Abstract
Hematophagous arthropods are medically important disease vectors that transmit a variety of pathogens. Unlike mammals that employ both innate and adaptive immunity to clear invading pathogens, these vectors rely mainly on an innate immune system to combat pathogens. Peptidoglycan recognition proteins (PGRPs) are important components of innate immune signaling pathways and are responsible for recognizing microbe-associated molecular patterns (MAMPs), thus regulating host immune interactions with both harmful and helpful microbes. Here we review a number of recent studies in different vectors that address the function of PGRPs in immune regulation. Further, we discuss the variation of PGRPs between vectors and Drosophila.
Keywords: Peptidoglycan recognition protein, innate immunity, mosquito, tsetse fly, ticks, vectors
1.1 Introduction
Hematophagous arthropods such as mosquitoes, tsetse flies and ticks are of medical significance as most of them transmit bacteria, viruses, and parasites that threaten human and animal health (WHO, 2016). Nearly 82 % of the entire human population is at risk of infection by at least one vector-borne disease; especially people in low-income areas (Golding et al., 2015). The ability of these invertebrates to transmit pathogens is determined by multiple factors, but the key to transmission is the vector’s innate immunity. Innate immunity is the front line of defense against any non-self or foreign microbes (Cheng et al., 2016; Clayton et al., 2014; International Glossina Genome, 2014; Kopacek et al., 2010; Lehane et al., 2004). It is activated upon recognition of microbial molecules, termed microbe-associated molecular patterns (MAMPs), by germline-encoded pattern recognition receptors (PRRs) (Chu and Mazmanian, 2013). After activation, various immune responses including cellular and humoral responses defend against pathogens. Cellular immune response is featured by phagocytosis and encapsulation that is mediated by hemocytes (Lavine and Strand, 2002). Humoral immune response includes cascades that regulate antimicrobial peptides synthesis, melanization, coagulation, and the production of reactive intermediates of oxygen and nitrogen (Hoffmann, 2003). The production of antimicrobial peptides is regulated by two signaling pathways; the Toll and the immune deficiency (Imd) pathway are hallmarks of invertebrate humoral immune response (Hoffmann and Reichhart, 2002). The Toll pathway is responsible for recognition and elimination of most Gram-positive (G+) bacteria and fungi. Whereas the Imd pathway is triggered by, and controls the defense against, Gram-negative (G−) bacteria. These two pathways are also present in most vector species and are vital in pathogen defense.
Peptidoglycan recognition proteins (PGRPs) are capable of recognizing bacteria cell wall peptidoglycans and as such are the receptors and regulators of Toll and Imd signaling pathway (Royet et al., 2011). Insect PGRPs are the largest and most versatile family of PRRs for the recognition of microbial products. The first PGRP was identified in silkworm (Bombyx mori) as binds peptidoglycans and initiates immune responses showing the critical role of PGRPs in the defense against invading microbes (Yoshida et al., 1996). PGRPs were then characterized in other insect species including moth, Drosophila, and mosquitoes, are now studied in mammals (Royet and Dziarski, 2007). These proteins are highly conserved between invertebrates and mammals. All of PGRPs have at least one PGRP domain, which is responsible for peptidoglycan recognition (Royet et al., 2011). Peptidoglycan (PGN) is an essential component of cell wall in almost all bacterial species. It is a polymer consisting of β-(1,4) linked N-acetylglucosamine NAG and N-acetylmuramic acid NAM crosslinked by short peptides (Schleifer and Kandler, 1972). PGN is classified into Lys-type PGNs if the 3rd amino acid of the short peptide is Lys and DAP-type PGN if the 3rd amino acid is diaminopimelic acid (DAP). Lys-type PGNs are found in G+ bacteria and DAP-type PGNs are in all G− bacteria and Bacillus. PGRPs are able to differentiate these two types of PGNs and initiate corresponding downstream signaling pathways (Choe et al., 2002; Gottar et al., 2002; Leulier et al., 2003; Michel et al., 2001; Ramet et al., 2002).
Emerging evidence shows that PGRPs in disease transmitting vectors are the key participants in the immune response against invading pathogens. Thus PGRPs are essential for maintenance of vector-pathogen homeostasis. In this review, we will summarize the recent findings regarding PGRPs in hematophagous vectors and describe their effects on immune defense.
1.2 Feature and gene organization of PGRPs
Peptidoglycan recognition proteins are a family of pathogen recognition receptors with at least one PGRP domain. The PGRP domain is homologous to bacteriophage and bacterial type 2 amidases (Kang et al., 1998). Based on their transcript size, PGRPs are divided into 2 subfamilies: short PGRPs (PGRP-S) and long PGRPs (PGRP-L) (Werner et al., 2000). Short PGRPs are secreted proteins with a signal peptide and one PGRP domain. Long PGRPs are at least double the size of short PGRPs with a N-terminal sequence of variable length and one or more PGRP domains. Long PGRPs can be secreted, transmembrane, or cytosolic proteins. Additionally, PGRPs based on function, can be divided into 2 subfamilies: catalytic PGRPs and non-catalytic PGRPs (Mellroth et al., 2003). Catalytic PGRPs as the name implies have enzymatic activity, these include PGRP-SB1, -SB2, -SC1, -SC2 and -LB in Drosophila, which function as the modulators of immune signaling pathways by sequestering PGN released by bacteria. The second group are PGRPs without amidase activity, including PGRP-SA, -SD, PGRP-LC and -LE, these initiate a cascade of immune responses by recognizing and binding to PGN. Another group of non-catalytic PGRPs, including PGRP-LF and rPGRP-LCs, have no amidase activity but negatively regulate immune response by interfering with the binding capacity of PGRP-LC to PGNs (Basbous et al., 2011; Neyen et al., 2016; Persson et al., 2007).
