Abstract
Amylin is a pancreatic β-cell hormone co-secreted with insulin, plays a role in normal glucose homeostasis, and forms amyloid in the pancreatic islets of individuals with type-2 diabetes. Aggregated amylin is also found in blood and extra-pancreatic tissues, including myocardium. Myocardial amylin accumulation is associated with myocyte Ca2+ dysregulation in diabetic rats expressing human amylin. Whether deposition of amylin in the heart is a consequence of or a contributor to diabetic cardiomyopathy remains unknown. We used amylin knockout (AKO) mice intravenously infused with either human amylin (i.e, the aggregated form) or non-amyloidogenic (i.e., monomeric) rodent amylin to test the hypothesis that aggregated amylin accumulates in the heart in the absence of diabetes. AKO mice infused with human, but not rodent amylin, showed amylin deposits in the myocardium. Cardiac amylin level was larger in males compared to females. Sarcolemmal Ca2+ leak and Ca2+ transients were increased in myocytes isolated from males infused with human amylin while no significant changes occurred in either females injected with human amylin or in rat amylin-infused mice. In isolated cardiac myocytes, the amylin receptor antagonist AC-187 did not effectively block the interaction of amylin with the sarcolemma. In conclusion, circulating aggregated amylin accumulates preferentially in male vs. female hearts and its effects on myocyte Ca2+ cycling do not require diabetic remodeling of the myocardium.
Keywords: Diabetic Cardiomyopathy, Amylin, Calcium, Type-2 Diabetes, Prediabetes, Hyperamylinemia
1. Introduction
Type-2 diabetes and insulin resistance are metabolic abnormalities that drive heart failure via multiple mechanisms [1–5]. To compensate for insulin resistance, pancreatic β-cells increase insulin secretion (hyperinsulinemia) [6]. Amylin is a ~4 kDa peptide hormone synthesized and co-secreted with insulin by pancreatic β-cells [7, 8]. Prior studies showed that amylin modulates ingestive behavior [9] and reduces insulin secretion [10–12] and sensitivity [13–16]. Conversely, amylin gene deletion improved glucose tolerance in mice [17] suggesting that amylin plays a role in energy homeostasis.
Amylin from humans aggregates, forming amyloid when overexpressed [8, 18, 19]. Aggregated amylin was shown to cause oxidative stress [20, 21], inflammation [22–24] and apoptosis [18, 25] in the pancreas. In contrast, amylin from species that do not develop type-2 diabetes spontaneously (i.e. mice and rats) has a different amino acid structure and a reduced propensity to form amyloid [26]. Pharmacological induction of insulin resistance in mice expressing human amylin caused amylin amyloid deposition, β-cell apoptosis and overt hyperglycemia [27]. These pathologic changes were replicated in rodents overexpressing human [25, 28], but not murine amylin [29]. Thus, hyperamylinemia, is an early contributor to type-2 diabetes.
Accumulating evidence (including our work [30–35]) demonstrates the presence of abundant deposits of aggregated amylin in failing hearts [30–32] from patients with type-2 diabetes or obesity, in brains [33–38] of patients suffering from Alzheimer’s disease and in kidneys [39] of patients with type-2 diabetes. The source of amylin deposition in the heart (and brain and kidneys) originates in the pancreas, as no amylin mRNA was found in cardiac [31] and brain [35] tissues. Rats expressing human amylin in the pancreatic β-cells (HIP rats) accumulate aggregated amylin in the pancreas [29], heart [30], brain [34, 40] and kidneys [41], similar to humans with type-2 diabetes [30–39]. HIP rats develop type-2 diabetes [29] and heart dysfunction characterized by diastolic dysfunction [30], cardiac hypertrophy [30, 31] and dilation [31]. At the myocyte level, we found increased cytosolic Ca2+ and sarcolemmal Ca2+ leak in HIP rats, but not in glucose- and age-matched diabetic rats that express endogenous rat amylin [30]. We also reported that mitigating myocardial accumulation of amylin improves myocyte Ca2+ handling and heart function in diabetic HIP rats [31]. However, it is unclear whether cardiac accumulation of amylin is promoted by diabetic remodeling of the heart or would arise independent of such remodeling.
In HIP rat hearts, we found that aggregated amylin accumulates both interstitially [32] and inside myocytes [31, 32]. A potential venue for the amylin uptake in cardiac myocytes may involve binding of amylin monomers to calcitonin gene-related peptide (CGRP) receptors. CGRP receptors appear to play a role in internalization of monomeric amylin in pancreatic β-cells [43] and neurons [44] and they are expressed in cardiac myocytes [42]. Alternatively, cardiac amylin accumulation may result from deposition of aggregated amylin circulating in the blood. This mechanism is consistent with our previously published data [32] showing that amylin aggregates detected in cardiac myocytes, pancreas and blood all have similar molecular weights.
Here, we tested the hypothesis that the cardiac buildup of amylin and its subsequent effects on myocyte function are independent of diabetes-mediated remodeling of the myocardium. To test this hypothesis, we measured cardiac amylin accumulation and myocyte Ca2+ cycling in amylin knockout (AKO) mice intravenously infused with either aggregated human amylin or non-amyloidogenic rat amylin. The reason for using AKO mice, not simply WT mice, for this study, is twofold. First, it eliminates the potential confound factor that rodent amylin and human amylin may form mixed oligomers. Second, comparing myocytes from WT vs. AKO mice will clarify whether lack of amylin affects myocyte Ca2+ cycling. Of note, previous investigations [17] demonstrated increased insulin secretion and improved glucose tolerance in AKO mice compared to WT littermates. Blood glucose elimination was enhanced in female compared to male AKO mice. Thus, lack of amylin appears to improve insulin secretion and glucose homeostasis in mice. Using isolated cardiac myocytes, we also investigated the efficacy of the CGRP receptor antagonist AC-187 to prevent the myocyte amylin uptake and downstream cytotoxicity. Our findings may contribute to the understanding of the complex molecular mechanisms underlying diabetic cardiomyopathy.
2. Materials and methods
2.1. Experimental animals
Animal studies conform to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996) and was approved by the Institutional Animal Care and Use Committees at University of Kentucky. Frozen embryos of amylin knockout mice (AKO mice; line B6.129P2-Iapptm1Sgm/Kctt) were purchased from EMMA (https://www.infrafrontier.eu/) and re-derived at Jackson Laboratory. Genotyping was performed as described in Ref [17]. A total of 55 AKO mice were used in the study (n=32 males and n=23 females), at an age of 8–9 months. AKO mice were infused with either aggregated human amylin (n=9 for each sex) or non-amyloidogenic rat amylin (n=5 per group/per sex) and used for measurements of myocyte amylin deposition, Ca2+ transients and sarcolemmal Ca2+ leak. N=9 AKO mice of both sexes were used as controls. Additionally, n=9 male AKO mice were used for assessing amylin accumulation in the myocardial tissue upon infusion of human or rat amylin (vs. control; 3 mice per group). WT littermate mice (n=4 males and n=4 females) were used to measure the baseline Ca2+ transient amplitude in cardiac myocytes and plasma interleukin (IL)-1β. Metabolic characterization of AKO vs. WT mice was previously published [17].
Intravenous infusion of amylin
AKO mice were intravenously infused, via the tail vein, with either aggregated human amylin (2 µg/g body weight; AS-60804, AnaSpec, CA) or rat amylin (2 µg/g body weight; 74-5-10, American Peptide, CA) for 7 days (q.d.). For inducing aggregation, human was dissolved in PBS (pH 7.4) at a concentration of 50 µM and maintained at 37°C for 72 hours with occasional shaking [34]. Our previous data [34] show that blood from AKO rats infused with human amylin under similar conditions (2 µg/g body weight, q.d., 7 days) has comparable distributions of amylin-positive molecular complexes with blood from HIP rats and humans with type-2 diabetes.
