect of treatment on two receptors regulated on NK cells by CD16-mediated activation: the stimulatory CD137 receptor and the inhibitory PD-1 receptor 17. We used samples from a different cohort of patients in the GALEN clinical study and investigated the effect of OBZ treatment at the following time points: 1) during LEN course, just before OBZ injection (D7 before OBZ); 2) 1 hour after the end of OBZ infusion (D7 after OBZ); 3) at D0 of cycle 2 before LEN (cycle 2); and 4) at assessment of clinical response after 6 cycles (end of induction). OBZ increased CD137 in both FL and DLBCL patients (Fig. 6). The effect was found within hours after OBZ treatment and increased until the end of treatment. In contrast, PD-1 expression was unchanged (Fig. 6). This suggests that OBZ induces NK CD137 receptor expression in the presence of LEN and that continued infusion of the mAb keeps levels of this activating receptor high. In contrast, under these conditions, the inhibitory PD-1 receptor was not expressed.
Figure 6.
NK cell increased expression of CD137, but not PD1, in FL and DLBCL patients. Expression of CD137 (TNFRSF9) and the exhaustion marker PD1 on NK cells before the treatment (day 0 – cycle 1), before the first infusion of OBZ (day 8 – cycle 1), before the treatment of 2nd cycle (end cycle 1) and finally at the end of the last cycle (end cycle 6). Statistical significance was determined by paired t-test between “day 0 – cycle 1” and the following time points; p values * * p ≤ 0.05, ** p ≤ 0.01 and *** p ≤ 0.001.
Discussion
Although RTX-based therapy is efficient in a large number of patients, there is still a need for improvement. The development of new mAbs such as OBZ aims to fulfill this demand. However, even the best-designed mAb could be inefficient in some patients if they lack proper effector cells. The use of LEN to activate NK cells could address this problem. Here we observe that treatment with LEN reverses the immature phenotype of patient NK cells (Figs. 2 and 3) and induces expression of activating ligands, i.e. NKG2D (Fig. 2) and CD137 (Fig. 6). During treatment and in the presence of target cells (at end of first cycle), NK cells from patients degranulated more than those from healthy donors (Fig. 4). Once target cells disappear (Fig. 1), the activated markers CD69 and CD45RO (Fig. 3), the degranulation marker CD107a (Fig. 4) and the marker of trogocytosis CD19 (Fig. 5) decrease on NK cell membrane. This suggests that is possible to follow disease development by studying NK cell markers; at least, when NK cells are the effectors of the therapy, e.g. some clinical mAb. At the end of first cycle, when target cells are still present in relative numbers, NK cells show increased cytotoxicity in vitro and ex vivo and low GrzB levels (Fig. 4). However, the NK cell number decreases (Fig. 1). We propose the following scenario. NK cells are constitutively killing target cells (Fig. 5 and9,10). Some NK cells die during this immune response generating an increase in immature cells. OBZ and LEN induce improved target cell recognition and NK cell activation. NK cells degranulate in larger numbers but also die in larger numbers. At the end of treatment, most targets cells have disappeared and NK cells are no longer dying, so there is less de novo formation of NK cells and they are becoming more mature. However, NK cells continue to show high Ki-67 levels. Perhaps this can be explained by adaptive differentiation of NK cells and subsequent growth of a larger population of mature “memory” NK cells, as has been suggested after CMV infection.37 Almost all phenotypic changes observed in NK cells disappear with the lack of target cells suggesting that treatment keeps NK activated only in the presence of target cells. Several populations are responsible for the changes in NK cell phenotype (Fig. 5B) although cytotoxicity ex vivo is almost exclusively associated to CD56dim cells (Fig. 4E). Unexpectedly, we observed that CD45RO+ NK cells showed low NKG2D expression (Fig. 5B). We speculate that once NK cells are engaged on killing, NKG2D expression is not anymore required.
