Abstract
Background
In observational studies, HIV patients have higher levels of soluble ST2 (sST2), galectin-3, growth differentiation factor-15 (GDF-15) than non-HIV controls. As statins exert pleiotropic immunomodulatory effects that may affect markers of myocardial fibrosis, the objective of the current study is to determine if biomarkers of myocardial fibrosis reflecting subclinical pathology may be modified by statin therapy in patients with HIV.
Setting and Methods
40 HIV+ men and women participated in a single center 12-month randomized, double-blind placebo controlled trial of atorvastatin 40mg qd vs. placebo. At baseline and 12-months sST2, GDF-15, galectin-3, were measured.
Results
The changes in sST2 were −0.310 [−4.195,2.075] vs. 1.163 [0.624, 4.715]ng/mL, median[IQR] atorvastatin vs. placebo (p=0.04). The change in sST2 was significantly related to changes in monocyte activation markers sCD14 (r=0.63, p<0.0001) and MCP (r=0.52, p=0.0009), markers of generalized inflammation hs-IL-6 (r=0.58, p=0.0002), oxLDL (r=0.49, p=0.002), and GDF-15 (r=0.54, p=0.0008).
Conclusion
sST2, a member of the IL-1 receptor family and a marker of fibrosis and inflammation increases over time among HIV patients and this increase is attenuated by statin therapy in HIV. This effect may relate to immunomodulatory mechanisms of statins.
Clinical Trial Registration
The trial is registered on http://clinicaltrials.gov registry number: (NCT00965185).
Keywords: HIV, soluble ST2 (sST2), growth differentiation factor-15 (GDF-15), coronary artery disease, statin
INTRODUCTION
Patients living with HIV (PLHIV) have an increased risk of myocardial infarction, heart failure and heightened inflammation1–4. We have previously shown that PLHIV have higher soluble ST2 (sST2), and galectin-3 than non-HIV controls5. sST2, thought to be a soluble decoy receptor to its cell membrane bound counterpart has been associated with atherosclerosis, fibrosis, inflammation, and immune activation possibly through inhibiting ST2/interleukin-33 (IL-33) signaling6–9. Growth differentiation factor-15 (GDF-15), a member of the transforming growth factor-β (TGF-β) superfamily, is secreted in the heart in response to ischemia and pressure overload10–12 with levels measured in blood predicting cardiac events and mortality in the general population13. Both GDF-15 and sST2 were found to be elevated and associated with mortality in PLHIV14. Galectin-3, a marker of inflammation and cardiac fibrosis, has been shown to predict incident heart failure and increased LV mass in the Framingham Offspring and Framingham Heart studies15. Statin therapy, a potent lipid lowering therapy, also significantly reduces measures of inflammation independent of low-density lipoprotein (LDL) lowering16. Statins in animal models can inhibit cardiac fibrosis independent of LDL cholesterol lowering thought to be mediated through Rho kinases (ROCKs) inhibition. ROCK mediated effects are thought to be responsible for several of the pleotropic effects of statins including limiting cardiac fibrosis, hypertrophy and pathologic remodeling in response to adverse stimuli such as angiotensin II17. In PLHIV, who have a greater burden of cardiac fibrosis than age and risk factor matched controls18, the influence of high potency statin therapy on these markers could provide important insights into the potential clinical effects of statin therapy.
METHODS
Study Design
This study was a single center 12-month randomized, double-blind, placebo-controlled clinical trial of atorvastatin vs. placebo, showing effects on coronary plaque volume as previously reported19. However, markers of fibrosis were not assessed previously nor are these results available from other statin studies in HIV. HIV-infected subjects 18 to 60 years of age, on stable antiretroviral therapy (ART) with no changes in ART regimen in the preceding six months, and LDL-cholesterol between 70–130 mg/dL (not meeting clinical guidelines criteria at the time for statin therapy) were enrolled. Exclusion criteria were prior history of CVD, evidence of subclinical obstructive ASCVD, defined by presence of one or more plaques on CCTA with significant stenosis (>50% left main stenosis or >70% stenosis in any major vessel), concurrent use of a statin, AST or ALT three times greater than the upper limit of normal and/or treatment for active liver disease, renal disease and/or creatinine >1.5 mg/dL, as well as other criteria as previously described19. Patients were randomized in 1:1 ratio to either atorvastatin (starting at dose of 20 mg per day and escalating to 40 mg per day at three months visit if study drug was well-tolerated) or placebo groups. All subjects received standardized lifestyle counseling based on National Cholesterol Education Program (NCEP) guidelines. All participants provided written informed consent and the study was approved by the MGH institutional IRB. The trial is registered on ClinicalTrials.gov (NCT00965185).
For the current analysis, we measured markers of myocardial fibrosis with inflammatory mechanisms (sST2, galectin-3 and GDF-15) previously found to be elevated in HIV that we hypothesized may be reduced by statin therapy. Secondarily, we sought to assess whether baseline and changes in sST2, galectin-3 and GDF-15 related to baseline and changes in circulating markers of inflammation and oxidized LDL.
