Summary
Metastases arising from tumors have the proclivity to colonize specific organs, suggesting that they must rewire their biology to meet the demands of the organ colonized, thus altering their primary properties. Each metastatic site presents distinct metabolic challenges to a colonizing cancer cell, ranging from fuel and oxygen availability to oxidative stress. Here, we discuss the organ-specific metabolic adaptations cancer cells must undergo, which provide the ability to overcome the unique barriers to colonization in foreign tissues and establish the metastatic tissue tropism phenotype.
While carcinogenesis initiates in the primary tumor, metastasis is the leading cause of death in cancer. 90% of mortality from solid tumors is due to this process of cellular evolution that allows cells to venture from their original niche, travel through blood and lymph and ultimately invade and colonize distant tissues and organs, causing organ failure and death (Mehlen and Puisieux, 2006). The complete set of factors that define how metastatic cells gain the ability to survive the arduous journey and distinctly hostile environments remains elusive, despite being of great interest towards the goal of finding novel and effective cancer therapies. Notably, the observation that some primary tumor types metastasize to specific organs (such as prostate cancers to the bone and pancreatic cancers to the liver) (Obenauf and Massague, 2015), while other primary tumor types metastasize promiscuously (such as breast cancers and lung cancers), presents an intriguing question. What defines the nature of the changes that cells undergo to transplant at different organs and display specific tropisms? Here, we propose that during their evolution, tumor cells acquire a metabolic signature adapted for survival at particular metastatic sites, which dictates where they are able to form distal colonies. This hypothesis is consistent with the growing body of evidence showing that the more metabolically flexible the primary tumor cells are, the more likely they are to survive the metastatic process and thrive in distant organs (Lehuede et al., 2016).
Metabolic Reprogramming: Different flavors depending on tumor stage?
The link between metabolism and cancer has long been apparent in epidemiological studies associating obesity, high fat diet and lack of exercise with the disease, although the molecular mechanisms governing this relationship have only begun to be uncovered in the last decade (Hojman et al., 2017; Holmes et al., 2005; Kenfield et al., 2011; Meyerhardt et al., 2006; Moore et al., 2016). Studies showing that exercise can suppress activation of molecular signaling pathways, such as the mTOR pathway, a central regulator of cellular metabolism, point to metabolism as being at the root of the cancer connection (Thompson et al., 2009). More recently, new findings have revealed a mechanism by which dietary lipids can drive metastasis by supporting the growth of metastasis-initiating cells expressing high levels of the fatty acid receptor CD36 (Pascual et al., 2017). Indeed, some of the most striking distinctions between tumors and non-transformed tissues are the differences in their metabolism (Pavlova and Thompson, 2016; Vander Heiden et al., 2009). These differences have been used to diagnose tumors, such as with the use of 18F-fluorodeoxyglucose PET imaging (Zhu et al., 2011). Changes in metabolism not only affect cellular energetics, but also highly influence signaling networks and gene expression patterns by limiting the availability of substrates and co-factors for post-translational modifications (Gomes and Blenis, 2015; Wellen and Thompson, 2012). Moreover, secreted metabolites can also function as signaling molecules that alter the environment and promote communication between different cell types (Martinez-Outschoorn et al., 2011; Romero-Garcia et al., 2016). Thus, it comes as no surprise that oncogenic mutations that enable tumor formation often do so by altering metabolic cellular processes (Nagarajan et al., 2016). One example of this is MYC, which is a major regulator of metabolism to promote growth and proliferation (Pusch et al., 1997; Shim et al., 1997). MYC-driven cancers are often addicted to glutamine, a property that has been used to develop more efficacious therapeutic strategies for these cancers (Shroff et al., 2015). Mutations in PI3K, RAS and PTEN, which can act as drivers of many cancers with poor prognoses, lead to the activation of the mTOR pathway and thereby to metabolic reprogramming (Brastianos et al., 2015). Interestingly, metabolic enzymes themselves can also function as oncogenes. For instance, neomorphic mutations in IDH1 or IDH2 have been identified in gliomas and AML (Mardis et al., 2009; Parsons et al., 2008). For these reasons, metabolic reprogramming has now been accepted as a hallmark of cancer (Ward and Thompson, 2012).
In addition to its role in primary tumors, metabolic reprogramming is also essential to the metastatic process. Recent studies demonstrating the potential to selectively target metabolic dependencies of metastases show promise for safer therapeutic options. In one example, inhibition of PRODH, which is important in proline catabolism, efficiently suppressed lung metastases without any adverse effects on normal cells and organ function (Elia et al., 2017). In order to accommodate drastically different environments with different oxygen abundances, energy sources and nutrient availabilities, extreme metabolic flexibility is required. The ability of oncogenes to direct metabolism is therefore likely to play a significant role in the selection of which cancer cells are able to metastasize. In solid tumors, the need to rewire metabolism begins at the very first step of metastasis, when cells undergo intravasation and detach from their primary site. A good example of this is the observation that dihydropyrimidine accumulation is required for the epithelial-to-mesenchymal transition (EMT) (Shaul et al., 2014). EMT is a reversible phenomenon that allows epithelial cells to become motile and invade adjacent tissues to enter the circulation, and is an important mechanism by which carcinoma cells are thought to acquire metastatic potential (Ye and Weinberg, 2015). Moreover, a growing body of evidence has shown that the Warburg effect, a phenomenon wherein cells upregulate glycolysis while downregulating oxidative phosphorylation, is a metabolic adaptation exhibited by many cancer cells that becomes indispensible during these initial steps of metastasis (Dong et al., 2013; Liu et al., 2016). The Warburg effect allows cells to maintain ATP levels and reduce reactive oxygen species (ROS) production, allowing them to avoid anoikis, a form of cell death associated with loss of anchorage, as they enter the circulation (Kamarajugadda et al., 2012; Lu et al., 2015). Once circulating tumor cells (CTCs) exit the circulation at their metastatic site, they again have to alter their metabolism to adapt to the new environment of the host organ. Interestingly, millions of cancer cells are shed into circulation by the primary tumors; however, only a very small portion have the ability to colonize and form metastatic lesions. This indicates that very few of these cells have the necessary traits to survive and thrive in new environments. As metabolism is essential for the ability of any given cell to survive and adjust to stress, the idea that the CTCs that extravasate and form micro-metastatic colonies have a specific metabolic signature that allows them to colonize a specific organ site is an attractive one, especially with regards to therapeutic potential.
