Abstract
Psychological depression is frequently linked to alcohol abuse and even serves as key indicators of an alcohol use disorder (AUD). This relationship is supported by preclinical findings in which depression-like phenotypes develop in animals exposed to chronic intermittent ethanol vapor, a common preclinical model of alcohol dependence. However, the emergence of these maladaptive phenotypes following repeated binge-like ethanol drinking remains relatively unexplored. The purpose of this study was to evaluate depression-like behaviors associated with binge-like consumption in mice. Using the drinking-in-the-dark (DID) paradigm, we examined the impact of multiple binge-like cycles (1, 3, or 6) on depression-like behaviors in the forced swim test (FST) and sucrose preference as a test for anhedonia. We also assessed the effect of repeated binge cycles on the consumption of bitter and sweet tastants over a range of concentrations. Results indicated that binge-like ethanol drinking did not lead to depression-like behavior as repeated cycles of DID did not alter sucrose consumption or preference nor did it impact time spent immobile during the FST. Animals that experienced six cycles of DID showed increased quinine consumption and increased quinine preference, which may be indicative of an escalated preference for tastants that resemble the gustatory aspects of ethanol. Interestingly, an unexpected ~20% increase in hypermobility was observed after three cycles of binge-like ethanol drinking. Although the FST is most frequently used to model depression-like behavior, emerging evidence suggests that increased hypermobility during the FST could be indicative of an inability to cope in a stressful situation, suggesting that repeated ethanol exposure in the present experiment transiently enhances stress reactivity.
Keywords: binge drinking, forced swim test, depression-like behavior, anhedonia, taste reactivity, stress reactivity
1. INTRODUCTION
Under the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5), alcohol use disorders (AUDs) are defined as “a problematic pattern of alcohol use leading to clinically significant impairment or distress… (American Psychiatric et al., 2013)”. Given that distress is a defining characteristic of AUDs, it is no surprise that AUDs rarely occur in isolation but are often accompanied by other disorders characterized by psychological distress. In fact, AUDs have been reported to have a relatively high comorbidity with both anxiety and major depressive disorders (Odlaug et al., 2016; Swendsen et al., 1998). It has even been argued that frequent alcohol (ethanol) use may precipitate these states of psychological distress as it is believed that repeated stressors- such as binge drinking episodes- may cause neuroadaptive changes that leaves the individual vulnerable to depression (Beauchaine et al., 2011; Brady and Sinha, 2005; McEwen, 2000).
In line with this reasoning, a well-established model of ethanol dependence, chronic intermittent ethanol (CIE) vapor exposure, leads to plastic changes in key neuropeptide systems such as corticotropin releasing factor (CRF) and neuropeptide Y (NPY; (Eisenhardt et al., 2015; Sommer et al., 2008; Walker et al., 2010). Not only are these alterations believed to be indicative of a transition to a dependence-like state (Koob, 2003), these neuropeptide systems have also been implicated in contributing to both stress/anxiety and depression (Amstadter et al., 2010; Binder and Nemeroff, 2010; Mickey et al., 2011; Soleimani et al., 2014). Accordingly, animals exposed to ethanol vapor subsequently display decreased latency to immobility and increased immobility time during the forced swim test (FST) (Ehlers et al., 2011; Walker et al., 2010) as well as reductions in the time spent in the open arms during an elevated zero maze procedure (Kliethermes et al., 2004; Valdez et al., 2002). Moreover, these animals exhibit elevated levels of ethanol intake following ethanol vapor exposure (Valdez et al., 2002).
