Abstract
Background
Fragile X premutation carriers are at increased risk for fragile X-associated tremor ataxia syndrome (FXTAS), but to date we know little about prediction of onset and rate of progression, and even less about treatment of this neurodegenerative disease. Thus, longitudinal study of carriers, and identification of potential biomarkers and prodromal states, is essential. Here, we present results of baseline assessments from an ongoing longitudinal project.
Methods
The cohort consisted of 73 males, 48 with the fragile X mental retardation 1 (FMR1) premutation (55–200 cytosine-cytosine-guanine, CGG repeats) and 25 well-matched controls (< 40 repeats) between 40 and 75 years. At enrollment, none met criteria for FXTAS or had any clinically-significant tremor or ataxia by blinded neurological examination. The battery consisted of measures of visual memory, spatial working memory, response inhibition, motor speed and control, planning and problem solving, sustained attention, and a standardized movement disorder evaluation.
Results
Contrary to expectations, there were no significant differences between premutation carriers and controls on any measure of executive function. However, premutation carriers had significantly longer manual movement and reaction times than controls, and the significant interaction between CGG repeat and age revealed the slowest movement times among older carriers with higher CGG repeat alleles. A subset of premutation carriers had marginally lower scores on the ataxia evaluation, and they performed no differently from controls on the parkinsonism assessment.
Conclusion
Early-developing cerebellar or fronto-motor tract white matter changes, previously documented in MRI studies, may underlie motor slowing that occurs before clinically observable neurological symptoms.
Keywords: tremor, FMR1 gene, neurodegeneration, ataxia, CANTAB
Introduction
Fragile X-associated tremor/ataxia syndrome (FXTAS) is a late-onset neurodegenerative disorder that affects many carriers of the fragile X premutation. The FXTAS phenotype is characterized by progressive gait ataxia, intention tremor, parkinsonism, dementia, autonomic dysfunction, and peripheral neuropathy.1 FXTAS demonstrates only partial penetrance; although larger studies are needed, one important survey suggested that 47% of males with the fragile X premutation will develop FXTAS by the 8th decade of life.2 Fragile X carriers harbor a trinucleotide expansion of the fragile X mental retardation 1 (FMR1) gene between 55 and 200 CGG repeats. There are two known molecular mechanisms of this disorder: 1) a toxic gain of function of the expanded CGG-repeat FMR1 mRNA, which results in the sequestration of the CGG-binding proteins contributing to inclusion formations in neurons and astrocytes; and (2) CGG repeat-associated non-AUG-initiated (RAN) translation, which generates a peptide toxic to cells.3 Also, recent studies have shed light on additional potential mechanisms of pathogenesis such as the antisense transcript ASFMR1 and mitochondrial dysfunction.4, 5 While the clinical, neuropathological and neuroanatomical features of FXTAS have been described extensively, the risk and protective factors for development of the disease are largely unknown. Currently there are no empirically validated treatments for FXTAS.
In their 2014 review of the cognitive phenotype of premutation carriers, Grigsby and colleagues stated that individuals with FXTAS show cognitive impairments in areas of executive functioning, working memory, and information processing, and Brega and Grigsby6, 7 referred to FXTAS as a “dysexecutive syndrome.” The cognitive phenotype of FXTAS appears to be consistent with fronto-cerebellar dysfunction and disease in a variety of white matter tracts, as variability in cognitive performance has been correlated with diffusion tensor imaging alterations in white matter, which are in turn related to FMR1 measures taken from blood samples in these carriers.1, 8, 9
The neuropsychological/cognitive abnormalities experienced by premutation carriers without FXTAS (or those who have not yet developed neurological symptoms; PFX-), on the other hand, are generally much more mild and usually below clinical significance, often requiring especially sensitive cognitive and brain measurements to detect effects and associations with FMR1 molecular measures.8–19 It is notable that in some studies a subgroup of premutation carriers who do not yet meet diagnostic criteria for FXTAS exhibit subtle weaknesses in executive function and frontal-executive motor control that are very similar to the more pervasive and robust deficits in patients with the fully developed syndrome.1 These observations raise a critical question whether milder EF weaknesses are in fact early markers of later frank neurodegeneration in FXTAS disease progression. An understanding of the key early markers and the actual pattern of emergence of FXTAS symptoms related to neuropsychological, neurological and brain changes should provide a foundation for monitoring during prodromal stages, earlier intervention and treatment, and later tracking of progression or stabilization.
The use of longitudinal studies is likely to be essential for evaluating whether such deficits are early signs of FXTAS and for understanding their progression over time. For example, longitudinal studies of preclinical Alzheimer’s disease (AD) have utilized the Cambridge Neuropsychological Test Automated Battery (CANTAB) as a neuropsychological marker, detecting dysfunction characteristic of probable AD diagnosis. In participants with ‘questionable dementia’ (QD) the baseline performance on CANTAB Paired Associates Learning (PAL) correlated with global cognitive decline over 8 months, and PAL scores allowed detection of a sub-group of QD participants who performed at the same range as diagnosed AD participants.20 Thirty-two months after the first assessment, 11 out of 43 QD participants were ‘converters’ who met criteria for probable AD. Performance on the PAL combined with the Graded Naming Test created a model that predicted diagnosis of probable AD with 100% accuracy for their sample of 40 participants.21 The predictive validity of the CANTAB in longitudinal studies of preclinical Alzheimer’s suggests that this battery may be a promising instrument for identifying at-risk carriers and monitoring early neuropsychological signs of pre-FXTAS progression.
