Abstract
Background
An issue of critical importance for psychiatry and women’s health is whether postpartum depression (PPD) represents a unique condition. The Diagnostic and Statistical Manual of Mental Disorders asserts that major depressive disorder (MDD) may present with peripartum onset, without suggesting any other differences between MDD and PPD. The absence of any distinct features calls into question the nosologic validity of PPD as a diagnostic category. The present study investigates whether symptom profiles differ between PPD and depression occurring outside the postpartum phase.
Methods
In a prospective, longitudinal study of parturient women (N=239), we examine the manifestation of depression symptoms. We assess factor structure of symptom profiles, and whether factors are differentially pronounced during and after the postpartum period.
Results
Factors were revealed representing: Worry, Emotional/Circadian/Energetic Dysregulation, Somatic/Cognitive, Appetite, Distress Display, and Anger symptoms. The factor structure was validated at postpartum and after-postpartum timepoints. Interestingly, the Worry factor, comprising anxiety and guilt, was significantly more pronounced during the postpartum timepoint, and the Emotional/Circadian/Energetic Dysregulation factor, which contained sadness and anhedonia, was significantly less pronounced during the postpartum period.
Conclusions
These results suggest that PPD may be a unique syndrome, necessitating research, diagnosis, and treatment strategies distinct from those for MDD. Results indicate the possibility that worry is an enhanced feature of PPD compared to depression outside the postpartum period, and the crucial role of sadness/anhedonia in MDD diagnosis may be less applicable to PPD diagnosis.
Keywords: Postpartum Depression, Diagnosis, Women’s Health, Classification, Symptom Cluster, Psychiatric Diagnosis, Statistical Factor Analysis, Prospective Studies, Diagnostic and Statistical Manual of Mental Disorders
1. INTRODUCTION
Depression conveys the greatest burden of any disease in the U.S. (C. J. Murray et al., 2013), and represents a unique public heath challenge due to the combined effects of its ubiquity, heterogeneity, bio-socio-psychological complexity, and the suffering it imposes on individuals, families, and communities. One of the most frequent yet understudied precipitators of depression is childbearing. It has been estimated that globally, 1 in 8 new mothers suffers from postpartum depression (PPD) (O’Hara & Swain, 1996). PPD inflicts an exceptionally pernicious impact because it poses serious threats to the well-being of both the mother (Goodman, 2007; O’Hara, 2009) and child. Infants of mothers suffering from PPD exhibit higher mortality rates and lifelong cognitive, social, and health detriments (Goodman, 2007; L. Murray et al., 2011; Verbeek et al., 2012).
Despite the high incidence and personally damaging repercussions of PPD, it is not known whether PPD represents a distinct syndrome from major depressive disorder (MDD) occurring outside the peripartum period (Bernstein et al., 2008; Hendrick, Altshuler, Strouse, & Grosser, 2000). As a result, we are severely limited in our ability to recognize or intervene in this devastating mental illness. Here, we address this major lacuna in our understanding of depression during the peripartum phase. Our approach focuses on symptom clustering and differential manifestation of particular symptom constellations in response to childbearing to determine the validity of PPD as a diagnostic category.
Whether PPD is different from MDD is a question of scientific and clinical relevance. In order to resolve its diagnostic status, it must be determined whether PPD has psychobiological mechanisms of risk, etiology, or manifestation that are unique from those of MDD. It is plausible to expect that PPD etiology is distinct from etiology of depression occurring outside the perinatal phase because endocrine regulation of mood has been implicated in a wide range of psychopathologies (Taylor, Maloney, Dearborn, & Weiss, 2009) and pregnancy involves endocrine fluctuations that are unparalleled in magnitude compared to the rest of the lifespan, even puberty and menopause (Tulchinsky & Little, 1994). Strong evidence from animal models and emerging evidence from human research suggests that pregnancy induces profound alterations to maternal brain structure and function (Glynn, 2010; Glynn, Davis, Sandman, & Goldberg, 2016; Yim et al., 2009). The distinctive endocrine experience and exceptional neuroplasticity associated with pregnancy justify the hypothesis that PPD may have distinct etiologic characteristics compared to depression occurring during other life phases.
Furthermore, PPD and MDD are associated with different neurobiological profiles. Women with PPD exhibit hypoactive resting-state neural activity in cortical and subcortical limbic regions compared with non-PPD mothers, while non-perinatal MDD men and women exhibit hypoactive resting-state neural activity in lateral brain regions and resting-state hyperactivity in medial affective and subcortical limbic regions compared with non-MDD individuals (reviewed in (Pawluski, Lonstein, & Fleming, 2017)). PPD and MDD are also associated with divergent activation profiles in response to non-infant emotional cues. Women with PPD exhibit lower activation in the amygdala and striatum (Barrett et al., 2012; Moses-Kolko, Horner, Phillips, Hipwell, & Swain, 2014), and individuals with MDD exhibit higher activation in the amygdala and striatum (Drevets, 2000; Phillips, Drevets, Rauch, & Lane, 2003). These differences in brain resting-state and activation profiles suggest that PPD could be a condition etiologically distinct from MDD, or have distinct characteristics.
The National Institutes of Mental Health (NIMH) recently instituted the Research Domain Criteria (RDoC) project in response to the limitations traditional diagnostic categories impose on the study of mental illness (Cuthbert & Insel, 2013). Our study design is compatible with the RDoC emphases on dimensionality and deconstruction of heterogeneous diagnostic categories (e.g., MDD) into fundamental components. In terms of dimensionality, we utilize a continuous scale measurement of each depressive symptom rather than the traditional binary medical approach, i.e., “depressed” versus “not depressed.” Exploring the essence and constitution of PPD requires a gradient quantitative analysis as a precondition of developing binary systems of participant recruitment and statistical analysis. In terms of deconstructing heterogeneous diagnostic categories into fundamental components, we assert that symptom-based approaches are necessary for clarifying how we define and classify mental illnesses (Calamari, Wiegartz, & Janeck, 1999) in order to identify biomarkers and genetic risk factors, and evaluate treatment efficacy (Bech, 2006; Fried & Nesse, 2015). For depression research, this necessity is born out in evidence that specific symptoms, compared with threshold scores, have demonstrated stronger relationships with allelic variants (Kendler, Aggen, & Neale, 2013; Myung et al., 2012), inflammatory (Duivis, Vogelzangs, Kupper, de Jonge, & Penninx, 2013) and hormone profiles (Lamers et al., 2013), and antidepressant responses (Dew et al., 1997). Subtyping of depression has been based upon symptom profiles (e.g., atypical), precipitating causes (e.g., seasonal affective disorder), or both (Keller & Nesse, 2006). This study will inform our understanding of whether the precipitating event of childbirth justifies a depression subtype or distinct syndrome based on symptom profiles, beyond just a precipitating event specifier (viz. Diagnostic and Statistical Manual of Mental Disorders (DSM)-5). While the PPD acronym may refer to either perinatal or postpartum depression, here we utilize the latter terminology because our timepoint of interest is during the postpartum phase.
Despite the necessity and benefits of symptom-based approaches for diagnosing and studying depression, many studies use categories based on depression rating scale sum score cutoffs, which amalgamate individuals into one undifferentiated classification. This procedure is based on the erroneous suppositions that depression is a monolithic syndrome, and all symptoms are interchangeable and contribute equivalently to diagnostic classification (Fried & Nesse, 2015). Psychometrically validated instruments that are used to assess depression in clinical research utilize the sum of self-reported Likert-scale assessments, with a threshold cutoff score for classifying individuals as depressed. The use of sum scores and cutoff thresholds imposes three major impediments to research. Firstly, a sum score reveals no information about symptomology, and this loss of information impairs the interpretability of the score. Secondly, cutoff thresholds treat all symptoms as having equivalent importance for diagnosis. This practice is contrary to the diagnostic criteria of the DSM and International Statistical Classification of Diseases (ICD), which highlight certain symptoms as necessary and others as ancillary (Table 2). Myriad combinations of symptom endorsements and severities can result in scores above the threshold. Thirdly, cutoff thresholds result in loss of the dimensionality that is valuable for statistically modeling depression’s relationships with risk factors and consequences.
Table 2.
