Abstract
In DNA, guanine oxidation yields diastereomers of 5-guanidinohydantoin (Gh) as one of the major products. In nucleosides and single-stranded DNA, Gh is in a pH-dependent equilibrium with its constitutional isomer iminoallantoin (Ia). Herein, the isomerization reaction between Gh and Ia was monitored in duplex DNA using a protein nanopore by measuring the ionic current when duplex DNA interacts with the pore under an electrophoretic force. Monitoring current levels in this single-molecule method proved to be superior for analysis of population distributions in an equilibrating mixture of four isomers in duplex DNA as a function of pH. The results identified Gh was a major isomer observed when base paired with A, C, or G at pH 6.4–8.4, and Ia was a minor isomer of the reaction mixture that was only observed when the pH was >7.4 in the duplex DNA context. The present results suggest that Gh will be the dominant isomer in duplex DNA under physiological conditions regardless of the base pairing partner in the duplex.
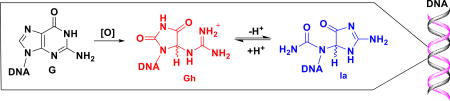
Graphical abstract
INTRODUCTION
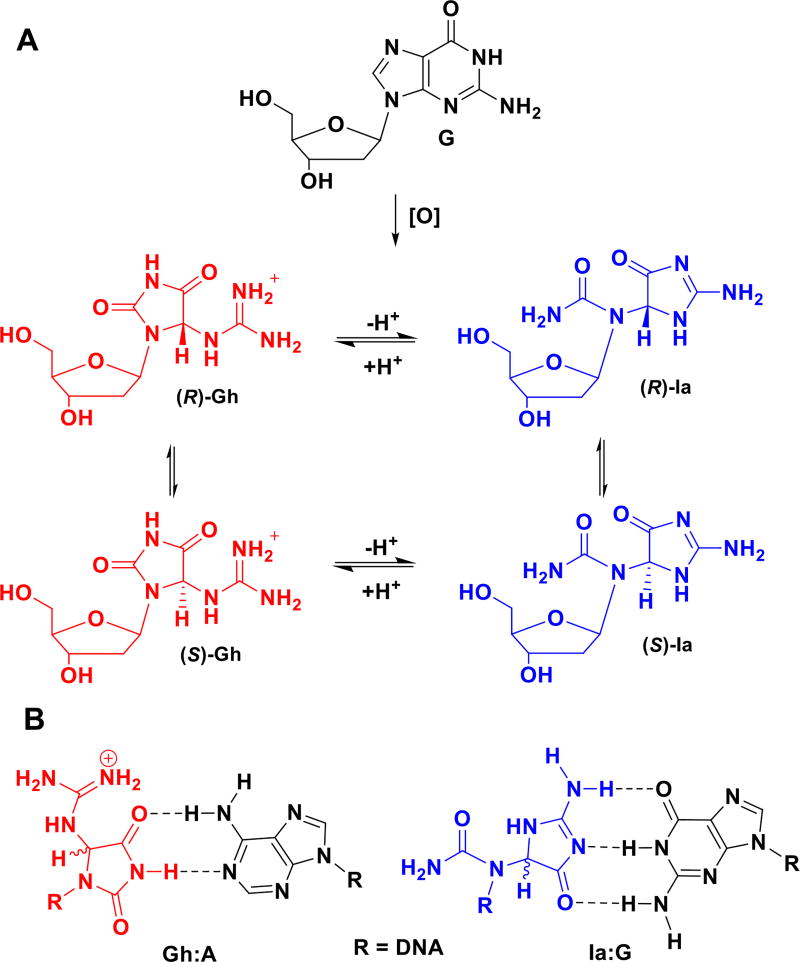
The heterocyclic bases of the four canonical DNA nucleotides have distinct hydrogen bonding properties utilized for information storage in biology that can be disrupted by oxidation or alkylation of the bases.1–4 The guanine (G) base is the most susceptible site in DNA toward oxidation yielding an array of products described by many different reaction trajectories.2,5,6 One oxidation channel of G in DNA furnishes the diastereotopic 5-guanidinohydantoin (Gh) products (Scheme 1A).7–9 The mechanism of this transformation has already been discussed7 and involves hydroxylation of G at C5 followed by hydration of C6 and decarboxylation. Gh forms in competition with another hydantoin, spiroiminodihydantoin, formed preferentially in nucleosides or in single-stranded oligonucleotides at elevated pH.10 Gh formation is preferred in the duplex context in which steric encumbrance likely hinders the competing spirocyclization.10 Depending upon which heterocycle is formed, base pairing and nucleotide insertion opposite the hydantoin will likely change.