Cardiac myocyte isolation
The experimental protocol was previously [30, 31] described. Briefly, mice were anesthetized by isoflurane (4.0%). When deep anesthesia was reached, hearts were excised quickly, placed on a Langendorff perfusion apparatus and perfused with buffer containing 1 mg/ml collagenase. When the heart became flaccid, the left ventricular tissue was cut into small pieces, dispersed, filtered and kept in a standard Tyrode’s solution containing (in mmol/L): 140 NaCl, 4 KCl, 1 MgCl2, 10 glucose, 5 HEPES, and 1 CaCl2 (pH=7.4). All experiments were performed at room temperature.
Amylin accumulation in cardiac myocytes
Isolated cardiac myocytes were lysed as described before [32] and amylin content was assessed by ELISA (EIA-AM, Raybiotech, GA) according with manufacturer’s protocol.
Immunofluorescence
Immunofluorescence was used in myocardial tissue and isolated myocytes from AKO mice infused with aggregated human amylin and control AKO mice (no amylin infusion). The primary antibodies were anti-human amylin antibody (1:200, SC-377530, Santa Cruz, TX; raised in rabbit) and CGRP antibody (ab47027, abcam, Cambridge, MA; raised in rabbit). The secondary antibody were Alexa Fluor 568 conjugated anti-rabbit IgG (A11036, Invitrogen, MA) and Texas red conjugated anti-rabbit IgG (SC-2780; Santa Cruz; TX).
Ca2+ transient measurements
Ca2+ transients were measured in isolated myocytes loaded with Fluo-4 (10 µM for 20 min) and electrically stimulated at 0.2, 0.5, 1 and 2 Hz.
Sarcolemmal Ca2+ leak
To measure the passive Ca2+ leak across sarcolemma, myocytes were loaded with Fura-2 (10 µM for 25 min) and pre-incubated (10 min) with 10 µM thapsigargin to block SERCA and empty the SR of Ca2+. Sarcolemmal Ca2+ leak was then measured the initial rate of [Ca2+]i decline upon reducing [Ca2+]o from 1 mM to 0, with the Na+/Ca2+ exchanger blocked by using a 0Na+/0Ca2+ external solution (Na+ was replaced by Li+, CaCl2 was omitted and 10 mM EGTA added to the Tyrode’s solution) and the plasma membrane Ca2+ ATPase inhibited with carboxyeosin (20 µM), as before [30].
IL-1β measurement
Plasma IL-1β was measured by ELISA (RAB0277, Sigma, MO, US) according with manufacturer’s protocol.
Statistical analysis
Comparison of two groups was done using unpaired Student’s t-test. One-way analysis of variance with Bonferroni’s post hoc test was used to compare multiple groups. Data are presented as means ± SEM. Difference between groups was considered significant when P < 0.05. All analyses were performed using GraphPad Prism 5.0 software.
3. Results
3.1. Lack of basal amylin has no effect on myocyte Ca2+ cycling
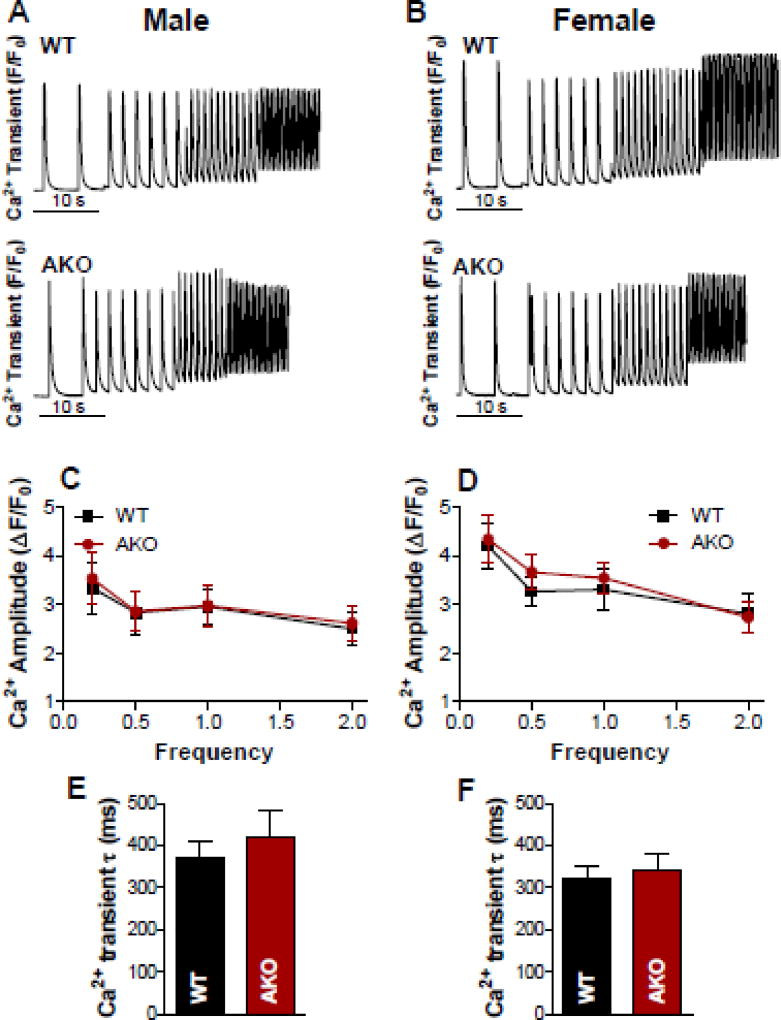
We have previously [30] shown that incubation of isolated cardiac myocytes with human amylin (50 µM; 2 hour incubation time) results in a significant increase of the Ca2+ transient amplitude [30]. A modest increase of Ca2+ transient amplitude was also measured in cardiac myocytes incubated with rat amylin 50 µM; 2 hour incubation time). To test whether lack of basal amylin expression alters myocyte Ca2+ handling, we compared Ca2+ transients in cardiac myocytes isolated from WT and AKO mice (both males and females; Fig 1). Our results (Fig 1) showed similar amplitude and decline time for Ca2+ transients in myocytes from WT and AKO mice. So, the presence of endogenous mouse amylin had no effect on basal myocyte Ca2+ cycling.
Figure 1. Basal amylin has no effect on myocyte Ca2+ cycling.
(A–B) Representative recordings of Ca2+ transients triggered at 0.2, 0.5, 1 and 2 Hz in cardiac myocytes from male (A) and female (B) WT and AKO mice. (C) Ca2+ transient amplitudes in cardiac myocytes from male WT mice (10 cells, 4 mice) vs. male AKO mice (11 myocytes, 4 mice). (D) Ca2+ transient amplitudes in cardiac myocytes from female WT mice (11 cells, 4 mice) vs. female AKO mice (13 myocytes, 3 mice). (E-F). The decline time of Ca2+ transients triggered by excitation at 0.5 Hz in myocytes from male (C) and female (D) WT and AKO mice. In all groups, data were recorded from 2–3 myocytes/mouse and are presented as the mean±SEM for myocytes.
