Abstract
Objectives
Population mixing patterns can greatly inform allocation of HIV prevention interventions such as treatment as prevention (TasP) or preexposure prophyloaxis (PrEP). Characterizing contact patterns among subgroups can help identify the specific combinations of contact expected to result in the greatest number of new infections.
Setting
Baseline data from an intervention to reduce HIV related risk behaviors in male PWID the northern Vietnamese province of Thai Nguyen was used for the analysis.
Methods
Egocentric network data was provided by PWID who reported any drug injection equipment sharing in the previous 3 months. Age-dependent mixing was assessed to explore its epidemiological implications on risk of HIV transmission risk (among those HIV infected) and HIV acquisition risk (among those not infected) in PWID.
Results
A total of 1,139 PWID collectively reported 2,070 equipment sharing partnerships in the previous 3 months. Mixing by age identified the 30–34 and 35–39 year age groups as the group from whom the largest number of new infections were transmitted, making them primary targets for TasP. Among the uninfected, 25–29, 30–35, and 35–39 year age groups had the highest HIV acquisition rate, making them the primary targets for PrEP.
Conclusions
Collection and analysis of contact patterns in PWID is feasible and can greatly inform infectious disease dynamics and targeting of appropriate interventions. Results presented also provide much needed empirical data on mixing to improve mathematical models of diseases transmission in this population.
Keywords: persons who inject drugs, contact mixing, HIV prevention, treatment as prevention, preexposure prophylaxis
INTRODUCTION
Novel biomedical tools for HIV prevention such as treatment as prevention (TasP) or pre-exposure prophylaxis (PrEP) show great promise in slowing HIV spread.1–3 Yet these tools depend on long term patient management, demand strict drug adherence, and are expected to require vast infrastructure and financial resources.4,5 Calls for finer-tuned and more cost-efficient approaches have led to proposals to target (i.e. prioritize) these interventions to subgroups with greater propensity to transmit or acquire HV.6–8 Proposed strategies for the targeting of TasP, for example, identify candidate subgroups based on biological or behavioral indications for enhanced HIV transmission, such as pregnancy in women or multiple concurrent sexual partnerships.9,10 Other proposals utilize molecular tools to construct HIV transmission networks based on genetic similarity among viral samples in order to identify HIV-infected persons of higher network centrality.11–13 For PrEP, drug indications and targeting recommendations—particularly in men who have sex with men (MSM)—have been informed by findings from clinical trials14 and mathematical models designed to identify targeting strategies that maximize prevention benefits.15–17
Such targeting approaches represent important first steps in the rollout of TasP and PrEP, but are not without their limitations. Targeting groups based on individual risk factors, for instance, fail to account for structural elements of HIV risk (syndemic patterns,18 social determinants of health,19 network characteristics,20 etc.) and may therefore overlook important target groups. And while molecular tools may provide more granularity in the tracking of transmissions, these approaches rely on large amounts of high quality viral sequence data, making it resource-prohibitive in many lower income settings.
Debates surrounding the appropriate use of PrEP and TasP to date have largely focused on sexual modes of HIV transmission. Despite growing interest in applications of these tools in persons who inject drugs (PWID), evidence on their effectiveness to date in this population is limited to two ongoing trials21,22 and several mathematical models.23–25 This underscores the need for easily measured data, along with low-cost methods to understand PWID population network structures to inform optimized implementation of preventive interventions. Given the costs and logistical barriers of mapping full networks—also known as sociocentric networks, or those which provide information on relationships among all nodes within a defined social network (see Figure S1 and Supplemental Digital Content for more detail)—particularly in populations such as PWID whose behaviors are stigmatized or criminalized, we focus on potential uses of egocentric network data which are comprised only of information on the immediate contacts of sampled individuals.
Here we present results of a contact mixing analysis in PWID in Vietnam using data from a baseline study of an HIV intervention trial28 in order to identify subgroups at relatively higher risk of HIV transmission (among those already infected) or HIV acquisition (among those uninfected). The network data presented in this analysis also provides an empirical basis to inform parameterization of population-level mixing in PWID transmission models (most often represented in the form of an “assortativity parameter,” also discussed in the Supplemental Digital Content), outcomes of which are highly sensitive to assumptions about population network structure.26,27 Our analysis also provides an instructional model to guide other network analyses that can inform targeting of PrEP and TasP in other settings and populations.
METHODS
Data used in this analysis arise from the baseline study of an HIV intervention trial examining the effectiveness a multi-level intervention to reduce sexual and injecting risk in HIV positive PWID.29 The study was conducted in the northern province of Thai Nguyen where injection use of opiates is prevalent and HIV prevalence among PWID estimated to be about 34%.30 Eligible PWID were recruited into the baseline study in 2009 through snowball sampling and outreach by study staff. Eligible participants were male, at least 18 years of age, sexually active in the last 3 months, had injected drugs in the past 3 months, and planned to remain in the study area for the next two years.31 HIV infection status was confirmed through two simultaneous rapid enzyme immunoassay HIV tests: Determine (Abbott Laboratories, Abbott Park, IL) and Bioline (SD, Toronto, Canada).