Scheme 1.
Oxidation of G to the isomers Gh and Ia and key base pairs for Gh and Ia.
The diastereomers of Gh slowly interconvert via enolization of the C4 carbonyl that was determined in the nucleoside context by NMR.7 The Gh diastereomers in single-stranded DNA (ssDNA) are resolvable by anion-exchange HPLC; however, the diastereomers interconvert on a time scale too fast to allow individual study (t1/2 < 12 h). Isomerization between the Gh diastereomers in duplex DNA has not been monitored because they cannot be resolved by anion-exchange HPLC in this context, there do not exist resolved NMR signals to monitor the reaction, and crystallography is not amendable to this analysis.
Recently it was confirmed that the two Gh diastereomers can also isomerize to a pair of constitutional isomers, (R,S)-iminoallantoin (Ia), supporting an earlier proposal regarding this reaction.11,12 In the nucleoside context, the transition pH (pHT) is 10.1, favoring Ia at high pH, while in the single-stranded DNA context the pHT is 8.2 (Scheme 1A).11 Monitoring the isomerization in the nucleoside context was achieved by HPLC; in contrast, in the DNA context, the reaction was indirectly monitored by a DNA polymerase insertion assay, working on the principal that Gh forms complementary hydrogen bonds with adenine (A) favoring dATP insertion opposite the modified site at pH < 8.2, while Ia makes complementary hydrogen bonds with G that favors dGTP insertion opposite the site at pH > 8.2 (Scheme 1B).
This approach monitored the equilibrium in single-strand DNA by polymerase insertion and did not directly report on the isomerization reaction in the context of duplex DNA; further, the impact of base pairing on the isomerization equilibrium could not be evaluated. Also, the diastereomers of Ia can interconvert via an enolization reaction.11 The Gh diastereomers are favored at low pH, due to protonation of the guanidine group, and the Ia diastereomers are favored at high pH. In light of the fact that the pHT between Gh and Ia appears to be context dependent, we sought to examine the pH dependency in the isomerization between the four Gh/Ia isomers in the context of duplex DNA, as well as how the base opposite the Gh/Ia site influenced the equilibrium.
RESULTS AND DISCUSSION
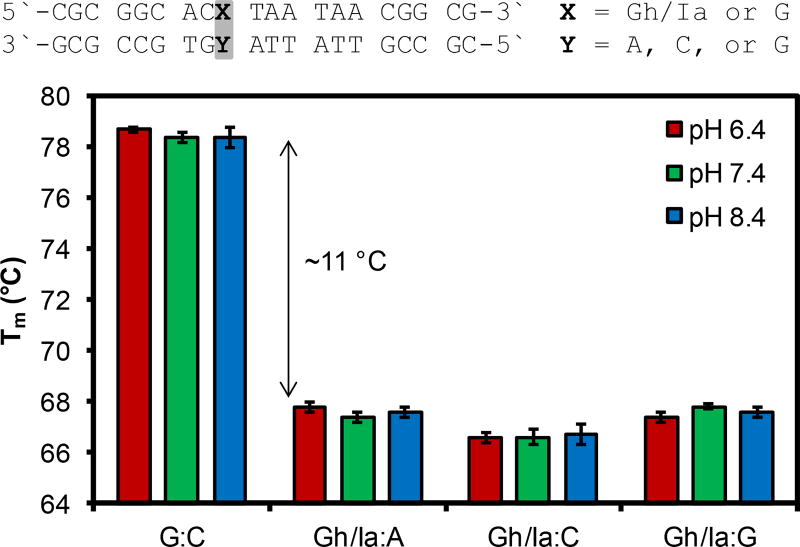
Initially, thermal melting values (Tm) for a 20-mer duplex with a centrally located Gh/Ia site opposite either A, C, or G were monitored as a function of pH and compared to a control duplex with a G:C base pair at the same location. We did not study duplexes containing T opposite the hydantoins because this nucleotide is not inserted by polymerases opposite Gh/Ia.11 The Tm values for the control duplex determined at pH 6.4, 7.4, and 8.4 produced values near 78 °C (Figure 1). These values indicated that the duplex thermal stability was not pH dependent in the range studied. Next, Tm values for duplexes of the same sequence but possessing a centrally located Gh/Ia site with either A, C, or G in the complementary strand were determined at pH 6.4, 7.4, and 8.4. Introduction of the Gh/Ia modification dropped the Tm values by ~11 °C in all cases (Figure 1), a similar change as was previously reported;13,14 however, pH dependency in the Tm values was not observed in the range studied. Thus, Tm analysis is not sensitive enough to monitor a difference between a duplex with a centrally located Gh vs. Ia base pair under the conditions studied.