Using “drinking-in-the-dark” (DID) procedures to model binge-like ethanol drinking, we and others have found that repeated episodes of binge-like ethanol drinking lead to similar changes in CRF and NPY systems (Lowery-Gionta et al., 2012; McClintick et al., 2015; Sparrow et al., 2012; Sparta et al., 2013). Given that alterations in these peptide systems following DID procedures are analogous to those stemming from CIE, it stands to reason that the DID model of binge-like ethanol drinking would produce animals that similarly exhibit anxiety-like or depression-like phenotypes, as well elevated levels of voluntary ethanol intake. Published data from our lab provided partial support for this hypothesis as repeated episodes of binge-like ethanol drinking using DID procedures augmented subsequent ethanol consumption, a phenotype also characteristic of models of ethanol dependence, but these animals did not display anxiety-like phenotypes across a variety of paradigms (Cox et al., 2013); however, depression-like behaviors were not assessed. Thus, the purpose of the present study was to investigate whether repeated episodes of ethanol DID promote a depression-like phenotype. Additionally, we employed procedures to assess anhedonia in mice with a history of binge-like ethanol drinking as anhedonia has been reported in individuals with AUDs (Hatzigiakoumis et al., 2011; Martinotti et al., 2008).
2. METHODS
2.1 Animals
Male C57BL/6J mice (n = 40), approximately 6–8 weeks old, were obtained from Jackson Laboratories (Bar Harbor, ME). Prior to experimentation, mice were given two weeks to acclimate to being individually housed in a vivarium with a reversed 12:12 hour light:dark cycle at approximately 22°C. The reversed light cycle allowed us to perform all experiments during the animals’ dark cycle. Mice were allowed ad libitum access to T.2920X diet (Harlan® Laboratories, Inc.; Indianapolis, IN) and water unless otherwise indicated in experimental procedures (Marshall et al., 2015). All experimental procedures were approved by the UNC IACUC and complied with the NIH Guide for Care and Use of Laboratory Animals (NRC, 2011).
2.2 Drinking-in-the-Dark (DID) Paradigm
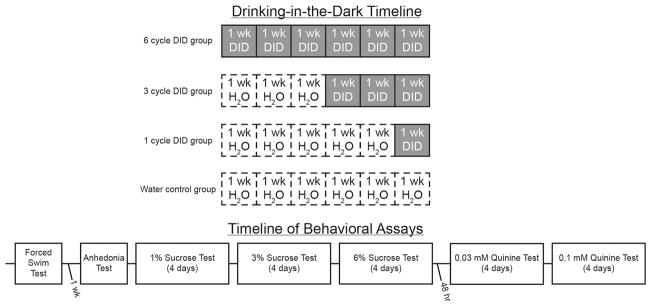
Binge-like drinking was modeled using the standard, four-day DID paradigm (Thiele and Navarro, 2014). Briefly, homecage water bottles were removed three hours into the dark cycle and replaced with sipper-tube bottles containing 20% (v/v) ethanol solution made from 95% ethanol (Decon Labs; King of Prussia, PA) and tap water. For days 1–3, ethanol bottles remained on the cages for two hours; however, on the fourth day, mice had four hours of access to the ethanol solution. Together, these four days constitute a single cycle of DID. Mice were randomly assigned to groups that experienced either 1, 3, or 6 cycles (n=10/group) of the 4-day binge with a three day rest period between cycles. The volume consumed was converted to grams of ethanol and divided by the body weight of the mouse to determine the ethanol dose (g/kg). A fourth group of control animals (n=10) had homecage water bottles replaced with sipper-tube bottles of tap-water for 6 cycles. Blood samples were not collected in an attempt to avoid any confounding effects of the stress of tail nicks and restraint on subsequent behavioral tasks (Harris et al., 2004; Tuli et al., 1995). A schematic outlining the procedure employed is presented in Figure 1
Figure 1. Timeline of experimental procedures.
Top: mice were randomly assigned to one of four groups that experienced six, three, or one week(s) of ethanol DID or a water control group that did not experience ethanol DID (n=10/group). Groups were staggered such that all animals completed DID on the same day. Bottom: 24 hours after the final DID session, mice began the battery of behavioral assays to assess depression-like phenotypes. Time between each assay was 24 hours unless otherwise stated. Abbreviations: DID = drinking-in-the-dark; wk = week.