Participant network data was collected using a name generating method in which participants listed names of all individuals who had played any kind of social support role in the most recent 3 months. Follow-up questions then collected information on each listed member including demographics, relationship to the participant, and frequency of equipment sharing between them. Equipment sharing was defined as using the same ampoule of water/Novocain; transferring drug solution, or using the same needle/syringe. Sharing frequency in the previous 3 months was assessed using prefixed categories (i.e. “less than once a month,” “2–3 times a month,” “weekly,” “2–3 times a week,” and “daily”) which were then multiplied by the number of days in the 3-month assessment period (90 days) to estimate total numbers of sharing episodes during that time. For sharing frequencies measured as a range (e.g. 2–3 times a week), we used the lower and upper bounds to calculate a minimum and maximum estimate of total sharing episodes over the 3-month duration (e.g. 24–36 times in 3 months), from which we randomly drew a value for each partnership. This approach was thought to better approximate a more realistic distribution of per-partnership sharing frequency in the past 3 months.
Mixing matrices were then constructed to describe contact patterns between participants and their drug sharing partners according to five-year age groups (age is thought to bear epidemiological implications for HIV risk in PWID32,33). An initial matrix assessed frequency of contacts between participants and their partners in the past 3 months (κij). A second matrix then assessed transmission risk resulting from contact between each age group by calculating expected numbers of new cases (κij * Ij/Nj * Si), with bootstrapped confidence intervals using resampling of partnerships over 1000 iterations. Finally, the sum of the relative number of new expected infections across columns was used to estimate the expected number of relative new infections due to sharing with contacts of the respective age group, and the sum across rows was used to estimate the relative number of expected new infections in each participant age group. All statistical analyses were performed in the R statistical package (R v. 3.1.1, www.cran.org).
The study was approved by the institutional review boards of the Johns Hopkins Bloomberg School of Public Health and the Thai Nguyen Center for Preventive Medicine. For each study visit participants were reimbursed 50,000 Vietnamese Dong (about $3 USD).
RESULTS
Participant and Network Characteristics
Of the 1674 participants enrolled in the study, 535 (32.0%) were excluded from this analysis for reporting no equipment sharing in the previous 3 months, leaving 1139 men in the final analysis set (Table 1). Compared to those who were HIV negative, fewer HIV infected men were employed either full or part time (84.9%, 95% CI, 81.3–88.6% versus 91.9%, 95% CI 89.9–93.8%) or had had unprotected sex in the past 3 months (69.0%, 95% CI, 64.3–73.8% versus 80.9%, 95% CI, 78.1–83.6%). However more of the HIV infected participants reported daily injection drug use in the past 3 months (51.2%, 95% CI, 46.1–56.4% versus 42.2%, 95% CI 38.89–45.7%) compared to their HIV uninfected counterparts.
Table 1.
Baseline characteristics of the 1139 male PWID participants, by HIV status.
| HIV Negative | HIV Positive | ||||
|---|---|---|---|---|---|
| N=774 (68.0%) | N=365 (32.0%) | ||||
|
|
|||||
| % | 95% CI | % | 95% CI | ||
| Size of sharing network* | |||||
|
| |||||
| 1 | 44.3 | (40.8–47.8) | 46.3 | (41.2–51.4) | |
| 2–3 | 49.9 | (46.3–53.4) | 48.5 | (43.4–53.6) | |
| 4 or more | 1.8 | (0.9–2.7) | 1.1 | (0–2.2) | |
|
| |||||
| Demographics | |||||
|
| |||||
| Age, in years (median, IQR) | 35 | (29–40) | 34 | (30–38) | |
| Years of education (median, IQR) | 9 | (7–12) | 9.0 | (7–11) | |
| Stable sexual partner† | 48.3 | (44.8–51.8) | 43.6 | (38.5–48.6) | |
| Employed‡ | 91.9 | (89.9–93.8) | 84.9 | (81.3–88.6) | |
| Income per month in millions of dong (median, IQR) | 2 | (1.5–2.5) | 1.5 | (1.0–2.5) | |
|
| |||||
| Drug use related behaviors | |||||
|
| |||||
| Injected daily, past 3 months§ | 42.2 | (38.8–45.7) | 51.2 | (46.1–56.4) | |
| Duration of drug use, in years (median, IQR)‖ | 12 | (7–18) | 12 | (9–18) | |
| Ever in drug treatment in lifetime | 29.8 | (26.6–33.1) | 30.7 | (26–35.4) | |
| Ever overdosed on drugs in lifetime | 19.3 | (16.5–22) | 21.1 | (16.9–25.3) | |
|
| |||||
| Other reported histories | |||||
|
| |||||
| Ever arrested in lifetime | 41.2 | (37.7–44.7) | 44.4 | (39.3–49.5) | |
| Ever in prison in lifetime¶ | 35.4 | (32–38.8) | 36.4 | (31.5–41.4) | |
| Ever spent a night in street, in a park, alley, or abandoned building in last 3 months | 20 | (17.2–22.8) | 16.4 | (12.6–20.2) | |
| Had any unprotected sex, past 3 months# | 80.9 | (78.1–83.6) | 69 | (64.3–73.8) | |
|
| |||||
| HIV related factors | |||||
|
| |||||
| Knew own HIV status at baseline | 16 | (13.4–18.6) | 20.5 | (16.4–24.7) | |
| CD4 cell count (median, IQR) | -- | 234 | (125–372) | ||
535 (32.0%) of participants reported not sharing with any partners in the past 3 months, and 46 (4.0%) were missing network information.