Biomarker Assessments
The following soluble protein biomarkers were measured at baseline and at 12-months for this analysis: sST2, for adverse cardiac remodeling and tissue fibrosis; galectin-3, for fibrosis and inflammation; and GDF-15. ELISA measurements for the following biomarkers were performed in strict accordance with manufacturer instructions: sST2 (Critical Diagnostics, San Diego, CA, USA); galectin-3 (BG-Medicine, Waltham, MA, USA); GDF-15 (R&D Systems, Minneapolis, MN). For sST2, the assay measurement range was 3.1 to 200.0 ng/mL; the inter-assay CVs ranged from 8.9% to 7.3% at values between 20 ng/mL and 79.0 ng/mL. The Galectin-3 assay’s measurement range is 0.96 to 130 ng/mL; inter-assay CVs ranged from <10% to 15% at values between 6 ng/mL and 70 ng/mL. For GDF-15, typical interassay imprecision is 6.0% at 225 pg/mL GDF-15 and 5.6% at 900 pg/mL. All laboratory assessments were obtained after a 12 hour fast and other analytes were measured as previously reported19, 20.
Statistical Analysis
Comparisons between the two groups (atorvastatin vs. placebo) were performed using Student t test for normally-distributed continuous variables and Wilcoxon rank sum test if the distribution was non-normal. To assess changes within each group, paired t-test or Wilcoxon signed rank test was performed, depending on normality of distribution. Pearson correlation coefficient was used to assess relationships of sST2, Galectin-3, GDF-15 with other parameters. Two-tailed probability values are reported, and statistical significance was assumed when p< .05. Means and standard deviations (SD) are reported to describe changes in continuous variables with normal distribution; otherwise, medians and interquartile ranges (IQR) are used. For analysis of GDF-15, two outliers based on the Dixon criteria were excluded. All statistical analyses were performed using SAS JMP version 11 (SAS Institute, Cary, North Carolina).
RESULTS
Characteristics of the Participants at Baseline
Eighty-one HIV-infected participants underwent screening for this study. 40 participants were randomized to atorvastatin or placebo. Demographic, clinical characteristics, lipids, immunologic and systemic inflammatory markers of these 40 study participants have previously been described and are also shown in Supplemental Table. Participants were all on antiretroviral therapy and most patients had undetectable viremia with similar immunological and virological indices between groups. At baseline, there were no significant differences in cardiac immunologic, inflammatory or cardiac biomarkers between the groups (Supplemental Table and Table 1). After randomization, 1 out of 21 patients in the placebo group and 2 out of 19 in the statin group discontinued and did not have a 12-month evaluation.
Table 1.
Lipids, Inflammatory Parameters, and Cardiac Biomarkers
| Baseline Placebo (n=21) |
Baseline Atorvastatin (n=19) |
Between Group P-value |
12-month Placebo (n=20) |
12-month Atorvastatin (n=17) |
Within Group (Placebo) P-value |
Within Group (Atorvastatin) P-value |
Change Placebo (n=20) |
Change Atorvastatin (n=17) |
Between Group P-value |
|