Our results show an increase in immature, CD56bright, NK cells in lymphoma patients (Fig. 2A). CD56bright cells produce high cytokine levels.28 In the context of anti-CD20-induced ADCC, we believed that NK cell cytotoxic function would be more relevant than cytokine production. In hematological cancer patients, cytotoxicity is mainly mediated by CD56dim NK cell subsets.9,10 This is confirmed in the current study (Fig. 4E). When we planned our analysis, we decided to maximize the study of cytotoxic, CD56dim, NK cells and did not investigate cytokine production because it is believed that CD56dim cells produce low cytokine levels.28 However, in view of our current results it would be interesting to investigate the cytokine profile of the immature NK cell populations that accumulate in lymphoma patients.
LEN targets the E3 ligase cereblon that degrades the Ikaros transcription factors IKZF1 and IKZF3.38 In vivo, LEN induces tumor cell apoptosis and blocks bone marrow stromal support,39 but also activates immune cells, e.g. NK cells.14,18–21 Our results support that NK cells are important mediators of the clinical benefits of LEN+OBZ.
It is noteworthy that PD-1 is absent on NK cells isolated from healthy donors but it is expressed on those from MM patients.40 We observe that there is a large heterogeneity of PD-1 expression in our patient cohort, and only a few of them constitutively express PD-1 (Fig. 6). As discussed above, there is probably continual production of mature NK cells to replace those dying during the immune response. These new cells are probably not exhausted and lack PD-1 expression. Hence, the continual renewal of NK cells might preclude PD-1 expression on NK cells in some patients. Treatment did not significantly affect PD-1 expression. This suggests that the role of PD-1 on treatment is minor. Perhaps LEN partially reversed the exhaustion of effector cells as previously suggested.41 LEN is probably the most active treatment (alone or combined with anti-PD-1/PD-L1 antibodies or other drugs) able to restore cytotoxic function to exhausted NK cells.14 Our results show the hypothesis that LEN in combination with OBZ increases several NK cell biological parameters associated with maturation and activation. However, because we did not obtain samples in monotherapy, i.e. LEN or OBZ alone, we cannot identify the relative contribution of these two drugs.
Cancer patients show NK cell subsets that are significantly different of those found in healthy donors.9,10 However, one question was unresolved: what is the fate of these NK cell subsets when their target cells disappear? Here we show for the first time that in our situation the NK cell subsets come back to a normal situation, i.e. similar to healthy donors, for the vast majority of markers. This is probably related to the disappearance of target cells because both LEN and OBZ are NK cell activating molecules that should not promote NK cell resting markers. This suggests that NK cells strongly react to effective treatment and that NK cell monitoring could be interesting to follow-up anti tumor treatments; mainly those involving mAb therapy.
Disclosure information
The authors declare the following conflict of interests: Roch Houot: Honoraria from Celgene and Roche; Guillaume Cartron: Honoraria and consultancy from Roche and Celgene ; Franck Morschhauser: honoraria Celgene, Roche advisory boards and scientific lectures; Karin Tarte: Celgene, Roche for advisory boards and scientific lectures; Cedric Menard: Celgene for scientific lectures.
Patients and methods
Patients
All patients belong to the BioGALEN study and signed specific informed consent form before biological samples collection of BioGALEN. This study is recorded in website ClinicalTrials.gov with number NCT01582776. Phase Ib was for follicular lymphoma (FL) patients and Phase II for follicular and aggressive (DLBCL and MCL) B-cell lymphoma patients. 3 × 3 ml of heparinized blood or 4 ml of bone marrow aspirate were collected at day 0. At the end of first cycle or at the end of treatment (supplemental Fig. 1) 3 × 3 ml of heparinized blood was collected.
Cell culture
The B cell lymphoblastoid Daudi cell line was maintained in logarithmic growth in RPMI 1640 medium (Gibco® GlutaMAX™ media) with 10% fetal bovine serum (FBS) (Gibco®). Cells were cultured at 37°C in a humidified chamber with 5% CO2 in air, and passaged 1:10 twice a week.