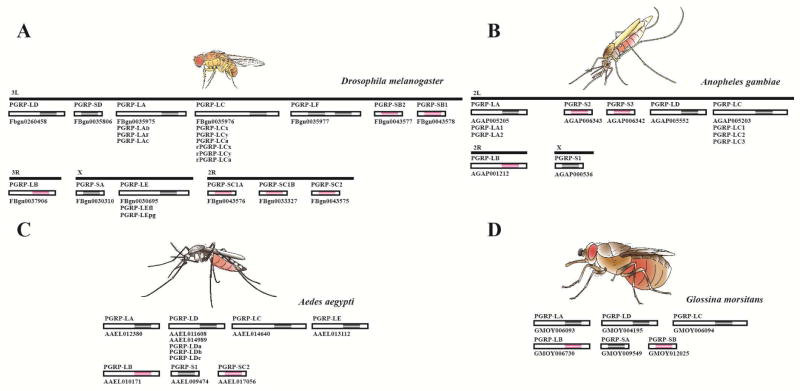
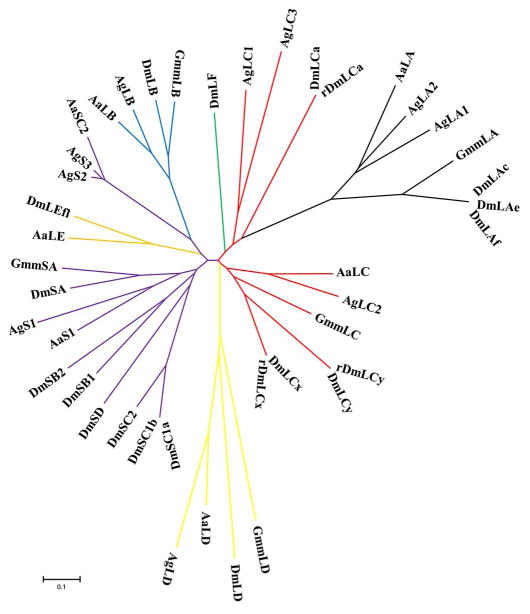
With the completion of genome annotation of three vectors, Anopheles, Aedes mosquitoes and tsetse fly, the genomic organization of PGRPs in these vectors becomes clear (Christophides et al., 2002; International Glossina Genome, 2014; Nene et al., 2007) (Fig. 1). In comparison to Drosophila that has 13 pgrp genes, encoding at least 22 PGRP proteins (Kurata, 2014; Neyen et al., 2016), mosquito and tsetse genomes have a limited repertoire, with only 7 and 6 genes present in each genome, respectively (Christophides et al., 2002; International Glossina Genome, 2014; Wang and Beerntsen, 2015) (Fig.1). Anopheles gambiae has 7 pgrp genes (pgrp-s1, -s2, -s3, -la, -lb, -lc and -ld) that encode at least 10 PGRP proteins. Aedes aegypti has the same number of pgrp genes as Anopheles, but different in family members with only 2 short pgrps, pgrp-s1 and -sc2, and 5 long pgrps including pgrp-la, -lb, -lc, -ld and -le. In tsetse fly, only six pgrp genes are identified, four in the long subfamily (pgrp-la, -lb, -lc, and -ld) and two in the short subfamily (pgrp-sa and -sb). Both mosquitoes and tsetse lack orthologs of PGRP-LF found in Drosophila, but have retained PGRP-LA, -LB, -LC and –LD. The Phylogenic analysis of PGRPs in Drosophila and vectors indicates that the same PGRP from different vectors are likely to cluster together with the counterparts of Drosophila (Fig. 2).
Figure 1.
Organization of PGRPs in Drosophila (Drosophila melanogaster) (A), mosquitoes (Anopheles gambiae (B), Aedes aegypti (C)) and tsetse fly (Glossina morsitans) (D). Non-catalytic PGRP domain is in black. Catalytic PGRP domain is in pink. Transcriptional isoforms that have been characterized are listed below of each gene.
Figure 2.
Phylogenic analysis of PGRPs in Drosophila (Dm), Anopheles gambiae (Ag), Aedes aegypti (Ae) and tsetse fly (Gmm) using neighbor-joining estimations. The tree was constructed using the MEGA program (v5) (http://www.megasoftware.met/mega.php) with default settings and 1000 bootstraps. PGRP-LAs (LA) from four insects were colored in black, PGRP-LBs (LB) were in blue, PGRP-LCs (LC) were in red, PGRP-LDs (LD) were in yellow, PGRP-LEs (LE) were in orange, PGRP-LF (LF) was in green, short PGRPs were in purple. Accession number of each sequence was indicated in Figure 1.
Decreasing diversities of PGRP proteins in mosquito and tsetse fly compared to Drosophila may be a result of their different life histories and reproductive physiologies. Drosophila eat mainly rotten fruit that contains abundant environmental microbes. Both species of female mosquitoes (Anopheles and Aedes) feed on plant nectar and need vertebrate blood for reproduction. While tsetse flies are obligate blood sucking insects. The relatively aseptic vertebrate blood may limit the opportunities of vectors to be in contact with environmental microbes, which may decrease the variety of PGRPs. Drosophila and mosquitoes have oviparous reproductive strategies, which allows their progeny (eggs and larva) to develop externally in the environment, independent of the female. Alternatively, the tsetse fly has a viviparous reproductive system such that offspring develop in the uterus of the mother, thus further preventing their exposure to a diverse environmental fauna of microbes (Benoit et al., 2015). Collectively, the diverse of PGRP organization may reflect the amount these insects are exposed to environmental microbes.
1.3 PGRPs in Drosophila
PGRPs are extensively studied using the Drosophila model system (Kurata, 2014; Royet and Dziarski, 2007; Royet et al., 2011). Drosophila has 13 pgrp genes. Four of them encoding recognition PGRPs, including PGRP-SA, -SD, -LC and LE, that act as receptors of immune signaling pathways; responsible for triggering immune responses. Five PGRPs encode catalytic PGRPs, including PGRP-SB1, -SB2, -SC1, -SC2 and -LB, which prevent the over-activation of immune signaling pathways via digestion of PGN into short, nonimmunogenic fragments. Another regulatory PGRP, PGRP-LF, although having no PGN binding activity, downregulates Imd pathway by antagonizing the ability of PGRP-LCa to bind to PGRP-LCx. The classical function of PGRPs in Drosophila are discussed in detail elsewhere (Kurata, 2014; Royet and Dziarski, 2007; Royet et al., 2011), so we will briefly discuss their recent discovered function here.
