Abstract
Objective(s)
This is the first clinical outcomes report of NRG Oncology RTOG 0539, detailing the primary endpoint, 3-year progression-free survival (3yPFS), compared to a predefined historical control for intermediate-risk meningioma, and secondarily evaluating overall survival (OS), local failure, and prospectively scored adverse events (AEs).
Methods
NRG Oncology RTOG 0539 was a phase II clinical trial allocating meningioma patients to 1 of 3 prognostic groups and management strategies according to WHO grade, recurrence status, and resection extent. For the intermediate-risk group (Group 2), eligible patients had either newly diagnosed WHO grade II meningioma with gross total resection (GTR, Simpson I-III) or recurrent WHO grade I of any resection extent. Pathology and imaging were centrally reviewed. Patients were treated with radiation therapy (RT), either intensity modulated (IMRT) or 3D conformal (3DCRT), 54 Gy in 30 fractions. The RT target volume was defined as the tumor bed and any nodular enhancement (e.g. recurrent WHO grade I patients) with a minimum 8 mm and maximum 15 mm margin, depending upon tumor locale and set-up reproducibility of RT method. The primary endpoint was 3yPFS. Results were compared to historical controls (3yPFS 70% following GTR alone and 90% with GTR + RT). AEs were scored using NCI Common Toxicity Criteria.
Results
Fifty-six patients enrolled in the intermediate-risk group; 3 were ineligible. Additionally, 1 did not receive RT, and 4 withdrew without recurrence before 3 years. Thus 52 patients received protocol therapy, and 48 were evaluable for the primary endpoint, 3y PFS which was 93.8% (p=.0003). Within 3 years there were 3 PFS events: 1 WHO grade II patient died of disease, 1 WHO grade II patient progressed and remained alive, and 1 recurrent WHO grade I patient died from undetermined cause without progression. Three-year local failure was 4.1%, and 3-year OS 96%. After 3 years 2 additional patients progressed: 1 recurrent WHO grade I, and the other WHO grade II; both remain alive. Among 52 evaluable patients who received protocol treatment, 36 (69.2%) were WHO II with GTR, and 16 (30.8%) recurrent WHO I. There was no significant difference in PFS between these subgroups (p=.52, HR 0.56, 95% CI 0.09 to 3.35), validating their consolidation. Of the 52 evaluable patients, 44 (84.6%) received IMRT, and 50 (96.2%) were treated per protocol or with acceptable variation. AEs (definitely, probably or possibly related to protocol treatment) were limited to grade 1 or 2, with no reported grade 3 events.
Conclusion
This is the first clinical outcomes report from NRG Oncology RTOG 0539. Patients with intermediate-risk meningioma treated with RT experienced excellent 3yPFS with a low rate of local failure, and a low risk of adverse events. These results support the use of post-operative RT for newly diagnosed gross totally resected WHO grade II, or recurrent WHO grade I meningioma irrespective of resection extent. They also document minimal toxicity and high rates of tumor control with IMRT.
This protocol (NRG Oncology RTOG 0539) is registered with ClinicalTrials.gov (https://clinicaltrials.gov/ct2/show/NCT00895622?term=RTOG+0539&rank=1). The ClinicalTrials.gov Identifier is NCT00895622.
Keywords: Meningioma, WHO Grade II (atypical), radiotherapy
Introduction
Treatment of patients with meningioma is most often based upon clinical judgment, personal experience, institutional tradition, and retrospective series, all in the absence of level 1 evidence. Practices have varied, and the establishment of a uniform approach has been hampered not only by the lack of prospective trials, but also, and rather importantly, by inconsistent grading criteria. In recent years, the latter has been addressed by the World Health Organization (WHO) with updated criteria in 2007 and 2016. Previous grading standards were not broadly accepted, but, based upon a recently published secondary endpoint analysis of pathology concordance from NRG Oncology RTOG 0539, the WHO 2000 and 2007 standards appear to have been broadly followed, at least among institutions enrolling patients on NRG Oncology RTOG cooperative group trials.42
Several cooperative group meningioma protocols have been launched, but have either met with disappointing results or have failed to reach accrual goals.10,21,25,27 The Southwest Oncology Group (SWOG-S9005) completed a phase III trial, published in 2015 by Ji and colleagues, assessing mifepristone, an antiprogestin. This study (SWOG-S9005) randomized patients with progressive or recurrent meningioma to receive either oral mifepristone or placebo, and found no improvement in either failure-free or overall survival with mifepristone.21
Regarding radiotherapy (RT), prior evidence has been limited to level IV or V.25 However, recently two phase II trials, this one from NRG Oncology RTOG and another from the EORTC (22042-26042), successfully completed accrual, and are undergoing analysis. This is the initial clinical outcomes report from NRG Oncology RTOG 0539, a phase II trial of observation for low-risk meningioma and of radiotherapy for intermediate and high-risk meningioma. The trial opened in June 2009, and closed ahead of schedule with full accrual in August 2012. The schema and enrollment data are depicted in Figure 1.
Figure 1. NRG Oncology RTOG 0539 Schema.
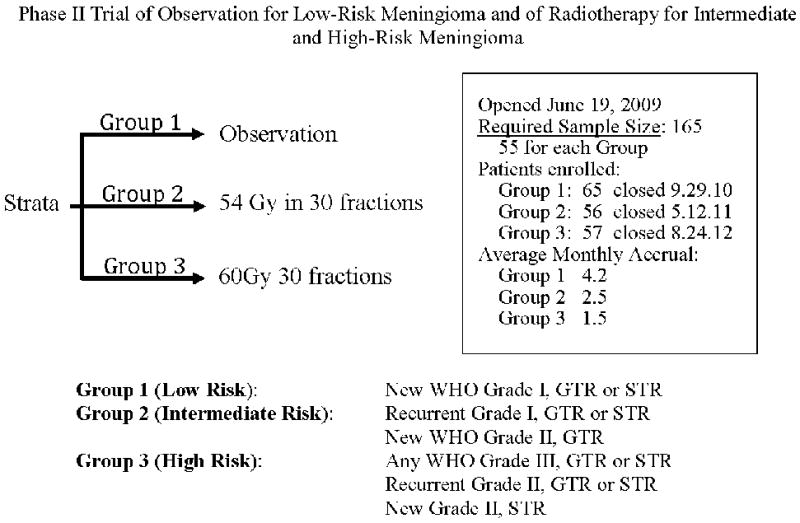
Protocol schema, accrual data, and subgroup definitions.
This report focuses on the intermediate-risk cohort (Group 2), comprised of patients with newly diagnosed WHO grade II meningioma after gross total resection (GTR), or recurrent WHO grade I meningioma, with or without resection of any extent. We address the study’s primary endpoint, 3-year progression-free survival (3yPFS), as well as the mature secondary endpoints of 3-year overall survival (3yOS) and acute and late adverse events (AEs).
Methods
Institutional Review Board (IRB) Approval, Consent, and Clinical Trial Registration
This cooperative group protocol was approved by the institutional review boards at each participating study site, and documentation was received at the Radiation Therapy Oncology Group (RTOG, now NRG Oncology) central office. Each patient signed an approved informed consent prior to trial enrollment. This protocol (NRG Oncology RTOG 0539) is registered with ClinicalTrials.gov (https://clinicaltrials.gov/ct2/show/NCT00895622?term=RTOG+0539&rank=1). The ClinicalTrials.gov Identifier is NCT00895622.
Selection criteria
Adults 18 years of age or older with a unifocal, histologically documented intracranial meningioma, with no prior history of cranial RT, with Zubrod performance status 0-1 and without severe, active comorbidity were eligible for enrollment. Histology, including WHO 2007 tumor grade and subtype, was confirmed for each patient via central pathology review by one of the authors (AP). Following central review, patients were partitioned according to specific criteria into three groupings: Group I (low risk), Group II (intermediate risk), and Group III (high risk), shown in Figure 1.
Protocol Registration
Registration took place in 2 steps. Step 1 was initial registration, followed by submission of pathology specimens for central review. Following central pathology review, step 2 registration entailed protocol group assignment, after which protocol-specified treatment began.