Brain metastases: From glucose to acetate and beyond
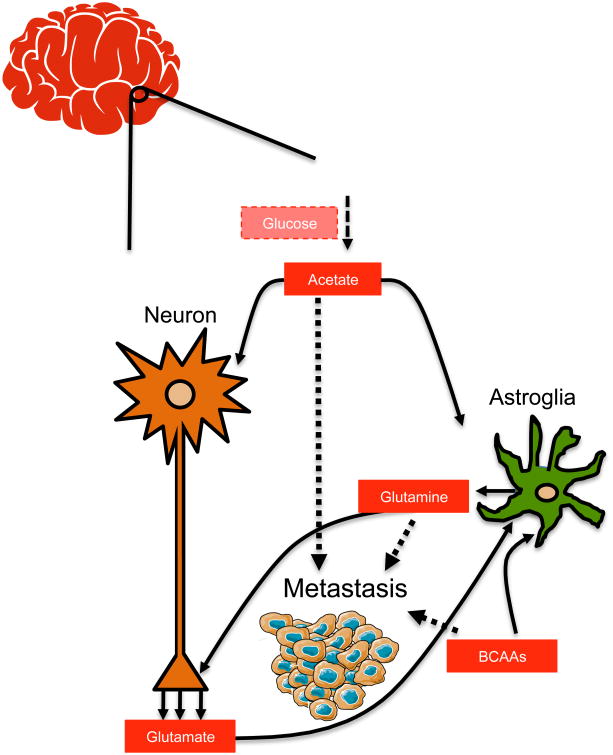
The brain has the highest energy demand of all the organs, consuming one fifth of the body's glucose-derived energy despite contributing a mere 2% to the total body weight (Erbsloh et al., 1958). Additionally, it possesses the unique ability to rewire its metabolism in response to varying metabolite availability, starvation or changing neuronal activity (Magistretti and Allaman, 2015). For example, when blood glucose is low, the brain is able to use acetate, ketone bodies, or short and medium chain fatty acids as alternative fuels (Ebert et al., 2003). Therefore, the idea that metastasizing cells that are able to mimic the brain in its ability to generate energy from non-glucose sources have the best chance to survive and thrive in this environment is appealing. In support of this, several studies have shown that brain metastases display a remarkable metabolic flexibility by utilizing acetate, glutamine and branched chain amino acids (BCAAs) as alternative sources of fuel (Figure 1).
Figure 1. Brain metastases are able to use acetate, branched chain amino acids and glutamine as alternative sources of fuel |.
As metastatic cells colonize the brain, they adapt their metabolism to be able to use locally available nutrients—acetate, branched chain amino acids and glutamine—when glucose becomes limiting.
The ability of brain metastases to utilize acetate as an energy source is illustrated in a study by Mashimo and colleagues. The authors observed that through upregulation of acetyl-CoA synthetase enzyme 2 (ACSS2), brain metastases are able to fuel the TCA cycle by converting acetate to acetyl-CoA (Mashimo et al., 2014). The study used 13C-labeled acetate to show that brain metastases originating from a wide variety of primary tumors, including breast cancer, non-small cell lung cancer, clear cell renal cell carcinoma, melanoma, and endometrial cancer, were able to efficiently oxidize acetate as an energy source, similarly to glioblastomas (Figure 1). This is in contrast to the observation that the organs from which tumors that usually metastasize to the brain arise show little 13C-acetate uptake on PET scans, indicating that this is a brain-specific adaptation. In addition to being able to use acetate as an alternate fuel, brain metastases have been observed to oxidize BCAAs and glutamine to survive and proliferate in the absence of glucose (Chen et al., 2015). Glutamine and BCAAs, like leucine, are vital to the maintenance of homeostasis in the brain. They are part of the astroglial-neuronal nitrogen shuttle and are responsible for maintaining an efficient response to subtle changes in glutamate levels in order to sustain neurotransmission (Figure 1). As such, they are highly abundant in the brain, making them important fuel sources that can be readily utilized by metastatic cancer cells (Albrecht et al., 2010). In addition to their ability to utilize other substrates available in the central nervous system as described above, brain metastases also have the ability to produce their own glucose through upregulation of fructose-1,6-bisphosphatase 2 (FBP2), a key enzyme within the gluconeogenesis pathway (Chen et al., 2015). Together, these findings show that cells that metastasize to the brain, just like the cells in the brain themselves, are remarkably flexible in their ability to utilize different fuels, and suggests that this metabolic flexibility is essential for their survival in this environment.
Lung metastases: Coping with an extreme pro-oxidant environment
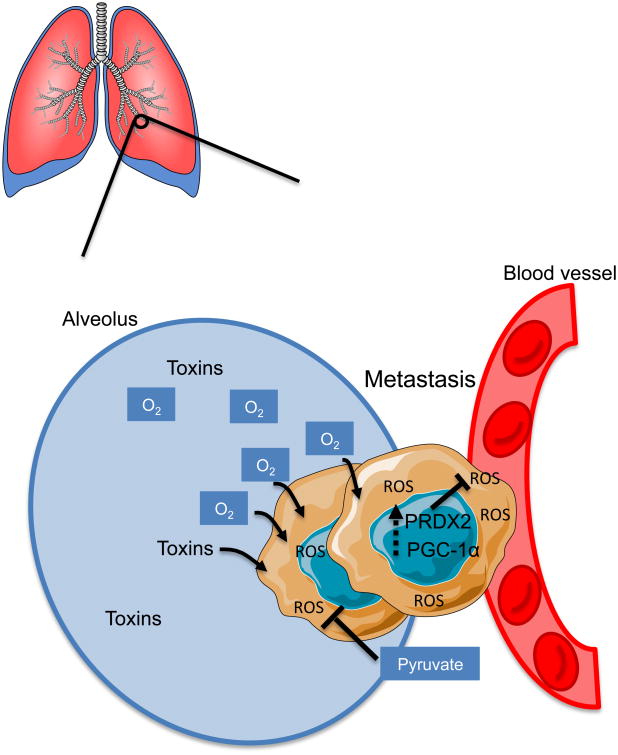
The lungs are the primary organs of respiration in humans (Alvarado and Arce, 2016). In addition to being exposed to high levels of oxygen, the lungs are also able to filter chemicals from the general circulation (Alvarado and Arce, 2016). High levels of both oxygen and toxic compounds contribute to increased oxidative stress, therefore requiring the lungs to have a high capacity to deal with ROS (Valavanidis et al., 2013), and may render the lung a uniquely problematic microenvironment for metastatic cells to colonize. Therefore, metastasizing cells must have a way to cope with oxidative damage. Indeed, LeBleu and colleagues have shown that metastatic cells derived from mammary epithelial tumors upregulate PPARγ coactivator 1 α (PGC-1α) expression in the circulation compared to the primary breast tumor, allowing them to preferentially metastasize to the lung (LeBleu et al., 2014). Additionally, silencing of PGC-1α in breast cancer cells drastically reduced their ability to metastasize to the lung (LeBleu et al., 2014). The authors suggest that this metastatic advantage is due to the role of PGC-1α as a transcriptional co-activator that induces mitochondrial biogenesis, thus increasing the total efficiency of the mitochondrial electron transport chain and consequently reducing electron leakage and ROS generation. However, a recent study showed that phenformin, an inhibitor of mitochondrial oxidative phosphorylation, had no significant effect on the lung metastatic burden resulting from injection of breast cancer cells overexpressing PGC-1α into mice (Andrzejewski et al., 2017). Rather, they suggest that PGC-1α promotes lung metastases through increased global metabolic flexibility of the cancer cells (Andrzejewski et al., 2017). Congruently, PGC-1α stimulates the expression of antioxidant genes such as GPx-1 and SOD2, which can help lung metastases cope with increased oxidative and chemical toxicity (St-Pierre et al., 2006). The fact that PGC-1α upregulation was also observed in CTCs (LeBleu et al., 2014) suggests that this particular metabolic adaptation may have been acquired upon intravasation into the bloodstream in order to ameliorate oxidative stresses, and then retained in the lung for its ability to confer fitness in the pulmonary environment (Figure 2). Another antioxidant mechanism found to play a role in lung metastasis is the upregulation of peroxiredoxins, small antioxidant proteins that shuttle electrons in order to reduce hydrogen peroxide. Stresing and colleagues discovered that lung metastases originating from breast tumors specifically upregulate the expression of peroxiredoxin 2 (PRDX2) (Stresing et al., 2013). Increased expression of PRDX2 facilitated survival in the lung where pro-oxidative stress is particularly prevalent (Figure 2). The authors found that compared with parental tumors, lung metastases were particularly sensitive to PRDX2 silencing due to ROS toxicity.
