Abstract
Deep brain stimulation of the subthalamic nucleus (STN DBS) significantly improves clinical motor symptoms, as well as intensive aspects of movement like velocity and amplitude in patients with Parkinson’s disease (PD). However, the effects of bilateral STN DBS on integrative and coordinative aspects of motor control are equivocal. The aim of this study was to investigate the effects of bilateral STN DBS on integrative and coordinative aspects of movement using a memory-guided sequential reaching task. The primary outcomes were eye and finger velocity and endpoint error. We expected that bilateral STN DBS would increase reaching velocity. More importantly, we hypothesized that bilateral STN DBS would increase eye and finger end-point error and this would not simply be the result of a speed accuracy trade-off. Ten patients with PD and bilaterally-implanted subthalamic stimulators performed a memory-guided sequential reaching task under 4 stimulator conditions (DBS-OFF, DBS-LEFT, DBS-RIGHT, and DBS-BILATERAL) over 4 days. DBS-BILATERAL significantly increased eye velocity compared to DBS-OFF, DBS-LEFT, and DBS-RIGHT. It also increased finger velocity compared to DBS-OFF and DBS-RIGHT. DBS-BILATERAL did not change eye endpoint error. The novel finding was that DBS-BILATERAL increased finger end-point error compared to DBS-OFF, DBS-LEFT, and DBS-RIGHT even after adjusting for differences in velocity. We conclude that bilateral STN DBS may facilitate basal ganglia-cortical networks that underlie intensive aspects of movement like velocity, but it may disrupt selective basal ganglia-cortical networks that underlie certain integrative and coordinative aspects of movement such as spatial accuracy.
Keywords: STN DBS, Parkinson disease, sequential reach, memory guided, subthalamic nucleus, deep brain stimulation
INTRODUCTION
Deep brain stimulation of the subthalamic nucleus (STN DBS) is highly efficacious at improving intensive aspects but not coordinative aspects of movement in patients with Parkinson’s disease (PD) (Hening 2009). Intensive aspects of movement are less complex than coordinative aspects in the sense that they possess one major dimension of intensity (Hening 2009; Mosier et al. 2011; Schettino et al. 2006; Snider et al. 2014). An example of an intensive aspect of movement is movement velocity. In contrast, STN DBS can impair performance or have no effect on coordinative aspects of movement. Coordinative aspects of movement are more complex in the sense that they require more than one dimension or process (Mosier et al. 2011; Schettino et al. 2006; Snider et al. 2014). In the context of a memory-guided sequential reach, coordinative aspects of movement begin with encoding and retaining locations and movement sequences using both visual and proprioceptive information. Next, the reach is planned by integrating sensory information and applying the required sensorimotor transformations. Finally, the movement is executed while continuing to rely on visual and/or proprioceptive information during feedforward and feedback control processes of execution.
The dorsolateral prefrontal cortex has been shown to be active during coordinative tasks that involve spatial working memory (Barbey et al. 2013; Owen et al. 2005), and basal ganglia-prefrontal and basal ganglia-fronto-parietal networks have been shown to be active during the initiation phase of motor sequences (Boecker et al. 2008). Interestingly, it has been shown that bilateral, but not unilateral, STN DBS can impair coordinative aspects of movement, for instance performance on a cognitive-motor dual-task (Alberts et al. 2008), and response inhibition on the antisaccade task (Goelz et al. 2017). Importantly, the cognitive task (n-back) implemented in the Alberts’ study and the antisaccade task implemented in the Goelz’ study both rely heavily upon prefrontal cortical areas (Alberts et al. 2008; Goelz et al. 2017). The antisaccade task employed in the Goelz et al. (2017) study investigated response inhibition that is more likely to be coordinative because in addition to reactively stopping a reflexive saccade, a subsequent saccade equal in amplitude but opposite to the direction of the target has to be executed. This has to be differentiated from only reactive stopping which is more likely to be intensive because the amplitude/gain is scaled to zero in response to an external cue and no subsequent movement is required. Several studies have in fact shown that inhibition is improved by bilateral stimulation (Mirabella et al. 2013; Mirabella et al. 2012; Swann et al. 2011; van den Wildenberg et al. 2006). It is possible that bilateral STN DBS favors certain processes involving prefrontal-basal-ganglia circuits underlying intensive control while disrupting coordinative control during cognitively-challenging motor tasks (Ballanger et al. 2009).
In order to further probe the effects of unilateral and bilateral STN DBS on intensive and coordinative aspects of movement, we implemented a memory-guided sequential reach task that relies heavily on prefrontal and fronto-parietal processes. Participants in the current study were required to look and reach to a sequence of memorized targets. With respect to intensive aspects, we hypothesized that bilateral and unilateral STN DBS would improve eye and limb movement velocity compared to no stimulation. With respect to coordinative aspects, we hypothesized that bilateral STN DBS, but not unilateral, would disrupt prefrontal and fronto-parietal processes, and that this would be evident as an increase in both eye and finger end-point error compared to no stimulation.
METHODS
Subjects
This study was conducted with approval from the Rush University Medical Center and University of Illinois Institutional Review Boards, and informed consent from all subjects. Data was collected from 10 patients (7 males; mean age 60.4 years SD± 8.34) with advanced PD and bilaterally implanted electrodes in the STN. Patients were recruited from the Rush University Medical Center Movement Disorders Clinic. All patients were examined by a movement disorders neurologist and included in the study if they: met the UK PD Society brain bank clinical diagnostic criteria for PD (Hughes et al. 1992), had successful response to STN DBS surgery, a score of 26 or greater on the Montreal Cognitive Assessment (MOCA) to rule out mild cognitive impairment (Nasreddine et al. 2005), and presented with no eye movement abnormalities and other known neurological disorders. In addition, electrode placement in the sensorimotor area of the STN was confirmed by microelectrode recording from multiple kinesthetic cells. All subjects were right-handed and used their right hand to complete the sequential reach task. The Edinburgh Handedness Inventory was used to confirm hand dominance (Oldfield 1971). Table 1 provides patient demographics including the Hoehn and Yahr scale (Hoehn and Yahr 1967), Levodopa equivalent dose (Tomlinson et al. 2010), stimulator parameters, and clinical rating scores.
Table 1.
