Abstract
Background
Hepatotoxicity associated with isoniazid preventive therapy (IPT) and antiretroviral therapy (ART) has not been well studied in severely immunosuppressed people with HIV. Our objective was to determine risk factors for hepatotoxicity in severely immunosuppressed individuals taking IPT and ART.
Setting
Multi-center study in resource limited settings with high burden of tuberculosis.
Methods
We conducted a secondary analysis of data from one randomized arm of the REMEMBER trial. The analysis includes participants with pre-ART CD4 cell counts of <50 cells/μl receiving IPT and ART for 24 weeks. Hepatotoxicity was defined as elevated aspartate aminotransferase (AST) or alanine aminotransferase (ALT) >5 × upper limit of normal or symptomatic hepatitis during IPT and ART.
Logistic regression was used to identify baseline risk factors for hepatotoxicity. Time to occurrence of hepatotoxicity was estimated by the Kaplan Meier method.
Results
Among 426 participants (53% male, median age 35 years, median CD4 count 19 cells/µL), 31 developed hepatotoxicity (7.3%). Raised pretreatment AST/ALT (OR 3.6, 95% CI 1.7–7.7) and hepatitis B surface antigen (HBsAg) sero-positivity at baseline (OR 4.7, 95% CI 1.7-12.9) were significantly associated with an increased risk of developing hepatotoxicity. Participants with both raised AST/ALT and positive HBsAg had a higher risk (OR 19.9, 95% CI 5.3-74.3) and earlier onset of hepatotoxicity than participants who did not have these conditions at baseline.
Conclusions
The incidence of hepatotoxicity during IPT and ART was high. Severely immunosuppressed individuals with raised pretreatment AST/ALT or HBsAg sero-positivity need closer monitoring for hepatotoxicity.
Keywords: isoniazid, ART, hepatotoxicity, HIV, immunosuppression
INTRODUCTION
Tuberculosis (TB) is a leading cause of death and morbidity in people living with the human immunodeficiency virus (PLHIV) (1,2). In 2015, TB accounted for 0.4 million of the 1.1 million deaths caused by HIV; most of these deaths occurred in resource limited settings (3). Sub-Saharan African and Southeast Asia are the epicenters of HIV-associated TB with 79% and 11% of the disease burden being in these regions (2).
The World Health Organization (WHO) recommends providing isoniazid preventive therapy (IPT) and antiretroviral therapy (ART) to prevent TB in PLHIV(4,5). When used concurrently, IPT and ART have greater efficacy at preventing TB than when either of them is used alone (6,7). In high TB burden countries, PLHIV should receive IPT and ART regardless of their level of immunosuppression (8,9). While ART has been widely adopted in national HIV programs, uptake of IPT has not been as successful (8). The WHO is encouraging national HIV/TB programs to increase uptake of IPT to parallel the success of ART programs (4,5).
Hepatotoxicity is a common life-threatening adverse event of both IPT and ART (9,10). When these two interventions are used concurrently, there are higher rates hepatotoxicity because of hepatic drug interactions and overlapping toxicities between drugs (11,12). While ART should be started as soon as possible, the best time of starting IPT relative to the ART is not clear (8,13,14). The current WHO guidelines do not precisely define how the interventions can optimally be used together. In the guidelines, WHO recognizes that there is an insufficient evidence on whether starting IPT at the same time as ART or delaying it is better in terms of efficacy, risk of toxicity or development of immune reconstitution inflammatory syndrome (8).
People with advanced HIV disease have a high incidence and mortality of TB (15,16). However, clinicians are reluctant to start IPT in this group because it is difficult to reliably exclude active TB and because of concerns that IPT may inadvertently increase the rate of hepatotoxicity and other drug related adverse events (14). Previous IPT studies typically enrolled healthier people with higher CD4 cell counts or whose CD4 counts were not known (17). As a result, we know little about safety of using IPT in PLHIV with very low CD4 counts.
We conducted a secondary data analysis of the REMEMBER trial to evaluate hepatotoxicity in participants who started IPT and ART at the same time. The REMEMBER trial compared mortality rates among people with advanced HIV disease living in high-burden settings who received either empiric TB treatment or IPT in addition to ART. The trial demonstrated similar mortality between the two arms and lower TB incidence in the IPT arm (18). We restricted our analysis to the IPT arm because empirical TB treatment would not be recommended in practice based on the REMEMBER trial findings. For this analysis, our objective was to examine the baseline risk factors of hepatotoxicity during concurrent IPT and ART use in people with advanced HIV disease.
METHODS
Study design and participants
The REMEMBER trial was an open-label randomized clinical trial (the clinical trial registration number is NCT0138008) that enrolled HIV-positive antiretroviral-naïve individuals from 18 outpatient research clinics in ten countries (Malawi, South Africa, Haiti, Kenya, Zambia, India, Brazil, Zimbabwe, Peru, and Uganda) sponsored and funded by the US National Institutes of Health.
Eligible participants were aged 13 years or older with CD4 counts less than 50 cells/μl and did not have evidence of active TB (18). Further inclusion criteria included having aspartate aminotransferase (AST), alanine aminotransferase (ALT) and total bilirubin (TBIL) less than 2.5 times the upper limit of normal, a creatinine clearance of at least 30mL/min and a Karnofsky score of at least 30 (18).
All participating sites obtained ethical approval from local ethics committees. All participants provided written informed consent.
Study procedures
The REMEMBER study procedures have been previously described (18). Potential participants were referred from local ART clinics to study clinics. At the study clinics, the potential participants were screened for TB using a symptom screen. Any positive symptom screen required further work-up per local standard of care. Individuals who were strongly suspected to have TB or for whom screening procedures identified confirmed or probable TB were excluded. Those with negative symptom screens or positive screens but no microbiological or presumptive diagnosis of TB were eligible for enrolment in the study.