Here, we present results from a baseline (time 1) assessment in the first longitudinal neuropsychological and neurological study of PFX- men and controls. We hypothesized that the PFX- group would demonstrate age-related deficits compared to matched controls on tasks involving visuo-spatial working memory, hippocampal-mediated memory recall, inhibition, and problem-solving linked to executive function. As FXTAS is predominantly a movement disorder, we also assessed various aspects of manual motor control and speed, and ataxia. We expected CGG repeat- and FMR1 mRNA-dependent effects on performance, such that individuals with both higher CGG repeats and older age would be most affected.
Method
Participants
The sample consisted of 77 males, 52 with the FMR1 premutation (55–200 FMR1 CGG repeats) and 25 healthy controls (< 40 CGG repeats) between the ages of 40 and 75 years. After enrollment and neurological exam review of all participants, 4 carriers were found to meet criteria for FXTAS and were excluded from analyses. Premutation and control groups did not differ significantly on age, IQ, education level, ethnicity/race, marital status or income (Table 1). The data reported here are from the first time point in a longitudinal project examining brain, neuropsychological and genetic markers of neurodegeneration in FMR1 premutation male carriers. The project protocol was approved by the Institutional Review Board at UC Davis and all participants provided signed consent. Participants in the premutation group were recruited from over 1200 extended pedigrees of probands with fragile X-associated disorders seen for research or clinical care, from flyers posted through the National Fragile X Foundation contact list, and from referrals by other clinical researchers focused on fragile X-associated disorders in the U.S. and Canada. None were ascertained based on clinical information. Participants in the control group were recruited from the local community of Sacramento, California, primarily through community and University-based flyers and from announcements at a variety of local clubs and organizations.
Table 1.
Participant demographics and molecular measures by group.
| Control (N=25) | Premutation (N=48) | Independent Samples t-Test Significance | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| Mean | SD | Mean | SD | p | ||
| Age | 55.99 | 9.07 | 59.61 | 8.45 | .09 | |
| FSIQ | 127.12 | 13.86 | 127.00 | 13.26 | .97 | |
| FMR1 mRNA | 0.52 | 0.12 | 0.85 | 0.36 | <.0001 | |
| FMR1 CGG repeats | 29.80 | 4.18 | 84.98 | 21.28 | <.0001 | |
|
|
||||||
| Within Group Percentages | Chi-Square Asymptotic Significance (2-sided) | |||||
|
|
||||||
| Marital Status | Married | 76.0% | 89.6% | p=.34 | ||
| Divorced | 4.0% | 4.2% | ||||
| Single | 16.0% | 4.2% | ||||
| Not Reported | 4.0% | 2.1% | ||||
| Education Level | High School/GED | 8.0% | 4.3% | p=.16 | ||
| Partial College | 8.0% | 23.4% | ||||
| BA/BS | 44.0% | 23.4% | ||||
| MA/MS/PhD/MD | 40.0% | 48.9% | ||||
| Race | White | 84.0% | 91.7% | p=.52 | ||
| Ethnicity | Hispanic or Latino | 12.0% | 2.1% | p=.18 | ||
| Not Hispanic or Latino | 68.0% | 68.8% | ||||
| Not Reported | 20.0% | 29.2% | ||||
Abbreviations: FSIQ, full-scale IQ.
Exclusion criteria included: acute renal, liver, or cardiac medical conditions; history of significant head trauma; substance abuse or dependence; use of medicine that impacts cerebral blood flow, such as beta blockers (due to effects on functional brain imaging aspects of the larger project); presence of metal implants of any kind which would preclude MRI; and non-English speaking. Finally, potential participants were excluded during screening if they had a history of tremor, ataxia or any other clear movement disorder symptom. (As mentioned previously, 4 carriers “passed” screening but were later found to have emerging or definite FXTAS symptoms and were excluded). This last exclusion was in place to allow the study to focus on the conversion to FXTAS, rather than on participants who already manifest the disorder. Only participants rated at FXTAS Stage 0 (no signs) or 1 (equivocal signs) based on the blinded neurological exam were allowed into the study and analyses.
Materials and Procedure
The Cambridge Neuropsychological Test Automated Battery (CANTAB; Cambridge Cognition, Cambridge, UK) is a touchscreen computer-administered battery providing a highly standardized and well-validated set of cognitive tests with excellent test-retest reliability. A forced randomization table was used to counterbalance the order of CANTAB test administrations across participants.
Memory
CANTAB Paired Associates Learning (PAL)
This task assesses hippocampal-mediated visual memory recall and is sensitive to changes in medial temporal lobe functioning.22 This measure was found to be the best for predicting ‘questionable dementia’ participants who converted to probable Alzheimer’s disease in a longitudinal study of neuropsychological markers in preclinical Alzheimer’s disease.21
CANTAB Spatial Working Memory (SWM)
In this task several boxes appear distributed around the screen and one of them contains a hidden token within. Participants must search through the boxes until the token is found. An error is made if the participant revisits a box where he found a token previously, or if he revisits an empty box that he already clicked on earlier in the same search. This test is sensitive to prefrontal and executive dysfunction.23, 24 Between errors – the number of times the participant revisits a box where he found a token previously – was the chosen dependent variable.