PGRP-LC is a well-known receptor with 3 isoforms, PGRP-LCa, -LCx and -LCy, and is responsible for the recognition of DAP-PGN and triggering signal transduction of the Imd pathway (Kaneko et al., 2004; Mellroth et al., 2005; Werner et al., 2003). Recently, a new regulatory approach has been found in PGRP-LC that is controlled by the insect steroid hormone ecdysone. This occurs through several ecdysone inducible transcription factors, including BR-C, Eip78C, Eip93F, Eip74EF, Eip75B, HR46, PNR and SRP (Rus et al., 2013). In this way, immune induction of all AMP genes through the IMD pathway is tightly controlled by both humoral and environmental factors. The complex interplay between innate signaling and steroid signaling pathway further ensures the proper function of immune system during development. PGRP-LC also functions as a receptor for retrograde, trans-synaptic signaling in nervous system (Harris et al., 2015). It participates in maintaining homeostasis of synaptic plasticity possibly through binding of Endostatin, a small peptide that is necessary for presynaptic homeostasis. Thus, PGRP-LC has dual roles in Drosophila, one of initiating immune defense against invading pathogens and the other of regulating homeostatic synaptic plasticity in the absence of infection. Three novel PGRP-LC alternative transcripts, rPGRP-LCx, -LCy and -LCa have been recently identified (Neyen et al., 2016). They have the same extracellular domain corresponding to PGRP-LCx, -LCy and -LCa but distinct intracellular domain. These rPGRP-LCs specifically recognize polymeric PGN released from dead bacteria and mediate endocytosis of PGRP-LC, thus terminate immune response via the endocytic pathway. Another recognition PGRP, PGRP-LE, is known as an intracellular receptor of the Imd pathway, which detects PGN within the cell (Kaneko et al., 2006; Yano et al., 2008). It is enriched in the larval and adult enterocytes (Neyen et al., 2012). PGRP-LE mediates the induction of intestinal peptidase expression upon colonization of Lactobacillus plantarum, a commensal bacterium in germ free Drosophila (Erkosar et al., 2015). This colonization promotes dietary protein digestion and increases host amino acid levels, which sustains systemic growth and maturation on a low nutrient diet. Mutation of PGRP-LE strongly impairs the induction of peptidases, Jon66Cii and CG18179 (Erkosar et al., 2015). As such PGRP-LE in addition to being an immune receptor may be involved in metabolic pathways, in the presence of commensal bacteria. PGRP-SD was originally characterized responsible for triggering Lys-type PGN mediated Toll signaling pathway by forming a complex with Gram-negative binding protein 1 (GNBP1) and PGRP-SA (Bischoff et al., 2004; Gobert et al., 2003; Wang et al., 2008). However, this PGRP has recently been identified to sense DAP-PGN and enhance PGRP-LC mediated Imd signaling activity (Iatsenko et al., 2016). It is highly possible that the binding affinity of PGRP-SD to Lys-PGN and DAP-PGN varies in different conditions allowing PGRP-SD to have multiple functions in regulating the immune activation. Altogether, these results suggest that PGRPs are key components of immune signaling pathways. PGRPs have evolved, to sense multiple factors, not limited to PGNs, to ensure the proper immune, neural, and metabolic functions.
1.4 PGRPs in mosquitoes
Mosquitoes, including Anopheles, Aedes and Culex, as the deadliest animals in the world, transmitting a variety of pathogens that threaten human and animal health. Emerging evidence shows that PGRPs in Anopheles and Aedes mosquitoes are critical in regulating immune activities in response to both invading pathogens and residential microbes.
Anopheles genus mosquitoes are the primary vector of malaria causing over 429 000 deaths globally in 2015, most of which, are children under 5 (WHO, 2016). PGRP-LC with 3 splicing isoforms, PGRP-LC1, -LC2 and -LC3, is the first PGRP characterized in Anopheles gambiae (Meister et al., 2009). The PGRP domain of each PGRP-LC isoform is encoded by a common exon 4 and an additional two other variable exons. Among these isoforms, PGRP-LC3 plays a major role in helping Anopheles defend against both invading bacteria and malaria parasites, Plasmodium. Knock-down of PGRP-LC not only increases the susceptibility of mosquito to malaria parasite infection, but also induces the proliferation of gut microbiota. In mosquitoes that native microbes are depleted by antibiotic treatment, knock-down of PGRP-LC has no influence on vector competence. These results suggest that PGRP-LC, through binding PGNs released from gut microbiota, primes the Imd signaling pathway then to clear invading Plasmodium. PGRP-LA has 2 isoforms, PGRP-LA1 and PGRP-LA2 (Gendrin et al., 2017). Both of the isoforms function similarly as PGRP-LC in that they inhibit Plasmodium infection by initiating the synthesis of downstream immune effectors of the Imd pathway. PGRP-LB was initially identified as being induced by gut microbiota (Dong et al., 2009). Depletion of PGRP-LB results in an increase in the number of the gut microbiota, suggesting a role of this protein in controlling gut microbial homeostasis. Recently, PGRP-LB has been shown to promote Plasmodium infection by negatively regulating systemic immune responses (Gendrin et al., 2017). Other PGPRs including PGRP-S2/S3 and -LD are also involved in parasite elimination, but mechanisms of how this occurs are still unknown (Gendrin et al., 2017).
Genome wide comparison between Anopheles and Drosophila reveals that the major components of Toll and Imd pathways are conserved in these two insects (Christophides et al., 2002). These two pathways are regulated by PGRPs and are of considerable importance for parasite defense (Clayton et al., 2014). Activation of the Toll and Imd pathways leads to the nuclear translocation of NF-κB transcription factors, Rel1 and Rel2, respectively, which initiates the synthesis of downstream anti-parasite immune effectors. Unlike in Drosophila where Toll responds specifically to G +/fungi and Imd specifically senses G− bacteria, the same Anopheles immune signaling pathway can be activated by both G+/G− bacteria and parasites (Frolet et al., 2006; Garver et al., 2012; Meister et al., 2005). Silencing either Rel1 or Rel2 abolishes resistance of Anopheles to Plasmodium. Upregulation of immune activities of either pathway, by knock-down of their respective negative regulators, Cactus or Caspar, confers almost complete resistance to malaria parasites (Frolet et al., 2006; Garver et al., 2009).
Aedes genus mosquitoes transmit viral diseases including dengue fever, chikungunya, and Zika fever. They are a continual global health threat putting billions of people at risk of infection causing large number of deaths (Bhatt et al., 2013; Caraballo and King, 2014). PGRPs mediated immune defense in Aedes mosquitoes, playing vital roles in viral and bacterial clearance (Cheng et al., 2016). Both G+ and G− bacterial infection induce the expression of 3 PGRPs, PGRP-S1, -SC2 and -LB, indicating these PGRPs respond directly to bacterial stimuli. Knock-down of PGRP-LC significantly reduces the survival rate of Ae. aegypti when challenged with both G+ and G− bacteria. PCRP-LC reduction also suppresses the synthesis of several antimicrobial peptides, including defensin, cecropin and gambicin. This strongly suggests that PGRP-LC mediates Aedes defense against both G+ and G− bacteria by positively regulating immune activities (Wang and Beerntsen, 2015). In Ae. aegypti, PGRP-LE, an intracellular receptor, is induced upon artificial colonization of Wolbachia, an insect endosymbiont (Pan et al., 2017). RNAi of this gene results in decreasing number of Wolbachia. While upregulation of immune signaling pathways, leads to elevated Wolbachia growth. These results suggest that PGRP-LE is involved in sensing intracellular Wolbachia and then mediates immune signaling pathways that facilitate colonization by this bacterium. PGRP-LA, -S1 and -LD also participate in the activation of immune responses, but their influence is marginal in response to bacterial infection (Wang and Beerntsen, 2015). PGRP-LB and -SC2 are the negative regulators of immune signaling pathways as knock-down of these genes significantly increases expression of defensin, cecropin and gambicin (Wang and Beerntsen, 2015).