All participants received efavirenz-containing ART with either study-provided tenofovir/emtricitabine (TDF/FTC) (donated by Gilead) or locally available nucleoside reverse transcriptase inhibitors. Participants in the IPT arm received 300 mg of isoniazid daily for 24 weeks, beginning within 7 days of starting ART. All participants received pyridoxine.
Participants were followed up for 96 weeks. In this analysis, we report results up to 24 weeks, the period of combined IPT and ART. Participants had study visits at screening, enrolment, and weeks 1, 2, 4, 8, 12, 16, 20, and 24. Clinical events, ART and anti-tuberculosis drug modification were reported at each visit. Blood samples for CD4 cell count and HIV-1 RNA level were collected at study entry and at weeks 4 and 24. Blood samples for hematology, liver function and renal function tests were collected at all visits except week 1. Participants who developed signs or symptoms of TB had investigations done per locally available diagnostics. Those diagnosed with TB received weight-adjusted, fixed-dose combination rifampin/isoniazid/ethambutol/pyrazinamide for 8 weeks, followed by fixed-dose combination rifampin/isoniazid for 16 weeks. In the event of missed visits, participants were traced by telephone calls and home visits if they or their contacts could not be reached by telephone. Those who could not be contacted after this process were defined as lost to follow-up.
For AST or ALT > 7.5× upper limit of normal (ULN), laboratory results were confirmed and isoniazid and ARVs were discontinued until levels fell to < Grade 2 at which time, the drugs could be introduced sequentially.
Variables
Outcome variable
We defined hepatotoxicity as occurrence of Grade 3 (5.1–10.0 × ULN) or Grade 4 (> 10.0 × ULN) AST or ALT per the Division of AIDS Toxicity Table (19) or symptomatic hepatitis after initiating IPT and ART.
Predictor variables
The following pre-treatment variables (baseline variables) were evaluated as possible risk factors for hepatotoxicity: age, sex, CD4 count, HIV-1 RNA, Karnofsky Performance Score, body mass index (BMI), hospitalization within 30 days prior to study entry, hepatitis B surface antigen (HBsAg) status, raised pretreatment level of AST/ALT, presence of anemia and use of alcohol within 30 days to study entry.
Raised pretreatment AST/ALT was defined as AST and/or ALT elevations at ≥1.25× ULN and <2.5×ULN at study entry.
The presence of fibrosis at baseline was assessed using the AST to Platelet Ratio Index (APRI score) calculated using the AST and platelet parameters at baseline. We used an APRI score >1.5 to indicate the presence of significant fibrosis or cirrhosis.
Other variables
Adherence to isoniazid was defined as the number of visits with 100% adherence divided by the number of visits with available adherence assessments over all visit weeks up to week 24.
Statistical analysis
Univariate logistic regression was used to examine the pre-treatment variables as possible risk factors for hepatotoxicity occurring within 24 weeks of entry into the study. All predictor variables listed above were considered for inclusion in a multivariate logistic regression, with variables selected using the stepwise method. P-values for inclusion and removal of a variable were set at 0.05. The time from start of ART and IPT to occurrence of hepatotoxicity was estimated by the Kaplan-Meier method. All analyses were performed using SAS version 9.4.
RESULTS
A total of 426 participants were randomized to the IPT arm in the REMEMBER trial; 77% were from sub-Saharan Africa; most were black African or of black African origin (Table 1). Half of the participants were male (226/426); the median age of the participants was 35 years (inter-quartile range [IQR] 30–42 years). The participants had advanced HIV disease with very low CD4 cell counts (median CD4 count 19 cells/µL; IQR 9–33 cells/µL). Among the study participants, 23% had raised pretreatment AST/ALT, 6% had HBsAg sero-positivity (HIV/HBV co-infection) and 3% had an APRI score >1.5 indicating the presence of significant fibrosis or cirrhosis at baseline. Most of the study participants 413/426 (97%) were on TDF/FTC including all participants who had HBsAg sero-positivity (data not shown in the table).
Table 1.
Baseline characteristics of participants started on isoniazid preventive therapy in the REMEMBER trial (N=426)
| Characteristics | n (%) or median (IQR)* |
|---|---|
| Age (years) | 35 (30–42) |
| Sex | |
| Male | 226 (53%) |
| Female | 200 (47%) |
| Race | |
| Black African or black of African origin | 388 (91%) |
| Other | 38 (9%) |
| Country | |
| Malawi | 98 (23%) |
| South Africa | 90 (21%) |
| Kenya | 75 (18%) |
| Haiti | 56 (13%) |
| Zimbabwe | 27 (6%) |
| Uganda | 24 (6%) |
| Peru | 19 (4%) |
| Zambia | 19 (4%) |
| India | 12 (3%) |
| Brazil | 6 (1%) |
| Pretreatment AST/ALT† | |
| Normal | 327 (77%) |
| Raised | 99 (23%) |
| Hepatitis B surface antigen‡ | |
| Negative | 384 (94%) |
| Positive | 26 (6%) |
| APRI score§ | |
| <=1.5 | 412 (97%) |
| >1.5 | 12 (3%) |
| Karnofsky Score | |
| 80–100 | 371 (87%) |
| 0–70 | 55 (13%) |
| CD4 cell count (cells per μL) | 19.0 (9–33) |
| Hemoglobin (g/dL) | 11.3 (10.2–12.6) |
| HIV-1 RNA (log10 copies per mL) | 5.3 (4·9–5·7) |
| Hospital admission within the past 30 days | 23 (5%) |
| Body-mass index <18·5 kg/m2 | 128 (30%) |
| Alcohol intake | |
| Never | 214 (50%) |
| Takes alcohol | 33 (8%) |
| No answer | 179 (42%) |
IQR = Interquartile range
Raised pretreatment AST/ALT defined as AST and/or ALT elevations at ≥1.25× ULN and <2.5×ULN at study entry.