Figure 2. Metastases to the lung must survive in a pro-oxidant environment |.
In order to cope with increased ROS, lung metastases upregulate PGC-1α and PRDX2. Additionally, metastases increase pyruvate consumption to fuel the TCA through anaplerosis and to alleviate strain on the mitochondrial electron chain.
Lung metastases may also take advantage of the same metabolic adaptations that occur in primary tumors of the lung. In non-small cell lung cancer (NSCLC), pyruvate carboxylase (PC) expression is upregulated in the primary tumors compared to normal tissues (Sellers et al., 2015). Increased PC-dependent anaplerosis has also been observed in growing lung metastases in vivo (Christen et al., 2016). In this study, it was shown that lung metastases upregulate PC expression and prefer utilizing pyruvate over glutamine to fuel the TCA, similar to what is observed in certain NSCLCs (Sellers et al., 2015). At first sight, this finding might seem contradictory, considering that glutamine is a very abundant carbon source in the lungs (van den Heuvel et al., 2012). However, it is now known that defects in the mitochondrial electron transport chain can be alleviated via exogenous addition of pyruvate, which serves as an electron acceptor to replenish NAD+ pools for TCA anaplerosis (Yin et al., 2016). Taking into consideration the added strain of high oxygen and limited glucose on the mitochondrial electron transport chain that upregulation of PGC-1α may not always fully ameliorate, the ability of lung metastases to increase pyruvate uptake might provide them with the ability to cope with an imbalance of reducing equivalents caused by a dysfunctional electron transport chain (Figure 2). Together, these studies underline the necessity for lung metastases, like lung cells, to manage the pro-oxidative environment of the lung in order to settle and thrive in this particular metastatic niche. Interestingly, despite the abundance of oxygen in this tissue, some primary tumors of the lung have been shown to contain hypoxic regions. These hypoxic regions are thought to promote metastases of these cancers outside of the lung, perhaps by providing tumor cells of those regions with a metabolic flexibility that allows them to thrive under low oxygen conditions (Zhang et al., 2014a; Zhang et al., 2014b).
Liver metastases: Competing for resources
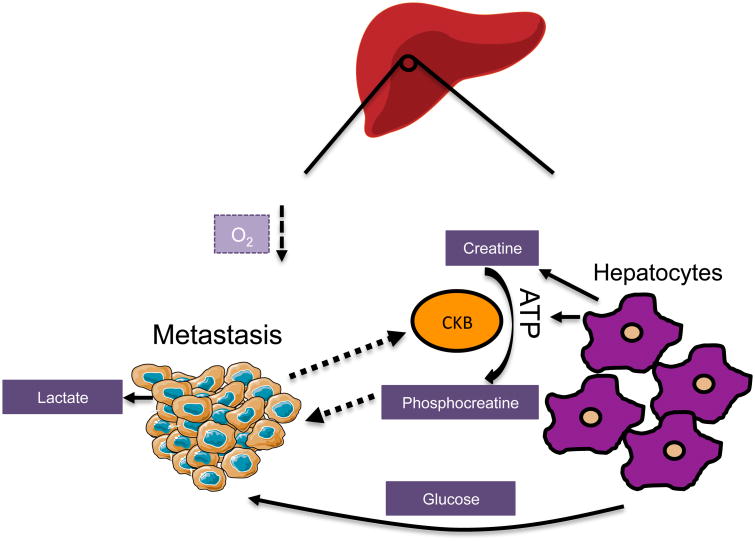
The liver is a key metabolic organ, governing the body's energy balance. It plays a critical role in maintaining blood glucose levels by tightly regulating glucose consumption and production, fatty acid and ketone body syntheses, as well as protein synthesis and break-down. In order to successfully carry out its various metabolic functions, the liver is divided by metabolic zonation, which corresponds with varying oxygen gradients, creating inhospitable regions for metastasizing cancer cells. The liver microenvironment is naturally more conducive to cells that display a high glycolytic profile and are adapted for a low-oxygen state. This is illustrated by the abundance of studies demonstrating that primary hepatocellular carcinomas preferentially engage in glycolytic metabolism to proliferate in the liver (Jiao et al., 2017; Lin et al., 2017; Song et al., 2015). It is therefore tempting to speculate that to colonize the liver, metastasizing cancer cells from other organs must be able to overcome this hypoxic barrier in order to adapt to the hepatic environment. In fact, liver metastases downregulate mitochondrial activity by upregulating pyruvate dehydrogenase kinase-1 (PDK-1) in a HIF-1α-dependent manner (Dupuy et al., 2015). This phenomenon was shown to be essential for the development of liver metastases as genetic silencing of PDK-1 completely blunts their formation. PDK-1 is the negative regulator of the pyruvate dehydrogenase complex and therefore of pyruvate entry into the TCA cycle. Dupuy and colleagues have shown that this increase in PDK-1 leads to a decreased flux into the TCA cycle (Dupuy et al., 2015), suggesting that this adaptation can help cope with conditions of metabolic stress, allowing metastases to maintain a glycolytic phenotype and survive in hypoxic regions of the liver (Figure 3). The decrease in the TCA flux caused by PDK-1 upregulation can also lead to an increased production of lactate, which can be taken up by hepatocytes and converted into other fuels to feed back to cancer cells (Dupuy et al., 2015).
Figure 3. Metastases to the liver face a unique biosynthetic milieu and must compete for resources |.
