Abstract
Background
The importance of Respiratory Syncytial Virus (RSV) is increasingly recognized in hospitalized adults, but mainly in those ≥ 65 years.
Objectives
We sought to describe the epidemiology and clinical severity of RSV compared to influenza in hospitalized adults ≥18 years.
Study Design
Adults hospitalized with acute respiratory illnesses (ARI) of ≤10 days duration were prospectively enrolled from two Michigan hospitals during two influenza seasons. Collected specimens were tested for RSV and influenza by real-time, reverse transcription polymerase chain reaction (RT-PCR). Viral load and subtype were determined for RSV-positive specimens. We evaluated factors associated with RSV and outcomes of infection using multivariable logistic regression. RSV-positive patients were separately compared to two reference groups: RSV-negative and influenza-negative, and influenza-positive patients.
Results
RSV was detected in 84 (7%) of 1259 hospitalized individuals (55 RSV-B, 29 RSV-A). The highest prevalence was found in 50-64 year olds (40/460; 8.7%); 98% of RSV cases in this age group had at least one chronic comorbidity. RSV detection was associated with obesity (OR: 1.71 95% CI: 0.99-3.06, p=0.03). Individuals with RSV were admitted to the hospital later in their illness and had a higher median Charlson comborbidity index (3 vs 2 p < 0.001) compared to those with influenza. Clinical severity of RSV-associated hospitalizations was similar to influenza-associated hospitalizations.
Discussion
In this study we observed the highest frequency of RSV-associated hospitalizations among adult 50-64 years old; many of whom had chronic comorbidities. Our results suggest the potential benefit of including these individuals in future RSV vaccination strategies.
Keywords: RSV, influenza, hospitalization, adults, acute respiratory illness
Background
Respiratory Syncytial Virus (RSV), commonly regarded as a childhood infection, is also an important contributor to respiratory illness among adults [1–8]. However, unlike influenza, in which serious morbidity has been clearly recognized for years, the relative impact of RSV infection in adults has more recently gained widespread recognition [9,10]. While primary RSV infections in infancy can result in severe disease, subsequent infections are often comparatively mild. Incomplete immunity results in continued susceptibility to reinfection through life. For example, we recently detected RSV in 4% of acute respiratory illnesses (ARI) in community dwelling adults 18-49 years old [11]. Current vaccine development efforts have identified prevention of severe RSV-associated illness in older adults, particularly those resulting in hospitalization, as a priority. Estimates of the frequency and severity of these RSV-associated hospitalizations have varied considerably, with some earlier studies using antibody titer rather than molecular methods to document infection. Most previous studies of RSV-associated hospitalization have concentrated on those 65 years of age and older, with five to ten percent of hospitalizations for ARI due to RSV infection [2,5,12–17]. Older adults with underlying cardiopulmonary disease such as chronic obstructive pulmonary disease (COPD) and congestive heart failure (CHF) have been shown to be at particular risk [2,18].
Because of these findings, and the known role of influenza in causing hospitalization, studies of RSV in hospitalized adults have often used influenza positive illnesses as a frame of reference. This was particularly true with regards to efforts to distinguish between symptoms of severe RSV and severe influenza prior to the availability of rapid, point-of-care diagnostics. RSV infections have been reported to present less frequently with fever and more frequently with wheezing, but otherwise can be difficult to distinguish clinically from influenza and, for that matter, from other viral respiratory illnesses [1,12]. In some studies, these comparative evaluations have found the frequency of RSV hospitalization to rival that of influenza in highly influenza vaccinated populations [1,3,4,12].
Objectives
Establishing the burden of severe RSV has added urgency given the accelerating development of RSV vaccines; a major issue for vaccination programs will be identifying target populations for rational use. We sought to characterize the frequency and clinical severity of RSV among hospitalized adults ≥18 years, overall and by subtype, for two respiratory illness seasons in two large Southeast Michigan hospitals.
Study Design
Study Design
We used specimens and data from a prospective study of adults hospitalized with ARI meeting a standardized case definition [19]. The ongoing, case-test negative study was designed to estimate influenza vaccine effectiveness (VE) in the prevention of influenza-associated hospitalization. Patients ≥18 years old hospitalized with ARI at one of two hospitals were prospectively identified from November 2014-March 2015 and November 2015-April 2016. These hospitals are two large tertiary care centers in Michigan serving primarily suburban (Hospital A) and urban (Hospital B) populations. Chief complaints and admission diagnoses for all new admissions were screened for evidence of an ARI of ≤10 days duration. Patients or a proxy/surrogate provided written informed consent to participate. This study was approved by the Institutional Review Boards at the University of Michigan Medical School and Henry Ford Health System.
Data collection
At enrollment, consented patients self-reported demographic characteristics, subjective health, frailty [20–22], influenza vaccination, and illness onset date via structured interviews with study staff. Throat and nasal swab specimens were collected and combined in universal transport media.
Electronic medical records (EMR) were reviewed to document evidence of COPD, CHF and other chronic conditions, for calculation of the Charlson Comorbidity Index (CCI) [23,24] and for determination of body mass index (BMI) [25]. Obesity was defined as a BMI ≥ 30. Measures of clinical severity were also collected from the EMR including: length of stay, admission to and duration of stay in the intensive care unit (ICU), requirement for invasive (e.g. intubation) and non-invasive (e.g. BiPAP/CPAP) mechanical ventilation, and discharge disposition.
Laboratory testing
Collected respiratory specimens were tested for RSV and influenza by real-time reverse transcription PCR (RT-PCR) using primers, probes, and a testing protocol developed by the CDC Division of Viral Diseases and Influenza division, respectively [26]. RSV positive specimens were subsequently quantified and subtyped using published methods [27]. Quantification was standardized using known quantities of plasmid-based standards containing the amplicon, and results were expressed in log copies/ml of media. All specimens were also tested for RNase P to assess sample quality [28].
Statistical analyses
Analyses were restricted to data from the first RSV-positive enrollment of an individual or the first overall enrollment if no RSV-positive episodes were identified. Characteristics and clinical outcomes of participants with RSV were compared to RSV-negative, influenza-negative subjects and, separately, to influenza-positive subjects using Chi-square, Fisher’s exact test, or Wilcoxon rank-sum tests, as appropriate. P-values less than 0.05 were considered statistically significant for all analyses.
