Abstract
PURPOSE
To evaluate the long-term alterations in corneal nerves in patients with herpes simplex virus (HSV) keratitis using in vivo confocal microscopy (IVCM).
DESIGN
Prospective longitudinal, cross sectional.
METHODS
This study included 16 patients with history of HSV keratitis and 15 age-matched normal controls. Slit-scanning IVCM was performed in all subjects at baseline and then after a mean follow-up of 37.3±1.7 months in the patient group. Corneal subbasal nerve density and corneal sensation were compared between groups at baseline and follow up.
RESULTS
At baseline, the mean subbasal nerve density was significantly lower in both affected eyes (1.4±0.6 mm/mm2) and contralateral unaffected eyes (6.4±0.7 mm/mm2) compared with the controls (14.1±1.6 mm/mm2; all P<0.001). At the end of follow-up, the mean nerve density in affected eyes increased to 2.8±0.7 mm/mm2 (P=0.006), with no significant change in contralateral unaffected eyes (6.5±1.0 mm/mm2, P=0.72). However, both eyes had lower nerve density than controls (all P<0.001). Corneal sensation was significantly lower in affected eyes (2.6±0.6 cm) than in the control group (6.0±0.0, P<0.001) and showed no significant change at the end of follow-up (2.5±0.6 cm, P=0.80). Corneal sensation in contralateral unaffected eyes was not different in comparison with controls at both baseline and follow up (all p>0.05).
CONCLUSIONS
Our results demonstrate that although corneal nerve regeneration occurs in patients with HSV keratitis, this change is not clinically significant and does not results in changes of corneal sensation. Therefore, these patients need to be followed closely for complications of neurotrophic keratopathy and might benefit from neuro-regenerative therapies.
Keywords: Corneal nerve regeneration, Corneal sensation, Herpes simplex keratitis, In vivo confocal microscopy
1. INTRODUCTION
Herpes simplex virus (HSV) keratitis is the most common type of infectious keratitis in the developed world [1, 2]. The incidence of HSV keratitis is estimated to be about 1.5 million cases per year worldwide, with 40,000 new cases of corneal blindness annually due to this disease [1]. All corneal layers can be involved in HSV keratitis, causing different forms of epithelial dendritic or geographic keratitis, stromal keratitis, and endotheliitis [1-3].
One of the complications of HSV keratitis is diminishment of corneal nerves, which results in neurotrophic keratopathy [4, 5]. Neurotrophic keratopathy may present as decreased corneal sensation, loss of epithelial integrity, and, in more severe cases, with corneal ulceration, melting, and even perforation [6, 7]. As corneal nerve damage is the underlying pathophysiologic mechanism for neurotrophic keratopathy, the long-term course of the disease depends, in part, on the regeneration of corneal nerves, which play a critical role in maintenance of corneal health and function [8, 9]. Although regeneration of corneal nerves and thus resolution of ocular surface disease have been observed after other forms of acute corneal nerve damage, such as that occurring after various corneal surgeries [9-14], it remains unclear whether there is regeneration of corneal nerves in patients with HSV keratitis.
Corneal nerves can be visualized using in vivo confocal microscopy (IVCM) [15-17]. IVCM is a non-invasive diagnostic tool, which allows real-time imaging of anterior segment structures at the cellular level. Using IVCM, density and morphology of corneal nerves have been evaluated in various corneal and systemic conditions such as dry eye disease, neurotrophic keratopathy, corneal infections, after corneal surgeries, and diabetes [15-17]. In patients with HSV keratitis, IVCM has shown significant changes in corneal subbasal nerves, including a decreased density along with morphologic changes [18-20]. Furthermore, it has been shown that such structural changes are correlated with changes in corneal sensation in these patients [18]. However, corneal nerve recovery after HSV keratitis has not been studied before.
As regeneration of corneal nerves has been noted after acute nerve injuries in conditions such as corneal surgeries and non-viral corneal infections [9-15, 21], we hypothesize that corneal nerves will also regenerate during long-term follow-up in patients with HSV keratitis. Therefore, this study was designed to evaluate such corneal nerve regeneration using IVCM.
2. METHODS
This prospective, longitudinal study included 16 patients with a history of HSV keratitis (HSV group) and 15 age-and gender-matched normal volunteers with healthy corneas and a normal tear film (control group). All patients and normal subjects were examined by slit-lamp biomicroscopy. The diagnosis of HSV keratitis was made according to the presence of epithelial dendritic lesions characteristic of epithelial HSV keratitis, by an experienced ophthalmologist in this field (DPL). All patients were initially diagnosed at the time of dendritic epithelial herpetic keratitis with no stromal involvement. Thus, any patient with a previous documented history of dendritic epithelial keratitis was included. Patients with history of contact lens wear, previous ocular surgery, or any history of ocular trauma were excluded from the study.
All subjects were recruited from the Cornea and Refractive Surgery Service, Massachusetts Eye and Ear Infirmary, Boston, Massachusetts, between November 2006 and December 2008. Prior to enrollment, all subjects signed an informed consent form. The protocol of the study was approved by the Human Studies Committee of the Massachusetts Eye and Ear Infirmary, Boston, Massachusetts, and the research was conducted according to the Health Insurance Portability and Accountability Act (HIPAA) and the tenets of the Declaration of Helsinki.
Primary outcome measures were corneal sensation and subbasal corneal nerve density (divided further by density of branches and trunks) in the affected eye. Secondary outcome measures were corneal sensation and subbasal corneal nerve density in the contralateral unaffected eyes, and total number of nerves (separated by number of branches and trunks) in each scan frame in both affected and unaffected contralateral eyes.
2.1. Corneal Sensation
Cochet-Bonnet esthesiometer (Luneau Ophthalmlogie, Chartres, France) was employed to measure central corneal sensation in both eyes. First, a length of 6 cm of the monofilament nylon thread was used to touch the cornea. If no sensation was felt by the patient the filament was progressively shortened by 1 cm until a positive response was obtained. Then, the filament was advanced by 0.5 cm to test again. The longest filament length which elicited a positive response in two times was considered as the corneal sensation threshold.