Figure 1.
Thermal melting values for duplex DNA with a Gh/Ia modification with respect to pH and base pairing partner.
A more sensitive technique for monitoring analyte stereochemistry and the progression of a reaction is the α-hemolysin (α-HL; Figure 2A) protein nanopore that profiles individual molecules.15–18 The α-HL protein nanopore has been harnessed as a nanoreactor to follow the reaction evolution for the click reaction,19 1,4-Michael additions,20 and an enzyme-catalyzed reaction on DNA.15,21 The sensitivity of the α-HL to monitor molecular structure allowed determination of epimers of carbohydrates,22 amino acid enantiomers,23 isomers of heteropolytungstates,24 and diastereomers in duplex DNA.25 Additionally, the latch zone of the α-HL protein was identified by the Burrows/White laboratories to possess high sensitivity for monitoring base pairs in duplex DNA.15,16,26 We chose to apply the sensitivity of the α-HL nanopore technique to analyze the isomerization between the four Gh and Ia diastereomers.
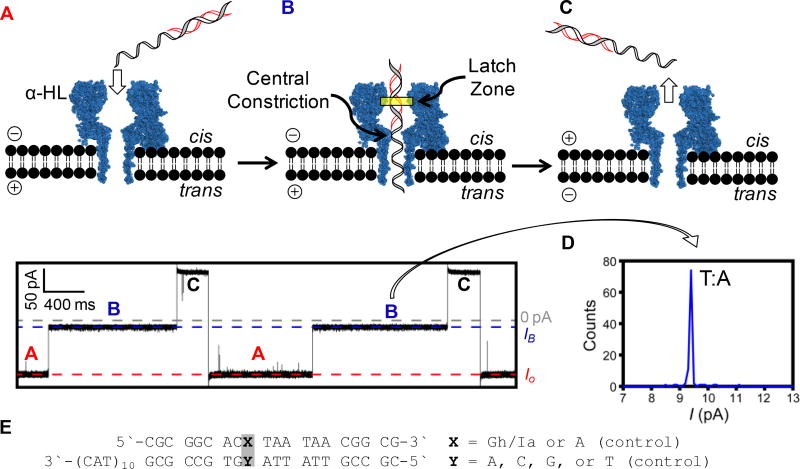
Figure 2.
Analysis of a DNA base pair utilizing the α-HL protein nanopore. (A) Electrophoretic capture of duplex DNA, (B) base pair interrogation, (C) ejection of the duplex DNA from the channel after current reversal, (D) histogram of current values for the control T:A duplex, and (E) sequences studied. The data were collected in 1.00 M KCl solution with 10 mM KPi buffer at pH 6.4, 7.4, or 8.4 at 22 ± 1 °C at 100 mV (trans vs. cis).
Nanopore analysis is initiated by embedding an α-HL protein into a lipid bilayer followed by applying an electric potential to the ion channel to establish an open channel current (Io, Figure 2A). When duplex DNA with a single-stranded tail is present, it is electrophoretically captured and trapped in the channel because duplex DNA cannot pass the central constriction of the protein while the single-stranded tail can easily thread through (Figure 2B). The diameter at the latch zone of the nanopore is only slightly larger than B-form DNA,27,28 and therefore, when duplex DNA is captured in the protein pore, a restriction to the current flow (IB) occurs (Figure 2B). The IB value is impacted by the base pair at the latch zone (Figure 2B),15,16 and alterations in IB and the noise of the current recorded report on the base pair type, dynamics, and stereochemistry.15,16,25,29 After analyzing a single duplex for 1 s, the voltage polarity is switched to eject the duplex from the protein channel and then reversed again to capture another duplex (Figure 2C); this cycle is repeated hundreds of times to obtain a population of IB values that are plotted as a histogram (Figure 2D). The number of peaks observed in a histogram in a population analysis and the average IB values report on the number of different DNA base pair structures at the latch zone of α-HL and can be used to identify the heterocycles in the base pair when the results are compared to known standards.
