Abstract
Maize (Zea mays L.) β-glucosidase was extracted from shoots of a wild-type (K55) and a “null” (H95) maize genotype. Enzyme activity assays and electrophoretic data showed that extracts from the null genotype had about 10% of the activity present in the normal genotype. Zymograms of the null genotype were devoid of any activity bands in the resolving gel, but had a smeared zone of activity in the stacking gel after native polyacrylamide gel electrophoresis. When extracts were made with buffers containing 0.5% to 2% sodium dodecyl sulfate, the smeared activity zone entered the resolving gel as a distinct band. These data indicated that the null genotypes have β-glucosidase activity, but the enzyme occurs as insoluble or poorly soluble large quaternary complexes mediated by a β-glucosidase-aggregating factor (BGAF). BGAF is a 35-kD protein and binds specifically to β-glucosidase and renders it insoluble during extraction. BGAF also precipitates β-glucosidase that is added exogenously to supernatant fluids of the null tissue extracts. The specific β-glucosidase-aggregating activity of BGAF is unequivocally demonstrated. These data clearly show that the monogenic inheritance reported for the null alleles at the β-glucosidase gene is actually for the BGAF protein, and BGAF is solely responsible for β-glucosidase aggregation and insolubility and, thus, the apparent null phenotype.
β-Glucosidase (β-d-glucoside glucohydrolase, EC 3.2.1.21) catalyzes the hydrolysis of aryl and alkyl β-d-glucosides as well as glucosides with a carbohydrate moiety such as cellobiose and other β-linked oligosaccharides (Reese, 1977). In maize (Zea mays L.), β-glucosidase occurs abundantly in young plant parts (e.g. root, mesocotyl, node, primordial leaves, coleoptile, silk, and ovule) and is localized in plastids (Esen and Stetler, 1993). The enzyme was initially thought to be encoded by a single nuclear gene (glu1) that maps to chromosome 10 (Pryor, 1978). The glu1 locus is the most polymorphic (>30 alleles) enzyme locus on record in maize or any other organism (Goodman and Stuber, 1983). cDNAs corresponding to the glu1 gene have been cloned and sequenced (Brzobohaty et al., 1993; A. Esen and M. Shahid, direct submission GenBank accession no. U25157). In addition, the genomic region containing the glu1 gene has been sequenced (H. Bandaranayake and A. Esen, submission GenBank accession no. U44773). The data show that the glu1 gene is about 5 kb in length, consisting of 12 exons interrupted by 11 introns. Moreover, a cDNA corresponding to another β-glucosidase gene (glu2) has been isolated and sequenced (Bandaranayake and Esen, 1996). This cDNA's putative protein product, Glu2, shows 90% sequence identity with those encoded by glu1 alleles. This second β-glucosidase gene (glu2) is expressed at low levels and only in leaves starting 6 d after germination. The Glu2 isozyme does not hydrolyze the artificial substrates commonly used for gel assays, and therefore it is not detected on zymograms unless one uses the fluorogenic substrate 4-methylumbelliferyl β-d-glucoside (M. Shahid and A. Esen, unpublished data).
The occurrence and activity of maize β-glucosidase is correlated with growth and certain desirable traits (Kahler and Wehrhahn, 1986). Castanospermin, a general glucosidase inhibitor, inhibits the growth of maize seedlings as much as 50% and the formation of secondary roots completely (Nagahashi et al., 1990). β-Glucosidases from different grasses, including maize, are implicated in phytohormone activation, the release of indole acetic acid (IAA) from its glucoconjugates (Wiese and Grambow, 1986; Campos et al., 1993). Maize β-glucosidase is also implicated in the activation of cytokinins during germination (Smith and van Staden, 1978). The major function of maize β-glucosidase, however, appears to be in the defense of young plant parts against pests by producing toxic hydroxamic acids from their glucosides. Hydroxamic acids, derivatives of 1,4-benzoxazin-3-one, are considered to be the major defense compounds in maize, wheat, rye, and barley (Niemeyer, 1988). They occur in fungi, yeast, bacteria, and plants, and are known to act as growth factors, antibiotics, antibiotic antagonists, tumor inhibitors, and cell division factors, and to play a role in iron metabolism (Nielands, 1967).
Hydroxamic acids were shown to be inhibitory to bacterial and fungal growth, as well as insect development and reproduction (Argandona et al., 1983; Sahi et al., 1990). The major hydroxamic acid glucoside in maize is 2-glucopyranosyl 4-dihydroxy-7-methoxy-1,4-benzoxazin-3-one (DIMBOAGlc) whose aglycone DIMBOA is the primary defense chemical against the European corn borer (Ostrinia nubilalis) and aphids. For example, a maize inbred, bxbx, is deficient in DIMBOA, and suffers from heavy infestation by the European corn borer. DIMBOAGlc constitutes up to 1% of the dry weight (4 mg/g fresh weight, or the equivalent of 10 mm final concentration) in young maize parts; thus, it is the most abundant hydroxamic acid glucoside (80%–90% of total). β-glucosidase and the substrate (DIMBOAGlc) are physically separated from each other by virtue of being in different compartments within the cell. Physical injury by a biting, chewing, or sucking insect or cell lysis after fungal and bacterial infections disrupts the compartmentalization and brings the enzyme and substrate in contact, releasing the toxic aglycone DIMBOA. Numerous studies show a high correlation between DIMBOA content of maize genotypes and the level of resistance to or inhibitory effect on insects and pathogens (Klun et al., 1967; Long et al., 1975; Argandona et al., 1983; Niemeyer, 1991).
In certain maize genotypes, β-glucosidase occurs as part of large, insoluble aggregates (Esen and Cokmus, 1990). The β-glucosidase zymograms of such genotypes are devoid of enzyme bands (Stuber et al., 1977). These genotypes were originally thought to be homozygous for a null allele at the glu1 locus. However, biochemical and immunological studies in our laboratory have clearly established that the so-called null genotypes have β-glucosidase activity when assayed in solution, and they have a 60-kD polypeptide reacting specifically with anti-β-glucosidase sera on immunoblots (Esen and Cokmus, 1990). The enzyme is not detected on zymograms because it occurs as large quaternary structures (>1.5 × 106 D), which fail to enter the gel. After dissociation of these structures by SDS, the enzyme can be detected on gels.