A "stable partner" was defined as someone to whom the participant is married or cohabitating.
1 person was missing injection frequency information.
7 people were missing information on duration of injection drug use.
1 person was missing information on past incarceration.
Employment either full or part time.
Unprotected sex was assessed for partners of either sex; 28 (2.4%) people were missing this information.
A collective total of 2070 equipment sharing partnerships were reported by participants, with most (45.0%) reporting only reporting one partner in the past 3 months and nearly half reporting monthly injection (Figure 1A).
Figure 1.
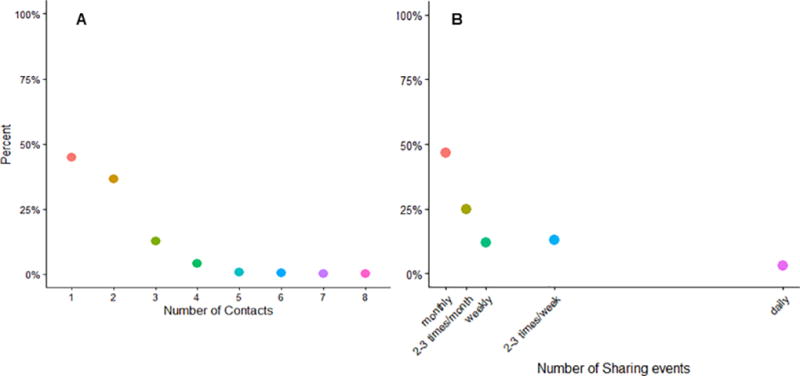
A) The graphs displays the proportion of study participants that reported each number of different drug injection equipment sharing partners in the past 3 months. The figure represents survey information provided by the 1139 male PWID participants of the parent study who reported at least one sharing event during that time. B) The graph displays the proportion of study participants that reported each frequency of injection in the past 3 months. This figures represents information provided by the same 1139 male PWID participants as in panel A.
Contact Patterns
Reported numbers of contacts between 5-year age groups in the 1139 participants are shown in Figure 2 (raw data provided in Figure S2). Higher cell values indicate more frequent mixing between members of the two age groups. The higher rates of contact between members of the same age (cells along the diagonal line) indicate that sharing in this population was relatively assortative, that is, sharing tended to take place between partners of the same or similar age groups (greater detailed discussion of assortativity provided in the Supplemental Digital Content and Figure S3).
Figure 2.
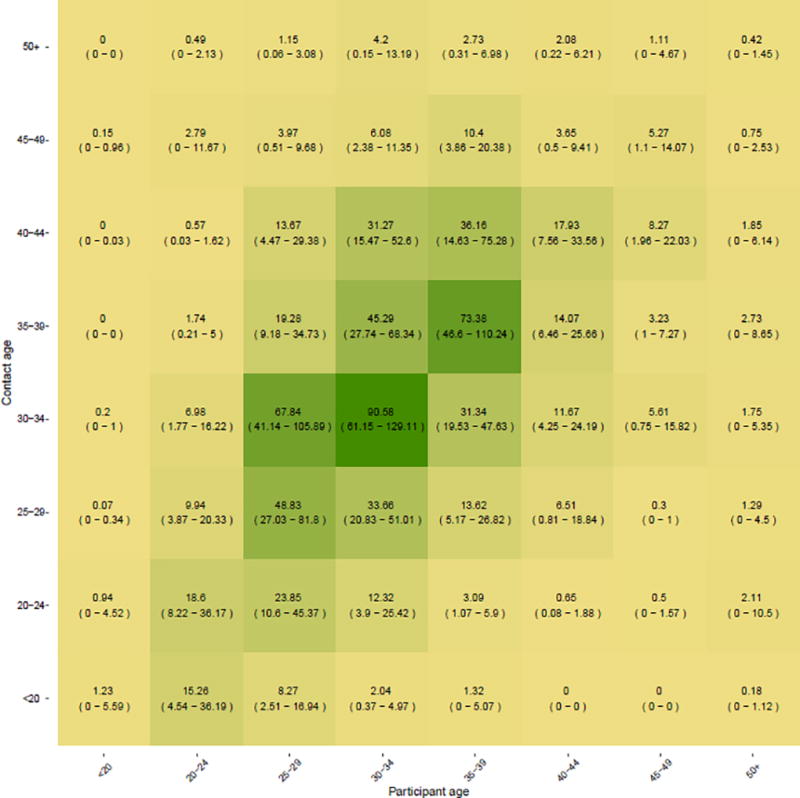
Age-dependent contact mixing matrix as reported by 1139 male PWID participants of the parent study. Cell values represent the 3 month average number of sharing events between each age group, with the ages of the study participants represented along the X-axis and those of their sharing partners’ ages represented along the Y-axis. 95% confidence intervals represent bootstrapped values estimated by sampling observed partnerships with replacement over 1000 iterations. Cell color shading corresponds to the intensity of sharing; that is, the more frequent the number of sharing episodes between members of two given age groups, the darker the corresponding cell.
