Abstract
Background
Theories of aberrant attentional processing in social anxiety, and anxiety disorders more broadly, have postulated an initial hypervigilance or facilitation to clinically relevant threats and consequent defensive avoidance. However, existing objective measurements utilized to explore this phenomenon lack the resolution to elucidate attentional dynamics, particularly covert influences.
Methods
We utilized a continuous measure of visuocortical engagement, the steady-state visual evoked potential in response to naturalistic angry, fearful, happy and neutral facial expressions. Participants were treatment-seeking patients with principal diagnoses of social anxiety circumscribed to performance situations (n=21) or generalized across interaction contexts (n=42), panic disorder with agoraphobia (n=25), and 17 healthy participants.
Results
At the principal disorder level, only circumscribed social anxiety patients showed sustained visuocortical facilitation to aversive facial expressions. Control participants as well as patients with panic disorder with agoraphobia and generalized social anxiety showed no bias. More finely stratifying the sample according to clinical judgment of social anxiety severity and interference revealed a linear increase in visuocortical bias to aversive expressions for all but the most severely impaired patients. This group showed an opposing sustained attentional disengagement.
Conclusions
Rather than shifts between covert vigilance and avoidance of aversive facial expressions, social anxiety appears to confer a sustained bias for one or the other. While vigilant attention reliably increases with social anxiety severity for the majority of patients, the most impaired show an opposing avoidance. These distinct patterns of attentional allocation could provide a powerful means of personalizing neuroscience-based interventions to modify attention bias and related impairment.
Keywords: ssVEP, EEG, social anxiety, panic disorder, agoraphobia, RDoC
Introduction
Heightened sensitivity to facial expressions, particularly those connoting threat or scrutiny, has often been observed in social anxiety disorder. This includes speeded behavioral responses to spatial cues (1), increased reflexive eye-movements (2) and enhanced early (i.e., 100–200 ms) and later (i.e., 300–500 ms) event-related potential components (ERPs) (3–5). Functional neuroimaging findings implicate excessive recruitment of limbic, paralimbic and medial prefrontal fear circuitry in conjunction with extrastriate visual cortex (6–8).
While heightened sensitivity to aversive facial expressions is common in social anxiety, a corpus of work has suggested marked inconsistency—at times revealing no bias for aversive faces or even a bias for neutral faces (9, 10). Aberrant perceptual sensitivities in social anxiety, and anxiety disorders more broadly, have often been interpreted in accordance with the vigilance-avoidance hypothesis—that perception of threat-relevant stimuli is characterized by initial hypervigilance and consequent defensive avoidance (11, 12). However, the measurements utilized to explore this phenomenon (i.e., fMRI, ERPs, reaction time, eye-tracking, self-report) lack the ability to continuously quantify threat-related changes in visuocortical engagement that may unfold at different latencies and for different durations. Opposing attentional shifts such as initial hypervigilance and reflexive avoidance, when averaged into a single epoch, may contribute to inconsistent findings.
To track dynamic attention to angry, fearful, happy and neutral facial expressions, here we used scalp-recorded steady-state visual evoked potentials (ssVEPs) as a continuous measure of selective attention (i.e., the attentional spotlight) with near optimal time resolution (13). The ssVEP is an oscillatory electrocortical response to a stimulus modulated in luminance or in contrast (i.e., flickered). It oscillates at the known, specific frequency of the driving stimulus (14, 15), allowing its separation from noise and quantification in the time-frequency domain (16). Generators of the ssVEP have been localized to extended visual cortex (14), with strong contributions from primary visual areas (17). Importantly, ssVEPs reflect repeated excitations of the visual system evoked by the same “flickered” stimulus. Temporal changes in driven neural mass activity indexed by the ssVEP reflect initial sensory processing as well as subsequent re-entrant, top-down modulation (18, 19) likely from fronto-parietal and limbic connections (20–22). In keeping with top-down contributions from these regions, modulation of the ssVEP by motivation has been observed as a function of instructed attention (23), fear conditioning (24, 25) and emotional arousal (26, 27) in patterns that vary with individual differences including depression (28), fearfulness (29), and anxiety (30, 31).
Unlike ssVEPs provoked by emotional scenes and fear conditioned cues, we have observed in a series of prior studies that ssVEPs to facial expressions are not modulated as a function of emotion—except in the case of social anxiety (30–32). For example, in a study of undergraduate students selected to be high and low on social anxiety, we observed no modulation in the low symptom group, and sustained enhancement to emotional (angry, fearful, happy) expressions relative to neutral that robustly increased with social fear and avoidance severity (32). These findings prompt the hypothesis that affective expressions should evoke heightened ssVEPs in those with social anxiety disorder. We have however found that treatment-seeking adult clinical samples are marked by substantial heterogeneity in defensive reactivity. Instead of uniform defensive hyper-reactivity, we have observed that a substantial portion of patients with social anxiety as well as other anxiety disorders, typically those with the most severe disorder-related distress and interference, show a paradoxical hypo-reactivity to threat cues (33–35). If visuocortical dynamics mirror these findings, we hypothesize that a portion of the sample with the most extreme levels of distress and impairment (36) would display attentional avoidance (either sustained or subsequent to initial hypervigilance) in response to angry faces.
While the latent structure of social anxiety appears dimensional (37, 38), a more discrete boundary between circumscribed (i.e., performance only) and generalized social anxiety subtypes has often been observed in subjective and objective measures (35, 36). Here we also examine if ssVEP modulation in response to facial expressions would vary by social anxiety subtype. Although facial expressions may hold particular salience for individuals with social anxiety, fears of scrutiny are prominent in many disorders. Limbic and visuocortical sensitivity to emotional facial expressions has been established across a range of disorders, particularly other anxiety disorders (39–41). To assess the specificity to principal social anxiety, we included a sample of individuals with principal panic disorder with agoraphobia, without comorbid social anxiety disorder. This clinical comparison was selected because persistent apprehension about experiencing panic symptoms in settings where escape is difficult and/or embarrassing renders cues connoting possible scrutiny especially pertinent (42).
Methods and Materials
Participants
Participants were assessed at the University of Florida Fear and Anxiety Disorders Clinic: 88 treatment-seeking adults with DSM-IV principal diagnoses of social anxiety disorder circumscribed to performance situations (n=21) or generalized across interaction contexts (n=42), principal panic disorder with agoraphobia (PDA; n=25), and 17 community control participants—all without psychosis, somatoform, substance use or eating disorders or major physical disease.
The University of Florida Institutional Review Board (IRB-01) approved the study. Participants provided informed consent, completed questionnaires and interview in the morning and psychophysiological assessment in the afternoon.
Diagnostic Classification
Diagnostic groups were established using the Anxiety Disorder Interview Schedule for DSM-IV (ADIS-IV) (43). For multiple Axis I disorders, diagnostic primacy was determined according to the severity rating of the Clinical Global Impression Scale (CGI-S) (44); ranging from 0, No features present, to 7, Most severely ill patients) reflecting both distress and interference for respective disorder presentations. The CGI-S was modified to consider functional interference and related distress not more appropriately subsumed under another disorder (see supplement). Interviewers rated CGI-S for all disorders assessed in the ADIS (anxiety, mood, adjustment, somatoform, substance use, and psychotic disorders) and any Axis II disorders assessed as warranted by SCID-II screener (45) elevations. Controls denied current or lifetime diagnoses of psychiatric illness and/or treatment and did not receive any CGI-S ratings that indicated more than minimal symptoms (i.e., severity rating=1). A doctoral level-clinical psychologist with expertise in anxiety disorders was present in all interviews (MCL) and inter-rater reliability (via videotape) was calculated for 25% of patients, yielding 100% agreement for principal diagnosis among three masters or doctoral-level clinical psychologists.
Consistent with the DSM-5 performance specifier, circumscribed social anxiety was operationalized as disabling and disturbing anxiety about negative evaluation limited to performance contexts1. Generalized social anxiety was defined as significant disturbance in at least two of the following domains: formal performances, informal speaking and interaction, observation of behavior, and assertive interaction2 (46, 47). To assess the specific nature of predominant symptom phenotypes on observed effects in ssVEP patterns, patients meeting criteria for social anxiety were exclusive of those with PDA and vice versa3.
Stimuli
Experimental methods were similar to those described previously (32). In brief, 96 pictures were selected from the Karolinska Directed Emotional Faces (KDEF) (48) of actors (12 female, 12 male) posing 4 different expressions (neutral, happy, fearful, angry) and were pre-processed to have equal overall luminance and color composition (mean luminance of 28 cd/m2; Michelson contrast of 0.83). To gather normative affective ratings on the Self-Assessment Manikin (SAM) (49), 242 unselected individuals rated the stimuli (Table S1).
Experimental Design
Participants were seated in a sound-attenuated, dimly lit room and the Electrical Geodesics (EGI) HydroCel EEG 129 sensor net was attached. Participants were instructed to view each picture for the duration of presentation, keeping their eyes comfortably focused on the center of the screen.
Faces were presented 116 cm from the participant on a 51 cm CRT monitor with a vertical refresh rate of 70 Hz, subtending a visual angle of 5° horizontally and 6.9° vertically. Using Psychtoolbox (50), faces appeared in a random order, each flickering at a rate of 17.5 Hz (28.57 ms on and 28.57 ms off) for 3428 ms (60 cycles), with a gray background set to the mean luminance of the faces. Faces were followed by a randomly variable 2–4 second inter-trial interval during which a central crosshair (1° visual angle) appeared. Each face was shown once, for 96 trials total over approximately 11 minutes.