Tumor Grade and Resection Extent
This report pertains to patients assigned to the intermediate-risk group (Group 2), which includes patients with a newly diagnosed gross totally resected WHO grade II meningioma or a recurrent WHO grade I meningioma irrespective of the resection extent. Resection extent was classified using Simpson criteria,43 and was based upon the neurosurgeons’ assessment and post-operative magnetic resonance imaging (MRI) findings, also centrally reviewed (by Bruce Dean – see acknowledgement – and authors JM, or AA). Gross totally resected tumors included Simpson grades I-III.
For patients with a newly diagnosed WHO grade II meningioma, initial registration and central pathology review must have been completed within 24 weeks of surgery. This interval was designed to permit sufficient time for post-operative imaging at 3 to 4 months to confirm gross total resection. In the setting of a recurrent WHO grade I tumor there were no such constraints, and although additional resection or biopsy was encouraged, it was not a prerequisite. For patients with WHO grade I meningioma, recurrence or progression leading to eligibility for protocol enrollment was defined clinically and radiographically at the enrolling institution, with documentary magnetic resonance imaging (MRI) submission as a pre-requisite. No patient with a newly diagnosed WHO grade I meningioma, irrespective of resection grade, was enrolled within group 2. If further biopsy or resection was performed for recurrent tumor, submission of such specimens was mandated. The diagnosis of recurrence solely on the basis of imaging findings was permitted, but if no additional resection was performed, submission of specimens from the prior resection was required, and tumor grade centrally confirmed from those specimens.
Pre- and postoperative MRIs were required for each patient with a WHO grade II meningioma. For those with recurrent WHO grade I meningioma, pre- and postoperative MRIs were required if surgery was done; however, only the follow-up imaging documenting recurrence was needed if additional surgery was not undertaken.
Radiation Therapy
Every Group 2 patient received radiation therapy (RT). Three-dimensional conformal RT (3D-CRT), intensity modulated RT (IMRT), or proton therapy was permitted. The dose was 54 Gy in 30 fractions of 1.8 Gy each, delivered on consecutive weekdays. The gross tumor volume (GTV) was the tumor or resection bed for all Group 2 patients, plus any nodular enhancement in the recurrent/progressive WHO grade I subgroup. The GTV was determined on the basis of pre- and post-operative MRIs, Multiplanar T1 post-contrast and pre-contrast T1, T2, and FLAIR images were required. Neither cerebral edema nor the dural tail were included within the GTV, however, hyperostotic bone or directly invaded bone was included. The clinical target volume (CTV) was the GTV + 1 cm. It was permissible to reduce the CTV margin to 0.5 cm around natural barriers to tumor growth such as uninvolved skull. The planning target volume (PTV) was the CTV + 3 to 5 mm, depending upon the daily radiation therapy localization method and reproducibility. A planning risk volume (PRV) was defined for each organ at risk (OAR), being the OAR + 3 mm. OAR dose limits were defined in terms of point dose (>0.03cc): lenses 5 Gy, retinae 45 Gy, optic nerves 50 Gy, optic chiasm 54 Gy, and brainstem 55 Gy.
In concept, a PTV accounts for variations in set-up and reproducibility. Thus altering PTV margins to reduce OAR dose is generally not approved in cooperative group trials. However, in this trial and in the interest of diminishing side effect risk in a tumor for which the absolute benefit of RT has not been established with level 1 evidence, a risk-adaptive modification was permitted. In the event that an OAR was in immediate proximity to a PTV such that dose to the OAR could not be constrained within protocol limits, a second PTV (termed the PTVPRV), could be fashioned. The PTVPRV was defined as the overlap between the PTV and the particular PRV of concern. If this modification was undertaken, then it was mandated that dose to the PTVPRV be as close as permissible to 54 Gy while not exceeding the OAR dose limit. Figure 2 provides an example of the use of a PTVPRV. Target volumes and organs at risk were reviewed centrally, but this was accomplished after treatment completion.
Figure 2. Permissible PTV Modification to Limit PRV Dose (PTVPRV).
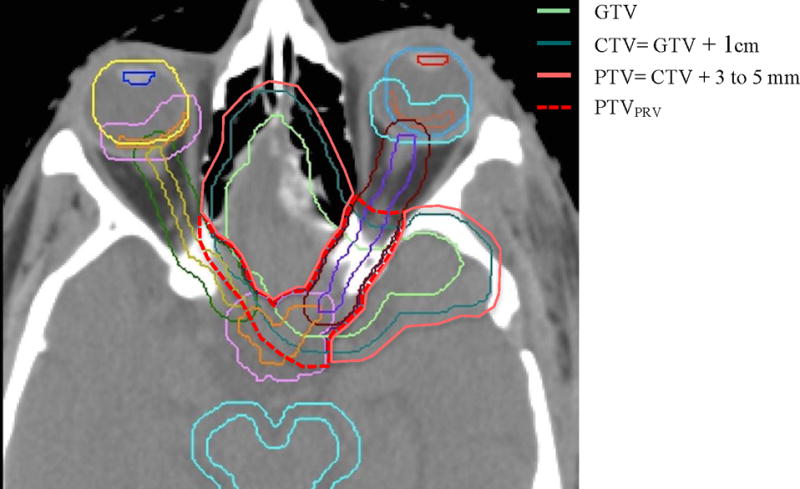
Planning CT with OAR, PRV, GTV, CTV, PTV, and PTVPRV contours in a patient whose WHO grade II meningioma surrounded the anterior optic apparatus. The PTV prescription dose was 54 Gy in 30 fractions. With this example, the PTVPRV prescription was lower in order to satisfy the optic nerve point dose constraint, while still prescribing 54 Gy to the larger PTV.
Patient Assessment
Pretreatment evaluation included a history and physical with neurological examination, documentation of steroid use and dose, documentation of other hormonal agents, and magnetic resonance imaging (MRI). Required MRI sequences were multiplanar T1 post-contrast and pre-contrast T1, T2, and FLAIR images. All patients were required to have had an MRI within 12 weeks prior to step 2 registration. Both preoperative and postoperative MRIs were required for all newly diagnosed patients. In the setting of recurrent or progressive meningioma without salvage surgery, MRI documentation of recurrence or progression was required. The determination of progression was at the discretion of the enrolling institution. Postoperative MRIs must have been completed within 12 weeks of surgery, although additional confirmatory imaging was permitted so long as initial registration and central pathology review were completed within 24 weeks of surgery.
Post-treatment clinical assessment was required at 1 month after RT, every 3 months for 3 years, then at least yearly for 10 years. Mini-Mental State Examination (MMSE) and documentation of corticosteroid and other hormonal agent use followed the same schedule. Response was evaluated according to criteria similar to RECIST (Response Evaluation Criteria in Solid Tumors), modified to better apply to meningioma. Continual no evidence of disease (CNED) was ascribed when there was no measurable meningioma, stable disease (SD) when measurable tumor remained unchanged or increased in maximum diameter by less than 20%, and progressive disease (PD) when tumor increased in any diameter by 20% or more. Neurologic progression was defined as new or progressive neurologic deficits without measurable growth; this was not observed in any patient. Adverse event (AE) evaluations and brain MRI were stipulated at 3 months post-RT, then at least every 6 months for 3 years, then at least yearly for 10 years.
Statistical Methodology
The primary endpoint of this phase II trial was to estimate the rate of progression-free survival at 3 years (3yPFS) after registration. For this initial evaluation we report the primary endpoint and secondary endpoints of 3yOS and AEs. The protocol opened to enrollment June 19, 2009. The full study closed August 24, 2012, but accrual to the intermediate-risk cohort (Group 2) was completed May 12, 2011 (see Figure 1). The analysis date for the present report was January 5, 2016. Findings regarding pathologic concordance have been published separately.42
For this intermediate-risk cohort, 3yPFS was estimated, based upon historical data, at 70% with GTR alone, and at 90% following GTR+RT.40 With a one-sided significance level of 0.05, a sample of 50 eligible patients would provide a statistical power of over 95% to detect the projected 20% absolute increase using a one-sample test on proportion, while providing a greater number of patients for the histopathologic and molecular correlative part of the study. Adjusting for a 10% ineligibility rate, the study required the accrual of 55 patients.