Depression symptoms full list and subset assessed in this study
| DSM-5 i | ICD-10 j | IDSk | SDQl | DSSm | BDIn | CIDIo | Scale with which we measure symptom | Item with which we measure symptom | |
|---|---|---|---|---|---|---|---|---|---|
| Sadness | x | x | x | x | x | x | x | CESDa | I felt sad |
| Fatigue | x | x | x | x | x | x | x | Phys Symc | Fatigue |
| Loss of energy | x | x | x | ||||||
| Sleepiness | x | x | |||||||
| Guilt | x | x | x | x | x | x | x | EPDSd | I have blamed myself unnecessarily when things went wrong |
| Worthlessness/loss of confidence | x | x | x | x | |||||
| Psychomotor retardation/feeling slowed down | x | x | x | x | x | CESDa | I felt that everything I did was an effort | ||
| Psychomotor agitation | x | x | |||||||
| Thoughts or desires of self-harm | x | EPDSd | The thought of harming myself has occurred to me | ||||||
| Agitated | x | x | CESDa | I was bothered by things that don’t usually bother me | |||||
| Diminished mental sharpness/ability to think | x | x | x | x | |||||
| Inability to focus/concentrate | x | x | x | x | CESDa | I had trouble keeping my mind on what I was doing | |||
| Indecisiveness | x | x | x | ||||||
| Weight loss | x | x | x | x | x | ||||
| Weight gain | x | x | |||||||
| Decreased appetite | x | x | x | x | x | Phys Symc | Poor appetite | ||
| Increased appetite | x | x | x | ||||||
| Anhedonia/diminished capacity for pleasure | x | x | x | x | x | CESDa | I enjoyed life (reverse scored) | ||
| Loss of interest | x | x | x | x | |||||
| Inability to fall asleep/insomnia | x | x | x | x | x | x | |||
| Oversleeping/hypersomnia | x | x | x | x | |||||
| Inability to stay asleep | x | x | x | CESDa | My sleep was restless. | ||||
| Positive mood reactivity | x | x | |||||||
| Negative mood reactivity | x | PSSe | How often have you been upset because of something that happened unexpectedly | ||||||
| Pessimism | x | x | x | x | x | STAIb | I am presently worrying over possible misfortunes | ||
| Anxiety | x | x | x | x | EPDSd | I have been anxious or worried for no good reason | |||
| Irritable | x | x | x | PSSe | How often have you been able to control irritations in your life? | ||||
| Heart palpitations | x | x | Phys Symc | Palpitations | |||||
| Pains/aches | x | x | Phys Symc | Back pain, Joint pain, Muscle pain, Leg paing | |||||
| Heavy limbs/weighted down | x | ||||||||
| GI problems | x | x | Phys Symc | Constipation, Diarrhea, Heartburn, Nausea, Vomitingg | |||||
| Sad affect display | x | CESDa | I felt that I could not shake off the blues even with help from my family and friends | ||||||
| Crying | x | x | x | x | EPDSd | I have been so unhappy that I have been crying | |||
| Sudden anger attacks | x | STAXIf | I feel like breaking things | ||||||
| Anger | x | STAIb | I feel angry | ||||||
| Diminished social functioning | x | ||||||||
| Lack of control/helplessness | x | ||||||||
| Past failure | x | ||||||||
| Punishment feelings | x | ||||||||
| Self-dislike | x | ||||||||
| Memory problems | x | ||||||||
| Ability to find words | x | ||||||||
| Ability to work/study/function at home | x | ||||||||
| Panic/phobic | x | x | |||||||
| Diurnal mood pattern | x | x | |||||||
| Diminished sexual interest | x | x | x | ||||||
| Diminished sexual functioning | x | ||||||||
| Interpersonal sensitivity | x | x | |||||||
| Unresponsive mood/flat affect | x | ||||||||
| Rumination | x | ||||||||
| Desire for social support | x | ||||||||
| Thoughts or desires of death | x | x | x | x | x | ||||
| Thoughts or desires of suicide | x | x | x | ||||||
CESD: Center for Epidemiologic Studies Depression Scale (Radloff, 1991). In this study the CESD Short Form (SF) (Santor & Coyne, 1997) was administered.
STAI: State-Trait Anxiety Inventory (Kvaal, Ulstein, Nordhus, & Engedal, 2005)
Phys Sym: Physical symptoms questionnaire for pregnancy and postpartum
EPDS: Edinburgh Postnatal Depression Scale (Cox, Holden, & Sagovsky, 1987)
PSS: Perceived Stress Scale (Cohen, Kamarck, & Mermelstein, 1983)
STAXI: State Trait Anger Expression Inventory (Fuqua et al., 1991)
Two depression symptoms were characterized by more than one item. “Pains and aches” was assessed by separate questions about back, joint, muscle, and leg pains. “GI (stomach or bowel) symptoms” was assessed by separate questions about constipation, diarrhea, heartburn, nausea, and vomiting. Each of these items was assessed from a questionnaire in which women described their frequency of each experience on Likert scales ranging from 1=never to 5=four or more times per week. These items were recoded as binary, with 0=never and 1=one or more times per week. Then, the sum was taken so that “Pain and aches” scores ranged from 0–4 and reflected how many kinds of pains women experienced per week, while “GI (stomach or bowel) symptoms” scores ranged from 0–5 and reflected how many kids of GI problems women experienced per week. Subsequently, these variables along with all symptom variables were unitized.
DSM-5: Diagnostic and Statistical Manual of Mental Disorders Fifth edition (American_Psychiatric_Association, 2003)
ICD-10: International Statistical Classification of Diseases and Related Health Problems 10th Revision (World_Health_Organization, 2016)
IDS: Inventory of Depressive Symptomatology (Rush, Carmody, & Reimitz, 2000)
SDQ: Symptoms of Depression Questionnaire (Pedrelli et al., 2014)
DSS: Depressive Symptoms Scale (Keller & Nesse, 2006)
BDI: Beck Depression Inventory (Beck, Steer, & Carbin, 1988)
CIDI: WHO-Composite International Diagnostic Interview (Wittchen, 1994)
Items that encompass more than one symptom (as defined by other scales that separate that item) are signified by a merged cell. The DSM-5 defines MDD as at least a two-week period of low mood or loss of pleasure, with no history of mania, alongside other daily symptoms including low appetite or weight loss, insomnia or hypersomnia, psychomotor retardation or agitation, fatigue, feelings of worthlessness or inappropriate guilt, diminished ability to think or concentrate, and/or suicidal behavior or ideation. ICD-10 proposes a similar definition involving at least one of these symptoms: persistent sadness or low mood, loss of interests or pleasure, fatigue or low energy, most days, most of the time for at least 2 weeks, alongside the following for a total of at least four symptoms: reduced enjoyment, reduced interest, reduced concentration, fatigue, tiredness, guilt, worthlessness, reduced self-esteem, and/or somatic symptoms such as loss of pleasure, sleep dysregulation, psychomotor retardation or agitation, loss of appetite, weight loss, and/or loss of libido.
We posit the widely-held assumption of PPD’s symptomatic similitude with MDD along with the wide use of sum score cutoffs to study PPD may have obfuscated unique aspects of perinatal mood dysregulation that could prove crucial for discerning the etiology of this ubiquitous form of mental illness. We therefore aim to discern whether depressive symptom constellations manifest differently during the postpartum phase compared with years later. We use a dimensional approach by measuring symptomology in the full cohort, and also repeat all analyses for the subset of women who meet traditional research study criteria for at least minor depression (Martens et al., 2006; S Matthey, Henshaw, Elliott, & Barnett, 2006), for the subset of women who did not use anti-depressant medications at the postpartum timepoint, and for the subsets of women who were and were not breastfeeding at the postpartum timepoint.
2. METHODS
2.1. Cohort and procedure
Study participants were 239 women participating in a longitudinal study on mother-child psychobiology and development at a university in California. Women were enrolled during early pregnancy if they met eligibility criteria of singleton pregnancy, English-speaking, non-smoking, age 18+, without use of steroid medications, drug or alcohol use during pregnancy. The study was conducted in compliance with the Code of Ethics of the World Medical Association and the Declaration of Helsinki. The protocol was approved by the institutional review boards of participating universities. All participants provided written, informed consent after receiving a complete description of study procedures.
To compare depression during postpartum and non-peripartum life-phases, we identified two timepoints in our protocol from which to draw data. We determined the timing of the postpartum phase by taking into consideration the inconsistent literature on this subject (Wisner, Moses-Kolko, & Sit, 2010), which has defined postpartum as spanning from parturition through 1-month (American_Psychiatric_Association, 1994), 1.5-months (World_Health_Organization, 2016), 6-months (Miller, 2002; Viguera et al., 2011) and 12-months afterwards (Munk-Olsen, Laursen, Pedersen, Mors, & Mortensen, 2006). PPD onset can occur at any time across several months after parturition (Stowe, Hostetter, & Newport, 2005), with peak phase of vulnerability at 3-months (Elliott, 2000; Munk-Olsen et al., 2006). Accordingly, we selected the assessment occurring 3-months after parturition to represent the “postpartum” timepoint, and the assessment occurring 24-months after parturition to represent the “after-postpartum” timepoint. Analyses herein compare depression symptom profiles assessed at these two timepoints. Participants were excluded if they had another pregnancy during the 24-month follow-up period (N=36). Details about the cohort are presented in Table 1.
Table 1.