2.2. In Vivo Confocal Microscopy
To measure the central corneal nerve density, all subjects in both groups underwent slit scanning IVCM (SSCM, Confoscan 4; Nidek Technologies, Gamagori, Japan) on the central cornea of both affected and unaffected eyes in the HSV group and only one randomly-selected eye in the control group. In addition to the initial imaging, the SSCM was repeated after a minimum of two years to evaluate the long-term changes in the central corneal nerve density in the HSV group.
The method of SSCM imaging has been previously described [18]. In brief, after topical anesthesia in both eyes, a drop of 0.3% hypromellose gel was placed on the objective lens for optical coupling, and then the lens was manually advanced until the gel contacted the central cornea. Full thickness confocal scans were acquired at a speed of 25 frames per second, obtaining 350 images per scan, every 7 μm. A second scan was obtained for the anterior cornea obtaining sections every 3 μm. A total of 4 to 8 scans were obtained for each cornea in all subjects, depending on full thickness or anterior scan mode.
The IVCM images of the subbasal layer were used to measure the central corneal subbasal nerve density. For this, three best-focused representative images of this layer from various locations in the central cornea were selected and anonymized for the analysis. For measuring subbasal nerve density, the nerves were traced using NeuronJ(http://www.imagescience.org/meijering/software/neuronj/), which is a semi-automated nerve analysis plug-in program of ImageJ (National Institutes of Health, USA). Nerve density was measured by tracing all visible nerve fibers in the image and calculating the length of the nerve fibers in millimeters (mm) and was expressed as mm/mm2 (Figs. 1 A and B). In addition, nerve numbers were counted manually per frame and were reported as number/frame. The subbasal nerves were further categorized as main nerves (those which did not branch from other nerves), branch nerves (those which branch off from main nerves), and total nerves (a combination of both main and branch nerves). In images, which showed the subbasal layer in only part of the frame, the area was considered in calculating the nerve density. Then, the average nerve density and the average nerve number of three different IVCM images were calculated. All measurements were performed by two independent observers and the average values from both observers were used for the analysis (intraclass correlation coefficient was >0.8 for all measured variables).
Fig. 1.
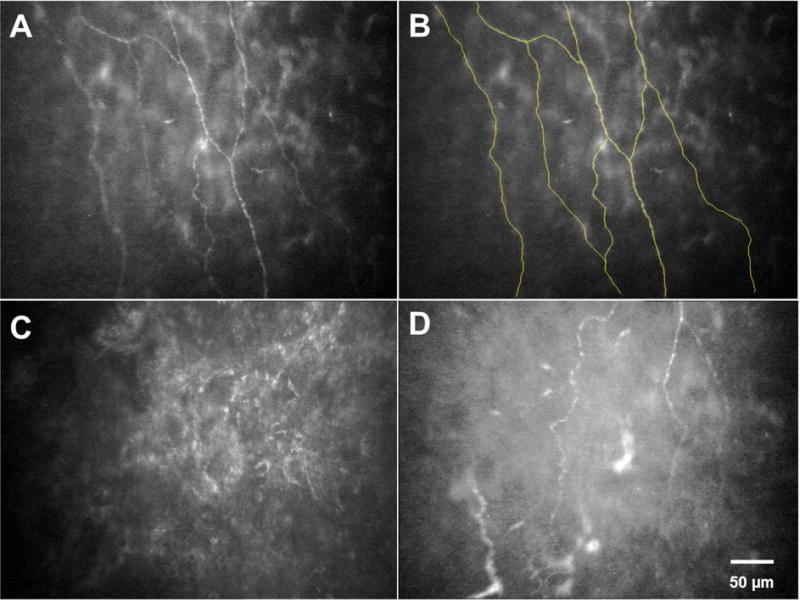
Slit scanning in vivo confocal microscopy for evaluation of corneal subbasal nerves. A) Normal subbasal nerve fibers. B) Using NeuronJ for tracing of subbasal nerves in A. C) Subbasal nerves in an eye with previous HSV keratitis. D) Subbasal nerves in the contralateral eye of a patient with HSV keratitis.
2.3. Statistical Analysis
Statistical analysis was performed with SPSS version 20 (SPSS Inc., Chicago, IL). Presence or absence of normal distribution of the data was first evaluated by Shapiro-Wilk test, which showed non-normal distribution of the data. Based on previous studies published by our group [21], the minimum sample size to detect difference (with confidence interval of 95%) with power of 80%, was calculated 9 patients. The following formula was used to calculate the sample size:
X1−X2 or delta was 9.79. Standard deviation (SD)1 was 9.98 and SD2 was 2.30. To compare the nerve density, nerve number, and sensation of the cornea between the HSV keratitis group and the control group, Mann-Whitney U test was used. To compare the nerve density, nerve number, and sensation of the cornea at the end of follow-up compared with the initial visit, related-samples Wilcoxon Signed Rank test was employed. Correlation studies were performed using Spearman’s rank correlation test. Nerve regeneration rate per month was calculated using this formula: (nerve density at baseline – nerve density at follow up)/months of follow-up. Data are presented as mean ± standard error of the mean (SEM). P values of less than 0.05 were considered as statistically significant.
3. RESULTS
The HSV group included 16 patients (10 women and 6 men) with a mean age of 61.7 ± 4.6 years (range, 21-84 years), and the control group was comprised of 15 subjects (7 women and 8 men) with a mean age of 59.1 ± 4.4 years (range, 42-76 years). There were no significant differences between the HSV group and the controls in regard to age and gender (Table 1). Two patients had a history of bilateral HSV keratitis, while all other 14 patients had a history of unilateral involvement. All patients in the HSV group had a history of corneal involvement by HSV for a mean duration of 17.9 ± 3.9 years (range, 0.75-56 years). During this time, they had a mean of 3.5 ± 0.6 episodes of recurrence (range, 1-10), with the last episode being 2.7 ± 0.6 years (range, 2 months to 9 years) prior to enrollment. During the follow-up period after enrollment in the study, 14 out of 18 eyes did not have any recurrences between the initial imaging and the follow-up imaging. However, 4 eyes had a recurrence of active stromal disease for 1.8 ± 0.5 times (range, 1-3). During the follow-up, all patients received prophylactic oral acyclovir with low dose steroids with different doses and variable periods of time.
Table 1.