16 values were missing
2 values were missing
During 24 weeks of follow up, 31 of 426 participants (7.3%) developed hepatotoxicity (Fig.1). 20/426 participants (4.7%) discontinued IPT after hepatotoxicity occurred; four subsequently restarted IPT following resolution of hepatotoxicity. At the site investigator’s discretion, eleven participants did not discontinue IPT when hepatotoxicity occurred. In some of these cases, the hepatotoxicity had changed to < Grade 3 by the time liver function tests were repeated and IPT was continued. 10/426 participants (2.4%) temporarily discontinued ART after hepatotoxicity occurred. All later resumed ART− three had modifications of their ART regimens while the rest resumed the same ART regimen they were on at the time of hepatotoxicity. Twenty-one participants did not have an interruption in their ART after hepatotoxicity occurred and they continued with the same regimen without modification. Three participants were hospitalized due to hepatotoxicity. Two participants who had hepatotoxicity died from TB while one died from hairy cell leukemia. These deaths were attributed to the TB and hairy cell leukemia.
Figure 1.
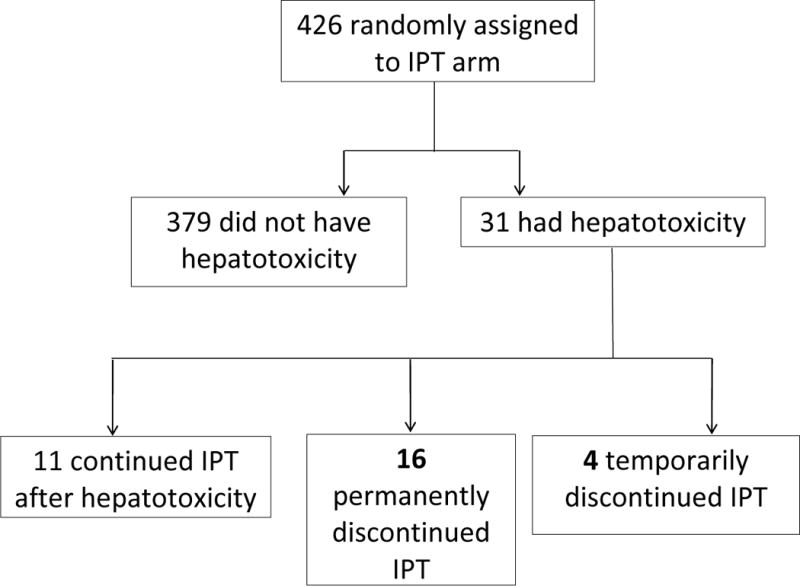
Occurrence of hepatotoxicity in the IPT arm in the REMEMBER trial
During follow up, 170/426 participants (39.9%) reported less than 100% adherence to IPT at their study visits. There was no difference in adherence to IPT between those who developed hepatotoxicity and those without hepatotoxicity (59.5% vs 67.7%; p-value 0.37) (data not in tables).
In univariate logistic regression analysis, raised pretreatment AST/ALT (OR 4.0, 95% CI 1.9-8.4) and HBsAg sero-positivity at baseline (n=410; OR 5.8, 95% CI 2.2-15.2) were significantly associated with increased risk of hepatotoxicity (Table 2).
Table 2.
Univariate odds ratios for having hepatotoxicity in the isoniazid preventive therapy arm within 24 weeks of follow up in the REMEMBER trial (N=426)
| Hepatotoxicity | Odds Ratio | 95% CI | P-value | ||
|---|---|---|---|---|---|
| Yes n=31 |
No n=395 |
||||
| Cd4 cell count (cells per µL) | |||||
| <25 | 16 (6%) | 241 (94%) | 1 | ||
| 25–50 | 15 (9%) | 154 (91%) | 1.5 | 0.7–3.1 | 0.31 |
| HIV-1 RNA (copies per mL) * | |||||
| <100,000 | 9 (7%) | 122 (93%) | 1 | ||
| 100,000–500,000 | 13 (7%) | 178 (93%) | 1.0 | 0.4–2.4 | 0.98 |
| >500,000 | 9 (9%) | 93 (91%) | 1.3 | 0.5–3.4 | 0.58 |
| Karnofsky Score | |||||
| 80–100 | 24 (7%) | 347 (94%) | 1 | ||
| 0–70 | 7 (13%) | 48 (87%) | 2.1 | 0.9–5.2 | 0.10 |
| Body mass index (kg/m2) | |||||
| ≥18.5 | 26 (9%) | 272 (91%) | 1 | ||
| <18.5 | 5 (4%) | 123 (96%) | 0.4 | 0.2–1.1 | 0.09 |
| Hospital admission within 30 days prior to study entry | |||||
| No | 28 (7%) | 375 (93%) | 1 | ||
| Yes | 3 (13%) | 20 (87%) | 2.0 | 0.6–7.2 | 0.28 |
| Age (years) | |||||
| ≤35 | 19 (9%) | 203 (91%) | 1 | ||
| >35 | 12 (6%) | 192 (94%) | 0.7 | 0.3–1.4 | 0.29 |
| Sex | |||||
| Male | 16 (7%) | 210 (93%) | 1 | ||
| Female | 15 (8%) | 185 (93%) | 1.1 | 0.5–2.2 | 0.87 |
| Hepatitis B surface antigen† | |||||
| Negative | 23 (6%) | 361 (94%) | 1 | ||
| Positive | 7 (27%) | 19 (73%) | 5.8 | 2.2–15.2 | <0.001 |
| Anemia | |||||
| No anemia | 7 (7%) | 98 (93%) | 1 | ||
| Anemia | 24 (8%) | 297 (93%) | 1.1 | 0.5–2.7 | 0.80 |
| Pretreatment AST/ALT‡ | |||||
| Normal | 15 (5%) | 312 (95%) | 1 | ||
| Raised | 16 (16%) | 83 (84%) | 4.0 | 1.9–8.4 | <0.001 |
| APRI score§ | |||||
| <=1.5 | 29 (7%) | 383 (93%) | 1 | ||
| >1.5 | 1 (8%) | 11 (92%) | 1.2 | 0.1–6.6 | 0.86 |
| Alcohol intake | |||||
| Never | 17 (8%) | 197 (92%) | 1 | ||
| Takes alcohol | 4 (12%) | 29 (88%) | 1.6 | 0.5–5.1 | 0.4 |
| No answer | 10 (6) | 169 (94%) | 0.7 | 0.3–1.5 | 0.36 |
2 results were not obtained
16 results were not obtained
Raised pretreatment AST/ALT defined as AST and/or ALT elevations at ≥1.25× ULN and <2.5×ULN at study entry.