There are examples of β-glucosidase aggregation and β-glucosidase-binding proteins in various plants. For example, flax and oat β-glucosidases occur in high molecular mass forms ranging from 245 to 1,200 kD (Nisius, 1988; Fieldes and Gerhardt, 1994; Gus-Mayer et al., 1994). Recently, Falk and Rask (1995) reported two myrosinase (β-thioglucosidase)-binding proteins (50 and 52 kD) from rapeseed. The objective of the present study was to elucidate the biochemical basis of the β-glucosidase aggregation and insolubility observed in certain maize genotypes. Our hypothesis is that the β-glucosidase “null” phenotype is due to another protein (β-glucosidase-aggregating factor or BGAF) that occurs in “null” genotypes and specifically interacts with the enzyme, rendering it insoluble by aggregation into large multimeric forms. We present data in this paper supporting the hypothesis.
MATERIALS AND METHODS
Plant Materials
Three to 5-d-old-etiolated seedling shoots from a normal (K55) and a “null” (H95) inbred of maize (Zea mays L.) were used for protein and β-glucosidase isolation and all other studies reported, unless otherwise indicated. Seeds were surface-sterilized by soaking in a 20% (v/v) solution of commercial household bleach for 30 min. They were then rinsed thoroughly with distilled water and germinated in wet vermiculate at 30°C in the dark. Etiolated shoots were harvested and used either fresh or after freezing or storing at −70°C. In addition, freeze-dried shoot powders were used in certain experiments.
Protein Extraction
Etiolated shoots or their freeze-dried shoot powders were ground in an ice-chilled mortar with a pestle in 50 mm sodium acetate buffer, pH 5.0 (referred to as extraction buffer), using a w/v ratio of 1 g/3 mL. The homogenates were transferred to centrifuge tubes and kept on ice for 30 to 60 min, during which time the tubes were gently swirled at intervals to suspend the settled material. The crude enzyme extract was recovered after centrifugation at 17,000g for 15 min. The supernatant was transferred to a fresh tube, the pellet was resuspended in one-half the volume of the buffer used for the first extraction, and the centrifugation was repeated. The two supernatants were combined, aliquoted, and used for enzyme activity assays in solution and gels as well as other experiments. When small amounts of extracts were needed, 0.5 g of fresh or frozen material was homogenized in 1.5 mL of extraction buffer in a small mortar and the homogenate was transferred to a 1.5-mL microfuge tube for centrifugation. In the case of freeze-dried powders, 5 to 50 mg was suspended and extracted with 30 volumes (0.15–1.5 mL) of extraction buffer.
Enzyme and Protein Assays
β-Glucosidase activity in crude extracts was measured using the chromogenic substrate p-nitrophenyl β-d-glucopyranoside (pNPG). Routinely, 30 μL of the enzyme solution (the extract) was diluted with 1,470 μL of 50 mm citrate-100 mm phosphate buffer, pH 5.5, in a 1.5-mL microfuge tube. Then 70 μL of diluted extract was incubated in the wells of 96-well microtiter plates with 70 μL of 5 mm pNPG in the same buffer at 25°C for 5 min. The reaction was stopped by adding 70 μL of 400 mm sodium carbonate, and the pNP liberated from pNPG was measured at 410 nm. β-Glucosidase activity was expressed as the A410 of pNP released. Protein determinations were performed colorimetrically (Bradford, 1976) with bovine serum albumin fraction V as a standard.
Analysis of β-Glucosidase Aggregates by Gel Filtration
To determine the nature of the interactions responsible for aggregation as well as the sizes of enzyme aggregates, β-glucosidase isolated from null and normal phenotypes were subjected to gel filtration analysis. Shoot extracts were made with extraction buffer from K55 (normal) and H95 (null) and subjected to size fractionation through a column (1.5 × 85 cm) of Sephacryl HR 300 (molecular mass cutoff 1.5 × 106 D) using the appropriate calibration standards (thyroglobulin, 670 kD; BSA, 66 kD; ovalbumin, 43 kD; carbonic anhydrase, 29 kD; soybean trypsin inhibitor, 20.1 kD; and myoglobin, 16 kD). Column fractions were assayed for enzyme activity and protein. The same fractions were assayed for peroxidase and catalase activity to use these enzymes as internal markers.
Dissociation of β-Glucosidase Aggregates by SDS
We discovered that maize β-glucosidase is stable and active in the presence of SDS concentrations up to 3.2%, and that zymogram profiles can be developed on SDS gels if samples are applied without boiling (Esen and Gungor, 1991, 1993). To determine the nature of interactions responsible for aggregation, the enzyme was extracted from H95 with extraction buffer containing SDS from 0% to 1%, or SDS was added to the supernatant fluid after extraction without SDS. These samples were electrophoresed through native, isoelectric focusing, and SDS gels and stained for enzyme activity.
Mixing and Homogenizing Tissues from “Null” and Normal Genotypes
To determine if seedling parts of a null genotype contain a substance that is capable of reducing the extractability of β-glucosidase from normal genotypes, equal amounts (weights) of shoots from H95 and K55 were mixed, homogenized, and extracted together with extraction buffer. K55 and H95 shoots were also extracted separately to serve as controls. Enzyme activity was measured in supernatants of mixed and control extracts. In addition, H95 and K55 supernatants were mixed in a ratio of 1:1 and assayed to serve as another control. Similarly, K55 and H95 shoots were homogenized separately, and then 0.8, 0.6, 0.4, and 0.2 mL of the H95 homogenate was mixed, respectively, with 0.2, 0.4, 0.6, and 0.8 mL of the K55 homogenate. The mixed homogenates and controls (pure homogenates) were incubated in microfuge tubes on ice for 15 min and centrifuged. The enzyme activity in supernatant fluids was assayed after centrifugation as usual. A variation of these experiments was performed by extracting the enzyme from mixtures of freeze-dried 4-d-old K55 and H95 shoot powders. In this case, 0, 5, 10, 15, 20, 25, 30, 35, and 40 mg of the H95 powder was mixed, respectively, with 40, 35, 30, 25, 20, 15, 10, 5, and 0 mg of the K55 powder such that the total in each mixture was 40 mg. The mixtures were extracted with 1.2 mL of extraction buffer, and the supernatants were assayed for activity.