PFS was measured from the date of study entry to the date of progression or death, or otherwise the date of the last follow-up on which the patient was reported alive and progression-free. OS was measured from the date of study entry to the date of death, or otherwise the date of the last follow-up on which the patient was reported alive. PFS and OS were estimated using the Kaplan-Meier method. Time to tumor progression was calculated using the cumulative incidence function, with death without progression treated as the competing risk. The incidence rates of grade 2+ acute and late AEs for dermatology/skin, neurology, and ocular/visual (excluding alopecia), individually and combined, were reported for all eligible patients who received protocol treatment. Acute AEs were defined as AEs that occurred ≤90 days from start of radiation, and late AEs as those that occurred >90 days from start of radiation.
We hypothesized that IMRT would minimize treatment-related late toxicities on dermatology/skin, neurology, and ocular/visual compared with 3DCRT. Recognizing that there are no reports of prospectively collected AEs using NCI common toxicity criteria for meningioma treated with 3DCRT, we determined to test the hypothesis of reduced toxicities following IMRT by prospectively comparing the late AEs following IMRT on this study with those following 3DCRT from the low grade glioma patients on NRG Oncology/RTOG 0424, which used the same dose and fractionation and similar definitions of treatment volume. Although 3D-CRT was allowed for treatment of intermediate-risk patients, it was expected that 80% to 90% of the intermediate-risk patients would be treated with IMRT. With 40 to 45 IMRT-treated patients, only large differences could be detected with a sufficient power. Therefore, a reduction of 10% or more in the worst overall grade 2+ AEs would be considered as supporting the hypothesis.
Results
Patient Characteristics, Protocol Enrollment, and Treatment Delivery
The study was activated in June 2009, and accrual for the intermediate-risk group was completed in February 2011, one year ahead of projected schedule. Out of the 56 patients enrolled, 52 (92.9%) were eligible and treated with protocol-specified RT (Figure 3). Pretreatment and tumor characteristics for the eligible patients are listed in Table 1. Out of the 52 patients, 36 (69.2%) had a gross totally resected (GTR) WHO grade II meningioma, and 16 (30.8%) had a recurrent WHO grade I tumor. With the RT technologies permitted in this study, 44 patients (84.6%) received IMRT, 8 (15.4%) received 3D-CRT, and none were treated with proton therapy. This is the first NRG Oncology RTOG brain trial with protocol-specific IMRT parameters. The majority of the patients were treated per protocol or with acceptable variations. A PTVPRV was used in 19 patients, principally to limit dose to the optic apparatus. No statistical association was found between protocol adherence or use of the PTVPRV target definition option and progression risk.
Figure 3. NRG Oncology/RTOG/0539 Group 2 Enrollment.
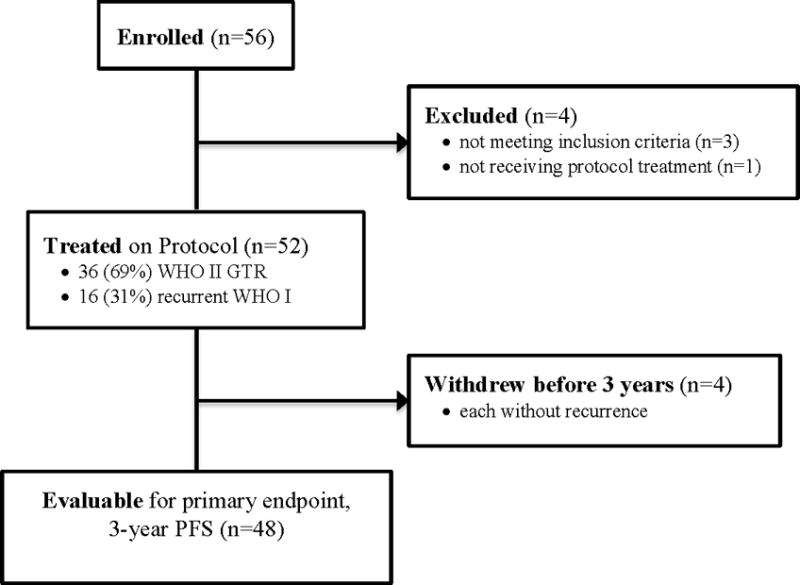
CONSORT diagram for the intermediate-risk group (Group 2).
Table 1. Patient and Tumor Characteristics.
Patient and tumor characteristics among 52 patients receiving protocol treatment. WHO grades are per central review. Simpson resection grades are per enrolling institution.
| Group II | ||
|---|---|---|
| n | % | |
| Age (years) | ||
| <50 | 20 | 38.5 |
| ≥ 50 | 32 | 61.5 |
| Gender | ||
| Male | 20 | 38.5 |
| Female | 32 | 61.5 |
| Race | ||
| American Indian/Alaska Native | 1 | 1.9 |
| Asian | 1 | 1.9 |
| Black or African American | 4 | 7.7 |
| Native Hawaiian or Other Pacific Islander | 0 | 0.0 |
| White | 44 | 84.6 |
| Unknown or not reported | 2 | 3.8 |
| Ethnicity | ||
| Hispanic or Latino | 4 | 7.7 |
| Not Hispanic or Latino | 45 | 86.5 |
| Unknown (Individuals not reporting ethnicity) | 3 | 5.8 |
| Pre-treatment Zubrod performance status | ||
| 0 | 39 | 75.0 |
| 1 | 13 | 25.0 |
| Pre-treatment Neurologic Function | ||
| No symptoms | 27 | 51.9 |
| Minor symptoms | 21 | 40.4 |
| Moderate symptoms | 4 | 7.7 |
| Status of Tumor | ||
| Initial diagnosis | 36 | 69.2 |
| Recurrent | 16 | 30.8 |
| Extent of Resection (Simpson Grade) | ||
| Initial - Grade I | 7 | 13.5 |
| Initial - Grade II | 17 | 32.7 |
| Initial - Grade III | 8 | 15.4 |
| Initial - Grade IV | 2 | 3.8 |
| Initial - unknown | 2 | 3.8 |
| Recurrent - Grade I | 0 | 0.0 |
| Recurrent - Grade II | 0 | 0.0 |
| Recurrent - Grade III | 0 | 0.0 |
| Recurrent - Grade IV | 3 | 5.8 |
| Recurrent - by imaging only | 13 | 25.0 |
| Histology | ||
| Initial - WHO grade I | 2 | 3.8 |
| Initial - WHO grade II | 32 | 61.5 |
| Initial - WHO grade III | 1 | 1.9 |
| Initial - unknown | 1 | 1.9 |
| Recurrent - WHO grade I | 2 | 3.8 |
| Recurrent - WHO grade II | 1 | 1.9 |
| Recurrent - WHO grade III | 0 | 0.0 |
| Recurrent - by imaging only | 13 | 25.0 |
| Lateralization of Tumor | ||
| Right | 20 | 38.5 |
| Left | 25 | 48.1 |
| Bilateral | 7 | 13.5 |
| Unknown | 0 | 0.0 |
|
| ||
| Total | 52 | 100.0 |
Progression-Free, Overall Survival & Local Failure
Median follow-up time for eligible patients still alive was 3.7 years, with a range from 0.4 to 4.9 years. For the 52 eligible patients who received protocol treatment, 4 (7.7%) withdrew less than 3 years after study entry without disease progression. Based on the 48 patients who were evaluable for the primary endpoint, 3yPFS was 93.8% (p-value = 0.0003). There was no difference in PFS between the intermediate-risk subgroups of WHO grade II with GTR and WHO grade I with recurrent/progressive meningioma (p-value=0.52, HR 0.56, 95% CI 0.09 to 3.35). Within 3 years, there were 3 PFS events: 1 WHO grade II patient who died of disease, 1 WHO grade II patient who progressed and remained alive, and 1 patient with recurrent WHO I meningioma who died of undetermined cause without progression. Neither median PFS nor median survival time was reached, with a 3yPFS of 93.8% and a 3yOS of 96.0%. The respective Kaplan-Meier PFS and OS curves are shown in Figures 4 and 5.
Figure 4.