Peripheral blood mononuclear cell (PBMC) purification
Bone marrow and peripheral blood samples were obtained from patients and total PBMC were isolated using Ficoll. Briefly, 3–6 ml of 1:2 diluted blood or 1:3 diluted bone marrow samples in RPMI were added on top of 5 ml of Histopaque (Sigma). Cells were centrifuged at 1600 rpm and at 20°C without break for 30 minutes. Mononuclear cells were collected from the white ring at the interface. After washing in RPMI, cells were cryopreserved in liquid nitrogen in medium comprise of FBS plus 10% culture-grade DMSO (CliniMACS) until analyzing.
Flow cytometry analysis
Isolated PBMCs were stained with 7AAD (Beckman) to identify viable cells and with the following -CD45RO-FITC, -CD161-FITC, -CD3-PE, -CD19-PE, -CD62 L-PE, -CD69-PE, -CD314(NKG2D)-PE, -CD3-ECD, -CD19-ECD, -CD56-PECy7, CD3-APC, -CD56-APC, -GzB-AlexaFluor700, -CD19-AlexaFluor700, -CD20-APC-AlexaFluor750, -CD45RA-APC-AlexaFluor750, -CD5-PacificBlue, -CD16-PacificBlue, -CD57-PacificBlue, -CD16-KromeOrange (Beckman), -CD158 a-V450, -CD158b-FITC, -CD158 a-PE, -CD107 a-HV500, -Ki-67-V450, HLA-ABC-BV711 (BD Biosciences), MIC-A/B-PE, -CD45RA-FITC, -CD45RO-PE, -CD159 a(NKG2 A)-PE, -CD94-PE-Vio770, -CD45RO-APC, -CD19-VioBlue, -CD158e-VioBlue (Miltenyi Biotec) antibodies against surface markers. Briefly, 1 to 10 × 106 cells were incubated with the different antibodies in PBS containing 2% FBS at 4 °C for 30 minutes. Cells were then washed with PBS and suspended in 200–250 µl PBS 2% FBS. Finally, sample acquisition was performed using Gallios flow cytometer (Beckman) or Fortessa (BD Biosciences). Acquired samples were later analyzed using Kaluza software v5.1 (Beckman).
In vitro CD107a degranulation assay
In vitro degranulation assay was performed to evaluate NK reactivity to the B cell target Daudi by measuring CD107a expression on the surface after cytotoxic granule release. In summary, isolated PBMC were pre-stained with CD3/CD56 to determine NK frequency in the sample. Next, PBMC were incubated with Daudi cells at a 1:10 ratio NK:Daudi in the presence of 1.5 ul of anti-CD107a (BD Biosciences, Franklin Lakes, NJ) and 1 ul Golgi-stop (BD Biosciences) (containing monensin) to inhibit vesicle trafficking. Cell mixture was then resuspended in RPMI Glutamax 10% supplemented with 10 IU/ml Interleukin 2 (eBiosciences) and incubated overnight. After stimulation, cell mixture was collected and stained for FACS using an antibody cocktail containing 7AAD, the anti-CD45RO-FITC, -CD69-PE, -CD19-ECD, -CD56-PECy7, -CD3-APC, -CD45RA-APCAlexaFluor750, -CD107a-HV500 and -CD16-KO antibodies (BD Biosciences, Beckman). A bivariate plot of CD56 versus CD3 was used to acquire at least 10,000 NK cells.