Adsorption of β-Glucosidase Activity from the Supernatant of Normal Genotypes by the Pellet of a “Null” Genotype
To determine if the β-glucosidase-aggregating activity resided in the insoluble fraction of the homogenates, the pellets of H95 and K55 homogenates were suspended and washed twice with a buffer volume equal to that used for the first extraction. Then aliquots of each pellet were weighed in amounts of 0.1, 0.2, 0.4, 0.6, 0.8, and 1.0 g and placed in separate tubes. Also, 1 mL of the K55 supernatant with known enzyme activity level was added to each tube, and pellets were suspended and incubated on ice for 15 min. Enzyme activity was determined in the supernatants after centrifugation.
Mixing and Incubating Extracts from “Null” and Normal Genotypes
To determine if null extracts contained a substance that can aggregate or inactivate the β-glucosidase present in the supernatant of normal extracts, 0.9, 0.8, 0.7, 0.6, 0.5, 0.4, 0.3, 0.2, and 0.1 mL of H95 extracts was mixed, respectively, with 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, and 0.9 mL of K55 extracts. The mixtures were incubated at 4°C for 16 h, and an aliquot was taken before centrifugation. Mixtures were then centrifuged at 14,000g at 4°C for 5 min, supernatants were transferred to fresh tubes, and the pellets were suspended in 50 mm Tris-HCl, pH 8.0, containing 0.5% SDS, and incubated for 1 h. Enzyme activity was assayed in the supernatants before and after centrifugation, as well as in the supernatant of the dissolved pellets. In addition, K55 and H95 supernatants were centrifuged at 17,000g for 10 min, and then a fixed volume (0.5 mL) of the H95 supernatant was mixed with the K55 supernatant ranging in volume from 0.1 to 1.0 mL. The mixtures were incubated at 4°C for 16 h, and enzyme activity in the supernatants and pellets of these mixtures was assayed as described above.
Solubilization and Recovery of β-Glucosidase Activity Adsorbed by H95 Pellets or Precipitated by H95 Supernatants
It was observed (see above) that the H95 pellets depleted of soluble protein adsorbed β-glucosidase activity from the K55 supernatants. Likewise, the H95 supernatants precipitated β-glucosidase activity from the K55 supernatants when they were mixed and incubated together. The question of whether or not the activity lost from the K55 supernatants can be recovered from the H95 pellet or the precipitate was addressed as follows. The pellet or precipitate was suspended and extracted sequentially with 50 mm Tris-HCl, pH 8.0, and with the same buffer containing 0.5% (w/v) SDS. These extracts were assayed for enzyme activity. Alternatively, the pellets were suspended and washed four times with buffer to remove any residual soluble protein. They were then suspended in SDS sample buffer (0.125 m Tris-HCl, pH 6.8, containing 2% [w/v] SDS, 5% [v/v] 2-ME [β-mercaptoethanol], and 10% [v/v] glycerol), heated at 95°C for 3 min, cooled, and the supernatants were recovered by centrifugation and used in electrophoretic analysis.
pH Dependence of β-Glucosidase Extractability and BGAF Activity
An earlier pilot experiment comparing BGAF activity (the ability to aggregate and precipitate β-glucosidase) and β-glucosidase aggregation and insolubility at pH 5.0 versus pH 8.0 suggested that both the activity of BGAF and the extractability of β-glucosidase were pH dependent. BGAF activity was higher at pH 5.0, whereas β-glucosidase extractability was higher at pH 8.0. To investigate this aspect further, 30 mg of freeze-dried K55 and H95 whole-shoot powders were each extracted in separate tubes four times with 0.9 mL of the following buffers: sodium citrate (pH 3.0 and 4.0), sodium acetate (pH 5.0), 2-(N-morpholino)-ethanesulfonic acid (MES) (pH 6.0), 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) (pH 7.0), Tris-HCl (pH 8.0), 2-(cyclohexylamino)ethanesulfonic acid (CHES) (pH 9.0), and sodium carbonate-bicarbonate (pH 10 and 11). The first, second, and third extracts were saved separately and assayed for enzyme activity. The fourth extract was discarded because it had little or no protein and activity. Because the first and second extracts contained about 90% to 95% of the total extractable activity at a given pH, they were pooled (1:1 volume) and reassayed for enzyme activity and protein so that the activity extracted at each pH could also be expressed as specific activity.
Determination of the Nature of BGAF and Its Specific Interaction with β-Glucosidase
The question of whether or not BGAF is a protein has also been addressed using another approach: a whole-shoot extract of H95 was subjected to gel filtration through a column of Sephacryl HR 300. Column fractions were tested for BGAF activity by mixing and incubating them with a K55 supernatant of a known amount of enzyme activity. Crude extracts and column fractions of H95 having BGAF activity were heated at 95°C and then mixed with K55 extracts and assayed for BGAF activity.
To determine if BGAF causes aggregation of other proteins in addition to β-glucosidase, the H95 and K55 supernatants were mixed at a volume ratio of 3:2, incubated overnight, centrifuged, and the supernatant and pellet fractions were subjected to SDS-PAGE along with unmixed controls. The same experiments were repeated using purified β-glucosidase from K55 or Escherichia coli lysates. In the latter case, the maize β-glucosidase isozyme Glu1 was cloned and expressed in E. coli (Cicek and Esen, 1999). The recombinant enzyme (r-Glu1) was purified and diluted with extraction buffer so as to have 0.2 A410 units/min p-nitrophenyl β-d-glucoside hydrolysis activity. The enzyme was added to 700 μL of H95 extract at amounts varying from 0 (control) to 300 μL, increasing at 50-μL increments, and the final volume was adjusted to 1 mL in all tubes by adding extraction buffer. The H95 extract was made with extraction buffer and subjected to a freeze-thaw-centrifugation cycle to remove nonspecifically precipitating proteins during which neither free β-glucosidase nor BGAF precipitates. Mixtures were incubated on ice for 2 h, and the enzyme activity was assayed in an aliquot of each mixture before and after centrifugation. The pellet was suspended in 100 μL of Laemmli buffer (1970) and subjected to SDS-PAGE analysis. The same experiments were also performed using K55 extracts.
RESULTS
The gel filtration analysis clearly shows that most of the β-glucosidase activity in the extracts of a null inbred (e.g. H95) occurs in a high molecular mass fraction (Table I). For example, when crude K55 and H95 extracts were fractionated by gel filtration, 22% of the activity in the H95 extract eluted as a dimer (120 kD), while 78% of the activity was in the flow-through fraction having an estimated molecular mass of 1.5 × 106 D or greater (Table I). In contrast, nearly all of the activity (98%) in extracts of a normal genotype (K55) eluted from the column as a dimer.