Response Inhibition
CANTAB Stop Signal Task (SST)
SST is a measure of response inhibition. Arrows appear on the screen and the participant must tap the correct button corresponding to the direction the arrow is pointing, left or right. If the auditory beep signal is heard the participant should withhold his response and refrain from button pressing. The task uses a staircase design for the stop signal delay, allowing the task to adapt to the performance of the participant and narrow in on the 50% success rate for inhibition. Mean stop signal reaction time during the last half of testing was chosen as the dependent variable.
Motor Speed and Control
CANTAB Reaction Time (RTI)
This task contains two variations, simple reaction time (SRT) and 5-choice reaction time (CRT). Both versions of the task involve cognitive constructs of vigilance, inhibitory control, and manual visual-motor speed. SRT measures the participant’s ability to quickly release his first finger from its resting position and accurately touch a bright circle stimulus as soon as it appears in a single, predictable location. Using the same resting position, CRT measures response to a stimulus that appears unpredictably in any one of five locations. We chose to examine CRT based on results of a previous study involving patients with Parkinson disease; while patients with parkinsonism were slower to initiate and carry out responses than control participants on both SRT and CRT, the difference was greater for CRT.25 Furthermore, the CRT taps visuospatial attention, and in previous studies we have found both impairments in reaction time in numerical visuospatial tasks26 and reduced right temporal-parietal junction activation associated with temporal processing in premutation carriers.27
Purdue Pegboard Test (Lafayette Instrument, Lafayette, IN)
This classic timed test measures gross movements of hands, fingers and arms, and fingertip dexterity, involving rapid placement of metal pegs into a series of holes. For this study, participants completed one trial each for the left hand, right hand, and both hands together.
Behavioral Dyscontrol Scale-2 (BDS-2).28
The BDS-2 is a 9-item, 19-point scale adapted from the work of A.R. Luria. It is a valid and reliable measure of the capacity for behavioral self-regulation involving intentional control of motor behavior. In addition, it has been documented to be sensitive to involvement in premutation carriers in prior studies. 1, 15
Planning and Problem Solving
CANTAB One Touch Stockings (OTS)
This subtest excercises the participant’s abilities of planning and problem solving, cognitive constructs related to executive function and mediated by prefrontal cortex activity.29 First the participant must move “billiard balls” in a lower picture to match the target pattern in an upper display. Balls toward the bottom of the stocking cannot be moved until the one above them is relocated. In the testing portion, participants are asked to work out mentally the number of moves required to solve the problem in their head and select a number accordingly. The dependent variable examined in this task was number of problems solved on first choice.
Sustained Visual Attention
CANTAB Rapid Visual Processing (RVP)
This task requires sustained attention, serves as a measure of general performance, and is sensitive to dysfunction in the parietal and frontal lobes of the brain. In RVP participants must attempt to detect three different target sequences of digits. The display shows a central box where digits (2–9) appear one at a time and change rapidly, at the rate of 100 digits per minute. When the participant recognizes a target sequence, he presses a button at the bottom of the screen. We selected A’ signal detection as the dependent variable. RVP A′ has been shown to be sensitive to both neurological damage (e.g., in Alzheimer’s disease), and pharmacological manipulation, such as by the cholinergic agonist, nicotine.22
Motor Examination
The Movement Disorder Society-Unified Parkinson Disease Rating Scale (MDS-UPDRS) is a validated widely used instrument that covers the relevant domains of Parkinson’s disease and is used to follow its longitudinal course. The bradykinesia subscale of the MDS-UPDRS motor examination also was examined and consisted of the following five items: finger taps (left and right), hand movement (left and right), rapid alternate movements of hands (right and left), leg agility (right and left), and body bradykinesia and hypokinesia.
General Intelligence
The Wechsler Adult Intelligence Scale, Third Edition (WAIS-III33) was used to measure overall cognitive ability.
Molecular Measures
Genomic DNA was isolated from 3ml of peripheral blood leukocytes using standard method (Qiagen, Valencia, CA). CGG sizing was determined using a combination of PCR and Southern Blot as previously described.34, 35 Total RNA was isolated from 3ml of whole blood, collected either in Tempus or PaXgene tubes, according to the manufacturer’s instructions (Applied Biosystems, Foster City, CA and Qiagen, Valencia, CA, respectively). Quantifications of FMR1 mRNA expression levels were performed using a 7900 Sequence detector (Applied Biosystems, Foster City, CA) using specific primers and probes as previously reported.36
Statistical Analyses
In the first set of analyses, we carried out statistical comparisons for each dependent variable of interest to quantify differences between PFX- and controls (Table 2). The second set of analyses examined the association between the outcomes and age, FMR1 CGG repeat size and FMR1 mRNA level. For measures with adequate range and distribution (all but UPDRS and ICARS) linear and quadratic regression models were used that included age, repeat size/mRNA, and the interaction between age and repeat size/mRNA as predictors. In addition, we examined whether use of psychotropic medication influenced the results of the analyses. Of the 48 participants in the premutation group, 15 (31.2%) reported taking psychotropic medication, as did 3 out of the 25 control participants (12.0%). Table 3 presents the parameter estimates and model information from the final model for each variable.
Table 2.