A second matrix (Figure 3) incorporates age-specific HIV prevalence and numbers of susceptible individuals, in which cell values represent estimates of the expected numbers of new infections due to contacts between each age group. This matrix suggests that contact among those aged 30–34 and 35–39 will likely produce the most new infections as compared to all other age combinations. A figure of age-specific HIV prevalence is provided in Supplemental Figure S4.
Figure 3.
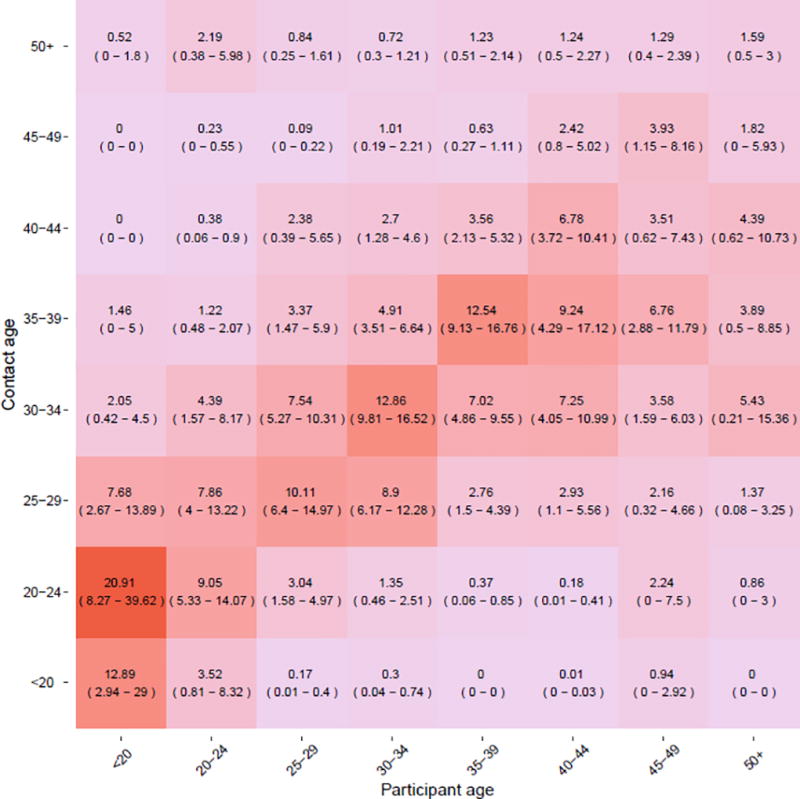
Age-dependent matrix for the expected relative number of additional people infected due to contacts in each age combination in the previous 3 months. The cell values provide the relative number of new infections expected to occur among sharing partners (the group on the Y-axis) due to sharing events with study participants (the group on the X-axis). 95% confidence intervals represent bootstrapped values estimated by sampling observed partnerships with replacement over 1000 iterations. Cell color shading corresponds to the relative number of newly infected individuals as a result of sharing between the two age groups; that is, the more new infections resulting from sharing between two age groups, the deeper the shading of the corresponding cell.
Total numbers of expected new infections in each age group, as well as total numbers of expected new infections in each age group attributable to contact with partners in every age group are shown in Figure 4. The dotted line shows the number of new infections expected as a result of sharing with partners in each group over a 3 month period. Among them, sharing with 30–34 year olds and 35–39 year olds would result in the most new infections compared to other age groups (N=14.1 and N=10.5, respectively). The solid line indicates the groups that would be expected to experience the highest force of infection in the same 3 month period, showing that those in the 30–34 and 35–39 year old age groups would be the most heavily impacted.
Figure 4.