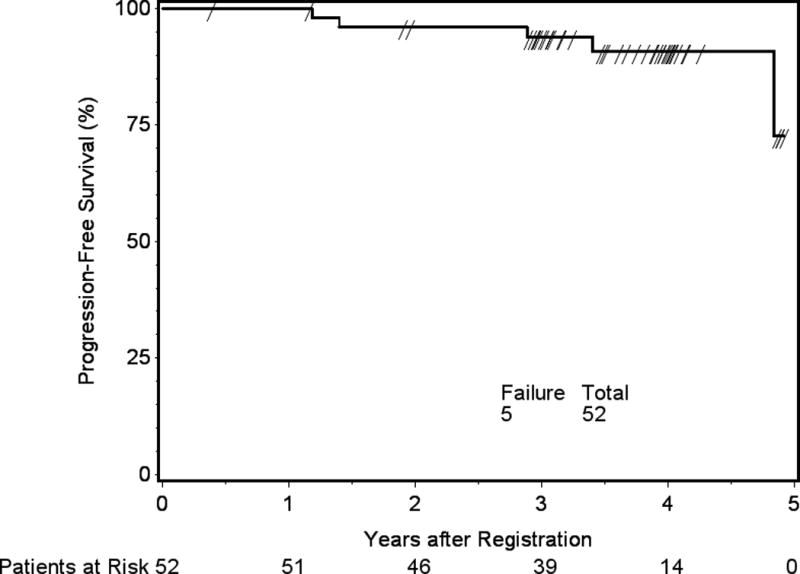
Progression-free survival (PFS), determined with progression and/or death as events. Three-year progression-free survival 93.8%, 95% confidence interval 87.2 to 100%.
Figure 5.
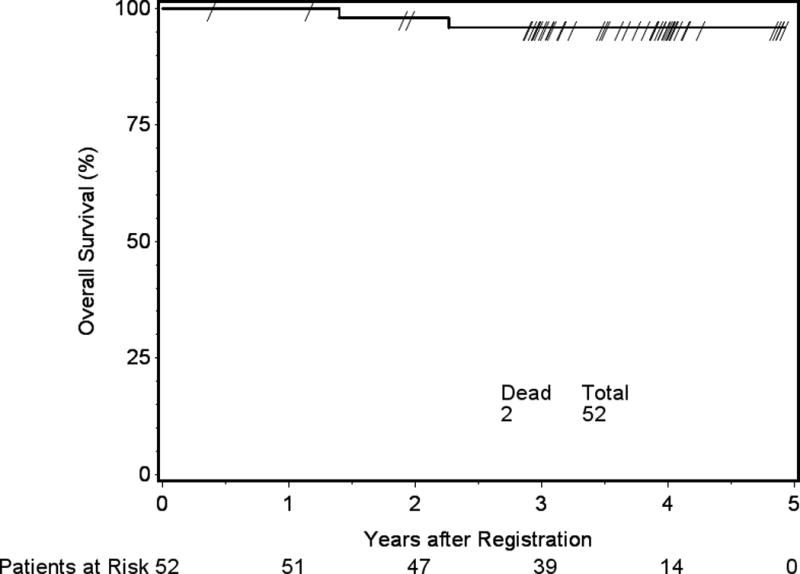
Overall survival (OS). Three-year overall survival 96%, 95% confidence interval 90.4 to 100%.
Two patients experienced local failure within 3 years, both WHO grade II following GTR. This corresponds to a 3y local failure rate of 4.1%. After 3 years, 2 additional patients experienced progression; one had a recurrent WHO grade I meningioma, and the other a newly diagnosed WHO grade II tumor. Both remain alive. The cumulative incidence curve for time to local failure is shown in Figure 6. Among the 4 patients with progression, 3 were treated with re-resection and systemic therapy. For the remaining patient, no off-protocol therapy was reported following progression.
Figure 6.
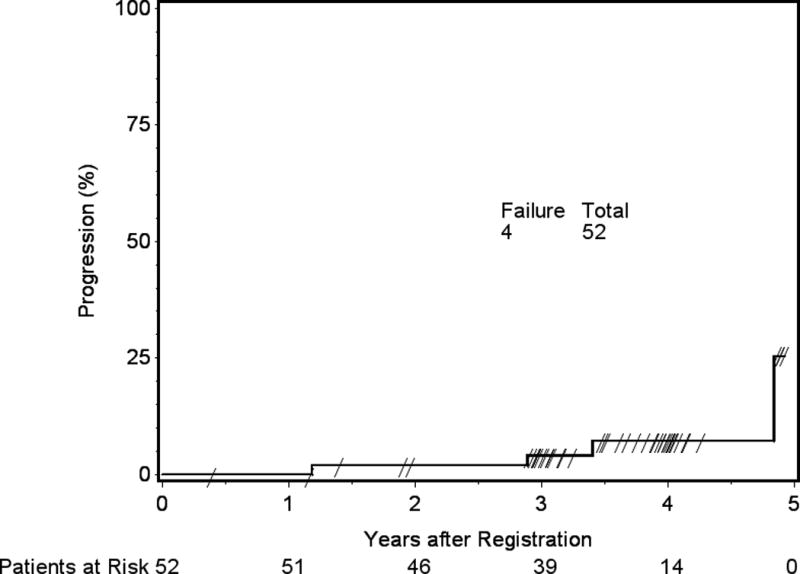
Local Failure (LF). Three-year local failure 4.1%, 95% confidence interval 0.7 to 12.5%.
Adverse Events (AEs)
According to the pre-specified analysis of treatment-related adverse events (dermatology/skin, neurology, and ocular/visual) definitely, probably or possibly related to protocol treatment, AEs were limited to grade 1 or 2, and there were no grade 3 events or higher. In particular, with respect to acute AEs, 5 (10.9%) patients experienced grade 2 AEs and 10 (21.7%) grade 1 AEs, as the AEs of highest grade. Regarding late AEs with the same reported relationship to protocol treatment, 13 (25.5%) and 7 (13.7%) experienced grade 2 and grade 1 AEs, respectively, as the AEs of highest grade. Of the 13 patients with late grade 2 AEs, 1 was dermatologic, and 12 neurologic. Some patients with grade 2 neurologic AEs experienced more than 1 AE. The most commonly reported late grade 2 neurologic events were seizure (n=6), speech disorder (n=3), depression (n=3), trigeminal nerve disorder (n=2), olfactory nerve disorder (n=2), peripheral sensory neuropathy (n=2), memory impairment (n=2), dizziness (n=2). Some patients with grade 1 late AEs experienced more than 1 AE as well. Seven patients had grade I late AEs, but the AEs themselves totaled 15: 2 dermatologic, 6 ocular/visual, and 7 neurologic. The most common grade 1 neurologic events, in descending order of likelihood, were dizziness, memory impairment, peripheral sensory neuropathy, and peripheral motor neuropathy. The most common ocular/visual events were blurred vision, flashing vision, dry eye, and diplopia.
For AEs with any relationship to protocol treatment, the reported highest grade AE was grade 1 in 4 (7.7%), grade 2 in 35 (67.3%) and grade 3 in 8 (15.4%) patients. There were no reported grade 4 or 5 events. Among the reported grade 3 AEs, 3 patients had auditory complaints without categorical evidence of audiometric loss, 2 had neurologic complaints, 1 had pain, 1 developed infection, 1 reported skin complaints, and 1 had gastrointestinal symptoms.
Functional outcomes were measured by Zubrod performance status, Mini-Mental State Examination (MMSE), and neurologic function score. The distributions of these outcomes at baseline, end of RT and year 3 are shown in Table 2. For each of these measures, majority of the patients had either stable or improved status at the end of RT and year 3.
Table 2. Functional Outcome Scores.