2.3 Forced-Swim Test (FST)
Approximately 24 hours following the last binge-like drinking session, mice were subjected to a forced-swim test (FST), which is a common method employed to assess depressive-like phenotypes in mice (Bogdanova et al., 2013; Porsolt et al., 1978). Procedures for the FST were similar to previous reports (Lowery et al., 2008; McCall et al., 2013). Briefly, mice were individually placed into a four-liter beaker (25 cm in height, 17.5 cm in diameter) filled with approximately 2.3 liters of room temperature water (21°C) for five minutes approximately three hours into the dark cycle to simulate the chronology of drinking conditions in the DID procedures. Mouse behavior was video-recorded and subsequently analyzed with EthoVision XT 7 (Noldus Information Technology, Inc.; Leesburg, VA). Using this EthoVision software, movement was characterized in three ways: immobility, mobility, and hyper-mobility. This procedure has been described previously (Hedou et al., 2001; Juszczak et al., 2008; Juszczak et al., 2006). Briefly, the software identifies the pixels within the frame belonging to the animal and tracks these pixels frame-by-frame. Movement is assessed by comparing the location of these pixels from one frame to the next. The percentage of pixels that have moved locations is used to determine mobility state (i.e. immobile, mobile, or hyper-mobile) based on predetermined thresholds. In short, the percentage of pixels that have relocated directly reflects the amount of movement such that the more pixels that have changed location, the more the animal is moving. Immobility was defined as movement below 11.5%; whereas hyper-mobility was defined as movement above 18% based on the parameters suggested by EthoVision and previous reports (Bigio et al., 2016; Juszczak et al., 2008).
2.4 Anhedonia Test
One week following the last binge-like drinking session, anhedonia-like behavior was determined using a two-bottle sweet preference test. Three hours into the dark cycle, mice were given six hour access to two drinking bottles containing either a 1% (w/v) sucrose solution or a second bottle containing tap water (Bachmanov et al., 2001). A sucrose preference ratio (sucrose consumed/sucrose + water consumed) was calculated for each animal. Reduced sucrose preference is considered to be reflective of an anhedonia-like state (Willner et al., 1987).
2.5 Two-Bottle Voluntary Consumption and Preference Tests
Twenty-four hours after the anhedonia test, mice were given continuous access to two bottles. One bottle contained water while the other had either a sweet (sucrose) or bitter (quinine) solution. Daily consumption (g/kg) was measured for each concentration of sucrose (1, 3, or 6%, w/v) over a four-day period, in an ascending order. On day one of the 1% sucrose test, the bottles were disturbed affecting the final volume measure; therefore, the 1% sucrose test was only measured over a three-day period with the first day being excluded from the analysis. After a two-day washout period, mice were then similarly tested with quinine solution (0.03mM or 0.1mM. In addition to consumption, preference for either sucrose or quinine was determined using the same formula used in the anhedonia test.
2.6 Statistical Analysis
Prism Version 7.02 (GraphPad Software, Inc. La Jolla, Ca) was used to analyze and graph all data reported herein. One-way analyses of variance (ANOVAs) were used to determine the effect of ethanol experience (control, 1 cycle DID, three cycle DID, and six cycle DID) on binge-like ethanol consumption and activity during the FST. Repeated measures ANOVAs were employed for all other tests. Specifically, concentration of the tastant (1%, 3%, and 6% sucrose, or 0.03mM and 0.1mM quinine) served as the within-subject variable during the tests of voluntary sucrose and quinine consumption, respectively, while test day (days 1 and 2 versus days 3 and 4) was the within-subject variable for the quinine preference test and time (hour 1, 2, 3, 4, 5, and 6) was the within-subject variable for the anhedonia test. In all of these analyses, ethanol experience (control, 1 cycle DID, three cycle DID, and six cycle DID) was the between-subject variable. Post hoc Bonferroni tests with a correction for multiple comparisons were conducted if a significant interaction or main effect of the number of DID cycles was observed. All data are reported as the mean ± standard error of the mean and considered significant if p < 0.05, two-tailed.