We determined risk factors associated with detection of RSV and assessed the association between RSV detection and extended length of stay (≥3 days) using regression models; comparison groups were defined similarly to the unadjusted analysis. Multivariable logistic regression models were used to analyze patient demographics and clinical outcomes, controlling for BMI, CCI, age ≥65 years, study site, study year, and time from illness onset to admission. All logistic models used Firth’s regression with profile-likelihood confidence intervals, p-values were calculated as Wald’s p-values. All analyses were performed using SAS software version 9.4.
Results
Detection of RSV and Influenza
1306 patients hospitalized for ARI were enrolled in the study between November 2014-March 2015 and November 2015-April 2016 (Figure 1). Subsequent enrollments of individuals enrolled multiple times (n=42) and subjects with missing or inconclusive influenza testing results (n=3) were excluded, resulting in a study population of 1,261 patients (726 in 2014-2015, 535 in 2015-2016). Figure 1 presents RSV and influenza epidemic curves by season. Overall, RSV was detected in 86 (7%) and influenza was detected in 236 patients (19%). Two individuals had co-detection of RSV and influenza in the same specimen [1 with RSV-B/unsubtypeable influenza A and 1 with RSV-A/Influenza A(H1N1)pdm09] and were excluded from further analysis. Overall study enrollment was lower in the second year of the study (726 in 2014-2015 and 535 in 3015-2016). The proportion of RSV detections was also lower in the second year (8% in 2014-2015 and 5% in 2015-2016, while the proportion of influenza infections remained consistent in both years (19% in 2014-2015 and 19% in 2015-2016).
Figure 1. Respiratory Syncytial Virus (RSV) and influenza detections and total number of specimens collected in adults hospitalized with acute respiratory illness (ARI) over two seasons.
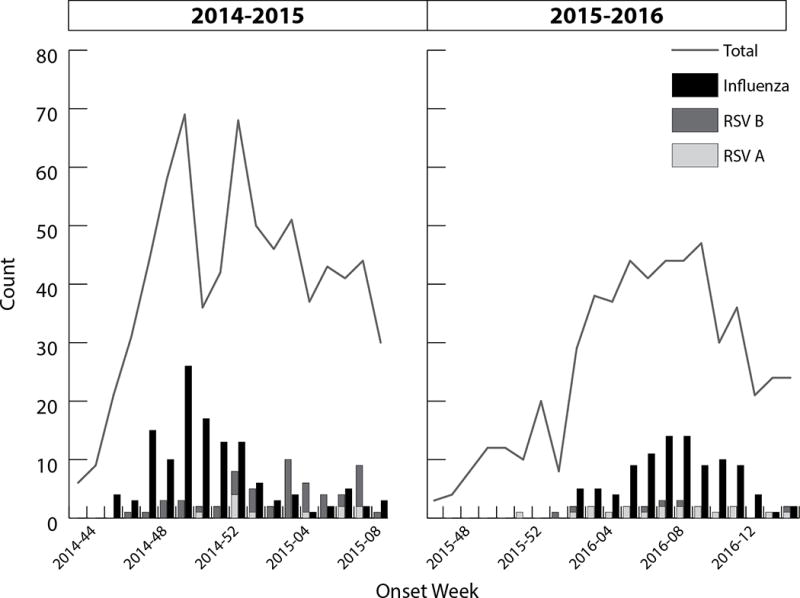
a 2014-2015 season: 62 RSV positive specimens, 11 RSV-A and 50 RSV-B. 136 Influenza positive specimens, 116 (85%) influenza A/H3N2. One individual was coinfected with RSV-B and unsubtypeable influenza A and was excluded from further analyses.
b 2015-2016 season: 25 total RSV positive specimens, 19 RSV-A and 6 RSV-B. 100 influenza positive specimens, 89 (89%) influenza A/H1N1. One individual in 2015-2016 was coinfected with RSV-A and influenza A/H1 and was excluded from further analyses.
RSV and influenza hospitalizations by participant characteristics
Nearly two-thirds of RSV cases detected in this study were among adults 18-64 years of age (n=56). The age-specific proportion of RSV detection (Table 1) was 4% among those 18-49, 9% among those 50-64, and 7% among those 65 years old and older (p = 0.06). In contrast, influenza was identified slightly less frequently in the middle age group (16%) than in older (20%) and younger adults (20%). Overall, 1118 (88%) of all enrolled patients had a CCI ≥1, indicating the presence of at least one major comorbid condition. On average, patients with RSV had a higher median CCI than patients with influenza (3 vs 2, p < 0.001). After stratifying by age group (Table 2), we further found that the higher average CCI of patients with RSV was specific to those 50-64 years old (median 4 vs 2, p =0.001). The median time from illness onset to admission among RSV-positive patients was 3 days (IQR: 2-4) and the median time from onset to specimen collection was 4 days (IQR: 3-6). Individuals with RSV had a longer interval from illness onset to admission (p=0.006) and to specimen collection (p=0.003) than individuals with influenza (Table 1).
Table 1.
Frequency of hospitalizations associated with respiratory syncytial virus (RSV), influenza, and RSV-negative, influenza-negative acute respiratory infection (ARI) by participant characteristics.