Neurologic function, Mini-Mental State Examination (MMSE), and Zubrod performance status scores.
| Time Point | |||
|---|---|---|---|
| Measure | Baseline | End of RT | Year 3 |
| Neurologic Function | n=52 | n=52 | n=42 |
| no symptoms | 27 (51.9%) | 21 (40.4%) | 22 (52.4%) |
| minor symptoms | 21 (40.4%) | 26 (50.0%) | 9 (21.4%) |
| moderate symptoms | 4 (7.7%) | 3 (5.8%) | 2 (4.8%) |
| Unknown | 0 | 2 (3.8%) | 9 (21.4%) |
| MMSE Total Score | n=52 | n=48 | n=27 |
| median | 30 | 29 | 30 |
| minimum - maximum | 24 – 30 | 23 – 30 | 26 – 30 |
| first – third quartile | 28 – 30 | 28 – 30 | 29 – 30 |
| Zubrod Performance Status | n=52 | n=52 | n=42 |
| 0 | 39 (75.0%) | 35 (67.3%) | 26 (61.9%) |
| 1 | 13 (25.0%) | 12 (23.1%) | 4 (9.5%) |
| 2 | 0 | 1 (1.9%) | 0 |
| unknown | 0 | 4 (7.7%) | 12 (28.6%) |
Intensity Modulated Radiation Therapy
We hypothesized that IMRT would minimize late toxicity compared with 3DCRT. Recognizing that there are no reports of prospectively collected AEs using NCI common toxicity criteria for 3DCRT for meningioma, we determined to prospectively test the hypothesis of reduced IMRT toxicity by prospectively comparing late AEs following IMRT on NRG Oncology RTOG 0539 to 3DCRT on NRG Oncology RTOG 0424, a high-risk low grade glioma study that used the same dose and fractionation and similar definitions of treatment volume. A reduction of 10% or more in worst overall grade 2+ AEs was considered supportive. Initial results of NRG Oncology RTOG 0424 have been published,11 with additional data supplied by NRG Oncology.
Of the 44 intermediate-risk patients who received IMRT, 43 were evaluable for late AEs. Eleven (25.6%) of them developed grade 2+ late AEs in the dermatology/skin, neurology, or ocular/visual realms that were deemed definitely, probably or possibly related to protocol treatment, with 1 (2.3%) who experienced a grade 2 AE on dermatology/skin and 10 (23.3%), grade 2 AEs in the neurology realm. This showed a 17.4% reduction in the rate of treatment related grade 2+ late AEs in those categories with IMRT (Table 3). According to the statistical design described above, these results are considered supportive of the hypothesis that IMRT minimizes late toxicity.
Table 3. Late Adverse Events IMRT versus 3DCRT NRG Oncology RTOG 0539 versus NRG Oncology RTOG 0424.
Comparison of CTCAE version 3 grade 2+ late adverse events from IMRT in RTOG-0539 (meningioma) versus 3DCRT on RTOG-0424 (low grade glioma).
| Trial | n | Grade 2+ | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|---|
| RTOG 0539 (IMRT) | 43 | 25.6% | 25.6% | 0 | 0 |
| RTOG 0424 (3DCRT) | 52 | 43% | 33% | 8% | 2% |
Discussion
Meningiomas are the most frequently reported primary intracranial neoplasm, accounting for 36.1% of intracranial tumors, compared with 15.4% for glioblastoma and 28.4% for all gliomas.33 The identification of meningiomas has been increasing over the last several decades, likely related to improved imaging and an aging population rather than actual changes in incidence.7
In older series approximately 90% of meningiomas were classified as benign, 5 to 10% atypical, and less than 5% anaplastic or malignant.20 Even from a recent CBTRUS (Central Brain Tumor Registry of the Unites States) report, WHO grade II accounted for only 4.2% of all newly diagnosed meningioma24. This was a database query and did not include regrading of pathology specimens. However, over its study period of 2004-2010, it did document an annual increase in WHO grade II meningioma of 3.6%. With improved adoption of modern WHO standards, an increased incidence of WHO grade II histology and improved correlations between histopathology and clinical outcomes have been documented. Perry and colleagues updated grading criteria, and found that approximately 20 to 25% of meningiomas fall into the intermediate prognostic group.37,38 These grading parameters were adopted by the world Health Organization (WHO) for their 2000 criteria.
The 2007 and most recent 2016 WHO criteria have added brain invasion as a criterion for grade II. With these new criteria, the proportion of WHO grade II meningioma has increased to approximately 25%,39,47 even reaching 35% in a single-institution report by Pearson et al,35 and 30% per Backer-Grøndahl and colleagues.3
Histopathologic grading is a critical element guiding management decisions for meningioma patients. Large series have independently confirmed tight association between WHO 2000/2007 histopathologic grade and patient outcomes.8,9,17,31,39 A secondary analysis from NRG Oncology RTOG 0539, a comparison of histopathologic concordance between the enrolling institution and central review, was recently published.42 We found a concordance rate of 87.8% for WHO grade II, statistically inferior to WHO grades I and III, for which the rates were, respectively 93.0% and 93.6% (p < .0001). Twenty-two cases were reclassified after central review. The most common reclassification was from WHO grade I at the enrolling institution to WHO grade II after central review (9 cases), although 8 WHO grade II cases were reclassified as WHO grade III, and 2 WHO grade II cases reclassified as WHO grade I. Additionally, 2 cases graded as WHO grade III by the enrolling institution were reclassified WHO grade II. In only 1 case did the reclassification not involve WHO grade II: a tumor diagnosed as WHO grade I at the enrolling hospital was identified as WHO grade III on central review.
These findings indicate that the current meningioma classification system is largely interpretable and congruous among pathologists at typically large institutions such as those accruing to this protocol. This was found to be the case with respect to overall meningioma grade, however, there remain subjectivities in component elements of grading. For instance, there was only slight agreement on focal papillary and focal clear cell; fair agreement for focal rhabdoid, chordoid, and small cells; and moderate agreement on hypercellularity, macronucleoli, sheeting, diffuse papillary, anaplasia, and mitoses ≥4 per 10 high power fields (HPF).42 The number of mitoses is a critical element, as it is the most common differentiating factor for meningioma grade. Improvements in concordance may require clarifications of criteria with lower rates of interobserver concordance, and the development of biomarkers predictive of clinical outcome. These are secondary goals of the present trial, awaiting further data maturity and recurrence events.
For WHO grade I tumors, a Simpson grade I resection is often curative. Control rates drop somewhat for Simpson grade II and III, although resections of Simpson grade I through III are often considered gross total and definitive. However, with sufficient follow-up, recurrence many years following a GTR is not uncommon. Retrospective studies with prolonged follow-up have shown progression rates of 15 to 40% at 10 years,44,45 and up to 60% at 15 years.44 Rates in this range have been confirmed in more recently published series as well.13,28
Recurrent meningiomas of any grade behave more aggressively than initially diagnosed tumors. After first salvage treatment for a WHO grade I meningioma, considerably higher rates of subsequent progression have been reported, particularly after surgery alone.29,30,36,45,46 For recurrent grade I meningiomas treated with re-resection, 3-year local progression risk of 55-60% is reported.30,46 Stafford identified a 25% 10-year local progression risk after initial diagnosis, and essentially the same risk at 2 years (24%) after first recurrence.45 With specific reference to sphenoid wing meningiomas involving the optic apparatus often treated with subtotal resection, Peele found a mean interval to first recurrence/progression of 4.4 years, but a considerably shorter mean interval of 14 months after first recurrence.36 Mehdorn published an experience with 463 patients noting that first recurrences are found at a mean 65 months, whereas second recurrences developed at a mean 34 months.29 This approximates the rates of first recurrence following gross total resection of a WHO grade II meningioma.
With such background data, we formulated an intermediate-risk group comprised of patients with a newly diagnosed gross totally resected WHO grade II meningioma or with a recurrent WHO grade I tumor irrespective of resection extent. These patients formed group 2 of the trial, and are the subjects of this report. The results we have observed to date support our decision to include both WHO grade II following GTR and recurrent WHO grade I tumors in the intermediate-risk group, albeit in recognition that there have been few recurrent events in this combined population to date. Moreover, the results of this prospective study corroborate retrospective analyses, which suggest that RT should play a role in the management of patients with intermediate-risk meningioma. 3yPFS from retrospective reports using WHO 2000-2007 grading criteria are shown in Figure 7, and include 10 reports following GTR alone and 7 from GTR + RT.
Figure 7. Atypical Meningioma – 3 year Local Control.