EEG Recording & Data Collection
EEG was continuously recorded and digitized at 250 Hz, using Cz as a recording reference. As suggested (51) for the EGI high input-impedance (200 MOhms) amplifier, electrode impedances were kept below 50 kΩ. Data were filtered online by 0.1-Hz high-pass and 100 Hz low-pass elliptic filters, and off-line at 30 Hz low-pass (48 dB/octave, 18th order Butterworth filter). An established procedure (52), as implemented in the EMEGS software suite (53) was used to identify artifact-free epochs, extracted relative to the onset of each picture, using 300 ms pre- and 4400 ms post-picture onset. See supplement for additional details.
ssVEP Analyses
To illustrate data quality, grand mean ssVEPs recorded over central occipital sensor Oz for the neutral face condition are shown in Figure 1. For ssVEP analysis, condition-based averages were submitted to time-frequency analysis using the Hilbert transform: Data were filtered with a 10th-order Butterworth band-pass filter (width: 0.5 Hz) around the driving frequency of 17.5 Hz. The time-varying ssVEP amplitude was extracted as the modulus of the band-pass filtered ssVEP signal and the Hilbert-transformed analytic signal.
Figure 1.
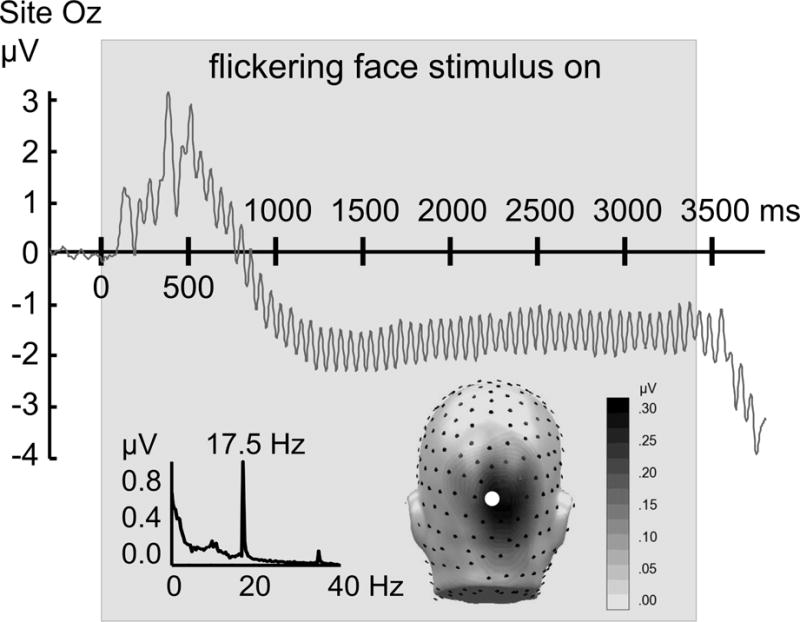
Top: Time domain representation of the ssVEP, averaged across participants (N=105) when viewing neutral faces flickering at a rate of 17.5 Hz, recorded from sensor Oz. The gray box indicates the duration of the flickering faces. Insets display the frequency spectrum of the same data (left lower panel), with a pronounced peak at the flickering frequency (17.5 Hz) and the first harmonic frequency (35 Hz) clearly visible. The inset on the right shows a back view of the spectral amplitude topography of this response as projected to a standard head. The location of sensor Oz is highlighted as a white circle. Note the focal parieto-occipital distribution of the ssVEP signal evoked by flickering pictures.
Statistical Analysis
Two complementary strategies were employed to evaluate group differences in ssVEP amplitude: an initial step assessed broad differences in time-averaged ssVEP amplitudes, the second step examined the ssVEP dynamics at each sample point. First, ssVEP amplitude was extracted as a posterior regional mean of the viewing period. For each participant and condition, the time-varying ssVEP amplitude was averaged between 800 and 3200 ms, across an occipital electrode cluster comprising electrode Oz and its 8 nearest neighbors. A linear mixed model analysis implemented in SPSS (SPSS, Inc., Chicago, Illinois) was conducted on the average ssVEP amplitude with fixed effects of facial expression (neutral, happy, fearful, angry), sex (male, female), and diagnostic group (control, circumscribed social anxiety, PDA, generalized social anxiety), and their interaction terms. Age was entered as a continuous covariate of interest, including its interaction terms with the categorical fixed effects. ssVEPs are sensitive to rated emotional arousal (26); thus contents were entered in order of increasing arousal for KDEF stimuli (i.e., neutral, happy, fearful, angry), demonstrated in the present normative sample and other studies (4). The subject factor was modeled as a random variable, nested within diagnostic group and nested within sex. Follow-up analyses to decompose omnibus effects were conducted with repeated measures analysis of variance (ANOVA), evaluating differences between facial expressions within each group and enabling planned contrast analyses of interactions between group and expression (54). Greenhouse-Geisser correction was applied where appropriate (55). Significant effects were followed up using paired t-tests or planned contrasts. Univariate ANOVAs and Tukey Honestly Significant Difference (HSD) tests for planned comparisons determined group differences in demographic and questionnaire data.
A second set of analyses capitalized on the rich temporal and spatial information contained in the dense array EEG recordings. In these analyses, t-values (comparing specific expressions) or F-contrasts comparing emotional (happy, fearful, angry) expressions to the neutral expression were determined for each EEG sensor, for the mean Hilbert-transformed ssVEP in the time window described above (800 to 3200 ms after face onset), and—importantly—for each time point individually. The latter analysis addresses hypotheses regarding hypervigilance-avoidance sequences vis-à-vis temporally sustained facilitation or suppression of threat cues. Thresholds for statistical significance were determined using a permutation technique (56, 57). Permutation distributions for t-values and F-values were generated based on randomly shuffling within each group. The maximum t- or F-value for each of 5000 random permutations entered a permutation distribution, and the top and bottom 2.5% tails of these distributions served as critical values for statistical significance.
Results
Principal Diagnosis and ssVEP Modulation
Linear mixed model analysis of ssVEP amplitude showed an interaction of facial expression and diagnosis, F(9,297)=2.54, p=0.0084. No further main effects or interactions were observed, including those of sex or age. Follow-up analyses to disentangle the omnibus interaction revealed no differences in ssVEP amplitude as a function of expression in control participants, F(3,48)=0.41, p=.72, or patients with generalized social anxiety, F(3,123)=0.38, p=.76, or panic disorder with agoraphobia, F(3,75)=0.83, p=.48. Meanwhile, patients with circumscribed social anxiety showed pronounced sensitivity to facial expression, F(3,60)=4.53, p=.019. Specifically, ssVEP amplitude was enhanced when patients with circumscribed social anxiety viewed fearful, t(20)=2.45, p=.024, and angry, t(20)=2.30, p=.032, compared to neutral expressions and fearful compared to happy expressions5, t(20)=2.50, p=.022 (Table 1). Between-group tests of emotional relative to neutral expressions (i.e., difference score) revealed a reliable difference specific to responses during fearful versus neutral expressions, F(3,101)=2.98, p=.035, with circumscribed showing greater enhancement than generalized social anxiety. In contrast, tests of between-group differences in ssVEP amplitude by expression revealed no differences, underscoring that modulation rather than raw amplitude of the ssVEP varied across groups.
Table 1.
Steady-state visual evoked potential (ssVEP) amplitude (means and standard deviations) by facial expression for Control, Social Anxiety and Panic Disorder with Agoraphobia Groups
| Facial Expression | Control | Principal Social Anxiety: Circumscribed | Principal Panic Disorder with Agoraphobia (PDA) | Principal Social Anxiety: Generalized |
|---|---|---|---|---|
| (n=17) | (n=21) | (n=25) | (n=42) | |
| Neutral | 0.24 (0.14) | 0.24 (0.21) | 0.28 (0.21) | 0.22 (0.14) |
| Happy | 0.23 (0.13) | 0.24 (0.23) | 0.28 (0.24) | 0.23 (0.13) |
| Fearful | 0.24 (0.13) | 0.29 (0.25)a | 0.29 (0.24) | 0.22 (0.13) |
| Angry | 0.25 (0.14) | 0.28 (0.22) | 0.27 (0.22) | 0.22 (0.13) |
=within-group comparison to neutral is significant at p< .05. No pairwise between-group comparisons were significant.
Regarding temporal dynamics contributing to these group effects, the time course for the occipital sensor cluster employed for statistical analysis is shown in Figure 2. Hypersensitivity to angry and fearful faces in circumscribed social anxiety patients emerged early in the viewing epoch and persisted. The lack of enhancement to emotional expressions in the other three groups was similarly persistent throughout viewing. As a follow-up to the circumscribed group effects, Figure 3 displays the ssVEP time course for each expression at sensor POz. To illustrate the finding that both angry and fearful expressions prompted temporally sustained ssVEP amplification, the white line shows the time-varying F-value of the contrast (angry=fearful > neutral) computed for this sensor.
Figure 2.