| Row Totals | RSV-positive (n=84)a |
Influenza-positive (n=234)a |
RSV-negative & Influenza-negative (n=941) | |
|---|---|---|---|---|
| Age group, n (%)b | ||||
| 18-49 | 372 | 16 (4.3) | 75 (20.2) | 281 (75.5) |
| 50-64 | 461 | 40 (8.7) | 75 (16.3) | 346 (75.1) |
| ≥65 | 426 | 28 (6.6) | 84 (19.7) | 314 (73.7) |
| BMI, n (%)c | ||||
| Normal/underweight | 351 | 19 (5.4) | 67 (19.1) | 265 (75.5) |
| Overweight | 311 | 18 (5.8) | 55 (17.7) | 238 (76.5) |
| Obese | 547 | 46 (8.4) | 103 (18.8) | 398 (72.8) |
| Charlson score, n (%)d | ||||
| 0 | 141 | 6 (4.3) | 36 (25.5) | 99 (70.2) |
| 1 | 301 | 15 (5.0) | 70 (23.3) | 216 (71.8) |
| 2 | 179 | 16 (8.9) | 37 (20.7) | 126 (70.4) |
| ≥3 | 637 | 47 (7.4) | 91 (14.3) | 499 (78.3) |
| CHF, n (%)d | 404 | 25 (6.2) | 54 (13.4) | 325 (80.4) |
| COPD, n(%)d | 769 | 58 (7.6) | 133 (17.3) | 578 (75.1) |
| Frailty score, n(%)e | ||||
| 0 | 267 | 23 (8.6) | 57 (21.3) | 187 (70.0) |
| 1 | 308 | 24 (7.8) | 56 (18.2) | 228 (74.0) |
| 2 | 261 | 8 (3.1) | 54 (20.7) | 199 (76.2) |
| 3 | 186 | 11 (5.9) | 34 (18.3) | 141 (75.8) |
| 4/5 | 198 | 15 (7.6) | 22 (11.1) | 161 (81.3) |
| Hospital B, n (%) | 556 | 43 (7.7) | 107 (19.2) | 406 (73.0) |
| Time to admission in days, med (IQR) | – | 3 (2-4) | 2 (1-3)f | 2 (1-4) |
| Time to specimen collection in days, med (IQR) | – | 4 (3-6) | 3 (2-5)f | 4 (2-6) |
| Antiviral prescribed, n (%) | 267 | 15 (5.6) | 160 (59.9) | 92 (34.5) |
| Antiviral prescribed before study specimen collection, n (%) | 184 | 12 (6.5) | 110 (59.8) | 62 (33.7) |
| Influenza vaccination (any), n(%)g | 792 | 57 (7.2) | 116 (14.6) | 619 (78.2) |
Abbreviations: BMI, body mass index; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; IQR, interquartile range
Two individuals were coinfected with RSV and influenza and excluded from analyses.
Chi-square p-value for frequency of detection by age group: 0.06 among RSV-positive; 0.40 among influenza positive; and 0.85 among RSV-negative, influenza-negative hospitalizions.
50 individuals were missing data on body mass index (BMI) – 40 RSV-negative/influenza-negative, 1 RSV-positive, 9 influenza-positive.
1 RSV-negative/influenza-negative individual was missing data on comorbid conditions.
39 individuals missing data on frailty score – 25 RSV-negative/influenza-negative, 3 RSV-positive, 11 influenza-positive.
P-value from Wilcoxon rank-sum test comparing RSV-positive and influenza-positive ARI for time to admission (0.003) and time to specimen collection (0.006)
64 individuals with vaccination status unknown – 49 RSV-negative/influenza-negative, 3 RSV-positive, 12 influenza-positive.
Table 2.
Frequency of RSV subtype by participant characteristics
| RSV-A positive (n=29) |
RSV-B positive (n=55) |
p-value | |
|---|---|---|---|
| Age group | 0.54 | ||
| 18-49 | 4 (13.8) | 12 (21.8) | |
| 50-64 | 16 (55.2) | 24 (43.6) | |
| ≥65 | 9 (31.0) | 19 (34.6) | |
| Male sex | 12 (41.4) | 21 (38.2) | 0.78 |
| BMI, median (IQR) | 31.45 (25.13-43.01) | 32.00 (26.08-37.46) | 0.53 |
| BMI categories, n (%)a | 0.82 | ||
| Underweight/normal (18.5-24.99) | 7 (25.0) | 12 (21.8) | |
| Overweight (25-29.99) | 5 (17.9) | 13 (23.6) | |
| Obese (≥ 30) | 16 (57.1) | 30 (54.6) | |
| Charlson score, n (%) | 0.41 | ||
| 0 | 1 (3.5) | 5 (9.1) | |
| 1 | 3 (10.3) | 12 (21.8) | |
| 2 | 7 (24.1) | 9 (16.4) | |
| ≥3 | 18 (62.1) | 29 (52.7) | |
| Time to specimen collection from onset day, median (IQR) | 5 (3-6) | 4 (3-6) | 0.97 |
| Time to admission from onset day, med (IQR) | 3 (2-4) | 3 (2-4) | 0.64 |
| Hospital B, n (%) | 12 (41.4) | 31 (56.4) | 0.19 |
| Year, n (%) | <0.0001 | ||
| 2014-2015 | 11 (37.9) | 49 (89.1) | |
| 2015-2016 | 18 (62.1) | 6 (10.9) | |
| Viral load (log10 copies/ml), median (IQR)b | 3.42 (2.93-4.06) | 4.86 (4.00-5.44) | <0.0001 |
Abbreviations: BMI, body mass index; IQR, interquartile range
P-value from Kruskal-Wallis test.
Results from linear regression model adjusted for time between illness onset to specimen collection (in days).
RSV subtyping & viral load
Among the 84 identified RSV infections, 29 were RSV-A and 55 were RSV-B; RSV-B predominated in 2014-2015 (n=49; 82%) and RSV-A predominated in 2015-2016 (n=19; 76%) (Figure 1). One individual, whose second enrollment was excluded from analysis, was hospitalized twice with RSV-B detected in specimens collected 29 days apart. Neither participant characteristics nor illness outcomes differed significantly between subtypes (Table 3). There was also no significant difference in time from illness onset to specimen collection by subtype.
Table 3.
Median (IQR) Charlson Comorbidity Index (CCI) among respiratory syncytial virus (RSV)-positive and influenza-positive hospitalizations, stratified by age-group
| RSV-positive | Influenza-positive | p-valuea | |
|---|---|---|---|
| 18-49 years | 1 (1-2) | 1 (1-2) | 0.97 |
| 50-64 years | 4 (2-6) | 2 (1-5) | 0.001 |
| 65+ years | 3 (2-5) | 2 (1-5) | 0.34 |
Abbreviations: IQR, interquartile range
P-value from Wilcoxon rank-sum test
Viral loads were obtained for 81 of 84 RSV-positive samples and are presented in table 4. Median viral load was 4.34 (IQR: 3.54-5.23; range: 1.95-7.86) log10 copies/ml. Median viral load of RSV-A infections was significantly lower than RSV-B infection [3.42 (2.93-4.06) vs 4.86 (4.00-5.44) log10 copies/ml; p<.0001]. We did not observe associations between viral load and increasing age, CCI, or BMI, in an analysis adjusting for time between illness onset and sample collection. The median viral load did not differ significantly for RSV-positive cases hospitalized for ≥3 days compared to those with a shorter length of stay [4.34 (3.69-5.23) vs 4.43 (3.33-5.33) log10 copies/ml; p=0.95]. Similarly, viral load did not differ for patients requiring mechanical ventilation or admission to the ICU, compared to those who did not.