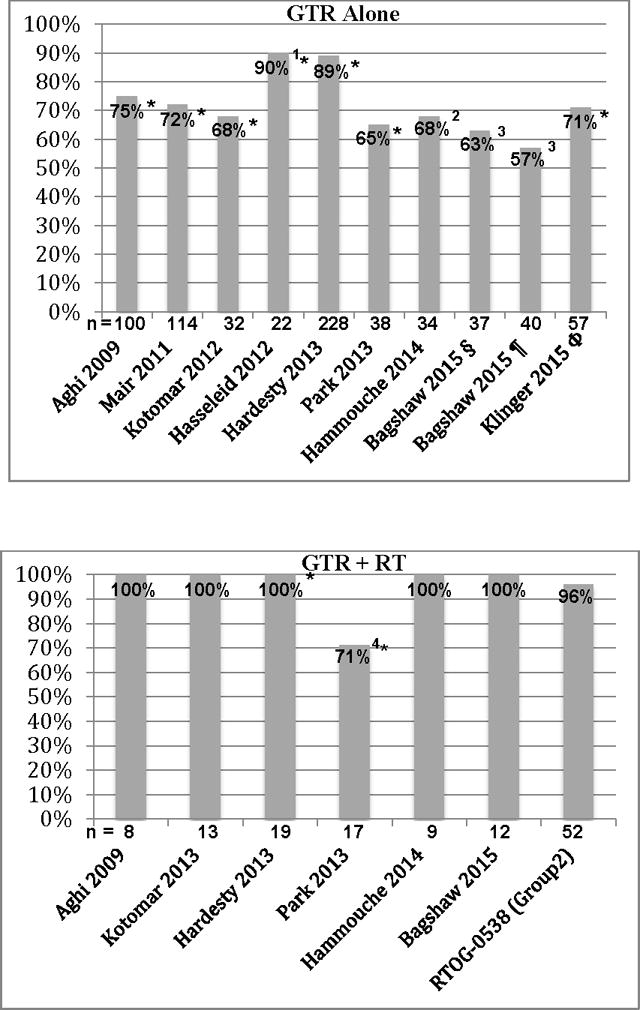
3-year local control following gross total resection (GTR) alone versus GTR + radiation therapy (RT). *taken from a graph; 1convexity only, 90% 3y retreatment-free survival; 2crude 4-year, 363% after Simpson 1, 57% after Simpson 1-3; 4 Pre-3D-CRT methods used in 18 of 27 (67%) patients who received RT from 1997-2011; § Simpson grade 1, ¶ Simpson 1-3, Φ Simpson 1 to 2, 3 of 57 patients received early adjuvant RT.
Aghi described 108 patients with atypical meningioma and Simpson Grade I resection, 100 of whom underwent surgery alone, and 8 who had surgery and external beam RT to a mean RT dose of 60.2 Gy. The 5-year recurrence with GTR alone was 45%, but 0% with surgery and RT (p=0.1).1 Komotar reported 45 patients with atypical meningioma and a Simpson grade I to II resection: 32 (71%) had a GTR alone, and 13 (29%) had a GTR and RT, median RT dose 59.4 Gy. After GTR alone 13 patients (41%) recurred at a median of 19 months. After GTR plus RT, 1 patient (8%) recurred at 52.5 months, for respective 6-year actuarial recurrence risks 65% versus 20% (p = 0.085).23
Park analyzed the role of RT following resection of atypical meningioma in 82 patients. Fifty-six were treated with initial surgery alone and 27 with surgery followed by RT. The median dose of RT was 61.2 Gy. Defining GTR as Simpson I to II, they found that post-operative RT significantly improved PFS for the entire cohort, although not for patients with GTR (p=.858). 3yPFS was 65% after GTR alone and 71% following GTR + RT, but at 5 years PFS remained 65% with GTR alone and fell to 53% after GTR + RT.34 This differs from the other recent reports, perhaps owing in part to patient selection, in part to the determination of GTR in their analysis based upon the surgeon’s report without the requirement for corroborating post-operative imaging, and in part to the fact that many of their patients (18 of 27, 67%) received RT prior to the incorporation of 3D image-based treatment planning. Goldsmith and colleagues reported that CT and MRI based treatment planning resulted in significantly superior tumor control.12
Reports using protons further illuminate outcomes with larger field RT following STR, and provide information that may be important for dose-response assessment for WHO grade II meningioma. Boskos detailed 24 patients with high-grade meningioma (79% WHO grade II), typically following STR. Cause-specific survival at 5 years was 80% with > 60 Gy versus 24% with ≤ 60 Gy (p = 0.01), with a trend toward further improvement with doses > 65 Gy (p = 0.06).5 Hug reported on 15 patients treated with 40 to 72 Cobalt Gray Equivalent (CGE). Five-year local control was 90% with > 60 CGE, and 0% with ≤ 60 CGE.19
Stereotactic radiosurgery (SRS) has become standard in the management of meningioma, and has resulted in favorable outcomes in the primary or adjuvant treatment of WHO grade I or presumed grade I tumors.41 We did not include radiosurgery as an option with this trial on four accounts: 1) patients with newly diagnosed WHO grade I meningioma were observed even following subtotal resection, 2) there was no impetus to include SRS as an option for gross totally resected WHO grade II meningioma in which setting there would be no traditional SRS target, 3) recurrent WHO grade I and newly diagnosed gross totally resected grade II meningiomas display similar recurrence rates and patterns of progression,32 and 4) SRS for WHO grade II meningioma has met with a high rates of recurrence outside the SRS volume, although in or near (e.g. within 2 cm of) the resection bed.2,41
Huffmann et al. reviewed 15 patients treated with SRS, median dose 16 Gy. At 18 to 36 months, crude local control was 60%. Six (40%) progressed, 1 in field, but all within the resection bed.18 Choi reviewed 25 patients with atypical meningioma, median SRS dose 22 Gy in 1–4 fractions. Recurrence occurred in 9: 3 within the targeted region, 5 elsewhere in the resection bed, and 1 regionally.6 These findings suggest that the appropriate target volume for atypical meningioma extends beyond the enhancing tumor, and includes the entire resection bed. Future patterns of failure analyses are needed before definitive guidelines can be developed.
NRG Oncology RTOG 0539 employed a fractionated external beam RT dose of 54 Gy in 30 fractions, and found that this is well tolerated, with a favorable adverse event (AE) profile and no serious AEs. This RT dose was chosen over a decade ago, at which time arguments against the use of RT for an intermediate-risk meningioma patient were even more vociferous than at present. However, we now have a larger volume of literature to draw from, suggesting, as described in the aforementioned reports, that higher doses may provide improved progression-free survival.
EORTC 22042-26042 (accrual completed, pending publication), a phase II trial of post-operative RT for patients with atypical or malignant meningioma employed 60 Gy following a GTR, and added a 10 Gy boost after subtotal resection. The definition of GTR as well as target volumes were very similar to the present trial (NRG Oncology RTOG 0539). GTR was defined as Simpson grades I to III. RT targets included the resection bed with any remaining enhancing tumor, adding a 10 mm margin for subclinical extension, and a planning margin of 5 mm with conformal or intensity modulated RT, permitting smaller margins (1 to 5 mm) if stereotactic methods were employed. This trial will provide further guidance regarding dosing for atypical meningioma, especially once long-term follow-up is available from both the EORTC and NRG Oncology RTOG trials and comparative outcomes can be assessed.
Conclusion
NRG Oncology RTOG 0539 has demonstrated that meningioma patients can be successfully enrolled to large cooperative group trials. Indeed accrual was ahead of schedule. It also shows that patients with intermediate-risk meningioma experience limited risk of local failure (4.1%), and excellent rates of progression-free survival (93.8%), and overall survival (96%) at 3 years when treated with radiation therapy (RT). Compared with historical controls, it suggests that 3-year progression-free survival for intermediate-risk patients is superior with RT than with observation. The results of this single-arm study support enrollment to a definitive, phase III trial that evaluates RT versus observation following gross total resection of a WHO grade II meningioma.
Acknowledgments
The late Dr. Bruce Dean, Barrow Neurological institute, was the neuroradiologist instrumental in trial design. He reviewed all images at protocol inception. It is with gratitude that we acknowledge his contributions.