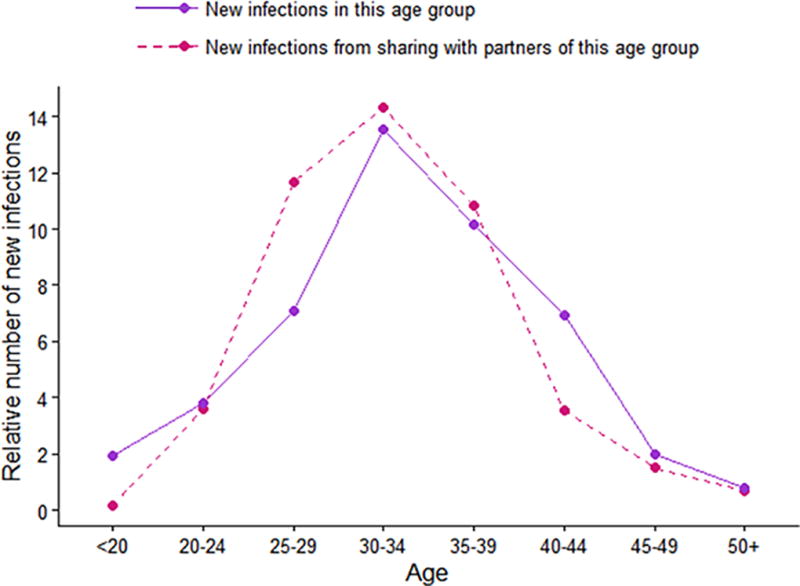
Plots show the number of new infections that one would expect to observe in the subsequent time period (i.e. following the initial 3 months of observed time) given the baseline age-specific HIV prevalence and age-dependent mixing patterns. The dotted line indicates the number of new infections that would be expected to occur as a result of contact with HIV infected individuals in the corresponding age group on the X-axis. This line is therefore indicative of the age groups that should be targeted for treatment as prevention (TasP). The solid line indicates the expected number of new infections acquired by the corresponding age groups on the X-axis, indicating the age groups that should be targeted for pre-exposure prophylaxis.
Implications for HIV Control
Contact patterns with drug sharing partners indicate assortative age-based mixing in this population. Accounting for age-specific HIV prevalence allows for identification of age groups expected to have the greatest likelihood of transmitting HIV to their partners, making them primary candidates for TasP. The same results also identify the age groups expected to acquire the most new infections, who in turn would be primary candidates for PrEP. It is noteworthy that similar age groups are both the primary transmitters and acquirers of new infection (30–34 and 35–39 year olds), a possible byproduct of the fact that this group has the combination of both high HIV prevalence, high rates of sharing, and sufficient numbers of uninfected persons still at risk of infection.
DICUSSION
Here we present a novel application of network analysis tools to address key questions to guide effective targeting of PrEP and TasP in high transmission settings. Growing interest in the use of TasP and PrEP for HIV control34,35—evidenced by several large scale trials on HIV combination prevention36—will bring with it the need for clearer insights into how infected and uninfected groups of people mix. Moreover as the epidemic in key populations continues to expand,37–40 effective use of these interventions in hard-to-reach populations such as PWID will become critical to global HIV control efforts. As promising results begin to emerge from early clinical trials on the effectiveness of PrEP41 (and imminently, for TasP42) in PWID populations, analyses such as this which inform allocation of biomedical intervention tools may play a key role in early program scale-up and implementation. Moreover, as part of a longstanding collaboration between US investigators and local Vietnamese health authorities, this study and its findings are well positioned to directly inform local HIV control policy and impact health outcomes in the near future.
Our findings also provide new insights into contact mixing patterns of PWID, particularly among those in LMIC settings. We are aware of only two studies to date that have quantified contact mixing in PWID, both conducted in the US and led by Dr. Mark A Williams. The earlier study, published in 1995, surveyed out-of-treatment PWID living in one of three US cities (Dayton OH, Houston TX, and Rio Piedras, Pureto Rico),43 and the latter, conducted in 2001, surveyed drug-using male sex workers in Houston, TX.44 Both studies corroborate our findings of assortative age-based mixing in PWID; however, the relatively few age groups considered in both Williams et al studies (3 in one and 4 in the other) limit our ability to make direct comparisons. The lack of biological outcome data in both Williams et al studies also limit their findings to descriptive results and so are unable to investigate expected transmission patterns as was done in this analysis.
It is our intention that the guidance provided here help inform future investigations in diverse settings and potentially in other populations, but only after careful considerations of its limitations. First, information bias such as recall or reporting bias, while a common problem in survey data, may compound existing measurement issues if the resulting bias is associated with misreporting of partner attributes. In our example of age-based mixing, for example, younger PWID may misreport or overestimate the ages of much older partners, a phenomenon known to affect other types of data,45 If reporting bias did in fact skew reported partner ages upwards, however, observed patterns of age-based mixing matrices would likely appear less assortative, suggesting robustness of the assortativity observed in our data. Second, the name generating approach used to enumerate participant contacts may have resulted in biased samples of each participant’s true drug sharing network. That is, by asking participants to first list names of people from whom they received social support, a list from which they would then identify network members with whom they had shared drug equipment, the resulting networks may overrepresent drug sharing partnerships with people to whom they are emotionally close. Little information is available on the differences across types sharing relationships, but findings from a 2001 study of Baltimore PWID reporting that partners who knew each other well (e.g. sexual partners or kin)46 may share with greater intensity. This may suggest that our sample captured key partnerships within which most sharing takes place, but more contextual background information would be needed to assess the impact of such biases on our observed result. A final limitation of this study was our lack of details on participants’ reported sexual partners, which precluded our ability to assess risk of sexual HIV transmission. Though future network analyses will greatly benefit from incorporation of this information, the outsized risk of HIV transmission associated with unsafe drug injection vis-à-vis vaginal intercourse (the dominant form of sexual behavior reported by this sample) retain some of public health significance of the results presented here.