Liver metastases become highly glycolytic through high consumption of local glucose. To replenish their ATP, metastases start expressing and secreting CKB which generates phosphocreatine from local ATP and creatine. Phosphocreatine is then imported into the metastatic cells and catabolized for energy.
The liver also plays an important role in creatine metabolism. Hepatic creatine production and export to other organs is an important system for ATP recycling and storage (da Silva et al., 2009). Creatine can hold a phosphate group as phosphocreatine, and acts as a phospho-donor to maintain a proper ADP:ATP ratio or replenish ATP in conditions of intense exercise or brain activity (Guimaraes-Ferreira, 2014). Liver metastases originating from colorectal tumors induce the secretion of creatine kinase, brain-type (CKB) into the microenvironment through downregulation of miR-483 and miR-551a (Loo et al., 2015). CKB phosphorylates extracellular creatine produced by hepatocytes, siphoning energy away from the liver in the form of phosphocreatine. Phosphocreatine is then imported into the metastatic cells, where it replenishes their intracellular ATP pools (Figure 3). Decreasing the expression of CKB or the creatine transporter, SLC6A8, in the metastatic cells decreased their ability to form colonies in the liver when re-injected into mice (Loo et al., 2015). Thus, those tumor cells that can maximize the resources available, such as creatine and glucose, to meet their energy demands, are the prime candidates to colonize the hepatic niche.
Bone metastases: Undercover agents to destroy and invade bone
The bone is a mineral-rich and heterogeneous tissue composed of many different cell types, as well as collagen, calcium, and phosphate. Bone metastases most commonly originate from breast and prostate tumors, and are generally associated with poor prognoses (Shiozawa et al., 2015). Osteotropic metastases can be characterized as osteolytic (stimulates bone destruction) or osteoblastic (stimulates bone formation), both of which are detrimental to the patient. The advantage for the cancer cells of osteolytic metastasis is easily deducible as the destruction of niche tissues renders more spatial and nutritional resources available to invading cells. In contrast, osteoblastic metastasis is best illustrated by the idea of “osteomimicry”, a phenomenon in which tumor cells acquire a bone cell phenotype, that is usually osteoblast-like, and express bone-specific secreted, cell-surface and matrix markers to avoid immune surveillance (Rucci and Teti, 2010).
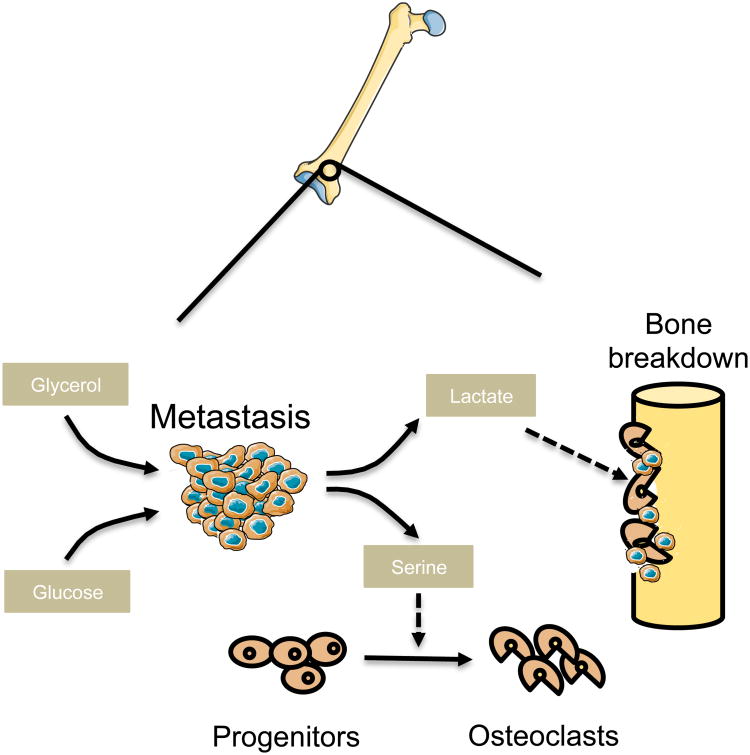
Bone metabolism is a continual, cyclic interplay of bone growth and resorption. The dynamic relationship between osteoclasts, osteoblasts, and an array of hormonal and regulatory factors orchestrates this process (Tanaka et al., 2005). Successful colonization of bone can be achieved by interaction of tumor cells with the microenvironment of the bone in order to blend in as well as to better penetrate and acquire nutrients in the bone. Regulatory pathways that modulate bone metabolism are, therefore, likely to be key in determining whether cancer cells can home to the bone and establish metastatic lesions. In support of this idea, de novo production of L-serine, an essential factor for differentiation of mesenchymal bone marrow precursors into osteoclasts (Iwamoto et al., 2005), has been observed in osteotropic breast cancer cells (Pollari et al., 2011) (Figure 4). Specifically, expression of the three enzymes required for de novo serine synthesis, phosphoglycerate dehydrogenase (PHGDH), phosphoserine aminotransferase 1 (PSAT1), and phosphoserine phosphatase (PSPH), as well as the serine transporter SLC1A4, was observed to be significantly higher in bone-metastatic variants of breast tumors (Pollari et al., 2011). Similarly, bone metastatic breast cancer cells, when compared to the non-osteotropic ones, have also been observed to release large amounts of lactate (Lemma et al., 2017). Lactate is an important fuel for osteoclasts suggesting that osteotropic tumor cells release lactate to support osteolysis (Figure 4). In fact, inhibition of the lactate transporter MCT-1 in the osteoclasts significantly impaired their osteolytic function (Lemma et al., 2017). Through the release of serine and lactate, cancer cells are able to form osteolytic bone metastases by promoting the differentiation and metabolic fitness of osteoclasts, allowing them to invade the metastatic niche and free up nutrients and space (Figure 4).
Figure 4. Bone metastases remodel the stroma to free up nutrients and space for growth |.
Osteotropic metastases secrete serine and lactate to facilitate osteoclastogenesis which results in bone resorption thereby releasing spatial and nutritional resources. Metastatic cells can also take on properties of local cells through the expression of bone-specific secreted, cell-surface and matrix markers, which can rewire their glucose and glycerol metabolism.