|---|---|---|---|---|---|---|---|---|---|---|
| Lipids | ||||||||||
|
| ||||||||||
| Total cholesterol (mg/dL) | 192 ± 27 | 199 ± 38 | 0.52 | 198 ± 29 | 153 ± 29 | 0.30 | <0.0001 | 5 ± 20 | −47 ± 23 | <0.0001 |
| HDL-Cholesterol (mg/dL) | 51 ± 15 | 52 ± 19 | 0.84 | 49 ± 15 | 55 ± 17 | 0.54 | 0.70 | −2 ± 11 | 1 ± 10 | 0.48 |
| Direct LDL-Cholesterol (mg/dL) | 125 ± 32 | 124 ± 37 | 0.93 | 137 ± 30 | 86 ± 30 | 0.03 | <0.0001 | 11 ± 21 | −38 ± 29 | <0.0001 |
| Triglycerides (mg/dL) | 113 (92 to 136) | 120 (97 to 204) | 0.39 | 117 (86 to 176) | 110 (81 to 134) | 0.93 | 0.49 | 8 (−41 to 34) | −9 (−41 to 39) | 0.64 |
| Oxidized LDL (U/L) | 63.5 ± 13.1 (n=21) | 62.2 ± 15.9 (n=18) | 0.78 | 67.7 ± 19.2 (n=21)* | 47.2 ± 11.3 (n=18)* | 0.24 | <0.0001 | 4.2 ± 15.8 (n=21)* | −14.9 ± 10.1 (n=18)* | <0.0001 |
|
| ||||||||||
| Inflammatory Parameters | (n=21) | (n=19) | ||||||||
|
| ||||||||||
| C-reactive protein (mg/L) | 1.1 (0.4 to 2.4) | 0.8 (0.3 to 1.9) | 0.36 | 1.5 (0.5 to 3.2) | 0.5 (0.3 to 1.2) | 0.26 | 0.27 | 0.2 (−0.1 to 1.5) | −0.1 (−1.1 to 0.2) | 0.07 |
| Interleukin-6 (ng/L) | 0.8 (0.5 to 1.2) | 0.6 (0.4 to 1.6) | 0.75 | 1.0 (0.5 to 1.8) | 0.8 (0.6 to 1.1) | 0.44 | 0.93 | 0.1 (−0.2 to 0.4) | 0.2 (−0.8 to 0.3) | 0.77 |
| sCD14 (ng/mL) | 1953 (350 to 2400) | 2100 (1088 to 2552) | 0.48 | 1848 (475 to 2652) | 1064 (509 to 2466) | 0.31 | 0.26 | 125 (−238 to 495) | −162 (−780 to 201) | 0.15 |
|
| ||||||||||
| Cardiac Biomarkers | (n=20) | (n=17) | ||||||||
|
| ||||||||||
| Galectin-3 (ng/ml) | 11.8 (10.3 to 15.2) (n=19) | 13.2 (10.4 to 15.4) | 0.63 | 13.9 (10.2 to 15.3) | 12.5 (11.0 to 16.9) | 0.26 | 0.43 | 0.5 (−1.1 to 1.8) (n=19) | 0.2 (−1.5 to 3.2) | 0.97 |
| sST2 (ng/ml) | 24.7 (20.4 to 30.9) | 27.7 (20.4 to 36.2) | 0.49 | 26.9 (21.4 to 31.1) | 27.0 (20.0 to 31.8) | 0.02 | 0.55 | 1.2 (0.6 to 4.7) | −0.3 (−4.2 to 2.1) | 0.04 |
| GDF-15 (pg/ml) | 2221 ± 2458 (n=19) | 1357 ± 836 (n=16) | 0.16 | 2482 ± 2372 (n=19) | 1339 ± 602 (n=16) | 0.04 | 0.86 | 262 ± 507 (n=19) | −19 ± 425 (n=16) | 0.08 |
Data reported as mean ± standard deviation or median (IQR).
Serum from last visit prior to study discontinuation used for 12-month oxidized LDL
Within group changes assessed by paired t-test for normally distributed variables or by Wilcoxon signed rank test as nonparametric version of paired t-test for non-normally distributed variables. Between group differences assessed by Student’s t-test for normally distributed variables or by Wilcoxon rank sum test for non-normally distributed variables.
Changes in cardiac biomarkers in the atorvastatin group vs. placebo
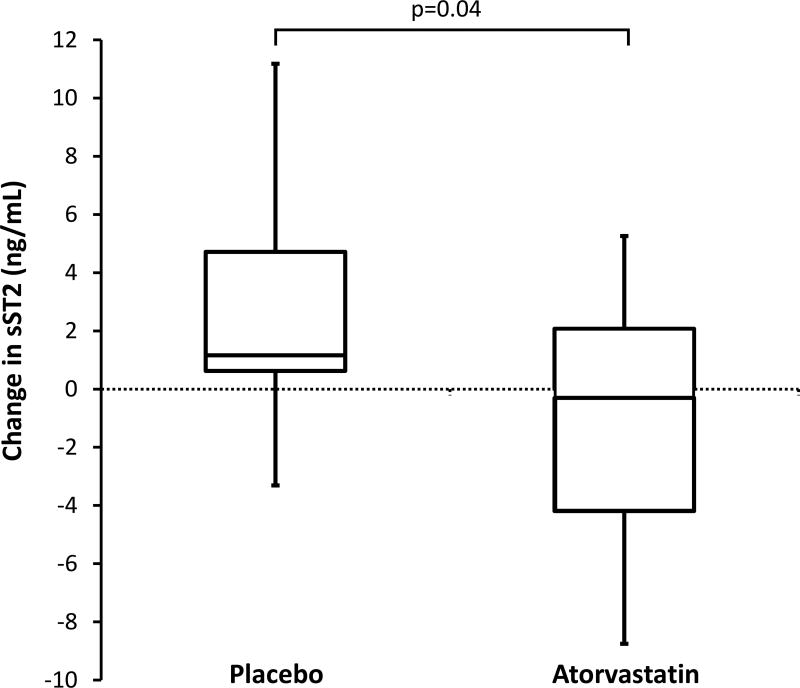
Changes in sST2 were −0.3 ng/mL (IQR −4.2 to 2.1) with atorvastatin vs. an increase of 1.2 ng/mL (0.6 to 4.7) with placebo (p=0.04 for comparison of change between groups) (Table 1 and Figure). Atorvastatin tended to lower GDF-15 with a change in GDF-15 of −19 ± 425 pg/mL with atorvastatin vs. a 262 ±507 pg/mL increase with placebo, but this did not reach statistical significance (p=0.08 for comparison of change between groups) (Table 1). In contrast, galectin-3 did not change significantly between groups with atorvastatin vs. placebo treatment.