Multicolor staining for intracellular markers
Cell permeablization and intracellular staining was performed as previous described.9,10 Briefly,1–10 million cells were incubated with 10% normal human serum at RT for 15 min and then stained with an antibody mix for cell surface markers (anti-CD45RO-FITC, -CD19-ECD, -CD56-PC7, -CD3-APC, -CD45RA-APCAlexaFluor750 and -CD16-KO antibodies) (BD Biosciences, Beckman). After surface staining, cells were washed twice and permeabilize with CytoFix/CytoPerm (BD Biosciences) reagent according to the manufacturer protocol. After fixation and permeablization, cells were washed twice in BD Perm/Wash solution and follow FACS staining for intracellular markers Granzyme B- PE (Miltenyi Biotec) and Ki-67-V450 (BD Biosciences) at 4°C for 30 minutes in the dark. Finally, cells were washed twice in BD Perm/Wash solution and resuspended in PBS 2% FBS prior to acquisition on flow cytometer Gallios (Beckman). A bivariate plot of CD56 versus CD3 was used to acquire at least 10,000 NK cells.
Statistics
Experimental figures and statistical analysis were performed using GraphPad Prism (v6.0). Statistical significance between day 0 and the following time-points was determined using paired Student t-test on the sample patients for each sampling point. To determine statistical significance between healthy donors and patients, one-way ANOVA test was used to compare between healthy donors versus patients at every time-points. All statistical values presented as *: p<0.05; **: p<0.01; ***: p<0.001. Average values were expressed as mean plus or minus the standard error (SD).
Supplementary Material
Funding Statement
This project has been conducted owing to the Bio_GALEN funding as a part of the ancillary GALEN study. DNV has received a fellowship from the Ministère de l'Enseignement Supérieur et de la Recherche (MESR).
Acknowledgments
FACs analysis was performed at the platform Montpellier Rio Imaging (MRI).
References
- 1.Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P, Watier H. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 2002;99:754–8. doi: 10.1182/blood.V99.3.754. PMID:11806974. [DOI] [PubMed] [Google Scholar]
- 2.Jefferis R. Glycosylation as a strategy to improve antibody-based therapeutics. Nat Rev Drug Discov. 2009;8:226–34. doi: 10.1038/nrd2804. PMID:19247305. [DOI] [PubMed] [Google Scholar]
- 3.Evans SS, Clemmons AB. Obinutuzumab: A Novel Anti-CD20 Monoclonal Antibody for Chronic Lymphocytic Leukemia. J Adv Pract Oncol. 2015;6:370–4. PMID:26705497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang W, Erbe AK, Hank JA, Morris ZS, Sondel PM. NK Cell-Mediated Antibody-Dependent Cellular Cytotoxicity in Cancer Immunotherapy. Front immunol. 2015;6:368. doi: 10.3389/fimmu.2015.00368. PMID:26284063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goede V, Fischer K, Busch R, Engelke A, Eichhorst B, Wendtner CM, Chagorova T, de la Serna J, Dilhuydy MS, Illmer T, et al.. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med. 2014;370:1101–10. doi: 10.1056/NEJMoa1313984. PMID:24401022. [DOI] [PubMed] [Google Scholar]
- 6.Salles GA, Morschhauser F, Solal-Celigny P, Thieblemont C, Lamy T, Tilly H, Gyan E, Lei G, Wenger M, Wassner-Fritsch E, et al.. Obinutuzumab (GA101) in patients with relapsed/refractory indolent non-Hodgkin lymphoma: results from the phase II GAUGUIN study. J Clin Oncol. 2013;31:2920–6. doi: 10.1200/JCO.2012.46.9718. PMID:23835715. [DOI] [PubMed] [Google Scholar]