Knowledge of the functions and dynamics of social networks can lend valuable insight into the design of effective network-based interventions.47 Our study demonstrates the utility of egocentric network data to investigate contact mixing patterns salient to decisions about targeting and allocation of HIV prevention resources in PWID. Although egocentric data is a relatively low-cost, fast, and feasible alternative to collection of sociocentric or viral sequence data (particularly in lower-income settings), researcher choice on network approaches should also be guided by their existing knowledge regarding things like epidemic stage or other local environmental factors. More sophisticated approaches may also be more appropriate for addressing more in-depth questions regarding things like long-term historical transmission trends,48,49 the relationship between network-wide behaviors and prevalence,50 and the role of network features on patterns of disease spread.51
Supplementary Material
Acknowledgments
Sources of Support:
Research reported in this manuscript was funded the National Institute on Drug Abuse (NIDA R01 DA032217) and the National Institute on Allergies and Infectious Diseases (NIAID; P30 AI094189). CAL received support from NIDA (R01DA031030, R01DA032217, and R01DA040488); MKS from NIAID (T32AI102623) and MG by the Bill and Melinda Gates Foundation (OPP1106427).
The authors would like to thank members of the study community for their generous participation in this research. They would also like to thank Wendy Davis for her logistical and substantive support in the preparation of this manuscript.
Footnotes
Contributions
MKS, CAL, and VLG conceptualized the original study. MKS and MG conducted the computational analyses. MKS composed the first draft of the manuscript, and substantial improvements were made by the other three coauthors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 Infection with Early Antiretroviral Therapy. N. Engl. J. Med. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodger AJ, Cambiano V, Bruun T, et al. Sexual Activity Without Condoms and Risk of HIV Transmission in Serodifferent Couples When the HIV-Positive Partner Is Using Suppressive Antiretroviral Therapy. Jama. 2016;316(2):171. doi: 10.1001/jama.2016.5148. [DOI] [PubMed] [Google Scholar]
- 3.Molina J-M, Capitant C, Spire B, et al. On-Demand Preexposure Prophylaxis in Men at High Risk for HIV-1 Infection. N. Engl. J. Med. 2015;373(23):2237–46. doi: 10.1056/NEJMoa1506273. [DOI] [PubMed] [Google Scholar]
- 4.Kessler J, Braithwaite RS. Modeling the cost–effectiveness of HIV treatment. Curr. Opin. HIV AIDS. 2013;8(6):544–549. doi: 10.1097/COH.0000000000000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McNairy ML, El-Sadr WM. Antiretroviral therapy for the prevention of HIV transmission: What will it take? Clin. Infect. Dis. 2014;58(7):1003–1011. doi: 10.1093/cid/ciu018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mikkelsen E, Hontelez JAC, Jansen MPM, et al. Evidence for scaling up HIV treatment in sub-Saharan Africa: A call for incorporating health system constraints. PLoS Med. 2017;14(2):1–6. doi: 10.1371/journal.pmed.1002240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaiser Family Foundation, UNAIDS. Financing the Response to HIV in Low-and Middle-Income Countries: International Assistance from Donor Governments in 2015. 2016 [Google Scholar]
- 8.Mills EJ, Nachega JB, Ford N. Can we stop AIDS with antiretroviral-based treatment as prevention? Glob. Heal. Sci. Pract. 2013;1(1):29–34. doi: 10.9745/GHSP-D-12-00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delva W, Eaton JW, Meng F, et al. HIV Treatment as Prevention: Optimising the Impact of Expanded HIV Treatment Programmes. PLoS Med. 2012;9(7):e1001258. doi: 10.1371/journal.pmed.1001258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The HIV Modelling Consortium Treatment as Prevention Editorial Writing Group. HIV Treatment as Prevention: Models, Data, and Questions—Towards Evidence-Based Decision-Making. PLoS Med. 2012;9(7):e1001259. doi: 10.1371/journal.pmed.1001259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Little SJ, Pond SLK, Anderson CM, et al. Using HIV networks to inform real time prevention interventions. PLoS One. 2014;9(6):1–8. doi: 10.1371/journal.pone.0098443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X, Wu Y, Mao L, et al. Targeting HIV prevention based on molecular epidemiology among deeply sampled subnetworks of men who have sex with men. Clin. Infect. Dis. 2015;61(9):1462–1468. doi: 10.1093/cid/civ526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dennis AM, Herbeck JT, Brown AL, et al. Phylogenetic studies of transmission dynamics in generalized HIV epidemics: an essential tool where the burden is greatest? J. Acquir. Immune Defic. Syndr. 