3.2. Circulating aggregated amylin accumulates in the myocardium in absence of diabetes
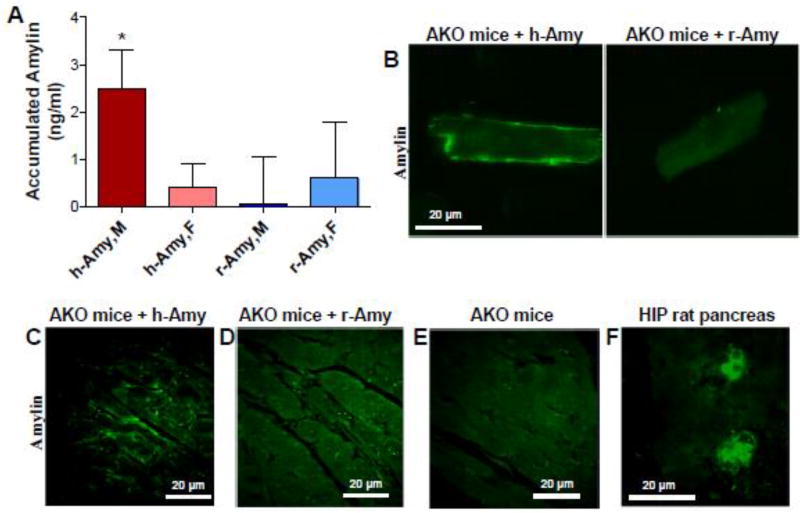
Male and female AKO mice were intravenously infused with either aggregated human amylin or non-amyloidogenic (i.e, monomeric) rat amylin (2 µg/g body weight) for 7 days (q.d.), as described in Methods. Mice were then euthanized for cardiac myocyte isolation. The level of amylin present in myocyte lysates from AKO mice infused with aggregated human amylin (h-Amy) or rodent amylin (r-Amy) were measured by ELISA (Fig 2A). Myocytes from males injected with aggregated human amylin show significant accumulation (P=0.02, One-sample t-test to compare the mean to zero) whereas no amylin was found in myocytes from females injected with human amylin (P=0.45). Infusion of rat amylin resulted in no significant myocardial amylin accumulation in either male (P=0.96) or female (P=0.64) mice.
Figure 2. Circulating aggregated (human) amylin, but not monomeric (rat) amylin, promotes myocardial amylin accumulation.
(A). ELISA data showing the average levels of amylin present in cardiac myocyte lysates from male and female AKO mice infused with either aggregated human amylin (h-Amy) or rat amylin (r-Amy). Measurements were done in 9 males and 5 females for both h-Amy and r-Amy. *P<0.05 using One-sample t-test to compare the mean to zero). (B). Immunofluorescence staining of amylin in cardiac myocytes isolated from amylin infused-male AKO mice vs. male AKO mice infused with rat amylin (r-Amy). (C–E). Immunofluorescence staining of amylin in cardiac sections from amylin infused-male AKO mice vs. control AKO mice (no amylin infusion). (F). Immunofluorescence staining of amylin in pancreatic tissue from HIP rats (positive control for amylin staining).
The presence of amylin in myocyte lysates from male AKO mice infused with aggregated human amylin indicates that amylin attached to the sarcolemma and/or incorporated into cardiac myocytes. To further test the attachment of circulating aggregated amylin to the sarcolemma from, we labeled isolated myocytes with an anti-amylin antibody and performed immunofluorescence (Fig 2B). Myocytes from human amylin-infused AKO male mice showed a strong immunofluorescence signal at the sarcolemma, consistent with the ELISA results in Fig 2A.
Fig 2C–E displays the results of immunofluorescent staining of amylin (green) in myocardial sections from AKO mice with or without amylin injection. Myocardial tissue from male AKO mice infused with aggregated human amylin showed a robust amylin immunofluorescence signal within the interstitial spaces or/and at the sarcolemma (Fig 2C). In contrast, immunofluorescence staining of myocardial tissues from male AKO mice infused with rodent amylin (Fig 2D) is similar to that in controls (AKO mice with no amylin infusion; Fig 2E), indicating the lack of amylin deposition in myocardium. In these experiments, HIP rat pancreatic tissue served as positive control for amylin deposition (Fig 2F).
Thus, acute intravenous infusion of aggregated human amylin (but not rodent amylin) caused myocardial amylin deposition and attachment to the sarcolemma in male AKO mice. Present results are consistent with our previous data from HIP rats [30–32] and isolated cardiac myocytes incubated with exogenous human amylin [30, 32].
3.3. Myocardial amylin accumulation alters myocyte Ca2+ cycling
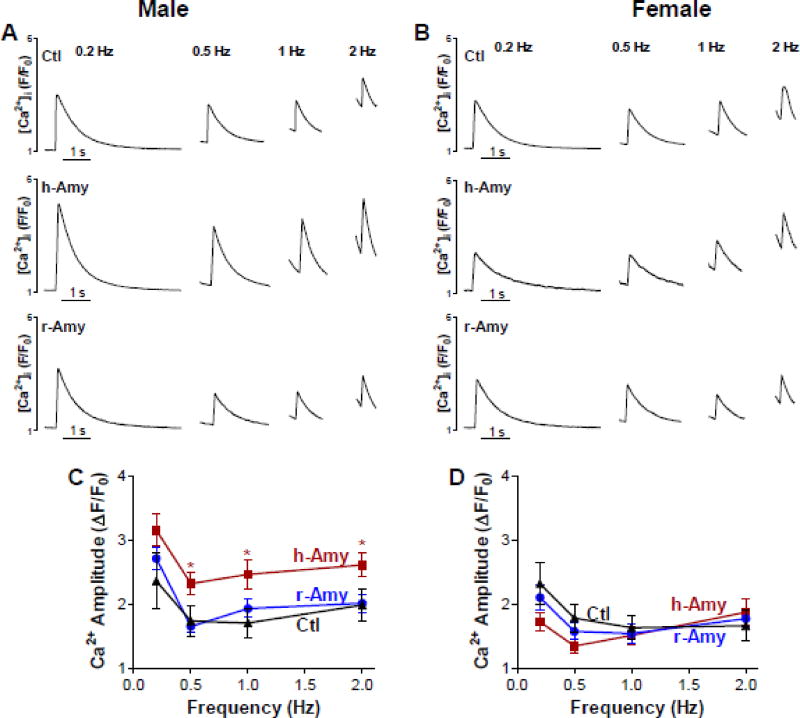
Next, we tested whether circulating amylin affects myocyte Ca2+ cycling by measuring Ca2+ transients in myocytes from male and female AKO mice infused with either aggregated human amylin or rat amylin versus control AKO mice (Fig 3). At all stimulation frequencies, Ca2+ transient amplitude was significantly larger in myocytes from male AKO mice infused with aggregated human amylin versus control AKO male (Fig 3A,C). In contrast, infusion of rat amylin in male AKO mice induced no effect on myocyte Ca2+ cycling (Fig 3A,C), in agreement with the lack of myocardial amylin deposition in this group (Fig 2A). Cardiac myocytes from AKO females infused with human or rat amylin, which did not accumulate amylin (Fig 2A), showed no change in Ca2+ transient amplitudes (Fig 3B,D). Neither human or rat amylin-infusion altered the decline of Ca2+ transients, consistent with our prior data in isolated myocytes incubated with amylin [34]. Thus, myocardial amylin accumulation is the cause for the larger Ca2+ transient amplitude in male AKO mice infused with aggregated human amylin, consistent with our previous results from HIP rats [30] and isolated cardiac myocytes incubated with exogenous human amylin [30].
Figure 3. Circulating aggregated amylin increases myocyte Ca2+ transient amplitude.
(A–B) Ca2+ transients recorded in myocytes from control AKO mice, AKO mice that were infused with aggregated human amylin (h-Amy) and AKO mice injected with monomeric rat amylin (r-Amy). Panel (A) shows recording in myocytes from males and panel (B) shows myocytes from females. (C) Mean Ca2+ transient amplitude in cardiac myocytes from male AKO mice infused with either aggregated human amylin (24 cells, 6 mice) or rat amylin (35 myocytes from 6 mice), and control AKO males (15 cells, 3 mice). (D) Mean Ca2+ transient amplitude in cardiac myocytes from female AKO mice infused with aggregated human amylin (29 cells, 6 mice) or rat amylin (32 cells, 6 mice) and control AKO females (14 cells, 3 mice).