FIGURE.
Bolded line inside box denotes median and top of box denotes 75th centile and lower end of box denotes 25th centile. Ends of whiskers denote 90th and 10th centile.
Correlation with changes in cardiac biomarker levels
In all patients, the change in sST2 positively correlated with changes in monocyte activation markers sCD14 (r=0.63, p<0.0001) and MCP-1 (r=0.52, p=0.0009), hsIL-6 (r=0.58, p=0.0002), oxLDL (r=0.49, p=0.002), % CD14+CD16+ monocytes (r=0.46, p=0.03), and change in GDF-15 (r=0.54, p=0.0008), whereas, the change in GDF-15 was positively correlated with changes in galectin-3 (r=0.51, p=0.002), MCP-1 (r=0.42, p=0.01), sCD14 (r=0.45, p=0.007), hsIL-6 (r=0.47, p=0.005), and change in %CD14+CD16+ monocytes (r=0.52, p=0.02).
DISCUSSION
In the current analysis, we found sST2 increased significantly more in the placebo treated patients than atorvastatin-treated PLHIV, suggesting a potential effect of statins to mitigate ongoing fibrosis in this population. We also demonstrate that overall changes in sST2 is related to changes in monocyte activation markers.
Cardiac fibrosis is increased in HIV patients18. Statin therapy is being increasingly contemplated for PLHIV, due to effect on subclinical atherosclerotic disease, but little is known regarding effects of statin therapy on markers of cardiac fibrosis in HIV. One marker of importance in this regard is sST2. sST2 has been shown to be higher in PLHIV compared to non-infected individuals5, 21. Furthermore, sST2 has also been shown to be associated with cardiovascular dysfunction and strongly predictive all-cause mortality in patients living with HIV14. In the general population sST2 predicts cardiovascular events and all-cause mortality13, and also predicts worse clinical outcomes in patients after acute myocardial infarction22 and in patients with chronic heart failure23.
ST2 is an interleukin-1 family receptor involved in inflammatory and immune responses8. The ST2 gene encodes for two isoforms of the ST2 protein: transmembrane ST2L, which is primarily expressed on dendritic cells, mast cells, macrophages, and T helper cells, and secreted soluble ST2 (sST2)6. The functional ligand of ST2 is interleukin-33 (IL-33), which has a variety of biological functions including its role as an alarmin which responds to cellular stress and infection24. IL-33 is released by myocardial endothelial cells and gut epithelial cells, and signals via binding to the ST2 receptor to mediate both proinflammatory and anti-inflammatory mechanisms,25–27. IL-33/ST2 signaling has been shown to reduce the development of atherosclerosis in mice, presumably through the induction of a Th1-to-Th2 shift and the production of antioxidized low-density lipoprotein (ox-LDL) and IL-5 antibodies6. Decreased production of ox-LDL antibodies may result from elevated sST2 levels, which would limit IL-33/ST2 signaling and elevate oxLDL, offering a possible explanation for the positive correlation we observed between sST2 and ox-LDL.
IL-33 signaling also has demonstrated antifibrotic effects in the myocardium, however endothelial cell production of IL-33 in response to myocardial pressure overload can induce a systemic inflammatory state25. IL-33/ST2 signaling is controlled by sST2, which acts as a decoy receptor to limit IL-33 activity, preventing the myocardial and vascular protective benefits of IL-3325. As such, elevated sST2 levels are associated with adverse prognosis in CVD and have been identified as a biomarker in heart disease, fibrosis, atherosclerosis, and inflammation8. IL-33 signaling via ST2 activates T cells and promotes Th2 lymphocyte responses, and has been shown to activate alternatively activated macrophages in ST2 deficient mice28, 29. The role of IL-33/ST2 signaling in monocyte and macrophage activation suggests that increased sST2 would result in increased inflammation.
The effect of statin therapy on IL-33/ST2 signaling has not yet been clearly elucidated, though previous studies have demonstrated that treatment of human umbilical vein endothelial cells (HUVEC) with statins affects expression of IL-33 and ST2 mRNA expression. DNA microarray studies have shown that atorvastatin and pitavastatin decrease ST2 mRNA expression30. These data suggest that statin therapy may directly affect the expression of IL-33 and ST2s31.
We demonstrated that the change in sST2 related significantly to the change hs-IL6 as well as to changes in MCP-1, sCD14 with statin therapy, further confirming the relationships between sST2 and markers of monocyte activation consistent with the known role of IL-33/ST2 signaling in monocyte and macrophage activation. Furthermore, change in sST2 related to the change in oxidized LDL. It is uncertain whether statins’ effects on lowering oxidized LDL may potentially mediate the reduction in sST2, however these relationships suggest that statins may reduce sST2 via direct anti-inflammatory effects on immune cells and possibly also via lowering of oxidized LDL. Given the prior strong association of sST2 with diastolic dysfunction and increased mortality in PLHIV prospective studies of statins such as the pitavastatin in the randomized trial to prevent vascular events in HIV (REPRIEVE, NCT02344290) will provide the opportunity to determine if a statin mediated decrease in sST2 is independently associated with a reduced incidence of heart failure and death as well as non-fatal ASCVD.