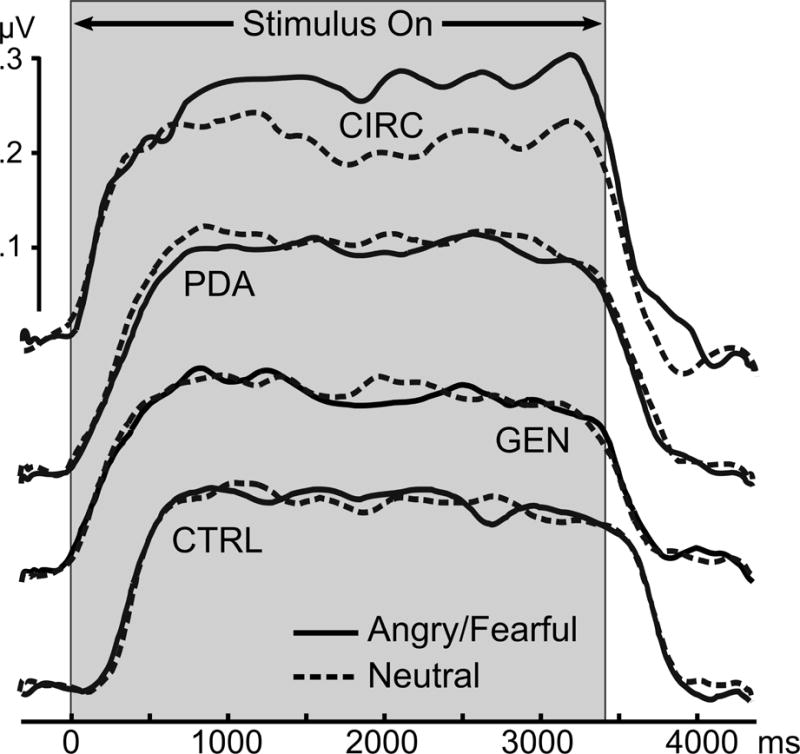
Grand mean time-varying envelope of the ssVEP signal (from a posterior sensor cluster including Oz and its 8 nearest neighbors) evoked by the flickering faces, comparing aversive expressions (averaged across angry and fearful expressions) with neutral expressions, for the four principal diagnostic groups. CTRL=Control (N=17); CIRC=Circumscribed social anxiety (N=21); GEN=Generalized social anxiety (N=42); PDA=Panic disorder with agoraphobia (N=25).
Figure 3.
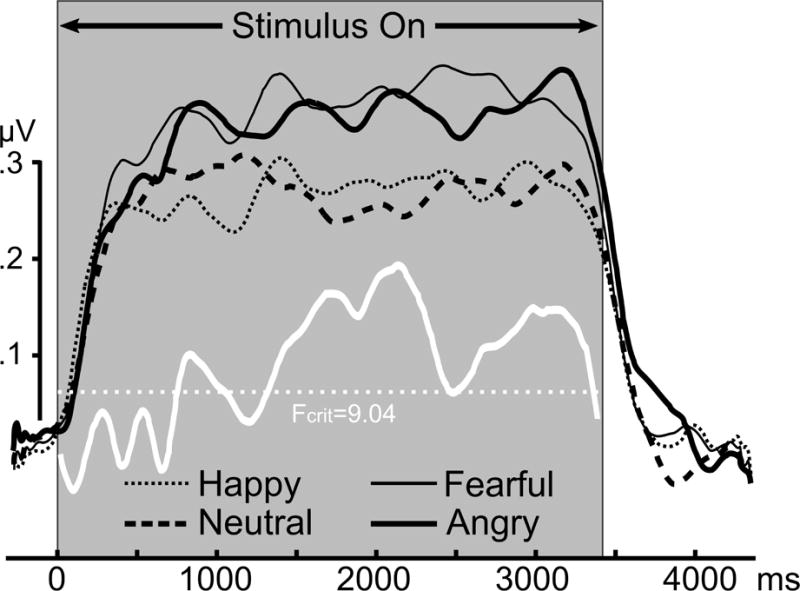
Grand mean time-varying envelope of the ssVEP signal evoked by the flickering faces with four different expressions, for participants diagnosed with circumscribed social anxiety (N=21) recorded from parieto-occipital electrode site POz. The white line shows the permutation-controlled F-contrast comparing neutral with angry and fearful contents (fearful = angry > neutral).
Topographical statistical mapping of permutation-controlled t-tests was consistent with mixed model and ANOVA results. Differences in ssVEP amplitude across posterior locations of the scalp were solely observed for circumscribed social anxiety patients, shown for comparisons between neutral versus angry and fearful faces, respectively (Figure 4). Topographical analyses also demonstrated that condition differences in the circumscribed social anxiety group were strongest at parieto-occipital sensors, superior to the maximum of the ssVEP, shown in Figure 1.
Figure 4.
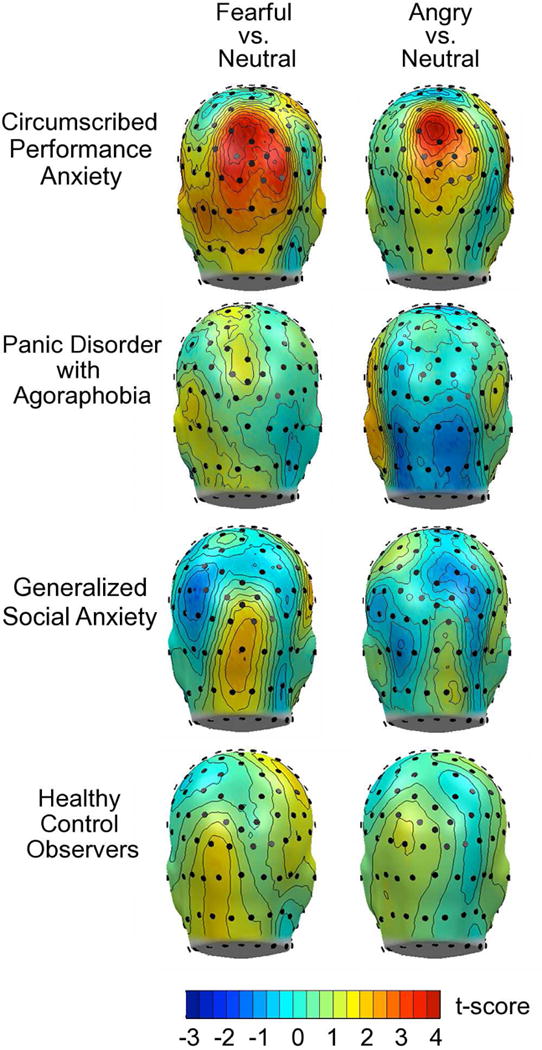
Mass univariate comparisons by principal disorder of mean ssVEP amplitude across the viewing epoch, evoked by fearful versus neutral and angry versus neutral faces. Red colors indicate significant differences (exceeding a critical t-value of 3.68). Note that reliable ssVEP enhancement during aversive relative to neutral face processing is observed only in participants in the principal circumscribed social anxiety group.
The pattern of ssVEP enhancement to aversive expressions specific to circumscribed social anxiety was not explained by self-reported symptoms. The results are reported in detail in Table 2 and the supplement. In short, we observed a linear increase in broad negative affectivity and functional impairment from control to circumscribed, PDA, and generalized social anxiety at the extreme. Anxious arousal/agoraphobia was most pronounced for PDA, followed by generalized and circumscribed social anxiety and lastly the control group. Social fear/avoidance was most extreme for the generalized followed by circumscribed social anxiety, PDA, and lastly the control group. Neither medication usage (see supplement), diagnostic comorbidity, nor demographics (Table 2) corresponded with ssVEP modulation.
Table 2.
Questionnaire, interview, and demographic responses (means and standard deviations) for Control, Social Anxiety and Panic Disorder with Agoraphobia (PDA) Groups
| Measure | Control | Principal Social Anxiety: Circumscribed | Principal Panic Disorder with Agoraphobia | Principal Social Anxiety: Generalized | Group Effect | |
|---|---|---|---|---|---|---|
| (n=17) | (n=21) | (n=25) | (n=42) | |||
| Social anxiety-related distress | ||||||
| LSAS-SR Total | 19.53 (15.90) b c d | 53.19 (22.49) a d | 55.96 (34.36) a d | 92.21 (19.63) a b c | F(3, 101)= 33.08, p< .001 | |
| LSAS-SR Fear | 10.59 (7.96) b c d | 29.38 (11.43) a d | 30.64 (15.90) a d | 48.57 (9.81) a b c | F(3, 101)=46.78, p< .001 | |
| LSAS-SR Avoidance | 8.94 (8.34) b c d | 23.81 (12.14) a d | 25.32 (18.79) a d | 43.64 (10.42) a b c | F(3, 101)= 33.08, p< .001 | |
| FSS Social | 37.35 (11.64) b c d | 63.05 (12.84) a d | 53.40 (17.97) a d | 78.67 (13.77) a b c | F(3, 101)=38.17, p< .001 | |
| Panic-related distress | ||||||
| PDSS Total | 0.06 (0.24) b c d | 5.71 (4.48) a c | 14.28 (5.30) a b d | 9.10 (6.78) a c | F(3, 101)=25.19, p< .001 | |
| ASI Total | 15.06 (9.56) c d | 25.14 (14.72) c | 39.16 (10.27) a b d | 28.88 (12.04) a c | F(3, 101)=14.52, p< .001 | |
| MASQ Anxious Arousal | 20.65 (3.43) c d | 26.05 (7.73) c | 34.64 (9.93) a b d | 28.76 (8.35) c | F(3, 101)=10.78, p< .001 | |
| FSS Agoraphobia | 22.59 (5.12) c d | 27.62 (8.24) c | 44.08 (11.75) a b d | 32.86 (10.98) a c | F(3, 101)=18.51, p< .001 | |
| Broad negative affectivity | ||||||
| MASQ Mixed Symptoms | 25.35 (5.44) b c d | 36.10 (13.23) a | 41.40 (14.75) a | 43.52 (10.42) a | F(3, 101)=10.68, p< .001 | |
| MASQ General Anxiety | 16.47 (4.52) c d | 22.0 (6.47) c d | 30.48 (5.80) a b | 28.05 (7.85) a b | F(3, 101)=18.99, p< .001 | |
| MASQ General Depression | 18.24 (4.49) b c d | 28.90 (11.98) a c | 31.84 (12.08) a | 37.12 (10.46) a b | F(3, 101)=13.40, p< .001 | |
| MASQ Anhedonia | 46.65 (10.91) b c d | 64.10 (16.37) a d | 73.24 (13.87) a | 76.24 (14.01) a b | F(3, 101)=19.60, p< .001 | |
| BDI-II Total | 3.18 (5.27) b c d | 11.52 (8.49) a d | 17.76 (10.73) a d | 18.98 (9.86) a b | F(3, 101)=13.53, p< .001 | |
| STAI-Trait | 34.0 (7.99) b c d | 48.24 (11.85) a d | 52.72 (10.03) a b | 58.31 (9.34) a b | F(3, 101)=25.33, p< .001 | |
| PSWQ | 37.35 (8.89) b c d | 52.67 (15.33) a d | 59.64 (11.34) a | 65.40 (10.33) a b | F(3, 101)=25.32, p< .001 | |
| Transdiagnostic functional interference | ||||||
| IIRS Total | 18.06 (9.48) b c d | 37.95 (16.19) a c d | 52.40 (15.75) a b | 51.98 (13.92) a b | F(3, 101)=27.21, p< .001 | |
| Clinician judgment of distress/impairment | ||||||
| CGI-S: Social Anxiety | 1.06 (0.24) b d | 4.43 (0.68) s c d | 1.48 (0.65) s b c | 5.07 (0.75) s b c | F(3, 101)=253.61, p< .001 | |
| CGI-S: Panic Disorder | 1.0 (0) c | 1.05 (0.22) c | 4.60 (0.58) a b d | 1.10 (0.48) c | F(3, 101)=432.32, p< .001 | |