3.4. Circulating aggregated amylin provokes sarcolemmal Ca2+ leak
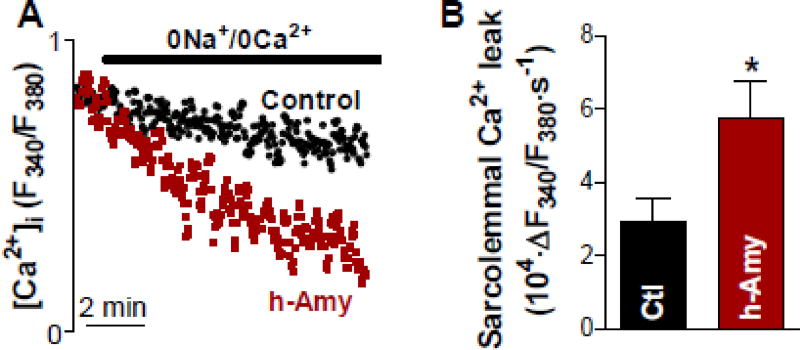
We hypothesized that myocardial amylin accumulation alters myocyte Ca2+ cycling by increasing sarcolemmal Ca2+ permeability. To test this hypothesis, we measured the passive sarcolemmal Ca2+ leak in myocytes from male AKO mice intravenously infused with aggregated human amylin control AKO mice (no amylin infusion). Myocytes were pre-incubated with thapsigargin to inhibit SERCA and thus empty the sarcoplasmic reticulum of Ca2+. Resting [Ca2+]i under these conditions was similar in myocytes from human amylin-infused and control AKO males (Fura-2 ratio of 2.63±0.10 vs. 2.57±0.10). We then measured the initial rate of [Ca2+]i decline upon reducing [Ca2+]0 from 1 to 0 mmol/L with Na+/Ca+ exchanger, Ca2+ influx and plasma membrane Ca2+-ATPase blocked (Fig 4A). Sarcolemmal Ca2+ leak was significantly larger in myocytes from human amylin-infused rats compared to controls (Fig 4B).
Figure 4. Circulating aggregated amylin provokes sarcolemmal Ca2+ leak.
(A) Representative examples of passive sarcolemmal Ca2+ leak measurements. (B) Mean data for the sarcolemmal Ca2+ leak in cardiac myocytes from male AKO mice infused with aggregated human amylin (h-Amy; 15 myocytes, 3 rats) and from male AKO mice with no amylin infusion (control; 12 myocytes, 3 mice). * P<0.05, Student’s t-test
3.5. Blocking CGRP receptors does not mitigate the amylin-mediated effects on myocytes, ex vivo
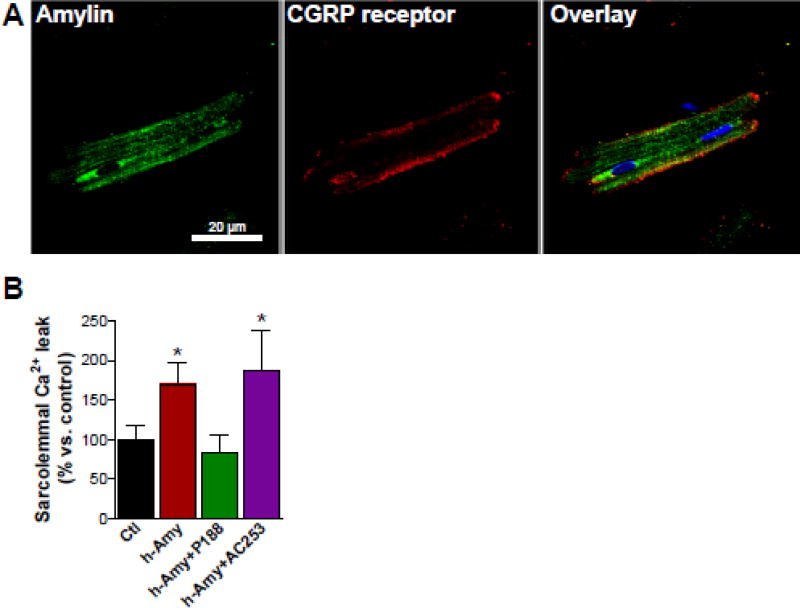
Previous work [42] suggested that amylin binds to the CGRP receptors on the sarcolemma. We tested whether blocking CGRP receptors by the AC-187 inhibitor reduces the interaction of amylin with cardiac myocytes, ex vivo. Immunoconfocal microscopy showed co-localization of amylin with CGRP receptors at the sarcolemma in cardiac myocytes incubated with human amylin (100 nM for 6 h; Fig 5A). However, co-incubation of isolated myocytes with the CGRP receptor AC-187 (100 nM) did not reduce the human amylin-provoked sarcolemmal Ca2+ leak (Fig 5B). In contrast the membrane sealant P188 prevented the increase in sarcolemmal Ca2+ leak in myocytes incubated with aggregated amylin (Fig 5B), which is consistent with our previous data [32] indicating that P188 efficiently reduces amylin-mediated peroxidative damage of the sarcolemma.
Figure 5. Inhibition of CGRP receptors has no significant effect on the ex vivo attachment of amylin to cardiac myocytes.
(A) Dual immunofluorescence staining of amylin (green) and CGRP receptor (red) in cardiac myocytes incubated ex vivo with human amylin (100 nM for 6 hours). (B) Passive sarcolemmal Ca2+ leak in cardiac myocytes from male AKO mice co-incubated with human amylin (100 nM) for 6 h and either the AC-187 inhibitor (100 nM) or the P188 membrane sealant (100 nM). Cardiac myocytes from male AKO mice (no amylin) were used as controls.For each group, measurements were done on ≥6 myocytes from 3 different mice. *P<0.05.
3.6. Circulating aggregated amylin triggers systemic inflammation in male AKO mice
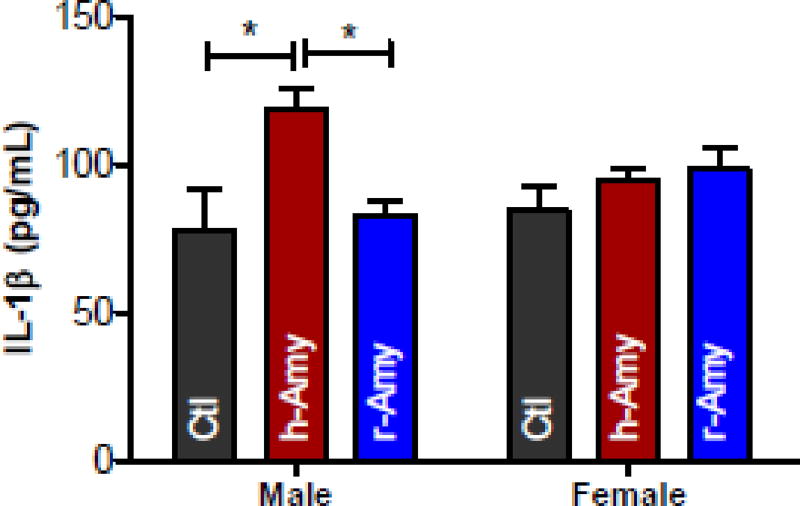
Accumulation of aggregated amylin in cells and tissues (including the heart) triggers a proinflammatory response [22–24, 32]. The plasma level of the proinflammatory cytokine IL-1β was elevated in male AKO mice infused with aggregated amylin (Fig 6). This systemic inflammatory response to aggregated amylin was not observed in female AKO mice. Infusion of rat amylin had no effect on plasma IL-1β in AKO mice (Fig 6).