3. RESULTS
3.1 DID Procedures Produce Similar Levels of Ethanol Consumption
The average ethanol consumption of all animals was 4.49 ± 0.2g/kg. A one-way ANOVA comparing each of the DID cycle groups revealed no change in ethanol consumption on the final test day of ethanol access ([F(2,27) = 1.42, p = 0.26]; Table 1). The similar levels of ethanol consumption between the groups experiencing different numbers of DID cycles is consistent with previous findings from our lab (Cox et al., 2013). However, the mice in the current study drank relatively less ethanol than mice from previous work (Cox et al., 2013; Olney et al., 2015; Sprow et al., 2015) but were similar to other DID reports with BECs above 80mg/dL (Lee et al., 2015; Marshall et al., 2016a; Marshall et al., 2016b). Differences in consumption between studies were most likely due to changes in rodent chow diet used at UNC (Marshall et al., 2015).
Table 1. Binge-like ethanol consumption (g/kg/4h) on the last day of ethanol access in mice that experienced 1, 3, or 6 cycles of DID.
No significant differences in ethanol consumption were observed as a function of DID history. All values are means ± SEM.
| Ethanol Consumption (g/kg/4h) | |
|---|---|
| 1-Cycle Ethanol | 4.05 ± 0.44 |
| 3-Cycle Ethanol | 4.62 ± 0.32 |
| 6-Cycle Ethanol | 4.82 ± 0.21 |
3.2 DID Cycle Experience alters High-Mobility in Forced-Swim Test
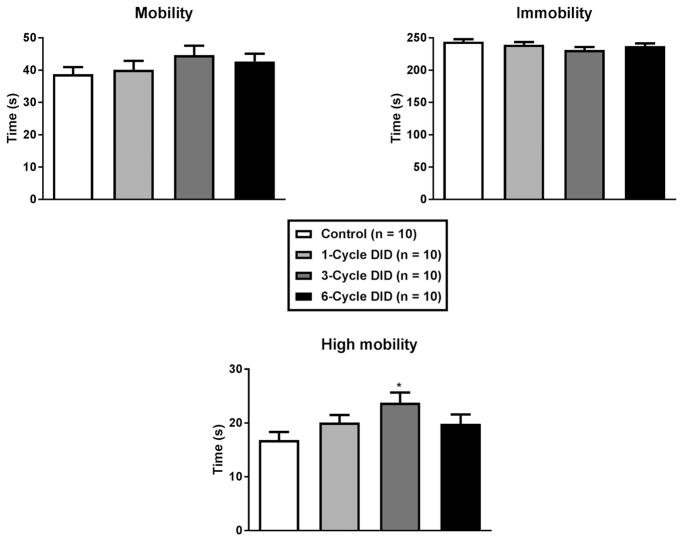
One-way ANOVAs indicated the number of DID cycles animals experience had no effect on either mobility (Figure 2A; [F(3,36) = 1.05, p = 0.38]) or immobility (Figure 2B; [F(3,36) = 1.75, p = 0.18]); however, in relation to high-mobility, a one-way ANOVA indicated that there was a significant effect of treatment (Figure 2C; [F(3,36) = 2.93, p = 0.047]). Post hoc Bonferroni tests indicate that after 3 cycles of DID mice exhibited greater hyper-mobility than mice of the control group but were no different from either of the other ethanol treatment groups.
Figure 2. Average mobility during the 5-minute forced-swim test.
Relative to water drinking control animals, there were no significant effects of binge-like drinking history (1 to 6 binge cycles) on mobility (A) or immobility (B) measures. Interestingly, relative to controls, mice that experience 3 DID cycles showed elevated levels of high mobility behavior (C; defined as 18% greater activity than the average of mobility behavior). All values are means ± SEM, and * p < 0.05 relative to control.