So far no PGRPs in Aedes mosquitoes have been linked to direct virus recognition or defense, however, PGRP mediated signaling pathways play important roles in fighting against viral infection (Cheng et al., 2016). The Toll pathway is triggered upon dengue virus infection. Upregulation of the Toll immune signaling pathway by RNAi mediated knock-down of its negative regulator, Cactus, reduces viral infection (Xi et al., 2008). This protective role relies on the presence of gut microbiota. In Aedes aegypti the Toll pathway is also activated by Wolbachia controlled induction of reactive oxygen species (ROS) (Pan et al., 2012). The induction of the Toll pathway reduces oxidative stress caused by Wolbachia and the Toll pathway is also responsible for inhibition of DENV proliferation in these mosquitoes. Final stages of Dengue infection, in salivary glands, elicits both Toll and Imd pathway (Luplertlop et al., 2011). One of the downstream antimicrobial peptides, Cecropin which is induced by Dengue virus, shows both antibacterial and anti-viral activities (Luplertlop et al., 2011). The Imd pathway also influences Sindbis virus infection indirectly, by controlling the proliferation of gut microbiota (Barletta et al., 2017). In addition, autophagy regulated by intracellular PRRs is crucial in elimination of intracellular virus, bacteria, and parasites in both insects and mammals (Yano and Kurata, 2011). In Drosophila, intracellular bacterium, Listeria monocytogenes infection induces PGRP-LE mediated autophagy, which in turn prevents its intracellular growth (Yano et al., 2008). It is probable that autophagy is also involved in viral clearance in Aedes mosquitoes, however, this needs to be further investigated.
In comparison to Drosophila, the function of PGRPs in mosquitoes, is generally conserved. PGRP-LC is the main receptor of Imd signaling pathway. PGRP-LA is also involved in initialing downstream antimicrobial synthesis, but its role is redundant in both mosquitoes and Drosophila. PGRP-LB is a negative regulator of the Imd signaling pathway although its catalytic activity needs further investigation in mosquitoes. In contrast to PGRPs that in Drosophila sense G+ and G− bacteria specifically, the same PGRP, such as PGRP-LC, in mosquitoes recognizes both G+ and G− bacteria, and regulating the synthesis of antimicrobial peptides downstream of both Toll and Imd pathways (Meister et al., 2009; Wang and Beerntsen, 2015). These two pathways work synergistically to protect Anopheles mosquito against G+, G− bacteria as well as Plasmodium infection (Clayton et al., 2014). PGRPs in Drosophila that recognize and digest of PGN are well studied, however the interaction between mosquito PGRPs and pathogens is far from complete. As in Anopheles, PGRPs mediated immune defense against malarial parasites still relies on the presence of gut microbiota (Gendrin et al., 2017; Meister et al., 2009). Clearance of gut microbiota through antibiotics treatment abolishes the protection of the immune response against parasites. It is highly possible that Plasmodium mediated immune signaling activation is via the recognition of PGNs, released from gut microbiota, by PGRPs. It is also possible that PGRPs in both Anopheles and Aedes mosquitoes adapted to have a broad recognition spectrum during a long term co-existence with pathogens.
1.5 PGRPs in Tsetse fly
The tsetse fly is the sole vector of African trypanosome that causes human African trypanomiasis (HAT) and animal African trypanosomiasis (AAT), a neglected tropical disease endemic in sub-Sahara African. PGRP-LC and -LB are the two PGRPs which functions are well characterized. PGRP-LC is the receptor of the Imd signaling pathway similar to what is observed in Drosophila (Wang et al., 2009). However, unlike Drosophila PGRP-LC which has 6 isoforms, no isoforms has been identified so far. Knock down of PGRP-LC abolishes the induction of downstream antimicrobial peptides which in turn increases susceptibility to trypanosome infection (Wang et al., 2009). Similar results are found in tsetse flies where the Imd pathway is blocked by the knock-down of Relish, the NF-κB transcription factor (Hu and Aksoy, 2006). PGRP-LB acts as both a negative regulator and an effector of Imd signaling pathway (Wang and Aksoy, 2012; Wang et al., 2009). Like Drosophila PGRP-LB, tsetse PGRP-LB is an amidase that degrades PGN, released from native microbes, and prevents the overactivation of immune responses. As a negative regulator of the Imd pathway, PGRP-LB protects the tsetse mutualistic symbiont, Wigglesworthia, from immune damage. This symbiont in turn stimulates the proper immune system development. Additionally, it evolved to have both bactericidal and trypanocidal activities that work synergistically with other antimicrobial peptides. PGRP-LB is also vertically transmitted from mother to offspring via milk secretion to ensure the proper development of immune system and symbionts colonization in next generation. Due to the limited PGRP repertoire and long term coexistence with trypanosome, PGRP-LB in tsetse flies has adapted to have both amidase and anti-pathogen activity to ensure a healthy symbiont community at the same time excluding harmful parasites. Although it is unclear how PGRP-LC and -LB recognize trypanosomes, these results suggest that the immune response mediated by the two PGRPs are vital in maintaining the fine tuned balance between tsetse fly, native microbes and trypanosomes. In comparison to Drosophila, tsetse flies lack the intracellular receptor, PGRP-LE. Possibly due to the relatively sterile environment during tsetse fly development. Functions of other PGRPs, PGRP-LA, -LD, -SA and -SB are still unknown. Further studies of these PGRPs will shed light on interactions between tsetse immune responses and trypanosomes.
1.6 PGRPs in other hematophagous vectors
Ticks are a large group of medically important vectors that transmit a variety of pathogens including viruses, bacteria and other parasites. Most disease transmitting ticks belong to suborder Ixodida that have a distinct life style compared to hematophagous insects (e.g., mosquitoes and tsetse flies). They only feed once at each developmental stage (larva, nymph and adult), when they feed they spend a prolonged period of time on a host. In addition, many bacteria that are transmitted by ticks are evolutionarily distinct and possess unique cell wall structures. For example, the peptidoglycan in Borrelia burgdorferi, which causes Lyme disease, is structurally different from regular bacteria as the third amino acid of the short peptide is L-ornithine instead of DAP (Schleifer and Kandler, 1972). Anaplasma phagocytophilum, the causative agent of Anaplasmosis lacks most of the genes for peptidoglycan biosynthesis (Rikihisa, 2010). The unique life cycles of ticks and the distinct cell wall structure of pathogens ticks transmit make the knowledge of PGRPs in other model insects difficult to apply to ticks. However, multiple PGRPs have been identified in several tick species (Rosa et al., 2016; Smith and Pal, 2014). For example, 9 PGRPs have been identified in I. Ricinus, 5 PGRPs in I. scapularis, and 4 PGRPs in R. pulchellus (Rosa et al., 2016). The role of the PGRPs mediated signaling pathways, Toll and Imd has also been examined in several tick species, including I. scapularis and Rhipicephalus microplus (Capelli-Peixoto et al., 2017; Rosa et al., 2016; Shaw et al., 2017; Smith and Pal, 2014). Ticks have a relatively conserved Toll pathway compared to Drosophila, alternatively ticks lack central components of the Drosophila Imd pathway (Oliva Chavez et al., 2017). Interfering with the signaling transduction of Imd pathway by silencing its regulatory component, Relish, Bendless or Uev1a, renders both R. microplus and I. scapularis increasingly susceptible to Anaplasma and Borrelia infection (Capelli-Peixoto et al., 2017; Severo et al., 2013; Shaw et al., 2017). These results suggest that ticks may have an evolutionarily divergent but functional Imd pathways. However, how PGRPs regulate humoral immune responses need to be further investigated.