Figure 6. Circulating aggregated amylin triggers systemic inflammation in male AKO mice.
ELISA data showing the average levels of IL-1β in plasma from male and female AKO mice infused with either aggregated human amylin (h-Amy) or rat amylin (r-Amy). Measurements were done in 9 males and 5 females for both h-Amy and r-Amy. Plasma from WT littermate mice (n=4 males and n=4 females) was used as control. *P<0.05 using One-sample t-test to compare the mean to zero).
4. Discussion
In summary, we found that intravenous infusion of aggregated human amylin leads to amylin accumulation in the myocardium and an increase in sarcolemmal Ca2+ leak and Ca2+ transient amplitudes in healthy, non-diabetic AKO mice. Alteration of sarcolemmal Ca2+ fluxes by circulating aggregated amylin in AKO mice is consistent with our previously published data [32] showing sarcolemmal peroxidative injury and cardiac inflammation in normal WT mice infused with aggregated amylin. These results suggest the following: i) the presence of amylin aggregates in hearts of humans with type-2 diabetes [30–32] is the result of higher levels of circulating aggregated amylin in the pre-diabetic state (when the secretion of insulin, and thus amylin, is greatly increased) rather than as a consequence of cardiac diabetic remodeling or heart failure; ii) human hearts undergoing the amylin stress are likely predisposed to a more rapid destabilization of sarcolemmal processes in diabetes.
In contrast to human (amyloidogenic) amylin, intravenous injection of monomeric rat amylin did not result in cardiac amylin deposition and had no effects on myocyte Ca2+ cycling. These results are consistent with previous studies [8, 18–22, 25–32] showing that amylin accumulation in cells and tissues is promoted by the amyloidogenicity of human amylin and increased propensity of aggregated amylin to incorporate in cellular membranes. Myocardial amylin accumulation may also be explained by an impaired clearance of aggregated amylin. Future studies need to investigate factors contributing to the impaired clearance of circulating aggregated amylin in vivo.
Blood glucose elimination is enhanced in female AKO mice compared to males [17]. We found that aggregated human amylin incorporated only in hearts from males but not female AKO mice, indicating a significant sex-dependent effect which might be estrogen-dependent. Indeed, we recently [34] showed that, compared to males, female HIP rats developed hyperglycemia and neurologic deficits later in life (i.e., ~12 months vs. ~18 months of age). These results are consistent with previous data [45] indicating that pancreatic amylin deposition is more abundant in men compared to women, which was attributed to increased insulin resistance in men [46, 47]. Ovariectomy or employing older female mice (as in our recent study in HIP rats [34]) might mitigate the sex difference in amylin-induced pathology. Indeed, previous studies demonstrated sex differences in cardiac myocyte ion channels [48–52], Ca2+ cycling [53, 54], contractions [53] and metabolism [55, 56], which may contribute to the differential effects of amylin stress. Elucidating sex-specific cardiac responses to amylin stress may help in understanding the development and progression of heart failure in men vs. women suffering from diabetes.
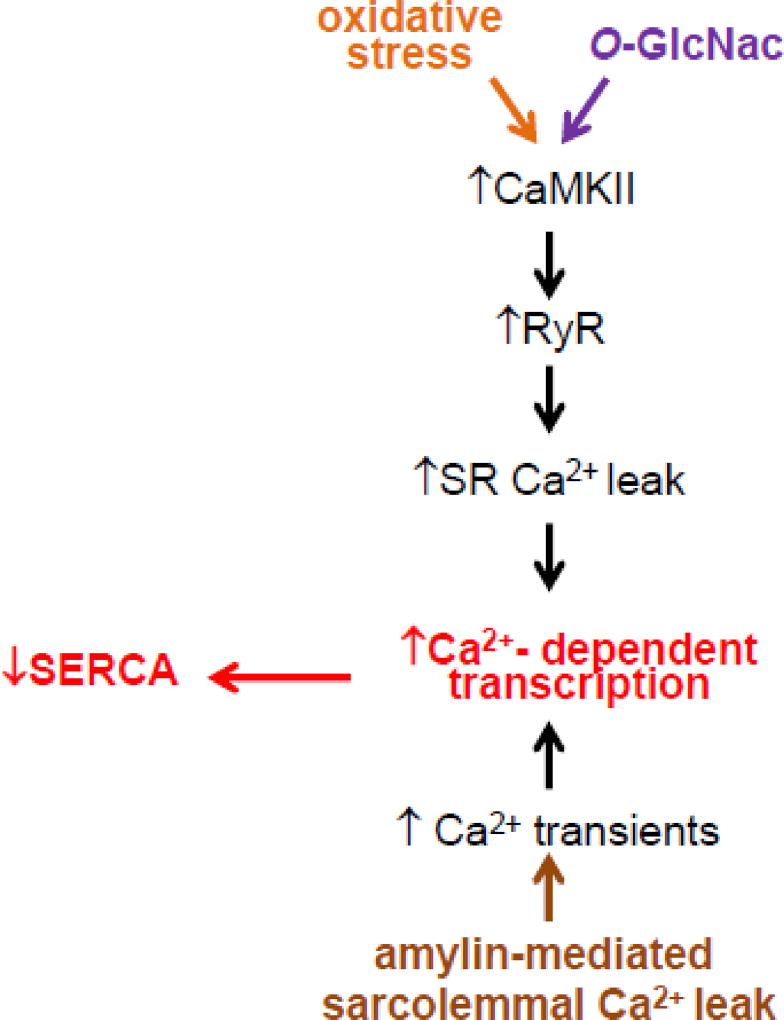
Previously published data showed that inducing diabetic states alters myocyte Ca2+ cycling [1, 57–59] in rodents, even in the absence of cardiac amylin deposition. The proposed mechanism for diabetes-induced Ca2+ dysregulation (see Fig 7; schematic mechanism) involves activation of CaMKII due to O-linked glycosylation (magenta pathway) [60] and oxidation (orange pathway) [61] of the kinase. CaMKII activation enhances sarcoplasmic reticulum (SR) Ca2+ leak through phosphorylation of ryanodine receptors (RyRs). Moreover, oxidative stress favors oxidation of the thiol residues of RyRs, which increases the opening probability of the channels [62]. Thus, the induction of diabetic states in rodents without cardiac amylin accumulation affects myocyte Ca2+ cycling mainly via the SR Ca2+ leak. Our results indicate that cardiac amylin accumulation alters myocyte Ca2+ cycling through provoking sarcolemmal Ca2+ leak (Fig 7; brown pathway), an effect that does not entail diabetic remodeling of myocardium. Our previous results [30, 31] showed that myocyte Ca2+ dysregulation caused by the enhanced sarcolemmal Ca2+ leak activates Ca2+-mediated transcriptional signaling leading to SERCA downregulation. Thus, amylin-induced sarcolemmal Ca2+ leak appears as a new and complementary mechanism of cardiac Ca2+ dysregulation in diabetic cardiomyopathy. While our previous study [30] showed that cardiac amylin accumulation is associated with diastolic dysfunction in HIP rats, the functional in vivo consequences of the infusion of aggregated amylin in AKO mice have not been explored, which is a limitation of the present work.
Figure 7. Proposed mechanism for the additive effect of amylin-induced sarcolemmal Ca2+ leak to myocyte Ca2+ dysregulation in diabetes.
Diabetes-induced Ca2+ dysregulation involves activation of CaMKII due to O-linked glycosylation (magenta pathway) [60] and oxidation (orange pathway) [61] of the kinase. CaMKII activation enhances sarcoplasmic reticulum (SR) Ca2+ leak through phosphorylation of ryanodine receptors (RyRs). Thus, the induction of diabetic states in rodents without cardiac amylin accumulation affects myocyte Ca2+ cycling mainly via the SR Ca2+ leak. Cardiac amylin accumulation alters myocyte Ca2+ cycling through provoking sarcolemmal Ca2+ leak (brown pathway), an effect that does not entail diabetic remodeling of myocardium.