In the nanopore studies, the same duplex as studied by Tm analysis was used, except that the complementary strand to Gh/Ia had a 30-mer single-stranded tail comprised of the sequence 5`-(TAC)10−3` appended to the 3` end (Figure 2E). The single-stranded overhang guided the entry of the duplex into α-HL to ensure that the Gh/Ia base pair was positioned correctly in the latch zone on the basis of previous studies (Figure 2B).15 First, a control duplex possessing a T:A base pair at the Gh/Ia site was studied to establish the pH dependency in the current of a normal DNA duplex when trapped by α-HL (Figures S1–S4). This analysis found the blocking current of the duplex and associated noise of the signal to remain constant at pH 6.4, 7.4, and 8.4; however, the Io was slightly altered as the pH changed, consistent with a literature report.30 Because the blocking current of the control duplex was not impacted by the pH, we anticipated this would also apply to the duplex with the Gh/Ia modification in the strand.
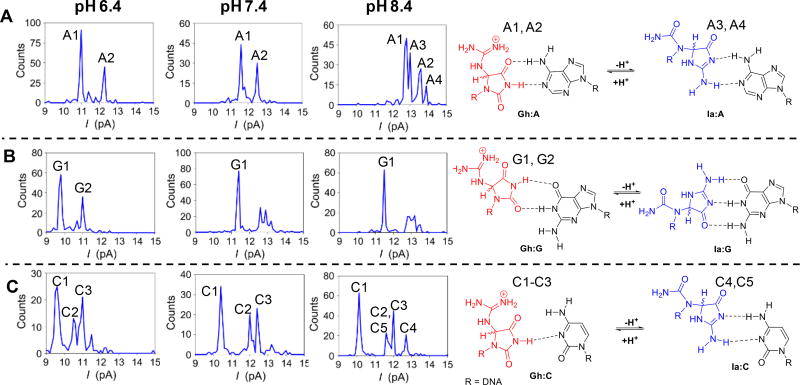
Next, the α-HL nanopore was used to analyze a Gh/Ia opposite A at pH 6.4, 7.4, or 8.4 (Figures 3A and S6–S13). All duplexes were annealed by heating at 90 °C followed by slow cooling to 22 °C where they were left to equilibrate for 24 h prior to nanopore analysis at 22 °C. The data in the text show only the events without an internal standard duplex present for brevity, while the identity of each peak was determined using an internal standard duplex DNA that did not overlap with the Gh or Ia peaks (see Supporting Information). At pH 6.4, a histogram constructed from the population of IB values revealed two baseline-resolved peaks (Figure 3A, peaks A1 and A2). When the pH was increased to 7.4, again a histogram of the population of IB values provided two similar baseline-resolved peaks. The data at pH 6.4 and 7.4 are consistent with only the diastereomers of Gh base paired with A being present in the duplex, as was found in the x-ray crystal structures reported for the Gh:A base pair in this pH range.31,32 When the equilibrium was monitored by a polymerase insertion assay, the results suggested that at pH 7.4, Ia was present at 30%;11 however, the nanopore results with A in the complementary strand find isomerization to Ia does not occur at pH 7.4 because the number of peaks and their ratios did not change between pH 6.4 and 7.4. This observation with the nanopore shows that base pairing between Gh and A influences the equilibrium to favor Gh, in which Gh:A is a more stable base pair than Ia:A (Figure 3A). The Gh peaks observed by the nanopore analysis exist in a 60:40 ratio that is consistent with the Gh diastereomer ratio observed in ssDNA by HPLC.11 Previous computational analysis identified the two stereoisomers of Gh base pair to A with similar energies.33 As a result of the relatively fast interconversion between the Gh diastereomers (t1/2 < 12 h), the absolute configurations for the two peaks observed in the nanopore cannot be made by this analytical approach.
Figure 3.
pH- and base pair-dependent analysis of a Gh/Ia site in duplex DNA via the α-HL nanopore. (A) Current histograms for Gh/Ia base pairs with A, (B) Gh/Ia base pairs with G, and (C) Gh/Ia base pairs with C. In panel C at pH 8.4, the C2 peak has two noise levels supporting two unresolved peaks (C2 and C5). Literature reports suggest the structures for the proposed base pairs.33–35 The data were collected in 1.00 M KCl solution with 10 mM KPi buffer at pH 6.4, 7.4, or 8.4 at 22 ± 1 °C at 100 mV (trans vs. cis).