High expression of osteopontin (OPN) can specifically predict osteotropism of multiple cancers (Kruger et al., 2014). OPN is a matrix glycoprotein that is expressed in the cells of the bone, and it helps facilitate bone mineralization and remodeling through paracrine and autocrine signaling via integrins (Kruger et al., 2014). In locally advanced nasopharyngeal carcinoma, OPN expression level was significantly higher in patients with bone metastases, and served as a prognostic biomarker for survival (Hou et al., 2015). A similar pattern is seen in breast cancer, where 83% of bone metastases exhibit high OPN expression, compared to 42% of all breast tumors (Carlinfante et al., 2003). OPN expression facilitates osteomimicry in tumor cells and the establishment of bone metastases at many levels: it facilitates the attachment of tumor cells to the bone matrix (Rodrigues et al., 2007), it stimulates bone resorption by releasing nutrients and space (Kahles et al., 2014), it increases glucose and glycerol influx into the metastatic cells, and it increases serine and glycine levels, all of which allow the tumor cells to conserve energy and avoid anoikis (Shi et al., 2014)
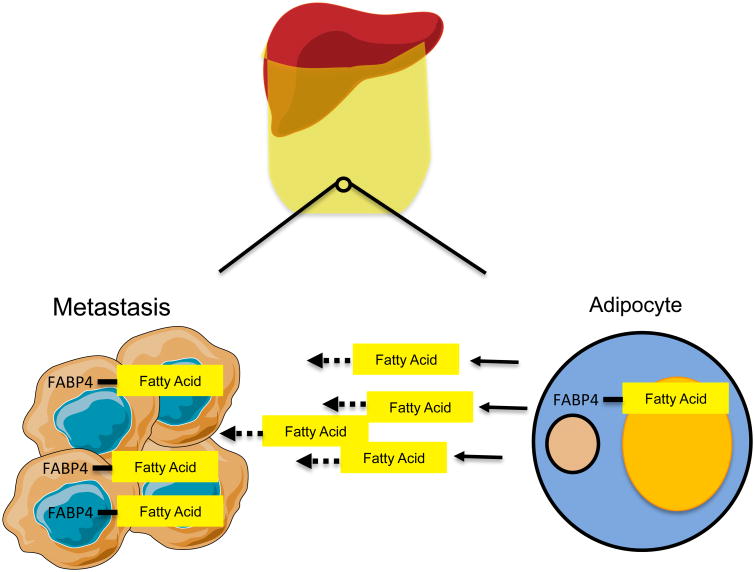
Omentum Metastasis: Hijacking adipocyte metabolism to thrive
80% of women diagnosed with ovarian cancer present metastases to the omentum. This fatty tissue functions as a metabolically active organ with endocrine characteristics, allowing it to respond to the body's nutritional needs by either producing or breaking down fatty acids (Choe et al., 2016). As with other tissues reviewed in this article, it is tempting to speculate that ovarian cancer cells metastasizing to the omentum display characteristics that allows them to thrive in such a specific metabolic environment. Indeed, Nieman and colleagues showed that omental metastases of ovarian cancer upregulate fatty acid binding protein 4 (FABP4), which is usually highly expressed in adipocytes (Nieman et al., 2011). FABP4 binds long-chain acids and has been shown to regulate lipolysis (Scheja et al., 1999). The authors showed that when co-cultured with ovarian cancer cells, adipocytes were stimulated to perform lipolysis and to directly transfer lipids to cancer cells. Cancer cells then underwent β-oxidation to utilize the fatty acids as an energy source (Figure 5). When FABP4 was deleted in mice, the omental tropism of ovarian cancer cells was significantly impaired, suggesting that FABP4 is key to fueling the omental metastases (Choe et al., 2016).
Figure 5. Metastases to omentum reprogram omental adipocytes to release fatty acids which supply metastatic cells with energy |.
Omental metastases stimulate lipolysis in adipocytes which releases fatty acids into the environment. Metastatic cells also upregulate FABP4 which is allows them to import and catabolize newly available fatty acids.
Conclusions and future perspectives
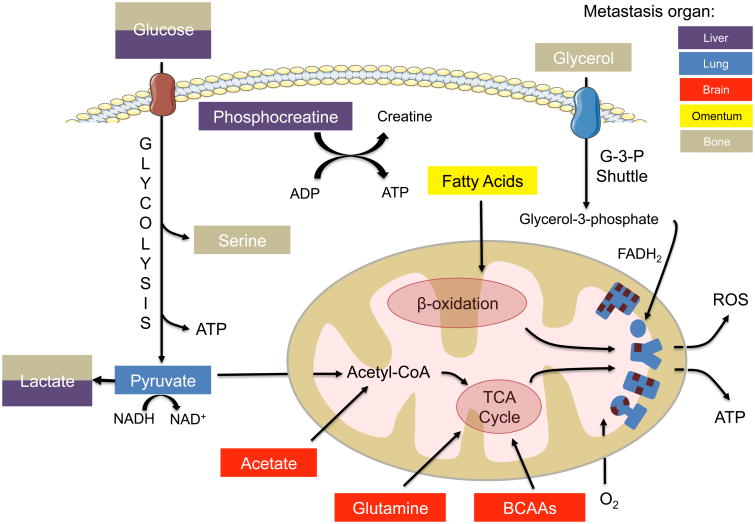
As growing success in treating primary cancers in the clinic parallels the development of targeted therapies, there is a push to expand our knowledge on how cancers metastasize. While acquisition of certain metastatic properties in the primary cancer cells and their ability to enter circulation are important events, the majority of CTCs do not actually go on to form metastases. Rather, the main determinant of a primary tumor cell forming a metastatic lesion lies in its ability to survive the circulation and specific distal organs. Metabolic plasticity governs a significant probability of a cancer cell surviving through the metastatic process, enabling it to meet stringent bioenergetic demands, unfavorable oxidative stresses and other barriers to colonization at its metastatic site. Here, we propose that meeting the metabolic demands of their host organ is essential for cancer cells to survive and thrive at such locations (Figure 6). When put into context, a vast body of data supports this idea and clearly demonstrates striking similarities between the metabolism of metastases and the metabolism of the tissue they home to. The concepts highlighted in this review may only skim the surface of an emerging understanding of tropism and a significant amount of work will need to be done to understand this process. If this hypothesis holds true, it opens the door to the identification of novel ways to target the spread of cancers at their weakest point -metastatic colonization - and there may come a day when treating metastases will no longer be a such a difficult task.
Figure 6. Metastases in different organs utilize distinct metabolic pathways to generate energy |.
Metastases that arise in different organs adapt to the specific conditions of their particular environment in order to generate ATP. In the brain (red), metastases are able to utilize glutamine, BCAAs and acetate as carbon sources to fuel the TCA cycle. In the lung (blue), pyruvate fuels the TCA cycle and additionally can replenish NAD+ pools for anaplerosis. The liver (purple) supports a glycolytic profile, and metastases to this location also use phosphocreatine to generate ATP. Omental (yellow) metastases bind fatty acids from adipocytes and oxidize them in order to fuel anaplerosis. Bone (gray) metastases rewire their metabolism through expression of osteopontin to differentially utilize glucose and glycerol to meet energetic demands and produce serine.