| CGI-S: Agoraphobia | 1.0 (0) c | 1.0 (0) c | 4.72 (0.79) a b d | 1.02 (0.15) c | F(3, 101)=550.38, p< .001 | |
| CGI-S: Total Axis I/II | 29.76 (0.90) c d | 32.23 (3.56) c d | 39.04 (5.06) s b | 36.83 (4.04) s b | F(3, 101)=25.34, p< .001 | |
| Treatment Prognosis | 2.43 (0.81) d | 2.80 (1.19) d | 3.52 (1.21) b c | F(2, 85)=7.54, p< .01 | ||
| Interview Measures | ||||||
| Axis I disorders (Count) | 0 (0) | 1.29 (0.46) d | 1.60 (0.96) b d | 1.83 (0.82) b | F(3, 101)=26.37, p< .001 | |
| Comorbid major depressive disorder (%) | 14.3 | 28.0 | 19.0 | X2 (2)=1.42, ns | ||
| Demographics | ||||||
| Age at assessment (Years) | 23.94 (6.07) b | 34.10 (11.71) d | 34.52 (11.34) b | 25.14 (7.38) b | F(3, 101)=9.21, p< .001 | |
| Gender (% Female) | 47.10 | 57.10 d | 60.0 d | 28.6 b | X2 (3)=8.14, p< .05 | |
| Race (% Caucasian) | 41.20 b c d | 76.20 a c | 96.0 a | 78.60 a | X2 (3)=17.03, p< .01 | |
| College graduate (%) | 41.20 | 66.70 d | 44.0 | 28.60 b | X2 (3)=8.41, p< .05 | |
Note. LSAS-SR= Liebowitz Social Anxiety Scale-Self-report Version (58); FSS=Fear Survey Schedule (59); PDSS=Panic Disorder Severity Scale (60); ASI=Anxiety Sensitivity Index (61); MASQ=Mood and Anxiety Symptom Questionnaire (62); BDI-II=Beck Depression Inventory-II (63); STAI= State-Trait Anxiety Inventory (64); PSWQ=Penn State Worry Questionnaire (65); IIRS=Illness Intrusiveness Rating Scale (66); CGI-S=Clinical Global Impressions-Severity rated for respective disorders (7-point scale ranging from 1, Normal/No illness or impairment, to 7, Among the most severely ill patients) (44); Total Axis I/II=sum of ratings of all Axis I disorders assessed on ADIS (anxiety, mood, adjustment, substance use, somatoform, psychosis) as well as Axis II disorders assessed based on elevated SCID-II screener scores (44). Treatment Prognosis=clinician-rated estimate of treatment outcome (scale ranging from 1, Excellent, to 7, Poor); Superscripts=Results of Tukey HSD or chi-square pairwise between-group comparisons:
=comparison to control is significant at p< .05;
=comparison to circumscribed social anxiety is significant at p< .05;
=comparison to PDA is significant at p< .05.
=comparison to generalized social anxiety is significant at p< .05.
Transdiagnostic Social Anxiety Severity and Impairment and ssVEP Modulation
Next, we considered whether finer-grade clinical judgments of social fear and avoidance severity and interference (as opposed to diagnostic grouping) might reveal distinctions in attentional patterns. These analyses were performed transdiagnostically. Participants across principal disorders were ranked according to CGI-S social anxiety ratings.
The mean expression-related differences in ssVEP amplitude during angry versus neutral expressions (expressed as % amplitude of neutral response) were examined according to CGI-S rankings (Figure 5; Figure S2–S3). The pie chart at each CGI rank reflects the proportion of each disorder contributing to a given severity and impairment level of social anxiety. As observed in Figure 5, no differences between angry and neutral expression-evoked ssVEPs were observed for individuals not impaired by social anxiety. A linear increase across groups was observed starting from the 1) not impaired to 2) minimally impaired to 3) moderately impaired, followed by individuals 4) markedly impaired by social anxiety showing the greatest bias to angry expressions. Patients rated as even more (i.e., severely) impaired showed a difference relative to neutral expressions nearly on par with the markedly impaired group—in the opposite direction (i.e., neutral evoking larger ssVEP amplitudes than angry expressions). Reliability of the overall pattern was confirmed by univariate ANOVA, which demonstrated ssVEP modulation as a function of CGI-S, F(4, 100)=3.02, p=.021, best described by a quadratic trend, F(1,100)=4.82, p=.031. No such trend was observed for fearful expressions, F(4, 100)=0.95, p=0.4386. The opposing patterns in the marked and severe groups represented sustained hyper- and hypo-sensitivity, respectively, to angry expressions as opposed to fluctuating attentional over- and under-engagement (Figure 6). Follow-up tests of all symptom severity, prognosis, and comorbidity indices in Table 2 suggested more similarities than differences between these groups.7 Follow-up tests of LSAS-SR total score, similarly separated into quintiles showed no reliable differences, suggesting that self-reported social anxiety did not track this attentional bias as closely as clinical judgment.
Figure 5.
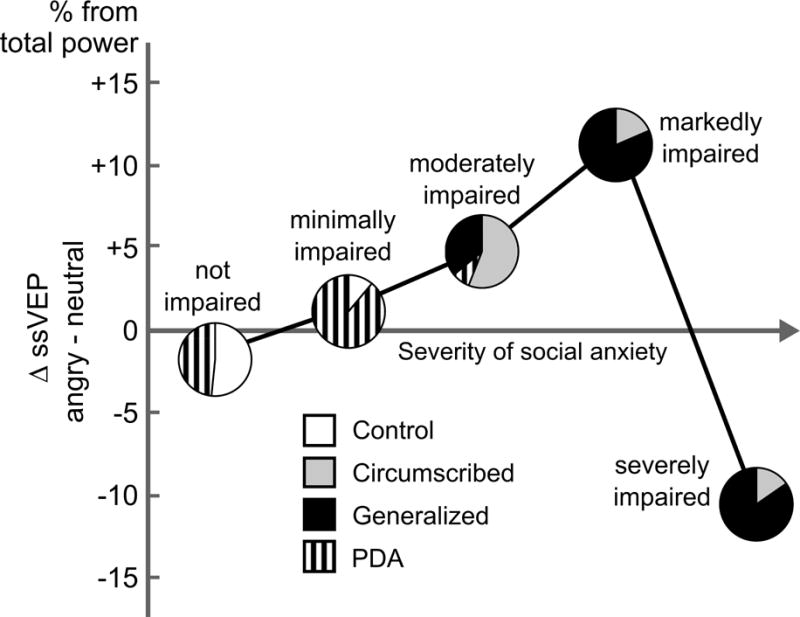
Differential sensitivity of the visual cortex to angry faces, as a function of social anxiety severity as rated on the Clinical Global Impression Scale (CGI-S) for all participants. Mean difference in ssVEP amplitude is shown transdiagnostically for increasing levels of social anxiety severity, for unimpaired (N =31), minimally impaired (N=9), moderately impaired (N=25), markedly impaired (N=27), and severely impaired (N=13) individuals. Relative contribution of each diagnostic category to the respective severity level is indicated by pie charts.
Figure 6.
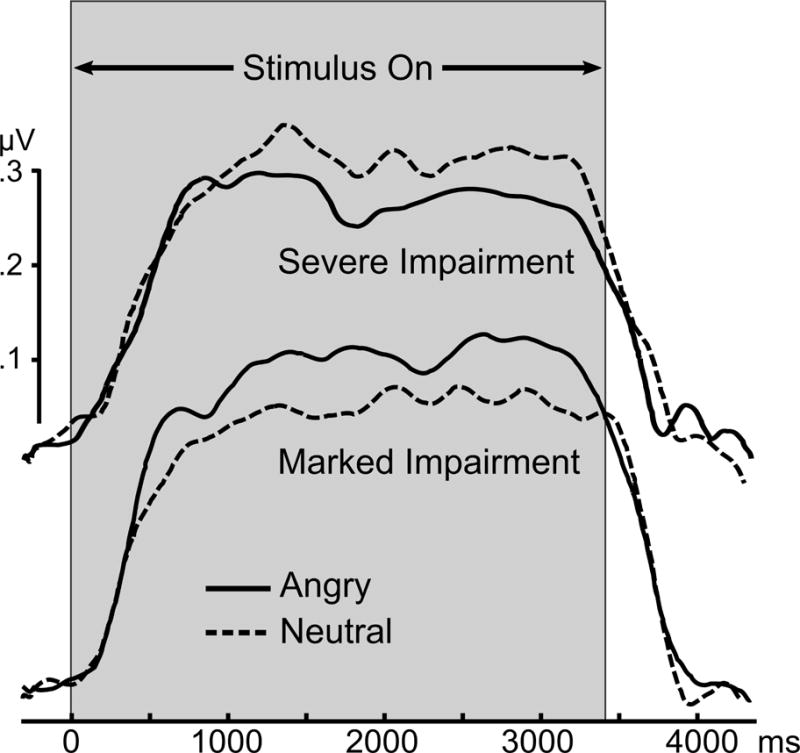
Grand mean time-varying envelope of the ssVEP signal (from a posterior sensor cluster including Oz and its 8 nearest neighbors) evoked by the flickering faces, comparing angry expressions with neutral expressions, for the two groups characterized as most impaired by social anxiety (markedly impaired (N=27) and severely impaired (N=13) individuals).