2014;67(2):181–95. doi: 10.1097/QAI.0000000000000271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buchbinder SP, Glidden DV, Liu AY, et al. HIV pre-exposure prophylaxis in men who have sex with men and transgender women: A secondary analysis of a phase 3 randomised controlled efficacy trial. Lancet Infect. Dis. 2014;14(6):468–475. doi: 10.1016/S1473-3099(14)70025-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kessler J, Myers JE, Nucifora KA, et al. Evaluating the impact of prioritization of antiretroviral pre-exposure prophylaxis in New York. AIDS. 2014;28(18):2683–2691. doi: 10.1097/QAD.0000000000000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen A, Dowdy D. Clinical effectiveness and cost-effectiveness of HIV pre-exposure prophylaxis in men who have sex with men: risk calculators for real-world decision-making. PLoS One. 2014;9(10):e108742. doi: 10.1371/journal.pone.0108742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jenness SM, Goodreau SM, Rosenberg E, et al. Impact of the Centers for Disease Control’s HIV Preexposure Prophylaxis Guidelines for Men Who Have Sex With Men in the United States. J. Infect. Dis. 2016;214(12):1800–1807. doi: 10.1093/infdis/jiw223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Starks TJ, Millar BM, Eggleston JJ, Parsons JT. Syndemic Factors Associated with HIV Risk for Gay and Bisexual Men: Comparing Latent Class and Latent Factor Modeling. AIDS Behav. 2014;18(11):2075–2079. doi: 10.1007/s10461-014-0841-9. [DOI] [PubMed] [Google Scholar]
- 19.Hoots BE, Finlayson T, Nerlander L, et al. Willingness to Take, Use of, and Indications for Pre-exposure Prophylaxis Among Men Who Have Sex With Men -- 20 US Cities, 2014. Clin. Infect. Dis. 2016;63(5):672–677. doi: 10.1093/cid/ciw367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hallfors DD, Iritani BJ, Miller WC, Bauer DJ. Sexual and Drug Behavior Patterns and HIV and STD Racial Disparities : The Need for New Directions. 2007;97(1):125–132. doi: 10.2105/AJPH.2005.075747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Y, Mâsse B, Wang L, et al. Statistical considerations for the HPTN 052 Study to evaluate the effectiveness of early versus delayed antiretroviral strategies to prevent the sexual transmission of HIV-1 in serodiscordant couples. Contemp. Clin. Trials. 2012;33(6):1280–1286. doi: 10.1016/j.cct.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choopanya K, Martin M, Suntharasamai P, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013;381(9883):2083–2090. doi: 10.1016/S0140-6736(13)61127-7. [DOI] [PubMed] [Google Scholar]
- 23.Alistar SS, Owens DK, Brandeau ML. Effectiveness and cost effectiveness of expanding harm reduction and antiretroviral therapy in a mixed HIV epidemic: A modeling analysis for Ukraine. PLoS Med. 2011;8(3) doi: 10.1371/journal.pmed.1000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Degenhardt L, Mathers B, Vickerman P, Rhodes T, Latkin CA, Hickman M. Prevention of HIV infection for people who inject drugs: why individual, structural, and combination approaches are needed. Lancet. 2010;376(9737):285–301. doi: 10.1016/S0140-6736(10)60742-8. [DOI] [PubMed] [Google Scholar]
- 25.Fraser H, Mukandavire C, Martin NK, et al. HIV treatment as prevention among people who inject drugs – a re-evaluation of the evidence. Int. J. Epidemiol. 2016 doi: 10.1093/ije/dyw180. dyw180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garnett GP, Anderson RM. Contact Tracing and the Estimation of Sexual Mixing Patterns: The Epidemiology of Gonococcal Infections. Sex. Transm. Dis. 1993 doi: 10.1097/00007435-199307000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Jacquez J, Koopman J, Simon C, Longini I. Role of the primary infection in epidemics of HIV infection in gay cohorts. J Acquir Immune Defic Syndr. 1994;7(11):1169–1184. [PubMed] [Google Scholar]
- 28.Zelaya CE, Le Minh N, Lau B, et al. The Effect of a Multi-Level Intervention on the Initiation of Antiretroviral Therapy (ART) among HIV-Infected Men Who Inject Drugs and Were Diagnosed Late in Thai Nguyen, Vietnam. PLoS One. 2016;11(8):e0161718. doi: 10.1371/journal.pone.0161718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Go VF, Frangakis C, Le Minh N, et al. Effects of an HIV peer prevention intervention on sexual and injecting risk behaviors among injecting drug users and their risk partners in Thai Nguyen, Vietnam: a randomized controlled trial. Soc. Sci. Med. 2013;96:154–64. doi: 10.1016/j.socscimed.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thai Nguyen Provincial HIV/AIDS Control Center. Bi-Annual Report of the Provincial HIV/AIDS Control Center, Thai Nguyen, Vietnam. 2014 [Google Scholar]