3.3 Ethanol Experience does not alter anhedonia state
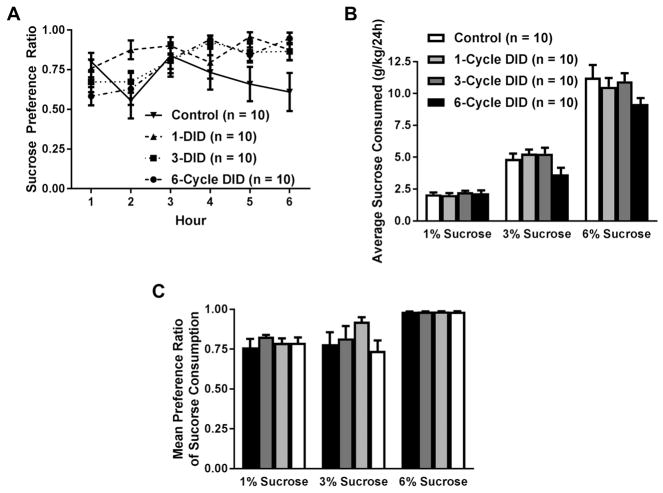
Over the course of six hours, each group had an average sucrose preference of at least 75%, suggesting that they continued to find the 1% sucrose solution rewarding following a history of DID. A two-way (time x ethanol experience) repeated measures ANOVA revealed that sucrose preference changed over the course of the six hour test period (Figure 3A; [main effect of time: F(5,180) = 3.360, p = 0.006]. However, further probing of this effect revealed that sucrose preference at any given time point did not reach statistical significance from any other time point after Bonferroni’s correction of the alpha level. Furthermore, our analysis revealed marginal, albeit nonsignificant, effects of ethanol experience [main effect of ethanol experience: F(3,36) = 2.493, p = 0.076] and an interaction effect [time × ethanol experience: F(15,180) = 1.635, p = 0.068].
Figure 3. Average hourly sucrose preference ratio (sucrose intake/total fluid intake) and daily sucrose consumption in groups of mice that drank water (control) or had a prior history of 1, 3, or 6 cycles of DID.
A prior history of binge-like ethanol drinking did not alter sucrose preference during the anhedonia test (A). No effect of ethanol experience was observed on sucrose consumption, but animals with access to 6% sucrose consumed more sucrose than the other concentrations (B). Moreover, all animals preferred the sweet taste of high concentration of sucrose (6%) more than either of the other two concentrations regardless of DID history (C). All values are means ± SEM.
3.4 Ethanol Experience Alters Tastant Consumption and Preference
In a two-bottle choice continuous access paradigm, mice with varied experiences with ethanol had distinct reactions to sweet and bitter solutions. A two-way repeated measures ANOVA of sucrose consumption showed a main effect of sucrose concentration [Figure 3B; F(2,72) = 383.640, p < 0.0001]. Further probing of this effect revealed that increasing the concentration of sucrose resulted in increased consumption (1% vs 3%: 1% vs 6%; and 3% vs 6% p<0.0001). There was a marginal, yet nonsignificant, effect of DID experience [F(3,36) = 2.420, p = 0.082]. No significant interaction effect of DID experience and sucrose concentration was observed [F(6,72) = 1.522, p = 0.183]. A two-way repeated measures ANOVA analyzing sucrose preference revealed a main effect of sucrose concentration [F(2,72) = 25.964, p < 0.0001]. Further probing of this effect indicated that the 6% sucrose solution was significantly more preferred than any other concentration of sucrose (Figure 3C; 1% vs 6%; and 3% vs 6% p < 0.0001)). However, no differences between the ethanol experience of groups [F(3,36) = 1.333, p = 0.279] or an interaction [F(6,72) = 1.265, p = 0.284] were observed.