Descriptive statistics, separated by the random partitioning for statistical tests (“Half 1” and “Half 2”), and the subset of the full cohort who meet traditional criteria for depression
| Full cohort | Cohort Half 1 | Cohort Half 2 | Half 1 vs Half 2a | Depressed subset | Euthymic vs Depresseda | |
|---|---|---|---|---|---|---|
|
| ||||||
| N | 239 | 119 | 120 | ns | 86 | ns |
|
| ||||||
| Maternal age at delivery (yrs, mean (SD)) | 29.9 (5.5) | 30.4 (5.4) | 29.5 (5.5) | ns | 29.5 (5.9) | ns |
|
| ||||||
| Household income before taxes (US$, mean (SD)) | 61276.2 (33872) | 63046.2 (33072.1) | 59520.8 (34696) | ns | 53488.4 (33237.7) | t=−2.7 ** |
|
| ||||||
| Gestational week at delivery (wks, mean (SD)) | 39.1 (2.1) | 39.1 (2.1) | 39.1 (2) | ns | 38.8 (2.3) | t=−1.8 + |
|
| ||||||
| Birth weight (g, mean (SD)) | 3373.9 (579.2) | 3386.3 (586.8) | 3361.5 (573.7) | ns | 3412.4 (613.3) | ns |
|
| ||||||
| CESD-SFb score (mean (SD)) | 3.6 (2.9) | 3.5 (2.8) | 3.6 (3) | ns | 6.8 (1.7) | t=23.3 **** |
|
| ||||||
| EPDSc score (mean (SD)) | 5.4 (4.5) | 5.3 (4.2) | 5.5 (4.7) | ns | 9.7 (4.1) | t=13.9 **** |
|
| ||||||
| Above depression cutoff threshold for CESD-SF d (N (%)) | 82 (34.3) | 38 (31.9) | 44 (36.7) | ns | 82 (95.3) | Xsq=220.0 **** |
|
| ||||||
| Above minor depression cutoff threshold for EPDSe (N (%)) | 50 (20.9) | 23 (19.3) | 27 (22.5) | ns | 50 (58.1) | Xsq=109.0 **** |
|
| ||||||
| Above major depression cutoff threshold for EPDSe (N (%)) | 20 (8.4) | 10 (8.4) | 10 (8.3) | ns | 20 (23.3) | Xsq=35.9 **** |
|
| ||||||
| Above any depression cutoff threshold (N (%)) | 86 (36) | 41 (34.5) | 45 (37.5) | ns | 86 (100) | Xsq=233.7 **** |
|
| ||||||
| Antidepressant medication f (N (%)) | ||||||
|
| ||||||
| Postpartum | 10 (4.2) | 6 (5) | 4 (3.3) | ns | 10 (11.6) | Xsq=11.0 *** |
|
| ||||||
| After-postpartum | 8 (3.3) | 2 (1.7) | 6 (5.0) | Xsq=4.4 * | 8 (9.3) | ns |
|
| ||||||
| History of mental illnessg (N (%)) | 12 (5) | 6 (5) | 6 (5) | ns | 10 (11.6) | Xsq=3.9+ |
|
| ||||||
| Maternal education (N (%)) | ||||||
| Less than high school | 7 (2.9) | 2 (1.7) | 5 (4.2) | ns | 4 (4.7) | ns |
| High school | 28 (11.7) | 12 (10.1) | 16 (13.3) | 11 (12.8) | ||
| Some college, vocational, or AA degree | 97 (40.6) | 52 (43.7) | 45 (37.5) | 38 (44.2) | ||
| Bachelors degree | 65 (27.2) | 31 (26.1) | 34 (28.3) | 22 (25.6) | ||
| Graduate degree | 41 (17.2) | 21 (17.6) | 20 (16.7) | 10 (11.6) | ||
| NA | 1 (0.4) | 1 (0.8) | 0 (0) | 1 (1.2) | ||
|
| ||||||
| Maternal ethnicity (N (%)) | ||||||
| White Non-Hispanic | 110 (46) | 51 (42.9) | 59 (49.2) | ns | 39 (45.3) | ns |
| Hispanic | 63 (26.4) | 35 (29.4) | 28 (23.3) | 19 (22.1) | ||
| Multi-ethnic | 31 (13) | 18 (15.1) | 13 (10.8) | 15 (17.4) | ||
| Asian | 22 (9.2) | 10 (8.4) | 12 (10) | 8 (9.3) | ||
| African-American | 8 (3.3) | 3 (2.5) | 5 (4.2) | 4 (4.7) | ||
| Other or NA | 4 (1.7) | 2 (1.7) | 2 (1.7) | 1 (1.2) | ||
|
| ||||||
| Cohabitation with baby’s father (N (%)) | ||||||
| No | 23 (9.6) | 13 (10.9) | 10 (8.3) | ns | 16 (18.6) | Xsq=10.9 *** |
| Yes | 216 (90.4) | 106 (89.1) | 110 (91.7) | 70 (81.4) | ||
|
| ||||||
| Parity (N (%)) | ||||||
| Nulliparous | 98 (41) | 49 (41.2) | 49 (40.8) | ns | 29 (33.7) | ns |
| Parous (all) | 141 (59) | 70 (58.8) | 71 (59.2) | 57 (66.3) | ||
| Parous, 1 previous delivery | 96 (40.2) | 44 (37) | 52 (43.3) | 35 (40.7) | ||
| Parous, 2 previous deliveries | 29 (12.1) | 17 (14.3) | 12 (10) | 13 (15.1) | ||
| Parous, 3 previous deliveries | 13 (5.4) | 7 (5.9) | 6 (5) | 7 (8.1) | ||
| Parous, 4 previous deliveries | 3 (1.3) | 2 (1.7) | 1 (0.8) | 2 (2.3) | ||
|
| ||||||
| Obstetric risk h (N (%)) | ||||||
| No | 169 (70.7) | 76 (63.9) | 93 (77.5) | Xsq=4.7 * | 60 (69.8) | ns |
| Yes | 70 (29.3) | 43 (36.1) | 27 (22.5) | 26 (30.2) | ||
|
| ||||||
| Delivery mode (N (%)) | ||||||
| Vaginal | 172 (72) | 83 (69.7) | 89 (74.2) | ns | 59 (68.6) | ns |
| Caesarean section | 67 (28) | 36 (30.3) | 31 (25.8) | 27 (31.4) | ||
|
| ||||||
| Preterm deliveryi (N (%)) | ||||||
| No | 219 (91.6) | 108 (90.8) | 111 (92.5) | ns | 75 (87.2) | ns |
| Yes | 20 (8.4) | 11 (9.2) | 9 (7.5) | 11 (12.8) | ||
|
| ||||||
| Low birthweightj (N (%)) | ||||||
| No | 224 (93.7) | 112 (94.1) | 112 (93.3) | ns | 82 (95.3) | ns |
| Yes | 15 (6.3) | 7 (5.9) | 7 (5.8) | 4 (4.7) | ||
|
| ||||||
| Infant sex (N (%)) | ||||||
| Female | 110 (46) | 51 (42.9) | 59 (49.2) | ns | 40 (46.5) | ns |
| Male | 129 (54) | 68 (57.1) | 61 (50.8) | 46 (53.5) | ||
|
| ||||||
| Breastfeeding (N (%)) | ||||||
| No | 75 (31.4) | 38 (31.9) | 38 (31.7) | ns | 27 (31.4) | ns |
| Yes | 152 (63.6) | 75 (63) | 75 (62.5) | 54 (62.8) | ||
| NA | 12 (5) | 6 (5) | 6 (5) | 5 (5.8) | ||
Data depict information for the postpartum timepoint unless otherwise noted.
The columns titled “Half 1 vs Half 2” and “Euthymic vs Depressed” present the results of significant t-tests and chi-squared tests with Yates’ correction.
CESD-SF: Center for Epidemiologic Studies Depression Scale (Radloff, 1991) Short Form (Santor & Coyne, 1997)
EPDS: Edinburgh Postnatal Depression Scale (Cox, Holden, & Sagovsky, 1987)
Women who met one or both traditional criteria for risk of depression based upon EPDS cutoff of 10/40 (Earls, 2010) and CESD-SF cutoff of 5/36 (Irwin, Artin, & Oxman, 1999; Martens et al., 2006).
Women who met one or both traditional criteria for risk of depression based upon EPDS cutoff of 12/40 (Earls, 2010) and CESD-SF cutoff of 5/36 (Irwin et al., 1999; Martens et al., 2006).
Antidepressant medications at postpartum timepoint included Paxil (N=1), Prozac (N=3), Wellbutrin (N=3), Zoloft (N=3), one of the latter also using Celexa, and at the after-postpartum timepoint Lexapro (N=1), Prozac (N=1), Zoloft (N=2), Celexa (N=1), Wellbutrin (N=3), and one of the latter also using Paxil.
History of mental illness defined as previous to this pregnancy they had ever been diagnosed with a psychological or emotional problem (N=10), had a serious mental illness, emotional problem, or nervous breakdown (N=5), if they had ever stayed overnight or longer in a hospital or treatment facility because of any mental or emotional problem (N=2).
Obstetric risk is defined as having any of the following gestational complications: hypertension; diabetes; severe infection; vaginal bleeding; anemia; oligohydramnios; placental abruption.
Preterm delivery is defined as delivery earlier than 37 weeks’ gestation.
Low birthweight is defined as lower than 10th percentile weight for gestational age and sex at birth.
NA=not available;
p < 0.10;
p <0.05;
p<0.01;
p<0.001;
p<0.0001; ns=not significant
2.2. Symptoms of depression
Our first step towards investigating the symptom profiles of PPD was compiling a comprehensive list of 53 depression symptoms based on authoritative sources (Table 2). Because the larger, longitudinal study of mother-child psychobiology was not designed with the present approach in mind, not all depression symptoms had a corresponding item. From the full list of possible symptoms, we found corresponding items by searching through the instruments administered in our longitudinal study, selecting items that were administered at both the postpartum and after-postpartum timepoints. The selection of items was discussed and refined by a group of clinical and academic researchers representing several academic disciplines, including Psychology, Psychiatry, and Biological Anthropology. Ultimately, selected items were agreed to reflect 21 symptoms of depression (Table 2).