In pancreatic β-cells [43] and neurons [44], binding of amylin to CGRP receptors is an important mechanism of internalization of monomeric amylin. Blocking CGRP receptors with the AC-187 inhibitor did not reduce amylin-mediated sarcolemmal Ca2+ leak in isolated cardiac myocytes suggesting that other mechanisms contribute to amylin-mediated myocyte Ca2+ dysregulation, i.e., sarcolemmal injury. Other possible explanations are that the 100 nM amylin concentration overcomes the CGRP receptor blockade or/and a lower selectivity of the AC-187 inhibitor for cardiac myocyte CGRP receptors compared to neurons and β-cells.
5. Conclusions
Cardiac amylin accumulation and subsequent amylin-mediated effects on myocyte Ca2+ cycling result from circulating aggregated (human) amylin, but not monomeric (rat) amylin and do not entail pre-existing diabetic remodeling of myocardium or heart failure. Lack of basal amylin has no effect on cardiac contractility; however, circulating aggregated amylin provokes myocyte Ca2+ mishandling by enhancing the sarcolemmal Ca2+ leak.
Hyperamylinemia is common in prediabetes [6–8] and promotes amylin aggregation [8, 25, 30, 31]. Aggregated amylin was identified in the blood [32] and cardiac tissue [30] of patients with type-2 diabetes or obesity. Present results demonstrate that circulating aggregated amylin induces cardiotoxicity independent of diabetic remodeling of myocardium. Thus, circulating aggregated amylin represents a potential therapeutic target in diabetic cardiomyopathy.
Highlights.
Basal amylin has no effect on myocyte Ca2+ cycling.
Aggregated amylin provokes myocyte Ca2+ dysregulation by enhancing sarcolemmal Ca2+ leak.
Myocyte uptake of amylin does not entail pre-existing diabetic remodeling of myocardium.
Circulating aggregated amylin, but not monomeric amylin, accumulates in myocardium.
Cardiac amylin accumulation is larger in male compared to female mice.
Acknowledgments
Funding: This research was supported by National Institutes of Health (R01HL118474, R01AG053999 and R01AG057290 to FD, and R01HL135000 to SD), Alzheimer’s Association (VMF-15-363458 to FD) and American Stroke Association (16GRNT310200 to FD).
Abbreviations
- AKO mice
amylin knockout mice
- CGRP
calcitonin gene-related peptide
- HIP rats
rats expressing the human islet amyloid polypeptide (IAPP; amylin)
- WT mice
wild-type mice
- h-Amy
human amylin
- r-Amy
rat amylin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bugger H, Abel ED. Molecular mechanisms of diabetic cardiomyopathy. Diabetologia. 2014;57:660–671. doi: 10.1007/s00125-014-3171-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lopaschuk GD. Metabolic abnormalities in the diabetic heart. Heart Fail Rev. 2002;7:149–159. doi: 10.1023/a:1015328625394. [DOI] [PubMed] [Google Scholar]
- 3.Taegtmeyer H, McNulty P, Young ME. Adaptation and maladaptation of the heart in diabetes: Part I: general concepts. Circulation. 2002;105:1727–1733. doi: 10.1161/01.cir.0000012466.50373.e8. [DOI] [PubMed] [Google Scholar]
- 4.Schulze PC, Drosatos K, Goldberg IJ. Lipid Use and Misuse by the Heart. Circ Res. 2016;118:1736–1751. doi: 10.1161/CIRCRESAHA.116.306842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taegtmeyer H, Young ME, Lopaschuk GD, Abel ED, Brunengraber H, Darley-Usmar V, Des Rosiers C, Gerszten R, Glatz JF, Griffin JL, Gropler RJ, Holzhuetter HG, Kizer JR, Lewandowski ED, Malloy CR, Neubauer S, Peterson LR, Portman MA, Recchia FA, Van Eyk JE, Wang TJ. American Heart Association Council on Basic Cardiovascular Sciences. Assessing Cardiac Metabolism: A Scientific Statement From the American Heart Association. Circ Res. 2016;118:1659–1701. doi: 10.1161/RES.0000000000000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim SH, Reaven GM. Insulin resistance and hyperinsulinemia: you can't have one without the other. Diabetes Care. 2008;31:1433–1438. doi: 10.2337/dc08-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kahn SE, D'Alessio DA, Schwartz MW, Fujimoto WY, Ensinck JW, Taborsky GJ, Jr, Porte D., Jr Evidence of cosecretion of islet amyloid polypeptide and insulin by beta-cells. Diabetes. 1990;39:634–638. doi: 10.2337/diab.39.5.634. [DOI] [PubMed] [Google Scholar]
- 8.Westermark P, Andersson A, Westermark GT. Islet amyloid polypeptide, islet amyloid, and diabetes mellitus. Physiol Rev. 2011;91:795–826. doi: 10.1152/physrev.00042.2009. [DOI] [PubMed] [Google Scholar]
- 9.Baldo BA, Kelley AE. Amylin infusion into rat nucleus accumbens potently depresses motor activity and ingestive behavior. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1232–R1242. doi: 10.1152/ajpregu.2001.281.4.R1232. [DOI] [PubMed] [Google Scholar]
- 10.Degano P, Silvestre RA, Salas M, Peiro E, Marco J. Amylin inhibits glucose-induced insulin secretion in a dose-dependent manner: study in the perfused rat pancreas. Regul Pept. 1993;43:91–96. doi: 10.1016/0167-0115(93)90411-z. [DOI] [PubMed] [Google Scholar]
- 11.Silvestre RA, Peiro E, Degano P, Miralles P, Marco J. Inhibitory effect of rat amylin on the insulin responses to glucose and arginine in the perfused rat pancreas. Regul Pept. 1990;31:23–31. doi: 10.1016/0167-0115(90)90192-y. [DOI] [PubMed] [Google Scholar]
- 12.Butler PC, Chou J, Carter WB, Wang YN, Bu BH, Chang D, Chang JK, Rizza RA. Effects of meal ingestion on plasma amylin concentration in NIDDM and nondiabetic humans. Diabetes. 1990;39:752–756. doi: 10.2337/diab.39.6.752. [DOI] [PubMed] [Google Scholar]
- 13.Leighton B, Cooper GJ. Pancreatic amylin and calcitonin gene-related peptide causes resistance to insulin in skeletal muscle in vitro. Nature. 1988;355:632–635. doi: 10.1038/335632a0. [DOI] [PubMed] [Google Scholar]
- 14.Zierath JR, Galuska D, Engstrom A, Johnson KH, Betsholtz C, Westermark P, Wallberg-Henriksson H. Human islet amyloid polypeptide at pharmacological levels inhibits insulin and phorbol ester-stimulated glucose transport in in vitro incubated human muscle strips. Diabetologia. 1992;35:26–31. doi: 10.1007/BF00400848. [DOI] [PubMed] [Google Scholar]
- 15.Molina JM, Cooper GJS, Leighton B, Olefsky JM. Induction of insulin resistance in vivo by amylin and calcitonin gene-related peptide. Diabetes. 1990;39:260–265. doi: 10.2337/diab.39.2.260. [DOI] [PubMed] [Google Scholar]
- 16.Cooper GJS, Leighton B, Dimitriadis GD, Parry-Billings M, Kowalchuck JM, Howland K, Rothbard JB, Willis AC, Reid KBM. Amylin found in amyloid deposits in human type 2 diabetes mellitus may be a hormone that regulates glycogen metabolism in skeletal muscle. Proc Natl Acad Sci USA. 1988;85:7763–7776. doi: 10.1073/pnas.85.20.7763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gebre-Medhin S, Mulder H, Pekny M, Westermark G, Törnell J, Westermark P, Sundler F, Ahrén B, Betsholtz C. Increased insulin secretion and glucose tolerance in mice lacking islet amyloid polypeptide (amylin) Biochem Biophys Res Commun. 1998;250:271–277. doi: 10.1006/bbrc.1998.9308. [DOI] [PubMed] [Google Scholar]