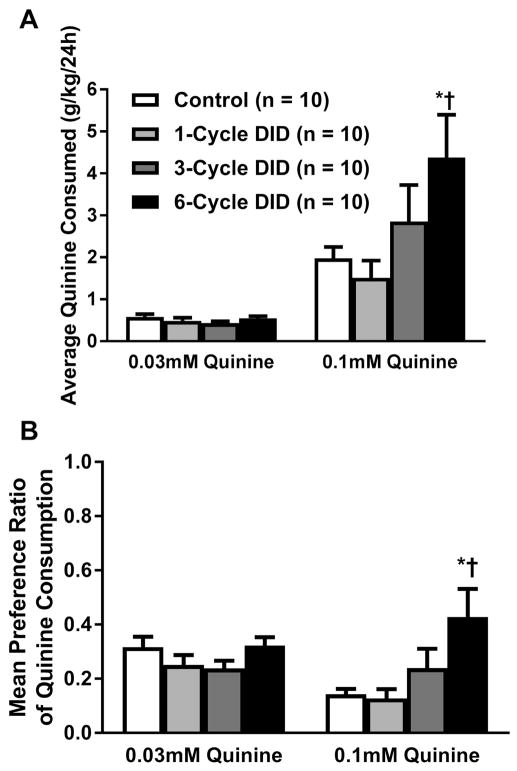
In regard to consumption of the bitter, quinine solution, a two-way repeated measures ANOVA revealed a significant main effect of ethanol experience (Figure 3A; [F(3,36) = 3.00, p = 0.043]), a significant main effect of quinine concentration [F(1,36) = 38.04, p <0.001], as well as a significant interaction effect between the two variables [F(3,36) = 3.19, p = 0.035]. Post hoc Bonferroni tests revealed that the six-cycle DID group consumed significantly more 0.1mM quinine than both the control group (p = 0.016) and one cycle DID group (p = 0.002) while no other comparison was significant. Examining the preference for quinine compared with water, a two-way repeated measures ANOVA, indicated a main effect of ethanol experience [F(3,36) = 3.87, p = 0.017] but not quinine concentration [F(1,36) = 2.14, p =0.15] and an interaction between the two variables ([F(3,36) = 8.92, p = 0.020]; Figure 3B. Further probing of the interaction using post hoc Bonferroni tests revealed that at the 0.1mM concentration, the six-cycle DID group preferred quinine more than both the water-control animals (p = 0.002) and the one cycle DID animals (p = 0.0008); however no effects of ethanol experience were observed at the 0.03mM concentration.
4. DISCUSSION
This study sought to elucidate the impact of binge-like alcohol consumption on depression and anhedonic-like phenotypes under non-dependent conditions. Findings from the present study suggest that repeated cycles of binge-like ethanol drinking using DID procedures did not induce a depression-like phenotype in the FST as measured by immobility nor did it alter anhedonia as measured by voluntary sucrose consumption. Ethanol consumption in the DID paradigm did not lead to a state of anhedonia as neither sucrose preference nor voluntary sucrose consumption was significantly altered as a function of binge-like ethanol drinking, similar to a previous report (Lee et al., 2015). However, it should be noted that anhedonia may not be a core feature of depression (Treadway and Zald, 2011). Indeed, when measured objectively, patients with depression display intact hedonic reactions to sweet tastes (Berlin et al., 1998; Dichter et al., 2010). Notably, these results suggest that our previous observation of increased voluntary ethanol intake stemming from prior binge-like ethanol drinking (Cox et al., 2013) is not likely related to altered sensitivity to salient reinforcers or caloric need. Despite the lack of group differences in sucrose intake, we did observe a trend for elevated consumption of the higher concentration of quinine (0.1 mM) which was further supported by an increase in preference among animals that experienced six cycles of DID. Although this may reflect a reduction in sensitivity to bitter tastants, these differences in quinine responding may arise from a learned association formed by these animals to the taste ethanol (Bachmanov et al., 1996). Indeed, using taste reactivity to measure gustatory perception, Kiefer and colleagues demonstrated that rodents perceive the taste of ethanol as possessing a bitter component (Kiefer et al., 1990). In this sense, repeated binge-like ethanol drinking may have promoted a learned association between the perceived bitter taste of ethanol and its reinforcing post-ingestive effects. When introduced to quinine, these animals may have perceived the bitter tasting solution as ethanol.