Disclosures
Dr. Rogers reports that NRG Oncology RTOG 0539 was supported by a grant received from the NIH, specifically the American Recovery and Reinvestment Act (ARRA). Dr. Vogelbaum reports other from Infuseon Therapeutics, Inc., personal fees from Neuralstem, personal fees from Pharmicokinesis, outside the submitted work. Dr. De Groot reports personal fees from Genentech, personal fees from VBL Therapeutics, grants and personal fees from Deciphera Therapeutics, personal fees from Celldex Therapeutics, grants from Novartis, grants from Eli Lilly, other from Ziopharm Oncology, personal fees from Foundation Medicine, personal fees from Monteris Medical, personal fees from Omniox, from Oxigene, outside the submitted work. Dr. Knisely reports support to attend scientific meetings from BrainLab AG, Elekta AB, and Cyber Medical Corporation and an honorarium from BrainLab AG. Dr. Mehta reports personal fees from Abbvie, grants and personal fees from Novelos, personal fees from Phillips, personal fees from BMS, personal fees from Celldex, personal fees from Roche, personal fees from Elekta, personal fees from Novartis, personal fees from Cavion, grants and personal fees from Novocure, personal fees from Pharmacyclics, personal fees from Monteris, outside the submitted work.
This project was supported by grants U10CA21661, U10CA180868 and U10CA180822 from the National Cancer Institute (NCI).
Footnotes
Previous presentation: ASTRO, 57rd Annual Meeting of the American Society of Therapeutic Radiology and Oncology, San Antonio, Texas, October 2015
References
- 1.Aghi MK, Carter BS, Cosgrove GR, Ojemann RG, Amin-Hanjani S, Martuza RL, et al. Long-term recurrence rates of atypical meningiomas after gross total resection with or without postoperative adjuvant radiation. Neurosurgery. 2009;64:56–60. doi: 10.1227/01.NEU.0000330399.55586.63. [DOI] [PubMed] [Google Scholar]
- 2.Attia A, Chan MD, Mott RT, Russell GB, Seif D, Bourland JD, et al. Patterns of failure after treatment of atypical meningioma with gamma knife radiosurgery. J Neurooncol. 2012;108:179–185. doi: 10.1007/s11060-012-0828-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Backer-Grøndahl T, Moen BH, Torp SH. The histopathological spectrum of human meningiomas. Int J Clin Exp Pathol. 2012;5:231–242. [PMC free article] [PubMed] [Google Scholar]
- 4.Bagshaw HP, Jensen RL, Palmer CA, Shrieve DC. Stereotactic Radiation Therapy and the Management of Atypical Meningiomas: Outcomes in the Upfront and Recurrent Setting. Int J Radiat Oncol Biol Phys. 2015;93 Abstract. [Google Scholar]
- 5.Boskos C, Feuvret L, Noel G, Habrand JL, Pommier P, Alapetite C, et al. Combined proton and photon conformal radiotherapy for intracranial atypical and malignant meningioma. Int J Radiat Oncol Biol Phys. 2009;75:399–406. doi: 10.1016/j.ijrobp.2008.10.053. [DOI] [PubMed] [Google Scholar]
- 6.Choi CY, Soltys SG, Gibbs IC, Harsh GR, Jackson PS, Lieberson RE, et al. Cyberknife stereotactic radiosurgery for treatment of atypical (WHO grade II) cranial meningiomas. Neurosurgery. 2010;67:1180–1188. doi: 10.1227/NEU.0b013e3181f2f427. [DOI] [PubMed] [Google Scholar]
- 7.Claus EB, Bondy ML, Schildkraut JM, Wiemels JL, Wrensch M, Black PM. Epidemiology of intracranial meningioma. Neurosurgery. 2005;57:1088–1095. doi: 10.1227/01.neu.0000188281.91351.b9. [DOI] [PubMed] [Google Scholar]
- 8.Combs SE, Schulz-Ertner D, Debus J, von Deimling A, Hartmann C. Improved correlation of the neuropathologic classification according to adapted World Health Organization classification and outcome after radiotherapy in patients with atypical and anaplastic meningiomas. Int J Radiat Oncol Biol Phys. 2011;81:1415–1421. doi: 10.1016/j.ijrobp.2010.07.039. [DOI] [PubMed] [Google Scholar]
- 9.Domingues PH, Sousa P, Otero A, et al. Proposal for a new risk stratification classification for meningioma based on patient age, WHO tumor grade, size, localization, and karyotype. Neuro Oncol. 2014;16:735–747. doi: 10.1093/neuonc/not325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.EORTC 26021-22021. Observation versus adjuvant conventional radiotherapy or radiosurgery after incompletely resected benign intracranial meningioma: a phase III study. http://groups.eortc.be/radio/2006.htm or https://clinicaltrials.gov/ct2/show/NCT00104936.
- 11.Fisher BJ, Hu C, Macdonald DR, Lesser GJ, Coons SW, Brachman DG, et al. Phase 2 study of temozolomide-based chemoradiation therapy for high-risk low-grade gliomas: Preliminary results of radiation therapy oncology Group 0424. Int J Radiat Oncol Biol Phys. 2015;91:497–504. doi: 10.1016/j.ijrobp.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldsmith BJ, Wara WM, Wilson CB, Larson DA. Postoperative irradiation for subtotally resected meningiomas. A retrospective analysis of 140 patients treated from 1967 to 1990. J Neurosurg. 1994;80:195–201. doi: 10.3171/jns.1994.80.2.0195. [DOI] [PubMed] [Google Scholar]
- 13.Gousias K, Schramm J, Simon M. The Simpson grading revisited: aggressive surgery and its place in modern meningioma management. J Neurosurg. 2016;125:551–560. doi: 10.3171/2015.9.JNS15754. [DOI] [PubMed] [Google Scholar]
- 14.Hammouche S, Clark S. Long-term survival analysis of atypical meningiomas: survival rates, prognostic factors, operative and radiotherapy treatment. Acta Neurochir. 2014;156:1475–1481. doi: 10.1007/s00701-014-2156-z. [DOI] [PubMed] [Google Scholar]
- 15.Hardesty DA, Wolf AB, Brachman DG, McBride HL, Youssef E, Nakaji P, et al. The impact of adjuvant stereotactic radiosurgery on atypical meningioma recurrence following aggressive microsurgical resection. J Neurosurg. 2013;119:475–481. doi: 10.3171/2012.12.JNS12414. [DOI] [PubMed] [Google Scholar]
- 16.Hasseleid BF, Meling TR, Rønning P, Scheie D, Helseth E. Surgery for convexity meningioma: Simpson Grade I as the goal. J Neurosurg. 2012;117:999–1006. doi: 10.3171/2012.9.JNS12294. [DOI] [PubMed] [Google Scholar]
- 17.Ho DM, Hsu CY, Ting LT, Chiang H. Histopathology and MIB-1 labeling index predicted recurrence of meningiomas: a proposal of diagnostic criteria for patients with atypical meningioma. Cancer. 2002;94:1538–1547. doi: 10.1002/cncr.10351. [DOI] [PubMed] [Google Scholar]
- 18.Huffmann BC, Reinacher PC, Gilsbach JM. Gamma knife surgery for atypical meningiomas. J Neurosurg. 2005;102:283–285. doi: 10.3171/jns.2005.102.s_supplement.0283. [DOI] [PubMed] [Google Scholar]
- 19.Hug EB, Devries A, Thornton AF, Munzenride JE, Pardo FS, Hedley-White ET, et al. Management of atypical and malignant meningiomas: role of high-dose, 3D-conformal radiation therapy. J Neurooncol. 2000;48:151–160. doi: 10.1023/a:1006434124794. [DOI] [PubMed] [Google Scholar]
- 20.Jaaskelainen J, Haltia M, Servo A. Atypical and anaplastic meningiomas: radiology, surgery, radiotherapy, and outcome. Surg Neurol. 1986;25:233–242. doi: 10.1016/0090-3019(86)90233-8. [DOI] [PubMed] [Google Scholar]