- 31.Go VF, Frangakis C, Minh NLe, et al. Efficacy of a Multi-level Intervention to Reduce Injecting and Sexual Risk Behaviors among HIV-Infected People Who Inject Drugs in Vietnam: A Four-Arm Randomized Controlled Trial. In: Chung MH, editor. PLoS One. 5. Vol. 10. 2015. pp. 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De P, Cox J, Boivin J-FF, Platt RW, Jolly AM. The importance of social networks in their association to drug equipment sharing among injection drug users: a review. Addiction. 2007;102(11):1730–9. doi: 10.1111/j.1360-0443.2007.01936.x. [DOI] [PubMed] [Google Scholar]
- 33.Tassiopoulos K, Bernstein J, Bernstein E. Age and sharing of needle injection equipment in a cohort of Massachusetts injection drug users: an observational study. Addict. Sci. Clin. Pract. 2013;8:20. doi: 10.1186/1940-0640-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan R. Biomedical strategies for human immunodeficiency virus (HIV) prevention? A new paradigm. Ann Acad Med Singapore. 2012;41(12):595–601. Available at: http://www.ncbi.nlm.nih.gov/pubmed/23303118. [PubMed] [Google Scholar]
- 35.Kurth AE, Celum C, Baeten JM, Vermund SH, Wasserheit JN. Combination HIV prevention: Significance, challenges, and opportunities. Curr. HIV/AIDS Rep. 2011;8(1):62–72. doi: 10.1007/s11904-010-0063-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shanaube K, Bock P. Innovative Strategies for Scale up of Effective Combination HIV Prevention Interventions in Sub-Saharan Africa. Curr. HIV/AIDS Rep. 2015;12(2):231–237. doi: 10.1007/s11904-015-0262-z. [DOI] [PubMed] [Google Scholar]
- 37.Baral S, Beyrer C, Muessig KE, et al. Burden of HIV among female sex workers in low-income and middle-income countries: a systematic review and meta-analysis. Lancet Infect. Dis. 2012;12(7):538–549. doi: 10.1016/S1473-3099(12)70066-X. [DOI] [PubMed] [Google Scholar]
- 38.Degenhardt L, Hall W. Extent of illicit drug use and dependence, and their contribution to the global burden of disease. Lancet. 2012;379(9810):55–70. doi: 10.1016/S0140-6736(11)61138-0. [DOI] [PubMed] [Google Scholar]
- 39.Baral SD, Poteat T, Strömdahl S, Wirtz AL, Guadamuz TE, Beyrer C. Worldwide burden of HIV in transgender women: A systematic review and meta-analysis. Lancet Infect. Dis. 2013;13(3):214–222. doi: 10.1016/S1473-3099(12)70315-8. [DOI] [PubMed] [Google Scholar]
- 40.Shannon K, Strathdee SA, Goldenberg SM, et al. Global epidemiology of HIV among female sex workers: influence of structural determinants. Lancet. 2015;385(9962):55–71. doi: 10.1016/S0140-6736(14)60931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choopanya K, Martin M, Suntharasamai P, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013;381(9883):2083–2090. doi: 10.1016/S0140-6736(13)61127-7. [DOI] [PubMed] [Google Scholar]
- 42.Miller WC, Hoffman IF, Burns DN. HPTN 074: Integrated Treatment and Prevention for People Who Inject Drugs: A Vanguard Study for a Network-Based Randomized HIV Prevention Trial Comparing an Integrated Intervention Including Supported Antiretroviral Therapy to the Standard of Care. Available at: http://www.hptn.org/research_studies/hptn074.asp#StudySummary.
- 43.Williams ML, Zhuo Z, Siegal HA, Robles RR, Trotter RT, Jones A. A comparison of drug use networks across three cities. NIDA Research Monograph Series: Social Networks, Drug Abuse and HIV Transmission. 1995:109–130. doi:8742763. [PubMed] [Google Scholar]
- 44.Williams ML, Bowen AM, Timpson S, Keel KB. Drug Injection and Sexual Mixing Patterns of Drug-Using Male Sex Workers. Sex. Transm. Dis. 2003;30(7):534–541. doi: 10.1136/sti.2007.027151. [DOI] [PubMed] [Google Scholar]
- 45.Bogue DJ, Arriaga EE, Anderton DL, Rumsey GW. Errors and bias in the reporting of ages in census data. Readings in Population Research Methodology. 1993:4-23–4-29. [Google Scholar]
- 46.Sherman SG, Latkin CA, Gielen aC. Social factors related to syringe sharing among injecting partners: a focus on gender. Subst. Use Misuse. 2001;36(14):2113–2136. doi: 10.1081/JA-100108439. [DOI] [PubMed] [Google Scholar]
- 47.Valente TW. Network Interventions. Science (80-.) 2012;337(6090):49–53. doi: 10.1126/science.1217330. [DOI] [PubMed] [Google Scholar]
- 48.Yebra G, Ragonnet-Cronin M, Ssemwanga D, et al. Analysis of the history and spread of HIV-1 in Uganda using phylodynamics. J. Gen. Virol. 2015;96(7):1890–1898. doi: 10.1099/vir.0.000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wertheim JO, Brown AJL, Hepler NL, et al. The Global Transmission Network of HIV-1. J. Infect. Dis. 2014;209:304–313. doi: 10.1093/infdis/jit524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dombrowski K, Curtis R, Khan B, Friedman SR. Topological and Historical Considerations for Infectious Disease Transmission among Injecting Drug Users in Bushwick, Brooklyn (USA) World J. AIDS. 2013;3:1–9. doi: 10.4236/wja.2013.31001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dombrowski K, Khan B, McLean K, et al. A reexamination of connectivity trends via exponential random graph modeling in two IDU risk networks. Subst. Use Misuse. 2013;48(14):1485–97. doi: 10.3109/10826084.2013.796987. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