2.3. Statistical methods
Data were unitized so each symptom scaled 0–1, where 0 is the lowest and 1 is the highest possible score even if those ends of the scale were not endorsed. The full cohort at the postpartum timepoint was randomly split into two subsets, Cohort-Half-1 and Cohort-Half-2, in order to perform exploratory factor analysis (EFA) on one half and confirmatory factory analysis (CFA) on the other half as a cross-validation of the determined factor structure. The groups did not significantly differ on socio-demographic or health-related traits, with the exception of number of obstetric risk factors (Table 1).
EFA was conducted on Cohort-Half-1 to assess whether particular symptoms segregate together to produce clusters in depression symptomology during the postpartum period. EFA was performed using psych, nFactors, and GPArotation packages for R. Kaiser’s eigenvalue criterion (Kaiser, 1960), parallel analysis (Horn, 1965), and Raiche’s optimal coordinates approach (Raîche, Walls, Magis, Riopel, & Blais, 2013) all suggested extracting 6 factors (Supporting Information Figure S1). The average residuals for the correlation matrix (root mean square of residuals) of the 6-factor model was 0.04, indicating excellent fit. These criteria converged to support the retention of 6 factors from the dataset.
Maximum Likelihood (ML) estimation was selected for factor extraction, because it estimates the level of shared variance for each item, and it has been shown experimentally to produce more generalizable and reproducible results than other methods (Osborne & Costello, 2009). The retained factors were rotated obliquely using a minimizing criterion.
Confirmatory factor analysis (CFA) was conducted to assess the stability of the factor structure suggested by EFA in three analyses. (1) CFA assessed the factor structure stability at the postpartum timepoint in Cohort-Half-1 and Cohort-Half-2. (2) CFA assessed the fit of the determined factor structure using the same cohort’s responses to the same items at the after-postpartum timepoint. (3) CFA was conducted on the subset of women (N=86) who met traditional research study criteria for at least minor depression at the postpartum timepoint (Table 1) (Irwin, Artin, & Oxman, 1999), and on the subset of women (N=229) who did not use anti-depressant medications at the postpartum timepoint (Table S3). Insufficient sample size prevented us from conducting separate analyses on the subset of women who met traditional criteria for major depression (N=20). For CFA analyses, we used the lavaan package for R, and assessed whether Heywood cases were detected based on error variances and squared multiple correlations (SMCs) for each item. We inspected standardized residual correlation tables, modification indices, and fit indices.
We assessed whether the prevalences of symptom clusters differ meaningfully between the postpartum and after-postpartum timepoints by measurement invariance modelling (using the semTools package for R) and paired t-tests. For paired t-tests, in order to avoid any influence from attrition in participation, after confirming the likelihood that data were missing at random within identifiable strata (Heitjan & Basu, 1996), we used multivariate imputation by chained equations (Azur, Stuart, Frangakis, & Leaf, 2011; Buuren & Groothuis-Oudshoorn, 2011), utilizing women’s demographic characteristics, obstetric history, marital and cohabitation status with the baby’s father, and depressive symptoms measured at 6-months and 12-months postpartum to impute missing symptom scores at 24-months postpartum (N=112) using predictive mean matching (PMM). PMM assures imputed values are plausible, and is robust for non-normally distributed data (Horton & Lipsitz, 2001). We followed standard procedure in which missing values are replaced with predictions derived from regression models over 10 cycles (Raghunathan, Solenberger, & Van Hoewyk, 2002), repeating this entire process 5 times (Graham, Olchowski, & Gilreath, 2007). T-test results were pooled (Harel & Zhou, 2007).
To validate the results obtained with the above procedures, we also employed a secondary methodology. Linear mixed effects models were fit by restricted maximum likelihood estimation (Verbeke & Molenberghs, 2009) with random intercepts for individual identity to assess the relation between symptom factor scores and timepoints. To describe results, we report pooled Pearson product-moment correlations between scores at the postpartum and after-postpartum timepoints for the factors. Analyses of imputed data utilize R packages Amelia, mice, and miceadds.
3. RESULTS
Results of the EFA indicated face validity of the resulting factor structure, with symptom clusters that had clinical meaning (Table 3). Factor 1 reflected a “Worry” cluster, consisting of anxiety and guilt. Factor 2 reflected an “Anger” symptom cluster. Factor 3 contained several items such as anhedonia, inability to stay asleep, and fatigue, and was deemed “Emotional/Circadian/Energetic Dysregulation.” Factor 4 reflected “Appetite.” Factor 5 included “Somatic/Cognitive” items. Factor 6 consisted of items that reflect the behavioral expression of negative affectivity, so we deem it “Distress display.”
Table 3.
Factor structure from exploratory factor analysis of postpartum depression symptoms
| Factor | Item | Loading on factor |
|---|---|---|
|
| ||
| Factor 1, “Worry” | Anxiety | 1.000 |
| Guilt | 0.418 | |
|
| ||
| Factor 2, “Anger” | Anger attacks | 0.906 |
| Anger | 0.458 | |
|
| ||
| Factor 3, “Emotional/Circadian/Energetic Dysregulation” | Agitated | 0.635 |
| Irritability | 0.551 | |
| Anhedonia | 0.515 | |
| Fatigue | 0.497 | |
| Sad | 0.471 | |
| Inability to stay asleep | 0.304 | |
| Psychomotor retardation | 0.300 | |
|
| ||
| Factor 4, “Appetite” | Low appetite | 1.000 |
|
| ||
| Factor 5, “Somatic/Cognitive” | GI problems | 0.718 |
| Pain | 0.597 | |
| Inability to focus | 0.427 | |
| Palpitations | 0.378 | |
|
| ||
| Factor 6, “Distress display” | Sad affect display | 0.601 |
| Crying | 0.540 | |
| Thoughts of self harm | 0.421 | |
|
| ||
| none | Negative reactivity | none |
| none | Pessimism | none |
Results of the EFA were highly parsimonious, with only one symptom loading at the 0.3 threshold onto more than one factor (Table 3). “Anger” loaded at 0.46 onto Factor 2 and .30 onto Factor 3, so the Factor with the higher loading was selected for this item. Because of the simple structure of our results, only the loading of each item on its respective factor are displayed. “Pessimism” and “Negative reactivity” had no loadings above 0.30, so were not included in the factor structure.
Results suggested no differences in symptom segregation profiles between the two random halves of the cohort at the postpartum timepoint. CFA supported the 6-factor model derived from the EFA as a good fit for the data in both Cohort-Half-1 and Cohort-Half-2. All fit indices indicated superior fit for the 6-factor compared with the null (one-factor) model (Table 4) and SMCs were higher in the 6-factor compared with the null model (M=0.2 higher for Cohort-Half-1, M=0.1 higher for Cohort-Half-2, Supporting Information Table S1).
Table 4.
Confirmatory factor analysis fit indices
| Cohort | Model structure | Model # | Relative chisqa | RMSEAb | CFIc | TLId | NFIe | SRMRf | χ2 difference test |
|---|---|---|---|---|---|---|---|---|---|
| Postpartum half #1 | 6-factor | Mod1 | 1.454 | 0.062 | 0.870 | 0.839 | 0.692 | 0.078 | |
| 1-factor | Mod2 | 1.924 | 0.088 | 0.708 | 0.671 | 0.551 | 0.091 | ||
| Is 6-factor better fit than 1-factor model? | Lower ✓ | Lower ✓ | Higher ✓ | Higher ✓ | Higher ✓ | Lower ✓ | χ2 diff=91.844 p=0.000 **** | ||
| Postpartum half #2 | 6-factor | Mod3 | 1.621 | 0.072 | 0.885 | 0.858 | 0.756 | 0.068 | |
| 1-factor | Mod4 | 1.635 | 0.073 | 0.871 | 0.854 | 0.729 | 0.071 | ||
| Is 6-factor better fit than 1-factor model? | Lower ✓ | Lower ✓ | Higher ✓ | Higher ✓ | Higher ✓ | Lower ✓ | χ2 diff=24.796 p=0.037 * |
||
| After-postpartum | 6-factor | Mod5 | 1.786 | 0.077 | 0.855 | 0.820 | 0.731 | 0.073 | |
| 1-factor | Mod6 | 2.066 | 0.090 | 0.783 | 0.756 | 0.658 | 0.084 | ||
| Is 6-factor better fit than 1-factor model? | Lower ✓ | Lower ✓ | Higher ✓ | Higher ✓ | Higher ✓ | Lower ✓ | χ2 diff=67.515 p=0.000 **** |
||
| Clinical depression postpartum | 6-factor | Mod7 | 1.237 | 0.052 | 0.853 | 0.818 | 0.566 | 0.079 | |
| 1-factor | Mod8 | 1.364 | 0.065 | 0.751 | 0.720 | 0.472 | 0.086 | ||
| Is 6-factor better fit than 1-factor model? | Lower ✓ | Lower ✓ | Higher ✓ | Higher ✓ | Higher ✓ | Lower ✓ | χ2 diff=28.081 p=0.014 * |
||
Relative chi-square is calculated as the chi-square statistic divided by the degrees of freedom. Lower values indicate better fit. Values < 2 are considered acceptable (Ullman, 2001), disqualifying Mod6.