Patient demographics, MDS-UPDRS scores, and stimulator settings
| A g e ( y r s ) |
Educ ation (yrs) |
S e x |
Disease duratio n (yrs) |
LE D (m g/ da y) |
Surgery duratio n (yrs) |
H o e h n & Y a hr R at in g (D B S- O FF ) |
MDS UPDRS motor score |
Left stimulator settings |
Right stimulator settings |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||||||||
| DB S- OF F |
DB S- LE FT |
DB S- RIG HT |
D B S - B L |
V o lt |
F r e q |
P ul s e w id t h |
Con tact lead s |
V o lt |
F r e q |
Puls e wid th |
Conta ct leads |
||||||||||
| + | − | + | − | ||||||||||||||||||
| 72 | 10 | M | 10 | 400 | 6 | 3 | 76 | 46 | 57 | 28 | 5 | 185 | 120 | 3 | 0,1 | 3.5 | 185 | 120 | 3 | 2,0 | |
| 51 | 19 | M | 16 | 300 | 7 | 3 | 71 | 49 | 60 | 46 | 3 | 185 | 60 | 3 | 0,1 | 2.6 | 185 | 60 | 3,c | 2 | |
| 64 | 14 | M | 10 | 1512.5 | 0.5 | 2 | 40 | 37 | 42 | 32 | 1.5 | 130 | 60 | 0,1 | 1 | 1.3 | 130 | 60 | 8 | 9 | |
| 52 | 13 | M | 3 | 150 | 0.75 | 2 | 51 | 35 | 47 | 17 | 4.4 | 240 | 150 | 2 | 0 | 4.3 | 240 | 120 | 11 | 8 | |
| 49 | 16 | F | 15 | 100 | 2 | 3 | 84 | 60 | 45 | 32 | 3.3 | 160 | 90 | 3 | 2 | 4.5 | 160 | 240 | 9 | 10,11 | |
| 67 | 13 | M | 25 | 400 | 8 | 2 | 63 | 46 | 50 | 42 | 2.4 | 185 | 90 | c | 2 | 2.9 | 185 | 90 | c | 5 | |
| 54 | 13 | F | 18 | 375 | 12 | 3 | 81 | 60 | 60 | 36 | 2.5 | 130 | 60 | c | 0 | 2.3 | 180 | 60 | 2 | 0 | |
| 60 | 12 | M | 8 | 400 | 1 | 3 | 19 | 21 | 24 | 11 | 2.3 | 130 | 60 | c | 2,3 | 2.3 | 130 | 60 | c | 9 | |
| 66 | 18 | M | 11 | 920 | 0.5 | 3 | 53 | 40 | 47 | 28 | 33 | 103 | 60 | 2 | 1 | 3 | 130 | 60 | C | 10 | |
| 69 | 18 | F | 25 | 175 | 10 | 3 | 57 | 39 | 49 | 26 | 3.5 | 185 | 90 | 3 | 1 | 3.5 | 185 | 90 | 3 | 1 | |
yrs, years; M, male; F, female; LED, Levodopa equivalent dose; mg, milligrams; DBS, Deep Brain Stimulation; BL, Bilateral; MDS UPDRS, Movement Disorders Society Unified Parkinson’s Disease Rating Scale; freq, frequency; +, positive contact; −, negative contact, c, case
Experimental conditions
Data collection took place over five days. On the first day of testing, patients arrived while on treatment and were consented and acclimatized to the laboratory and experimental tasks. Patients performed the experimental task in four different stimulation conditions over the next four days (one stimulation condition per day): both stimulators off (DBS-OFF), right unilateral on (DBS-RIGHT), left unilateral on (DBS-LEFT) and both on (DBS-BILATERAL). Stimulators were turned off three hours prior to testing, which provided sufficient time for the patient’s condition to stabilize (Temperli et al. 2003). Patients refrained from PD medications 12 hours prior to testing. The order of testing conditions was randomized for the first five patients and this order was reversed for the last five patients. Figure 1 illustrates the participant schedule and the experimental tasks used.
Fig 1.
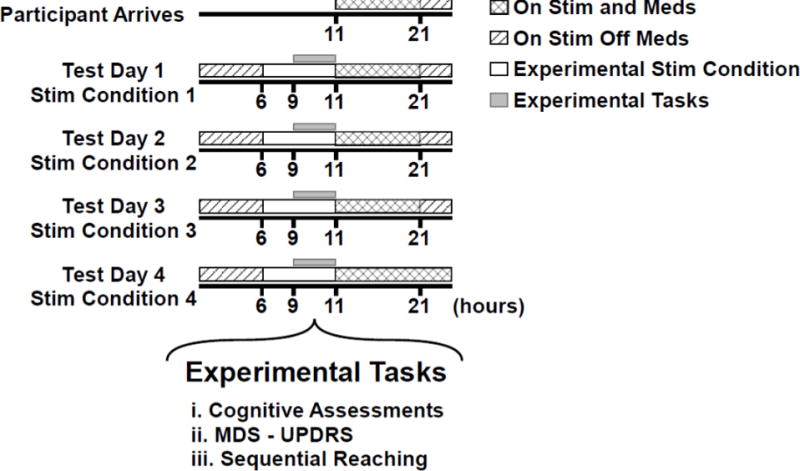
Participant’s schedule from the day of arrival through the 4 days of experimental testing is shown. It shows overnight withdrawal from anti-Parkinsonian medication. Every morning at 6 am, the stimulation settings for that day were set. Each session occurred between 9 am and 11 am. Medication and stimulation were resumed as prescribed after the testing session.
Cognitive assessments
The cognitive assessments administered were used to determine if there were differential effects of STN DBS on cognition, as well as to determine if the outcomes of the sequential reaching task correlated with known measures of spatial working memory. The cognitive assessments administered were the Wechsler Memory Scale-4 (WMS-IV) Flexible Approach, the Stockings of Cambridge (SOC) test, and the Spatial Span (SSP) test. The WMS-IV Flexible Approach is a version of the WMS-IV with a shorter administration time and reduced motor demands (Wechsler 2010). The WMS-IV Flexible Approach consisted of the Logical Memory test and the Design test. The Logical Memory (LM) test assessed subjects’ narrative memory, while the Design (DE) test assessed content memory and spatial memory for unfamiliar visual material. The Logical Memory and Design Content and Spatial test consisted of an immediate recall, a delayed recall, and a recognition trial. The immediate and delayed recall raw scores from each of the tests were used to calculate the respective scaled scores for the Logical Memory (LM I and II) and Design Content (DE Content I and II), and Design Spatial (DE Spatial I and II) tests. LM I and II, Designs Content I and II, and Designs Spatial I and II were used as the primary outcomes for the WMS-IV Flexible Approach test.
The SOC is a spatial planning task (CANTABeclipse® [Cognitive assessment software] Cambridge Cognition 2012). On a touch screen monitor, the participant was shown two displays containing three colored balls. The display on the top row was the target display and the display on the bottom row was the display that the participant could move and manipulate. The participant was required to arrange the balls in the bottom display by moving them one at a time such that it was identical to the target display. The primary outcomes used were minimum number of moves required to complete the task and the mean number of moves for a 4-move problem.
The SSP is a test that assesses visuospatial working memory capacity (CANTABeclipse® [Cognitive assessment software] Cambridge Cognition 2012). On a touch screen monitor, the participant was shown randomly placed white squares. Some of these squares briefly changed color in a random sequence. After a tone that indicated the completion of trial presentation, the participant was required to touch the squares that changed color in the order that they were presented. The number of boxes that changed color progressed from 2 to 9. The primary outcomes used were spatial span length and the total number of errors.
Instrumentation for the sequential reaching task
The sequential reach task was conducted in a completely darkened room, seated upright on an adjustable chair, and with the chin on a chin rest to minimize head movement. Consequently, vestibular contributions to proprioception were minimized. Head and finger movements were captured with a 3-D motion capture system (Northern Digital). Eye movements were captured at 500 Hz with a head-mounted video-based eye-tracking system (SR Research). An active infrared emitting diode was taped to the patient’s index finger to track finger movements (Northern Digital). Figure 2 shows the sequential reaching task.
Fig 2.