Table I.
Distribution of β-glucosidase activity between two size classes in normal and “null” genotypes of maize
| Genotype | Estimated Molecular Mass | |
|---|---|---|
| D | ||
| 120,000 | >1.5 × 106 | |
| “Null” (H95) | 22% | 78% |
| Normal (K55) | 98% | 2% |
Estimated by gel filtration (Sephacryl HR 300).
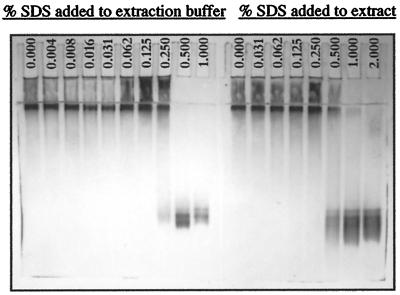
The electrophoretic data (Fig. 1) show that the enzyme extracted from a typical null genotype such as H95 does not enter the 6% (w/v) resolving gel during native PAGE, remaining mostly in the sample well and stacking gel, with only its leading front barely entering the top of the resolving gel. The situation does not change significantly until SDS is added up to a final concentration of 0.5% (w/v) to the extraction buffer. At 0.25% (w/v) SDS in the extraction buffer, the occurrence of dimeric enzyme in the extract and its entry into the resolving gel are evident, while at 0.5% (w/v) SDS, all of the enzyme is dimeric and enters the resolving gel. In contrast, most of the activity remained in the stacking gel and at the top of the resolving gel when SDS is added later to a final concentration of 0.5% (w/v) to the extract after it has been made in the absence of SDS. In the latter case, complete entry into the resolving gel occurs after SDS is added to a final concentration of 2% (w/v).
Figure 1.
Zymogram (6% [w/v] native PAGE gel; anode at bottom) showing the entry of β-glucosidase from a null genotype (H95) into the gel after SDS was added to the extract or the extraction buffer at a final concentration of 0.5% (w/v) or higher. Note that enzyme-BGAF aggregates remain in the stacking gel or form a band at the top of the resolving gel at lower than a 0.5% (w/v) SDS concentration.
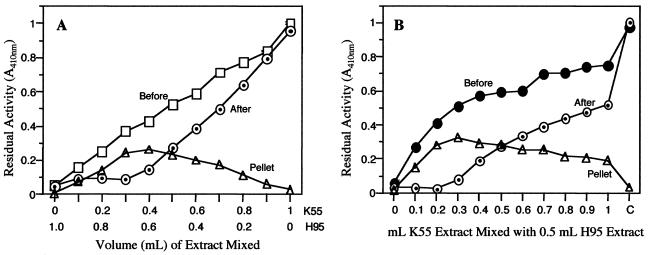
When enzyme activity was assayed in the supernatants of K55, H95, and H95 plus K55 (homogenized and extracted together), the H95 and H95 plus K55 supernatants had only 6% and 11%, respectively, of the activity present in the K55 supernatant. In contrast, when H95 and K55 supernatants were mixed in a ratio of 1:1 after they had been extracted separately, the activity in the supernatant mix was equal to the arithmetic mean of the activity present in the individual supernatants used to prepare the mix.
Figure 2 shows the expected and observed enzyme activities in the supernatants of pure H95 and K55 homogenates and of their mixtures incubated for 15 min before centrifugation. The data indicate that enzyme activity in the supernatant of the mixed H95 and K55 homogenate is drastically reduced. For example, only 12% of the expected activity was present in the supernatant resulting from the homogenate mix containing three parts H95 and two parts K55, and in all cases enzyme activity in the supernatant decreased as the percentage of the H95 homogenate in the mixture increased (Fig. 2A). The data from extraction of mixtures of H95 and K55 freeze-dried shoot powders also indicated that the extractability of β-glucosidase decreased as the amount of the H95 powder in the mixture increased. For example, the greatest reduction in extractability occurred when 25 mg of the H95 powder was mixed with 15 mg of the K55 powder. In this case, only 13% of the expected activity (sum of the activities expected if 25 mg of the H95 and 15 mg of the K55 powder were extracted separately) was found in the supernatant.
Figure 2.
A, Reduced extractability of β-glucosidase from K55 (wild-type) maize after its homogenate was mixed with that of H95 (null) and centrifuged. □, Expected activity in the mixture if the homogenate of each genotype were centrifuged separately and the resulting supernatants mixed at the indicated ratios. ⊙, Observed activity in the supernatant after homogenates were mixed at the indicated ratios and centrifuged, showing a decrease in enzyme activity as the amount of H95 homogenate in the mixture increases. B, Adsorption of β-glucosidase activity from K55 (wild-type) maize extract by the H95 (⊙) protein-depleted pellet after suspension and incubation with K55 extract. The K55 pellet (□) adsorbed little or no β-glucosidase activity.
When soluble protein-depleted pellets of H95 and K55 homogenates were checked for BGAF activity, the K55 pellet had little or no β-glucosidase-adsorbing activity (Fig. 2B). Even when the highest amount of pellet (1 g) was incubated with 1 mL of supernatant, only a 25% reduction in activity was observed. Such reduction was attributed to dilution of the supernatant activity by the buffer retained in the wet K55 pellet. In contrast, the H95 pellet adsorbed β-glucosidase activity in a weight-dependent manner (Fig. 2B). For example, 0.1- and 1.0-g H95 pellets, respectively, removed 60% and 96% of β-glucosidase activity from 1 mL of K55 supernatant after 15 min of incubation. Assay of protein content in the K55 supernatant before and after incubation with the H95 pellet showed little or no detectable changes. Freeze-dried H95 shoot powders also adsorbed β-glucosidase activity from a fixed volume of K55 supernatant with a known amount of enzyme activity after they were suspended and incubated in the K55 supernatant. Again, the amount of activity loss from the supernatant in this case was also directly proportional to the amount of the H95 shoot powder used.