Acknowledgments
We apologize to those whose work was not discussed and cited in this review due to limitations in space and scope. We thank Dr. Edouard Mullarky, Dr. Didem Ilter, Dr. Anders Mutvei and Bryan Ngo for kindly providing helpful discussions on this topic and/or comments on this manuscript. A.P.G. is supported by a Susan G. Komen Postdoctoral Fellowship and a Pathway to Independence Award from NCI (K99CA218686-01). T.S. is supported by the NIH F31 pre-doctoral fellowship 1F31CA220750-01. NIH Grants GM51405, CA46595 and HL121266 provide research support for the Blenis laboratory.
References
- Albrecht J, Sidoryk-Wegrzynowicz M, Zielinska M, Aschner M. Roles of glutamine in neurotransmission. Neuron Glia Biol. 2010;6:263–276. doi: 10.1017/S1740925X11000093. [DOI] [PubMed] [Google Scholar]
- Alvarado A, Arce I. Metabolic Functions of the Lung, Disorders and Associated Pathologies. J Clin Med Res. 2016;8:689–700. doi: 10.14740/jocmr2668w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrzejewski S, Klimcakova E, Johnson RM, Tabaries S, Annis MG, McGuirk S, Northey JJ, Chenard V, Sriram U, Papadopoli DJ, et al. PGC-1alpha Promotes Breast Cancer Metastasis and Confers Bioenergetic Flexibility against Metabolic Drugs. Cell Metab. 2017;26:778–787 e775. doi: 10.1016/j.cmet.2017.09.006. [DOI] [PubMed] [Google Scholar]
- Brastianos PK, Carter SL, Santagata S, Cahill DP, Taylor-Weiner A, Jones RT, Van Allen EM, Lawrence MS, Horowitz PM, Cibulskis K, et al. Genomic Characterization of Brain Metastases Reveals Branched Evolution and Potential Therapeutic Targets. Cancer Discov. 2015;5:1164–1177. doi: 10.1158/2159-8290.CD-15-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlinfante G, Vassiliou D, Svensson O, Wendel M, Heinegard D, Andersson G. Differential expression of osteopontin and bone sialoprotein in bone metastasis of breast and prostate carcinoma. Clin Exp Metastasis. 2003;20:437–444. doi: 10.1023/a:1025419708343. [DOI] [PubMed] [Google Scholar]
- Chen J, Lee HJ, Wu X, Huo L, Kim SJ, Xu L, Wang Y, He J, Bollu LR, Gao G, et al. Gain of glucose-independent growth upon metastasis of breast cancer cells to the brain. Cancer Res. 2015;75:554–565. doi: 10.1158/0008-5472.CAN-14-2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe SS, Huh JY, Hwang IJ, Kim JI, Kim JB. Adipose Tissue Remodeling: Its Role in Energy Metabolism and Metabolic Disorders. Front Endocrinol (Lausanne) 2016;7:30. doi: 10.3389/fendo.2016.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christen S, Lorendeau D, Schmieder R, Broekaert D, Metzger K, Veys K, Elia I, Buescher JM, Orth MF, Davidson SM, et al. Breast Cancer-Derived Lung Metastases Show Increased Pyruvate Carboxylase-Dependent Anaplerosis. Cell Rep. 2016;17:837–848. doi: 10.1016/j.celrep.2016.09.042. [DOI] [PubMed] [Google Scholar]
- da Silva RP, Nissim I, Brosnan ME, Brosnan JT. Creatine synthesis: hepatic metabolism of guanidinoacetate and creatine in the rat in vitro and in vivo. Am J Physiol Endocrinol Metab. 2009;296:E256–261. doi: 10.1152/ajpendo.90547.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C, Yuan T, Wu Y, Wang Y, Fan TW, Miriyala S, Lin Y, Yao J, Shi J, Kang T, et al. Loss of FBP1 by Snail-mediated repression provides metabolic advantages in basal-like breast cancer. Cancer Cell. 2013;23:316–331. doi: 10.1016/j.ccr.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuy F, Tabaries S, Andrzejewski S, Dong Z, Blagih J, Annis MG, Omeroglu A, Gao D, Leung S, Amir E, et al. PDK1-Dependent Metabolic Reprogramming Dictates Metastatic Potential in Breast Cancer. Cell Metab. 2015;22:577–589. doi: 10.1016/j.cmet.2015.08.007. [DOI] [PubMed] [Google Scholar]
- Ebert D, Haller RG, Walton ME. Energy contribution of octanoate to intact rat brain metabolism measured by 13C nuclear magnetic resonance spectroscopy. J Neurosci. 2003;23:5928–5935. doi: 10.1523/JNEUROSCI.23-13-05928.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlund M, Sung SY, Chung LW. Modulation of prostate cancer growth in bone microenvironments. J Cell Biochem. 2004;91:686–705. doi: 10.1002/jcb.10702. [DOI] [PubMed] [Google Scholar]
- Elia I, Broekaert D, Christen S, Boon R, Radaelli E, Orth MF, Verfaillie C, Grunewald TGP, Fendt SM. Proline metabolism supports metastasis formation and could be inhibited to selectively target metastasizing cancer cells. Nat Commun. 2017;8:15267. doi: 10.1038/ncomms15267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbsloh F, Bernsmeier A, Hillesheim H. The glucose consumption of the brain & its dependence on the liver. Arch Psychiatr Nervenkr Z Gesamte Neurol Psychiatr. 1958;196:611–626. doi: 10.1007/BF00344388. [DOI] [PubMed] [Google Scholar]
- Gomes AP, Blenis J. A nexus for cellular homeostasis: the interplay between metabolic and signal transduction pathways. Curr Opin Biotechnol. 2015;34:110–117. doi: 10.1016/j.copbio.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimaraes-Ferreira L. Role of the phosphocreatine system on energetic homeostasis in skeletal and cardiac muscles. Einstein (Sao Paulo) 2014;12:126–131. doi: 10.1590/S1679-45082014RB2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojman P, Gehl J, Christensen JF, Pedersen BK. Molecular Mechanisms Linking Exercise to Cancer Prevention and Treatment. Cell Metab. 2017 doi: 10.1016/j.cmet.2017.09.015. [DOI] [PubMed] [Google Scholar]
- Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293:2479–2486. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- Hou X, Wu X, Huang P, Zhan J, Zhou T, Ma Y, Qin T, Luo R, Feng Y, Xu Y, et al. Osteopontin is a useful predictor of bone metastasis and survival in patients with locally advanced nasopharyngeal carcinoma. Int J Cancer. 2015;137:1672–1678. doi: 10.1002/ijc.29540. [DOI] [PubMed] [Google Scholar]