Consistent with these observations in ticks, Rhodnius prolixus, a major vector of Chagas disease caused by American trypanosomes (Trypanosoma cruzi), also lacks the key components of Imd pathway. Specifically these bugs lack the IMD, Fas-associated protein with death domain (Fadd), death-related ced-3/Nedd2-like caspase (Dredd), and Caspar, but retains the relatively conserved Toll pathway (Mesquita et al., 2015). Multiple PGRPs have been identified in Rhodnius prolixus, although their function is unknown. Perturbing the activity of either the Toll or the Imd pathway fails to influence the vector competence of this bug, but significantly increases proliferation of its symbiont, Rhodococcus rhodnii. In contrast to the tsetse fly and mosquitoes whose humoral immune responses control both symbiotic homeostasis and pathogens infection, PGRP mediated signaling pathways in Rhodnius prolixus contribute primarily to symbiont regulation.
Taken together, results in other hematophagous vectors suggest an atypical but functional immune signaling pathway may exist such that PGRPs in these vectors may have distinctive functions in limiting pathogen infection.
2. Conclusion
In this review we summarize the current state of knowledge regarding PGRPs on hematophagous arthropods. In comparison to Drosophila, functions of PGRPs in both mosquito species, An. gambiae and Ae. Aegypti as well as the tsetse fly display a high degree of conservation as PGRPs all participate in the regulation of host immune signaling pathways. In contrast, the PGRP gene families are relatively small among these vectors, likely due to the different levels of microbial exposures during the different species’ life cycle. Although the knowledge of PGRP functions in other vectors including ticks and reduviid bugs is limited, they still do have all the functional humoral immune signaling pathways similar to those of Drosophila. The presence of PGRPs in these vectors indicates that they might play roles in immune regulation.
Hematophagous vectors pose serious public health threats by transmitting a variety of disease causing agents. Most of these diseases lack effective vaccines and rely on vector control to prevent pathogen transmission. Our knowledge of interactions between vectors and pathogens, especially vector immune responses to pathogens, has been advancing at an accelerating pace. However, mechanisms underlining interactions between pathogens and PGRPs are not fully studied. Thus, expanding the knowledge of PGRPs, the major PRRs involved in pathogen recognition, will provide fundamental information about vectors-pathogens interaction, and thus contribute to development of improved vector associated disease treatments.
Highlights.
Summarize the latest findings of PGRPs in Drosophila
Summarize the latest findings of PGRPs in major disease transmitting vectors
Compare the organization and functions of PGRPs between vectors and Drosophila
Acknowledgments
Funding: This work was supported by National Research and Development Plan of China (No. 2016YFC1200500), National Natural Science Foundation of China (31472039 and 81601793), National Institutes of Health Grant (R01AI129819) and the Research Fund of the State Key Laboratory of Genetic Engineering, Fudan University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barletta AB, Nascimento-Silva MC, Talyuli OA, Oliveira JH, Pereira LO, Oliveira PL, Sorgine MH. Microbiota activates IMD pathway and limits Sindbis infection in Aedes aegypti. Parasit Vectors. 2017;10:103. doi: 10.1186/s13071-017-2040-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basbous N, Coste F, Leone P, Vincentelli R, Royet J, Kellenberger C, Roussel A. The Drosophila peptidoglycan-recognition protein LF interacts with peptidoglycan-recognition protein LC to downregulate the Imd pathway. EMBO Rep. 2011;12:327–333. doi: 10.1038/embor.2011.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit JB, Attardo GM, Baumann AA, Michalkova V, Aksoy S. Adenotrophic viviparity in tsetse flies: potential for population control and as an insect model for lactation. Annu Rev Entomol. 2015;60:351–371. doi: 10.1146/annurev-ento-010814-020834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GR, Simmons CP, Scott TW, Farrar JJ, Hay SI. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff V, Vignal C, Boneca IG, Michel T, Hoffmann JA, Royet J. Function of the drosophila pattern-recognition receptor PGRP-SD in the detection of Gram-positive bacteria. Nat Immunol. 2004;5:1175–1180. doi: 10.1038/ni1123. [DOI] [PubMed] [Google Scholar]
- Capelli-Peixoto J, Carvalho DD, Johnson WC, Scoles GA, Fogaca AC, Daffre S, Ueti MW. The transcription factor Relish controls Anaplasma marginale infection in the bovine tick Rhipicephalus microplus. Dev Comp Immunol. 2017;74:32–39. doi: 10.1016/j.dci.2017.04.005. [DOI] [PubMed] [Google Scholar]
- Caraballo H, King K. Emergency department management of mosquito-borne illness: malaria, dengue, and West Nile virus. Emerg Med Pract. 2014;16:1–23. quiz 23–24. [PubMed] [Google Scholar]
- Cheng G, Liu Y, Wang P, Xiao X. Mosquito Defense Strategies against Viral Infection. Trends Parasitol. 2016;32:177–186. doi: 10.1016/j.pt.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe KM, Werner T, Stoven S, Hultmark D, Anderson KV. Requirement for a peptidoglycan recognition protein (PGRP) in Relish activation and antibacterial immune responses in Drosophila. Science. 2002;296:359–362. doi: 10.1126/science.1070216. [DOI] [PubMed] [Google Scholar]
- Christophides GK, Zdobnov E, Barillas-Mury C, Birney E, Blandin S, Blass C, Brey PT, Collins FH, Danielli A, Dimopoulos G, Hetru C, Hoa NT, Hoffmann JA, Kanzok SM, Letunic I, Levashina EA, Loukeris TG, Lycett G, Meister S, Michel K, Moita LF, Muller HM, Osta MA, Paskewitz SM, Reichhart JM, Rzhetsky A, Troxler L, Vernick KD, Vlachou D, Volz J, von Mering C, Xu J, Zheng L, Bork P, Kafatos FC. Immunity-related genes and gene families in Anopheles gambiae. Science. 2002;298:159–165. doi: 10.1126/science.1077136. [DOI] [PubMed] [Google Scholar]