Although the focus of these studies was on depressive-like symptoms induced by binge-like consumption, it is important to recognize that we did observe a peculiar increase in high-mobility behavior among mice that experienced three cycles of ethanol DID. It is difficult to truly interpret this finding given that the increased hypermobility observed herein was transient and not seen in animals that experienced six cycles of ethanol. Although the FST is a widely used paradigm for depressive-like behaviors, the validity and translatability of this test to human behaviors continues to be of interest. A review by Commons and colleagues indicates that many of the behaviors previously attributed to depression may actually reflect coping mechanisms in response to the stress during the FST (Commons et al., 2017). Although rather unexpected, the increase in hypermobility observed in these studies may actually reflect altered stress-reactivity in these mice. More specifically, it could indicate that a history of binge-like ethanol drinking promotes maladaptive stress responding of the mice, which caused them to display hyper-reactive behavior (i.e. hypermobility) in response to the stress associated with the FST (de Kloet and Molendijk, 2016). This interpretation is supported by the fact that increases in CRF immunoreactivity within the central nucleus of the amygdala (CeA), an area known to be integrally involved in both alcohol consumption and stress/anxiety (Gilpin et al., 2015; Lowery et al., 2010; Rinker et al., 2016), were evident following three cycles of ethanol DID (Lowery-Gionta et al., 2012). Moreover, alterations in the NPY system within the CeA emerge after a single cycle of DID and peak after three cycles (Sparrow et al., 2012). Given that our previous study did not suggest altered stress-related phenotypes following DID using the elevated-plus maze or open field locomotor chambers (Cox et al., 2013), we believe the hypermobility observed herein may suggest a maladaptive response to a stressor or panic-like phenotype (Becker et al., 2011; Breese et al., 2005; Lowery et al., 2008). Moreover, a recent report by Somkuwar and colleagues demonstrate through both biological and behavioral indicators that irritability responses are heightened after withdrawal, suggesting that ethanol dependence may sensitize stress-reactivity (Somkuwar et al., 2017). Interestingly, the effects on irritability and anxiety observed appear to be dependent upon the duration of abstinence (Somkuwar et al., 2017; Zhao et al., 2007). Whether the stress associated with FST compounds stress reactivity similar to the stress of withdrawal remains to be determined. To truly understand this anomalous result, future studies will have to compare stress reactivity within the context of non-dependent ethanol consumption during intoxication as well as during different durations of abstinence using tests such as acute startle response or tail suspension task.
Together with the previous findings from our lab (Cox et al., 2013), the present study indicates that a history of binge-like ethanol drinking resulting from DID procedures is not significantly associated with anhedonia, anxiety-, or depression-like behavior. Intriguingly, these findings contrast with a recent study that used similar methods yet found drastically different results. Using modified DID procedures, Lee and colleagues reported that a history of binge-like ethanol drinking produced increases in anxiety-like behavior as measured by a variety of assays, including the elevated plus maze, as well as signs of depression-like phenotypes via the FST (Lee et al., 2015). Notably, these authors did not measure hypermobility during FST. Whether or not the DID paradigm employed by Lee and colleagues similarly induced a hyperactive state during the FST remains unknown. Although the procedures of the behavioral assessments used in our studies versus those previously used were slightly different (Lee et al., 2015), the contrasting behavioral outcomes are most likely a result of the different DID procedures used by each research group. Indeed, we employed the standard, 4-day DID model that consists 2-h access to ethanol during the first three days and 4-h access on the fourth day followed by three days of rest between cycles in our studies. On the other hand, Lee and colleagues used a modified, 5-day procedure in which animals had 2-h of access to ethanol across the five days with only two days of rest between cycles (Lee et al., 2015). Moreover, our 5-minute FST was done 24 hours after the last ethanol cycle, but Lee and colleagues first used a 15-minute FST and report that the anxiety measures increased with prolonged abstinence (22 days after ethanol exposure) (Lee et al., 2015). It is possible that by using a longer FST or by putting animals through prolonged forced ethanol abstinence, Lee’s studies may have observed heightened depression and anxiety. It is also possible that a depression-like phenotype may have emerged had we performed the FST temporally more proximal to the removal of ethanol.