Demographic data of normal and patients with herpes simplex keratitis
| Control group | Herpes simplex keratitis group | P value | |
|---|---|---|---|
| Number of patients | 15 | 16 | – |
| Age (years) | 59.1 ± 4.4 | 61.7 ± 4.6 | 0.65 |
| Gender (male/female) | 8/7 | 6/10 | 0.48 |
| Disease duration (years) | – | 17.9 ± 3.9 | – |
| Number of episodes | – | 3.5 ± 0.6 | – |
| Mean follow up time (months) | – | 37.3 ± 1.7 | – |
3.1. Affected Eyes in the HSV Group
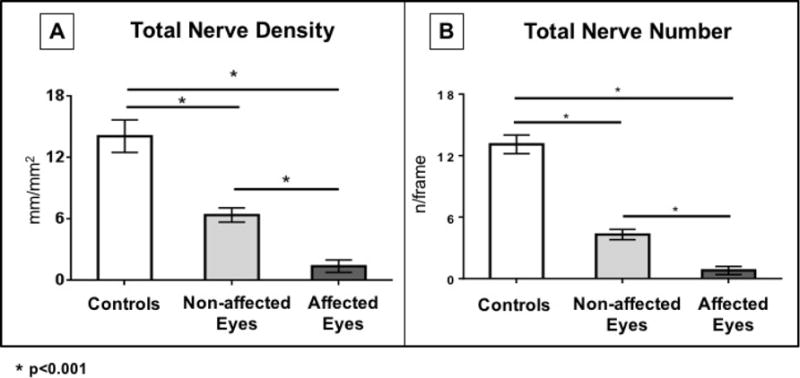
At the initial imaging visit in this study, all affected eyes (n=16) in the HSV group had corneal stromal scar or haze. At the initial visit, these eyes had a mean corneal sensation of 2.6 ± 0.6 cm which was significantly lower than the controls (6.0 ± 0.0 cm, P<0.001). In addition, at baseline, the total subbasal nerve density in the central cornea of the affected eyes was significantly lower (1.4 ± 0.6 mm/mm2) compared with controls (14.1 ± 1.6 mm/mm2, P<0.001, Figures 1 and 2). Total nerve number was also significantly lower in the affected eyes (0.8 ± 0.4 nerves per frame) compared with controls (13.1 ± 0.9 nerves per frame, P<0.001, Table 2, Figs. 1 and 2).
Fig. 2.
Total corneal subbasal nerve density (A) and number (B) in the affected and unaffected eyes of patients with HSV keratitis compared with the age-matched control group.
Table 2.
Corneal sensation, nerve density and nerve number in normal controls as well as in patients with HSV keratitis.
| Controls | HSV Keratitis Group | ||||||
|---|---|---|---|---|---|---|---|
| Affected Eyes (n=16) |
Contralateral Non-affected Eyes (n=14)§ |
||||||
| Initial Visit | Final Follow-up | P value | Initial Visit | Final Follow-up | P value | ||
| Number of Eyes | 15 | 16 | 16 | – | 14 | 14 | – |
| Mean Central Corneal Sensation (cm) | 6.0 ± 0 | 2.6 ± 0.6* | 2.5 ± 0.6* | 0.80 | 5.4 ± 0.2*† | 5.2 ± 0.2*† | 0.26 |
| Total Nerve Number (per frame) | 13.1 ± 0.9 | 0.8 ± 0.4 | 1.3 ± 0.5 | 0.02 | 4.3 ± 0.5 | 4.0 ± 2.9 | 0.88 |
| Total Nerve Density (mm/mm2) | 14.1 ± 1.6 | 1.4 ± 0.6* | 2.8 ± 0.7* | 0.006 | 6.4 ± 0.7*† | 6.5 ± 4.0*† | 0.72 |
Statistically significant difference compared with the control group
Statistically significant difference compared with the affected eye
Of the 16 patients with herpes simplex keratitis 2 had bilateral disease and therefore their contralateral eyes were not considered in this group.
Patients in the HSV group were followed for a mean duration of 37.3 ± 1.7 months (range, 29-46 months). At the end of follow-up, the corneal sensation was 2.5 ± 0.6 cm in the affected eye with no significant changes compared with the initial visit (P=0.80, Table 2). However, the central total corneal nerve density in the affected eyes increased to 2.8 ± 0.7 mm/mm2 (P=0.006), with a nerve regeneration rate of 37.5 ± 13.2 μm/mm2 per month (Figures 3 and 4). Total nerve density was further divided into branch and main nerve density. Branch density increased from 0.6 ± 0.2 to 1.5 ± 0.4 mm/mm2 (P=0.05). On the other hand, increase in main nerves density was not significant (from 0.8 ± 0.4 to 1.3 ± 0.5 mm/mm2, P=0.09). Total nerve number increased significantly at the end of follow up as compared to the baseline (from 0.8 ± 0.4 to 1.3 ± 0.5 nerves/frame, P=0.02). Main nerve number also increased significantly from 0.4 ± 0.2 nerves/frame at baseline to 0.9 ± 0.2 nerves/frame at follow up (P=0.02). However, branch number did not increase significantly at the end of follow-up as compared to baseline (from 0.3 ± 0.2 to 0.5 ± 0.2 nerves/frame, P=0.21) (Figs. 3 and 4). Despite the presence of nerve regeneration, at the end of follow-up, the densities and numbers of main nerves, branches, and total nerves in affected eyes still remained significantly lower compared with the control group (all P<0.001).
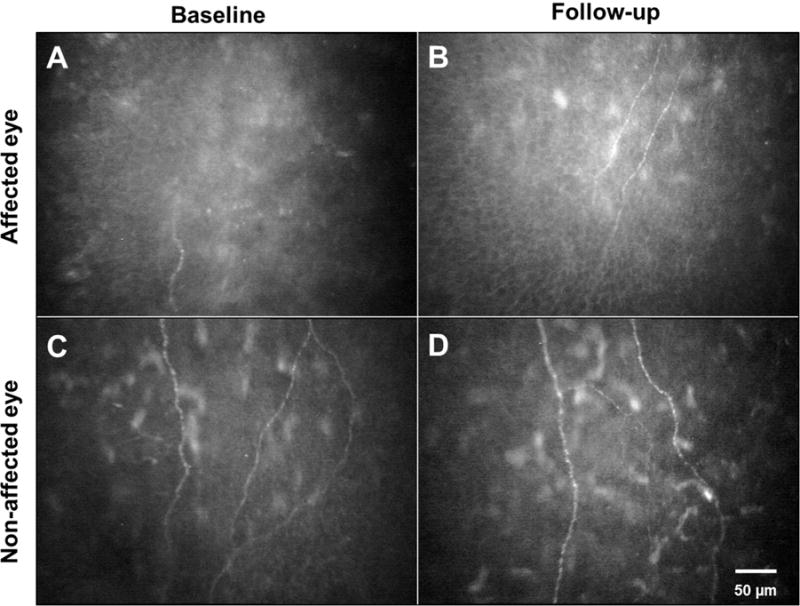
Fig. 3.