- Iwamoto T, Mamiya N, Masushige S, Kida S. PLCgamma2 Activates CREB-dependent Transcription in PC12 Cells Through Phosphorylation of CREB at Serine 133. Cytotechnology. 2005;47:107–116. doi: 10.1007/s10616-005-3763-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao L, Zhang HL, Li DD, Yang KL, Tang J, Li X, Ji J, Yu Y, Wu RY, Ravichandran S, et al. Regulation of Glycolytic Metabolism by Autophagy in Liver Cancer Involves Selective Autophagic Degradation of HK2 (hexokinase 2) Autophagy. 2017:0. doi: 10.1080/15548627.2017.1381804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahles F, Findeisen HM, Bruemmer D. Osteopontin: A novel regulator at the cross roads of inflammation, obesity and diabetes. Mol Metab. 2014;3:384–393. doi: 10.1016/j.molmet.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarajugadda S, Stemboroski L, Cai Q, Simpson NE, Nayak S, Tan M, Lu J. Glucose oxidation modulates anoikis and tumor metastasis. Mol Cell Biol. 2012;32:1893–1907. doi: 10.1128/MCB.06248-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenfield SA, Stampfer MJ, Giovannucci E, Chan JM. Physical activity and survival after prostate cancer diagnosis in the health professionals follow-up study. J Clin Oncol. 2011;29:726–732. doi: 10.1200/JCO.2010.31.5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger TE, Miller AH, Godwin AK, Wang J. Bone sialoprotein and osteopontin in bone metastasis of osteotropic cancers. Crit Rev Oncol Hematol. 2014;89:330–341. doi: 10.1016/j.critrevonc.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBleu VS, O'Connell JT, Gonzalez Herrera KN, Wikman H, Pantel K, Haigis MC, de Carvalho FM, Damascena A, Domingos Chinen LT, Rocha RM, et al. PGC-1alpha mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat Cell Biol. 2014;16:992–1003. 1001–1015. doi: 10.1038/ncb3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehuede C, Dupuy F, Rabinovitch R, Jones RG, Siegel PM. Metabolic Plasticity as a Determinant of Tumor Growth and Metastasis. Cancer Res. 2016;76:5201–5208. doi: 10.1158/0008-5472.CAN-16-0266. [DOI] [PubMed] [Google Scholar]
- Lemma S, Di Pompo G, Porporato PE, Sboarina M, Russell S, Gillies RJ, Baldini N, Sonveaux P, Avnet S. MDA-MB-231 breast cancer cells fuel osteoclast metabolism and activity: A new rationale for the pathogenesis of osteolytic bone metastases. Biochim Biophys Acta. 2017 doi: 10.1016/j.bbadis.2017.08.030. [DOI] [PubMed] [Google Scholar]
- Lin YH, Wu MH, Huang YH, Yeh CT, Cheng ML, Chi HC, Tsai CY, Chung IH, Chen CY, Lin KH. Taurine upregulated gene 1 functions as a master regulator to coordinate glycolysis and metastasis in hepatocellular carcinoma. Hepatology. 2017 doi: 10.1002/hep.29462. [DOI] [PubMed] [Google Scholar]
- Liu M, Quek LE, Sultani G, Turner N. Epithelial-mesenchymal transition induction is associated with augmented glucose uptake and lactate production in pancreatic ductal adenocarcinoma. Cancer Metab. 2016;4:19. doi: 10.1186/s40170-016-0160-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo JM, Scherl A, Nguyen A, Man FY, Weinberg E, Zeng Z, Saltz L, Paty PB, Tavazoie SF. Extracellular metabolic energetics can promote cancer progression. Cell. 2015;160:393–406. doi: 10.1016/j.cell.2014.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Tan M, Cai Q. The Warburg effect in tumor progression: mitochondrial oxidative metabolism as an anti-metastasis mechanism. Cancer Lett. 2015;356:156–164. doi: 10.1016/j.canlet.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magistretti PJ, Allaman I. A cellular perspective on brain energy metabolism and functional imaging. Neuron. 2015;86:883–901. doi: 10.1016/j.neuron.2015.03.035. [DOI] [PubMed] [Google Scholar]
- Mardis ER, Ding L, Dooling DJ, Larson DE, McLellan MD, Chen K, Koboldt DC, Fulton RS, Delehaunty KD, McGrath SD, et al. Recurring mutations found by sequencing an acute myeloid leukemia genome. N Engl J Med. 2009;361:1058–1066. doi: 10.1056/NEJMoa0903840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Outschoorn UE, Pavlides S, Howell A, Pestell RG, Tanowitz HB, Sotgia F, Lisanti MP. Stromal-epithelial metabolic coupling in cancer: integrating autophagy and metabolism in the tumor microenvironment. Int J Biochem Cell Biol. 2011;43:1045–1051. doi: 10.1016/j.biocel.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlen P, Puisieux A. Metastasis: a question of life or death. Nat Rev Cancer. 2006;6:449–458. doi: 10.1038/nrc1886. [DOI] [PubMed] [Google Scholar]
- Meyerhardt JA, Giovannucci EL, Holmes MD, Chan AT, Chan JA, Colditz GA, Fuchs CS. Physical activity and survival after colorectal cancer diagnosis. J Clin Oncol. 2006;24:3527–3534. doi: 10.1200/JCO.2006.06.0855. [DOI] [PubMed] [Google Scholar]
- Moore SC, Lee IM, Weiderpass E, Campbell PT, Sampson JN, Kitahara CM, Keadle SK, Arem H, Berrington de Gonzalez A, Hartge P, et al. Association of Leisure-Time Physical Activity With Risk of 26 Types of Cancer in 1.44 Million Adults. JAMA Intern Med. 2016;176:816–825. doi: 10.1001/jamainternmed.2016.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarajan A, Malvi P, Wajapeyee N. Oncogene-directed alterations in cancer cell metabolism. Trends Cancer. 2016;2:365–377. doi: 10.1016/j.trecan.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieman KM, Kenny HA, Penicka CV, Ladanyi A, Buell-Gutbrod R, Zillhardt MR, Romero IL, Carey MS, Mills GB, Hotamisligil GS, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. 2011;17:1498–1503. doi: 10.1038/nm.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obenauf AC, Massague J. Surviving at a distance: organ specific metastasis. Trends Cancer. 2015;1:76–91. doi: 10.1016/j.trecan.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Siu IM, Gallia GL, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual G, Avgustinova A, Mejetta S, Martin M, Castellanos A, Attolini CS, Berenguer A, Prats N, Toll A, Hueto JA, et al. Targeting metastasisinitiating cells through the fatty acid receptor CD36. Nature. 2017;541:41–45. doi: 10.1038/nature20791. [DOI] [PubMed] [Google Scholar]
- Pavlova NN, Thompson CB. The Emerging Hallmarks of Cancer Metabolism. Cell metabolism. 2016;23:27–47. doi: 10.1016/j.cmet.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollari S, Kakonen SM, Edgren H, Wolf M, Kohonen P, Sara H, Guise T, Nees M, Kallioniemi O. Enhanced serine production by bone metastatic breast cancer cells stimulates osteoclastogenesis. Breast Cancer Res Treat. 2011;125:421–430. doi: 10.1007/s10549-010-0848-5. [DOI] [PubMed] [Google Scholar]
- Pusch O, Soucek T, Hengstschlager-Ottnad E, Bernaschek G, Hengstschlager M. Cellular targets for activation by c-Myc include the DNA metabolism enzyme thymidine kinase. DNA Cell Biol. 1997;16:737–747. doi: 10.1089/dna.1997.16.737. [DOI] [PubMed] [Google Scholar]