Another well-validated cardiac biomarker assessed in the current study is GDF-15, which we found to increase significantly over time in the placebo group but not in the atorvastatin group. GDF-15, also known as MIC-1, PTGF-β, PDF, PLAB, NAG-1, and PL74 is a member of the transforming growth factor-β (TGF-β) superfamily10 that is expressed in the heart and other tissues in response to pro-inflammatory cytokines and cardiovascular stress-related stimuli10–12. Though the exact mechanisms are unclear, these effects appear to be mediated via Akt- and SMAD2/3-related signaling pathways11, 12.
Levels of GDF-15 have been shown to correlate with cardiovascular mortality, and all-cause mortality in the general population32–34. In the Framingham heart study, both GDF-15 and sST2 were found to be predictive of death, heart failure, and major cardiac events13. Though GDF-15 is increased in settings of inflammation and cardiovascular injury, Bonaca et al. found no significant difference in the effect of high dose vs. low dose statin therapy on GDF-15 levels in the PROVE-IT TIMI 22 trial35. Their null findings may be because both groups had received statin therapy so it would be harder to detect a difference. Although we did not find a significant decrease in GDF-15 with atorvastatin in our study either, our data suggest that statin therapy may help to prevent increases in GDF-15 in the HIV population which has increased GDF-15 at baseline. To our knowledge, no prior studies have examined the effects of statins on GDF-15 compared to placebo in HIV.
Only one prior study has assessed GDF-15 in HIV-infected participants. In that prospective cohort study, GDF-15 levels were elevated in HIV patients compared to controls, were associated with pulmonary hypertension and independently predicted all-cause mortality in the HIV group14. In our study, we demonstrated an increase in GDF-15 in the placebo group over 1 year and showed that atorvastatin tended to lower GDF-15 compared to placebos. GDF-15 may be induced by LPS in intestinal myofibroblasts36, 37. Further research is needed to determine if GDF-15 is increased in HIV by similar mechanisms.
In our study, we observed significant correlations between change in GDF-15 and markers of immune activation and inflammation, suggesting that GDF-15 may act primarily via immune and inflammatory pathways.38. In addition, we found the changes in GDF-15 and sST2 to be positively correlated. This finding is consistent with other studies13, 39, 40 and suggests that GDF-15 and sST2 may act via a common biological pathway.
This study has limitations, including its small size. Despite this, results from this randomized placebo-controlled trial are informative regarding potential effects of statins on myocardial fibrosis in HIV, which may be modulated by effects on immune pathways. Data from our randomized trial suggest for the first time that statin therapy may prevent ongoing progression of fibrosis as indicated by sST2 in PLHIV and thus help to preserve the cardioprotective mechanisms of the IL-33/ST2 signaling system. Statin therapy may also help to prevent the progression of rise in GDF-15. Further larger studies are now needed to confirm these findings and ultimately determine if such changes relate to improved measurements of cardiac fibrosis, inflammation and events and whether PLHIV with higher sST2 or GDF-15 should be targeted for such studies.
Supplementary Material
Acknowledgments
We thank the patients who generously donated their time to participate in this study and the nurses and bionutritionists at the MGH Clinical Research Center of the Harvard Clinical and Translational Science Center.
FUNDING SOURCES
This work was supported by NIH K23HL092792 (JL), NIH RO1HL123351 (JL), NIH 5T32HL076136 (MTL), NIH 5K24HL113128 (UH), NIH R01HL095123 (SKG), NIH P30 DK040561 (SKG). The project described was also supported by Grant Number 1 UL1 RR025758-04, Harvard Clinical and Translational Science Center, from the National Center for Research Resources.
DISCLOSURES
Unrelated to this study, Dr. deFilippi has received research support and consulted for Roche Diagnostics. He also consults for Alere, Ortho Diagnostics, Metanomics, and Siemens healthcare diagnostics; serves on an endpoint committee for Radiometer and Qunitiles; and receives royalties from UpToDate. Dr. Lo has served as consultant for Gilead Sciences. Dr. Hoffmann received grant support from Siemens Healthcare and HeartFlow Inc., unrelated to this manuscript. Dr. Grinspoon has consulted with Navidea and Theratechnologies, and received grant support from Gilead, KOWA Pharmaceuticals, and Theratechnologies, unrelated to this manuscript.