Table 4.
Median respiratory syncytial virus (RSV) viral load by participant characteristics and regression coefficients and 95% confidence intervals (CI) from linear regression models
| Independent variable | Viral load (log10 copies/ml), median (IQR) | p-valuea | Adjusted linear regression modelsb, β (95% CI) | p-value |
|---|---|---|---|---|
| Age group | 0.79 | |||
| 18-49 | 4.36 (3.86-5.54) | Ref | ||
| 50-64 | 4.59 (3.31-5.40) | −0.20 (−0.98-0.57) | .45 | |
| ≥65 | 4.29 (3.54-4.86) | −0.31 (−1.13-0.51) | .60 | |
| BMI | 0.23 | |||
| Underweight/normal (18.5-24.99) | 5.00 (3.90-5.73) | Ref | ||
| Overweight (25-29.99) | 4.10 (3.18-4.75) | −0.70 (−1.55-0.15) | .11 | |
| Obese (≥ 30) | 4.55 (3.53-5.24) | −0.30 (−1.02-0.41) | .40 | |
| Charlson score | 0.07 | |||
| 0 | 4.33 (4.03-6.06) | Ref | ||
| 1 | 5.06 (4.05-5.33) | 0.36 (−0.86-1.59) | .56 | |
| 2 | 3.91 (3.53-4.41) | −0.84 (−2.05-0.38) | .17 | |
| ≥3 | 4.59 (3.28-5.40) | −0.18 (−1.28-0.91) | .74 |
Abbreviations: BMI, body mass index
P-value from Kruskal-Wallis test.
All models include the independent variable listed and viral load as the dependent variable adjusted for time between illness onset to specimen collection (in days).
RSV-positive compared with influenza-positive and RSV-negative, influenza-negative patients
1174 (94%) patients with complete data for all covariates were included in multivariable logistic regression models comparing RSV-positive patients to RSV-negative, influenza positive and, separately, RSV-negative, influenza-negative patients. After adjusting for potential confounders, RSV detection was associated with obesity (OR 1.71, 95% CI: 0.99-3.06; p=0.03) when compared to RSV-negative, influenza-negative patients (Table 5). In analyses comparing individuals with RSV to those with influenza, obesity was not significantly associated with RSV detection but participants admitted to the hospital later in their course of illness were more likely to have RSV than influenza (OR 1.16 95% CI: 1.03-1.30 per illness day; p=0.02) (Table 6).
Table 5.
Factors associated with RSV hospitalization compared to RSV-negative, influenza-negative hospitalization in unadjusted and adjusted logistic regression models.
| RSV compared to RSV-negative and influenza-negative | |||||
|---|---|---|---|---|---|
| Unadjusted OR (95% CI)a | Adjusted OR (95% CI)a,b | Pc | Adjusted OR (95% CI)a,b | Pc | |
| BMI | |||||
| Underweight/normal (18.5-24.99) | Ref | Ref | |||
| Overweight (25-29.99) | 1.06 (0.54-2.05) | .53 | 1.08 (0.55-2.11) | .49 | |
| Obese (≥ 30) | 1.59 (0.93-2.81) | .06 | 1.71 (0.99-3.06) | .03 | |
| Charlson score | |||||
| 0 | Ref | Ref | |||
| 1 | 1.10 (0.44-3.03) | .40 | 0.73 (0.29-2.05) | .25 | |
| 2 | 2.00 (0.81-5.52) | .09 | 1.23 (0.48-3.51) | .28 | |
| ≥3 | 1.46 (0.67-3.73) | .63 | 0.92 (0.40-2.46) | .86 | |
| Age group | |||||
| 18-49 | Ref | Ref | |||
| 50-64 | 1.99 (1.12-3.70) | .04 | 1.77 (0.97-3.36) | .10 | |
| ≥65 | 1.55 (0.84-2.95) | .71 | 1.45 (0.75-2.86) | .75 | |
| Hospital B | 1.38 (0.89-2.16) | .16 | 1.40 (0.88-2.10) | .15 | |
| Year (2015-2016) | 0.52 (0.32-0.84) | .01 | 0.53 (0.31-0.87) | .01 | |
| Time to admission, d | 1.08 (0.98-1.18) | .12 | 1.07 (0.97-1.17) | .17 | |
Abbreviations: BMI, body mass index
Firth penalized logistic regression models with profile-likelihood confidence intervals.
Adjusted for BMI, Charlson score, age, study site, year, and time from illness onset to admission.
Wald p-values.
Table 6.
Factors associated with RSV hospitalization compared to influenza hospitalization unadjusted and adjusted logistic regression models.
| RSV compared to Influenza | ||||
|---|---|---|---|---|
| Unadjusted OR (95% CI)a | Pc | Adjusted OR (95% CI)a,b | Pc | |
| BMI | ||||
| Underweight/normal (18.5-24.99) | Ref | Ref | ||
| Overweight (25-29.99) | 1.15 (0.55-2.40) | .80 | 1.01 (0.47-2.18) | .66 |
| Obese (≥ 30) | 1.56 (0.85-2.91) | .15 | 1.38 (0.73-2.66) | .25 |
| Charlson score | ||||
| 0 | Ref | Ref | ||
| 1 | 1.24 (0.47-3.57) | .18 | 0.84 (0.30-2.54) | .11 |
| 2 | 2.47 (0.93-7.26) | .17 | 1.76 (0.63-5.36) | .26 |
| ≥3 | 2.92 (1.25-7.83) | .01 | 1.87 (0.76-5.20) | .08 |
| Age group | ||||
| 18-49 | Ref | Ref | ||
| 50-64 | 2.46 (1.29-4.83) | .01 | 1.87 (0.93-3.84) | .05 |
| ≥65 | 1.54 (0.79-3.10) | .96 | 1.16 (0.56-2.45) | .57 |
| Hospital B | 1.24 (0.76-2.05) | .39 | 1.25 (0.73-2.14) | .42 |
| Year (2015-2016) | 0.55 (0.32-0.93) | .03 | 0.57 (0.32-0.99) | .05 |
| Time to admission, days | 1.16 (1.04-1.31) | .01 | 1.16 (1.03-1.30) | .02 |
Abbreviations: BMI, body mass index
Firth penalized logistic regression models with profile-likelihood confidence intervals.