Experiments addressing the question of whether the β-glucosidase activity that was precipitated by BGAF could be resolubilized showed that the extraction of the pellet with 50 mm Tris-HCl, pH 8.0, released only about 20% of the pellet-bound activity. However, the extraction using the same buffer plus 0.5% (w/v) SDS released essentially all of the activity from the pellet (Fig. 3). In fact, when activities were normalized for about 15% activity loss due to exposure to SDS and the insoluble activity present in the H95 pellet prior to incubation with the K55 supernatant, essentially all of the activity removed by BGAF from the K55 supernatant was recovered.
Figure 3.
A, Precipitation of β-glucosidase activity from K55 (wild-type) maize extract by H95 (null) extract after incubation and centrifugation of extract mixtures at the indicated volumes. Shown are activity in the mixture before centrifugation □; activity after centrifugation, ⊙; and activity in the suspended pellet ▵. Note that the highest precipitation of activity occurs at two to three parts of K55 (wild-type) to five parts of H95 (null extract), and the activity lost from the supernatant is recovered from the pellet. B, β-Glucosidase activity profile of extracts after mixing a fixed volume of H95 (null, 0.5 mL) extract with varying volumes of K55 (wild-type, 0–1.0 mL). Shown are activity in the mixture before centrifugation, ●; activity after centrifugation ⊙; and activity in the suspended pellet ▵. C, Control (K55 extract). Again note that the highest precipitation of enzyme activity occurs at two to three parts of K55 (wild-type) to five parts of H95 (null extract), and the activity lost from the supernatant is recovered from the pellet.
When supernatants of H95 and K55 extracts instead of homogenates were mixed in similar experiments, the expected and observed enzyme activities in supernatant mixes were the same. However, if the supernatant mixes were incubated overnight and then centrifuged, the observed activity in the supernatant decreased after centrifugation, for example, by 55% in the 1:1 mixture and by 80% in the 2:3 mixture (K55:H95) (Fig. 3). In all cases, the amount of activity decrease was directly proportional to the amount of the H95 extract present in the mix.
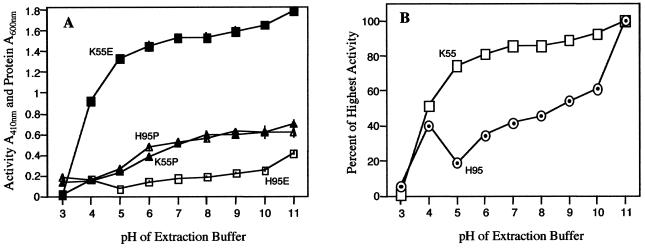
The experiments testing the effect of pH on the extractability of β-glucosidase and the activity of BGAF (Fig. 4A) showed that the amount of enzyme and extractable protein increased with pH in K55, being lowest or negligible at pH 3.0 and highest at pH 11. When the highest β-glucosidase activity extractable at pH 11 is set as 100%, relative extractabilities were 52% at pH 4.0 and increased from 74% at pH 6.0 to 93% at pH 10 (Fig. 4B). However, in terms of specific activity (expressed here as A410 units or the absorbance of pNP produced per milligram of protein), the pH 4.0 and 5.0 extracts had the highest activities (92 and 89 units), and in the pH range 6.0 to 11, specific activity decreased as the pH of the extraction buffer increased (data not shown). The H95 (“null”) extracts exhibited the following striking differences compared with those of K55: (a) the amount of total extractable β-glucosidase activity was about 3 to 20 times lower, depending on pH, resulting in drastic decreases in specific activities; and (b) more surprisingly, relative extractabilities in the pH range 7.0 to 10 increased from 42% to 61%, in stark contrast to the little or no increase (86%–93%) for K55 in the same pH range. Another surprising result was the decrease of relative extractability to 19% at pH 5.0 and to 35% at pH 6.0 after 40% at pH 4.0 (Fig. 4B).
Figure 4.
A, pH-dependent extractability of β-glucosidase (▪) and protein (▴) from K55 (wild-type) and β-glucosidase (□) and protein (▵) from H95 (null) maize genotypes. Note that extractability increases with pH, and the two genotypes do not differ with respect to total extractable protein, but do with respect to extractable β-glucosidase. B, β-Glucosidase extractability as percent of pH of highest activity from K55 (□, wild-type) and H95 (⊙, null). Note that the amount of extractable β-glucosidase is drastically reduced in H95 tissue, especially at pH 5.0.
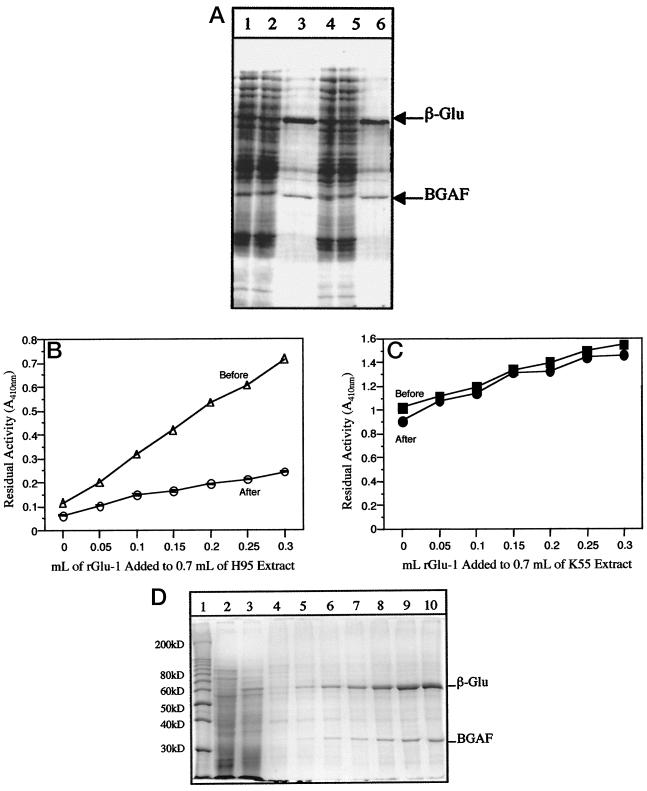
Association of BGAF activity with a protein fraction has been confirmed by assaying fractions of a whole shoot extract of H95 (null) that was subjected to gel filtration chromatography through a column of Sephacryl HR 300. Again, essentially all of the β-glucosidase activity appeared in the excluded fraction (>1.5 × 106 D) due to aggregation by BGAF. When column fractions were assayed for BGAF activity by mixing and incubating them with a K55 supernatant of known enzyme activity level, two fractions (41 and 42) had β-glucosidase-precipitating activity. Both fractions were positive for protein based on spectrophotometric and gel assays. SDS-PAGE analysis of these fractions revealed that both had a 35-kD polypeptide whose intensity correlated with the amount of BGAF activity. The same polypeptide appeared as a minor component in the excluded fraction, presumably representing the BGAF associated with the aggregated β-glucosidase. Moreover, when an aliquot of fractions 41 and 42 were boiled for 10 min, they completely lost their BGAF activity.