2 values were missing
Using multivariate logistic regression, raised pretreatment AST/ALT (OR 3.6, 95% CI 1.7-7.7) and HBsAg sero-positivity at baseline (OR 4.7, 95% CI 1.7-12.9) remained as the statistically significant predictors for increased risk of hepatotoxicity (Table 3A). The other baseline factors including the presence of significant fibrosis at baseline as indicated by the APRI scores at baseline were not associated with increased risk of hepatotoxicity. We further examined the interplay between raised pretreatment AST/ALT and HBsAg sero-positivity at baseline by fitting a univariate model of a combined three-level variable comprised of these two variables. The categories within this variable were defined as: none (normal AST/ALT and HBsAg negative), raised AST/ALT or HBsAg positive, and both (raised AST/ALT and HBsAg positive) (Table 3B). The incidence of hepatotoxicity was 5/11 (45%) in participants with both raised pretreatment AST/ALT and HBsAg sero-positivity while it was 12/299 (4%) in those with normal pretreatment AST/ALT and HBsAg negative at baseline.
Table 3.
| A. Predictors of hepatotoxicity using multivariate logistic regression (N=410)*
| ||||||
|---|---|---|---|---|---|---|
| Characteristics | Hepatotoxicity | Odds Ratio | 95% CI | P-value | ||
| Yes (n=30) | No (n=380) | |||||
| Hepatitis B surface antigen | ||||||
| Negative | 23 (6%) | 361 (94%) | 1 | |||
| Positive | 7 (27%) | 19 (73%) | 4.7 | 1.7–12.9 | 0.002 | |
| Pretreatment AST/ALT† | ||||||
| Normal | 15 (5%) | 312 (95%) | 1 | |||
| Raised | 16 (16%) | 83 (84%) | 3.6 | 1.7–7.7 | 0.001 | |
|
B. Univariate logistic regression for the combined variable for raised pretreatment AST/ALT and HBsAg positive at baseline (N=410)* | ||||||
| Neither raised AST/ALT nor HBsAg positive | 12 (4%) | 287 (96%) | 1 | |||
| Raised AST/ALT only or HBsAg positive only | 13 (13%) | 87 (87%) | 3.4 | 1.5–7.3 | 0.003 | |
| Both raised AST/ALT and HBsAg positive | 5 (45%) | 6 (55%) | 19.9 | 5.3–74.3 | <0.001 | |
16 results were not obtained
Participants with both raised pretreatment AST/ALT and HBsAg sero-positivity had the highest risk of developing hepatotoxicity against those who had none of these conditions (OR 19.9, 95% CI 5.3-74.3). Those who had raised AST/ALT or HBsAg sero-positivity also had a high risk of developing hepatotoxicity (OR 3.4, 95% CI 1.5-7.3). There were differences in the time to occurrence of hepatotoxicity by the pretreatment AST/ALT and HBsAg status over 24 weeks of study follow up (Fig.2A).
Figure 2.

(A) Time to hepatotoxicity during IPT and ART by pretreatment AST/ALT and HbsAg status. (B) Cross-tabulation showing the distribution of hepatotoxicity events by pretreatment AST/ALT and HBsAg status
The median time to hepatotoxicity in all those who had hepatotoxicity was 9.9 weeks (IQR 4-16 weeks). Earlier hepatotoxicity cases occurred among participants with raised pretreatment AST/ALT 6.4 weeks (IQR 3.8-12.3 weeks) whereas other cases were distributed more evenly over the 24 weeks. Five cases of hepatotoxicity in participants with HBsAg sero-positivity occurred within 12 weeks (3 months) of starting IPT and ART. The other 2 cases occurred at 19 weeks and 24 weeks of starting IPT and ART.
DISCUSSION
We report a high incidence of hepatotoxicity in a severely immunosuppressed cohort on IPT and ART. Hepatotoxicity resulted in an IPT interruption rate of 4.7% and ART interruption rate of 2.4%. HepahhRaised pretreatment AST/ALT and HBsAg sero-positivity at baseline were associated with developing hepatotoxicity.
The incidence of hepatotoxicity in this study was higher than in other studies where IPT and ART were started at the same time. These other studies reported lower rates of hepatotoxicity: similar to rates in HIV-uninfected populations(13,20). In contrast with our study, these studies involved participants with higher CD4 cell counts (12,13). Thus our finding suggests that there are higher rates of hepatotoxicity during highly advanced HIV than at higher CD4 cell counts which is consistent with previous studies (14,21). However it is difficult to make direct comparisons of hepatotoxicity rates between studies due to differences in the definition of hepatotoxicity used (22). The level of elevated liver function tests used to define hepatotoxicity ranges from >2 to >10 × ULN in various studies. In this study, we used a high threshold of definition at >5 × ULN and we still found a high incidence of hepatotoxicity.