Discussion
In the current study, continuous visuocortical responses to naturalistic emotional and neutral facial expressions were assessed in individuals with circumscribed and generalized social anxiety disorder and panic disorder with agoraphobia as well as healthy control participants. At the principal disorder level, only circumscribed or performance social anxiety groups showed attentional facilitation to static aversive facial expressions. Furthermore, these group-level patterns were consistent throughout viewing. Circumscribed social anxiety patients showed a sustained pattern of hypervigilance. The other groups, despite elevated self-reported social anxiety and broad distress, showed no visuocortical sensitivity to emotional expressions.
Finer-grade clinical judgments of social anxiety severity and impairment were more predictive of visuocortical anomalies to facial expressions. By stratifying—transdiagnostically—on the basis of CGI severity ratings of social anxiety, we observed that interpersonal apprehension and related interference predicted a linear increase in sustained perceptual sensitivity to aversive facial expressions. That is, with the exception of the most impaired group, which showed sustained attentional disengagement or avoidance of aversive relative to neutral facial expressions. Despite the opposing patterns of attentional bias observed in the two most extreme ranks, both were composed of principal social anxiety patients. Surprisingly, the percentage at each of these two CGI ranks belonging to circumscribed versus generalized subtypes was equivalent, despite the substantial differences in prognosis and symptomatic distress typical of these classes (36). Considering subjective symptom severity, further suggested that while the severely impaired group reported more social fearfulness, anxious arousal, and non-specific anxiety on select measures6, that these groups were predominantly similarly extreme in their subjective distress. The difference in CGI ranking was based on the extent of impairment in psychosocial functioning. That is, marked impairment was characterized by significant (but not gross) impairment in important areas of functioning. Severe impairment was characterized by at least severe impairment in several or total impairment in one domain. Essentially, clinician judgment of psychosocial impairment was uniquely related to reliable and opposing patterns of selective attention to aversive expressions.
Individuals who demonstrated heightened sensitivity to angry expressions also demonstrated facilitation to fearful expressions. Vigilance to other aversive expressions has frequently been observed in social anxiety in both ERP and hemodynamic imaging studies (10, 67), particularly disgust expressions (68). Rather than specific expressions, aversive expressions as a whole may prompt threat of scruitiny and contempt in social anxiety.
Proposals that affective modulation of primary visual responses result from re-entrant signals ultimately originating in fronto-parietal and limbic cortices (20, 67), have borne out in recent concurrent ssVEP-fMRI investigations (21, 69). Chronic social anxiety may tune visuocortical neurons sensitive to facial cues via altering thresholds and gains in the networks representing expectations of interpersonal failure and its consequences. Work with ERPs may assist in identifying the extra-visual processing stages that contribute to these effects (70). Limbic and paralimbic regions shown to drive re-entrant modulation of the ssVEP are those consistently shown during fMRI studies to be hyper-reactive to social cues in social anxiety patients (10, 68).
Similar to posttraumatic stress (71, 72), there may be subtypes of social anxiety with different corticolimbic biases, correspondingly different patterns of re-entrant modulation of visual cortex, and thus different attentional phenotypes. Our prior work with startle, autonomic, and facial expressivity measures (35) has suggested that hyper-versus hypo-reactivity to social threat cues in social anxiety is related to disorder duration, with more enduring dysfunction related to response attenuation. Whether hyper-versus hypo-reactivity may reflect a transition in response dispositions as a function of chronicity or is more reflective of invariant trait dispositions will await a longitudinal investigation. Regardless of the respective pathogenesis of the attentional biases, fMRI studies of social anxiety treatment outcome hint that reactivity patterns may relate to prognosis: Successful cognitive-behavioral intervention for social anxiety down-regulates defensive activation of limbic and visuocortical regions to clinically relevant cues while up-regulating dorsolateral and medial prefrontal areas suggestive of enhanced control (73–75). Notably, symptom amelioration is more pronounced among those patients who at pre-treatment show stronger visuocortical and paralimbic reactivity to aversive cues (73, 76) as well as weaker fronto-parietal activation and connectivity during simultaneous cognitive demands (76–78). Steady-state visuocortical response to social cues may be a prognostic indicator of whether a patient is primed for the sensory, cognitive, and emotional engagement optimal for CBT response.
The finer discernment of visuocortical anomalies observed on the basis of transdiagnostic CGI ratings of social anxiety highlights the relevance of these symptoms and related attentional biases across disorders. Interpersonal apprehension and avoidance are elevated in a range of psychiatric disorders including other anxiety (79, 80), eating (81), personality (82) and substance use disorders (83), autism (84), unipolar and bipolar depression (85), and psychosis (86), as well as numerous physical health conditions (87). Relative to discrete diagnosis, the graded clinical impression weighting the extent of interpersonal fear, distress, and interference yielded superior prediction of attentional dysregulation—a potential intermediate phenotype.
With the rollout of the Research Domain Criteria (RDoC) initiative (88) to promote a science of psychopathology based around dimensions of brain-behavior relationships as opposed to subjectively based diagnostic categories, numerous clinical scientists have called for clearer specification of the clinical targets (89). This has included calls for incorporating the metrics of prognosis, caseness, and disability (90) while also attending to the need for improved reliability of experimental indices, brain and behavior alike (91). The current findings suggest a potential point of reconciliation—linking objective measures to subjective dimensional indices that account for not only symptom domain severity but also broader impairment. In the current study, follow-up analyses utilizing self-reported social fear and avoidance (i.e., LSAS-SR Total) to stratify patients in a manner akin to the CGI rankings obscured the attentional patterns revealed by clinician judgment. The present study requires replication and extension, particularly in light of the established inconsistency of impairment ratings such as the Global Assessment of Functioning, which contributed to its exclusion from DSM-5. Furthermore, although inter-rater reliability for principal disorders was 100% in the current sample, reliability was not calculated for CGI ratings. In summary, systematic efforts to operationalize and clarify clinical judgment of global severity and impairment in relation to more objective RDoC-style domains could be especially productive (92, 93).
The steady-state visual evoked potential is a strong candidate measure for advancing clinical science at the intersection of brain and behavior. With selective sensitivity to visuocortical processing ssVEPs are limited in reflecting processes occurring in other brain areas. However, their fine temporal and dynamic resolution for covert attention fluctuations during sustained stimulation (13), its prediction of behavioral performance (13), and its established reliability (94–96), even at the single-trial level (97, 98) highlight their usefulness in assessing biased visual processing. Single-trial analyses of ssVEPs have revealed attentional differences as a function of nuanced cues in static facial expressions (i.e., direct versus averted gaze (99)). The fidelity of ssVEPs could be especially productive for individually tailoring attention bias modification interventions, and thus potentially reconcile inconsistent findings (100–102). Similarly, dysfunctional attentional biases might be pliable to highly resolved, ssVEP-guided real-time neurofeedback (103). Real-time feedback of overt eye movements has recently shown promise to ameliorate biases to aversive faces and symptoms in social anxiety (104). The added capability to index covert biases in real-time with ssVEPs could enhance novel neuroscience-guided interventions.
Conclusion
Rather than initial hypervigilance or facilitation to aversive facial expressions and consequent defensive avoidance, social anxiety appears to confer a sustained bias for hypervigilance or avoidance. Furthermore, while the extent of sustained vigilance to aversive expressions reliably increases with social anxiety severity for the majority of patients, the most impaired show an opposing avoidance. These distinct patterns of attentional allocation could provide a powerful means of personalizing neuroscience-based interventions to modify attention bias and related impairment.
Supplementary Material
Acknowledgments
This work was supported in part by National Institute of Mental Health grants R01 MH084932 and R01 MH097320 to Andreas Keil, K23 MH104849 to Lisa M. McTeague and R01 MH084932, R01 MH097320, and R21 MH082702 to Peter J. Lang. Special thanks to Christopher T. Sege, M.S. for comments on this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
The authors declare no competing financial interests relevant to this research. Dr. McTeague reports receiving stock options in Joyable.com for consulting unrelated to this work. All other authors report no biomedical financial interests or potential conflicts of interest.
For example, idiographic performance fears included taking examinations, musical performance, athletic participation, speaking in group meetings at work, giving a speech, or interviewing.
Circumscribed social anxiety patients endorsed fear (ADIS-IV Fear Severity Rating, 4 and above) and/or avoidance (Avoidance Severity Rating, 4 and above) of at least one formal, structured performance situation (i.e., public speaking, participating in meetings and classes, or idiographic situations). In addition, these individuals exceeded the same threshold for distress and/or functional interference regarding apprehension/avoidance of performance situations, but did not similarly rate other social contexts.
As only one participant was excluded owing to comorbid generalized social anxiety and PDA, this criterion likely did not impact the generalizability to a naturally occurring treatment-seeking sample.
Initial inspection of means and distributions indicated that age was not evenly distributed across diagnostic categories. When age was added as an additional random factor nested in group, the pattern of modulation of ssVEP amplitude by diagnosis did not differ.