- Chu H, Mazmanian SK. Innate immune recognition of the microbiota promotes host-microbial symbiosis. Nat Immunol. 2013;14:668–675. doi: 10.1038/ni.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton AM, Dong Y, Dimopoulos G. The Anopheles innate immune system in the defense against malaria infection. J Innate Immun. 2014;6:169–181. doi: 10.1159/000353602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Manfredini F, Dimopoulos G. Implication of the mosquito midgut microbiota in the defense against malaria parasites. PLoS Pathog. 2009;5:e1000423. doi: 10.1371/journal.ppat.1000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erkosar B, Storelli G, Mitchell M, Bozonnet L, Bozonnet N, Leulier F. Pathogen Virulence Impedes Mutualist-Mediated Enhancement of Host Juvenile Growth via Inhibition of Protein Digestion. Cell Host Microbe. 2015;18:445–455. doi: 10.1016/j.chom.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frolet C, Thoma M, Blandin S, Hoffmann JA, Levashina EA. Boosting NF-kappaB-dependent basal immunity of Anopheles gambiae aborts development of Plasmodium berghei. Immunity. 2006;25:677–685. doi: 10.1016/j.immuni.2006.08.019. [DOI] [PubMed] [Google Scholar]
- Garver LS, Bahia AC, Das S, Souza-Neto JA, Shiao J, Dong Y, Dimopoulos G. Anopheles Imd pathway factors and effectors in infection intensity-dependent anti-Plasmodium action. PLoS Pathog. 2012;8:e1002737. doi: 10.1371/journal.ppat.1002737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garver LS, Dong Y, Dimopoulos G. Caspar controls resistance to Plasmodium falciparum in diverse anopheline species. PLoS Pathog. 2009;5:e1000335. doi: 10.1371/journal.ppat.1000335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendrin M, Turlure F, Rodgers FH, Cohuet A, Morlais I, Christophides GK. The Peptidoglycan Recognition Proteins PGRPLA and PGRPLB Regulate Anopheles Immunity to Bacteria and Affect Infection by Plasmodium. J Innate Immun. 2017;9:333–342. doi: 10.1159/000452797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobert V, Gottar M, Matskevich AA, Rutschmann S, Royet J, Belvin M, Hoffmann JA, Ferrandon D. Dual activation of the Drosophila toll pathway by two pattern recognition receptors. Science. 2003;302:2126–2130. doi: 10.1126/science.1085432. [DOI] [PubMed] [Google Scholar]
- Golding N, Wilson AL, Moyes CL, Cano J, Pigott DM, Velayudhan R, Brooker SJ, Smith DL, Hay SI, Lindsay SW. Integrating vector control across diseases. BMC Med. 2015;13:249. doi: 10.1186/s12916-015-0491-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottar M, Gobert V, Michel T, Belvin M, Duyk G, Hoffmann JA, Ferrandon D, Royet J. The Drosophila immune response against Gram-negative bacteria is mediated by a peptidoglycan recognition protein. Nature. 2002;416:640–644. doi: 10.1038/nature734. [DOI] [PubMed] [Google Scholar]
- Harris N, Braiser DJ, Dickman DK, Fetter RD, Tong A, Davis GW. The Innate Immune Receptor PGRP-LC Controls Presynaptic Homeostatic Plasticity. Neuron. 2015;88:1157–1164. doi: 10.1016/j.neuron.2015.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann JA. The immune response of Drosophila. Nature. 2003;426:33–38. doi: 10.1038/nature02021. [DOI] [PubMed] [Google Scholar]
- Hoffmann JA, Reichhart JM. Drosophila innate immunity: an evolutionary perspective. Nat Immunol. 2002;3:121–126. doi: 10.1038/ni0202-121. [DOI] [PubMed] [Google Scholar]
- Hu C, Aksoy S. Innate immune responses regulate trypanosome parasite infection of the tsetse fly Glossina morsitans morsitans. Mol Microbiol. 2006;60:1194–1204. doi: 10.1111/j.1365-2958.2006.05180.x. [DOI] [PubMed] [Google Scholar]
- Iatsenko I, Kondo S, Mengin-Lecreulx D, Lemaitre B. PGRP-SD, an Extracellular Pattern-Recognition Receptor, Enhances Peptidoglycan-Mediated Activation of the Drosophila Imd Pathway. Immunity. 2016;45:1013–1023. doi: 10.1016/j.immuni.2016.10.029. [DOI] [PubMed] [Google Scholar]
- International Glossina Genome I. Genome sequence of the tsetse fly (Glossina morsitans): vector of African trypanosomiasis. Science. 2014;344:380–386. doi: 10.1126/science.1249656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko T, Goldman WE, Mellroth P, Steiner H, Fukase K, Kusumoto S, Harley W, Fox A, Golenbock D, Silverman N. Monomeric and polymeric gram-negative peptidoglycan but not purified LPS stimulate the Drosophila IMD pathway. Immunity. 2004;20:637–649. doi: 10.1016/s1074-7613(04)00104-9. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Yano T, Aggarwal K, Lim JH, Ueda K, Oshima Y, Peach C, Erturk-Hasdemir D, Goldman WE, Oh BH, Kurata S, Silverman N. PGRP-LC and PGRP-LE have essential yet distinct functions in the drosophila immune response to monomeric DAP-type peptidoglycan. Nat Immunol. 2006;7:715–723. doi: 10.1038/ni1356. [DOI] [PubMed] [Google Scholar]
- Kang D, Liu G, Lundstrom A, Gelius E, Steiner H. A peptidoglycan recognition protein in innate immunity conserved from insects to humans. Proc Natl Acad Sci U S A. 1998;95:10078–10082. doi: 10.1073/pnas.95.17.10078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopacek P, Hajdusek O, Buresova V, Daffre S. Tick innate immunity. Adv Exp Med Biol. 2010;708:137–162. [PubMed] [Google Scholar]
- Kurata S. Peptidoglycan recognition proteins in Drosophila immunity. Dev Comp Immunol. 2014;42:36–41. doi: 10.1016/j.dci.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavine MD, Strand MR. Insect hemocytes and their role in immunity. Insect Biochem Mol Biol. 2002;32:1295–1309. doi: 10.1016/s0965-1748(02)00092-9. [DOI] [PubMed] [Google Scholar]