References
- 1.Freiberg MS, Chang CC, Kuller LH, Skanderson M, Lowy E, Kraemer KL, Butt AA, Bidwell Goetz M, Leaf D, Oursler KA, Rimland D, Rodriguez Barradas M, Brown S, Gibert C, McGinnis K, Crothers K, Sico J, Crane H, Warner A, Gottlieb S, Gottdiener J, Tracy RP, Budoff M, Watson C, Armah KA, Doebler D, Bryant K, Justice AC. Hiv infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013;173:614–622. doi: 10.1001/jamainternmed.2013.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lo J, Plutzky J. The biology of atherosclerosis: General paradigms and distinct pathogenic mechanisms among hiv-infected patients. J Infect Dis. 2012;205(Suppl 3):S368–374. doi: 10.1093/infdis/jis201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kearns A, Gordon J, Burdo TH, Qin X. Hiv-1-associated atherosclerosis: Unraveling the missing link. J Am Coll Cardiol. 2017;69:3084–3098. doi: 10.1016/j.jacc.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freiberg MS, Chang CH, Skanderson M, Patterson OV, DuVall SL, Brandt CA, So-Armah KA, Vasan RS, Oursler KA, Gottdiener J, Gottlieb S, Leaf D, Rodriguez-Barradas M, Tracy RP, Gibert CL, Rimland D, Bedimo RJ, Brown ST, Goetz MB, Warner A, Crothers K, Tindle HA, Alcorn C, Bachmann JM, Justice AC, Butt AA. Association between hiv infection and the risk of heart failure with reduced ejection fraction and preserved ejection fraction in the antiretroviral therapy era: Results from the veterans aging cohort study. JAMA Cardiol. 2017;2:536–546. doi: 10.1001/jamacardio.2017.0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fitch KV, DeFilippi C, Christenson R, Srinivasa S, Lee H, Lo J, Lu MT, Wong K, Petrow E, Sanchez L, Looby SE, Hoffmann U, Zanni M, Grinspoon SK. Subclinical myocyte injury, fibrosis and strain in relationship to coronary plaque in asymptomatic hiv-infected individuals. AIDS. 2016;30:2205–2214. doi: 10.1097/QAD.0000000000001186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller AM, Xu D, Asquith DL, Denby L, Li Y, Sattar N, Baker AH, McInnes IB, Liew FY. Il-33 reduces the development of atherosclerosis. J Exp Med. 2008;205:339–346. doi: 10.1084/jem.20071868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Porsbjerg C, Baines K, Gibson P, Bergqvist A, Erjefalt JS, Sverrild A, Backer V. Il-33 is related to innate immune activation and sensitization to hdm in mild steroid-free asthma. Clin Exp Allergy. 2016;46:564–574. doi: 10.1111/cea.12702. [DOI] [PubMed] [Google Scholar]
- 8.Sanada S, Hakuno D, Higgins LJ, Schreiter ER, McKenzie AN, Lee RT. Il-33 and st2 comprise a critical biomechanically induced and cardioprotective signaling system. J Clin Invest. 2007;117:1538–1549. doi: 10.1172/JCI30634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, Gorman DM, Bazan JF, Kastelein RA. Il-33, an interleukin-1-like cytokine that signals via the il-1 receptor-related protein st2 and induces t helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 10.Bootcov MR, Bauskin AR, Valenzuela SM, Moore AG, Bansal M, He XY, Zhang HP, Donnellan M, Mahler S, Pryor K, Walsh BJ, Nicholson RC, Fairlie WD, Por SB, Robbins JM, Breit SN. Mic-1, a novel macrophage inhibitory cytokine, is a divergent member of the tgf-beta superfamily. Proc Natl Acad Sci U S A. 1997;94:11514–11519. doi: 10.1073/pnas.94.21.11514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kempf T, Eden M, Strelau J, Naguib M, Willenbockel C, Tongers J, Heineke J, Kotlarz D, Xu J, Molkentin JD, Niessen HW, Drexler H, Wollert KC. The transforming growth factor-beta superfamily member growth-differentiation factor-15 protects the heart from ischemia/reperfusion injury. Circ Res. 2006;98:351–360. doi: 10.1161/01.RES.0000202805.73038.48. [DOI] [PubMed] [Google Scholar]
- 12.Xu J, Kimball TR, Lorenz JN, Brown DA, Bauskin AR, Klevitsky R, Hewett TE, Breit SN, Molkentin JD. Gdf15/mic-1 functions as a protective and antihypertrophic factor released from the myocardium in association with smad protein activation. Circ Res. 2006;98:342–350. doi: 10.1161/01.RES.0000202804.84885.d0. [DOI] [PubMed] [Google Scholar]