Descriptive statistics of the dependent measures and group differences.
| FMR1 Premutation | Controls | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Mean (SD) | Mean (SD) | df | t/Z | Effect Size | p | |
| Memory | ||||||
| PAL total errors | 24.52 (23.75) | 18.84 (17.32) | 71 | −1.06 | .25 | .295 |
| SWM between errors | 44.81 (23.35) | 36.12 (19.56) | 71 | −1.59 | .38 | .116 |
| Response Inhibition | ||||||
| SST reaction time | 225.8 (56.44) | 216.4 (57.29) | 71 | −0.68 | .16 | .501 |
| Motor Control and Speed | ||||||
| CRT movement time | 270.9 (83.43) | 229.1 (43.95) | 71 | −2.34 | .56 | .022 |
| CRT reaction time | 335.5 (42.59) | 306.1 (32.32) | 71 | −3.02 | .72 | .003 |
| Purdue Pegboard (R+L+Both) | 35.77 (4.90) | 39.60 (5.11) | 70 | 3.12 | .77 | .003 |
| BDS-2 | 19.60 (2.52) | 19.84 (2.14) | 71 | 0.40 | .10 | .691 |
| Planning and Problem Solving | ||||||
| OTS problems solved | 10.21 (2.32) | 10.68 (1.97) | 71 | 0.86 | .20 | .390 |
| Sustained Visual Attention | ||||||
| RVP A′ signal detection | .926 (0.05) | .941 (0.04) | 71 | 1.20 | .28 | .235 |
| Neurological Examination | ||||||
| UPDRS | 0.69 (0.95) | 0.56 (0.87) | 71 | 0.56 | .07 | .558 |
| ICARS | 3.38 (1.69) | 2.46 (1.06) | 69 | 2.31 | .27 | .021 |
Abbreviations: PAL, Paired Associates Learning; SWM, Spatial Working Memory; SST, Stop Signal Task; CRT, Choice Reaction Time; OTS, One-Touch Stockings; RVP, Rapid Visual Processing; BDS-2, Behavioral Dyscontrol Scale, Second Edition; UPDRS, Unified Parkinson’s Disease Rating Scale; ICARS, International Cooperative Ataxia Rating Scale.
Table 3.
Parameter estimates from regression analyses examining relation between age/CGG repeat length and dependent variables.
| Estimate (SE) | t-value | p | R2 | |
|---|---|---|---|---|
| Memory | ||||
| PAL total errors | .064 | |||
| CGG | .716 (.334) | 2.14 | .035 | |
| CGG2 | −.005 (.002) | −2.18 | .032 | |
| SWM between errors | ||||
| age | .645 (.291) | 2.22 | .029 | .085 |
| CGG | .100 (.081) | 1.24 | .221 | |
| Response Inhibition | ||||
| SST reaction time | .030 | |||
| age | .798 (.758) | 1.05 | .296 | |
| CGG | .217 (.211) | 1.03 | .307 | |
| Motor Control and Speed | ||||
| CRT movement time | .225 | |||
| age | −1.417 (2.226) | −0.64 | .527 | |
| CGG | −3.248 (1.819) | −1.79 | .079 | |
| age*CGG | 0.069 (.032) | 2.12 | .038 | |
| CRT reaction time | .130 | |||
| age | .668 (.528) | 1.27 | .210 | |
| CGG | .435 (.147) | 2.96 | .004 | |
| BDS-2 | .109 | |||
| age | -.089 (.030) | −2.91 | .005 | |
| CGG | -.003 (.008) | −0.34 | .733 | |
| Purdue Pegboard | .309 | |||
| age | -.221 (.060) | −3.70 | .001 | |
| CGG | -.068 (.016) | −4.12 | .001 | |
| Planning and Problem Solving | ||||
| OTS problems solved | .069 | |||
| age | -.066 (.029) | −2.30 | .025 | |
| Sustained Visual Attention | ||||
| RVP A′ signal detection | .116 | |||
| age | -.001 (.001) | −2.18 | .033 | |
| Medication | -.025 (.015) | −1.97 | .053 | |
Abbreviations: PAL, Paired Associates Learning; SWM, Spatial Working Memory; SST, Stop Signal Task; CRT, Choice Reaction Time; OTS, One-Touch Stockings; RVP, Rapid Visual Processing
Results
Visual memory
In the Paired Associates Learning (PAL) test, the analyses for Total Errors (Adjusted) indicated that observed differences in task performance between PFX- and controls were not statistically significant. The best model from regression analyses included both linear and quadratic terms of CGG repeat size. Parameter estimates were statistically significant for both terms, indicating a nonlinear relation across the CGG repeat span in the form of an increase in the total errors for those with mid-length expansions, followed by a decrease.
Spatial working memory
Similarly, analyses for between errors made during SWM yielded a nonsignificant comparison between groups. The best model was one that included linear components of age and CGG. This model indicated that the errors increased linearly with age but were not associated with CGG.
Response inhibition
No significant differences were found between groups for the SST reaction time measure. Neither linear nor quadratic regression analyses indicated a relation to age or CGG repeat size.
Planning and problem solving
Analyses regarding problems solved on first choice within the OTS test showed no significant differences between PFX- and controls. Score on this measure was linearly and negatively related to age. Neither a higher-order age function nor CGG were statistically associated with performance on the test.
Sustained visual attention
The groups did not significantly differ on performance of A’ signal detection within the RVP test. A similar pattern to OTS was found for the RVP A’ signal detection measure, namely this variable was linearly and negatively related to age.