When the pH was increased to 8.4, four peaks were observed from a histogram of IB values (Figure 3A, peaks A1–A4). The pH 8.4 observation supports the presence of both diastereomers of Gh and Ia at the latch zone in α-HL. The two peaks that align with the Gh diastereomers paired with A have a 60:40 ratio and these two peaks represented 70% of the peak area. The two Ia:A peaks represent 30% of the peak area (error based on triplicate trials was ~10% of the reported values), and the two diastereomer peaks were split in a 70:30 ratio. The nanopore results for the ratio of Gh to Ia opposite A favoring Gh are different than the previous polymerase assay results because at pH 8.4, 60% of the sample was Ia. In contrast, when monitoring the Gh/Ia equilibrium with A opposite, the equilibrium favors Gh even at pH 8.4, suggesting that the base pairing partner influences the equilibrium. Additionally, these results identify the diastereomer ratios of Gh and Ia in the duplex DNA context that is nearly impossible to determine by any other method, illustrating that the latch zone of the α-HL nanopore is more sensitive to stereochemistry than the Tm analysis (Figure 1). Further, the base pair partner aids in establishing where the Gh/Ia equilibrium resides, which was not detectable in the polymerase insertion assay that we previously reported.11
The equilibrium between Gh and Ia opposite G in the duplex was then analyzed by the α-HL nanopore at pH 6.4 (Figures 3B and S14–S21). The histogram produced from the population of IB events revealed two major populations of structures present in a 60:40 ratio (Figure 3B, peaks G1 and G2). The pattern of peaks in the histogram at pH 6.4 with a G opposite the modification site was similar to the pattern observed when A was the base pairing partner. Thus, at pH 6.4 with A or G opposite the modification site, the equilibrium is shifted toward the Gh diastereomers almost exclusively. When the equilibrium between Gh and Ia opposite G was monitored at pH 7.4, the histogram of IB recordings showed one major peak and three closely spaced minor peaks (Figure 3B, peak G1). A similar peak pattern and ratio of peaks was observed for the Gh/Ia modification opposite G at pH 8.4 (Figure 3B, peak G1). These data at pH 7.4 and 8.4 identify the isomerization reaction equilibrates to similar species under these pH conditions. The major peak observed aligns with a single Gh diastereomer (G1), while the other three peaks may reflect one Gh diastereomer and the two Ia diastereomers; however, even with an internal standard duplex present, the identities of the three minor peaks could not be made with confidence (Figure S14–S21). Also, it was surprising that a change in the peak ratios did not occur when the pH was increased to 8.4, in which the Ia:G base pair is expected to dominate. The results with G opposite the Gh/Ia site are not as clear as observed when A was the base pairing partner; although, the results do identify four possible species present under the analysis conditions that is consistent with the presence of the four interconverting Gh and Ia isomers existing at pH 7.4 and 8.4 when G is the base pairing partner.
In the final analysis, the Gh/Ia equilibrium, as a function of pH while base pairing with C, was evaluated using the α-HL protein nanopore (Figures 3C and S22–S31). At pH 6.4, the histogram of IB events found three major populations (Figure 3C, C1, C2, and C3). A consistent observation in the studies reported above and the polymerase report11 found the Gh diastereomers were the only isomers observed at pH < 7; therefore, the peaks likely represent C base pairs with the Gh diastereomers in different orientations. The smaller C heterocycle is anticipated to form poor base pairs with Gh (Figure 3C) resulting in greater flexibility of the base pair and a greater number of possible orientations. When the pH was increased to 7.4, three major peaks were observed in the histogram derived from the IB values (C1, C2, and C3). The similarity of the distribution of peaks in the histograms for analysis at pH 6.4 and 7.4 suggests the isomerization reaction was not impacted by the change in pH. When the pH was increased to pH 8.4, four peaks were observed in the histogram derived from the population of IB events (Figure 3C, C1–C4). The new peak observed at pH 8.4 is consistent with isomerization of Gh to Ia; however, at least two peaks are expected for the two diastereomers. A strong possibility that the two Ia:C isomer base pairs are not resolvable is suggested when looking at the noise of the events for peak C2. The C2 peak had one consistent associated noise level at pH 6.4 and 7.4, while at pH 8.4 two populations of noise (C2 and C5) in the IB were found (Figure S29). This observation suggests more than one species was present in this peak in the histogram. Thus, the data suggest Gh and Ia exist at pH 8.4 when paired opposite C, and the dominant species present are the Gh diastereomers. The results of these studies identify the duplex context in which π stacking with adjacent base pairs and interactions with a base pair partner result in the isomerization equilibrium favoring Gh under the conditions of these nanopore studies. Further, the present data suggest Gh is better accommodated within the duplex context relative to Ia; however, an x-ray crystal structure of Ia within the duplex context will need to be solved in order support this claim.