Regeneration of corneal nerves in patients with HSV keratitis. In an eye with previous HSV keratitis, there is a significant loss of subbasal nerves (A). The same eye after 3 years of follow-up shows regeneration of corneal subbasal nerves (B). The contralateral eye of a patient with previous HSV keratitis demonstrates a significant subbasal nerve loss (C) with no significant change in nerve density after 3 years of follow-up (D).
Fig. 4.
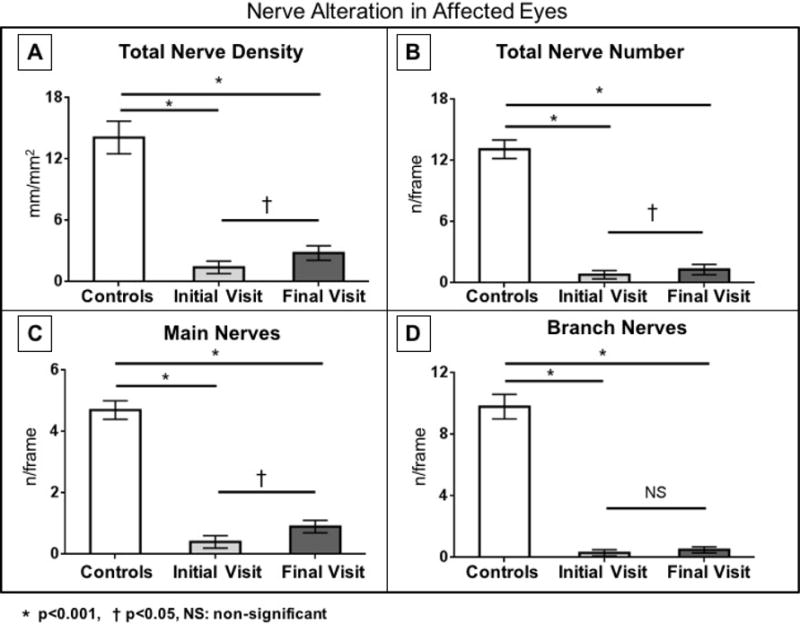
Corneal subbasal nerve density and number in the affected eyes of patients with HSV keratitis at baseline and after a mean follow-up of more than 3 years. There are significant increases in total nerve density, total nerve number, and main nerve number in the affected eyes.
There were no significant correlations between nerve regeneration and the following: age, gender, and interval from the first episode to enrollment, number of previous episodes of the disease, or the number of recurrences during the follow-up. A sub-group analysis of those four patients who had recurrences during the follow up period showed an increased nerve density at follow up visit as compared with their baseline, however it was not a significant change (3.2 ± 1.1 vs. 2.0 ± 1.0 mm/mm2, p=0.37). Total nerve number was also slightly increased at the follow up as compared with baseline in this subgroup of patients (2.2 ± 0.7 vs. 1.1 ± 0.5 n/frame, p=0.25).
3.2. Non-Affected Eyes in the HSV Group
At the initial imaging visit, there was no clinical corneal involvement in the non-affected eyes of the HSV group (n=14). At this visit, the corneal sensation in these eyes (5.4 ± 0.2 mm) was lower than the controls (6.0 ± 0.0 mm, P=0.03). Furthermore, at baseline, the mean central total corneal subbasal nerve density was significantly lower in the non-affected eyes of the HSV group (6.4 ± 0.7 mm/mm2) compared with the control group (14.1 ± 1.6 mm/mm2, P<0.001, Figs. 1 and 2). Total nerve number was also significantly lower in the non-affected eyes (4.3 ± 0.5 nerves/frame) compared with controls (13.1 ± 0.9 nerves/frame, P<0.001, Figs. 1 and 2). In addition, there were also significant differences between affected and non-affected eyes in main nerve, branch, and total nerve densities (P=0.001, P=0.002, and P<0.001, respectively [Figs. 1 and 2]). Similarly, the number of main nerve, branch, and total nerves were significantly lower in affected eyes as compared to non-affected eyes (all P<0.001).
At the end of follow-up in the non-affected eyes (Figs. 3 and 5), the corneal nerve density was 6.5 ± 1.0 mm/mm2, which was not statistically significant from the initial visit (P=0.72), but still significantly lower compared with the control group (P<0.001). At the end of follow-up, there were also no significant changes in main nerve (4.2 ± 0.9 mm/mm2, P=0.72) and branch nerve (2.6 ± 0.5 mm/mm2, P=0.65) densities. There were also no significant changes at the end of follow-up in numbers of main nerves (2.2 ± 0.4 nerves/frame, P=0.20), branches (1.7 ± 0.4 nerves/frame, P=0.51), and total nerves (4.0 ± 0.7 nerves/frame, P=0.88) (Figures 3 and 5). Furthermore, at the end of follow-up, the corneal sensation in the non-affected eyes was 5.2 ± 0.2 mm, with no significant changes compared with the initial visit (P=0.28, Table 2).
Fig. 5.