Motor control and speed
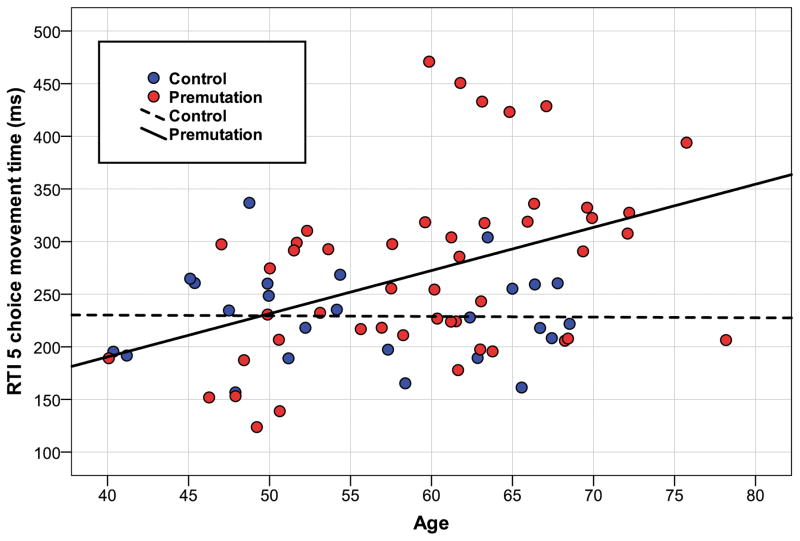
For the motor tests, analyses yielded significant differences for both the CANTAB reaction time and movement time measures, and for the Purdue Pegboard. Between-group comparisons indicated that PFX- were statistically slower in manual reaction time and movement time, and they placed fewer Purdue pegs than controls. No significant group differences were found for the BDS-2. The best model for RTI movement time was one that included linear terms for age and CGG repeats, as well as their interaction, which indicated that increased movement time was observed primarily in older carriers with larger CGG repeat size (Figure 1). Unlike the previously discussed variables, the total explained variance by this model was moderate (R2 = .23). For RTI reaction time, a similar model with linear trends for age and CGG was also best. Likewise on Purdue Pegboard there were linear effects of both age and CGG. However, no significant interaction of CGG and age was observed on these variables.
Figure 1. Manual movement time and age.
The association between age and CANTAB 5-choice manual movement time in fragile X premutation carriers asymptomatic for FXTAS and healthy controls. This test involves resting the dominant hand forefinger on a rectangle at the bottom of a tablet screen until one of 5 locations is illuminated, at which time the participant touches the target as quickly as possible. Linear regression modeling showed a significant interaction between FMR1 CGG length and age, demonstrating that older carriers with high CGG alleles had the slowest movement times.
Neurological examination
Although carriers with FXTAS were excluded from the study, given the results of slowed manual reaction and movement times, we were interested to examine whether any participants had even subtle or equivocal tremor or bradykinesia by neurological exam that might explain the findings. Indeed, none of the carriers or controls had any signs of dominant or non-dominant hand tremor according to the UPDRS37 or the finger-to-nose test of the ICARS32, and there was no significant group difference on the UPDRS bradykinesia subscale (p=.34). Group comparisons revealed skewed data with few subjects in either group with elevated scores, necessitating non-parametric (Mann-Whitney) tests for the UPDRS and ICARS. Results showed no differences on the UPDRS, and a statistically significant, but modest elevation on the ICARS for carriers compared to controls (see Table 2).
FMR1 mRNA (in place of CGG repeat length in the above regression models) had no significant associations with the dependent variables of interest, either alone or in interaction with age, except for with Purdue Pegboard (higher mRNA associated with worse performance; t=−2.42, p = .018).
Discussion
Fragile X premutation carriers are confronted by many health risks as they age, and often express concern and questions about whether they will develop FXTAS, how soon, and how quickly it will progress. Although limited evidence suggests that higher CGG repeat size is associated with an earlier age of FXTAS onset and age of death,38 little is known about risk and protective factors, and there is no consensus on early clinical or biological markers for onset or progression. These markers may turn out to be key brain imaging signs, neurological or neuropsychological changes, specific molecular markers, or a combination of the above. What is clear is that longitudinal studies are essential to identify such markers and their relative prognostic value. Here, we presented baseline neuropsychological and neurological data from a well-characterized cohort of premutation carriers at risk for FXTAS using a widely-validated computer-based test battery.
Based on prior published studies highlighting executive function in carriers with and without FXTAS, we hypothesized significant weaknesses in these areas, and predicted that they would be associated with older age and higher CGG repeat length. These hypotheses were not confirmed. In fact, carriers were no worse than controls in several executive function domains including response inhibition, sustained visual attention, frontal-mediated problem solving, and visual spatial memory. Also the lack of differences on the BDS-2, involving executive control of movement was surprising, given prior literature and the instrument’s clear sensitivity to FXTAS EF deficits. One possibility is that deficits in these domains may occur at a later stage or only in older carriers, as they approach typical age of FXTAS onset. However, in this cohort, a lack of interaction of CGG size and age for these metrics does not support this interpretation. The lack of prominent effects on the key EF domains in our cohort does not necessarily exclude the possibility that deficits in these areas may coincide with, or even pre-date FXTAS onset. The inconsistency across published studies regarding EF deficits in PFX- appears related to cohort differences and/or subtle differences in the tests used to tap these domains. Subtle weaknesses in hippocampus-mediated visual memory do appear to be present in carriers with mid-length CGG alleles, a finding that may be consistent with alterations in hippocampus, and co-activation of the hippocampus and frontal regions in memory recall and encoding tasks.9, 39 To clarify the prodrome, we and others will need to examine the trajectories of cognitive and motor functions over time in carriers as they transition into and fully manifest the disorder.