The root mean square error of approximation (RMSEA) is a noncentrality-based index, for which lower values indicate better fit. RMSEA values < 0.05 indicates close fit (Steiger, 1990), and values < 0.08 are considered acceptable (Browne, Cudeck, Bollen, & Long, 1993; Cangur & Ercan, 2015; Hooper, Coughlan, & Mullen, 2008), disqualifying Mod2 and Mod6.
The comparative fit index (CFI) is another noncentrality-based index, for which higher values indicate better fit (Bentler, 1990). Values > 0.80 are considered acceptable (Sharma, Mukherjee, Kumar, & Dillon, 2005), disqualifying Mod2,6,8.
The Tucker-Lewis index (TLI) is a relative fit index, for which higher values indicate better fit (Tucker & Lewis, 1973).
The Normed Fit Index (NFI) is an incremental fit measure for which larger values indicate better fit (Bentler & Bonett, 1980).
The standardized root mean square residual (SRMR) (Joreskog & Sorbom, 1981) is an absolute fit index for which lower values indicate better fit. Values ≤ .08 indicate good fit (L.-t. Hu & Bentler, 1998; L. t. Hu & Bentler, 1999), disqualifying Mod2,6,8.
Inspection of the standardized residual correlation tables revealed no patterns of fit problems, as no more than 2 of the 18 correlations for any individual item were significant at the 0.05 level. Modification indices were all small (<25.00) indicating no need to alter the models’ designs.
p<0.05
p<0.01
p<0.001
Results suggested no differences in how symptoms segregate between the postpartum and after-postpartum timepoints. We claim the same factor structure holds across timepoints based on achievement of configural, metric, and scalar invariance (Meredith, 1993; Vandenberg & Lance, 2000). For configural invariance, CFA supported the 6-factor model derived from the EFA as a good fit for the data at the after-postpartum timepoint. All fit indices indicated superior fit for the 6-factor compared with the null model (Table 4) and SMCs were higher in the 6-factor compared with the null model (M=0.1 higher, Supporting Information Table S1). Configural invariance indicates that the same pattern of relationships between indicators and latent variables holds across timepoints. Metric invariance suggests that in addition to latent variables measured by the same indicators, factor loadings are equivalent across administrations. For metric invariance (Supporting Information Table S4), chi-square change between the configural and metric models is nonsignificant (p=0.12) indicating that factor loadings are invariant across timepoints. For scalar invariance (Supporting Information Table S4), chi-square change between the metric and scalar models is nonsignificant (p=0.36) indicating that item intercepts are equivalent across timepoints. Configural, metric, and scalar invariance justify comparison of factor means across timepoints. The model did not exhibit strict invariance (chi-square p<0.00, Supporting Information Table S4), indicating variance in residual variances across timepoints, but strict invariance is highly constrained and rarely achieved in practice (Bialosiewicz, Murphy, & Berry, 2013; Millsap & Meredith, 2007).
The same symptom profile structure also robustly applied to the subset of data from women who met traditional depression criteria at the postpartum timepoint (Table 1). CFA supported the 6-factor model derived from EFA as a good fit for the data for women above the traditional cutoff thresholds for at least minor depression at the postpartum timepoint. All fit indices indicated superior fit for the 6-factor compared with the null model (Table 4) and SMCs were higher in the 6-factor compared with the null model (M=0.1 higher, Supporting Information Table S1). The same symptom profile structure also robustly applied to the subsets of women who did not use antidepressant medications, and those who were and were not breastfeeding at the postpartum timepoint (Supporting Information Table S3).
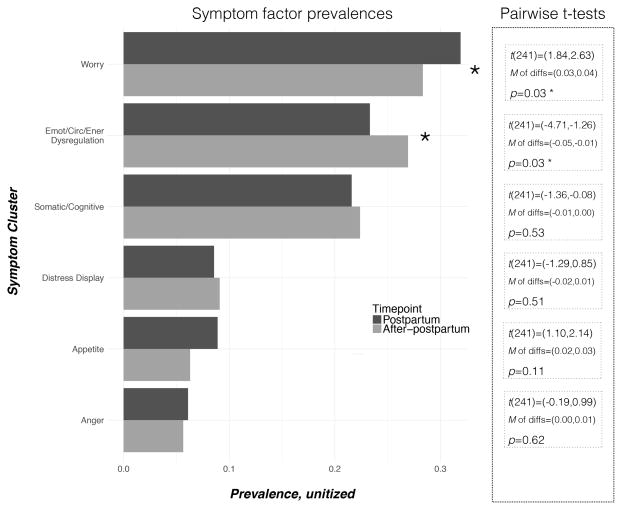
Two particular symptom factors exhibited meaningful differences in prevalence between the postpartum and after-postpartum timepoints (Figure 1). Pairwise t-tests revealed that “Worry” scores were more pronounced during the postpartum timepoint, compared with the after-postpartum (t(241)=(1.84,2.63), M of differences=0.04, p=0.03), and “Emotional/Circadian/Energetic Dysregulation” scores were less pronounced during the postpartum timepoint, compared with the after-postpartum (t(241)=(−4.71,−1.26), M of differences=−0.01, p=0.03). These results were further validated using a different method of analysis. Mixed-effects models supported our calculation that “Worry” scores were more pronounced during the postpartum timepoint (β=0.03, p=0.08), and “Emotional/Circadian/Energetic Dysregulation” scores were less pronounced during the postpartum timepoint (β=−0.03, p=0.04) (Supporting Information Table S2).
Figure 1.
Differences in symptom cluster prevalences between the postpartum and after-postpartum timepoints. Results suggest that the “Worry” factor is more prevalent during the postpartum compared with the after-postpartum timepoint, and the “Emotional/Circadian/Energetic Dysregulation” factor is less pronounced during the postpartum compared with the after-postpartum timepoint. Pooled Pearson product-moment correlations between timepoints: factor 1 r=0.49, p<0.00***; factor 2 r=0.27; p=0.01**; factor 3 r=0.24, p=0.01*; factor 4 r=0.48, p<0.00***; factor 5 r=0.62, p<0.00***; factor 6 r=0.12, p=0.11.
Among the subset of women who met traditional research study criteria for at least minor depression at the postpartum timepoint, “Worry” and “Emotional/Circadian/Energetic Dysregulation” scores differed meaningfully between the two timepoints. Pairwise t-tests confirmed that “Worry” scores were more pronounced during the postpartum timepoint, compared with the after-postpartum (t(85)=(3.44,4.36), p<0.00). “Emotional/Circadian/Energetic Dysregulation” scores exhibited no significant difference between the two timepoints. It should be noted that subsetting the cohort based on traditional research criteria cutoff for minor depression is redundant with “Emotional/Circadian/Energetic Dysregulation” scores (logistic regression χ2=20.1, p<0.00, Nagelkerke’s R2=0.7), so differences between timepoints would not be expected.
4. DISCUSSION
Depression symptom profiles are known to vary between individuals, as well as within individuals over time (Oquendo et al., 2004). Here, we address the question of whether depression occurring during the postpartum phase is characterized by a unique symptom profile, compared with depression outside the postpartum phase. In a longitudinal cohort of 239 women assessed at postpartum (3-months after parturition) and after-postpartum (24-months after parturition) timepoints, we conducted exploratory factor analyses of individual symptoms to investigate whether particular symptoms are more likely to present together, confirmatory factor analysis and longitudinal invariance modelling (Meredith, 1993; Vandenberg & Lance, 2000) to investigate whether the segregation of these symptoms differs between the two timepoints, and t-tests to investigate whether symptom cluster prevalence varies between timepoints. Results suggest that the structure of symptom profiles is not different between the postpartum and after-postpartum timepoints, but the prevalence of two particular symptom clusters differs. “Worry” is more pronounced during the postpartum, and “Emotional/Circadian/Energetic Dysregulation” is less pronounced during the postpartum. Our observation that PPD may have a symptomatic signature that is distinct from depression during other life phases is consistent with the possibility that depression occurring during the perinatal phase of life is a condition separate from MDD, or a condition with unique characteristics.