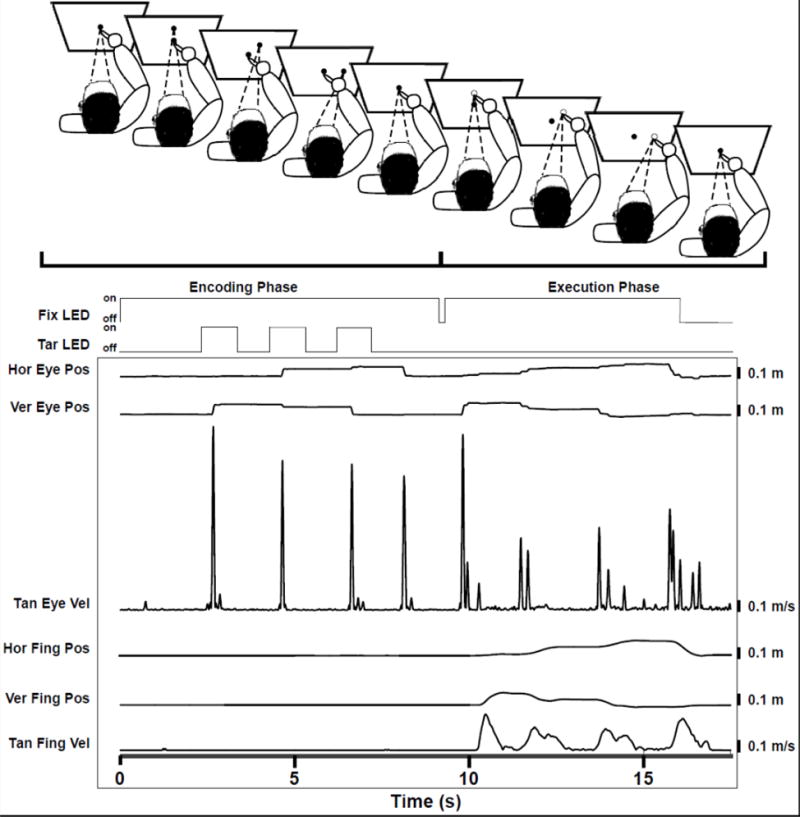
Shows the sequential reaching task divided into the encoding and executing phase. The subject starts the encoding phase with the eyes fixated on the fixation LED (solid central circle) and the finger immediately below the fixation LED. The subject then moves his/her eyes to the target LED (solid peripheral circle) as it is presented thereby visually encoding target location and sequence. This is followed by the execution phase where the subject looks and points to the remembered targets (unfilled circles) and then returns to the central fixation LED. Below that, the timing of the fixation LED (Fix LED)and Target LED (Tar LED) onsets are shown with an example horizontal and vertical eye position (Hor Eye Pos, Ver Eye Pos), tangential eye velocity (Tan Eye Vel), horizontal and vertical finger position (Hor Fing Pos, Ver Fing Pos), and tangential finger velocity (Tan Fing Vel).
Three targets were presented to the subjects using a five-degree of freedom (DOF) robot arm (Thermo CRS). An initial fixation light (3mm green LED, 70 mcd) was situated 42 cm away from the subject. This served as the starting point for the subject’s pointing motion. The robot presented targets (3mm green LED, 70 mcd) in a plane that was 42 cm from the chin rest. Figure 2 illustrates the eye and finger traces.
Head, eye, finger and robot movements were synchronized and stored using the Motion Monitor system (Innovative Sports Training 2010).
Protocol
Each testing session began with the administration of the WMS-IV Flexible Approach, the SOC, the SSP, and The Movement Disorders Society – Unified Parkinson’s disease Rating Scale, motor subscale (Goetz et al. 2008), followed by the sequential task.
Subjects performed the sequential reach task in complete darkness to eliminate visual cues or other visual stimuli. The sequential reach task began with subjects fixating on the central fixation light (0° visual angle) with their finger on the fixation stand. This was done to ensure that the finger and the eye focal point were at the same location. With the central fixation still lit, the robotic arm flashed three sequential targets in the subjects’ visual field. The duration of target presentation and the duration between each target presentation was 1000 ms. Targets were located on a circle with a 10 cm radius. The horizontal target was located at 0°, the diagonal target was located at 45°, and the vertical target was located at 90°. During the encoding phase, subjects were asked to look at each target as it was presented, and to remember their location and the order in which they appeared. After a 2000 ms delay, the central light fixation was extinguished for 100 ms and then lit again. This served as the cue for subjects to initiate pointing to the remembered sequential targets. The central fixation light stayed on for 5000 ms as the subjects executed the pointing movement. When the pointing movement was completed, subjects returned their finger to the central fixation stand. Targets were presented in random order. Subjects performed one block of 30 trials for each of the stimulation conditions. The first 5 trials were practice trials and were discarded.
Analysis
The data were analyzed using a custom Matlab script (The MathWorks 2014). A 20 Hz low-pass 2nd order, zero-phase Butterworth filter was applied to the eye and finger position signals. The filtered position data was then differentiated to calculate velocity.
The following procedure was used to determine eye error. First, saccade fixation end-points to the three targets were visually marked. From each of the three visually determined saccade end-points, an algorithm searched backwards to determine the first peak in the saccade velocity profile. This peak was associated with the saccade that brought the gaze to the target location. Thus, time points corresponding to these three peaks were established. Next, for each of these peaks, the algorithm searched forwards to detect the first time point when saccade velocity went below 5% of peak velocity and stayed below this threshold for 200ms. The first time point when this condition was met was designated as the point in time when the subjects’ eyes fixated on the targets and was defined as the eye end-point. Next, for the first peak, the algorithm searched backwards to detect the first time point when saccade velocity went below 5% of peak velocity and stayed below this threshold for 200ms. The first time point when this condition was met was designated as the onset of the first saccade. The difference between this point in time and the cue to initiate the movement was defined as the eye latency. The same process was used to determine finger end-points and finger latency. Once the locations of eye and finger end-points were determined, error was calculated by subtracting the values of these locations from the values of the corresponding target locations. Error was calculated only in the vertical and horizontal dimensions for eye movements because our analysis was confined to eye movements in the target plane. Error was calculated in all three dimensions for the finger end-points.
The magnitude of eye error was calculated using the following equation:
The magnitude of finger end-point error was calculated using the following equation:
Statistical Analysis
MDS UPDRS III
A mixed-effect regression model was used to determine differences in the MDS-UPDRS III as a function of stimulation condition. The fixed effect was stimulation condition (DBS-OFF, DBS-LEFT, DBS-RIGHT, and DBS-BILATERAL). The random effect was subject. If the omnibus F-test was significant for condition, then post-hoc pairwise comparisons were performed using t-tests.
Cognitive assessments
Preliminary analyses revealed that few of the cognitive outcomes met the distributional assumptions for parametric testing. In order to be consistent across all cognitive outcomes, Friedman’s test was performed as the omnibus test to address the effect of stimulation condition on all cognitive outcomes. If the omnibus test was significant for condition, the Wilcoxon Signed Rank test was used to conduct post-hoc pairwise comparisons.
Sequential reach
Each of the sequential reach outcomes were subject to a mixed-effect regression model. The fixed effects were stimulation condition (DBS-OFF, DBS-LEFT, DBS-RIGHT, and DBS-BILATERAL). The random effect was subject. In addition, for the eye and finger end-point error outcomes, peak gaze velocity and peak finger velocity were used as time varying covariates in our respective mixed-effect regression models. This was done because first, there is a well-known speed accuracy trade-off, i.e., faster movements are likely to be associated with greater errors. Second, it is also established that STN DBS is very effective at improving movement speed. Therefore, we wanted to control for any differences in velocity as a consequence of stimulation condition that might contribute to increase in error. If the omnibus F-test was significant for condition, then post-hoc pairwise comparisons were performed using t-tests.
All statistical analyses were performed using SAS™ (version 9.4; SAS Institute, Cary, NC). All statistical tests were two-sided, critical alpha was 0.05, and p values associated with all pairwise comparisons were corrected using the Bonferroni method.
RESULTS
Substantial variability in disease duration and motor impairment was observed as shown in Table 1. The age was 60.4±8.3 years (mean ± SD), disease duration (14.1±7.2 years), Levodopa equivalent dose (473±430 mg/day), and MDS-UPDRS III (DBS-BL-ON, 29.8±10.5).
Clinical measures: MDS-UPDRS III
Compared to DBS-OFF, DBS-LEFT (estimated mean difference, −16.2; p < 0.001), DBS-RIGHT (−11.4; p = 0.009), and DBS-BILATERAL (−29.7; p < 0.001) significantly reduced the MDS-UPDRS III. DBS-BILATERAL reduced the MDS-UPDRS III to a greater extent than DBS-LEFT (13.5; p = 0.002) and DBS-RIGHT (18.3; p < 0.001). The significant reduction in the MDS-UPDRS III is consistent with electrode placement within the STN.