The high incidence of hepatotoxicity in this study is likely multi-factorial. Firstly, the population was severely immunosuppressed with a median CD4 cell count of 19 cells/µL. Advanced HIV disease is a risk factor for drug toxicities including hepatotoxicity (14,21). Opportunistic infections with hepatic involvement during advanced HIV disease such as, mycobacterium avium complex, invasive bacterial diseases and fungal infections may cause increased susceptibility to hepatotoxicity (23). Another explanation is that during advanced HIV primary manifestation of HIV in the liver increases susceptibility to hepatotoxicity (21,24). People with advanced HIV are also likely to have other risk factors for hepatotoxicity i.e. malnutrition with low BMI and hypoalbuminemia (10). In this study low BMI was not associated with hepatotoxicity and we did not evaluate hypoalbuminemia as a risk factor for hepatotoxicity.
The WHO does not require laboratory monitoring for hepatotoxicity during IPT (8). However based on our findings, people with advanced HIV should have targeted monitoring with either laboratory or clinical monitoring for signs and symptoms of liver disease. Clinical monitoring is as effective as laboratory monitoring and can be useful in resource limited settings without laboratory resources (9,12).
Since combining IPT and ART is associated with more hepatotoxicity than when IPT or ART are used alone, some studies suggest that these two interventions should not be started at the same time (11,12,17). In our study, IPT was initiated at the same time as ART. Because there was no placebo group, we cannot comment on the excess toxicity attributable to the IPT.
Our study included some participants who had modestly raised AST and ALT (≤2.5×ULN) and/or were HBsAg positive before starting IPT and ART. These are known risk factors for hepatotoxicity (23,25). Other studies exclude participants with raised liver function tests and HBsAg sero-positivity at baseline (13). As expected, the incidence of hepatotoxicity in those with both raised pretreatment AST/ALT and HBsAg sero-positivity was high at 46%. In comparison, we observed a lower rate of hepatotoxicity (4%) in the participants who had normal AST/ALT and who were HBsAg negative. This lower rate is still higher than the other similar studies where participants taking IPT and ART had higher CD4 cell counts (13). People with severe immunosuppression should be considered for baseline evaluation for liver function and HBsAg status and have targeted monitoring to monitor for hepatotoxicity. Most health care providers in resource limited settings are not able to test for liver function and HBsAg routinely. However when such routine testing is available, it should be done.
In our study participants, HIV/HBV coinfection was at 6% which is within expected rates of HIV/HBV co-infection that are reported globally at 5-20% (26). HIV infection modifies the course of HBV infection by decreasing HBV clearance and increasing the risk of chronic HBV. In turn, chronic HBV complicates treatment of HIV by increasing the risk of ART-related hepatotoxicity and by causing HBV-IRIS (27,28). Death and other liver morbidities including fibrosis, cirrhosis and hepatocellular carcinoma are increased in co-infected individuals (29). All 26 HIV/HBV co-infected participants in our study were on TDF/FTC, which has dual anti-HIV and anti-HBV activity and is the preferred regiment for treating HIV/HBV co-infection (30,31).
Among study participants who had HIV/HBV coinfection, 7/26 (27%) developed hepatotoxicity. An important cause of increased LFTs in these participants is HBV immune reconstitution syndrome (HBV-IRIS): an early complication of ART in individuals with HIV/HBV co-infection. During HBV-IRIS, there is a significant rise in serum transaminases (a hepatitis flare) characteristic of acute hepatitis even while using an ART regimen with anti-HBV activity such as TDF/FTC as was the case in our study (28,32). HBV-IRIS is observed in 20-25% of patients with HBV/HIV coinfection and typically occurs within 3 months of starting ART although atypical cases have been reported at more than 3 months after starting ART(33,34). The timing of hepatotoxicity in 5/7 of HIV/HBV co-infected participants in our study is consistent with the timing expected for HBV-IRIS. The other 2 cases of hepatotoxicity occurred at 19 weeks and 24 weeks which could still be an atypical presentation of HBV-IRIS (33). Other than the timing of hepatotoxicity, there was no other way of differentiating IRIS from hepatotoxicity caused by isoniazid or ART. Similar to our study findings, Avihingsanon et al reported an incidence of HBV-IRIS of 22% in participants who had HIV/HBV co-infection; were severely immunosuppressed and who started ART but not IPT (33).
Before initiating treatment in participants with HBV infection, baseline tests should be done to stage HBV disease (i. e. HBeAg, Anti-HBe, HBV DNA) and to assess pre-existing liver disease and fibrosis (i.e. liver transaminases, bilirubin, aspartate transaminase-to-platelet ratio index (APRI), fibrosis index based on four factors (FIB-4), ultrasound elastography and liver biopsy) (35). Liver biopsy is the gold standard for diagnosing and staging fibrosis. However it is too invasive (36). Instead, noninvasive tests such as elastography and serum markers (APRI and FIB-4) are validated to assess liver fibrosis in HIV-infected and HIV/HBV co-infected individuals. These tests can be used where liver biopsy is unavailable or not necessary (31,36). APRI and FIB-4 tests have lower costs, do not require particular expertise to interpret and can be used in an outpatient setting. APRI is the preferred test in resource-limited settings (31). Despite that fibrosis at baseline was not predictive of hepatotoxicity in our study, the recommendation is that liver fibrosis should be monitored at least annually in individuals during treatment of HBV because it is an important risk factor for hepatotoxicity (31).
Treating HIV/HBV co-infected individuals requires special considerations of the risk hepatotoxicity in this group. Following assessment of HBV disease and liver fibrosis, HBV carriers (those who are HBsAg positive, HBeAg negative, have low HBV DNA levels and normal ALT) are not at higher risk of developing hepatotoxicity (35). These individuals could start ART and IPT with monitoring of hepatotoxicity (31). As extra precaution, ART can be started weeks earlier to suppress the HBV DNA levels before introducing IPT after checking for serum transaminases. However, those individuals with active HBV and advance liver fibrosis would probably not tolerate ART and IPT and should be excluded from taking IPT. ART should still be started regardless of advanced liver disease (31).