- Rodrigues LR, Teixeira JA, Schmitt FL, Paulsson M, Lindmark-Mansson H. The role of osteopontin in tumor progression and metastasis in breast cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:1087–1097. doi: 10.1158/1055-9965.EPI-06-1008. [DOI] [PubMed] [Google Scholar]
- Romero-Garcia S, Moreno-Altamirano MM, Prado-Garcia H, Sanchez-Garcia FJ. Lactate Contribution to the Tumor Microenvironment: Mechanisms, Effects on Immune Cells and Therapeutic Relevance. Front Immunol. 2016;7:52. doi: 10.3389/fimmu.2016.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucci N, Teti A. Osteomimicry: how tumor cells try to deceive the bone. Front Biosci (Schol Ed) 2010;2:907–915. doi: 10.2741/s110. [DOI] [PubMed] [Google Scholar]
- Scheja L, Makowski L, Uysal KT, Wiesbrock SM, Shimshek DR, Meyers DS, Morgan M, Parker RA, Hotamisligil GS. Altered insulin secretion associated with reduced lipolytic efficiency in aP2-/- mice. Diabetes. 1999;48:1987–1994. doi: 10.2337/diabetes.48.10.1987. [DOI] [PubMed] [Google Scholar]
- Sellers K, Fox MP, Bousamra M, 2nd, Slone SP, Higashi RM, Miller DM, Wang Y, Yan J, Yuneva MO, Deshpande R, et al. Pyruvate carboxylase is critical for non-small-cell lung cancer proliferation. J Clin Invest. 2015;125:687–698. doi: 10.1172/JCI72873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaul YD, Freinkman E, Comb WC, Cantor JR, Tam WL, Thiru P, Kim D, Kanarek N, Pacold ME, Chen WW, et al. Dihydropyrimidine accumulation is required for the epithelial-mesenchymal transition. Cell. 2014;158:1094–1109. doi: 10.1016/j.cell.2014.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z, Mirza M, Wang B, Kennedy MA, Weber GF. Osteopontin-a alters glucose homeostasis in anchorage-independent breast cancer cells. Cancer Lett. 2014;344:47–53. doi: 10.1016/j.canlet.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim H, Dolde C, Lewis BC, Wu CS, Dang G, Jungmann RA, Dalla-Favera R, Dang CV. c-Myc transactivation of LDH-A: implications for tumor metabolism and growth. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:6658–6663. doi: 10.1073/pnas.94.13.6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiozawa Y, Eber MR, Berry JE, Taichman RS. Bone marrow as a metastatic niche for disseminated tumor cells from solid tumors. Bonekey Reports. 2015;4 doi: 10.1038/bonekey.2015.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shroff EH, Eberlin LS, Dang VM, Gouw AM, Gabay M, Adam SJ, Bellovin DI, Tran PT, Philbrick WM, Garcia-Ocana A, et al. MYC oncogene overexpression drives renal cell carcinoma in a mouse model through glutamine metabolism. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:6539–6544. doi: 10.1073/pnas.1507228112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song K, Kwon H, Han C, Zhang J, Dash S, Lim K, Wu T. Active glycolytic metabolism in CD133(+) hepatocellular cancer stem cells: regulation by MIR-122. Oncotarget. 2015;6:40822–40835. doi: 10.18632/oncotarget.5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jager S, Handschin C, Zheng K, Lin J, Yang W, et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- Stresing V, Baltziskueta E, Rubio N, Blanco J, Arriba MC, Valls J, Janier M, Clezardin P, Sanz-Pamplona R, Nieva C, et al. Peroxiredoxin 2 specifically regulates the oxidative and metabolic stress response of human metastatic breast cancer cells in lungs. Oncogene. 2013;32:724–735. doi: 10.1038/onc.2012.93. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Nakayamada S, Okada Y. Osteoblasts and osteoclasts in bone remodeling and inflammation. Curr Drug Targets Inflamm Allergy. 2005;4:325–328. doi: 10.2174/1568010054022015. [DOI] [PubMed] [Google Scholar]
- Thompson HJ, Jiang W, Zhu Z. Candidate mechanisms accounting for effects of physical activity on breast carcinogenesis. IUBMB Life. 2009;61:895–901. doi: 10.1002/iub.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valavanidis A, Vlachogianni T, Fiotakis K, Loridas S. Pulmonary oxidative stress, inflammation and cancer: respirable particulate matter, fibrous dusts and ozone as major causes of lung carcinogenesis through reactive oxygen species mechanisms. Int J Environ Res Public Health. 2013;10:3886–3907. doi: 10.3390/ijerph10093886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel AP, Jing J, Wooster RF, Bachman KE. Analysis of glutamine dependency in non-small cell lung cancer: GLS1 splice variant GAC is essential for cancer cell growth. Cancer Biol Ther. 2012;13:1185–1194. doi: 10.4161/cbt.21348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward PS, Thompson CB. Metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer Cell. 2012;21:297–308. doi: 10.1016/j.ccr.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellen KE, Thompson CB. A two-way street: reciprocal regulation of metabolism and signalling. Nat Rev Mol Cell Biol. 2012;13:270–276. doi: 10.1038/nrm3305. [DOI] [PubMed] [Google Scholar]
- Ye X, Weinberg RA. Epithelial-Mesenchymal Plasticity: A Central Regulator of Cancer Progression. Trends Cell Biol. 2015;25:675–686. doi: 10.1016/j.tcb.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin C, He D, Chen S, Tan X, Sang N. Exogenous pyruvate facilitates cancer cell adaptation to hypoxia by serving as an oxygen surrogate. Oncotarget. 2016;7:47494–47510. doi: 10.18632/oncotarget.10202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Cao J, Ma S, Dong R, Meng W, Ying M, Weng Q, Chen Z, Ma J, Fang Q, et al. Tumor hypoxia enhances Non-Small Cell Lung Cancer metastasis by selectively promoting macrophage M2 polarization through the activation of ERK signaling. Oncotarget. 2014a;5:9664–9677. doi: 10.18632/oncotarget.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Hulver MW, McMillan RP, Cline MA, Gilbert ER. The pivotal role of pyruvate dehydrogenase kinases in metabolic flexibility. Nutr Metab (Lond) 2014b;11:10. doi: 10.1186/1743-7075-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu A, Lee D, Shim H. Metabolic positron emission tomography imaging in cancer detection and therapy response. Semin Oncol. 2011;38:55–69. doi: 10.1053/j.seminoncol.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]