We found that the Worry symptom cluster was the most pronounced at the postpartum timepoint (Figure 1), and was a hallmark differentiating depression profiles at the postpartum compared with the after-postpartum timepoint. These results were validated (and even stronger) among the subset of women who met traditional research criteria for at least minor depression. The Worry symptom cluster comprises anxiety and guilt. While previous studies have not assessed symptomology using multiple instruments, previous studies have recognized anxiety as a distinctive feature of PPD (Stephen Matthey, Barnett, Howie, & Kavanagh, 2003), with multiple reports that postpartum depressed women exhibit enhanced anxiety symptoms (C. T. Beck & Indman, 2005; Hendrick et al., 2000). Two studies conducting principal components analysis with the EPDS have found that the three anxiety-related items comprise a distinct subscale (Brouwers, van Baar, & Pop, 2001; L. Ross, S. G. Evans, E. Sellers, & M. Romach, 2003). Furthermore, the 3-item EPDS anxiety subscale exhibits strong validity as a predictor of overall EPDS score (Kabir, Sheeder, & Kelly, 2008). A cross-sectional study comparing pregnant and postpartum women using anxiety, depressed mood, and anhedonia EPDS sub-scales (Stephen Matthey, Fisher, & Rowe, 2013; L. E. Ross, S. G. Evans, E. Sellers, & M. Romach, 2003; Tuohy & McVey, 2008) found that anxiety and anhedonia were more likely to have postpartum rather than antepartum onset (Putnam et al., 2017). Another cross-sectional study found enhanced worthlessness/guilt in pregnant compared with non-peripartum women (Hoertel et al., 2015).
We found that prevalence of “Emotional/Circadian/Energetic Dysregulation,” the symptom factor that contains sadness, anhedonia, and psychomotor retardation among other symptoms, was significantly lower during the postpartum compared with the after-postpartum timepoint (Figure 1). In other words, women exhibited less emotional/circadian/energetic dysregulation, during the postpartum compared with the after-postpartum timepoint. One previous study observed among postpartum women the sadness symptom exhibited significantly weaker correlation with overall depression than it did among non-postpartum women (Bernstein et al., 2008). Further, the only previous study known to us comparing postpartum and non-postpartum depression symptomology documented that psychomotor symptoms were relatively reduced among the postpartum cohort, and that this was true for women both with and without a clinical diagnosis of depression (Hoertel et al., 2015). These findings parallel our observation that the factor containing psychomotor retardation was reduced during the postpartum in women who did and did not meet the threshold for minor depression.
As expected, among the subset of women who met traditional research criteria for at least minor depression, no differences in prevalence of “Emotional/Circadian/Energetic Dysregulation” between the timepoints were revealed. Traditional research instruments for assessment of depression rely heavily on symptoms contained in this factor, so “Emotional/Circadian/Energetic Dysregulation” scores statistically behave as a close surrogate for the cutoff criterion itself.
Given the principal role of sadness/anhedonia in diagnostic determination of MDD (American_Psychiatric_Association, 2003), our observation that a distinguishing feature of depression during the postpartum phase is lower prevalence of emotional/circadian/energetic dysregulation supports the case for different diagnostic criteria to identify MDD and PPD. For example, the DSM-5 requires either depressed mood or loss of interest to contribute towards five or more symptoms to diagnose MDD (American_Psychiatric_Association, 2003). If Worry is, instead, the most pronounced feature of PPD, then it could be efficacious to consider diagnostic criteria for PPD that emphasize Worry symptoms, rather than necessitating presence of depressed mood or loss of interest.
Despite the theoretical supposition from evolutionary anthropology that social signals of distress might be a key feature of PPD (Hagen, 1999), we observed no overrepresentation of “Distress Display” (the symptom factor that includes crying, sad affect display, and thoughts of self-harm) in our data. We found no significant differences in prevalence of this factor between the postpartum and after-postpartum timepoints (Figure 1). Previous authors have described similar observations in this regard. One study of PPD symptomology found that the symptoms with the five lowest prevalence scores were all related to suicidal thoughts (C. T. Beck & Indman, 2005), another found that postpartum women exhibited less suicidal thoughts than non-postpartum women (Bernstein et al., 2008), and another observed less suicidality in postpartum compared with non-peripartum women (Hoertel et al., 2015). Future evolutionary frameworks for understanding the potential function or selective consequences of PPD should consider the symptom profiles that characterize the condition.
Determining whether PPD is a distinct syndrome from MDD has important implications for public health surveillance as well as designing and assessing prevention strategies and treatment practices. Hendrick et al. found differences in treatment efficacy for depression among postpartum compared with non-postpartum women (Hendrick et al., 2000). Postpartum women exhibited longer time to response for pharmacological treatment, and greater need to be on two or more antidepressant agents to achieve response. Beck and Indman suggest that “[c]linicians need to be cognizant of the fact that anxiety and not depression may be the presenting symptom of mothers suffering from postpartum depression” (C. T. Beck & Indman, 2005). In this vein, an important question for future research will be to determine how anxiety in the postpartum period may differ from that characterizing generalized anxiety disorder or anxiety manifesting outside of the peripartum period.
A major strength of this investigation is the longitudinal study design and the broad characterization of depressive symptoms, which allows us to observe each postpartum woman compared to herself during a later life-phase. This life-course, trajectory approach to investigating depressive symptomology is unprejudiced by bias or random differences between cohorts. A limitation of the study is that we are not able to compare these results to a non-perinatal cohort. However, we were able to ensure no pregnancies or miscarriages over a two-year period in an adult female cohort, which would pose logistical challenges if a cross-sectional comparison cohort were sought. Another limitation is the latest after-postpartum timepoint we were able to assess in this study is two-years-post-parturition, and future studies are needed to confirm that two years is sufficiently representative of depressive symptoms not associated with perinatal experiences. Future studies with a larger sample size of individuals followed across a longer time scale are needed to validate our findings.
5. CONCLUSION
Our results suggest that PPD has a symptomatic signature involving greater prevalence of Worry and lower prevalence of Emotional/Circadian/Energetic Dysregulation. This observation is consistent with the possibility that PPD represents a unique syndrome from MDD occurring outside the perinatal phase. Future studies should compare psychobiological mechanisms of symptom manifestation in PPD and MDD to further investigate the validity of these diagnostic categories.
Supplementary Material
Acknowledgments
All authors report no competing interests. All authors report no financial relationships with commercial interests. The authors gratefully acknowledge Hal Stern for his feedback and help on the statistical analyses. This research was supported by NIH grants National Institute of Diabetes and Digestive and Kidney Diseases award K01 DK105110 to Dr. Fox, National Institute of Child Health and Human Development HD-40967 to Dr. Glynn, National Institute of Neurological Disorders and Stroke award NS-41298 to Dr. Sandman, and a National Institute of Mental Health Silvio O. Conte Center award MH-96889.
Footnotes
Conflicts of interest: None.
References
- American_Psychiatric_Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-4. 1994. [Google Scholar]
- American_Psychiatric_Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. 2003. [Google Scholar]
- Azur MJ, Stuart EA, Frangakis C, Leaf PJ. Multiple imputation by chained equations: what is it and how does it work? International Journal of Methods in Psychiatric Research. 2011;20(1):40–49. doi: 10.1002/mpr.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett J, Wonch KE, Gonzalez A, Ali N, Steiner M, Hall GB, Fleming AS. Maternal affect and quality of parenting experiences are related to amygdala response to infant faces. Social Neuroscience. 2012;7(3):252–268. doi: 10.1080/17470919.2011.609907. [DOI] [PubMed] [Google Scholar]
- Bech P. Rating scales in depression: limitations and pitfalls. Dialogues in Clinical Neuroscience. 2006;8(2):207. doi: 10.31887/DCNS.2006.8.2/pbech. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Carbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clinical Psychology Review. 1988;8(1):77–100. [Google Scholar]
- Beck CT, Indman P. The many faces of postpartum depression. Journal of Obstetric, Gynecologic, and Neonatal Nursing. 2005;34(5):569–576. doi: 10.1177/0884217505279995. [DOI] [PubMed] [Google Scholar]