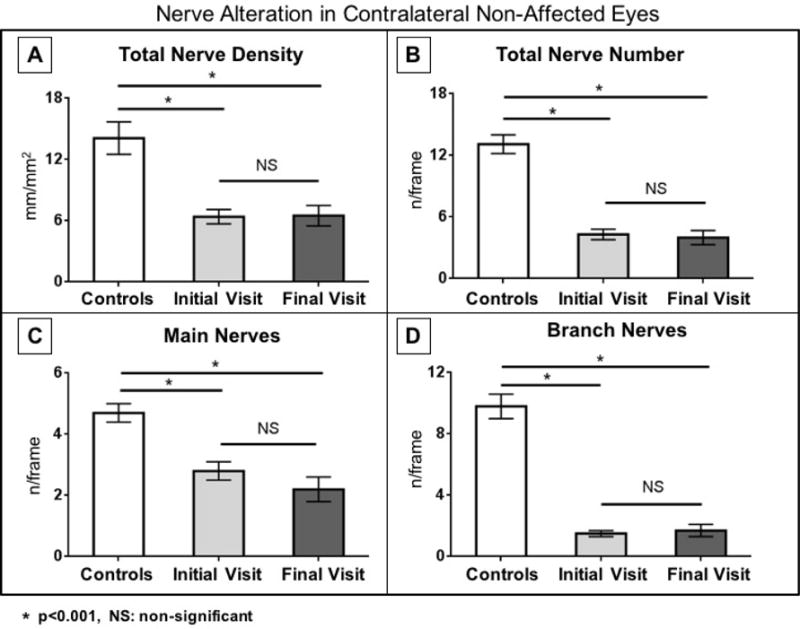
Corneal subbasal nerve density and number in the non-affected eyes of patients with HSV keratitis at baseline and after a mean follow-up of more than 3 years. There are no significant changes in nerve density and number in the non-affected eyes.
4. DISCUSSION
Herpes simplex keratitis is a known cause of neurotrophic keratopathy [4, 5]. This prospective, longitudinal study is the first to evaluate central corneal nerve changes in patients with HSV keratitis. Over a mean follow-up of more than 3 years, although we observe a statistically significant regeneration of central corneal subbasal nerves in the affected eyes, the level of regeneration is not clinically significant. At the end of follow-up, corneal nerve density remains significantly lower compared with the age-matched control group. In addition, no significant change in their low corneal sensation was observed during this time period, confirming the lack of clinically significant improvement in nerve function.
Using slit-scanning IVCM in this study, we demonstrate a significantly lower density and number of central corneal subbasal nerves in the affected eyes as well as in the clinically unaffected contralateral eyes of the patients with HSV keratitis compared with an age-matched control group, as has been demonstrated before [18-20, 22]. Although neurotrophic keratopathy has been reported in 6% of patients with HSV keratitis [6, 23], the incidence of corneal nerve damage in these cases may be much higher. This is due to the fact that changes in corneal sensation are less sensitive than those in corneal nerve density, detected by IVCM. This is supported by our previous studies, which have shown that significant nerve damage (approximately 70%) would occur before development of reduced corneal sensation and associated neurotrophic complications [18]. Therefore, clinical use of IVCM for evaluation of corneal nerves could provide a better insight to the frequency of this complication in patients with HSV keratitis and will detect those with nerve damage much earlier.
During a mean follow-up of 3.1 years in our study, the central corneal subbasal nerve density in the affected eyes shows a nerve regeneration rate of 37.5 ± 13.2 μm/mm2 per month. Surprisingly, disease recurrence did not have a negative impact on corneal nerve regeneration as we demonstrated by subgroup analysis of four patients with recurrent disease during follow up. Interestingly, even those four patients demonstrated some non-significant corneal nerve regeneration capacity with nerve regeneration rate of 39.2 ± 28.2 μm/mm2 per month.
Separating main nerve and branch density and number, our data suggest that the increase in nerve density was more probably due to increased branch length (not number of new branches) and increased number of main nerves (but not main nerve density) at follow up as compared to baseline. Although regeneration of corneal nerves after an acute injury has been well documented after corneal laser refractive surgeries [10, 11] and cataract surgery [14, 24], our study is the first to show such a nerve regeneration after corneal nerve loss in patients with HSV keratitis. Similarly, in a mouse model of HSV keratitis, Chucair-Elliott and colleagues demonstrated re-innervation of HSV infected corneas 30 days post infection after an initial loss [25]. The rate of nerve regeneration in our patients with HSV keratitis is much slower than the rate reported for non-viral corneal infections (1.02 ± 0.52 mm/mm2 per month) [21]. The reasons for such slow regeneration after corneal infections by HSV are not clear. However, in HSV encephalitis it has been shown that patients develop autoantibodies against NMDA receptors expressed by nerves [26-29]. It may be speculated that such autoantibodies, are also developed after HSV keratitis, preventing or slowing down the corneal nerve regeneration.
The exact mechanisms involved in corneal nerve regeneration in eyes with HSV keratitis and the factors affecting this regeneration are not well understood. Various neurotrophins, neurotrophic factors, and nerve guidance factors such as semaphorins, regeneration-associated genes, and inflammatory mediators have been implicated in corneal nerve regeneration [9]; however, their role in re-innervation after HSV keratitis remains to be elucidated. A recent study [25] on a murine model of HSV keratitis demonstrated that semaphorin 7A, a nerve guidance molecule which enhances axonal outgrowth and regeneration [30], plays a role in nerve regeneration after HSV keratitis. Another pre-clinical study has also shown that corneal nerve damage is regulated by CD4+ T cells and that in a CD4+ T cell-deficient mice, the nerve degeneration and loss of blink reflex is reversible [31].
Despite significant regeneration of corneal nerves in eyes with HSV keratitis during long-term follow-up, both affected and non-affected eyes still had significantly lower corneal nerve densities compared with the control group. In photorefractive keratectomy (PRK), where only subbasal and superficial nerves are ablated, normal nerve density is achieved by 2 years after the surgery [32]. In laser in situ keratomileusis (LASIK), where the stromal nerves are cut, the nerve regeneration may take more than 5 years [33, 34]. On the other hand, after penetrating keratoplasty, which transects the stromal nerves 360°, re-innervation is very limited even decades after the procedure [12, 13, 35]. Therefore, the regeneration rate seems to be dependent on the depth and extent of initial corneal nerve injury. As the stromal keratitis in HSV may involve stromal nerves to a variable degree, the regeneration may be very slow and protracted. Therefore, it may be more important to measure the density of stromal nerves as a predictor for regeneration. However, as quantification of stromal nerves by IVCM is challenging [36], published studies on HSV keratitis to date have evaluated the subbasal nerves, which can be quantified in a standardized fashion. Additional research is required to relate the extent of stromal nerve involvement by the rate of regeneration.