The reliable ssVEP enhancement to fearful in relation to happy expressions among circumscribed social anxiety patients was consistent with the reliably increased arousal ratings obtained for the fearful relative to happy expressions (see supplement for details).
A posthoc test of the interaction of CGI rank and expression (neutral, angry) underscored the reliability of this effect, F(4,100) = 2.79, p=.03. Additional follow-up pairwise comparisons of the ssVEP amplitude difference in response to angry relative to neutral expressions across groups, F(4,100) = 3.02, p=.02, further revealed that the markedly impaired group showed ssVEP enhancement to angry relative to neutral expressions that exceeded the responses of the not impaired, p=.044, and severely impaired groups, p=.02. All other pairwise comparisons did not differ.
Consistent with the worse CGI-S score for social anxiety conferred to the severely impaired relative to markedly impaired group, the severely impaired group endorsed more social fearfulness as rated on the LSAS-SR (Total: Markedly M=86.96; SD=17.21; Severely M=100.38; SD=21.57, p=.04; Fear: Markedly M=45.67; SD=8.95; Severely M=52.92; SD=10.57, p=.03). Repeating symptom, comorbidity, and prognosis analyses in Table 2 between these subgroups, however, revealed additional differences only in MASQ Anxious Arousal (Markedly M=26.22; SD=7.37; Severely M=34.54; SD=8.81, p=.003) and General Anxiety (Markedly M=26.41; SD=7.16; Severely M=31.69; SD=8.79, p=.049). To reduce the array of questionnaires to underlying dimensions, we also conducted a principal components analysis using the dimensional symptom measures and then compared the two groups on the resulting factors: 1) general distress/negative affectivity, anxious/hyperarousal, and 3) social fear and anxiety (details in Supplemental Results). The two most impaired groups did not differ on these broad factors.
References
- 1.Klumpp H, Amir N. Examination of vigilance and disengagement of threat in social anxiety with a probe detection task. Anxiety Stress Coping. 2009;22:283–296. doi: 10.1080/10615800802449602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wieser MJ, Pauli P, Muhlberger A. Probing the attentional control theory in social anxiety: an emotional saccade task. Cogn Affect Behav Neurosci. 2009;9:314–322. doi: 10.3758/CABN.9.3.314. [DOI] [PubMed] [Google Scholar]
- 3.Wieser MJ, Pauli P, Reicherts P, Muhlberger A. Don’t look at me in anger! Enhanced processing of angry faces in anticipation of public speaking. Psychophysiology. 2010;47:271–280. doi: 10.1111/j.1469-8986.2009.00938.x. [DOI] [PubMed] [Google Scholar]
- 4.Muhlberger A, Wieser MJ, Herrmann MJ, Weyers P, Troger C, Pauli P. Early cortical processing of natural and artificial emotional faces differs between lower and higher socially anxious persons. J Neural Transm (Vienna) 2009;116:735–746. doi: 10.1007/s00702-008-0108-6. [DOI] [PubMed] [Google Scholar]
- 5.Wangelin BC, Bradley MM, Kastner A, Lang PJ. Affective engagement for facial expressions and emotional scenes: the influence of social anxiety. Biol Psychol. 2012;91:103–110. doi: 10.1016/j.biopsycho.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bas-Hoogendam JM, Blackford JU, Bruhl AB, Blair KS, van der Wee NJ, Westenberg PM. Neurobiological candidate endophenotypes of social anxiety disorder. Neurosci Biobehav Rev. 2016;71:362–378. doi: 10.1016/j.neubiorev.2016.08.040. [DOI] [PubMed] [Google Scholar]
- 7.Binelli C, Subira S, Batalla A, Muniz A, Sugranyes G, Crippa JA, et al. Common and distinct neural correlates of facial emotion processing in social anxiety disorder and Williams syndrome: A systematic review and voxel-based meta-analysis of functional resonance imaging studies. Neuropsychologia. 2014;64:205–217. doi: 10.1016/j.neuropsychologia.2014.08.027. [DOI] [PubMed] [Google Scholar]
- 8.Bruhl AB, Delsignore A, Komossa K, Weidt S. Neuroimaging in social anxiety disorder-a meta-analytic review resulting in a new neurofunctional model. Neurosci Biobehav Rev. 2014;47:260–280. doi: 10.1016/j.neubiorev.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Bantin T, Stevens S, Gerlach AL, Hermann C. What does the facial dot-probe task tell us about attentional processes in social anxiety? A systematic review. J Behav Ther Exp Psychiatry. 2016;50:40–51. doi: 10.1016/j.jbtep.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 10.Schulz C, Mothes-Lasch M, Straube T. Automatic neural processing of disorder-related stimuli in social anxiety disorder: faces and more. Front Psychol. 2013;4:282. doi: 10.3389/fpsyg.2013.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bogels SM, Mansell W. Attention processes in the maintenance and treatment of social phobia: hypervigilance, avoidance and self-focused attention. Clin Psychol Rev. 2004;24:827–856. doi: 10.1016/j.cpr.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Mogg K, Bradley BP. A cognitive-motivational analysis of anxiety. Behav Res Ther. 1998;36:809–848. doi: 10.1016/s0005-7967(98)00063-1. [DOI] [PubMed] [Google Scholar]
- 13.Muller MM, Teder-Salejarvi W, Hillyard SA. The time course of cortical facilitation during cued shifts of spatial attention. Nat Neurosci. 1998;1:631–634. doi: 10.1038/2865. [DOI] [PubMed] [Google Scholar]
- 14.Muller MM, Teder W, Hillyard SA. Magnetoencephalographic recording of steady-state visual evoked cortical activity. Brain Topogr. 1997;9:163–168. doi: 10.1007/BF01190385. [DOI] [PubMed] [Google Scholar]
- 15.Regan D. Human brain electrophysiology : evoked potentials and evoked magnetic fields in science and medicine. New York: Elsevier; 1989. [Google Scholar]
- 16.Wang J, Clementz BA, Keil A. The neural correlates of feature-based selective attention when viewing spatially and temporally overlapping images. Neuropsychologia. 2007;45:1393–1399. doi: 10.1016/j.neuropsychologia.2006.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Russo F, Pitzalis S, Aprile T, Spitoni G, Patria F, Stella A, et al. Spatiotemporal analysis of the cortical sources of the steady-state visual evoked potential. Hum Brain Mapp. 2007;28:323–334. doi: 10.1002/hbm.20276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keil A, Gruber T, Muller MM. Functional correlates of macroscopic high-frequency brain activity in the human visual system. Neurosci Biobehav Rev. 2001;25:527–534. doi: 10.1016/s0149-7634(01)00031-8. [DOI] [PubMed] [Google Scholar]
- 19.Silberstein RB, Ciorciari J, Pipingas A. Steady-state visually evoked potential topography during the Wisconsin card sorting test. Electroencephalography and clinical neurophysiology. 1995;96:24–35. doi: 10.1016/0013-4694(94)00189-r. [DOI] [PubMed] [Google Scholar]
- 20.Leppanen JM, Nelson CA. Tuning the developing brain to social signals of emotions. Nature reviews Neuroscience. 2009;10:37–47. doi: 10.1038/nrn2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petro NM, Gruss LF, Yin S, Huang H, Miskovic V, Ding M, et al. Multimodal Imaging Evidence for a Frontocortical Modulation of Visual Cortex during the Selective Processing of Conditioned Threat. Journal of cognitive neuroscience. 2017:1–15. doi: 10.1162/jocn_a_01114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pourtois G, Schettino A, Vuilleumier P. Brain mechanisms for emotional influences on perception and attention: what is magic and what is not. Biol Psychol. 2013;92:492–512. doi: 10.1016/j.biopsycho.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 23.Muller MM, Malinowski P, Gruber T, Hillyard SA. Sustained division of the attentional spotlight. Nature. 2003;424:309–312. doi: 10.1038/nature01812. [DOI] [PubMed] [Google Scholar]
- 24.McTeague LM, Gruss LF, Keil A. Aversive learning shapes neuronal orientation tuning in human visual cortex. Nature communications. 2015;6:7823. doi: 10.1038/ncomms8823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miskovic V, Keil A. Perceiving threat in the face of safety: excitation and inhibition of conditioned fear in human visual cortex. J Neurosci. 2013;33:72–78. doi: 10.1523/JNEUROSCI.3692-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keil A, Gruber T, Muller MM, Moratti S, Stolarova M, Bradley MM, et al. Early modulation of visual perception by emotional arousal: evidence from steady-state visual evoked brain potentials. Cogn Affect Behav Neurosci. 2003;3:195–206. doi: 10.3758/cabn.3.3.195. [DOI] [PubMed] [Google Scholar]
- 27.Keil A, Costa V, Smith JC, Sabatinelli D, McGinnis EM, Bradley MM, et al. Tagging cortical networks in emotion: a topographical analysis. Hum Brain Mapp. 2012;33:2920–2931. doi: 10.1002/hbm.21413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moratti S, Rubio G, Campo P, Keil A, Ortiz T. Hypofunction of right temporoparietal cortex during emotional arousal in depression. Arch Gen Psychiatry. 2008;65:532–541. doi: 10.1001/archpsyc.65.5.532. [DOI] [PubMed] [Google Scholar]