- 7.Morschhauser FA, Cartron G, Thieblemont C, Solal-Celigny P, Haioun C, Bouabdallah R, Feugier P, Bouabdallah K, Asikanius E, Lei G, et al.. Obinutuzumab (GA101) monotherapy in relapsed/refractory diffuse large b-cell lymphoma or mantle-cell lymphoma: results from the phase II GAUGUIN study. J Clin Oncol. 2013;31:2912–9. doi: 10.1200/JCO.2012.46.9585. PMID:23835718. [DOI] [PubMed] [Google Scholar]
- 8.Cartron G, Watier H. Obinutuzumab: what is there to learn from clinical trials? Blood. 2017;130:581–9. doi: 10.1182/blood-2017-03-771832. PMID:28584136.26629531 [DOI] [PubMed] [Google Scholar]
- 9.Krzywinska E, Allende-Vega N, Cornillon A, Vo D, Cayrefourcq L, Panabieres C, Vilches C, Déchanet-Merville J, Hicheri Y, Rossi JF, et al.. Identification of anti tumor cells carrying natural killer (NK) cell antigens in patients with hematological cancers. EBioMedicine. 2015;2:1364–76. doi: 10.1016/j.ebiom.2015.08.021. PMID:26629531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krzywinska E, Cornillon A, Allende-Vega N, Vo DN, Rene C, Lu ZY, Pasero C, Olive D, Fegueux N, Ceballos P, et al.. CD45 Isoform Profile Identifies Natural Killer (NK) Subsets with Differential Activity. PLoS One. 2016;11:e0150434. doi: 10.1371/journal.pone.0150434. PMID:27100180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Villalba M, Rathore MG, Lopez-Royuela N, Krzywinska E, Garaude J, Allende-Vega N. From tumor cell metabolism to tumor immune escape. Int J Biochem Cell Biol. 2013;45:106–13. doi: 10.1016/j.biocel.2012.04.024. PMID:22568930. [DOI] [PubMed] [Google Scholar]
- 12.Villalba M, Lopez-Royuela N, Krzywinska E, Rathore MG, Hipskind RA, Haouas H, Allende-Vega N. Chemical metabolic inhibitors for the treatment of blood-borne cancers. Anti-Cancer Agents Med Chem. 2014;14:223–32. doi: 10.2174/18715206113136660374. PMID:24237221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baier C, Fino A, Sanchez C, Farnault L, Rihet P, Kahn-Perles B, Costello RT. Natural Killer Cells Modulation in Hematological Malignancies. Front Immunol. 2013;4:459. doi: 10.3389/fimmu.2013.00459. PMID:24391641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giuliani M, Janji B, Berchem G. Activation of NK cells and disruption of PD-L1/PD-1 axis: two different ways for lenalidomide to block myeloma progression. Oncotarget. 2017;8:24031–44. PMID:28199990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ambrosini P, Loiacono F, Conte R, Moretta L, Vitale C, Mingari MC. IL-1beta inhibits ILC3 while favoring NK-cell maturation of umbilical cord blood CD34(+) precursors. Eur J Immunol. 2015;45:2061–71. doi: 10.1002/eji.201445326. PMID:25847448. [DOI] [PubMed] [Google Scholar]
- 16.Moretta L, Pietra G, Vacca P, Pende D, Moretta F, Bertaina A, Mingari MC, Locatelli F, Moretta A. Human NK cells: From surface receptors to clinical applications. Immunol lett. 2016;178:15–9. doi: 10.1016/j.imlet.2016.05.007. PMID:27185471. [DOI] [PubMed] [Google Scholar]
- 17.Muntasell A, Ochoa MC, Cordeiro L, Berraondo P, Lopez-Diaz de Cerio A, Cabo M, López-Botet M, Melero I. Targeting NK-cell checkpoints for cancer immunotherapy. Curr Opin Immunol. 2017;45:73–81. doi: 10.1016/j.coi.2017.01.003. PMID:28236750. [DOI] [PubMed] [Google Scholar]