- 18.Jurgens CA, Toukatly MN, Fligner CL, Udayasankar J, Subramanian SL, Zraika S, Aston-Mourney K, Carr DB, Westermark P, Westermark GT, Kahn SE, Hull RL. β-cell loss and β-cell apoptosis in human type 2 diabetes are related to islet amyloid deposition. Am J Pathol. 2011;178:2632–2640. doi: 10.1016/j.ajpath.2011.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Höppener JWM, Ahren B, Lips CJM. Islet amyloid and type 2 diabetes mellitus. N Engl J Med. 2000;343:411–419. doi: 10.1056/NEJM200008103430607. [DOI] [PubMed] [Google Scholar]
- 20.Zraika S, Hull RL, Udayasankar J, Aston-Mourney K, Subramanian SL, Kisilevsky R, Szarek WA, Kahn SE. Oxidative stress is induced by islet amyloid formation and time-dependently mediates amyloid-induced beta cell apoptosis. Diabetologia. 2009;52:626–35. doi: 10.1007/s00125-008-1255-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janciauskiene S, Ahrén B. Fibrillar islet amyloid polypeptide differentially affects oxidative mechanisms and lipoprotein uptake in correlation with cytotoxicity in two insulin-producing cell lines. Biochem Biophys Res Commun. 2000;267(2):619–625. doi: 10.1006/bbrc.1999.1989. [DOI] [PubMed] [Google Scholar]
- 22.Westwell-Roper C, Dai DL, Soukhatcheva G, Potter KJ, van Rooijen N, Ehses JA, Verchere CB. IL-1 blockade attenuates islet amyloid polypeptide-induced proinflammatory cytokine release and pancreatic islet graft dysfunction. J Immunol. 2011;187:2755–2765. doi: 10.4049/jimmunol.1002854. [DOI] [PubMed] [Google Scholar]
- 23.Masters SL, Dunne A, Subramanian SL, Hull RL, Tannahill GM, Sharp FA, Becker C, Franchi L, Yoshihara E, Chen Z, Mullooly N, Mielke LA, Harris J, Coll RC, Mills KH, Mok KH, Newsholme P, Nunez G, Yodoi J, Kahn SE, Lavelle EC, O'Neill LA. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1beta in type 2 diabetes. Nat Immunol. 2010;11:897–904. doi: 10.1038/ni.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Netea MG, van de Veerdonk FL, van der Meer JW, Dinarello CA, Joosten LA. Inflammasome-independent regulation of IL-1-family cytokines. Annu Rev Immunol. 2015;33:49–77. doi: 10.1146/annurev-immunol-032414-112306. [DOI] [PubMed] [Google Scholar]
- 25.Matveyenko AV, Butler PC. β-cell deficit due to increased apoptosis in the human islet amyloid polypeptide transgenic (HIP) rat recapitulates the metabolic defects present in type-2 diabetes. Diabetes. 2006;55:2106–2114. doi: 10.2337/db05-1672. [DOI] [PubMed] [Google Scholar]
- 26.Westermark P, Engström U, Johnson KH, Westermark GT, Betsholtz C. Islet amyloid polypeptide: pinpointing amino acid residues linked to amyloid fibril formation. Proc. Natl. Acad. Sci. USA. 1990;87:5036–5040. doi: 10.1073/pnas.87.13.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Couce M, Kane LA, O'Brien TD, Charlesworth J, Soeller W, McNeish J, Kreutter D, Roche P, Butler PC. Treatment with growth hormone and dexamethasone in mice transgenic for human islet amyloid polypeptide causes islet amyloidosis and beta-cell dysfunction. Diabetes. 1996;45:1094–1101. doi: 10.2337/diab.45.8.1094. [DOI] [PubMed] [Google Scholar]
- 28.Matveyenko AV, Butler PC. Islet amyloid polypeptide (IAPP) transgenic rodents as models for Type 2 Diabetes. ILAR Journal. 2006;47:225–233. doi: 10.1093/ilar.47.3.225. [DOI] [PubMed] [Google Scholar]
- 29.Huang CJ, Haataja L, Gurlo T, Butler AE, Wu X, Soeller WC, Butler PC. Induction of endoplasmic reticulum stress-induced beta-cell apoptosis and accumulation of polyubiquitinated proteins by human islet amyloid polypeptide. Am J Physiol Endocrinol Metab. 2007;293:E1656–E1662. doi: 10.1152/ajpendo.00318.2007. [DOI] [PubMed] [Google Scholar]
- 30.Despa S, Margulies KB, Chen L, Knowlton AA, Havel PJ, Taegtmeyer H, Bers DM, Despa F. Hyperamylinemia contributes to heart dysfunction in obesity and diabetes, a study in humans and rats. Circ Res. 2012;110:598–608. doi: 10.1161/CIRCRESAHA.111.258285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Despa S, Sharma S, Harris TR, Dong H, Li N, Chiamvimonvat N, Taegtmeyer H, Margulies K, Hammock BD, Despa F. Cardioprotection by controlling hyperamylinemia in a “humanized” diabetic rat model. J Am Heart Assoc. 2014;3:e001015. doi: 10.1161/JAHA.114.001015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu M, Verma N, Peng X, Srodulski S, Morris A, Chow M, Hersh LB, Chen J, Zhu H, Netea MG, Margulies KB, Despa S, Despa F. Hyperamylinemia increases IL-1beta synthesis in the heart via peroxidative sarcolemmal injury. Diabetes. 2016;65:2772–2783. doi: 10.2337/db16-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jackson K, Barisone G, Jin L-W, DeCarli C, Despa F. Amylin deposition in the brain: A second amyloid in Alzheimer disease? Ann Neurol. 2013;74:517–526. doi: 10.1002/ana.23956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ly H, Verma N, Wu F, Liu M, Saatman KE, Nelson PT, Slevin JT, Goldstein LB, Biessels GJ, Despa F. Brain microvascular injury and white matter disease provoked by diabetes-associated hyperamylinemia. Ann Neurol. 2017;82:208–222. doi: 10.1002/ana.24992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verma N, Ly H, Liu M, Chen J, Zhu H, Chow M, Hersh LB, Despa F. Intraneuronal amylin deposition, peroxidative membrane injury and increased IL-1beta synthesis in brains of Alzheimer's disease patients with type-2 diabetes and in diabetic HIP rats. J Alzheimers Dis. 2016;53:259–272. doi: 10.3233/JAD-160047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fawver JN, Ghiwot Y, Koola C, Carrera W, Rodriguez-Rivera J, Hernandez C, Dineley KT, Kong Y, Li J, Jhamandas J, Perry G, Murray IV. Islet amyloid polypeptide (IAPP): a second amyloid in Alzheimer's disease. Curr Alzheimer Res. 2014;11:928–940. doi: 10.2174/1567205011666141107124538. [DOI] [PubMed] [Google Scholar]
- 37.Oskarsson ME, Paulsson JF, Schultz SW, Ingelsson M, Westermark P, Westermark GT. In vivo seeding and cross-seeding of localized amyloidosis: A molecular link between type 2 diabetes and Alzheimer disease. Am J Pathol. 2015;185:834–846. doi: 10.1016/j.ajpath.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 38.Schultz N, Byman E, Fex M, Wennström M. Amylin alters human brain pericyte viability and NG2 expression. J Cereb Blood Flow Metab. 2017;37:1470–1482. doi: 10.1177/0271678X16657093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gong W, Liu ZH, Zeng CH, Peng A, Chen HP, Zhou H, Li LS. Amylin deposition in the kidney of patients with diabetic nephropathy. Kidney International. 2001;72:213–218. doi: 10.1038/sj.ki.5002305. [DOI] [PubMed] [Google Scholar]