- 21.Ji Y, Rankin C, Grunberg S, Sherrod AE, Ahmadi J, Townsend JJ, et al. Double-blind phase III randomized trial of the antiprogestin agent mifepristone in the treatment of unresectable meningioma: SWOG S9005. J Clin Oncol. 2015;33:4093–4098. doi: 10.1200/JCO.2015.61.6490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klinger DR, Flores BC, Lewis JJ, Hatanpaa K, Choe K, Mickey B, et al. Atypical Meningiomas: Recurrence, Reoperation, and Radiotherapy. World Neurosurgery. 2015;84:839–845. doi: 10.1016/j.wneu.2015.04.033. [DOI] [PubMed] [Google Scholar]
- 23.Komotar RJ, Iorgulescu JB, Raper DMS, Holland EC, Beal K, Bilsky MH, et al. The role of radiotherapy following gross-total resection of atypical meningiomas. J Neurosurg. 2012;117:679–686. doi: 10.3171/2012.7.JNS112113. [DOI] [PubMed] [Google Scholar]
- 24.Kshettry VR, Ostrom QT, Kruchko C, Al-Mefty O, Barnett GH, Barnholtz-Sloan JS. Descriptive epidemiology of World Health Organization grades II and III intracranial meningiomas in the United States. Neuro-Oncology. 2015;17:1166–1173. doi: 10.1093/neuonc/nov069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maclean J, Fersht N, Short S. Controversies in radiotherapy for meningioma. Clinical Oncology. 2014;26:51–64. doi: 10.1016/j.clon.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 26.Mair R, Morris K, Scott I, Carroll TA. Radiotherapy for atypical meningiomas. J Neurosurg. 2011;115:811–819. doi: 10.3171/2011.5.JNS11112. [DOI] [PubMed] [Google Scholar]
- 27.Mazza E, Brandes A, Zanon S, Eoli M, Lombardi G, Faedi Meta, et al. Hydroxyurea with or without imatinib in the treatment of recurrent or progressive meningiomas: a randomized phase II trial by Gruppo Italiano Cooperativo di Neuro-Oncologia (GICNO) Cancer Chemother Pharmacol. 2015;77:115–120. doi: 10.1007/s00280-015-2927-0. [DOI] [PubMed] [Google Scholar]
- 28.McGovern SL, Aldape KD, Munsell MF, Mahajan A, DeMonte F, Woo SY. A comparison of World Health Organization tumor grades at recurrence in patients with non–skull base and skull base meningiomas. J Neurosurg. 2010;112:925–933. doi: 10.3171/2009.9.JNS09617. [DOI] [PubMed] [Google Scholar]
- 29.Mehdorn HM. Intracranial meningiomas: A 30-year experience and literature review. Adv Tech Stand Neurosurg. 2016;43:139–184. doi: 10.1007/978-3-319-21359-0_6. [DOI] [PubMed] [Google Scholar]
- 30.Miralbell R, Linggood RM, De la Monte S, Convery K, Munzenrider JE, Mirimanoff RO. The role of radiotherapy in the treatment of subtotally resected benign meningiomas. Journal of Neuro-Oncology. 1992;13:157–164. doi: 10.1007/BF00172765. [DOI] [PubMed] [Google Scholar]
- 31.Olar A, Wani KM, Sulman EP, Mansouri A, Zadeh G, Wilson CD, et al. Mitotic Index is an Independent Predictor of Recurrence-Free Survival in Meningioma. Brain Pathol. 2015;25:266–275. doi: 10.1111/bpa.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Onodera S, Aoyama H, Katoh N, Taguchi H, Yasuda K, Yoshida D, et al. Long-term outcomes of fractionated stereotactic radiotherapy for intracranial skull base benign meningiomas in single institution. Jpn J Clin Oncol. 2011;41:462–468. doi: 10.1093/jjco/hyq231. [DOI] [PubMed] [Google Scholar]
- 33.Ostrom QT, Gittleman H, Liao P, Rouse C, Chen Y, Dowling J, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007-2011. Neuro Oncol. 2014;16:iv1–iv63. doi: 10.1093/neuonc/nou223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park HJ, Kang HC, Kim IH, Park SH, Kim DG, Park CK, et al. The role of adjuvant radiotherapy in atypical meningioma. J Neurooncol. 2013;115:241–247. doi: 10.1007/s11060-013-1219-y. [DOI] [PubMed] [Google Scholar]
- 35.Pearson BE, Markert JM, Fisher WS, Guthrie BL, Fiveash JB, Palmer CA, et al. Hitting a moving target: evolution of a treatment paradigm for atypical meningiomas amid changing diagnostic criteria. Neurosurg Focus. 2008;24:E3. doi: 10.3171/FOC/2008/24/5/E3. [DOI] [PubMed] [Google Scholar]
- 36.Peele KA, Kennerdell JS, Maroon JC, Kalnicki S, Kazim M, Gardner T, et al. The role of postoperative irradiation in the management of sphenoid wing meningiomas. A preliminary report. Ophthalmology. 1996;103:1761–1766. doi: 10.1016/s0161-6420(96)30430-2. [DOI] [PubMed] [Google Scholar]
- 37.Perry A, Stafford SL, Scheithauer BW, Suman VJ, Lohse CM. Meningioma grading: an analysis of histologic parameters. Am J Surg Pathol. 1997;21:1455–1465. doi: 10.1097/00000478-199712000-00008. [DOI] [PubMed] [Google Scholar]
- 38.Perry A, Scheithauer BW, Stafford SL, Lohse CM, Wollan PC. “Malignancy” in meningiomas: a clinicopathologic study of 116 patients. Cancer. 1999;85:2046–2056. doi: 10.1002/(sici)1097-0142(19990501)85:9<2046::aid-cncr23>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 39.Perry A. Meningiomas. In: McLendon R, Rosenblum M, Bigner DD, editors. Russell & Rubinstein’s Pathology of Tumors of the Nervous System. 7th. London, England: Hodder Arnold; 2006. pp. 427–474. [Google Scholar]
- 40.Rogers L, Mehta M. Role of radiation therapy in treating intracranial meningiomas. Neurosurg Focus. 2007;23:E4. doi: 10.3171/FOC-07/10/E4. [DOI] [PubMed] [Google Scholar]
- 41.Rogers Leland, Barani Igor, Chamberlain Marc, Kaley Thomas, McDermott Michael, Raizer Jeffrey, et al. Meningioma: Knowledge Base, Treatment Outcomes, and Uncertainties: A RANO Meningioma Group Review. J Neurosurg. 2015;122:4–23. doi: 10.3171/2014.7.JNS131644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rogers CL, Perry A, Pugh S, Brachman D, McMillan W, Jenrette J, et al. Pathology Concordance Levels for Meningioma Classification and Grading in RTOG Trial 0539. Neuro Oncol. 2016;18:565–574. doi: 10.1093/neuonc/nov247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simpson D. The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry. 1957;20:22–39. doi: 10.1136/jnnp.20.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soyuer S, Chang EL, Selek U, Shi W, Maor MH, DeMonte F. Radiotherapy after surgery for benign cerebral meningioma. Radiother Oncol. 2004;71:85–90. doi: 10.1016/j.radonc.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 45.Stafford S, Perry A, Suman V, Meyer FB, Scheithauer BW, Lohse CM, et al. Primarily resected meningiomas: outcome and prognostic factors in 581 Mayo Clinic patients, 1978 through 1988. Mayo Clin Proc. 1998;73:936–942. doi: 10.4065/73.10.936. [DOI] [PubMed] [Google Scholar]
- 46.Taylor BW, Jr, Marcus RB, Jr, Friedman WA, Ballinger WE, Jr, Million RR. The meningioma controversy: postoperative radiation therapy. Int J Radiat Oncol Biol Phys. 1988;15:299–304. doi: 10.1016/s0360-3016(98)90008-6. [DOI] [PubMed] [Google Scholar]
- 47.Willis J, Smith C, Ironside JW, Erridge S, Whittleà IR, Everington D. The accuracy of meningioma grading: a 10-year retrospective audit. Neuropathol Appl Neurobiol. 2005;31:141–149. doi: 10.1111/j.1365-2990.2004.00621.x. [DOI] [PubMed] [Google Scholar]