- Bentler PM. Comparative fit indexes in structural models. Psychological Bulletin. 1990;107(2):238–246. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- Bentler PM, Bonett DG. Significance tests and goodness of fit in the analysis of covariance structures. Psychological Bulletin. 1980;88(3):588–600. [Google Scholar]
- Bernstein IH, Rush AJ, Yonkers K, Carmody TJ, Woo A, McConnell K, Trivedi MH. Symptom features of postpartum depression: are they distinct? Depression and Anxiety. 2008;25(1):20–26. doi: 10.1002/da.20276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialosiewicz S, Murphy K, Berry T. Do our Measures Measure up? The Critical Role of Measurement Invariance. Washington, DC: American Evaluation Association; 2013. [Google Scholar]
- Brouwers EP, van Baar AL, Pop VJ. Does the Edinburgh Postnatal Depression Scale measure anxiety? Journal of Psychosomatic Research. 2001;51(5):659–663. doi: 10.1016/s0022-3999(01)00245-8. [DOI] [PubMed] [Google Scholar]
- Browne MW, Cudeck R, Bollen KA, Long JS. Alternative ways of assessing model fit. Sage focus editions. 1993;154:136–136. [Google Scholar]
- Buuren S, Groothuis-Oudshoorn K. mice: Multivariate imputation by chained equations in R. Journal of statistical software. 2011;45(3):1–67. [Google Scholar]
- Calamari JE, Wiegartz PS, Janeck AS. Obsessive–compulsive disorder subgroups: a symptom-based clustering approach. Behaviour Research and Therapy. 1999;37(2):113–125. doi: 10.1016/s0005-7967(98)00135-1. doi: http://dx.doi.org/10.1016/S0005-7967(98)00135-1. [DOI] [PubMed] [Google Scholar]
- Cangur S, Ercan I. Comparison of Model Fit Indices Used in Structural Equation Modeling Under Multivariate Normality. Journal of Modern Applied Statistical Methods. 2015;14(1):152–167. [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24(4):385–396. [PubMed] [Google Scholar]
- Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. The British Journal of Psychiatry. 1987;150(6):782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Medicine. 2013;11(1):126–134. doi: 10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dew MA, Reynolds CF, Houck PR, Hall M, Buysse DJ, Frank E, Kupfer DJ. Temporal profiles of the course of depression during treatment: predictors of pathways toward recovery in the elderly. Archives of General Psychiatry. 1997;54(11):1016–1024. doi: 10.1001/archpsyc.1997.01830230050007. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Functional anatomical abnormalities in limbic and prefrontal cortical structures in major depression. Progress in Brain Research. 2000;126:413–431. doi: 10.1016/S0079-6123(00)26027-5. doi: http://dx.doi.org/10.1016/S0079-6123(00)26027-5. [DOI] [PubMed] [Google Scholar]
- Duivis HE, Vogelzangs N, Kupper N, de Jonge P, Penninx BW. Differential association of somatic and cognitive symptoms of depression and anxiety with inflammation: findings from the Netherlands Study of Depression and Anxiety (NESDA) Psychoneuroendocrinology. 2013;38(9):1573–1585. doi: 10.1016/j.psyneuen.2013.01.002. [DOI] [PubMed] [Google Scholar]
- Earls MF. Incorporating Recognition and Management of Perinatal and Postpartum Depression Into Pediatric Practice. Pediatrics. 2010;126(5):1032–1039. doi: 10.1542/peds.2010-2348. [DOI] [PubMed] [Google Scholar]
- Elliott S. Report on the Satra Bruk workshop on classification of postnatal mental disorders. Archives of Women’s Mental Health. 2000;3:27–33. [Google Scholar]
- Fried EI, Nesse RM. Depression sum-scores don’t add up: why analyzing specific depression symptoms is essential. BMC Medicine. 2015;13(72):1–11. doi: 10.1186/s12916-015-0325-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuqua DR, Leonard E, Masters MA, Smith RJ, Campbell JL, Fischer PC. A structural analysis of the state-trait anger expression inventory. Educational and Psychological Measurement. 1991;51(2):439–446. [Google Scholar]
- Glynn LM. Giving birth to a new brain: Hormone exposures of pregnancy influence human memory. Psychoneuroendocrinology. 2010;35(8):1148–1155. doi: 10.1016/j.psyneuen.2010.01.015. [DOI] [PubMed] [Google Scholar]
- Glynn LM, Davis EP, Sandman CA, Goldberg WA. Gestational hormone profiles predict human maternal behavior at 1-year postpartum. Hormones and Behavior. 2016;85:19–25. doi: 10.1016/j.yhbeh.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman SH. Depression in mothers. Annu Rev Clin Psychol. 2007;3:107–135. doi: 10.1146/annurev.clinpsy.3.022806.091401. [DOI] [PubMed] [Google Scholar]
- Graham JW, Olchowski AE, Gilreath TD. How many imputations are really needed? Some practical clarifications of multiple imputation theory. Prevention Science. 2007;8(3):206–213. doi: 10.1007/s11121-007-0070-9. [DOI] [PubMed] [Google Scholar]
- Hagen EH. The functions of postpartum depression. Evolution and Human Behavior. 1999;20(5):325–359. [Google Scholar]
- Harel O, Zhou XH. Multiple imputation: review of theory, implementation and software. Statistics in Medicine. 2007;26(16):3057–3077. doi: 10.1002/sim.2787. [DOI] [PubMed] [Google Scholar]
- Heitjan DF, Basu S. Distinguishing “missing at random” and “missing completely at random”. The American Statistician. 1996;50(3):207–213. [Google Scholar]
- Hendrick V, Altshuler L, Strouse T, Grosser S. Postpartum and nonpostpartum depression: differences in presentation and response to pharmacologic treatment. Depression and Anxiety. 2000;11(2):66–72. doi: 10.1002/(sici)1520-6394(2000)11:2<66::aid-da3>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Hoertel N, López S, Peyre H, Wall MM, González-Pinto A, Limosin F, Blanco C. Are symptom features of depression during pregnancy, the postpartum period and outside the peripartum period distinct? Results from a nationally representative sample using item response theory (IRT) Depression and Anxiety. 2015;32(2):129–140. doi: 10.1002/da.22334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper D, Coughlan J, Mullen M. Structural equation modelling: Guidelines for determining model fit. Electronic Journal of Business Research Methods. 2008;6(1):53–60. [Google Scholar]
- Horn JL. A rationale and test for the number of factors in factor analysis. Psychometrika. 1965;30(2):179–185. doi: 10.1007/BF02289447. [DOI] [PubMed] [Google Scholar]
- Horton NJ, Lipsitz SR. Multiple imputation in practice: comparison of software packages for regression models with missing variables. The American Statistician. 2001;55(3):244–254. [Google Scholar]
- Hu L-t, Bentler PM. Fit indices in covariance structure modeling: Sensitivity to underparameterized model misspecification. Psychological Methods. 1998;3(4):424–453. [Google Scholar]
- Hu Lt, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural equation modeling: a multidisciplinary journal. 1999;6(1):1–55. [Google Scholar]
- Irwin M, Artin KH, Oxman MN. Screening for depression in the older adult: criterion validity of the 10-item Center for Epidemiological Studies Depression Scale (CES-D) Archives of Internal Medicine. 1999;159(15):1701–1704. doi: 10.1001/archinte.159.15.1701. [DOI] [PubMed] [Google Scholar]
- Joreskog KG, Sorbom D. LISREL: Analysis of linear structural relationships by the method of maximum likelihood. Chicago: National Educational Resources; 1981. [Google Scholar]
- Kabir K, Sheeder J, Kelly LS. Identifying postpartum depression: are 3 questions as good as 10? Pediatrics. 2008;122(3):e696–e702. doi: 10.1542/peds.2007-1759. [DOI] [PubMed] [Google Scholar]
- Kaiser HF. The application of electronic computers to factor analysis. Educational and Psychological Measurement. 1960;20:141–151. [Google Scholar]
- Keller MC, Nesse RM. The evolutionary significance of depressive symptoms: different adverse situations lead to different depressive symptom patterns. Journal of Personality and Social Psychology. 2006;91(2):316–330. doi: 10.1037/0022-3514.91.2.316. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Aggen SH, Neale MC. Evidence for multiple genetic factors underlying DSM-IV criteria for major depression. JAMA psychiatry. 2013;70(6):599–607. doi: 10.1001/jamapsychiatry.2013.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvaal K, Ulstein I, Nordhus IH, Engedal K. The Spielberger state-trait anxiety inventory (STAI): the state scale in detecting mental disorders in geriatric patients. International Journal of Geriatric Psychiatry. 2005;20(7):629–634. doi: 10.1002/gps.1330. [DOI] [PubMed] [Google Scholar]
- Lamers F, Vogelzangs N, Merikangas K, De Jonge P, Beekman A, Penninx B. Evidence for a differential role of HPA-axis function, inflammation and metabolic syndrome in melancholic versus atypical depression. Molecular Psychiatry. 2013;18(6):692–699. doi: 10.1038/mp.2012.144. [DOI] [PubMed] [Google Scholar]
- Martens MP, Parker JC, Smarr KL, Hewett JE, Ge B, Slaughter JR, Walker SE. Development of a shortened Center for Epidemiological Studies Depression scale for assessment of depression in Rheumatoid Arthritis. Rehabilitation Psychology. 2006;51(2):135–139. [Google Scholar]
- Matthey S, Barnett B, Howie P, Kavanagh DJ. Diagnosing postpartum depression in mothers and fathers: whatever happened to anxiety? Journal of Affective Disorders. 2003;74(2):139–147. doi: 10.1016/s0165-0327(02)00012-5. [DOI] [PubMed] [Google Scholar]
- Matthey S, Fisher J, Rowe H. Using the Edinburgh postnatal depression scale to screen for anxiety disorders: conceptual and methodological considerations. Journal of Affective Disorders. 2013;146(2):224–230. doi: 10.1016/j.jad.2012.09.009. [DOI] [PubMed] [Google Scholar]