Adjusted for BMI, Charlson score, age, study site, year, and time from illness onset to admission.
Wald p-values.
Clinical severity of RSV hospitalizations
Sixty-six percent of RSV-positive participants were admitted for ≥3 days, compared to 54% of influenza-positive participants (p = 0.048). Mechanical ventilation was required in 6 (7%) individuals with RSV. Five individuals with RSV (6%) were admitted to the ICU. There were no in-hospital deaths among the RSV group, 2 in the influenza group (1%) and 13 in the RSV-negative, influenza-negative group (1%). In multivariable analysis controlling for age, CCI, study site, year, and time from illness onset, no significant differences were found between RSV and influenza with respect to these clinical indicators of severity (Table 7).
Table 7.
Clinical outcomes of respiratory syncytial virus (RSV)-positive, RSV-negative and influenza-negative, and influenza-positive hospitalization.
| N, (%) | Adjusted logistic regression models OR (95% CI)a |
||||||
|---|---|---|---|---|---|---|---|
| RSV-positive (n=84) |
RSV-negative & influenza-negative (n=941) |
Influenza-positive (n=234) |
RSV vs RSV-negative, influenza-negative | P-valuec | RSV vs Influenza | p-valuec | |
| LOS ≥ 3 daysb | 56 (66.7) | 593 (63.2) | 127 (54.3) | 1.12 (0.70-1.84) | .64 | 1.53 (0.90-2.67) | .13 |
| ICU admission | 5 (6.0) | 89 (9.5) | 19 (8.1) | 0.76 (0.28-1.73) | .56 | 0.88 (0.29-2.31) | .79 |
| Mechanical ventilationd | 6 (7.1) | 116 (12.3) | 24 (10.3) | 0.65 (0.26-1.41) | .32 | 0.65 (0.23-1.63) | .38 |
Abbreviations: OR, odds ratio; CI, confidence interval; LOS, length of stay; ICU, intensive care unit
Firth penalized logistic regression models with profile-likelihood confidence intervals, adjusted for age ≥65, categorized Charlson score (0, 1, 2, ≥3), site, year, and time from illness onset to admission (in days).
2 RSV-negative/influenza-negative individuals were missing data on LOS.
Wald p-values.
Any mechanical ventilation (invasive or noninvasive). 1 RSV-negative/influenza-negative individual was missing information on mechanical ventilation status.
Discussion
Adults ≥65 years old have long been the focus of targeted efforts to reduce the burden of hospitalization due to severe respiratory illness. Even now that yearly influenza vaccination recommendations have been extended to adults of all ages, efforts still continue to improve vaccines for older individuals including the introduction of high dose formulations. Thus, it is no surprise that recent initiatives to develop an RSV vaccine for adults have targeted those 60-65 years and older. However, in this study conducted over two respiratory seasons in two different hospitals, we have demonstrated that a substantial proportion of RSV infections was detected in younger adults. We also observed that patients with RSV had a higher median CCI than patients with influenza, and that this association was driven by individuals in the 50-64 year old age group. This may suggest that influenza infection results in hospitalization for a relatively healthier group of adults 50-64 years old while RSV infection is more likely to result in hospitalization for those with multiple chronic conditions. A similarly designed study conducted in France over 3 seasons found that RSV cases were more likely to have cancer or be on immunosuppressive therapy than influenza cases [10]. Our findings provide evidence in support of focused development of RSV vaccines and therapies targeted toward an expanded age group of adults, particularly those 50-64 and those of any age with chronic comorbidities.
This study was conducted, in part, during the 2014-2015 respiratory illness season, which was notable for the circulation of an antigenically drifted influenza strain and unusually high numbers of hospitalizations for influenza-associated ARI [29]. Even in this context, we found RSV to be associated with a substantial proportion of ARI hospitalizations among adults of all ages. A number of previous studies of RSV in adults have enrolled an older study population [1,2,12,30,31]. An exception was a recent US study examining RSV in medically attended illnesses of all severities [32] which extended down to age 50, as well as previous studies from the United Kingdom that examined visits to general practitioners among individuals of all ages [33]. Modeling studies suggest a modest burden of RSV-associated medical visits, but little burden of hospitalizations, in those 18-49 years, especially for those with underlying risk conditions [6].
Much effort has been dedicated to comparing the clinical severity of RSV and influenza [1,3,4,12,17,34]. As with previous studies, we found that morbidity due to RSV in this adult population was comparable to that of influenza, as defined by need for mechanical ventilation and ICU admission; albeit in relatively few patients with severe endpoints. Importantly, this study was conducted in communities where influenza vaccination was common. Recent results from case-test negative studies, including the 2014-2015 season of this study, have suggested that influenza vaccine may be more effective in preventing severe rather than mild infections [19,35,36], thus our estimates of influenza severity may be reduced accordingly. While the occurrence of severe morbidity was comparable to that of influenza in this study, we were unable to adequately compare age-specific effects on severity between the two diseases due to sample size. We also observed that individuals with RSV were admitted later in their illness, on average, than individuals with influenza. While not significant in multivariable analyses, this finding could suggest a slower overall progression to acute illness for RSV versus influenza, as has been found in challenge studies [37].
We found that the predominant RSV subtype, as determined through molecular methods, varied from year to year. This is a pattern that has been observed elsewhere in studies of community-based RSV epidemiology [38,39]. In our modern era of rapid molecular methods, the rapid determination of RSV subtype is potentially significant on two fronts: early prediction of disease burden in a given season and eventual monitoring of RSV vaccine effectiveness. It is well-established that varied strains of influenza A are associated with differing disease burden and morbidity [1,40] and require the selection of type-specific vaccine strains. Our analysis, unlike previous work in children [41,42], did not find any evidence of a corresponding difference in morbidity by RSV strain type. The antigenic target of RSV vaccines currently in development is fairly well conserved between the subtypes [43]; nevertheless, it remains to be seen to what degree subtype variability in RSV will present challenges for a broadly effective vaccine.