- 29.Deweese MM, Bradley MM, Lang PJ, Andersen SK, Muller MM, Keil A. Snake fearfulness is associated with sustained competitive biases to visual snake features: hypervigilance without avoidance. Psychiatry Res. 2014;219:329–335. doi: 10.1016/j.psychres.2014.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wieser MJ, McTeague LM, Keil A. Competition effects of threatening faces in social anxiety. Emotion (Washington, DC) 2012;12:1050–1060. doi: 10.1037/a0027069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wieser MJ, McTeague LM, Keil A. Sustained preferential processing of social threat cues: bias without competition? Journal of cognitive neuroscience. 2011;23:1973–1986. doi: 10.1162/jocn.2010.21566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McTeague LM, Shumen JR, Wieser MJ, Lang PJ, Keil A. Social vision: sustained perceptual enhancement of affective facial cues in social anxiety. Neuroimage. 2011;54:1615–1624. doi: 10.1016/j.neuroimage.2010.08.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lang PJ, McTeague LM, Bradley MM. RDoC, DSM, and the reflex physiology of fear: A biodimensional analysis of the anxiety disorders spectrum. Psychophysiology. 2016;53(3):336–47. doi: 10.1111/psyp.12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McTeague LM, Lang PJ. The anxiety spectrum and the reflex physiology of defense: from circumscribed fear to broad distress. Depress Anxiety. 2012;29(4):264–81. doi: 10.1002/da.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McTeague LM, Lang PJ, Laplante MC, Cuthbert BN, Strauss CC, Bradley MM. Fearful imagery in social phobia: generalization, comorbidity, and physiological reactivity. Biol Psychiatry. 2009;65:374–382. doi: 10.1016/j.biopsych.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hook JN, Valentiner DN. Are Specific and Generalized Social Phobias Qualitatively Distinct? Cliincal Psychology: Science & Practice. 2002;9:379–395. [Google Scholar]
- 37.Kollman DM, Brown TA, Liverant GI, Hofmann SG. A taxometric investigation of the latent structure of social anxiety disorder in outpatients with anxiety and mood disorders. Depress Anxiety. 2006;23:190–199. doi: 10.1002/da.20158. [DOI] [PubMed] [Google Scholar]
- 38.Ruscio AM. The latent structure of social anxiety disorder: consequences of shifting to a dimensional diagnosis. J Abnorm Psychol. 2010;119:662–671. doi: 10.1037/a0019341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pine DS, Klein RG, Mannuzza S, Moulton JL, Lissek S, Guardino M, et al. Face-Emotion Processing in Offspring at Risk for Panic Disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2005;44(7):664–72. doi: 10.1097/01.chi.0000162580.92029.f4. [DOI] [PubMed] [Google Scholar]
- 39.Lai CH. Patterns of cortico-limbic activations during visual processing of sad faces in depression patients: a coordinate-based meta-analysis. The Journal of neuropsychiatry and clinical neurosciences. 2014;26:34–43. doi: 10.1176/appi.neuropsych.12060143. [DOI] [PubMed] [Google Scholar]
- 40.Li H, Chan RC, McAlonan GM, Gong QY. Facial emotion processing in schizophrenia: a meta-analysis of functional neuroimaging data. Schizophr Bull. 2010;36:1029–1039. doi: 10.1093/schbul/sbn190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McCleery A, Lee J, Joshi A, Wynn JK, Hellemann GS, Green MF. Meta-analysis of face processing event-related potentials in schizophrenia. Biol Psychiatry. 2015;77:116–126.s. doi: 10.1016/j.biopsych.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 42.Pine DS, Klein RG, Mannuzza S, Moulton JL, Lissek S, Guardino M, et al. Face-Emotion Processing in Offspring at Risk for Panic Disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2005;44(7):664–72. doi: 10.1097/01.chi.0000162580.92029.f4. [DOI] [PubMed] [Google Scholar]
- 43.Brown TA, DiNardo PA, Barlow DH. The Anxiety Disorder Interview Schedule for DSM-IV. Albany, NY: State University of New York Center for Stress and Anxiety Disorders; 1984. [Google Scholar]
- 44.Guy W. Clinical Global Impression. ECDEU Assessment Manual for Psychopharmacology, revised 1976 [Google Scholar]
- 45.First MB, Spitzer RL, Gibbon M, WIlliams JBW, Benjamin L. Structured clinical interview for DSM-IV axis II personality disorders (SCID-II) New York: Biometrics Research Department, New York State Psychiatric Institute; 1994. [Google Scholar]
- 46.Weerts TC, Lang PJ. Psychophysiology of fear imagery: differences between focal phobia and social performance anxiety. J Consult Clin Psychol. 1978;46:1157–1159. doi: 10.1037//0022-006x.46.5.1157. [DOI] [PubMed] [Google Scholar]
- 47.Holt CS, Heimberg RG, Hope DA. Avoidant personality disorder and the generalized subtype of social phobia. J Abnorm Psychol. 1992;101:318–325. doi: 10.1037//0021-843x.101.2.318. [DOI] [PubMed] [Google Scholar]
- 48.Lundqvist D, Flykt A, Öhman A. Karolinska directed emotional faces. Stockholm: Karolinska Institutet; 1998. [Google Scholar]
- 49.Bradley MM, Lang PJ. Measuring emotion: the Self-Assessment Manikin and the Semantic Differential. Journal of behavior therapy and experimental psychiatry. 1994;25:49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 50.Brainard DH. The Psychophysics Toolbox. Spatial vision. 1997;10:433–436. [PubMed] [Google Scholar]
- 51.Ferree TC, Luu P, Russell GS, Tucker DM. Scalp electrode impedance, infection risk, and EEG data quality. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2001;112:536–544. doi: 10.1016/s1388-2457(00)00533-2. [DOI] [PubMed] [Google Scholar]
- 52.Junghofer M, Elbert T, Tucker DM, Rockstroh B. Statistical control of artifacts in dense array EEG/MEG studies. Psychophysiology. 2000;37:523–532. [PubMed] [Google Scholar]
- 53.Peyk P, De Cesarei A, Junghofer M. ElectroMagnetoEncephalography software: overview and integration with other EEG/MEG toolboxes. Computational intelligence and neuroscience. 2011;2011:861705. doi: 10.1155/2011/861705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kristjansson SD, Kircher JC, Webb AK. Multilevel models for repeated measures research designs in psychophysiology: an introduction to growth curve modeling. Psychophysiology. 2007;44:728–736. doi: 10.1111/j.1469-8986.2007.00544.x. [DOI] [PubMed] [Google Scholar]
- 55.Vasey MW, Thayer JF. The continuing problem of false positives in repeated measures ANOVA in psychophysiology: a multivariate solution. Psychophysiology. 1987;24:479–486. doi: 10.1111/j.1469-8986.1987.tb00324.x. [DOI] [PubMed] [Google Scholar]
- 56.Blair RC, Karniski W. An alternative method for significance testing of waveform difference potentials. Psychophysiology. 1993;30:518–524. doi: 10.1111/j.1469-8986.1993.tb02075.x. [DOI] [PubMed] [Google Scholar]
- 57.Keil A, Moratti S, Sabatinelli D, Bradley MM, Lang PJ. Additive effects of emotional content and spatial selective attention on electrocortical facilitation. Cereb Cortex. 2005;15:1187–1197. doi: 10.1093/cercor/bhi001. [DOI] [PubMed] [Google Scholar]
- 58.Fresco DM, Coles ME, Heimberg RG, Liebowitz MR, Hami S, Stein MB, et al. The Liebowitz Social Anxiety Scale: a comparison of the psychometric properties of self-report and clinician-administered formats. Psychol Med. 2001;31:1025–1035. doi: 10.1017/s0033291701004056. [DOI] [PubMed] [Google Scholar]
- 59.Wolpe J. Fear Survey Schedule. San Diego: Educational and Industrial Testing Service; 1969. [Google Scholar]
- 60.Houck PR, Spiegel DA, Shear MK, Rucci P. Reliability of the self-report version of the panic disorder severity scale. Depress Anxiety. 2002;15:183–185. doi: 10.1002/da.10049. [DOI] [PubMed] [Google Scholar]
- 61.Reiss S, Peterson RA, Gursky DM, McNally RJ. Anxiety sensitivity, anxiety frequency and the prediction of fearfulness. Behaviour research and therapy. 1986;24:1–8. doi: 10.1016/0005-7967(86)90143-9. [DOI] [PubMed] [Google Scholar]
- 62.Watson D, Weber K, Assenheimer JS, Clark LA, Strauss ME, McCormick RA. Testing a tripartite model: I. Evaluating the convergent and discriminant validity of anxiety and depression symptom scales. J Abnorm Psychol. 1995;104:3–14. doi: 10.1037//0021-843x.104.1.3. [DOI] [PubMed] [Google Scholar]
- 63.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory. 2nd. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 64.Spielberger CD, Gorsuch RL, Lushene RL, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory (STAI) Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 65.Meyer TJ, Miller ML, Metzger RL, Borkovec TD. Development and validation of the Penn State Worry Questionnaire. Behaviour research and therapy. 1990;28:487–495. doi: 10.1016/0005-7967(90)90135-6. [DOI] [PubMed] [Google Scholar]
- 66.Devins GM. Using the illness intrusiveness ratings scale to understand health-related quality of life in chronic disease. Journal of Psychosomatic research. 2010;68:591–602. doi: 10.1016/j.jpsychores.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 67.Blair KS, Geraci M, Korelitz K, Otero M, Towbin K, Ernst M, et al. The pathology of social phobia is independent of developmental changes in face processing. Am J Psychiatry. 2011;168:1202–1209. doi: 10.1176/appi.ajp.2011.10121740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gentili C, Cristea IA, Angstadt M, Klumpp H, Tozzi L, Phan KL, et al. Beyond emotions: A meta-analysis of neural response within face processing system in social anxiety. Experimental biology and medicine (Maywood, NJ) 2016;241:225–237. doi: 10.1177/1535370215603514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Keil A, Sabatinelli D, Ding M, Lang PJ, Ihssen N, Heim S. Re-entrant projections modulate visual cortex in affective perception: evidence from Granger causality analysis. Hum Brain Mapp. 2009;30:532–540. doi: 10.1002/hbm.20521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weinberg A, Hajcak G. Electrocortical evidence for vigilance-avoidance in generalized anxiety disorder. Psychophysiology. 2011;48(6):842–51. doi: 10.1111/j.1469-8986.2010.01149.x. [DOI] [PubMed] [Google Scholar]