The most robust finding of the present study was the slowing of manual motor reaction and movement times while reaching for a target and while performing a manual dexterity task in carriers relative to controls that were correlated with both higher CGG repeat length and older age. This slowing could not be explained by any observable tremor or bradykinesia by blinded neurological exam by an experienced movement disorder specialist. One interpretation of this finding is that early-developing cerebellar or fronto-cerebellar tract changes in carriers 8, 40 underlie the motor slowing seen in the RTI tasks. These changes could be occurring before the appearance of intention tremor in high risk individuals. This interpretation is consistent with several studies of upper limb movement in cerebellar ataxia neurodegenerative diseases. For example, an early comparative study of motor reaction time in Parkinson’s, Huntington’s, and cerebellar diseases showed that patients with cerebellar disease had slower movement times compared to the patients with Parkinson’s disease and Huntington’s disease, whose times did not differ.41 Day and colleagues42 examined spatial and temporal characteristics of free reaching movements of the arm in 17 patients with ataxic syndromes due to disease of the cerebellum. Participants were required to reach out and touch a visually presented target (similar to the CRT test in our study) either in the dark or with the target and their finger visible. Overall, patients had prolonged reaction and reaching movement times, and the spatial paths described by their fingertips were more circuitous. The authors suggested that these spatial errors and delays arise because the cerebellum normally contributes either directly or indirectly to preparatory motor processes which compute the pattern of muscle activity required to launch the limb accurately towards a target. This abnormality would have implications for both upper limb movement, but also leg movements that would impact gait and balance that is also seriously impacted in FXTAS. Given that movement problems also typically precede cognitive decline in Parkinson’s disease, it would be reasonable to explore the potential impact of basal ganglia changes in the pathogenesis of FXTAS, as it is known that the characteristic inclusion formations do occur in this region43. The forthcoming longitudinal data from our project, including MRI delineating brain changes will determine the specificity and neural basis of motor slowing in prodromal FXTAS, in particular the possibility that subtle reaching movement abnormalities precede more obvious and clinically significant neurological problems.
This study was limited by a smaller control group; it was necessary to devote limited resources to the recruitment, travel, and imaging of carriers. Also, the molecular markers were taken from blood samples, which provide an indirect estimate of these markers in brain. It also would have also been advantageous to study associations between measures of mitochondrial function and RAN translation, as these have been implicated in the FXTAS pathogenesis. In addition, individuals participating in the study were very high functioning, primarily Caucasian, and well educated. Thus the results may not generalize to the full population of premutation carriers. We focused on males due to the much higher risk of FXTAS in males in this X-linked condition, and as such, these results may not generalize to women.
The results of the longitudinal project may provide information about the early markers of neurodegeneration that will aid in identifying carriers most in need of preventive care and treatment as these interventions become available. This research also may identify important measures for tracking response to interventions in the future. The analysis of combined molecular, brain, neurological and neuropsychological data, as well as a variety of indicators of risk and resilience across mid to late adulthood is essential for understanding the pathogenesis and treatment of FXTAS.
Acknowledgments
Funding Sources: This work was supported by National Institute of Health Grants MH078041 to Drs. Hessl and Rivera; HD036071 and AG032115 to Dr. Randi Hagerman; HD02274 to Dr. Tassone; 90DD0596 from Health and Human Services Administration of Developmental Disabilities; and by the MIND Institute Intellectual and Developmental Disabilities Research Center (U54 HD079125).
We thank the participants and their families for their effort and dedication to this research. We also thank Dr. Randi Hagerman for her clinical examinations and guidance, and Drs. Andrea Schneider, Corrisa Jacomini and Sundas Pasha for neuropsychological and psychiatric examinations of participants.
Footnotes
Authors’ Roles
1. Research Project: A. Conception, B. Organization, C. Execution; 2. Statistical Analysis: A. Design, B. Execution, C. Review and Critique; 3. Manuscript Preparation: A. Writing the First Draft, B. Review and Critique.
Ryan Shickman: 2B, 2C, 3A;
Jessica Famula: 1B, 1C, 3B
Flora Tassone: 1A, 1C, 3B
Maureen Leehey: 1A, 1C, 3B
Emilio Ferrer: 1A, 2A, 2B, 2C, 3B
Susan Rivera: 1A, 1B, 2C, 3B
David Hessl: 1A, 1B, 1C, 2B, 2C, 3A, 3B
Financial Disclosures
The authors have no financial disclosures to report for the past 12 months.
Financial Disclosure/Conflict: Dr. Hessl has received support and consulting fees from Novartis, Roche, and Seaside Therapeutics for fragile X syndrome clinical trials.