Cognitive Assessments
The effect of stimulation on cognitive outcomes
There were no differences between stimulation conditions on any of the WMS-IV Flexible Approach, the SOC, and the SSP measures (table 2).
Table 2.
Cognitive outcomes descriptive and inferential statistics
| Cognitive test | Condition | Mean ± SD | Median (IQR) | Omnibus Test |
|---|---|---|---|---|
| WMS-IV: Logical Memory I scaled score | DBS-OFF | 14.6 ± 2.41 | 14.5 (13, 16) | χ2 = 0.6 p = 0.89 |
| DBS-LEFT | 14.6 ± 2.63 | 14.5 (13, 17) | ||
| DBS-RIGHT | 14.8 ± 1.99 | 15 (14, 16) | ||
| DBS-BILATERAL | 15.2 ± 2.49 | 16 (14, 17) | ||
| WMS-IV: Logical Memory II scaled score | DBS-OFF | 15.9 ± 2.56 | 16 (15, 18) | χ2 = 1.8 p = 0.62 |
| DBS-LEFT | 15.7 ± 2.21 | 15.5 (15, 18) | ||
| DBS-RIGHT | 16.4 ± 1.96 | 16.5 (16, 18) | ||
| DBS-BILATERAL | 15.8 ± 3.36 | 17 (15, 18) | ||
| WMS-IV: Design Content I scaled score | DBS-OFF | 13.7 ± 3.27 | 14.5 (11, 16) | χ2 = 2.03 p = 0.57 |
| DBS-LEFT | 14.7 ± 3.86 | 15.5 (11, 18) | ||
| DBS-RIGHT | 13.8 ± 4.16 | 15 (9, 17) | ||
| DBS-BILATERAL | 14 ± 4.16 | 15 (13, 16) | ||
| WMS-IV: Design Content I Iscaled score | DBS-OFF | 14.1 ± 3.25 | 14.5 (13, 16) | χ2 = 0.95 p = 0.81 |
| DBS-LEFT | 14.5 ± 5.08 | 17 (9, 19) | ||
| DBS-RIGHT | 14.2 ± 4.08 | 12.5 (11, 19) | ||
| DBS-BILATERAL | 14.6 ± 4.03 | 13.5 (11, 19) | ||
| WMS -IV: Design Spatial I scaled score | DBS-OFF | 12.1 ±3.45 | 12 (10, 15) | χ2 = 2.8 p = 0.42 |
| DBS-LEFT | 12.8 ± 1.55 | 13 (12, 14) | ||
| DBS-RIGHT | 12.1 ± 3.54 | 11.5 (9, 16) | ||
| DBS-BILATERAL | 12.8 ± 2.7 | 13 (11, 14) | ||
| WMS-IV: Design Spatial I Iscaled score | DBS-OFF | 13.2 ± 3.29 | 13 (11, 15) | χ2 = 2.6 p = 0.45 |
| DBS-LEFT | 13.9 ±2.42 | 13 (13, 15) | ||
| DBS-RIGHT | 14.3 ± 2.79 | 15 (12, 16) | ||
| DBS-BILATERAL | 15.1 ± 3.38 | 15.5 (13, 18) | ||
| Stockings of Cambridge: Problems solved in minimum moves | DBS-OFF | 9.9 ± 2.28 | 11 (9, 11) | χ2 = 1.6 p = 0.67 |
| DBS-LEFT | 9.4 ± 2.59 | 10 (7, 12) | ||
| DBS-RIGHT | 10.4 ± 0.7 | 10.5 (10, 11) | ||
| DBS-BILATERAL | 10.3 ± 1.95 | 11 (10, 12) | ||
| Stockings of Cambridge: Mean moves for a 4 move problem | DBS-OFF | 4.8 ± 1.09 | 4 (4, 5) | χ2 = 1.6 p = 0.67 |
| DBS-LEFT | 4.9 ± 0.94 | 4.8 (4, 6) | ||
| DBS-RIGHT | 4.4 ± 0.59 | 4 (4, 5) | ||
| DBS-BILATERAL | 4.8 ±0.9 | 4.9 (4, 5) | ||
| Spatial Span: Span length | DBS -OFF | 5.8 ± 1.4 | 5.5 (5, 7) | χ2 = 5.4 p = 0.15 |
| DBS-LEFT | 6 ± 1.33 | 6 (5, 7) | ||
| DBS-RIGHT | 6.3 ± 1.49 | 6.5 (5, 8) | ||
| DBS-BILATERAL | 5.7 ± 1.42 | 5.5 (5, 6) | ||
| Spatial Span: Total Errors | DBS-OFF | 13.4 ± 6.62 | 13.5 (6, 19) | χ2 = 5.2 p = 0.16 |
| DBS-LEFT | 13.5 ± 4.38 | 13 (10, 18) | ||
| DBS-RIGHT | 13.4 ± 5.02 | 14 (11, 16) | ||
| DBS-BILATERAL | 11.1 ± 4.82 | 10 (8, 13) |
SD, standard deviation; IQR, inter quartile range; WMS, Wechsler Memory Scale;
Sequential Reach
Eye and finger latency
DBS-RIGHT significantly increased eye latency compared to DBS-OFF (0.05s; p = 0.01, figure 3a). No other differences were observed.
Fig 3.
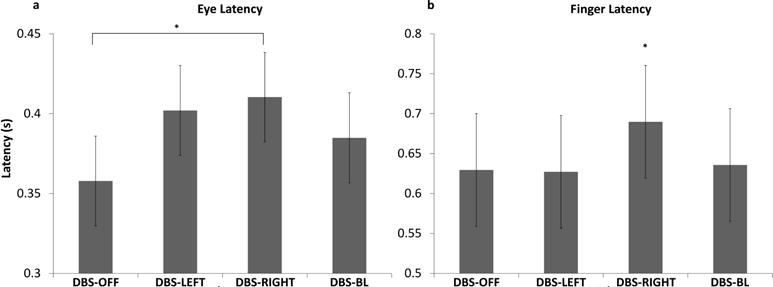
These plots show the estimated mean (± standard error) for eye latency (a) and finger latency (b) for all stimulation conditions. * indicate significant differences between stimulation conditions
DBS-RIGHT significantly increased finger latency compared to DBS-OFF (0.06s; p = 0.01), DBS-LEFT (0.06s; p = 0.01), and DBS-BILATERAL (0.05s; p = 0.04) (see figure 3b). No other differences were observed.
Eye and finger velocity
Figure 4a shows eye velocity for the DBS-OFF, DBS-LEFT, DBS-RIGHT, and DBS-BILATERAL conditions during the execution of the sequential reach. DBS-LEFT (0.09 m/s; p < 0.001), DBS-RIGHT (0.07 m/s; p = 0.009), and DBS-BILATERAL (0.23 m/s; p < 0.001) significantly increased eye velocity relative to DBS-OFF. Relative to DBS-BILATERAL, eye velocity was significantly slower during DBS-LEFT (−0.14 m/s; p <0.001) and DBS-RIGHT (−0.16 m/s; p < 0.001).
Fig 4.