Electrophoretic analysis of before and after centrifugation of K55 and H95 supernatant mixes and their pellets further confirmed the association between β-glucosidase aggregation and a 35-kD polypeptide. These data revealed that the difference between the before and after centrifugation supernatant protein profiles was reduction in the intensity of the 60-kD β-glucosidase monomer band in the after centrifugation supernatant (Fig. 5A). In addition, the pellet fraction contained the 60-kD β-glucosidase monomer as the most predominant protein band along with some minor polypeptides, one of which (35 kD) was visible only in the H95 profile but considerably enriched in the pellet. This 35-kD polypeptide is identified a putative BGAF (Fig. 5A).
Figure 5.
A, SDS-PAGE profiles of H95 (null, 3 volumes) and K55 (wild-type, 2 volumes) maize extract mixtures and of their pellet. Lanes 1 to 3, After incubation on ice. Lane 1, Before centrifugation; lane 2, post-centrifugation; and lane 3, post-centrifugation pellet. Lanes 4 to 6, After incubation at 4°C. Lane 4, Before centrifugation; lane 5, post-centrifugation; and lane 6, post-centrifugation pellet. Note the decrease in the intensity of a 35-kD polypeptide in the H95 plus K55 mixture supernatant profile after centrifugation. The pellet profile includes primarily two polypeptides, the 60-kD β-glucosidase monomer and the 35-kD BGAF monomer, indicating the specific interaction between β-glucosidase and BGAF and precipitation of the resulting complex upon centrifugation. B, Precipitation of exogenously added purified β-glucosidase (r-Glu1) to extracts of H95 (null) by BGAF as a function of the volume of purified β-glucosidase solution (0.05–0.3 mL) added to a fixed volume (0.7 mL) of the H95 extract. C, As in B except that the wild-type extract (K55) was used in the experiment. Note that there is little or no detectable BGAF activity in the wild-type extract. D, SDS-PAGE gel showing the specific interaction between β-glucosidase and BGAF and its quantitative precipitation of the resulting complex upon addition of purified β-glucosidase to H95 (null) extracts and centrifugation (refer to B). Lane 1, Molecular mass markers; lane 2, H95 extract before addition of purified enzyme; lane 3, K55 extract before addition of purified enzyme; lanes 4 to 10, post-centrifugation pellets after 0, 0.05, 0.1, 0.15, 0.20, 0.25, and 0.30 mL of purified β-glucosidase solution was added, respectively, to 0.7 mL of H95 (null) extract (lane 2), incubated for 2 h, and centrifuged. Note the intensities of the 60-kD β-glucosidase monomer and the 35-kD BGAF monomers in the pellet fraction increases as the amount of β-glucosidase added to the H95 extract increases.
More unequivocal evidence associating BGAF activity with the 35-kD polypeptide was obtained by incubating purified β-glucosidase with whole-shoot extracts of H95. In this case, β-glucosidase activity was lost from the supernatant in proportion to the amount of added β-glucosidase after centrifugation and recovered in the pellet fraction (Fig. 5B). SDS-PAGE analysis of the pellet fraction showed essentially two polypeptides, the 60-kD β-glucosidase monomer and the 35-kD BGAF monomer (Fig. 5D). However, no enzyme activity was lost from the supernatant after centrifugation when the same amount of purified β-glucosidase was added to the K55 extract (Fig. 5C). Consequently, there was no detectable amount of the 60-kD β-glucosidase monomer and the 35-kD BGAF monomer in the pellet fraction (data not shown).
DISCUSSION
It has been reported that certain maize genotypes were devoid of β-glucosidase activity based on the absence of any activity zones (bands) on zymograms after starch gel electrophoresis (Stuber et al., 1977). Therefore, such genotypes were classified as “null.” However, the original paper from our laboratory on the subject reported that the β-glucosidase “null” phenotypes had β-glucosidase activity when assayed spectrophotometrically, and an immunoreactive protein in the 60-kD region of the gel when assayed by immunoblotting (Esen and Cokmus, 1990). The null phenotype appeared to be an artifact in that the enzyme of null genotypes occurred in large aggregates, was poorly soluble, did not enter the gel, and was not detected when zymogram techniques were used for analysis and scoring.
Our gel filtration data support the conclusion that the null characteristic is a consequence of poor enzyme extractability and insolubility due to aggregation. The data indicate that about 80% of β-glucosidase activity is in aggregates of 1.5 × 106 D or greater in size and thus, does not enter the gel (Table I; Fig. 1). In fact, this is likely to be underestimated because the extract used for gel filtration was freshly made and applied onto the column immediately after centrifugation. In extracts stored 24 h or longer, nearly all of the β-glucosidase was in the aggregated state, suggesting that the interaction between β-glucosidase dimers and BGAF continue as long as there is free β-glucosidase and BGAF in the extract. Interestingly, the gel filtration data did not reveal any distinct intermediate size classes between β-glucosidase dimers, and the >1.5 × 106 D aggregates detected by gel filtration. This may suggest the kinetics of aggregation are rather fast and if intermediate forms indeed occur, they must have a short half-life.
Our activity staining data indicated that extracts of null genotypes did not yield any bands on 5% to 7% polyacrylamide gels, confirming the results of Stuber et al. (1977) on starch gels. Instead, we found a diffuse zone of activity extending from sample wells to the boundary between the stacking gel and the resolving gel, indicating the presence of large aggregates that failed to enter the resolving gel (Fig. 1). The data clearly show that the enzyme from a typical null genotype such as H95 stays in the aggregated form and does not enter a native alkaline gel unless SDS is added to the extract to a final concentration of 0.5% (w/v) or the extraction is made with a buffer containing SDS at or above 0.5% (w/v).