Oxidation of the G heterocycle in DNA to Gh results in a product with a stereocenter in the base existing as a pair of epimers,7,12 and the new heterocycle can also undergo a pH- dependent isomerization to the Ia constitutional isomers.11 The present studies interrogated the pH-dependent and base pair partner impact on the isomerization between Gh and Ia in duplex DNA. Analysis of the reaction by Tm failed to identify the presence of the isomers and HPLC analysis of duplexes is impractical (Figure 1); therefore, the α-HL protein nanopore was harnessed to monitor the reaction when the base pair was positioned at the latch zone of the protein. The nanopore-based method allowed us to determine that Gh is the major isomer in duplex DNA when base paired with A, G, or C a pH 6.4 and 7.4. The conversion to Ia was observed when the pH was increased to 8.4, but the equilibrium still favored Gh. The present observations extend our understanding of the Gh/Ia isomerization in the context of duplex DNA beyond our previous studies in nucleosides and ssDNA.11 The data suggest that the duplex context with a base in the complementary strand alters the equilibrium to favor Gh even at higher pH. These observations in duplex DNA could not be made using traditional analytical methods such as HPLC, NMR, or MS. The single-molecule profiling nature and high sensitivity to DNA structure that the α-HL protein nanopore affords renders this analytical technique superior for monitoring isomerization reactions within the context of duplex DNA.
EXPERIMENTAL SECTION
Oligodeoxynucleotide (ODN) Preparation
All ODNs were synthesized via solid-phase synthesis following established phosphoramidite chemistry. The ODNs containing OG were prepared utilizing commercially available 8-oxo-7,8-dihydro-2`-deoxyguanosine (OG) phosphoramidite following the manufacturer’s protocol. The ODNs containing Gh/Ia were synthesized by literature protocol.14 Briefly, synthesis of Gh/Ia was achieved by selectively oxidizing OG to Gh. The OG-containing strands (5 nmole, 50 µM) where allowed to react with Na2IrCl6 (30 nmole, 300 µM) in 100 µL of ddH2O for 20 min at ambient temperature. The reaction was quenched by addition of EDTA (300 nmole, 3 mM) after the 20 min reaction. The Gh/Ia product single-stranded DNA was HPLC purified using an analytical anion-exchange column running a flow rate of 1 mL/min with mobile phase compositions comprised of A = 10% CH3CN/90% ddH2O and B = 1.5 M NaOAc, pH 7.0 in 10% CH3CN/90% ddH2O. The elution of the DNA from the column was monitored by following the absorbance at 260 nm. The Gh diastereomer peaks were collected from the HPLC and then dialyzed against ddH2O for 36 h at 4 °C while changing the ddH2O every 12 h for removal of the purification salts. The dialyzed samples were lyophilized to dryness and resuspended in ddH2O. The concentration for the stock solution was determined measuring the UV absorbance at 260 nm and using the primary sequence to determine the extinction coefficient by the nearest neighbor model. The Gh nucleotide was omitted from the sequence for calculation of the extinction coefficient because the base does not absorb at 260 nm.11 The purity of the synthesized Gh-containing single strands of DNA was determined by anion-exchange HPLC (Figure S33).
The DNA duplexes were prepared by annealing the two complementary single strands of DNA at a 100 µM concentration for each strand in nanopore buffer consisting of 1.00 M KCl and 10 mM KPi (pH 6.4, 7.4, or 8.4) at 90 °C for 10 min and then slowly cooling to 22 °C over ~4 h. The samples were left for another 24 h at 22 °C to allow the interconversion between Gh and Ia to reach equilibrium before analysis.
Thermal melting analysis
The melting temperatures (Tm) were measured on a UV-vis spectrophotometer that was coupled to a temperature controller unit. The Tm values were determined by monitoring the temperature induced change in absorbance at 260 nm. The data were analyzed with the manufacture’s software. The Tm analysis was conducted on duplex samples of 0.75 µM by heating them at a rate of 1 °C/min in 1.00 M KCl, 10 mM KPi (pH 6.4, 7.4, or 8.4) buffer. For the Tm studies the long single-stranded tails needed for the nanopore studies was removed from the strands.