- Lehane MJ, Aksoy S, Levashina E. Immune responses and parasite transmission in blood-feeding insects. Trends Parasitol. 2004;20:433–439. doi: 10.1016/j.pt.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Leulier F, Parquet C, Pili-Floury S, Ryu JH, Caroff M, Lee WJ, Mengin-Lecreulx D, Lemaitre B. The Drosophila immune system detects bacteria through specific peptidoglycan recognition. Nat Immunol. 2003;4:478–484. doi: 10.1038/ni922. [DOI] [PubMed] [Google Scholar]
- Luplertlop N, Surasombatpattana P, Patramool S, Dumas E, Wasinpiyamongkol L, Saune L, Hamel R, Bernard E, Sereno D, Thomas F, Piquemal D, Yssel H, Briant L, Misse D. Induction of a peptide with activity against a broad spectrum of pathogens in the Aedes aegypti salivary gland, following Infection with Dengue Virus. PLoS Pathog. 2011;7:e1001252. doi: 10.1371/journal.ppat.1001252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister S, Agianian B, Turlure F, Relogio A, Morlais I, Kafatos FC, Christophides GK. Anopheles gambiae PGRPLC-mediated defense against bacteria modulates infections with malaria parasites. PLoS Pathog. 2009;5:e1000542. doi: 10.1371/journal.ppat.1000542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister S, Kanzok SM, Zheng XL, Luna C, Li TR, Hoa NT, Clayton JR, White KP, Kafatos FC, Christophides GK, Zheng L. Immune signaling pathways regulating bacterial and malaria parasite infection of the mosquito Anopheles gambiae. Proc Natl Acad Sci U S A. 2005;102:11420–11425. doi: 10.1073/pnas.0504950102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellroth P, Karlsson J, Hakansson J, Schultz N, Goldman WE, Steiner H. Ligand-induced dimerization of Drosophila peptidoglycan recognition proteins in vitro. Proc Natl Acad Sci U S A. 2005;102:6455–6460. doi: 10.1073/pnas.0407559102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellroth P, Karlsson J, Steiner H. A scavenger function for a Drosophila peptidoglycan recognition protein. J Biol Chem. 2003;278:7059–7064. doi: 10.1074/jbc.M208900200. [DOI] [PubMed] [Google Scholar]
- Mesquita RD, Vionette-Amaral RJ, Lowenberger C, Rivera-Pomar R, Monteiro FA, Minx P, Spieth J, Carvalho AB, Panzera F, Lawson D, Torres AQ, Ribeiro JM, Sorgine MH, Waterhouse RM, Montague MJ, Abad-Franch F, Alves-Bezerra M, Amaral LR, Araujo HM, Araujo RN, Aravind L, Atella GC, Azambuja P, Berni M, Bittencourt-Cunha PR, Braz GR, Calderon-Fernandez G, Carareto CM, Christensen MB, Costa IR, Costa SG, Dansa M, Daumas-Filho CR, De-Paula IF, Dias FA, Dimopoulos G, Emrich SJ, Esponda-Behrens N, Fampa P, Fernandez-Medina RD, da Fonseca RN, Fontenele M, Fronick C, Fulton LA, Gandara AC, Garcia ES, Genta FA, Giraldo-Calderon GI, Gomes B, Gondim KC, Granzotto A, Guarneri AA, Guigo R, Harry M, Hughes DS, Jablonka W, Jacquin-Joly E, Juarez MP, Koerich LB, Lange AB, Latorre-Estivalis JM, Lavore A, Lawrence GG, Lazoski C, Lazzari CR, Lopes RR, Lorenzo MG, Lugon MD, Majerowicz D, Marcet PL, Mariotti M, Masuda H, Megy K, Melo AC, Missirlis F, Mota T, Noriega FG, Nouzova M, Nunes RD, Oliveira RL, Oliveira-Silveira G, Ons S, Orchard I, Pagola L, Paiva-Silva GO, Pascual A, Pavan MG, Pedrini N, Peixoto AA, Pereira MH, Pike A, Polycarpo C, Prosdocimi F, Ribeiro-Rodrigues R, Robertson HM, Salerno AP, Salmon D, Santesmasses D, Schama R, Seabra-Junior ES, Silva-Cardoso L, Silva-Neto MA, Souza-Gomes M, Sterkel M, Taracena ML, Tojo M, Tu ZJ, Tubio JM, Ursic-Bedoya R, Venancio TM, Walter-Nuno AB, Wilson D, Warren WC, Wilson RK, Huebner E, Dotson EM, Oliveira PL. Genome of Rhodnius prolixus, an insect vector of Chagas disease, reveals unique adaptations to hematophagy and parasite infection. Proc Natl Acad Sci U S A. 2015;112:14936–14941. doi: 10.1073/pnas.1506226112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel T, Reichhart JM, Hoffmann JA, Royet J. Drosophila Toll is activated by Gram-positive bacteria through a circulating peptidoglycan recognition protein. Nature. 2001;414:756–759. doi: 10.1038/414756a. [DOI] [PubMed] [Google Scholar]
- Nene V, Wortman JR, Lawson D, Haas B, Kodira C, Tu ZJ, Loftus B, Xi Z, Megy K, Grabherr M, Ren Q, Zdobnov EM, Lobo NF, Campbell KS, Brown SE, Bonaldo MF, Zhu J, Sinkins SP, Hogenkamp DG, Amedeo P, Arensburger P, Atkinson PW, Bidwell S, Biedler J, Birney E, Bruggner RV, Costas J, Coy MR, Crabtree J, Crawford M, Debruyn B, Decaprio D, Eiglmeier K, Eisenstadt E, El-Dorry H, Gelbart WM, Gomes SL, Hammond M, Hannick LI, Hogan JR, Holmes MH, Jaffe D, Johnston JS, Kennedy RC, Koo H, Kravitz S, Kriventseva EV, Kulp D, Labutti K, Lee E, Li S, Lovin DD, Mao C, Mauceli E, Menck CF, Miller JR, Montgomery P, Mori A, Nascimento AL, Naveira HF, Nusbaum C, O’Leary S, Orvis J, Pertea M, Quesneville H, Reidenbach KR, Rogers YH, Roth CW, Schneider JR, Schatz M, Shumway M, Stanke M, Stinson EO, Tubio JM, Vanzee JP, Verjovski-Almeida S, Werner D, White O, Wyder S, Zeng Q, Zhao Q, Zhao Y, Hill CA, Raikhel AS, Soares MB, Knudson DL, Lee NH, Galagan J, Salzberg SL, Paulsen IT, Dimopoulos G, Collins FH, Birren B, Fraser-Liggett CM, Severson DW. Genome sequence of Aedes aegypti, a major arbovirus vector. Science. 2007;316:1718–1723. doi: 10.1126/science.1138878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyen C, Poidevin M, Roussel A, Lemaitre B. Tissue- and ligand-specific sensing of gram-negative infection in drosophila by PGRP-LC isoforms and PGRP-LE. J Immunol. 2012;189:1886–1897. doi: 10.4049/jimmunol.1201022. [DOI] [PubMed] [Google Scholar]