- 18.Benson DM Jr., Cohen AD, Jagannath S, Munshi NC, Spitzer G, Hofmeister CC, Efebera YA, Andre P, Zerbib R, Caligiuri MA. A Phase I Trial of the Anti-KIR Antibody IPH2101 and Lenalidomide in Patients with Relapsed/Refractory Multiple Myeloma. Clin Cancer Res. 2015;21:4055–61. doi: 10.1158/1078-0432.CCR-15-0304. PMID:25999435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grossenbacher SK, Aguilar EG, Murphy WJ. Leveraging natural killer cells for cancer immunotherapy. Immunotherapy. 2017;9:487–97. doi: 10.2217/imt-2017-0013. PMID:28472904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lagrue K, Carisey A, Morgan DJ, Chopra R, Davis DM. Lenalidomide augments actin remodeling and lowers NK-cell activation thresholds. Blood. 2015;126:50–60. doi: 10.1182/blood-2015-01-625004. PMID:26002964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paiva B, Mateos MV, Sanchez-Abarca LI, Puig N, Vidriales MB, Lopez-Corral L, Corchete LA, Hernandez MT, Bargay J, de Arriba F, et al.. Immune status of high-risk smoldering multiple myeloma patients and its therapeutic modulation under LenDex: a longitudinal analysis. Blood. 2016;127:1151–62. doi: 10.1182/blood-2015-10-662320. PMID:26668134. [DOI] [PubMed] [Google Scholar]
- 22.Chanan-Khan AA, Chitta K, Ersing N, Paulus A, Masood A, Sher T, Swaika A, Wallace PK, Jr Mashtare TL, Wilding G, et al.. Biological effects and clinical significance of lenalidomide-induced tumour flare reaction in patients with chronic lymphocytic leukaemia: in vivo evidence of immune activation and antitumour response. Br J Haematol. 2011;155:457–67. doi: 10.1111/j.1365-2141.2011.08882.x. PMID:22010965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hagner PR, Chiu H, Ortiz M, Apollonio B, Wang M, Couto S, Waldman MF, Flynt E, Ramsay AG, Trotter M, et al.. Activity of lenalidomide in mantle cell lymphoma can be explained by NK cell-mediated cytotoxicity. Br J Haematol. 2017;179 :399–409. doi: 10.1111/bjh.14866. PMID:28771673. [DOI] [PubMed] [Google Scholar]
- 24.Shanafelt TD, Ramsay AG, Zent CS, Leis JF, Tun HW, Call TG, LaPlant B, Bowen D, Pettinger A, Jelinek DF, et al.. Long-term repair of T-cell synapse activity in a phase II trial of chemoimmunotherapy followed by lenalidomide consolidation in previously untreated chronic lymphocytic leukemia (CLL). Blood. 2013;121:4137–41. doi: 10.1182/blood-2012-12-470005. PMID:23493782. [DOI] [PubMed] [Google Scholar]
- 25.Gribben JG, Fowler N, Morschhauser F. Mechanisms of Action of Lenalidomide in B-Cell Non-Hodgkin Lymphoma. J Clin Oncol. 2015;33:2803–11. doi: 10.1200/JCO.2014.59.5363. PMID:26195701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kritharis A, Coyle M, Sharma J, Evens AM. Lenalidomide in non-Hodgkin lymphoma: biological perspectives and therapeutic opportunities. Blood. 2015;125:2471–6. doi: 10.1182/blood-2014-11-567792. PMID:25736312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fionda C, Abruzzese MP, Zingoni A, Cecere F, Vulpis E, Peruzzi G, Soriani A, Molfetta R, Paolini R, Ricciardi MR, et al.. The IMiDs targets IKZF-1/3 and IRF4 as novel negative regulators of NK cell-activating ligands expression in multiple myeloma. Oncotarget. 2015;6:23609–30. doi: 10.18632/oncotarget.4603. PMID:26269456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bryceson YT, Chiang SC, Darmanin S, Fauriat C, Schlums H, Theorell J, Wood SM. Molecular mechanisms of natural killer cell activation. J Innate Immun. 2011;3:216–26. doi: 10.1159/000325265. PMID:21454962. [DOI] [PubMed] [Google Scholar]