Hepatotoxicity causes treatment interruption. With a high incidence of hepatotoxicity in this study, the rates of IPT and ART interruption due to hepatotoxicity were expectedly high. IPT was interrupted in 4.7% of the participants and ART in 2.8% due to hepatotoxicity. The rate for IPT interruption is higher than expected compared to other studies where IPT and ART were used concurrently. For example Rangaka et al reported a rate of 2.2% with concurrent IPT and ART use (20). Treatment interruption can lead to suboptimal treatment options for both IPT and ART by compromising the effectiveness at preventing TB and treating HIV (22).
Other TB prophylactic therapy regimens with different toxicity profiles are available. Compared with the 6 months isoniazid regimen used in this study, regimens using longer durations of isoniazid are associated with more drug toxicities (11,37,38) . Therefore, these regimens might be unsuited for use during advanced HIV. Intermittent use of isoniazid plus rifapentine over 12 weeks is as effective as isoniazid alone at preventing TB and also has less hepatotoxicity and higher treatment-completion rates in PLHIV (8,37,39). With its lower toxicity profile, this regimen is an ideal alternative for use in people with advanced HIV. However, the WHO also cautions on use of this regimen with ART due to concerns of potential drug-to-drug interactions (40).
Our analysis had the following limitations. Firstly, people with advanced HIV are usually taking other concomitant drugs including cotrimoxazole and fluconazole for opportunistic conditions and traditional medication. We could not analyze concomitant drugs taken at the time of hepatotoxicity even though these are risk factors for hepatotoxicity(25,41). Secondly, it is difficult to attribute the cause of hepatotoxicity to a particular drug. However, since our interest was to find the overall incidence of hepatotoxicity and its risk factors, our approach was pragmatic considering the variety of concomitant drugs that would have been used by study participants. Finally, while this was a large cohort, only 5 of the 11 participants that had both raised AST and ALT and HBsAg positivity at baseline developed hepatotoxicity While this is a significant result, there is limited precision in that measurement.
In conclusion, we report a high incidence of hepatotoxicity in people with advanced HIV taking IPT and ART. Those with raised AST/ALT or with HBsAg sero-positivity at baseline need closer monitoring for hepatotoxicity. There is need for further research on alternative TB preventive regimens that are less hepatotoxic in people with advanced HIV.
Acknowledgments
We would like to acknowledge the contribution of all participants and participating sites in the study. A5274 was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award numbers UM1 AI068634, UM1 AI068636, and UM1 AI106701 and also supported by National Institute of Mental Health (NIMH), National Institute of Dental and Craniofacial Research (NIDCR) through the AIDS Clinical Trials Group (ACTG). The primary author was supported by the UM1 AI068634 grant (ACTG-SDAC Internship program at the Center for Biostatistics AIDS Research). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Pharmaceutical support was provided by Gilead Sciences, but Gilead had no influence in the study design or the analysis of the data.
Source of Funding
A5274 was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award numbers UM1 AI068634, UM1 AI068636, and UM1 AI106701 and also supported by National Institute of Mental Health (NIMH), National Institute of Dental and Craniofacial Research (NIDCR). The primary author was supported by the ACTG-SDAC Internship program at the Center for Biostatistics AIDS Research under award number UM1 AI068634.
Footnotes
Contribultors: MN did the literature search. MN, SM, XS, JAL analyzed the data and generated the figures. MN, SM, MDH, XS, JAL, TST, interpreted the data. MN, SM, MDH, MCH drafted the manuscript. MN, SM, XS, TST, JAL, MN, KK, JK, AG, GBP, MDH, MCH revised the manuscript and contributed intellectually.
Presented at the Conference of Retroviruses and Opportunistic Infections (CROI) 2017, Seattle, 14 February 2017.
Conflict of Interest
The authors have no conflict of interest to declare.
References
- 1.Zumla A, Raviglione M, Hafner R, et al. Tuberculosis. N Engl J Med. 2013;268(8):745–55. doi: 10.1056/NEJMra1200894. [DOI] [PubMed] [Google Scholar]
- 2.WHO. Global tuberculosis report 2015. [Internet] Vol. 1. Geneva: 2015. Available from: http://www.who.int/tb/publications/global_report/en/ [Google Scholar]
- 3.WHO. Global tuberculosis report 2016. [Internet] Geneva: 2016. [cited 2017 May 11]. Available from: http://apps.who.int/iris/bitstream/10665/250441/1/9789241565394-eng.pdf?ua=1. [Google Scholar]
- 4.WHO. Report of a joint WHO HIV/AIDS and TB Department Meeting [Internet] Geneva: 2008. WHO three I’s meeting. Intensifi ed case fi nding (ICF), isoniazid preventive therapy (IPT) and TB infection control (IC) for people living with HIV. Available from: http://www.who.int/hiv/pub/meetingreports/WHO_3Is_meeting_report.pdf?ua=1. [Google Scholar]
- 5.WHO. Progress report 2009. World Health Organization; Geneva: 2009. Towards universal access: scaling up priority HIV/AIDS interventions in the health sector. [Google Scholar]
- 6.Golub JE, Saraceni V, Cavalcante SC, et al. The impact of antiretroviral therapy and isoniazid preventive therapy on tuberculosis incidence in HIV-infected patients in Rio de Janeiro, Brazil. AIDS. 2007;21(11):1441–8. doi: 10.1097/QAD.0b013e328216f441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dodd LE, Wilkinson RJ. Isoniazid preventive therapy in HIV infection. Lancet. 2011;377(9777):1548–50. doi: 10.1016/S0140-6736(11)60434-0. [DOI] [PubMed] [Google Scholar]