Our current data suggest that there is more hospitalization attributable to RSV in younger adults than has been previously thought, and that most of these individuals have underlying risk conditions. Our study was conducted only among hospitalized individuals and, therefore, does not provide population-based incidence rates. Still, more than 67% of RSV identifications were in individuals under 65 years of age, suggesting the potential benefit of including these individuals in future RSV vaccination strategies. The fact that most have multiple underlying conditions makes it possible to target vaccination programs similar to the strategies used for influenza vaccination in countries without have a universal recommendation.
Key points.
RSV-associated hospitalizations were highest among adults 50-64 years and those with chronic illness, suggesting these individuals should be targeted in future RSV vaccination strategies.
Acknowledgments
Conflict of Interest Declaration
REM, ETM and ASM report a grant from MUGAS outside the current work. ETM reports grants from Merck and Pfizer outside the current work. ASL reports grants from the Doris Duke Charitable Foundation outside the current work. ASM reports grants and personal fees from Sanofi-Pasteur, and personal fees from Novartis and Protein Sciences outside the submitted work
Funding
This work was supported by the Centers for Disease Control and Prevention (CDC) through two co-operative agreements with the University of Michigan [grant numbers U01IP000474 and U01 IP000974].
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or funding agency.
References
- 1.Falsey AR, McElhaney JE, Beran J, van Essen GA, Duval X, Esen M, Galtier F, Gervais P, Hwang SJ, Kremsner P, Launay O, Leroux-Roels G, McNeil SA, Nowakowski A, Richardus JH, Ruiz-Palacios G, Rose SS, Devaster JM, Oostvogels L, Durviaux S, Taylor S. Respiratory Syncytial Virus and Other Respiratory Viral Infections in Older Adults With Moderate to Severe Influenza-like Illness. J Infect Dis. 2014;209:1873–1881. doi: 10.1093/infdis/jit839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory Syncytial Virus Infection in Elderly and High-Risk Adults. N Engl J Med. 2005;352:1749–1759. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 3.Falsey AR, McCann RM, Hall WJ, Tanner MA, Criddle MM, Formica MA, Irvine CS, Kolassa JE, Barker WH, Treanor JJ. Acute Respiratory Tract Infection in Daycare Centers for Older Persons. J Am Geriatr Soc. 1995;43:30–36. doi: 10.1111/j.1532-5415.1995.tb06238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Falsey AR, Treanor JJ, Betts RF, Walsh EE. Viral Respiratory Infections in the Institutionalized Elderly: Clinical and Epidemiologic Findings. J Am Geriatr Soc. 1992;40:115–119. doi: 10.1111/j.1532-5415.1992.tb01929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Widmer K, Zhu Y, Williams JV, Griffin MR, Edwards KM, Talbot HK. Rates of Hospitalizations for Respiratory Syncytial Virus, Human Metapneumovirus, and Influenza Virus in Older Adults. J Infect Dis. 2012;206:56–62. doi: 10.1093/infdis/jis309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fleming DM, Taylor RJ, Lustig RL, Schuck-Paim C, Haguinet F, Webb DJ, Logie J, Matias G, Taylor S. Modelling estimates of the burden of Respiratory Syncytial virus infection in adults and the elderly in the United Kingdom. BMC Infect Dis. 2015;15 doi: 10.1186/s12879-015-1218-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldstein E, Greene SK, Olson DR, Hanage WP, Lipsitch M. Estimating the hospitalization burden associated with influenza and respiratory syncytial virus in New York City, 2003–2011, Influenza Other Respir. Viruses. 2015;9:225–233. doi: 10.1111/irv.12325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glezen W, Taber LH, Frank AL, Kasel JA. RIsk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child. 1986;140:543–546. doi: 10.1001/archpedi.1986.02140200053026. [DOI] [PubMed] [Google Scholar]
- 9.Branche AR, Falsey AR. Respiratory Syncytial Virus Infection in Older Adults: An Under-Recognized Problem. Drugs Aging. 2015;32:261–269. doi: 10.1007/s40266-015-0258-9. [DOI] [PubMed] [Google Scholar]
- 10.Loubet P, Lenzi N, Valette M, Foulongne V, Krivine A, Houhou N, Lagathu G, Rogez S, Alain S, Duval X, Galtier F, Postil D, Tattevin P, Vanhems P, Carrat F, Lina B, Launay O, Seddik K, Lesieur Z, Bonmarin I, Loulergue P, Bodilis H, Servera-Miyalou M, Sadler I, Momcilovic S, Kanaan R, Coolent N, Boun KT, Blanche P, Charpentier J, Daviaud F, Mongardon N, Bretagnol A, Claessens YE, Rozenberg F, Yazdanpanah Y, Burdet C, Harent S, Lachatre M, Rioux C, Bleibtreu A, Casalino E, Choquet C, Leleu A, Belghalem K, Colosi L, Ranaivoson M, Verry V, Pereira L, Dupeyrat E, Bernard J, Emeyrat N, Chavance P, Debit A, Aubier M, Pradere P, Justet A, Mal H, Brugiere O, Papo T, Goulenok T, Boisseau M, Jouenne R, Alexandra JF, Raynaud-Simon A, Lilamand M, Cloppet-Fontaine A, Becheur K, Pelletier AL, Fidouh N, Ralaimazava P, Beaumale F, Costa Y, Munier E, Betend F, Amour S, Loeffert S, Francourt K, Merle C, Letois F, Géraud P, Driss V, Noslier S, Ray M, Sebbane M, Konaté A, Bourdin A, Klouche K, Léglise MS, Couve-Deacon E, Fruit D, Fenerol C, Vallejo C, Jouneau S, Lainé F, Thébault E, Fillatre P, Pape CL, Beuzit L, Chau F, Carrat F, Chau F, Goderel I. Clinical characteristics and outcome of respiratory syncytial virus infection among adults hospitalized with influenza-like illness in France. Clin Microbiol Infect. 2017;23:253–259. doi: 10.1016/j.cmi.2016.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monto AS, Malosh RE, Petrie JG, Thompson MG, Ohmit SE. Frequency of Acute Respiratory Illnesses and Circulation of Respiratory Viruses in Households With Children Over 3 Surveillance Seasons. J Infect Dis. 2014 doi: 10.1093/infdis/jiu327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Falsey AR, Cunningham CK, Barker WH, Kouides RW, Yuen JB, Menegus M, Weiner LB, Bonville CA, Betts RF. Respiratory Syncytial Virus and Influenza A Infections in the Hospitalized Elderly. J Infect Dis. 1995;172:389–394. doi: 10.1093/infdis/172.2.389. [DOI] [PubMed] [Google Scholar]