- 13.Wang TJ, Wollert KC, Larson MG, Coglianese E, McCabe EL, Cheng S, Ho JE, Fradley MG, Ghorbani A, Xanthakis V, Kempf T, Benjamin EJ, Levy D, Vasan RS, Januzzi JL. Prognostic utility of novel biomarkers of cardiovascular stress: The framingham heart study. Circulation. 2012;126:1596–1604. doi: 10.1161/CIRCULATIONAHA.112.129437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Secemsky EA, Scherzer R, Nitta E, Wu AH, Lange DC, Deeks SG, Martin JN, Snider J, Ganz P, Hsue PY. Novel biomarkers of cardiac stress, cardiovascular dysfunction, and outcomes in hiv-infected individuals. JACC Heart Fail. 2015;3:591–599. doi: 10.1016/j.jchf.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ho JE, Liu C, Lyass A, Courchesne P, Pencina MJ, Vasan RS, Larson MG, Levy D. Galectin-3, a marker of cardiac fibrosis, predicts incident heart failure in the community. J Am Coll Cardiol. 2012;60:1249–1256. doi: 10.1016/j.jacc.2012.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Albert MA, Danielson E, Rifai N, Ridker PM. Effect of statin therapy on c-reactive protein levels: The pravastatin inflammation/crp evaluation (prince): A randomized trial and cohort study. JAMA. 2001;286:64–70. doi: 10.1001/jama.286.1.64. [DOI] [PubMed] [Google Scholar]
- 17.Oesterle A, Laufs U, Liao JK. Pleiotropic effects of statins on the cardiovascular system. Circ Res. 2017;120:229–243. doi: 10.1161/CIRCRESAHA.116.308537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thiara DK, Liu CY, Raman F, Mangat S, Purdy JB, Duarte HA, Schmidt N, Hur J, Sibley CT, Bluemke DA, Hadigan C. Abnormal myocardial function is related to myocardial steatosis and diffuse myocardial fibrosis in hiv-infected adults. J Infect Dis. 2015;212:1544–1551. doi: 10.1093/infdis/jiv274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lo J, Lu MT, Ihenachor EJ, Wei J, Looby SE, Fitch KV, Oh J, Zimmerman CO, Hwang J, Abbara S, Plutzky J, Robbins G, Tawakol A, Hoffmann U, Grinspoon SK. Effects of statin therapy on coronary artery plaque volume and high-risk plaque morphology in hiv-infected patients with subclinical atherosclerosis: A randomised, double-blind, placebo-controlled trial. The Lancet HIV. 2015;2:e52–e63. doi: 10.1016/S2352-3018(14)00032-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nou E, Lu MT, Looby SE, Fitch KV, Kim EA, Lee H, Hoffmann U, Grinspoon SK, Lo J. Serum oxidized low-density lipoprotein decreases in response to statin therapy and relates independently to reductions in coronary plaque in patients with hiv. AIDS. 2016;30:583–590. doi: 10.1097/QAD.0000000000000946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyagaki T, Sugaya M, Yokobayashi H, Kato T, Ohmatsu H, Fujita H, Saeki H, Kikuchi Y, Tamaki T, Sato S. High levels of soluble st2 and low levels of il-33 in sera of patients with hiv infection. J Invest Dermatol. 2011;131:794–796. doi: 10.1038/jid.2010.366. [DOI] [PubMed] [Google Scholar]
- 22.Shimpo M, Morrow DA, Weinberg EO, Sabatine MS, Murphy SA, Antman EM, Lee RT. Serum levels of the interleukin-1 receptor family member st2 predict mortality and clinical outcome in acute myocardial infarction. Circulation. 2004;109:2186–2190. doi: 10.1161/01.CIR.0000127958.21003.5A. [DOI] [PubMed] [Google Scholar]
- 23.Weinberg EO, Shimpo M, Hurwitz S, Tominaga S, Rouleau JL, Lee RT. Identification of serum soluble st2 receptor as a novel heart failure biomarker. Circulation. 2003;107:721–726. doi: 10.1161/01.cir.0000047274.66749.fe. [DOI] [PubMed] [Google Scholar]
- 24.Haraldsen G, Balogh J, Pollheimer J, Sponheim J, Kuchler AM. Interleukin-33 - cytokine of dual function or novel alarmin? Trends Immunol. 2009;30:227–233. doi: 10.1016/j.it.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Chen WY, Hong J, Gannon J, Kakkar R, Lee RT. Myocardial pressure overload induces systemic inflammation through endothelial cell il-33. Proc Natl Acad Sci U S A. 2015;112:7249–7254. doi: 10.1073/pnas.1424236112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin NT, Martin MU. Interleukin 33 is a guardian of barriers and a local alarmin. Nat Immunol. 2016;17:122–131. doi: 10.1038/ni.3370. [DOI] [PubMed] [Google Scholar]