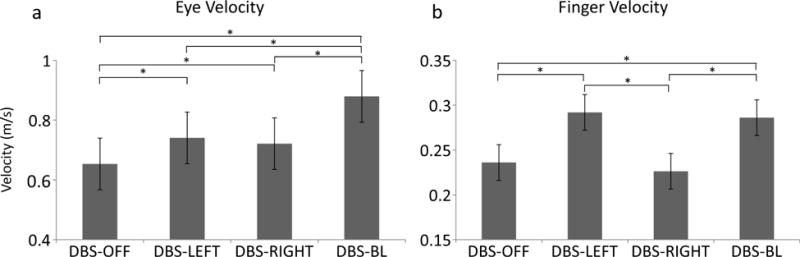
These plots show the estimated mean (± standard error) for eye velocity (a) and finger velocity (b) for all stimulation conditions. * indicate significant differences between stimulation conditions
Peak finger velocity was significantly faster during DBS-LEFT (0.06 m/s; p < 0.001) and DBS-BILATERAL (0.05 m/s; p < 0.001) relative to DBS-OFF (figure 4b). There were no differences in finger velocity between DBS-RIGHT and DBS-OFF (0.01 m/s; p = 0.08; only the right hand was tested). Compared to DBS-BILATERAL, finger velocity was similar during DBS-LEFT (0.01 m/s; p = 0.86) and significantly slower during DBS-RIGHT (−0.06 m/s; p < 0.001).
Eye and finger end-point error covarying (adjusting) for velocity
There were no differences in eye error adjusting for differences in velocity during execution between stimulation conditions (table 3, figure 5a).
Table 3.
Statistical tests comparing the effect of stimulation on eye and finger velocity and error during the execution phase of the sequential reaching task
| DBS Condition | Latency Estimated Mean ± SE (s) | Velocity Estimated Mean ± SE (m/s) | Error1 Estimated Mean ± SE (m) | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Eye | Finger | Eye | Finger | Eye | Finger | |
| DBS-OFF | 0.358 ± 0.03 | 0.630 ± 0.07 | 0.659 ± 0.08 | 0.237 ± 0.02 | 0.036 ± 0.002 | 0.043 ± 0.004 |
| DBS-LEFT | 0.402 ± 0.03 | 0.627 ± 0.07 | 0.746 ± 0.08 | 0.29 3 ± 0.02 | 0.038 ± 0.002 | 0.046 ± 0.004 |
| DBS-RIGHT | 0.41 0 ± 0.03 | 0.69 0 ± 0.07 | 0.727 ± 0.08 | 0.227 ± 0.02 | 0.038 ± 0.002 | 0.04 5 ± 0.004 |
| DBS-BL | 0.385 ± 0.03 | 0.636 ± 0.07 | 0.885 ± 0.08 | 0.287 ± 0.02 | 0.037 ± 0.002 | 0.049 ± 0.004 |
| Omnibus Test | F3,879 = 3.87 p = 0.009* |
F3,885 = 4.57 p = 0.004* |
F3,2663 = 37.2 | F3,2681 = 141.72 p < 0.001* p < 0.001* |
F3,2662 = 1.01 p = 0.389 |
F3,2680 = 12.07 p < 0.001* |
| Pairwise comparisons2 | ||||||
| Off - Left | Diff = −0.04 t = −2.62 p = 0.054 |
Diff = 0 .002 t = 0.12 p = 1 |
Diff = −0.09 t = −4.03 p < 0.001* |
Diff = −0.06 t = −13.87 p < 0.001* |
Diff = −0 .001 t = −1.04 p = 1 |
Diff = −0 .003 t = −3.04 p = 0.014* |
| Off - Right | Diff = −0.05 t = −3.17 p = 0.009* |
Diff = −0.06 t = −3.06 p = 0.014* |
Diff = −0.07 t = −3.19 p = 0.009* |
Diff = 0.01 t = 2.47 p = 0.08 |
Diff = −0 .002 t = −1.66 p = 0.58 |
Diff = −0 .002 t = −1.7 p = 0.53 |
| Off - BL | Diff = - 0.03 t = −1.59 p = 0.678 |
Diff = −0.01 t = −0.32 p = 1 |
Diff = −0.23 t = −10.32 p < 0.001* |
Diff = −0.05 t = −12.37 p < 0.001* |
Diff = −0 .001 t = −0.52 p = 1 |
Diff = −0.0 06 t = −5.88 p < 0.001* |
| Left – Right | Diff = −0.01 t = −0.51 p = 1 |
Diff = −0.06 t = −3.16 p = 0.01* |
Diff = 0.02 t = 0.9 p = 1 |
Diff = 0.07 t = 16.49 p < 0.001* |
Diff = −0 .001 t = −0.6 p = 1 |
Diff = 0 .001 t = 1.42 p = 0.94 |
| Left - BL | Diff = 0.02 t = 1 p = 1 |
Diff = −0.01 t = −0.43 p = 1 |
Diff = −0.14 t = −6.31 p < 0.001* |
Diff = 0.01 t = 1.47 p = 0.85 |
Diff = 0 .001 t = 0.5 p = 1 |
Diff = −0 .003 t = −2.89 p = 0.02* |
| Right - BL | Diff = 0.03 t = 1.51 p = 0.782 |
Diff = 0.05 t = 2.71 p = 0.041* |
Diff = −0.16 t = −7.27 p < 0.001* |
Diff = −0.06 t = −14.95 p < 0.001* |
Diff = 0 .001 t = 1.09 p = 1 |
Diff = −0 .004 t = −4.24 p < 0.001* |
DBS, Deep Brain Stimulation; SE, Standard Error; s, seconds; m/s, meters/second; m, meters; BL, Bilateral; Diff, Difference
estimated means are adjusted for peak velocity
p-values are adjusted for multiple comparisons using the Bonferroni method
denotes statistical significance
Fig 5.
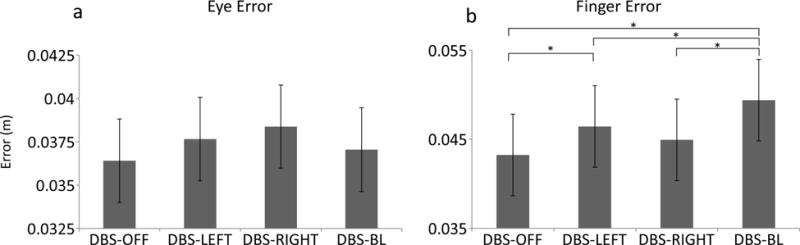
These plots show the estimated mean (± standard error) for eye error (a) and finger end-point error (b) for all stimulation conditions. * indicate significant differences between stimulation conditions.
Finger end-point error adjusting for differences in velocity was significantly greater during DBS-LEFT (0.003; p = 0.01) and DBS-BILATERAL (0.006; p < 0.001) compared to DBS-OFF (figure 5b). However, there was no difference in finger endpoint error adjusting for differences in velocity between DBS-OFF and DBS-RIGHT (0.002; p = 0.53) and no difference between DBS-RIGHT and DBS-LEFT (0.001; p=0.93). Compared to DBS-BILATERAL, finger end-point error adjusting for differences in velocity was significantly lower during DBS-LEFT (−0.003 m; p = 0.02) and DBS-RIGHT (−0.004 m; p < 0.001).
Relationship between cognitive outcomes measuring spatial memory and pointing error
In order to obtain accurate estimates of correlations we used SAS Proc Mixed and treated the variables as repeated measurements that were linked as suggested by Hamlett Et al. (2003). We did this because the variables were repeated measurements derived from the same person under 4 different stimulation conditions. The correlation between pointing error and the Designs Spatial Immediate recall was significant (r = −0.32; p = 0.03). The negative correlation observed for Designs Spatial Immediate recall indicates that improved performance on spatial memory tests was moderately associated with reduced pointing error. This correlation falls in the moderate to substantial range as defined by Cohen (1988) and implies that pointing error is associated with spatial memory.
DISCUSSION
We investigated the effects of unilateral (DBS-LEFT, DBS-RIGHT) and bilateral (DBS-BILATERAL) STN DBS on eye and limb latency, velocity, and accuracy during a memory-guided sequential reach task. STN DBS had very little effect on eye and limb latency which is consistent with previous literature showing a lack of an effect of STN DBS on the latency of visually-guided reaches to a single target (Mirabella et al. 2013; Mirabella et al. 2012). Our findings with respect to intensive aspects of movement supported our hypothesis, i.e., DBS improved eye and finger velocity. Our findings with respect to coordinative aspects of movement i.e., end-point error adjusting for differences in velocity, partially supported our hypothesis. Eye end-point was unaffected by STN DBS, while finger end-point error was adversely affected, especially during DBS-BILATERAL.