- 29.Domaica CI, Fuertes MB, Uriarte I, Girart MV, Sardanons J, Comas DI, Di Giovanni D, Gaillard MI, Bezrodnik L, Zwirner NW. Human natural killer cell maturation defect supports in vivo CD56(bright) to CD56(dim) lineage development. PLoS One. 2012;7:e51677. doi: 10.1371/journal.pone.0051677. PMID:23240056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moretta L. Dissecting CD56 dim human NK cells. Blood. 2010;116:3689–91. doi: 10.1182/blood-2010-09-303057. PMID:21071612. [DOI] [PubMed] [Google Scholar]
- 31.Freud AG, Yu J, Caligiuri MA. Human natural killer cell development in secondary lymphoid tissues. Semin Immunol. 2014;26:132–7. doi: 10.1016/j.smim.2014.02.008. PMID:24661538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Montaldo E, Del Zotto G, Della Chiesa M, Mingari MC, Moretta A, De Maria A Moretta L. Human NK cell receptors/markers: a tool to analyze NK cell development, subsets and function. Cytometry Part A: the journal of the International Society for Analytical Cytology. 2013;83:702–13. doi: 10.1002/cyto.a.22302. PMID:23650273. [DOI] [PubMed] [Google Scholar]
- 33.Juelke K, Killig M, Luetke-Eversloh M, Parente E, Gruen J, Morandi B, Ferlazzo G, Thiel A, Schmitt-Knosalla I, Romagnani C. CD62 L expression identifies a unique subset of polyfunctional CD56 dim NK cells. Blood. 2010;116:1299–307. doi: 10.1182/blood-2009-11-253286. PMID:20505160. [DOI] [PubMed] [Google Scholar]
- 34.Lopez-Verges S, Milush JM, Pandey S, York VA, Arakawa-Hoyt J, Pircher H, Norris PJ, Nixon DF, Lanier LL. CD57 defines a functionally distinct population of mature NK cells in the human CD56dimCD16+ NK-cell subset. Blood. 2010;116:3865–74. doi: 10.1182/blood-2010-04-282301. PMID:20733159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pegram HJ, Andrews DM, Smyth MJ, Darcy PK, Kershaw MH. Activating and inhibitory receptors of natural killer cells. Immunol Cell Biol. 2011;89:216–24. doi: 10.1038/icb.2010.78. PMID:20567250. [DOI] [PubMed] [Google Scholar]
- 36.Grzywacz B, Kataria N, Verneris MR. CD56(dim)CD16(+) NK cells downregulate CD16 following target cell induced activation of matrix metalloproteinases. Leukemia. 2007;21:356–9; author reply 9. doi: 10.1038/sj.leu.2404499. PMID:17251901. [DOI] [PubMed] [Google Scholar]
- 37.Lopez-Botet M, Vilches C, Redondo-Pachon D, Muntasell A, Pupuleku A, Yelamos J, Pascual J, Crespo M. Dual Role of Natural Killer Cells on Graft Rejection and Control of Cytomegalovirus Infection in Renal Transplantation. Front Immunol. 2017;8:166. doi: 10.3389/fimmu.2017.00166. PMID:28261220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stewart AK. Medicine. How thalidomide works against cancer. Science. 2014;343:256–7. doi: 10.1126/science.1249543. PMID:24436409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vallet S, Palumbo A, Raje N, Boccadoro M, Anderson KC. Thalidomide and lenalidomide: Mechanism-based potential drug combinations. Leuk Lymphoma. 2008;49:1238–45. doi: 10.1080/10428190802005191. PMID:18452080. [DOI] [PubMed] [Google Scholar]
- 40.Benson DM Jr., Bakan CE, Mishra A, Hofmeister CC, Efebera Y, Becknell B, Baiocchi RA, Zhang J, Yu J, Smith MK, et al.. The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: a therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibody. Blood. 2010;116:2286–94. doi: 10.1182/blood-2010-02-271874. PMID:20460501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gorgun G, Samur MK, Cowens KB, Paula S, Bianchi G, Anderson JE, White RE, Singh A, Ohguchi H, Suzuki R, et al.. Lenalidomide Enhances Immune Checkpoint Blockade-Induced Immune Response in Multiple Myeloma. Clin Cancer Res. 2015;21:4607–18. doi: 10.1158/1078-0432.CCR-15-0200. PMID:25979485. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.