The β-glucosidase present in null extracts, having dissociated from BGAF by SDS added at a final concentration of 0.5% to 1.0% (w/v) to extracts, enters and focuses in a position in isoelectric focusing gels similar to the positions of β-glucosidase allozymes found in normal extracts (data not shown). Thus, the so-called null genotypes do not have a specific mutant allele coding for an allozyme unique to them that have propensity to aggregate. When zymograms were developed after SDS-PAGE, all “null” genotypes had a band in the 120-kD region of the gel (data not shown) whether or not the samples were treated with SDS prior to electrophoresis. This indicates that the combination of high pH and 0.1% (w/v) SDS (the concentration in the gel and running buffer) is sufficient to dissociate the β-glucosidase aggregates into dimers. Alternatively, dissociation occurs when the SDS ion front (much higher concentration of SDS) passes through the sample zone during stacking. It is clear that SDS alone is able to dissociate the β-glucosidase-BGAF aggregates, yielding the dimeric form of the enzyme as it occurs in wild-type genotypes. This observation suggests that the aggregates are formed and stabilized by non-covalent (e.g. hydrophobic) interactions.
The most plausible interpretation of the presented data is that the β-glucosidase null phenotype observed in certain maize genotypes is caused not by specific mutant alleles of the β-glucosidase gene, but by an allele of another gene that encodes BGAF. BGAF (a protein factor present in all null genotypes) interacts specifically either in vivo or during extraction or both with native β-glucosidase dimers, causing them to aggregate into multimeric forms 1.5 × 106 D or larger in size. Analysis of isolated aggregates (precipitates) by electrophoresis indicates that they are made up of primarily β-glucosidase and BGAF (Fig. 5, A and D) and may include other minor protein components that are probably nonspecifically associated with or trapped in aggregates.
Evidence for the conclusion that the β-glucosidase null phenotype is caused by another protein (i.e. BGAF) rather than by a β-glucosidase null allele is as follows: (a) drastically reduced extractability of the enzyme from the normal genotype after its shoot material is mixed and homogenized together with the shoot material of the so-called null genotype (Fig. 2A); (b) reduction or loss of enzyme activity from supernatant fluids of the normal genotype after suspension of freeze-dried tissue powders or post-extraction and centrifugation pellets of null tissues in a manner directly proportional to the amount of null tissue powders or post-centrifugation pellets (Fig. 2B); (c) reduction or loss of enzyme activity from homogenates and supernatant fluids of the normal genotype after mixing and incubating them with homogenates and supernatants of the null genotype and then centrifuging them—again, the activity loss being directly proportional to the amount of null homogenate or supernatant (Figs. 2A and 3); (d) recovery of lost (adsorbed or precipitated) enzyme activity from post-centrifugation pellets after suspension in buffers containing SDS (Fig. 3); (e) loss of β-glucosidase-precipitating activity in null homogenates, supernatants, and pellets after heating at 70°C or above; (f) co-precipitation of a 35-kD polypeptide with β-glucosidase after mixing and incubating supernatants of the normal genotype or purified β-glucosidase solutions with supernatants of the null genotype (Fig. 5, A and D); and (g) isolation of the above-mentioned 35-kD polypeptide from null extracts by gel filtration through a Sephacryl S-300 column and demonstration of its β-glucosidase aggregation and precipitating activity and loss of such an activity upon heating at 70°C or above.
The significance of what appears to be the pH dependence of the interaction between β-glucosidase and BGAF is not fully understood. The fact that β-glucosidase from tissues of the null genotype is least extractable at pH 5.0 (Fig. 4) suggests that this pH is optimum for β-glucosidase-BGAF interaction and the resulting aggregation and insolubility of the enzyme. Moreover, the estimated pI of β-glucosidase is 5.2, meaning that charge repulsion will be near a minimum at and around pH 5.0, thus promoting non-covalent (e.g. hydrophobic) interactions between β-glucosidase molecules, as well as those between β-glucosidase and BGAF. The same result can also be explained by low solubility of β-glucosidase-BGAF complexes at and around pH 5.0, rather than pH 5.0 being optimum for β-glucosidase-BGAF interaction. The latter interpretation is supported by the zymogram data, which show the same enzyme activity profile in extracts made with buffers ranging in pH from 4.0 to 11. In other words, the activity was detected only in the stacking gel and at the boundary between the stacking and resolving gel, indicating the occurrence of enzyme-BGAF aggregates at all pHs tested.
The stoichiometry of the interaction between β-glucosidase and BGAF could not be determined because we could not purify sufficient amounts of free BGAF. However, densitometric analysis of β-glucosidase and BGAF monomer intensities after correction for size differences suggests about two molecules of β-glucosidase (dimer) to one molecule of BGAF stoichiometry. The fact that BGAF and β-glucosidase precipitate together and are the most abundant proteins in the precipitate suggests that BGAF mediates enzyme aggregation by directly binding to β-glucosidase rather than by catalyzing aggregation between β-glucosidase molecules. We postulate that BGAF has at least two binding sites for β-glucosidase, as does an antibody molecule for its specific antigen, which will lead to the formation of large multimeric complexes (>1.5 × 106). If BGAF were monovalent, it could bind only one β-glucosidase dimer, resulting in a quaternary association of approximately 150 to 160 kD whose existence is doubtful. This interpretation is consistent with our gel filtration data in that about 80% of the enzyme activity was in the excluded fraction as β-glucosidase-BGAF aggregates while about 20% of the enzyme activity eluted from the column as a dimer (approximately 120 kD) (Table I). Moreover, free BGAF eluted from the column as a monomer (approximately 35 kD), and its mobility was the same through a SDS-PAGE gel whether the sample was applied onto the gel after denaturation (i.e. boiling in Laemmli [1970] buffer) or directly.
It appears that binding of BGAF to β-glucosidase has no detectable effect on enzyme activity and kinetic parameters, suggesting that either BGAF binding does not sterically block the active site or does not change the conformation to affect enzyme activity.
In conclusion, the data we present clearly establish that the so-called β-glucosidase null genotypes of maize are not null. Instead, they express and contain a 35-kD protein, which specifically binds β-glucosidase, leading to the formation of large, insoluble quaternary associations. The nature of the interaction between β-glucosidase and BGAF is specific and non-covalent, reminiscent of that of an antigen-antibody interaction. This presents an excellent model system with which to study protein-protein interactions and their mechanism. It is conceivable that the specific interaction between β-glucosidase and BGAF has physiological relevance, or it may simply be fortuitous. This remains to be determined.
Footnotes
This research was supported in part by a Jeffress Foundation grant.