- 27.Guabiraba R, Besnard AG, Menezes GB, Secher T, Jabir MS, Amaral SS, Braun H, Lima-Junior RC, Ribeiro RA, Cunha FQ, Teixeira MM, Beyaert R, Graham GJ, Liew FY. Il-33 targeting attenuates intestinal mucositis and enhances effective tumor chemotherapy in mice. Mucosal Immunol. 2014;7:1079–1093. doi: 10.1038/mi.2013.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 29.Kurowska-Stolarska M, Stolarski B, Kewin P, Murphy G, Corrigan CJ, Ying S, Pitman N, Mirchandani A, Rana B, van Rooijen N, Shepherd M, McSharry C, McInnes IB, Xu D, Liew FY. Il-33 amplifies the polarization of alternatively activated macrophages that contribute to airway inflammation. J Immunol. 2009;183:6469–6477. doi: 10.4049/jimmunol.0901575. [DOI] [PubMed] [Google Scholar]
- 30.Morikawa S, Takabe W, Mataki C, Wada Y, Izumi A, Saito Y, Hamakubo T, Kodama T. Global analysis of rna expression profile in human vascular cells treated with statins. J Atheroscler Thromb. 2004;11:62–72. doi: 10.5551/jat.11.62. [DOI] [PubMed] [Google Scholar]
- 31.Broch K, Ueland T, Nymo SH, Kjekshus J, Hulthe J, Muntendam P, McMurray JJ, Wikstrand J, Cleland JG, Aukrust P, Gullestad L. Soluble st2 is associated with adverse outcome in patients with heart failure of ischaemic aetiology. Eur J Heart Fail. 2012;14:268–277. doi: 10.1093/eurjhf/hfs006. [DOI] [PubMed] [Google Scholar]
- 32.Rohatgi A, Patel P, Das SR, Ayers CR, Khera A, Martinez-Rumayor A, Berry JD, McGuire DK, de Lemos JA. Association of growth differentiation factor-15 with coronary atherosclerosis and mortality in a young, multiethnic population: Observations from the dallas heart study. Clin Chem. 2012;58:172–182. doi: 10.1373/clinchem.2011.171926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Daniels LB, Clopton P, Laughlin GA, Maisel AS, Barrett-Connor E. Growth-differentiation factor-15 is a robust, independent predictor of 11-year mortality risk in community-dwelling older adults: The rancho bernardo study. Circulation. 2011;123:2101–2110. doi: 10.1161/CIRCULATIONAHA.110.979740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eggers KM, Kempf T, Wallentin L, Wollert KC, Lind L. Change in growth differentiation factor 15 concentrations over time independently predicts mortality in community-dwelling elderly individuals. Clin Chem. 2013;59:1091–1098. doi: 10.1373/clinchem.2012.201210. [DOI] [PubMed] [Google Scholar]
- 35.Bonaca MP, Morrow DA, Braunwald E, Cannon CP, Jiang S, Breher S, Sabatine MS, Kempf T, Wallentin L, Wollert KC. Growth differentiation factor-15 and risk of recurrent events in patients stabilized after acute coronary syndrome: Observations from prove it-timi 22. Arterioscler Thromb Vasc Biol. 2011;31:203–210. doi: 10.1161/ATVBAHA.110.213512. [DOI] [PubMed] [Google Scholar]
- 36.Asmuth DM, Pinchuk IV, Wu J, Vargas G, Chen X, Mann S, Albanese A, Ma ZM, Saroufeem R, Melcher GP, Troia-Cancio P, Torok NJ, Miller CJ, Powell DW. Role of intestinal myofibroblasts in hiv-associated intestinal collagen deposition and immune reconstitution following combination antiretroviral therapy. AIDS. 2015;29:877–888. doi: 10.1097/QAD.0000000000000636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen W, Frank ME, Jin W, Wahl SM. Tgf-beta released by apoptotic t cells contributes to an immunosuppressive milieu. Immunity. 2001;14:715–725. doi: 10.1016/s1074-7613(01)00147-9. [DOI] [PubMed] [Google Scholar]
- 38.de Jager SC, Bermudez B, Bot I, Koenen RR, Bot M, Kavelaars A, de Waard V, Heijnen CJ, Muriana FJ, Weber C, van Berkel TJ, Kuiper J, Lee SJ, Abia R, Biessen EA. Growth differentiation factor 15 deficiency protects against atherosclerosis by attenuating ccr2-mediated macrophage chemotaxis. J Exp Med. 2011;208:217–225. doi: 10.1084/jem.20100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mueller T, Leitner I, Egger M, Haltmayer M, Dieplinger B. Association of the biomarkers soluble st2, galectin-3 and growth-differentiation factor-15 with heart failure and other non-cardiac diseases. Clin Chim Acta. 2015;445:155–160. doi: 10.1016/j.cca.2015.03.033. [DOI] [PubMed] [Google Scholar]
- 40.Pfetsch V, Sanin V, Jaensch A, Dallmeier D, Mons U, Brenner H, Koenig W, Rothenbacher D. Increased plasma concentrations of soluble st2 independently predict mortality but not cardiovascular events in stable coronary heart disease patients: 13-year follow-up of the karola study. Cardiovasc Drugs Ther. 2017;31:167–177. doi: 10.1007/s10557-017-6718-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.