In spite of corneal nerve regeneration in the affected eyes during the follow-up in this study, there was no clinically significant change in corneal sensation as measured by Cochet-Bonnet esthesiometer. Similarly, in a murine model of HSV keratitis, no recovery of sensation was observed 30 days after infection despite corneal nerve regeneration [25]. We have previously shown that the correlation between corneal subbasal nerve density and the corneal sensation is not linear, and thus a significant increase in corneal nerves is required to note an increase in corneal sensation [18]. Therefore, it is plausible that in the current study the nerve density at follow up did not reach to a certain threshold to affect corneal sensation. In addition, it can be speculated that the newly regenerated nerves may not be fully functional. This has been confirmed in a murine model of HSV keratitis in which despite nerve regeneration there was no recovery of corneal sensation as measured by blink reflex [25]. In addition, the latter investigation showed that the regenerated fibers were disorganized and lacked both the fine nerve bundles and substance P reactivity, with areas of abnormally myelinated fibers [25]. Moreover, in a more recent preclinical study, it has been demonstrated that nerve regeneration after HSV keratitis is mainly from sympathetic nerves rather than sensory nerves [37]. Therefore, if this is also true in humans, it may also explain the lack of an increase in corneal sensation despite an increased nerve density as was seen in our study. Future studies are necessary to determine whether with longer follow-up, full recovery of corneal nerve structure and function may occur.
As has been shown before in our patients with unilateral involvement, the corneal nerve density and number, as well as corneal sensation, were significantly lower in non-affected contralateral eyes as well. Such structural and functional changes persisted after follow-up [18, 20, 38]. In addition, it has been noted that such eyes are prone to develop dry eye disease [39, 40]. Therefore, it is important that both eyes of patients with seemingly unilateral HSV disease are monitored for any associated ocular surface disease. Our hypothesis on the mechanisms of contralateral involvement has been previously described in details [18]. In brief, it can be due to: i) asymmetric bilateral disease, ii) HSV crossing between nerve anastomosis or from the trigeminal ganglia to the mesencephalic trigeminal nucleus, causing contralateral damage to the distal nerve plexus, and more likely iii) due to central nervous system downregulation in the contralateral eye. Interestingly, during our follow up we did not observe any significant change in the corneal nerve density or number in the contralateral un-affected eyes. This can be explained by lower extent of damage at the baseline and different mechanism of nerve damage in the contralateral corneas.
One of the limitations of this study is that slit-scanning IVCM, which has a lower resolution than laser-scanning IVCM [48], was used in this study, as the latter was not available when the initial imaging was performed. Moreover, subbasal nerve density was measured only in the central cornea and not peripheral cornea, which might not be representative of whole cornea. Furthermore, morphologic parameters of cornea nerves, such as tortuosity, were not evaluated. Additional research is required to investigate the morphology and topographic distribution of the regenerated nerves relative to the site of previous corneal involvement by HSV. Finally, we used Cochet-Bonnet esthesiometer for measuring corneal sensation. Additional studies using other types of esthesiometer such as Belmonte, which is not commercially available, may provide more information on changes in corneal sensation after corneal HSV disease.
Because nerve regeneration after HSV keratitis can be very slow, measures that facilitate corneal nerve growth may be valuable in these patients. This may be of particular importance for those with significantly low corneal nerve density, as they have a high risk of developing the complications of neurotrophic keratopathy, such as neurotrophic ulcers, corneal melting, or perforations. Previously, various measures have been used for promoting nerve regeneration and epithelial healing in patients with neurotrophic keratopathy [9]. Examples include nerve growth factor (NGF) [41, 42], autologous serum eye drops [43, 44], and umbilical cord serum eye drops [45]. However, the effects of these measures on nerve regeneration after HSV keratitis remain unknown. On the other hand, the role of anti-inflammatory treatment on this regeneration is also challenging, as it has been shown that some degrees of inflammation may enhance the nerve regeneration in neurotrophic keratopathy [46, 47]. The optimal measure for enhancing the nerve regeneration in patients with HSV keratitis remains to be elucidated in future studies.
5. CONCLUSION
This study demonstrates modest regeneration of corneal nerves during long-term follow-up in eyes with HSV keratitis. Despite this regeneration, these corneas had still significantly lower nerve density compared with the control group even years after the last episode of active recurrence and the corneal sensitivity did not recover. Close monitoring of these patients for complications of neurotrophic keratopathy, and measures to further facilitate the regeneration is recommended for patients with HSV keratitis likely indefinitely.
Acknowledgments
Funding support: NIH K08-eye020575 (to PH), NIH R01-EY022695 (PH), Falk Medical Research Trust (to PH), New England Corneal Transplant Research Fund (to PH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosures: Hamid-Reza Moein: none; Ahmad Kheirkhah: none; Rodrigo T. Muller: none; Andrea C. Cruzat: none; Deborah Pavan-Langston: none; Pedram Hamrah has a patent, INFLAMMATORY EYE DISORDERS, issued on the use of IVCM to assess ocular inflammatory disorders.