LITERATURE CITED
- Argandona VH, Corcuera LJ, Niemeyer HM, Cambell BC. Toxicity and feeding deterrency of hydroxamic acids from Gramineae in synthetic diets against the green bug, Schizaphis graminum. Ned Entomol Ver Amsterdam. 1983;34:134–138. [Google Scholar]
- Bandaranayake H, Esen A. Nucleotide sequence of a beta-glucosidase (glu2) cDNA from maize (accession no. U44087) (PGR 96-009) Plant Physiol. 1996;110:1048. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brzobohaty B, Moore I, Christofferson P, Bako L, Campos N, Schell J, Palme K. Release of active cytokinin by a β-glucosidase localized to the maize root meristem. Science. 1993;262:1051–1054. doi: 10.1126/science.8235622. [DOI] [PubMed] [Google Scholar]
- Campos N, Bako L, Brzobohaty B, Feldwisch J, Zettl R, Boland W, Palme K. Identification and characterization of a novel phytohormone conjugate specific β-glycosidases activity from maize. In: Esen A, editor. β-Glucosidases: Biochemistry and Molecular Biology. ACS Symp Ser 533: 204–212. 1993. [Google Scholar]
- Cicek M, Esen A. Expression of soluble and catalytically active plant (monocot) β-glucosidases in E. coli. Biotech Bioenerg. 1999;63:392–400. doi: 10.1002/(sici)1097-0290(19990520)63:4<392::aid-bit2>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Esen A, Cokmus C. Maize genotypes classified as null at the glu locus have β-glucosidase activity and immunoreactive protein. Biochem Genet. 1990;28:319–336. doi: 10.1007/BF02401422. [DOI] [PubMed] [Google Scholar]
- Esen A, Gungor G. Detection of β-glucosidase activity on SDS-polyacrylamide gels. Theor Appl Electrophysiol. 1991;2(2–3):63–69. [PubMed] [Google Scholar]
- Esen A, Stetler DA. Subcellular localization of maize β-glucosidase. Maize Genet Coop News Lett. 1993;67:19. [Google Scholar]
- Falk AJ, Rask L. Expression of a zeatin-O-glucoside-degrading β-glucosidase in Brassica napus. Plant Physiol. 1995;108:1369–1377. doi: 10.1104/pp.108.4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fieldes MA, Gerhardt KE. An examination of the β-glucosidase (linamarase) banding pattern in flax seedlings using Ferguson plots and SDS-PAGE. Electrophoresis. 1994;15:654–661. doi: 10.1002/elps.1150150192. [DOI] [PubMed] [Google Scholar]
- Goodman MM, Stuber CW. Maize. In: Tanksley SD, Orton TJ, editors. Isozymes in Plant Genetics and Breeding, Part B. New York: Elsevier Science Publishing; 1983. pp. 1–33. [Google Scholar]
- Gus-Mayer S, Brunner H, Scneider-Poetsch HAW, Rudiger W. Avenacosidase from oat: purification, sequence analysis and biochemical characterization of a new member of the BGA family of β-glucosidases. Plant Mol Biol. 1994;26:909–921. doi: 10.1007/BF00028858. [DOI] [PubMed] [Google Scholar]
- Kahler AL, Wehrhahn CF. Associations between quantitative traits and enzyme loci in the F2 population of a maize hybrid. Theor Appl Genet. 1986;72:15–26. doi: 10.1007/BF00261448. [DOI] [PubMed] [Google Scholar]
- Klun JA, Titon CL, Brindley TA. 2:4-Dihydroxy-7-methoxy-1,4-benzoxazin-3-one (DIMBOA), an active agent in the resistance of maize to the European corn borer. J Econ Entomol. 1967;60:1529–1533. [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;277:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Long BJ, Dunn GM, Routley DG. Relationship of hydroxamic acid content in maize and resistance to northern corn leaf blight. Crop Sci. 1975;15:333–335. [Google Scholar]
- Nagahashi G, Tu S-I, Fleet G, Namgoong SK. Inhibition of cell wall-associated enzymes in vitro and in vivo with sugar analogs. Plant Physiol. 1990;92:413–418. doi: 10.1104/pp.92.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielands JB. Hydroxamic acids in nature. Science. 1967;156:1443–1447. doi: 10.1126/science.156.3781.1443. [DOI] [PubMed] [Google Scholar]
- Niemeyer HM. Hydroxamic acids (4-hydroxy-1,4-benzoxazin-3-ones), defense chemicals in the Gramineae. Phytochemistry. 1988;27:3349–3358. [Google Scholar]
- Niemeyer HM. Secondary plant chemicals in aphid-host interactions. In: Peters DC, Webster JD, Chlouber CJ, editors. Aphid-Plant Interactions: Populations to Molecules. Oklahoma State University, Stillwater, OK; 1991. p. 101. [Google Scholar]
- Nisius A. The stromacentre in Avenaplastids: an aggregation of β-glucosidase responsible for the activation of oat-leaf saponins. Planta. 1988;173:474–481. doi: 10.1007/BF00958960. [DOI] [PubMed] [Google Scholar]
- Pryor AJ. Mapping of glucosidase on chromosome 10. Maize Genet Coop News Lett. 1978;52:14. [Google Scholar]
- Reese ET. Degradation of polymeric carbohydrates by microbial enzymes. Recent Adv Phytochem. 1977;11:311–368. [Google Scholar]
- Sahi SV, Chilton M-D, Chilton WS. Corn metabolites affect growth and virulence of Agrobacterium tumefaciens. Proc Natl Acad Sci USA. 1990;87:3879–3883. doi: 10.1073/pnas.87.10.3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AR, van Staden J. Changes in endogenous cytokinin levels in kernels of Zea maysL. during imbibition and germination. J Exp Bot. 1978;29:1067–1073. [Google Scholar]
- Stuber CW, Goodman MM, Johnson FM. Genetic control and racial variation of β-glucosidase isozymes in maize (Zea maysL.) Biochem Genet. 1977;15:383–394. doi: 10.1007/BF00484468. [DOI] [PubMed] [Google Scholar]
- Wiese G, Grambow H. Indole-3-methanol-β-d-glucoside and indole-3-carboxylic acid β-d-glucoside are products of indole-3-acetic acid degradation in wheat leaf segments. Phytochemistry. 1986;25:2451–2455. [Google Scholar]