- 8.WHO. Guidelines for intensified tuberculosis case-finding and isoniazid preventive therapy for people living with HIV in resource-constrained settings [Internet] Geneva: 2011. Available from: http://www.who.int/hiv/pub/tb/9789241500708/en/ [Google Scholar]
- 9.Akolo C, Adetifa I, Shepperd S, et al. Treatment of latent tuberculosis infection in HIV infected persons. Cochrane Database Syst Rev [Internet] 2010;(1) doi: 10.1002/14651858.CD000171.pub3. Available from: http://doi.wiley.com/10.1002/14651858.CD000171.pub2. [DOI] [PMC free article] [PubMed]
- 10.Jussi J, David L, Robert M. An official ATS Statement: Hepatotoxicity of antituberculosis therapy. Am J Respir Crit Care Med [Internet] 2006;174(8):935–52. doi: 10.1164/rccm.200510-1666ST. Available from: www.thoracic.org/statements/resources/mtpi/hepatotoxicity-of-antitub. [DOI] [PubMed] [Google Scholar]
- 11.Ayele HT, Van Mourik MSM, Debray TPA, et al. Isoniazid prophylactic therapy for the prevention of tuberculosis in HIV infected adults: A systematic review and meta-analysis of randomized trials. PLoS One [Internet] 2015;10(11):1–16. doi: 10.1371/journal.pone.0142290. Available from: http://dx.doi.org/10.1371/journal.pone.0142290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Griensven J, Choun K, Chim B, et al. Implementation of isoniazid preventive therapy in an HIV clinic in Cambodia: High rates of discontinuation when combined with antiretroviral therapy. Trop Med Int Heal. 2015;20(12):1823–31. doi: 10.1111/tmi.12609. [DOI] [PubMed] [Google Scholar]
- 13.Tedla Z, Nyirenda S, Peeler C, et al. Isoniazid-associated Hepatitis and Antiretroviral Drugs during Tuberculosis Prophylaxis in HIV-infected Adults in Botswana. Am J Respir Crit Care Med. 2010;182:278–85. doi: 10.1164/rccm.200911-1783OC. [DOI] [PubMed] [Google Scholar]
- 14.Mosimaneotsile B, Mathoma A, Chengeta B, et al. Isoniazid Tuberculosis Preventive Therapy in HIV-Infected Adults Accessing Antiretroviral Therapy: A Botswana Experience. J Acquir Immune Defic Syndr. 2010;54:71–7. doi: 10.1097/QAI.0b013e3181c3cbf0. [DOI] [PubMed] [Google Scholar]
- 15.Lawn SD, Badri M, Wood R, et al. Tuberculosis among HIV-infected patients receiving HAART : long term incidence and risk factors in a South African cohort. AIDS. 2005 Aug;19:2109–16. doi: 10.1097/01.aids.0000194808.20035.c1. [DOI] [PubMed] [Google Scholar]
- 16.Gupta A, Nadkarni G, Yang W-T, et al. Early mortality in adults initiating antiretroviral therapy (ART) in low- and middle-income countries (LMIC): a systematic review and meta-analysis. PLoS One [Internet] 2011;6(12):e28691. doi: 10.1371/journal.pone.0028691. Available from: http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0028691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lawn SD, Wood R, De Cock KM, et al. Antiretrovirals and isoniazid preventive therapy in the prevention of HIV-associated tuberculosis in settings with limited health-care resources. Lancet Infect Dis. 2010;10(7):489–98. doi: 10.1016/S1473-3099(10)70078-5. [DOI] [PubMed] [Google Scholar]
- 18.Hosseinipour MC, Bisson GP, Miyahara S, et al. Empirical tuberculosis therapy versus isoniazid in adult outpatients with advanced HIV initiating antiretroviral therapy (REMEMBER): A multicountry open-label randomised controlled trial. Lancet. 2016;387(10024):1198–209. doi: 10.1016/S0140-6736(16)00546-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.U.S. Department of Health and Human Services, National Institutes of Health, National Institute of Allergy and Infectious Diseases D of A. The Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Event version 1.0 [Updated August 2009] [cited 2017 Apr 3]; Available from: https://rsc.tech-res.com/docs/default-source/safety/table_for_grading_severity_of_adult_pediatric_adverse_events.pdf?sfvrsn=6.
- 20.Rangaka MX, Wilkinson RJ, Boulle A, et al. Isoniazid plus antiretroviral therapy to prevent tuberculosis: a randomised double-blind, placebo-controlled trial. Lancet (London, England) [Internet] 2014;384(9944):682–90. doi: 10.1016/S0140-6736(14)60162-8. Available from: http://www.sciencedirect.com/science/article/pii/S0140673614601628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Towner WJ, Xu L, Leyden WA, et al. The Effect of HIV Infection, Immunodeficiency, and Antiretroviral Therapy on the Risk of Hepatic Dysfunction. J Acquir Immune Defic Syndr. 2012;60(3):321–7. doi: 10.1097/QAI.0b013e31824e9ef2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coca NSM, Oliveira SM, Voieta I, et al. Antituberculosis drug-induced hepatotoxicity : a comparison between patients with and without human immunodeficiency virus seropositivity. Rev Soc Bras Med Trop. 2010;43(6):624–8. doi: 10.1590/s0037-86822010000600004. [DOI] [PubMed] [Google Scholar]
- 23.Price JC, Thio CL. NIH Public Access. Clin Gastroenterol Hepatol. 2010;8(12):1002–12. doi: 10.1016/j.cgh.2010.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ansari N, Kombe A, Kenyon T, et al. Pathology and causes of death in a group of 128 HIV-positive patients in Boswana, 1997-1998. Int J Tuberc Lung Dis. 2002;6(1):55–63. [PubMed] [Google Scholar]
- 25.Hoffmann CJ, Charalambous S, Thio CL, et al. Hepatotoxicity in an African antiretroviral therapy cohort: the effect of tuberculosis and hepatitis B. AIDS [Internet] 2007 Jun;21(10):1301–8. doi: 10.1097/QAD.0b013e32814e6b08. [cited 2017 Jul 30] Available from: http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00002030-200706190-00009. [DOI] [PubMed] [Google Scholar]
- 26.UNAIDS. Global Update AIDS 2016. 2016 [cited 2017 Dec 4]; Available from: http://www.unaids.org/sites/default/files/media_asset/global-AIDS-update-2016_en.pdf.