- 40.Srodulski S, Savita S, Bachstetter AB, Brelsfoard JM, Pascual C, Xie XS, Saatman KE, Van Eldik LJ, Despa F. Neuroinflammation and neurologic deficits in diabetes linked to brain accumulation of amylin. Mol Neurodegener. 2014;9:30. doi: 10.1186/1750-1326-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Srodulski S, Loria A, Despa S, Despa F. Hyperamylinemia, a potential therapeutic target in diabetic cardiorenal syndrome. Circulation. 2013;130:A13963. [Google Scholar]
- 42.Bell D, Schlüter KD, Zhou XJ, McDermott BJ, Piper HM. Hypertrophic effects of calcitonin gene-related peptide (CGRP) and amylin on adult mammalian ventricular cardiomyocytes. J Mol Cell Cardiol. 1995;27:2433–2443. doi: 10.1006/jmcc.1995.0231. [DOI] [PubMed] [Google Scholar]
- 43.Trikha S, Jeremic AM. Distinct internalization pathways of human amylin monomers and its cytotoxic oligomers in pancreatic cells. PLoS One. 2013;8:e73080. doi: 10.1371/journal.pone.0073080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jhamandas JH, MacTavish D. Antagonist of the amylin receptor blocks beta-amyloid toxicity in rat cholinergic basal forebrain neurons. J. Neurosci. 2004;24:5579–5584. doi: 10.1523/JNEUROSCI.1051-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao HL, Sui Y, Guan J, He L, Lai FM, Zhong DR, Yang D, Baum L, Tong PC, Tomlinson B, Chan JC. Higher islet amyloid load in men than in women with type 2 diabetes mellitus. Pancreas. 2008;37:e6873. doi: 10.1097/MPA.0b013e3181788e18. [DOI] [PubMed] [Google Scholar]
- 46.Kahn SE, Prigeon RL, McCulloch DK, Boyko EJ, Bergman RN, Schwartz MW, Neifing JL, Ward WK, Beard JC, Palmer JP. Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects. Evidence for a hyperbolic function. Diabetes. 1993;42:1663–1672. doi: 10.2337/diab.42.11.1663. [DOI] [PubMed] [Google Scholar]
- 47.Geer EB, Shen W. Gender differences in insulin resistance, body composition, and energy balance. Gend Med. 2009;6:60–75. doi: 10.1016/j.genm.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu Y, Ai X, Oster RA, Bers DM, Pogwizd SM. Sex differences in repolarization and slow delayed rectifier potassium current and their regulation by sympathetic stimulation in rabbits. Pflugers Arch. 2013;465:805–818. doi: 10.1007/s00424-012-1193-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yan S, Chen Y, Dong M, Song W, Belcher SM, Wang HS. Bisphenol A and 17β-estradiol promote arrhythmia in the female heart via alteration of calcium handling. PLoS One. 2011;6:e25455. doi: 10.1371/journal.pone.0025455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stauffer BL, Sobus RD, Sucharov CC. Sex differences in cardiomyocyte connexin43 expression. J Cardiovasc Pharmacol. 2011;58:32–39. doi: 10.1097/FJC.0b013e31821b70b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barajas-Martinez H, Haufe V, Chamberland C, Roy MJ, Fecteau MH, Cordeiro JM, Dumaine R. Larger dispersion of INa in female dog ventricle as a mechanism for gender-specific incidence of cardiac arrhythmias. Cardiovasc Res. 2009;81:82–89. doi: 10.1093/cvr/cvn255. [DOI] [PubMed] [Google Scholar]
- 52.Sims C, Reisenweber S, Viswanathan PC, Choi BR, Walker WH, Salama G. Sex, age, and regional differences in L-type calcium current are important determinants of arrhythmia phenotype in rabbit hearts with drug-induced long QT type 2. Circ Res. 2008;102:e86–e100. doi: 10.1161/CIRCRESAHA.108.173740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Farrell SR, Ross JL, Howlett SE. Sex differences in mechanisms of cardiac excitation-contraction coupling in rat ventricular myocytes. Am J Physiol Heart Circ Physiol. 2010;299:H36–H45. doi: 10.1152/ajpheart.00299.2010. [DOI] [PubMed] [Google Scholar]
- 54.Wasserstrom JA, Kapur S, Jones S, Faruque T, Sharma R, Kelly JE, Pappa A, Ho W, Kadish AH, Aistrup GL. Characteristics of intracellular Ca2+ cycling in intact rat heart: a comparison of sex differences. Am J Physiol Heart Circ Physiol. 2008;295:H1895–H1904. doi: 10.1152/ajpheart.00469.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lagranha CJ, Deschamps A, Aponte A, Steenbergen C, Murphy E. Sex differences in the phosphorylation of mitochondrial proteins result in reduced production of reactive oxygen species and cardioprotection in females. Circ Res. 2010;106:1681–1691. doi: 10.1161/CIRCRESAHA.109.213645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang F, He Q, Sun Y, Dai X, Yang XP. Female adult mouse cardiomyocytes are protected against oxidative stress. Hypertension. 2010;55:1172–1178. doi: 10.1161/HYPERTENSIONAHA.110.150839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wold LE, Dutta K, Mason MM, Ren J, Cala SE, Schwanke ML, Davidoff AJ. Impaired SERCA function contributes to cardiomyocyte dysfunction in insulin resistant rats. J Mol Cell Cardiol. 2005;39:297–307. doi: 10.1016/j.yjmcc.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 58.Shah MS, Brownlee M. Molecular and Cellular Mechanisms of Cardiovascular Disorders in Diabetes. Circ Res. 2016;118:1808–1829. doi: 10.1161/CIRCRESAHA.116.306923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stølen TO, Høydal MA, Kemi OJ, Catalucci D, Ceci M, Aasum E, Larsen T, Rolim N, Condorelli G, Smith GL, Wisløff U. Interval training normalizes cardiomyocyte function, diastolic Ca2+ control, and SR Ca2+ release synchronicity in a mouse model of diabetic cardiomyopathy. Circ Res. 2009;105:527–536. doi: 10.1161/CIRCRESAHA.109.199810. [DOI] [PubMed] [Google Scholar]
- 60.Erickson JR, Pereira L, Wang L, Han G, Ferguson A, Dao K, Copeland RJ, Despa F, Hart GW, Ripplinger CM, Bers DM. Diabetic hyperglycaemia activates CaMKII and arrhythmias by O-linked glycosylation. Nature. 2013;502:372–376. doi: 10.1038/nature12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Luo M, Guan X, Luczak ED, Lang D, Kutschke W, Gao Z, Yang J, Glynn P, Sossalla S, Swaminathan PD, Weiss RM, Yang B, Rokita AG, Maier LS, Efimov IR, Hund TJ, Anderson ME. Diabetes increases mortality after myocardial infarction by oxidizing CaMKII. J Clin Invest. 2013;123:1262–1274. doi: 10.1172/JCI65268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bovo E, Lipsius SL, Zima AV. Reactive oxygen species contribute to the development of arrhythmogenic Ca2+ waves during β-adrenergic receptor stimulation in rabbit cardiomyocytes. J Physiol. 2012;590:3291–3304. doi: 10.1113/jphysiol.2012.230748. [DOI] [PMC free article] [PubMed] [Google Scholar]