- 13.Gilca R, Amini R, Douville-Fradet M, Charest H, Dubuque J, Boulianne N, Skowronski DM, De Serres G. Other Respiratory Viruses Are Important Contributors to Adult Respiratory Hospitalizations and Mortality Even During Peak Weeks of the Influenza Season. Open Forum Infect Dis. 2014;1 doi: 10.1093/ofid/ofu086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naorat S, Chittaganpitch M, Thamthitiwat S, Henchaichon S, Sawatwong P, Srisaengchai P, Lu Y, Chuananon S, Amornintapichet T, Chantra S, Erdman DD, Maloney SA, Akarasewi P, Baggett HC. Hospitalizations for Acute Lower Respiratory Tract Infection Due to Respiratory Syncytial Virus in Thailand, 2008–2011. J Infect Dis. 2013;208:S238–S245. doi: 10.1093/infdis/jit456. [DOI] [PubMed] [Google Scholar]
- 15.Pastula ST, Hackett J, Coalson J, Jiang X, Villafana T, Ambrose C, Fryzek J. Hospitalizations for Respiratory Syncytial Virus Among Adults in the United States, 1997–2012. Open Forum Infect Dis. 2017;4 doi: 10.1093/ofid/ofw270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Volling C, Hassan K, Mazzulli T, Green K, Al-Den A, Hunter P, Mangat R, Ng J, McGeer A. Respiratory syncytial virus infection-associated hospitalization in adults: a retrospective cohort study. BMC Infect Dis. 2014;14 doi: 10.1186/s12879-014-0665-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou H, Thompson WW, Viboud CG, Ringholz CM, Cheng PY, Steiner C, Abedi GR, Anderson LJ, Brammer L, Shay DK. Hospitalizations Associated With Influenza and Respiratory Syncytial Virus in the United States, 1993–2008. Clin Infect Dis. 2012;54:1427–1436. doi: 10.1093/cid/cis211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Falsey AR, Formica MA, Hennessey PA, Criddle MM, Sullender WM, Walsh EE. Detection of Respiratory Syncytial Virus in Adults with Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2006;173:639–643. doi: 10.1164/rccm.200510-1681OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petrie JG, Ohmit SE, Cheng CK, Martin ET, Malosh RE, Lauring AS, Lamerato LE, Reyes KC, Flannery B, Ferdinands JM, Monto AS. Influenza Vaccine Effectiveness Against Antigenically Drifted Influenza Higher Than Expected in Hospitalized Adults: 2014-2015. Clin Infect Dis Off Publ Infect Dis Soc Am. 2016;63:1017–1025. doi: 10.1093/cid/ciw432. [DOI] [PubMed] [Google Scholar]
- 20.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA. Cardiovascular Health Study Collaborative Research Group, Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 21.Romero-Ortuno R, Walsh CD, Lawlor BA, Kenny RA. A frailty instrument for primary care: findings from the Survey of Health, Ageing and Retirement in Europe (SHARE) BMC Geriatr. 2010;10:57. doi: 10.1186/1471-2318-10-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.NHIS - Questionnaires, (n.d.) http://www.cdc.gov/nchs/nhis/quest_doc.htm (accessed June 2, 2016.
- 23.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 24.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 25.N.O.E.I.E.P. on the I. (US) Evaluation, and Treatment of Obesity in Adults. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults, National Heart, Lung, and Blood Institute. 1998 [Google Scholar]
- 26.Fry AM, Chittaganpitch M, Baggett HC, Peret TCT, Dare RK, Sawatwong P, Thamthitiwat S, Areerat P, Sanasuttipun W, Fischer J, Maloney SA, Erdman DD, Olsen SJ. The Burden of Hospitalized Lower Respiratory Tract Infection due to Respiratory Syncytial Virus in Rural Thailand. PLOS ONE. 2010;5:e15098. doi: 10.1371/journal.pone.0015098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuypers J, Wright N, Morrow R. Evaluation of quantitative and type-specific real-time RT-PCR assays for detection of respiratory syncytial virus in respiratory specimens from children. J Clin Virol. 2004;31:123–129. doi: 10.1016/j.jcv.2004.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shu B, Wu KH, Emery S, Villanueva J, Johnson R, Guthrie E, Berman L, Warnes C, Barnes N, Klimov A, Lindstrom S. Design and Performance of the CDC Real-Time Reverse Transcriptase PCR Swine Flu Panel for Detection of 2009 A (H1N1) Pandemic Influenza Virus. J Clin Microbiol. 2011;49:2614–2619. doi: 10.1128/JCM.02636-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Appiah GD, Blanton L, D’Mello T, Kniss K, Smith S, Mustaquim D, Steffens C, Dhara R, Cohen J, Chaves SS, Bresee J, Wallis T, Xu X, Abd Elal AI, Gubareva L, Wentworth DE, Katz J, Jernigan D, Brammer L. Centers for Disease Control and Prevention (CDC), Influenza activity - United States, 2014-15 season and composition of the 2015-16 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2015;64:583–590. [PMC free article] [PubMed] [Google Scholar]