Intensive aspects of movement
In general, STN DBS improves intensive aspects of eye and limb movements relative to DBS-OFF. These findings are consistent with several previous studies (Lohnes and Earhart 2012; Vaillancourt et al. 2006; Vaillancourt et al. 2004). More specifically, we found that unilateral STN DBS increased eye velocity and that DBS-BILATERAL increased eye velocity to an even greater extent (figure 4a). Similarly, we found that DBS-LEFT increased limb velocity and that DBS-BILATERAL increased limb velocity to an even greater extent (figure 4b). These findings are also in line with previous studies showing that bilateral STN DBS improves certain motor processes to a greater extent than unilateral STN DBS (Bastian et al. 2003; Kumar et al. 1999). Finally, as expected, DBS-RIGHT had no effect on right (ipsilateral) limb velocity.
Coordinative aspects of movement
The memory-guided sequential reaching task requires the subject to first identify and encode the location and sequence of the targets, then maintain the location and sequence of targets in memory until cued to point to the memorized targets. Given that the analyses of end-point error are adjusted for differences in velocity, velocity as a contributor to error is statistically eliminated. Therefore, it follows that the increase in finger end-point error during DBS-BILATERAL may have arisen from DBS-induced alterations in target location encoding, spatial memory, and/or the planning and execution of the limb movement to the target. We will discuss each of these options in turn.
Target location and spatial memory
Relative to DBS-OFF, STN DBS did not affect eye error during execution of finger pointing (figure 5a). Patients were able to retrieve target location from memory in order to generate spatially accurate eye movements. These results suggest that STN DBS did not affect the spatial memory function involved with the task. Further, the cognitive tests that were specifically sensitive to aspects of spatial memory function showed no adverse effect of stimulation on performance. Taken together, we can conclude that STN DBS did not interfere with the spatial memory components of the memory-guided reach task.
Planning and execution of reach to targets
The planning and execution of reaching movements rely on both visual and proprioceptive information during both feedforward and feedback control processes (Medina et al. 2009; Sarlegna and Sainburg 2009). Feedforward mechanisms are responsible for the initial ballistic phase of the reach (Medina et al. 2009) and include a transformation from an extrinsic plan (i.e., trajectory of movement) guided primarily by the visual system to an intrinsic movement plan (i.e., muscle activation pattern) guided primarily by the proprioceptive system (Sarlegna and Sainburg 2009). Feedback mechanisms utilize sensory information to control the terminal stage of the reach (Medina et al. 2009). It should be noted that in the current study, only proprioceptive information was available for closed-loop control of the terminal stage of reaching because the reaching movements were performed in total darkness. The increase in finger end-point error during DBS-BILATERAL may have arisen from a disruption in feedforward and/or feedback mechanisms.
Feedforward mechanisms
With respect to the feedforward mechanisms underlying reach, previous work supports the idea that the kinematics of the reach (i.e., trajectory) are initially planned in extrinsic visual space and then transformed into an intrinsic kinetic plan that is used to send motor commands to the muscles (Sarlegna and Sainburg 2009). Whereas visual information is critical to the extrinsic planning of the reach, proprioception is critical for the transfer to an intrinsic plan that will dictate the appropriate force output patterns to achieve the kinematic goal (Sarlegna and Sainburg 2009). Furthermore, the transformation from the extrinsic kinematic plan to the intrinsic kinetic plan first requires a transfer between an eye-centered coordinate system to a limb-centered coordinate system. The increase in finger end-point error during DBS-BILATERAL may have arisen from disruptions in one or more of these feedforward processes. It is unclear whether or not DBS affects visual processes involved with extrinsic space planning and/or proprioceptive processes underlying intrinsic mechanisms of the reaching. As discussed in the following paragraph, it is also possible that the increase in finger end-point error during DBS-BILATERAL resulted from disruptions in the transfer between the eye-centered and limb-centered coordinate systems.
It has been proposed that the posterior parietal cortex (PPC) is involved with the transformation from visual target locations to hand-centered coordinates (Batista et al. 1999; Buneo et al. 2002). It is possible that DBS-BILATERAL interferes with sensorimotor transformations of this area. Although there are no known projections between the basal ganglia and PPC, previous studies have shown that DBS-BILATERAL alters the activity in the PPC (Hilker et al. 2004; Trost et al. 2006; Vafaee et al. 2004). However, there were no simultaneous neurophysiological recordings performed during the current study to determine if STN DBS alters PPC activity during a memory-guided sequential reaching task.
Alternatively, it has also been proposed that a direct sensorimotor transformation occurs in the premotor dorsal cortex (PMDc) (Graziano 2006; Pesaran et al. 2006). Neuronal activity in the PMDc encodes the spatial relationships between the target and eye, the target and hand, and the eye and hand suggesting that multiple coordinate frames are represented in the PMDc (Graziano 2006). Considering the known projection from the PMDc to the STN (Nambu et al. 1997), it is also possible that DBS-BILATERAL could be interfering with the transformation between eye- and hand-centered coordinates in the PMDc via antidromic stimulation of its projections to the STN. However, it is also known that the PMDc is involved with reactive inhibition (Mattia et al. 2013; Mattia et al. 2012; Mirabella et al. 2011), and several studies have shown an improvement in reactive inhibition during STN DBS (Mirabella et al. 2013; Mirabella et al. 2012; Swann et al. 2011; van den Wildenberg et al. 2006). If indeed STN DBS is antidromically affecting the PMDc, this differential effect on tasks could be explained by the differential oscillatory patterns associated with various tasks. For instance, changes in beta band oscillatory activity have been associated with motor preparatory processes, while changes in alpha band oscillatory patterns have been associated with reactive stopping (Pani et al. 2014). It is therefore plausible that high frequency stimulation of the STN could improve one PMDc process while impairing another.
Feedback mechanisms
In the current study, the terminal stage of reaching involves feedback mechanisms that solely depend on proprioceptive feedback because the reaching movements are performed in total darkness (Medina et al. 2009). The goal of the terminal stage is to move the finger to the memorized target location. Proprioceptive feedback about arm configuration, specifically finger position, must be compared to the target location held in memory. It is possible that STN DBS interferes with these proprioceptive processes. It should be noted that, if STN DBS was in fact interrupting proprioceptive processing, this interference would also affect feedforward processing since proprioception is also necessary for the transformation from an extrinsic kinematic plan to an intrinsic kinetic plan (Sarlegna and Sainburg 2009). Our data does not provide enough information to determine whether STN DBS interfered with proprioceptive processing in the current task, but previous work suggests that bilateral STN DBS may actually improve proprioception (Lee et al. 2013). Lee et al. (2013) investigated the effects of bilateral STN DBS on reaching to proprioceptively defined targets in PD and found that stimulation significantly reduced localization errors. In contrast to the task in the current study, the reaching task utilized did not involve transfer between coordinate systems. Whereas our subjects encoded target location with vision, then executed the reach with proprioception, the subjects in the Lee study both encoded and executed the reach with proprioception. Further, the subjects in the Lee study were on anti-parkinsonian medication which may have contributed to the results. Due to the fact that we did not measure proprioception directly, we cannot eliminate the possibility that bilateral STN DBS interfered with proprioceptive processes underlying the memory-guided sequential reach. Future work should investigate whether STN DBS has an effect on proprioception while patients are withdrawn from their anti-parkinsonian medication.