Nanopore analysis
The glass nanopore membrane (GNM) system was constructed based on a literature method.36 After a single α-HL channel was formed, duplex-DNA samples with a 30-mer single-stranded tail were added into the chamber to achieve a concentration of 25 µM. The voltage was set at 100 mV (trans vs. cis) to trap the DNA tail first that positions the Gh/Ia site in the duplex at the latch zone of the α-HL protein. Each trapped DNA was held in the α-HL protein nanopore for ~1 s to collect the current signature (IB) for the base pair at the latch zone. A transient reversal of the voltage to −100 mV (trans vs. cis) for ~200 ms was used to eject the duplex and clear the channel for capture of another DNA construct once the voltage was returned to its original settings. In each experiment, over 200 DNA duplexes were analyzed for the data analysis. The current–time (i–t) traces were recorded at a 50-kHz sampling rate and filtered with a 10-kHz low-pass filter. All nanopore experiments were performed in 1.00 M KCl, 10 mM KPi, pH 6.4, 7.4, or 8.4 at 100 mV (trans vs. cis) and 22 ± 1 °C.
A control α-HL nanopore experiment was conducted with a duplex containing a T:A base pair at the latch zone was studied at pH 6.4, 7.4, and 8.4 to determine how the pore and duplex respond to the change in pH. Additionally, the same duplex was utilized as an internal standard in all experiments to support the identification of each peak. These data are provided in the Supporting Information, but were omitted from the figures in the text.
Data analysis
Blockade events with residual currents (IB) associated with tail entry and correct orientation of the base pair of interest at the latch zone of α-HL were determined as previously described.25 Current histograms, curve fitting, and current–noise density plots were constructed as previously described25 and with software provided by Electronic BioSciences (EBS, San Diego, CA), in which the current interval was set to 0.1 pA and the current noise (IRMS) interval 0.01 pA. Power spectral density (PSD) plots were generated using the EBS software.
Supplementary Material
Acknowledgments
Financial support from the National Institutes of Health (R01 GM093099) is acknowledged. Electronic BioSciences (EBS, San Diego, CA) provided the instrument and software used to record the current vs. time traces, and the oligonucleotides were provided by the DNA/Peptide core facility at the University of Utah that is supported in part by a NCI Cancer Center Support Grant (P30 CA042014).
Footnotes
The Supporting Information is available free of charge on the ACS Publications website at DOI:XYZ.
Representative data for each duplex analyzed under the three pH conditions and nucleotide in the complementary strand with the α-HL protein nanopore.
A.M.F. consults for EBS and all other authors declare no competing interests.
References
- 1.Gates K. Chem. Res. Toxicol. 2009;22:1747–1760. doi: 10.1021/tx900242k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cadet J, Wagner JR, Shafirovich V, Geacintov NE. Int. J. Radiat. Biol. 2014;90:423–432. doi: 10.3109/09553002.2013.877176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cadet J, Douki T, Ravanat J-L. Acc. Chem. Res. 2008;41:1075–1083. doi: 10.1021/ar700245e. [DOI] [PubMed] [Google Scholar]
- 4.Gahlon HL, Sturla SJ. Chemistry. 2013;19:11062–11067. doi: 10.1002/chem.201204593. [DOI] [PubMed] [Google Scholar]
- 5.Fleming AM, Burrows CJ. Free Radic. Biol. Med. 2017;107:35–52. doi: 10.1016/j.freeradbiomed.2016.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steenken S, Jovanovic SV. J. Am. Chem. Soc. 1997;119:617–618. [Google Scholar]
- 7.Ye Y, Muller JG, Luo W, Mayne CL, Shallop AJ, Jones RA, Burrows CJ. J. Am. Chem. Soc. 2003;125:13926–13927. doi: 10.1021/ja0378660. [DOI] [PubMed] [Google Scholar]
- 8.Niles JC, Wishnok JS, Tannenbaum SR. Chem. Res. Toxicol. 2004;17:1510–1519. doi: 10.1021/tx0400048. [DOI] [PubMed] [Google Scholar]