- 30.Walsh EE, Peterson DR, Falsey AR. Is clinical Recognition of Respiratory Syncytial Virus Infection in Hospitalized Elderly and High-Risk. J Infect Dis. 2007;195:1046–1051. doi: 10.1086/511986. [DOI] [PubMed] [Google Scholar]
- 31.Lee N, Lui GCY, Wong KT, Li TCM, Tse ECM, Chan JYC, Yu J, Wong SSM, Choi KW, Wong RYK, Ngai KLK, Hui DSC, Chan PKS. High Morbidity and Mortality in Adults Hospitalized for Respiratory Syncytial Virus Infections. Clin Infect Dis. 2013;57:1069–1077. doi: 10.1093/cid/cit471. [DOI] [PubMed] [Google Scholar]
- 32.Sundaram ME, Meece JK, Sifakis F, Gasser RA, Belongia EA. Medically attended respiratory syncytial virus infections in adults aged ≥ 50 years: clinical characteristics and outcomes. Clin Infect Dis Off Publ Infect Dis Soc Am. 2014;58:342–349. doi: 10.1093/cid/cit767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zambon MC, Stockton JD, Clewley JP, Fleming DM. Contribution of influenza and respiratory syncytial virus to community cases of influenza-like illness: an observational study. Lancet Lond Engl. 2001;358:1410–1416. doi: 10.1016/s0140-6736(01)06528-x. [DOI] [PubMed] [Google Scholar]
- 34.Nicholson KG, Kent J, Hammersley V, Cancio E. Acute viral infections of upper respiratory tract in elderly people living in the community: comparative, prospective, population based study of disease burden. BMJ. 1997;315:1060–1064. doi: 10.1136/bmj.315.7115.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petrie JG, Cheng C, Malosh RE, VanWormer JJ, Flannery B, Zimmerman RK, Gaglani M, Jackson ML, King JP, Nowalk MP, Benoit J, Robertson A, Thaker SN, Monto AS, Ohmit SE. Illness Severity and Work Productivity Loss Among Working Adults With Medically Attended Acute Respiratory Illnesses: US Influenza Vaccine Effectiveness Network 2012-2013. Clin Infect Dis Off Publ Infect Dis Soc Am. 2016;62:448–455. doi: 10.1093/cid/civ952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Castilla J, Godoy P, Domínguez Á, Martínez-Baz I, Astray J, Martín V, Delgado-Rodríguez M, Baricot M, Soldevila N, Mayoral JM, Quintana JM, Galán JC, Castro A, González-Candelas F, Garín O, Saez M, Tamames S, Pumarola T, Azor E, Carrillo J, Moyano R, Navarro JA, Vázquez M, Zafra F, Bautista MF, Navarro JM, Pedrosa I, Pérez M, Gallardo V, Pérez E, Maldonado JR, Morillo A, Ubago MC, Carriedo D, Díez F, Fernández I, Fernandez S, Castrodeza J, Rodríguez C, Sanz P, Ortiz de Lejarazu R, Pérez A, Redondo P, Seco A, Pueyo A, Viejo JL, Fernández T, Molina A, Barbé F, Blanch L, Navarro G, Bonfill X, López-Contreras J, Pomar V, Puig MT, Borràs E, Martínez A, Torner N, Calafell F, Alonso J, Caylà J, Tortajada C, Garca I, Ruiz J, García JJ, Gea J, Horcajada JP, Hayes N, Moraga F, Dorca J, Agustí A, Trilla A, Vilella A, Génova R, García Barquero M, Gil E, Jiménez S, Martín F, Martínez ML, Sánchez S, Cantón R, Robustillo A, Álvarez C, Hernández A, Pozo F, Paño JR, Martínez A, Martínez L, Ruiz M, Fanlo P, Gil F, Martínez-Artola V, Ursua ME, Sota M, Virto MT, Gamboa J, Pérez-Afonso F, Aguirre U, Caspelastegui A, España PP, García S, Arístegui J, Bilbao A, Escobar A, Astigarraga I, Antoñana JM, Cilla G, Korta J, Pérez Trallero E, Lobo JL, Troya FJ, Morales M. Influenza Vaccine 387 Effectiveness in Preventing Outpatient, Inpatient, and Severe Cases of Laboratory-Confirmed 388 Influenza. Clin Infect Dis. 2013;57:167–175. doi: 10.1093/cid/cit194. [DOI] [PubMed] [Google Scholar]
- 37.Bagga B, Woods CW, Veldman TH, Gilbert A, Mann A, Balaratnam G, Lambkin-Williams R, Oxford JS, McClain MT, Wilkinson T, Nicholson BP, Ginsburg GS, DeVincenzo JP. Comparing influenza and RSV viral and disease dynamics in experimentally infected adults predicts clinical effectiveness of RSV antivirals. Antivir Ther. 2013;18:785–791. doi: 10.3851/IMP2629. [DOI] [PubMed] [Google Scholar]
- 38.Monto AS, Ohmit S. Respiratory Syncytial Virus in a Community Population: Circulation of Subgroups A and B since 1965. J Infect Dis. 1990;161:781–783. doi: 10.1093/infdis/161.4.781. [DOI] [PubMed] [Google Scholar]
- 39.Peret TC, Hall CB, Schnabel KC, Golub JA, Anderson LJ. Circulation patterns of genetically distinct group A and B strains of human respiratory syncytial virus in a community. J Gen Virol. 1998;79:2221–2229. doi: 10.1099/0022-1317-79-9-2221. [DOI] [PubMed] [Google Scholar]
- 40.Chaves SS, Aragon D, Bennett N, Cooper T, D’Mello T, Farley M, Fowler B, Hancock E, Kirley PD, Lynfield R, Ryan P, Schaffner W, Sharangpani R, Tengelsen L, Thomas A, Thurston D, Williams J, Yousey-Hindes K, Zansky S, Finelli L. Patients Hospitalized With Laboratory-Confirmed Influenza During the 2010–2011 Influenza Season: Exploring Disease Severity by Virus Type and Subtype. J Infect Dis. 2013;208:1305–1314. doi: 10.1093/infdis/jit316. [DOI] [PubMed] [Google Scholar]
- 41.McConnochie KM, Hall CB, Walsh EE, Roghmann KJ. Variation in severity of respiratory syncytial virus infections with subtype. J Pediatr. 1990;117:52–62. doi: 10.1016/S0022-3476(05)82443-6. [DOI] [PubMed] [Google Scholar]
- 42.Walsh EE, McConnochie KM, Long CE, Hall CB. Severity of respiratory syncytial virus infection is related to virus strain. J Infect Dis. 1997;175:814–820. doi: 10.1086/513976. [DOI] [PubMed] [Google Scholar]
- 43.Anderson LJ, Hierholzer JC, Tsou C, Hendry RM, Fernie BF, Stone Y, McIntosh K. Antigenic characterization of respiratory syncytial virus strains with monoclonal antibodies. J Infect Dis. 1985;151:626–633. doi: 10.1093/infdis/151.4.626. [DOI] [PubMed] [Google Scholar]