Unilateral stimulation: Compensation from the non-stimulated side
Although we found that DBS-LEFT increased finger end-point error relative to OFF, this error was no different from DBS-RIGHT and was significantly lower than the error during DBS-B ILATERAL (figure 5b). The disruption in finger end-point control manifested itself most when DBS-BILATERAL was used. As previously discussed, one possibility is that while high frequency stimulation (≥ 130 Hz) improves motor function, this high frequency could disrupt pre-frontal processes (Hershey et al. 2008). The STN is a common node in the motor and cognitive networks and STN DBS differentially affects the motor and cognitive network (Alhourani et al. 2015). While high frequency facilitates the motor network, the same high frequency could be disruptive to the cognitive network. During unilateral stimulation, pre-frontal regions in the non-stimulated side could compensate for this ‘cognitive’ disruption from the stimulated side and reduce end-point error.
CONCLUSION
The current study extends previous work that has shown that bilateral STN DBS improves intensive parameters of movement such as velocity (table 3, figure 4), but also may impair coordinative aspects of movement that affect spatial error (table 3, figure 5). Our findings, along with those previously reported (Alberts et al. 2008; Goelz et al. 2017), supports the idea that bilateral STN DBS may facilitate basal-ganglia function with respect to the control of intensive aspects of movement but may disrupt basal ganglia function with respect to coordination. This idea is quite consistent with previous suggestions that the basal ganglia are involved both in gain control (Prodoehl et al. 2009; Spraker et al. 2007) and also in coordination (Hening 2009; Mosier et al. 2011; Schettino et al. 2006; Snider et al. 2014). Future work should investigate the neural patterns underlying these differential effects induced by bilateral DBS in addition to studying the effect of STN DBS on reaching tasks designed to measure proprioception. This work will provide better insight into some of the disruptive effects of bilateral STN DBS on motor programming and execution in patients with PD.
Acknowledgments
The authors thank the participants and our professional colleagues, Maya Cottongim and Christiane Alford for their important contributions to the successful implementation of this project.
Sources of funding: This study was supported by National Institutes of Health (R56NS040902 and R01NS09295001). The sponsors were not involved in the design, conduct, collection, management, analysis, and/or interpretation of the study results and preparation, review, or approval of the manuscript. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of NIH. Statistical analysis: Conducted by FJD
DMC is a Full Professor at Northwestern University and receives a salary, has additional NIH funding (5R01NS074343, 5R01HD075777, 1R01DK110699, 5T15HD074546, and receives honoraria and/or consults for the following: University of Florida, Ohio University Athens, Temple University, Iowa State University, University of Alabama, Birmingham, Oregon Health Sciences Institute, University of Westminster, University of Waterloo, University of Colorado, Denver, Several NIH Study Sections, ACRM, ASNR, University of New Hampshire, University of Minnesota, Movement Disorders Society. LVM has foundation research support from Michael J. Fox Foundation; commercial research support from Medtronic, Inc., US WorldMeds LLC, Pfizer Inc, Boston Scientific, Avanir Pharmaceuticals, Inc., and Adamas Pharmaceuticals, Inc.; is on the scientific advisory board of St. Jude Medical, AbbVie, Inc., and Britannia Pharmaceuticals Ltd.; and consults for St. Jude Medical, AbbVie, Inc., Medtronic, Inc., and Boston Scientific.
Footnotes
Conflict of Interest: FJD, RZT, and LCG have nothing to report.
Contributor Information
Fabian J. David, Department of Physical Therapy and Human Movement Sciences, Northwestern University, Chicago, USA
Lisa C. Goelz, Department of Kinesiology and Nutrition, University of Illinois; Department of Physical Therapy and Human Movement Sciences, Northwestern University, Chicago, USA.
Ruth Z. Tangonan, Department of Kinesiology and Nutrition, University of Illinois; Department of Physical Therapy and Human Movement Sciences, Northwestern University, Chicago, USA
Leonard Verhagen Metman, Department of Neurological Sciences, Section of Parkinson Disease and Movement Disorders, Rush University Medical Center, Chicago, USA.
Daniel M. Corcos, Department of Physical Therapy and Human Movement Sciences, Northwestern University; Department of Neurological Sciences, Rush University Medical Center, Chicago, USA
References
- Alberts JL, Voelcker-Rehage C, Hallahan K, Vitek M, Bamzai R, Vitek JL. Bilateral subthalamic stimulation impairs cognitive-motor performance in Parkinson’s disease patients. Brain. 2008;131:3348–3360. doi: 10.1093/brain/awn238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhourani A, et al. Network effects of deep brain stimulation. J Neurophysiol. 2015;114:2105–2117. doi: 10.1152/jn.00275.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballanger B, et al. Stimulation of the subthalamic nucleus and impulsivity: release your horses. Ann Neurol. 2009;66:817–824. doi: 10.1002/ana.21795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbey AK, Koenigs M, Grafman J. Dorsolateral prefrontal contributions to human working memory. Cortex. 2013;49:1195–1205. doi: 10.1016/j.cortex.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastian AJ, Kelly VE, Revilla FJ, Perlmutter JS, Mink JW. Different effects of unilateral versus bilateral subthalamic nucleus stimulation on walking and reaching in Parkinson’s disease. Mov Disord. 2003;18:1000–1007. doi: 10.1002/mds.10493. [DOI] [PubMed] [Google Scholar]
- Batista AP, Buneo CA, Snyder LH, Andersen RA. Reach plans in eye-centered coordinates. Science. 1999;285:257–260. doi: 10.1126/science.285.5425.257. [DOI] [PubMed] [Google Scholar]
- Boecker H, Jankowski J, Ditter P, Scheef L. A role of the basal ganglia and midbrain nuclei for initiation of motor sequences. Neuroimage. 2008;39:1356–1369. doi: 10.1016/j.neuroimage.2007.09.069. [DOI] [PubMed] [Google Scholar]
- Buneo CA, Jarvis MR, Batista AP, Andersen RA. Direct visuomotor transformations for reaching. Nature. 2002;416:632–636. doi: 10.1038/416632a. [DOI] [PubMed] [Google Scholar]
- CANTABeclipse® [Cognitive assessment software] Cambridge Cognition. 2012 All rights reserved. www.cantab.com.