- Neyen C, Runchel C, Schupfer F, Meier P, Lemaitre B. The regulatory isoform rPGRP-LC induces immune resolution via endosomal degradation of receptors. Nat Immunol. 2016;17:1150–1158. doi: 10.1038/ni.3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva Chavez AS, Shaw DK, Munderloh UG, Pedra JH. Tick Humoral Responses: Marching to the Beat of a Different Drummer. Front Microbiol. 2017;8:223. doi: 10.3389/fmicb.2017.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X, Pike A, Joshi D, Bian G, McFadden MJ, Lu P, Liang X, Zhang F, Raikhel AS, Xi Z. The bacterium Wolbachia exploits host innate immunity to establish a symbiotic relationship with the dengue vector mosquito Aedes aegypti. ISME journal. 2017 doi: 10.1038/ismej.2017.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X, Zhou G, Wu J, Bian G, Lu P, Raikhel AS, Xi Z. Wolbachia induces reactive oxygen species (ROS)-dependent activation of the Toll pathway to control dengue virus in the mosquito Aedes aegypti. Proc Natl Acad Sci U S A. 2012;109:E23–31. doi: 10.1073/pnas.1116932108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson C, Oldenvi S, Steiner H. Peptidoglycan recognition protein LF: a negative regulator of Drosophila immunity. Insect Biochem Mol Biol. 2007;37:1309–1316. doi: 10.1016/j.ibmb.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Ramet M, Manfruelli P, Pearson A, Mathey-Prevot B, Ezekowitz RA. Functional genomic analysis of phagocytosis and identification of a Drosophila receptor for E. coli. Nature. 2002;416:644–648. doi: 10.1038/nature735. [DOI] [PubMed] [Google Scholar]
- Rikihisa Y. Anaplasma phagocytophilum and Ehrlichia chaffeensis: subversive manipulators of host cells. Nat Rev Microbiol. 2010;8:328–339. doi: 10.1038/nrmicro2318. [DOI] [PubMed] [Google Scholar]
- Rosa RD, Capelli-Peixoto J, Mesquita RD, Kalil SP, Pohl PC, Braz GR, Fogaca AC, Daffre S. Exploring the immune signalling pathway-related genes of the cattle tick Rhipicephalus microplus: From molecular characterization to transcriptional profile upon microbial challenge. Dev Comp Immunol. 2016;59:1–14. doi: 10.1016/j.dci.2015.12.018. [DOI] [PubMed] [Google Scholar]
- Royet J, Dziarski R. Peptidoglycan recognition proteins: pleiotropic sensors and effectors of antimicrobial defences. Nat Rev Microbiol. 2007;5:264–277. doi: 10.1038/nrmicro1620. [DOI] [PubMed] [Google Scholar]
- Royet J, Gupta D, Dziarski R. Peptidoglycan recognition proteins: modulators of the microbiome and inflammation. Nat Reviews Immunol. 2011;11:837–851. doi: 10.1038/nri3089. [DOI] [PubMed] [Google Scholar]
- Rus F, Flatt T, Tong M, Aggarwal K, Okuda K, Kleino A, Yates E, Tatar M, Silverman N. Ecdysone triggered PGRP-LC expression controls Drosophila innate immunity. EMBO J. 2013;32:1626–1638. doi: 10.1038/emboj.2013.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleifer KH, Kandler O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev. 1972;36:407–477. doi: 10.1128/br.36.4.407-477.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severo MS, Choy A, Stephens KD, Sakhon OS, Chen G, Chung DW, Le Roch KG, Blaha G, Pedra JH. The E3 ubiquitin ligase XIAP restricts Anaplasma phagocytophilum colonization of Ixodes scapularis ticks. J Infect Dis. 2013;208:1830–1840. doi: 10.1093/infdis/jit380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw DK, Wang X, Brown LJ, Chavez AS, Reif KE, Smith AA, Scott AJ, McClure EE, Boradia VM, Hammond HL, Sundberg EJ, Snyder GA, Liu L, DePonte K, Villar M, Ueti MW, de la Fuente J, Ernst RK, Pal U, Fikrig E, Pedra JH. Infection-derived lipids elicit an immune deficiency circuit in arthropods. Nat Commun. 2017;8:14401. doi: 10.1038/ncomms14401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AA, Pal U. Immunity-related genes in Ixodes scapularis--perspectives from genome information. Front Cell Infect Microbiol. 2014;4:116. doi: 10.3389/fcimb.2014.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Aksoy S. PGRP-LB is a maternally transmitted immune milk protein that influences symbiosis and parasitism in tsetse’s offspring. Proc Natl Acad Sci U S A. 2012;109:10552–10557. doi: 10.1073/pnas.1116431109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wu Y, Yang G, Aksoy S. Interactions between mutualist Wigglesworthia and tsetse peptidoglycan recognition protein (PGRP-LB) influence trypanosome transmission. Proc Natl Acad Sci U S A. 2009;106:12133–12138. doi: 10.1073/pnas.0901226106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Gilbert RJ, Atilano ML, Filipe SR, Gay NJ, Ligoxygakis P. Peptidoglycan recognition protein-SD provides versatility of receptor formation in Drosophila immunity. Proc Natl Acad Sci U S A. 2008;105:11881–11886. doi: 10.1073/pnas.0710092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Beerntsen BT. Functional implications of the peptidoglycan recognition proteins in the immunity of the yellow fever mosquito, Aedes aegypti. Insect Mol Biol. 2015;24:293–310. doi: 10.1111/imb.12159. [DOI] [PubMed] [Google Scholar]
- Werner T, Borge-Renberg K, Mellroth P, Steiner H, Hultmark D. Functional diversity of the Drosophila PGRP-LC gene cluster in the response to lipopolysaccharide and peptidoglycan. J Biol Chem. 2003;278:26319–26322. doi: 10.1074/jbc.C300184200. [DOI] [PubMed] [Google Scholar]
- Werner T, Liu G, Kang D, Ekengren S, Steiner H, Hultmark D. A family of peptidoglycan recognition proteins in the fruit fly Drosophila melanogaster. Proc Natl Acad Sci U S A. 2000;97:13772–13777. doi: 10.1073/pnas.97.25.13772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. World Malaria Report 2016. 2016. [Google Scholar]
- Xi Z, Ramirez JL, Dimopoulos G. The Aedes aegypti toll pathway controls dengue virus infection. PLoS Pathog. 2008;4:e1000098. doi: 10.1371/journal.ppat.1000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano T, Kurata S. Intracellular recognition of pathogens and autophagy as an innate immune host defence. J Biochem. 2011;150:143–149. doi: 10.1093/jb/mvr083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano T, Mita S, Ohmori H, Oshima Y, Fujimoto Y, Ueda R, Takada H, Goldman WE, Fukase K, Silverman N, Yoshimori T, Kurata S. Autophagic control of listeria through intracellular innate immune recognition in drosophila. Nat Immunol. 2008;9:908–916. doi: 10.1038/ni.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H, Kinoshita K, Ashida M. Purification of a peptidoglycan recognition protein from hemolymph of the silkworm, Bombyx mori. J Biol Chem. 1996;271:13854–13860. doi: 10.1074/jbc.271.23.13854. [DOI] [PubMed] [Google Scholar]