- 27.Konopnicki D, Mocroft A, de Wit S, et al. Hepatitis B and HIV: prevalence, AIDS progression, response to highly active antiretroviral therapy and increased mortality in the EuroSIDA cohort. AIDS [Internet] 2005 Mar 24;19(6):593–601. doi: 10.1097/01.aids.0000163936.99401.fe. [cited 2017 Dec 4] Available from: http://www.ncbi.nlm.nih.gov/pubmed/15802978. [DOI] [PubMed] [Google Scholar]
- 28.Drake A, Mijch A, Sasadeusz J. Immune Reconstitution Hepatitis in HIV and Hepatitis B Coinfection, Despite Lamivudine Therapy as Part of HAART. Clin Infect Dis. 2004;39:129–32. doi: 10.1086/421386. [DOI] [PubMed] [Google Scholar]
- 29.Joshi D, O’Grady J, Dieterich D, et al. Increasing burden of liver disease in patients with HIV infection. Lancet (London, England) [Internet] 2011 Apr 2;377(9772):1198–209. doi: 10.1016/S0140-6736(10)62001-6. [cited 2017 Dec 6] Available from: http://www.ncbi.nlm.nih.gov/pubmed/21459211. [DOI] [PubMed] [Google Scholar]
- 30.Matthews GV, Avihingsanon A, Lewin SR, et al. A randomized trial of combination hepatitis B therapy in HIV/HBV coinfected antiretroviral naïve individuals in Thailand. Hepatology [Internet] 2008 Oct 1;48(4):1062–9. doi: 10.1002/hep.22462. [cited 2017 Dec 6] Available from: http://doi.wiley.com/10.1002/hep.22462. [DOI] [PubMed] [Google Scholar]
- 31.WHO. Guidelines for the prevention, care and treatment of persons with chronic Hepatitis B infection. 2015 [cited 2017 Dec 6]; Available from: http://apps.who.int/iris/bitstream/10665/154590/1/9789241549059_eng.pdf?ua=1&ua=1. [PubMed]
- 32.Crane M, Oliver B, Matthews G, et al. Immunopathogenesis of Hepatic Flare in HIV/Hepatitis B Virus (HBV)– Coinfected Individuals after the Initiation of HBV-Active Antiretroviral Therapy. J Infect Dis. 2009;199(7):974–81. doi: 10.1086/597276. [DOI] [PubMed] [Google Scholar]
- 33.Avihingsanon A, Matthews GV, Lewin SR, et al. Assessment of HBV flare in a randomized clinical trial in HIV/HBV coinfected subjects initiating HBV-active antiretroviral therapy in Thailand. AIDS Res Ther [Internet] 2012 Mar 9;9(1):6. doi: 10.1186/1742-6405-9-6. [cited 2017 Dec 4] Available from: http://www.ncbi.nlm.nih.gov/pubmed/22405335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andrade BB, Hullsiek KH, Boulware DR, et al. Biomarkers of inflammation and coagulation are associated with mortality and hepatitis flares in persons coinfected with HIV and hepatitis viruses. J Infect Dis [Internet] 2013 May 1;207(9):1379–88. doi: 10.1093/infdis/jit033. [cited 2017 Dec 4] Available from: http://www.ncbi.nlm.nih.gov/pubmed/23335804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patel N, Singh S. Antituberculosis therapy in patients with hepatitis B viral infection. Hepat B Annu [Internet] 2012;9(1):16. [cited 2017 Dec 6] Available from: http://www.hepatitisbannual.org/text.asp?2012/9/1/16/193287. [Google Scholar]
- 36.Terrault NA, Bzowej NH, Chang K-M, et al. AASLD Guidelines for Treatment of Chronic Hepatitis B Objectives and Guiding Principles. Hepatotology [Internet] 2016;63(1):261–83. doi: 10.1002/hep.28156. [cited 2017 Dec 4] Available from: https://www.aasld.org/sites/default/files/Terrault_et_al-2016-Hepatology.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stagg HR, Zenner D, Harris RJ, et al. Treatment of latent tuberculosis infection a network meta-analysis. Annals of Internal Medicine. 2014 doi: 10.7326/M14-1019. [DOI] [PubMed] [Google Scholar]
- 38.Martinson NA, Barnes GL, Moulton LH, et al. New Regimens to Prevent Tuberculosis in Adults with HIV Infection. N Eng J Med. 2011;365(1):11–20. doi: 10.1056/NEJMoa1005136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sterling TR, Villarino ME, Borisov AS, et al. Three Months of Rifapentine and Isoniazid for Latent Tuberculosis Infection. N Engl J Med. 2011;36523365(8):2155–66. doi: 10.1056/NEJMoa1104875. [DOI] [PubMed] [Google Scholar]
- 40.WHO. Guidelines on the management of latent tuberculosis infection [Internet] WHO Library Cataloguing-in-Publication Data. 2015 [cited 2017 Jul 30]. Available from: www.who.int/about/licensing/copyright_form/en/index.html.
- 41.Breen RAM, Miller RF, Gorsuch T, et al. Adverse events and treatment interruption in tuberculosis patients with and without HIV co-infection. Thorax [Internet] 2006 Sep;61(9):791–4. doi: 10.1136/thx.2006.058867. [cited 2017 Jun 24] Available from: http://www.ncbi.nlm.nih.gov/pubmed/16844730. [DOI] [PMC free article] [PubMed] [Google Scholar]