References
- 1.Grigsby J, Cornish K, Hocking D, et al. The cognitive neuropsychological phenotype of carriers of the FMR1 premutation. J Neurodev Disord. 2014;6(1):28. doi: 10.1186/1866-1955-6-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacquemont S, Farzin F, Hall D, et al. Aging in individuals with the FMR1 mutation. Am J Ment Retard. 2004;109(2):154–164. doi: 10.1352/0895-8017(2004)109<154:AIIWTF>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kong HE, Zhao J, Xu S, Jin P, Jin Y. Fragile X-Associated Tremor/Ataxia Syndrome: From Molecular Pathogenesis to Development of Therapeutics. Front Cell Neurosci. 2017;11:128. doi: 10.3389/fncel.2017.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ross-Inta C, Omanska-Klusek A, Wong S, et al. Evidence of mitochondrial dysfunction in fragile X-associated tremor/ataxia syndrome. Biochem J. 2010;429(3):545–552. doi: 10.1042/BJ20091960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song G, Napoli E, Wong S, et al. Altered redox mitochondrial biology in the neurodegenerative disorder fragile X-tremor/ataxia syndrome: use of antioxidants in precision medicine. Mol Med. 2016:22. doi: 10.2119/molmed.2016.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brega AG, Goodrich G, Bennett RE, et al. The primary cognitive deficit among males with fragile X-associated tremor/ataxia syndrome (FXTAS) is a dysexecutive syndrome. J Clin Exp Neuropsychol. 2008;30(8):853–869. doi: 10.1080/13803390701819044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grigsby J, Brega AG, Engle K, et al. Cognitive profile of fragile X premutation carriers with and without fragile X-associated tremor/ataxia syndrome. Neuropsychology. 2008;22(1):48–60. doi: 10.1037/0894-4105.22.1.48. [DOI] [PubMed] [Google Scholar]
- 8.Wang JY, Hessl D, Schneider A, Tassone F, Hagerman RJ, Rivera SM. Fragile X-associated tremor/ataxia syndrome: influence of the FMR1 gene on motor fiber tracts in males with normal and premutation alleles. JAMA Neurol. 2013;70(8):1022–1029. doi: 10.1001/jamaneurol.2013.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang JM, Koldewyn K, Hashimoto R, et al. Male carriers of the FMR1 premutation show altered hippocampal-prefrontal function during memory encoding. Front Hum Neurosci. 2012;6:297. doi: 10.3389/fnhum.2012.00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cornish KM, Li L, Kogan CS, et al. Age-dependent cognitive changes in carriers of the fragile X syndrome. Cortex. 2008;44(6):628–636. doi: 10.1016/j.cortex.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodrich-Hunsaker NJ, Wong LM, McLennan Y, et al. Adult Female Fragile X Premutation Carriers Exhibit Age- and CGG Repeat Length-Related Impairments on an Attentionally Based Enumeration Task. Front Hum Neurosci. 2011;5:63. doi: 10.3389/fnhum.2011.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodrich-Hunsaker NJ, Wong LM, McLennan Y, et al. Young adult female fragile X premutation carriers show age- and genetically-modulated cognitive impairments. Brain Cogn. 2011;75(3):255–260. doi: 10.1016/j.bandc.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gossett A, Sansone S, Schneider A, et al. Psychiatric disorders among women with the fragile X premutation without children affected by fragile X syndrome. Am J Med Genet B Neuropsychiatr Genet. 2016;171(8):1139–1147. doi: 10.1002/ajmg.b.32496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grigsby J, Brega AG, Jacquemont S, et al. Impairment in the cognitive functioning of men with fragile X-associated tremor/ataxia syndrome (FXTAS) J Neurol Sci. 2006;248(1–2):227–233. doi: 10.1016/j.jns.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 15.Grigsby J, Brega AG, Leehey MA, et al. Impairment of executive cognitive functioning in males with fragile X-associated tremor/ataxia syndrome. Mov Disord. 2007;22(5):645–650. doi: 10.1002/mds.21359. [DOI] [PubMed] [Google Scholar]
- 16.Hashimoto R, Backer KC, Tassone F, Hagerman RJ, Rivera SM. An fMRI study of the prefrontal activity during the performance of a working memory task in premutation carriers of the fragile X mental retardation 1 gene with and without fragile X-associated tremor/ataxia syndrome (FXTAS) J Psychiatr Res. 2011;45(1):36–43. doi: 10.1016/j.jpsychires.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hessl D, Rivera S, Koldewyn K, et al. Amygdala dysfunction in men with the fragile X premutation. Brain. 2007;130(Pt 2):404–416. doi: 10.1093/brain/awl338. [DOI] [PubMed] [Google Scholar]
- 18.Hessl D, Wang JM, Schneider A, et al. Decreased fragile X mental retardation protein expression underlies amygdala dysfunction in carriers of the fragile X premutation. Biol Psychiatry. 2011;70(9):859–865. doi: 10.1016/j.biopsych.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schneider A, Johnston C, Tassone F, et al. Broad autism spectrum and obsessive-compulsive symptoms in adults with the fragile X premutation. Clin Neuropsychol. 2016;30(6):929–943. doi: 10.1080/13854046.2016.1189536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swainson R, Hodges JR, Galton CJ, et al. Early detection and differential diagnosis of Alzheimer’s disease and depression with neuropsychological tasks. Dement Geriatr Cogn Disord. 2001;12(4):265–280. doi: 10.1159/000051269. [DOI] [PubMed] [Google Scholar]
- 21.Blackwell AD, Sahakian BJ, Vesey R, Semple JM, Robbins TW, Hodges JR. Detecting dementia: novel neuropsychological markers of preclinical Alzheimer’s disease. Dement Geriatr Cogn Disord. 2004;17(1–2):42–48. doi: 10.1159/000074081. [DOI] [PubMed] [Google Scholar]