References
- 1.Farooq AV, Shukla D. Herpes simplex epithelial and stromal keratitis: an epidemiologic update. Surv Ophthalmol. 2012;57(5):448–62. doi: 10.1016/j.survophthal.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rowe AM, St Leger AJ, Jeon S, Dhaliwal DK, Knickelbein JE, Hendricks RL. Herpes keratitis. Prog Retin Eye Res. 2013;32:88–101. doi: 10.1016/j.preteyeres.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hill GM, Ku ES, Dwarakanathan S. Herpes simplex keratitis. Dis Mon. 2014;60(6):239–46. doi: 10.1016/j.disamonth.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Cavanagh HD, Pihlaja D, Thoft RA, Dohlman CH. The pathogenesis and treatment of persistent epithelial defects. Trans Sect Ophthalmol Am Acad Ophthalmol Otolaryngol. 1976;81(5):754–69. [PubMed] [Google Scholar]
- 5.Davis EA, Dohlman CH. Neurotrophic keratitis. Int Ophthalmol Clin. 2001;41(1):1–11. doi: 10.1097/00004397-200101000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Semeraro F, Forbice E, Romano V, Angi M, Romano MR, Filippelli ME, et al. Neurotrophic keratitis. Ophthalmologica. 2014;231(4):191–7. doi: 10.1159/000354380. [DOI] [PubMed] [Google Scholar]
- 7.Bonini S, Rama P, Olzi D, Lambiase A. Neurotrophic keratitis. Eye (London) 2003;17(8):989–95. doi: 10.1038/sj.eye.6700616. [DOI] [PubMed] [Google Scholar]
- 8.Müller LJ, Marfurt CF, Kruse F, Tervo TM. Corneal nerves: structure, contents and function. Exp Eye Res. 2003;76(5):521–42. doi: 10.1016/s0014-4835(03)00050-2. [DOI] [PubMed] [Google Scholar]
- 9.Shaheen BS, Bakir M, Jain S. Corneal nerves in health and disease. Surv Ophthalmol. 2014;59(3):263–85. doi: 10.1016/j.survophthal.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Latvala T, Linna T, Tervo T. Corneal nerve recovery after photorefractive keratectomy and laser in situ keratomileusis. Int Ophthalmol Clin. 1996;36(4):21–7. doi: 10.1097/00004397-199603640-00005. [DOI] [PubMed] [Google Scholar]
- 11.Linna TU, Pérez-Santonja JJ, Tervo KM, Sakla HF, Alió y Sanz JL, Tervo TM. Recovery of corneal nerve morphology following laser in situ keratomileusis. Exp Eye Res. 1998;66(6):755–63. doi: 10.1006/exer.1998.0469. [DOI] [PubMed] [Google Scholar]
- 12.Cho H, Ide C, Tazawa Y. Nerve regeneration in cornea after penetrating keratoplasty in rabbit, with special reference to relationship between regenerating nerves and basal laminae. Jpn J Ophthalmol. 1988;32(3):255–63. [PubMed] [Google Scholar]
- 13.Richter A, Slowik C, Somodi S, Vick HP, Guthoff R. Corneal reinnervation following penetrating keratoplasty–correlation of esthesiometry and confocal microscopy. German J Ophthalmol. 1996;5(6):513–7. [PubMed] [Google Scholar]
- 14.Kohlhaas M. Corneal sensation after cataract and refractive surgery. J Cataract Refract Surg. 1998;24(10):1399–409. doi: 10.1016/s0886-3350(98)80237-x. [DOI] [PubMed] [Google Scholar]
- 15.Patel DV, McGhee CN. In vivo confocal microscopy of human corneal nerves in health, in ocular and systemic disease, and following corneal surgery: a review. Br J Ophthalmol. 2009;93(7):853–60. doi: 10.1136/bjo.2008.150615. [DOI] [PubMed] [Google Scholar]
- 16.Cruzat AD, Pavan-Langston D, Hamrah P. In vivo confocal microscopy of corneal nerves: analysis and clinical correlation. Semin Ophthalmol. 2010;25(5–6):171–7. doi: 10.3109/08820538.2010.518133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang EF, Misra SL, Patel DV. In vivo confocal microscopy of the human cornea in the assessment of peripheral neuropathy and systemic diseases. BioMed Res Int. 2015;2015:951081. doi: 10.1155/2015/951081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamrah P, Cruzat A, Dastjerdi MH, Zheng L, Shahatit BM, Bayhan HA, et al. Corneal sensation and subbasal nerve alterations in patients with herpes simplex keratitis: an in vivo confocal microscopy study. Ophthalmology. 2010;117(10):1930–6. doi: 10.1016/j.ophtha.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagasato D, Araki-Sasaki K, Kojima T, Ideta R, Dogru M. Morphological changes of corneal subepithelial nerve plexus in different types of herpetic keratitis. Jpn J Ophthalmol. 2011;55(5):444–50. doi: 10.1007/s10384-011-0068-5. [DOI] [PubMed] [Google Scholar]
- 20.Hamrah P, Sahin A, Dastjerdi MH, Shahatit BM, Bayhan HA, Dana R, et al. Cellular changes of the corneal epithelium and stroma in herpes simplex keratitis: an in vivo confocal microscopy study. Ophthalmology. 2012;119(9):1791–7. doi: 10.1016/j.ophtha.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Müller RT, Abedi F, Cruzat A, Witkin D, Baniasadi N, Cavalcanti BM, et al. Degeneration and regeneration of subbasal corneal nerves after infectious keratitis: a longitudinal in vivo confocal microscopy study. Ophthalmology. 2015;122(11):2200–9. doi: 10.1016/j.ophtha.2015.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martone G, Alegente M, Balestrazzi A, Nuti E, Traversi C, Pichierri P, et al. In vivo confocal microscopy in bilateral herpetic keratitis: a case report. Eur J Ophthalmol. 2008;18(6):994–7. doi: 10.1177/112067210801800622. [DOI] [PubMed] [Google Scholar]
- 23.Sacchetti M, Lambiase A. Diagnosis and management of neurotrophic keratitis. Clin Ophthalmol. 2014;8:571–9. doi: 10.2147/OPTH.S45921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Cillà S, Fogagnolo P, Sacchi M, Orzalesi N, Carini E, Ceresara G, et al. Corneal involvement in uneventful cataract surgery: an in vivo confocal microscopy study. Ophthalmologica. 2014;231(2):103–10. doi: 10.1159/000355490. [DOI] [PubMed] [Google Scholar]