- 71.Lanius RA, Vermetten E, Loewenstein RJ, Brand B, Schmahl C, Bremner JD, et al. Emotion modulation in PTSD: Clinical and neurobiological evidence for a dissociative subtype. Am J Psychiatry. 2010;167:640–647. doi: 10.1176/appi.ajp.2009.09081168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lanius RA, Brand B, Vermetten E, Frewen PA, Spiegel D. The dissociative subtype of posttraumatic stress disorder: rationale, clinical and neurobiological evidence, and implications. Depress Anxiety. 2012;29:701–708. doi: 10.1002/da.21889. [DOI] [PubMed] [Google Scholar]
- 73.Doehrmann O, Ghosh SS, Polli FE, Reynolds GO, Horn F, Keshavan A, et al. Predicting treatment response in social anxiety disorder from functional magnetic resonance imaging. JAMA Psychiatry. 2013;70:87–97. doi: 10.1001/2013.jamapsychiatry.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Goldin PR, Ziv M, Jazaieri H, Hahn K, Heimberg R, Gross JJ. Impact of cognitive behavioral therapy for social anxiety disorder on the neural dynamics of cognitive reappraisal of negative self-beliefs: randomized clinical trial. JAMA Psychiatry. 2013;70:1048–1056. doi: 10.1001/jamapsychiatry.2013.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mansson KN, Frick A, Boraxbekk CJ, Marquand AF, Williams SC, Carlbring P, et al. Predicting long-term outcome of Internet-delivered cognitive behavior therapy for social anxiety disorder using fMRI and support vector machine learning. Translational psychiatry. 2015;5:e530. doi: 10.1038/tp.2015.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Klumpp H, Fitzgerald DA, Phan KL. Neural predictors and mechanisms of cognitive behavioral therapy on threat processing in social anxiety disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2013;45:83–91. doi: 10.1016/j.pnpbp.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Klumpp H, Fitzgerald DA, Piejko K, Roberts J, Kennedy AE, Phan KL. Prefrontal control and predictors of cognitive behavioral therapy response in social anxiety disorder. Social cognitive and affective neuroscience. 2016;11:630–640. doi: 10.1093/scan/nsv146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Klumpp H, Roberts J, Kennedy AE, Shankman SA, Langenecker SA, Gross JJ, et al. Emotion regulation related neural predictors of cognitive behavioral therapy response in social anxiety disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2017;75:106–112. doi: 10.1016/j.pnpbp.2017.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Craske MG, Stein MB. Anxiety. Lancet (London, England) 2016;388:3048–3059. doi: 10.1016/S0140-6736(16)30381-6. [DOI] [PubMed] [Google Scholar]
- 80.Gros DF, McCabe RE, Antony MM. Using a hybrid model to investigate the comorbidity and symptom overlap between social phobia and the other anxiety disorders and unipolar mood disorders. Psychiatry Res. 2013;210:188–192. doi: 10.1016/j.psychres.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 81.Kaye WH, Bulik CM, Thornton L, Barbarich N, Masters K. Comorbidity of anxiety disorders with anorexia and bulimia nervosa. Am J Psychiatry. 2004;161:2215–2221. doi: 10.1176/appi.ajp.161.12.2215. [DOI] [PubMed] [Google Scholar]
- 82.Latas M, Milovanovic S. Personality disorders and anxiety disorders: what is the relationship? Current opinion in psychiatry. 2014;27:57–61. doi: 10.1097/YCO.0000000000000025. [DOI] [PubMed] [Google Scholar]
- 83.Buckner JD, Schmidt NB, Lang AR, Small JW, Schlauch RC, Lewinsohn PM. Specificity of social anxiety disorder as a risk factor for alcohol and cannabis dependence. J Psychiatr Res. 2008;42:230–239. doi: 10.1016/j.jpsychires.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kuusikko S, Pollock-Wurman R, Jussila K, Carter AS, Mattila ML, Ebeling H, et al. Social anxiety in high-functioning children and adolescents with Autism and Asperger syndrome. Journal of autism and developmental disorders. 2008;38:1697–1709. doi: 10.1007/s10803-008-0555-9. [DOI] [PubMed] [Google Scholar]
- 85.Dold M, Bartova L, Souery D, Mendlewicz J, Serretti A, Porcelli S, et al. Clinical characteristics and treatment outcomes of patients with major depressive disorder and comorbid anxiety disorders - results from a European multicenter study. J Psychiatr Res. 2017;91:1–13. doi: 10.1016/j.jpsychires.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 86.Michail M, Birchwood M. Social anxiety disorder in first-episode psychosis: incidence, phenomenology and relationship with paranoia. Br J Psychiatry. 2009;195:234–241. doi: 10.1192/bjp.bp.108.053124. [DOI] [PubMed] [Google Scholar]
- 87.Altintas E, Yerdelen VD, Taskintuna N. Social Anxiety Level in Adult Patients With Epilepsy and Their First-Degree Cohabiting Relatives. The Journal of neuropsychiatry and clinical neurosciences. 2015;27:339–344. doi: 10.1176/appi.neuropsych.15030061. [DOI] [PubMed] [Google Scholar]
- 88.Kozak MJ, Cuthbert BN. The NIMH Research Domain Criteria Initiative: Background, Issues, and Pragmatics. Psychophysiology. 2016;53:286–297. doi: 10.1111/psyp.12518. [DOI] [PubMed] [Google Scholar]
- 89.Patrick CJ, Hajcak G. RDoC: Translating promise into progress. Psychophysiology. 2016;53:415–424. doi: 10.1111/psyp.12612. [DOI] [PubMed] [Google Scholar]
- 90.Weinberger DR, Glick ID, Klein DF. Whither Research Domain Criteria (RDoC)?: The Good, the Bad, and the Ugly. JAMA Psychiatry. 2015;72:1161–1162. doi: 10.1001/jamapsychiatry.2015.1743. [DOI] [PubMed] [Google Scholar]
- 91.Lilienfeld SO. The Research Domain Criteria (RDoC): an analysis of methodological and conceptual challenges. Behav Res Ther. 2014;62:129–139. doi: 10.1016/j.brat.2014.07.019. [DOI] [PubMed] [Google Scholar]
- 92.Kadouri A, Corruble E, Falissard B. The improved Clinical Global Impression Scale (iCGI): development and validation in depression. BMC Psychiatry. 2007;7:7. doi: 10.1186/1471-244X-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Targum SD, Busner J, Young AH. Targeted scoring criteria reduce variance in global impressions. Human psychopharmacology. 2008;23:629–633. doi: 10.1002/hup.966. [DOI] [PubMed] [Google Scholar]
- 94.Miskovic V, Keil A. Reliability of event-related EEG functional connectivity during visual entrainment: magnitude squared coherence and phase synchrony estimates. Psychophysiology. 2015;52:81–89. doi: 10.1111/psyp.12287. [DOI] [PubMed] [Google Scholar]
- 95.Wieser MJ, Miskovic V, Keil A. Steady-state visual evoked potentials as a research tool in social affective neuroscience. Psychophysiology. 2016;53:1763–1775. doi: 10.1111/psyp.12768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Miskovic V, Keil A. Reliability of event-related EEG functional connectivity during visual entrainment: magnitude squared coherence and phase synchrony estimates. Psychophysiology. 2015;52:81–89. doi: 10.1111/psyp.12287. [DOI] [PubMed] [Google Scholar]
- 97.Wieser MJ, Miskovic V, Keil A. Steady-state visual evoked potentials as a research tool in social affective neuroscience. Psychophysiology. 2016;53:1763–1775.s. doi: 10.1111/psyp.12768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Keil A, Smith JC, Wangelin BC, Sabatinelli D, Bradley MM, Lang PJ. Electrocortical and electrodermal responses covary as a function of emotional arousal: a single-trial analysis. Psychophysiology. 2008;45:516–523. doi: 10.1111/j.1469-8986.2008.00667.x. [DOI] [PubMed] [Google Scholar]
- 99.Wieser MJ, Miskovic V, Rausch S, Keil A. Different time course of visuocortical signal changes to fear-conditioned faces with direct or averted gaze: a ssVEP study with single-trial analysis. Neuropsychologia. 2014;62:101–110. doi: 10.1016/j.neuropsychologia.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 100.Heeren A, Mogoase C, Philippot P, McNally RJ. Attention bias modification for social anxiety: A systematic review and meta-analysis. Clin Psychol Rev. 2015;40:76–90. doi: 10.1016/j.cpr.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 101.Clarke PJ, Notebaert L, MacLeod C. Absence of evidence or evidence of absence: reflecting on therapeutic implementations of attentional bias modification. BMC Psychiatry. 2014;14:8. doi: 10.1186/1471-244X-14-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kuckertz JM, Amir N. Attention bias modification for anxiety and phobias: current status and future directions. Current psychiatry reports. 2015;17:9. doi: 10.1007/s11920-014-0545-x. [DOI] [PubMed] [Google Scholar]
- 103.Ordikhani-Seyedlar M, Lebedev MA, Sorensen HB, Puthusserypady S. Neurofeedback Therapy for Enhancing Visual Attention: State-of-the-Art and Challenges. Frontiers in neuroscience. 2016;10:352. doi: 10.3389/fnins.2016.00352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lazarov A, Pine DS, Bar-Haim Y. Gaze-Contingent Music Reward Therapy for Social Anxiety Disorder: A Randomized Controlled Trial. Am J Psychiatry. 2017 doi: 10.1176/appi.ajp.2016.16080894. appiajp201616080894. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