- 9.Delaney S, Delaney JC, Essigmann JM. Chem. Res. Toxicol. 2007;20:1718–1729. doi: 10.1021/tx700273u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fleming AM, Muller JG, Dlouhy AC, Burrows CJ. J. Am. Chem. Soc. 2012;134:15091–15102. doi: 10.1021/ja306077b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu J, Fleming AM, Orendt AM, Burrows CJ. J. Org. Chem. 2016;81:351–359. doi: 10.1021/acs.joc.5b02180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo W, Muller JG, Rachlin EM, Burrows CJ. Chem. Res. Toxicol. 2001;14:927–938. doi: 10.1021/tx010072j. [DOI] [PubMed] [Google Scholar]
- 13.Yennie CJ, Delaney S. Chem. Res. Toxicol. 2012;25:1732–1739. doi: 10.1021/tx300190a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kornyushyna O, Berges AM, Muller JG, Burrows CJ. Biochemistry. 2002;41:15304–15314. doi: 10.1021/bi0264925. [DOI] [PubMed] [Google Scholar]
- 15.Jin Q, Fleming AM, Johnson RP, Ding Y, Burrows CJ, White HS. J. Am. Chem. Soc. 2013;135:19347–19353. doi: 10.1021/ja410615d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson RP, Fleming AM, Perera RT, Burrows CJ, White HS. J. Am. Chem. Soc. 2017;139:2750–2756. doi: 10.1021/jacs.6b12284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howorka S, Cheley S, Bayley H. Nat. Biotechnol. 2001;19:636–639. doi: 10.1038/90236. [DOI] [PubMed] [Google Scholar]
- 18.Maglia G, Heron AJ, Stoddart D, Japrung D, Bayley H. Methods in Enzymology. 2010;475:591–623. doi: 10.1016/S0076-6879(10)75022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee J, Bayley H. Proc. Natl. Acad. Sci. U. S. A. 2015;112:13768–13773. doi: 10.1073/pnas.1510565112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu S, Li WW, Rotem D, Mikhailova E, Bayley H. Nat. Chem. 2010;2:921–928. doi: 10.1038/nchem.821. [DOI] [PubMed] [Google Scholar]
- 21.Tan CS, Riedl J, Fleming AM, Burrows CJ, White HS. ACS Nano. 2016;10:11127–11135. doi: 10.1021/acsnano.6b05995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramsay WJ, Bayley H. Angew. Chem. Int. Ed. Engl. 2018 doi: 10.1002/anie.201712740. [DOI] [PubMed] [Google Scholar]
- 23.Boersma AJ, Bayley H. Angew. Chem. Int. Ed. Engl. 2012;51:9606–9609. doi: 10.1002/anie.201205687. [DOI] [PubMed] [Google Scholar]
- 24.Ettedgui J, Kasianowicz JJ, Balijepalli A. J. Am. Chem. Soc. 2016;138:7228–7231. doi: 10.1021/jacs.6b02917. [DOI] [PubMed] [Google Scholar]
- 25.Zeng T, Fleming AM, Ding Y, White HS, Burrows C. J. Biochemistry. 2017;56:1596–1603. doi: 10.1021/acs.biochem.6b01175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ding Y, Fleming AM, White HS, Burrows CJ. ACS Nano. 2015;9:11325–11332. doi: 10.1021/acsnano.5b05055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song L, Hobaugh M, Shustak C, Cheley S, Bayley H, Gouaux J. Science. 1996;274:1859–1866. doi: 10.1126/science.274.5294.1859. [DOI] [PubMed] [Google Scholar]
- 28.Drew HR, Wing RM, Takano T, Broka C, Tanaka S, Itakura K, Dickerson RE. Proc. Natl. Acad. Sci. U.S.A. 1981;78:2179–83. doi: 10.1073/pnas.78.4.2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y, Luan BQ, Yang Z, Zhang X, Ritzo B, Gates K, Gu LQ. Sci. Rep. 2014;4:5883. doi: 10.1038/srep05883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Zoysa RS, Krishantha DM, Zhao Q, Gupta J, Guan X. Electrophoresis. 2011;32:3034–3041. doi: 10.1002/elps.201100216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aller P, Ye Y, Wallace SS, Burrows CJ, Doublie S. Biochemistry. 2010;49:2502–2509. doi: 10.1021/bi902195p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beckman J, Wang M, Blaha G, Wang J, Konigsberg WH. Biochemistry. 49:8554–8563. doi: 10.1021/bi100913v. 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jena NR, Gaur V, Mishra PC. Phys. Chem. Chem. Phys. 2015;17:18111–18120. doi: 10.1039/c5cp02636a. [DOI] [PubMed] [Google Scholar]
- 34.Zhao X, Muller JG, Halasyam M, David SS, Burrows CJ. Biochemistry. 2007;46:3734–3744. doi: 10.1021/bi062214k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jena NR, Bansal M, Mishra PC. Phys. Chem. Chem. Phys. 2016;18:12774–12783. doi: 10.1039/c6cp02212j. [DOI] [PubMed] [Google Scholar]
- 36.Zhang B, Galusha J, Shiozawa P, Wang G, Bergren A, Jones R, White R, Ervin E, Cauley C, White H. Anal Chem. 2007;79:4778–4787. doi: 10.1021/ac070609j. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.