- Matthey S, Henshaw C, Elliott S, Barnett B. Variability in use of cut-off scores and formats on the Edinburgh Postnatal Depression Scale–implications for clinical and research practice. Archives of Women’s Mental Health. 2006;9(6):309–315. doi: 10.1007/s00737-006-0152-x. [DOI] [PubMed] [Google Scholar]
- Meredith W. Measurement invariance, factor analysis and factorial invariance. Psychometrika. 1993;58(4):525–543. [Google Scholar]
- Miller LJ. Postpartum depression. JAMA: Journal of the American Medical Association. 2002;287(6):762–765. doi: 10.1001/jama.287.6.762. [DOI] [PubMed] [Google Scholar]
- Millsap RE, Meredith W. Factorial invariance: Historical perspectives and new problems. In: Cudeck R, MacCallum RC, editors. Factor analysis at 100: historical developments and future directions. Mahwah, New Jersey: Lawrence Erlbaum Associates Publishers; 2007. pp. 131–152. [Google Scholar]
- Moses-Kolko EL, Horner MS, Phillips ML, Hipwell AE, Swain JE. In Search of Neural Endophenotypes of Postpartum Psychopathology and Disrupted Maternal Caregiving. Journal of Neuroendocrinology. 2014;26(10):665–684. doi: 10.1111/jne.12183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munk-Olsen T, Laursen TM, Pedersen CB, Mors O, Mortensen PB. New parents and mental disorders: a population-based register study. JAMA: Journal of the American Medical Association. 2006;296(21):2582–2589. doi: 10.1001/jama.296.21.2582. [DOI] [PubMed] [Google Scholar]
- Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, … Abdalla S. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. The Lancet. 2013;380(9859):2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- Murray L, Arteche A, Fearon P, Halligan S, Goodyer I, Cooper P. Maternal postnatal depression and the development of depression in offspring up to 16 years of age. Journal of the American Academy of Child & Adolescent Psychiatry. 2011;50(5):460–470. doi: 10.1016/j.jaac.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Myung W, Song J, Lim SW, Won HH, Kim S, Lee Y, … Carroll BJ. Genetic association study of individual symptoms in depression. Psychiatry Research. 2012;198(3):400–406. doi: 10.1016/j.psychres.2011.12.037. [DOI] [PubMed] [Google Scholar]
- O’Hara MW. Postpartum depression: what we know. Journal of Clinical Psychology. 2009;65(12):1258–1269. doi: 10.1002/jclp.20644. [DOI] [PubMed] [Google Scholar]
- O’Hara MW, Swain AM. Rates and risk of postpartum depression—a meta-analysis. International Review of Psychiatry. 1996;8(1):37–54. [Google Scholar]
- Oquendo MA, Barrera A, Ellis SP, Li S, Burke AK, Grunebaum M, … Mann JJ. Instability of symptoms in recurrent major depression: a prospective study. American Journal of Psychiatry. 2004;161(2):255–261. doi: 10.1176/appi.ajp.161.2.255. [DOI] [PubMed] [Google Scholar]
- Osborne JW, Costello AB. Best practices in exploratory factor analysis: Four recommendations for getting the most from your analysis. Pan-Pacific Management Review. 2009;12(2):131–146. [Google Scholar]
- Pawluski JL, Lonstein JS, Fleming AS. The Neurobiology of Postpartum Anxiety and Depression. Trends in Neurosciences. 2017;40(2):106–120. doi: 10.1016/j.tins.2016.11.009. [DOI] [PubMed] [Google Scholar]
- Pedrelli P, Blais MA, Alpert JE, Shelton RC, Walker RS, Fava M. Reliability and validity of the Symptoms of Depression Questionnaire (SDQ) CNS spectrums. 2014;19(6):535–546. doi: 10.1017/S1092852914000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: implications for major psychiatric disorders. Biological Psychiatry. 2003;54(5):515–528. doi: 10.1016/S0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- Putnam KT, Wilcox M, Robertson-Blackmore E, Sharkey K, Bergink V, Munk-Olsen T, … Newport J. Clinical phenotypes of perinatal depression and time of symptom onset: analysis of data from an International Consortium. The Lancet Psychiatry. 2017;4(6):477–485. doi: 10.1016/S2215-0366(17)30136-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The use of the Center for Epidemiologic Studies Depression Scale in adolescents and young adults. Journal of youth and adolescence. 1991;20(2):149–166. doi: 10.1007/BF01537606. [DOI] [PubMed] [Google Scholar]
- Raghunathan TE, Solenberger PW, Van Hoewyk J. IVEware: Imputation and variance estimation software. Ann Arbor, MI: Survey Methodology Program, Survey Research Center, Institute for Social Research, University of Michigan; 2002. http://www.isr.umich.edu/src/smp/ive/ [Google Scholar]
- Raîche G, Walls TA, Magis D, Riopel M, Blais JG. Non-graphical solutions for Cattell’s scree test. Methodology: European Journal of Research Methods for the Behavioral and Social Sciences. 2013;9(1):23–29. [Google Scholar]
- Ross L, Evans SG, Sellers E, Romach M. Measurement issues in postpartum depression part 1: anxiety as a feature of postpartum depression. Archives of Women’s Mental Health. 2003;6(1):51–57. doi: 10.1007/s00737-002-0155-1. [DOI] [PubMed] [Google Scholar]
- Ross LE, Evans SG, Sellers E, Romach M. Measurement issues in postpartum depression part 1: anxiety as a feature of postpartum depression. Archives of Women’s Mental Health. 2003;6(1):51–57. doi: 10.1007/s00737-002-0155-1. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Carmody T, Reimitz PE. The Inventory of Depressive Symptomatology (IDS): Clinician (IDS-C) and Self-Report (IDS-SR) ratings of depressive symptoms. International Journal of Methods in Psychiatric Research. 2000;9(2):45–59. [Google Scholar]
- Santor DA, Coyne JC. Shortening the CES-D to improve its ability to detect cases of depression. Psychological Assessment. 1997;9(3):233–243. [Google Scholar]
- Sharma S, Mukherjee S, Kumar A, Dillon WR. A simulation study to investigate the use of cutoff values for assessing model fit in covariance structure models. Journal of Business Research. 2005;58(7):935–943. [Google Scholar]
- Steiger JH. Structural model evaluation and modification: An interval estimation approach. Structural equation modeling: a multidisciplinary journal. 1990;5:411–419. doi: 10.1207/s15327906mbr2502_4. [DOI] [PubMed] [Google Scholar]
- Stowe ZN, Hostetter AL, Newport DJ. The onset of postpartum depression: Implications for clinical screening in obstetrical and primary care. American Journal of Obstetrics and Gynecology. 2005;192(2):522–526. doi: 10.1016/j.ajog.2004.07.054. [DOI] [PubMed] [Google Scholar]
- Taylor GT, Maloney S, Dearborn J, Weiss J. Hormones in the mentally disturbed brain: steroids and peptides in the development and treatment of psychopathology. Central Nervous System Agents in Medicinal Chemistry (Formerly Current Medicinal Chemistry-Central Nervous System Agents) 2009;9(4):331–360. doi: 10.2174/187152409789630398. [DOI] [PubMed] [Google Scholar]
- Tucker LR, Lewis C. A reliability coefficient for maximum likelihood factor analysis. Psychometrika. 1973;38(1):1–10. [Google Scholar]
- Tulchinsky D, Little AB. Maternal-fetal endocrinology. 2. Philadelphia: W.B. Saunders; 1994. [Google Scholar]
- Tuohy A, McVey C. Subscales measuring symptoms of non-specific depression, anhedonia, and anxiety in the Edinburgh Postnatal Depression Scale. British Journal of Clinical Psychology. 2008;47(2):153–169. doi: 10.1111/j.2044-8260.2008.tb00463.x. [DOI] [PubMed] [Google Scholar]
- Ullman JB. Structural equation modeling. In: Tabachnick BG, Fidell LS, editors. Using Multivariate Statistics. 4. Needham Heights, MA: Allyn & Bacon; 2001. pp. 653–771. [Google Scholar]
- Vandenberg RJ, Lance CE. A review and synthesis of the measurement invariance literature: Suggestions, practices, and recommendations for organizational research. Organizational Research Methods. 2000;3(1):4–70. [Google Scholar]
- Verbeek T, Bockting CL, van Pampus MG, Ormel J, Meijer JL, Hartman CA, Burger H. Postpartum depression predicts offspring mental health problems in adolescence independently of parental lifetime psychopathology. Journal of Affective Disorders. 2012;136(3):948–954. doi: 10.1016/j.jad.2011.08.035. [DOI] [PubMed] [Google Scholar]
- Verbeke G, Molenberghs G. Linear mixed models for longitudinal data. New York: Springer Science & Business Media; 2009. [Google Scholar]
- Viguera AC, Tondo L, Koukopoulos AE, Reginaldi D, Lepri B, Baldessarini RJ. Episodes of mood disorders in 2,252 pregnancies and postpartum periods. American Journal of Psychiatry. 2011 doi: 10.1176/appi.ajp.2011.11010148. [DOI] [PubMed] [Google Scholar]
- Wisner KL, Moses-Kolko EL, Sit DK. Postpartum depression: a disorder in search of a definition. Archives of Women’s Mental Health. 2010;13(1):37–40. doi: 10.1007/s00737-009-0119-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittchen HU. Reliability and validity studies of the WHO-Composite International Diagnostic Interview (CIDI): a critical review. Journal of Psychiatric Research. 1994;28(1):57–84. doi: 10.1016/0022-3956(94)90036-1. [DOI] [PubMed] [Google Scholar]
- World_Health_Organization. International Statistical Classification of Diseases and Related Health Problems 10th Revision. 2016. [Google Scholar]
- Yim IS, Glynn LM, Schetter CD, Hobel CJ, Chicz-DeMet A, Sandman CA. Risk of postpartum depressive symptoms with elevated corticotropin-releasing hormone in human pregnancy. Archives of General Psychiatry. 2009;66(2):162–169. doi: 10.1001/archgenpsychiatry.2008.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.