- 25.Chucair-Elliott AJ, Zheng M, Carr DJ. Degeneration and regeneration of corneal nerves in response to HSV-1 infection. Invest Ophthalmol Vis Sci. 2015;56(2):1097–107. doi: 10.1167/iovs.14-15596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bradshaw MJ, Venkatesan A. Herpes simplex virus-1 encephalitis in adults: pathophysiology, diagnosis, and management. Neurotherapeutics. 2016;13(3):493–508. doi: 10.1007/s13311-016-0433-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yushvayev-Cavalier Y, Nichter C, Ramirez-Zamora A. Possible autoimmune association between herpes simplex virus infection and subsequent anti-N-methyl-d-aspartate receptor encephalitis: a pediatric patient with abnormal movements. Pediatr Neurol. 2015;52(4):454–6. doi: 10.1016/j.pediatrneurol.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 28.Desena A, Graves D, Warnack W, Greenberg BM. Herpes simplex encephalitis as a potential cause of anti-N-methyl-D-aspartate receptor antibody encephalitis: report of 2 cases. JAMA Neurol. 2014;71(3):344–6. doi: 10.1001/jamaneurol.2013.4580. [DOI] [PubMed] [Google Scholar]
- 29.Hacohen Y, Deiva K, Pettingill P, Waters P, Siddiqui A, Chretien P, et al. N-methyl-D-aspartate receptor antibodies in post-herpes simplex virus encephalitis neurological relapse. Mov Disord. 2014;29(1):90–6. doi: 10.1002/mds.25626. [DOI] [PubMed] [Google Scholar]
- 30.Pasterkamp RJ, Peschon JJ, Spriggs MK, Kolodkin AL. Semaphorin 7A promotes axon outgrowth through integrins and MAPKs. Nature. 2003;424(6947):398–405. doi: 10.1038/nature01790. [DOI] [PubMed] [Google Scholar]
- 31.Yun H, Rowe AM, Lathrop KL, Harvey SA, Hendricks RL. Reversible nerve damage and corneal pathology in murine herpes simplex stromal keratitis. J Virol. 2014;88(14):7870–80. doi: 10.1128/JVI.01146-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Erie JC, McLaren JW, Hodge DO, Bourne WM. Recovery of corneal subbasal nerve density after PRK and LASIK. Am J Ophthalmol. 2005;140(6):1059–1064. doi: 10.1016/j.ajo.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 33.Darwish T, Brahma A, O’Donnell C, Efron N. Subbasal nerve fiber regeneration after LASIK and LASEK assessed by noncontact esthesiometry and in vivo confocal microscopy: prospective study. J Cataract Refract Surg. 2007;33(9):1515–21. doi: 10.1016/j.jcrs.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 34.Moilanen JA, Holopainen JM, Vesaluoma MH, Tervo TM. Corneal recovery after lasik for high myopia: a 2-year prospective confocal microscopic study. Br J Ophthalmol. 2008;92(10):1397–402. doi: 10.1136/bjo.2007.126821. [DOI] [PubMed] [Google Scholar]
- 35.Mather, Jester JV, Lemp MA. Return of human corneal sensitivity after penetrating keratoplasty. Arch Ophthalmol. 1988;106(2):210–1. doi: 10.1001/archopht.1988.01060130220030. [DOI] [PubMed] [Google Scholar]
- 36.Patel DV, McGhee CN. Quantitative analysis of in vivo confocal microscopy images: a review. Surv Ophthalmol. 2013;58(5):466–75. doi: 10.1016/j.survophthal.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 37.Yun H, Lathrop KL, Hendricks RL. A central role for sympathetic nerves in herpes stromal keratitis in mice. Invest Ophthalmol Vis Sci. 2016;57(4):1749–56. doi: 10.1167/iovs.16-19183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Müller RT, Pourmirzaie R, Pavan-Langston D, Cavalcanti BM, Aggarwal S, Colón C, et al. In vivo confocal microscopy demonstrates bilateral loss of endothelial cells in unilateral herpes simplex keratitis. Invest Ophthalmol Vis Sci. 2015;56(8):4899–906. doi: 10.1167/iovs.15-16527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simard-Lebrun A, Boisjoly H, Al-Saadi A, Choremis J, Mabon M, Chagnon M. Association between unilateral quiescent stromal herpetic keratitis and bilateral dry eyes. Cornea. 2010;29(11):1291–5. doi: 10.1097/ICO.0b013e3181cbf9f5. [DOI] [PubMed] [Google Scholar]
- 40.Jabbarvand M, Hashemian H, Khodaparast M, Rafatnejad A, Beheshtnejad A, Salami A. Do unilateral herpetic stromal keratitis and neurotrophic ulcers cause bilateral dry eye? Cornea. 2015;34(7):768–72. doi: 10.1097/ICO.0000000000000425. [DOI] [PubMed] [Google Scholar]
- 41.Bonini S, Lambiase A, Rama P, Caprioglio G, Aloe L. Topical treatment with nerve growth factor for neurotrophic keratitis. Ophthalmology. 2000;107(7):1347–51. doi: 10.1016/s0161-6420(00)00163-9. discussion 1351-2. [DOI] [PubMed] [Google Scholar]
- 42.Esquenazi S, Bazan HE, Bui V, He J, Kim DB, Bazan NG. Topical combination of NGF and DHA increases rabbit corneal nerve regeneration after photorefractive keratectomy. Invest Ophthalmol Vis Sci. 2005;46(9):3121–7. doi: 10.1167/iovs.05-0241. [DOI] [PubMed] [Google Scholar]
- 43.Aggarwal S, Kheirkhah A, Cavalcanti BM, Cruzat A, Colon C, Brown E, et al. Autologous serum tears for treatment of photoallodynia in patients with corneal neuropathy: efficacy and evaluation with in vivo confocal microscopy. Ocul Surf. 2015;13(3):250–262. doi: 10.1016/j.jtos.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matsumoto Y, Dogru M, Goto E, Ohashi Y, Kojima T, Ishida R, et al. Autologous serum application in the treatment of neurotrophic keratopathy. Ophthalmology. 2004;111(6):1115–20. doi: 10.1016/j.ophtha.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 45.Yoon KC, You IC, Im SK, Jeong TS, Park YG, Choi J. Application of umbilical cord serum eyedrops for the treatment of neurotrophic keratitis. Ophthalmology. 2007;114(9):1637–42. doi: 10.1016/j.ophtha.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 46.Namavari A, Chaudhary S, Chang JH, Yco L, Sonawane S, Khanolkar V, et al. Cyclosporine immunomodulation retards regeneration of surgically transected corneal nerves. Invest Ophthalmol Vis Sci. 2012;53(2):732–40. doi: 10.1167/iovs.11-8445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee HK, Ryu I, Seo KY, Hong S, Kim HC, Kim EK. Topical 0.1% prednisolone lowers nerve growth factor expression in keratoconjunctivitis sicca patients. Ophthalmology. 2006;113(2):198–205. doi: 10.1016/j.ophtha.2005.09.033. [DOI] [PubMed] [Google Scholar]
- 48.Szaflik JP. Comparison of in vivo confocal microscopy of human cornea by white light scanning slit and laser scanning systems. Cornea. 2007;26(4):438–45. doi: 10.1097/ICO.0b013e31803dcd11. [DOI] [PubMed] [Google Scholar]