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. (2nd) 1988 [Google Scholar]
- Goelz LC, David FJ, Sweeney JA, Vaillancourt DE, Poizner H, Metman LV, Corcos DM. The effects of unilateral versus bilateral subthalamic nucleus deep brain stimulation on prosaccades and antisaccades in Parkinson’s disease. Exp Brain Res. 2017;235:615–626. doi: 10.1007/s00221-016-4830-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz CG, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23:2129–2170. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- Graziano MS. Progress in understanding spatial coordinate systems in the primate brain. Neuron. 2006;51:7–9. doi: 10.1016/j.neuron.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Hamlett ARL, Serrano-Trespalacios P, Wolfinger R. Mixed Models for Assessing Correlation in the Presence of Replication. Journal of the Air & Waste Management Association. 2003;53:442–450. doi: 10.1080/10473289.2003.10466174. [DOI] [PubMed] [Google Scholar]
- Hening W, Harrington D, Poizner H. Encyclopedia of Neuroscience Motor functions of the basal ganglia. Springer Verlag; 2009. [Google Scholar]
- Hershey T, Wu J, Weaver PM, Perantie DC, Karimi M, Tabbal SD, Perlmutter JS. Unilateral vs. bilateral STN DBS effects on working memory and motor function in Parkinson disease. Exp Neurol. 2008;210:402–408 d. doi: 10.1016/j.expneurol.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilker R, et al. Subthalamic nucleus stimulation restores glucose metabolism in associative and limbic cortices and in cerebellum: evidence from a FDG-PET study in advanced Parkinson’s disease. J Cereb Blood Flow Metab. 2004;24:7–16. doi: 10.1097/01.WCB.0000092831.44769.09. [DOI] [PubMed] [Google Scholar]
- Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innovative Sports Training. The Motion Monitor. Vol. 8. Innovative Sports Training, Inc.; Chicago, IL: 2010. 99 edn. [Google Scholar]
- Kumar R, Lozano AM, Sime E, Halket E, Lang AE. Comparative effects of unilateral and bilateral subthalamic nucleus deep brain stimulation. Neurology. 1999;53:561–566. doi: 10.1212/wnl.53.3.561. [DOI] [PubMed] [Google Scholar]
- Lee D, Henriques DY, Snider J, Song D, Poizner H. Reaching to proprioceptively defined targets in Parkinson’s disease: effects of deep brain stimulation therapy. Neuroscience. 2013;244:99–112. doi: 10.1016/j.neuroscience.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohnes CA, Earhart GM. Effect of subthalamic deep brain stimulation on turning kinematics and related saccadic eye movements in Parkinson disease. Exp Neurol. 2012;236:389–394. doi: 10.1016/j.expneurol.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattia M, Pani P, Mirabella G, Costa S, Del Giudice P, Ferraina S. Heterogeneous attractor cell assemblies for motor planning in premotor cortex. J Neurosci. 2013;33:11155–11168. doi: 10.1523/JNEUROSCI.4664-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattia M, et al. Stop-event-related potentials from intracranial electrodes reveal a key role of premotor and motor cortices in stopping ongoing movements Front. Neuroeng. 2012;5:12. doi: 10.3389/fneng.2012.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina J, Jax SA, Coslett HB. Two-component models of reaching: evidence from deafferentation in a Fitts’ law task. Neurosci Lett. 2009;451:222–226. doi: 10.1016/j.neulet.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirabella G, Iaconelli S, Modugno N, Giannini G, Lena F, Cantore G. Stimulation of subthalamic nuclei restores a near normal planning strategy in Parkinson’s patients. PLoS One. 2013;8:e62793. doi: 10.1371/journal.pone.0062793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirabella G, Iaconelli S, Romanelli P, Modugno N, Lena F, Manfredi M, Cantore G. Deep brain stimulation of subthalamic nuclei affects arm response inhibition in Parkinson’s patients Cereb. Cortex. 2012;22:1124–1132. doi: 10.1093/cercor/bhr187. [DOI] [PubMed] [Google Scholar]
- Mirabella G, Pani P, Ferraina S. Neural correlates of cognitive control of reaching movements in the dorsal premotor cortex of rhesus monkeys. J Neurophysiol. 2011;106:1454–1466. doi: 10.1152/jn.00995.2010. [DOI] [PubMed] [Google Scholar]
- Mosier K, Lau C, Wang Y, Venkadesan M, Valero-Cuevas FJ. Controlling instabilities in manipulation requires specific cortical-striatal-cerebellar networks. J Neurophysiol. 2011;105:1295–1305. doi: 10.1152/jn.00757.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambu A, Tokuno H, Inase M, Takada M. Corticosubthalamic input zones from forelimb representations of the dorsal and ventral divisions of the premotor cortex in the macaque monkey: comparison with the input zones from the primary motor cortex and the supplementary motor area. Neurosci Lett. 1997;239:13–16. doi: 10.1016/s0304-3940(97)00877-x. [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- Northern Digital Optotrak 3020 Active-Marker 3D Optical Tracking System. Northern Digital Inc.; Waterloo, Canada: [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pani P, Di Bello F, Brunamonti E, D’Andrea V, Papazachariadis O, Ferraina S. Alpha- and beta-band oscillations subserve different processes in reactive control of limb movements. Front Behav Neurosci. 2014;8:383. doi: 10.3389/fnbeh.2014.00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesaran B, Nelson MJ, Andersen RA. Dorsal premotor neurons encode the relative position of the hand, eye, and goal during reach planning. Neuron. 2006;51:125–134. doi: 10.1016/j.neuron.2006.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prodoehl J, Corcos DM, Vaillancourt DE. Basal ganglia mechanisms underlying precision grip force control. Neurosci Biobehav Rev. 2009;33:900–908. doi: 10.1016/j.neubiorev.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarlegna FR, Sainburg RL. The roles of vision and proprioception in the planning of reaching movements. Adv Exp Med Biol. 2009;629:317–335. doi: 10.1007/978-0-387-77064-2_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schettino LF, Adamovich SV, Hening W, Tunik E, Sage J, Poizner H. Hand preshaping in Parkinson’s disease: effects of visual feedback and medication state. Exp Brain Res. 2006;168:186–202. doi: 10.1007/s00221-005-0080-4. [DOI] [PubMed] [Google Scholar]
- Snider J, Lee D, Harrington DL, Poizner H. Scaling and coordination deficits during dynamic object manipulation in Parkinson’s disease. J Neurophysiol. 2014;112:300–315. doi: 10.1152/jn.00041.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spraker MB, Yu H, Corcos DM, Vaillancourt DE. Role of individual basal ganglia nuclei in force amplitude generation. J Neurophysiol. 2007;98:821–834. doi: 10.1152/jn.00239.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SR Research Eyelink II. SR Research Ltd.; Ottawa, Canada: [Google Scholar]
- Swann N, et al. Deep brain stimulation of the subthalamic nucleus alters the cortical profile of response inhibition in the beta frequency band: a scalp EEG study in Parkinson’s disease. J Neurosci. 2011;31:5721–5729. doi: 10.1523/jneurosci.6135-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temperli P, Ghika J, Villemure JG, Burkhard PR, Bogousslavsky J, Vingerhoets FJ. How do parkinsonian signs return after discontinuation of subthalamic DBS? Neurology. 2003;60:78–81. doi: 10.1212/wnl.60.1.78. [DOI] [PubMed] [Google Scholar]
- The MathWorks. Matlab, R2014b. The MathWorks, Inc; Natick, MA: 2014. edn. [Google Scholar]
- Thermo CRS Catalyst 5 Robot Arm. Thermo CRS Ltd.; Burlington, Canada: [Google Scholar]
- Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord. 2010;25:2649–2653. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- Trost M, et al. Network modulation by the subthalamic nucleus in the treatment of Parkinson’s disease. Neuroimage. 2006;31:301–307. doi: 10.1016/j.neuroimage.2005.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vafaee MS, K OS, Sunde N, Gjedde A, Dupont E, Cumming P. Focal changes of oxygen consumption in cerebral cortex of patients with Parkinson’s disease during subthalamic stimulation. Neuroimage. 2004;22:966–974. doi: 10.1016/j.neuroimage.2004.02.019. [DOI] [PubMed] [Google Scholar]
- Vaillancourt DE, Prodoehl J, Sturman MM, Bakay RA, Metman LV, Corcos DM. Effects of deep brain stimulation and medication on strength, bradykinesia, and electromyographic patterns of the ankle joint in Parkinson’s disease. Mov Disord. 2006;21:50–58. doi: 10.1002/mds.20672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaillancourt DE, Prodoehl J, Verhagen Metman L, Bakay RA, Corcos DM. Effects of deep brain stimulation and medication on bradykinesia and muscle activation in Parkinson’s disease. Brain. 2004;127:491–504. doi: 10.1093/brain/awh057. [DOI] [PubMed] [Google Scholar]
- van den Wildenberg WP, van Boxtel GJ, van der Molen MW, Bosch DA, Speelman JD, Brunia CH. Stimulation of the subthalamic region facilitates the selection and inhibition of motor responses in Parkinson’s disease. J Cogn Neurosci. 2006;18:626–636. doi: 10.1162/jocn.2006.18.4.626. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Flexible Approach Manual. San Antonio, TX: NCS Pearson Inc.; 2010. Wechsler Memory Scale—Fourth Edition (WMS–IV) [Google Scholar]