- 22.Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry. 2000;57(10):925–935. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- 23.Owen AM, Morris RG, Sahakian BJ, Polkey CE, Robbins TW. Double dissociations of memory and executive functions in working memory tasks following frontal lobe excisions, temporal lobe excisions or amygdalo-hippocampectomy in man. Brain. 1996;119( Pt 5):1597–1615. doi: 10.1093/brain/119.5.1597. [DOI] [PubMed] [Google Scholar]
- 24.Baddeley A. Working memory: looking back and looking forward. Nat Rev Neurosci. 2003;4(10):829–839. doi: 10.1038/nrn1201. [DOI] [PubMed] [Google Scholar]
- 25.Jahanshahi M, Brown RG, Marsden CD. Simple and choice reaction time and the use of advance information for motor preparation in Parkinson’s disease. Brain. 1992;115( Pt 2):539–564. doi: 10.1093/brain/115.2.539. [DOI] [PubMed] [Google Scholar]
- 26.Wong LM, Goodrich-Hunsaker NJ, McLennan Y, et al. Young adult male carriers of the fragile X premutation exhibit genetically modulated impairments in visuospatial tasks controlled for psychomotor speed. J Neurodev Disord. 2012;4(1):26. doi: 10.1186/1866-1955-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim SY, Tassone F, Simon TJ, Rivera SM. Altered neural activity in the ‘when’ pathway during temporal processing in fragile X premutation carriers. Behav Brain Res. 2014;261:240–248. doi: 10.1016/j.bbr.2013.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grigsby J, Kaye K, Robbins LJ. Reliabilities, norms and factor structure of the Behavioral Dyscontrol Scale. Percept Mot Skills. 1992;74(3 Pt 1):883–892. doi: 10.2466/pms.1992.74.3.883. [DOI] [PubMed] [Google Scholar]
- 29.Goel V, Grafman J. Are the frontal lobes implicated in “planning” functions? Interpreting data from the Tower of Hanoi Neuropsychologia. 1995;33(5):623–642. doi: 10.1016/0028-3932(95)90866-p. [DOI] [PubMed] [Google Scholar]
- 30.Movement Disorder Society Task Force on Rating Scales for Parkinson’s D. The Unified Parkinson’s Disease Rating Scale (UPDRS): status and recommendations. Mov Disord. 2003;18(7):738–750. doi: 10.1002/mds.10473. [DOI] [PubMed] [Google Scholar]
- 31.Buck PO, Wilson RE, Seeberger LC, Conner JB, Castelli-Haley J. Examination of the UPDRS bradykinesia subscale: equivalence, reliability and validity. J Parkinsons Dis. 2011;1(3):253–258. doi: 10.3233/JPD-2011-11035. [DOI] [PubMed] [Google Scholar]
- 32.Trouillas P, Takayanagi T, Hallett M, et al. International Cooperative Ataxia Rating Scale for pharmacological assessment of the cerebellar syndrome. The Ataxia Neuropharmacology Committee of the World Federation of Neurology. J Neurol Sci. 1997;145(2):205–211. doi: 10.1016/s0022-510x(96)00231-6. [DOI] [PubMed] [Google Scholar]
- 33.Wechsler D. Wechsler Adult Intelligence Scale. 3. San Antonio, TX: Pearson; 1997. [Google Scholar]
- 34.Filipovic-Sadic S, Sah S, Chen L, et al. A novel FMR1 PCR method for the routine detection of low abundance expanded alleles and full mutations in fragile X syndrome. Clin Chem. 2010;56(3):399–408. doi: 10.1373/clinchem.2009.136101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tassone F, Pan R, Amiri K, Taylor AK, Hagerman PJ. A rapid polymerase chain reaction-based screening method for identification of all expanded alleles of the fragile X (FMR1) gene in newborn and high-risk populations. J Mol Diagn. 2008;10(1):43–49. doi: 10.2353/jmoldx.2008.070073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tassone F, Hagerman RJ, Taylor AK, Gane LW, Godfrey TE, Hagerman PJ. Elevated levels of FMR1 mRNA in carrier males: a new mechanism of involvement in the fragile-X syndrome. Am J Hum Genet. 2000;66(1):6–15. doi: 10.1086/302720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23(15):2129–2170. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- 38.Tassone F, Adams J, Berry-Kravis EM, et al. CGG repeat length correlates with age of onset of motor signs of the fragile X-associated tremor/ataxia syndrome (FXTAS) Am J Med Genet B Neuropsychiatr Genet. 2007;144B(4):566–569. doi: 10.1002/ajmg.b.30482. [DOI] [PubMed] [Google Scholar]
- 39.Koldewyn K, Hessl D, Adams J, et al. Reduced Hippocampal Activation During Recall is Associated with Elevated FMR1 mRNA and Psychiatric Symptoms in Men with the Fragile X Premutation. Brain Imaging Behav. 2008;2(2):105–116. doi: 10.1007/s11682-008-9020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang JY, Hessl DH, Hagerman RJ, Tassone F, Rivera SM. Age-dependent structural connectivity effects in fragile x premutation. Arch Neurol. 2012;69(4):482–489. doi: 10.1001/archneurol.2011.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jahanshahi M, Brown RG, Marsden CD. A comparative study of simple and choice reaction time in Parkinson’s, Huntington’s and cerebellar disease. J Neurol Neurosurg Psychiatry. 1993;56(11):1169–1177. doi: 10.1136/jnnp.56.11.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Day BL, Thompson PD, Harding AE, Marsden CD. Influence of vision on upper limb reaching movements in patients with cerebellar ataxia. Brain. 1998;121( Pt 2):357–372. doi: 10.1093/brain/121.2.357. [DOI] [PubMed] [Google Scholar]
- 43.Greco CM, Berman RF, Martin RM, et al. Neuropathology of fragile X-associated tremor/ataxia syndrome (FXTAS) Brain. 2006;129(Pt 1):243–255. doi: 10.1093/brain/awh683. [DOI] [PubMed] [Google Scholar]