It is important to continue to differentiate the neurobiological maladaptations and behavioral consequences induced by alcohol dependence compared with binge drinking to understand the transition between alcohol misuse and dependence. Reports continue to demonstrate that neurobiological changes that underlie the transition to a dependent-like state may occur as a result of repeated binge exposure. For example, CIE, a model of alcohol dependence, and the DID binge-model have been reported to lead to similar changes in key neuromodulatory systems associated with dependence and psychological distress (Eisenhardt et al., 2015; Lowery-Gionta et al., 2012; McClintick et al., 2015; Sommer et al., 2008; Sparrow et al., 2012; Sparta et al., 2013; Walker et al., 2010). However, the behavioral outcomes resulting from these two paradigms are not identical. Although each of these models promotes increases in subsequent ethanol consumption (Cox et al., 2013; Gilpin et al., 2011), animals that experience CIE display stress-related phenotypes (Ehlers et al., 2011; Kliethermes et al., 2004; Somkuwar et al., 2017; Valdez et al., 2002) while those that experience DID do not consistently show stress-related phenotypes (Cox et al., 2013; Lee et al., 2015). Findings from the present study extend upon these findings by demonstrating that, unlike CIE procedures (Walker et al., 2010), repeated cycles of binge-like ethanol drinking using DID procedures did not support a depression-like phenotype in the FST. Importantly, both models promote subsequent increases of voluntary ethanol consumption. When taken together, these findings support DID as an ideal model for studying the early stages of neuroplasticity the emerge with repeated bouts of binge-like ethanol intake and that continue to develop over the transition to dependence.
Figure 4. Average daily consumption (g/kg/24h) of each concentration of quinine solution, or average preference ratio of the 0.1 mM quinine solution, by control mice or mice that had a prior history of 1, 3, or 6 cycles of DID.
Animals in the 6-cycle DID group displayed significantly elevated voluntary consumption (A) and preference (B) of quinine at the 0.1 mM concentration relative to both the control and 1-cycle DID groups. All values are mean ± SEM; * signifies that p < 0.05 relative to the water-drinking control group; † denotes that p < 0.05 relative to 1-cycle DID group.
Highlights.
Non-dependent binge-like consumption did not alter depressive-like behaviors
Binge drinking resulted in increased quinine consumption
Binge drinking caused a transient increased hyper-reactivity response in the forced-swim test
Acknowledgments
This work was funded by National Institute of Health grants AA022048, AA013573, GM000678, and AA015148. The authors thank Ms. Rhiannon D. Thomas and Ms. Marian G. Mersmann for their expert technical assistance in these studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric, A., American Psychiatric, A., Force, D.S.M.T. Diagnostic and statistical manual of mental disorders: DSM-5 2013 [Google Scholar]
- Amstadter AB, Koenen KC, Ruggiero KJ, Acierno R, Galea S, Kilpatrick DG, Gelernter J. NPY moderates the relation between hurricane exposure and generalized anxiety disorder in an epidemiologic sample of hurricane-exposed adults. Depress Anxiety. 2010;27(3):270–275. doi: 10.1002/da.20648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmanov AA, Tordoff MG, Beauchamp GK. Ethanol consumption and taste preferences in C57BL/6ByJ and 129/J mice. Alcohol Clin Exp Res. 1996;20(2):201–206. doi: 10.1111/j.1530-0277.1996.tb01630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmanov AA, Tordoff MG, Beauchamp GK. Sweetener preference of C57BL/6ByJ and 129P3/J mice. Chem Senses. 2001;26(7):905–913. doi: 10.1093/chemse/26.7.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine TP, Neuhaus E, Zalewski M, Crowell SE, Potapova N. The effects of allostatic load on neural systems subserving motivation, mood regulation, and social affiliation. Dev Psychopathol. 2011;23(4):975–999. doi: 10.1017/S0954579411000459. [DOI] [PubMed] [Google Scholar]
- Becker HC, Lopez MF, Doremus-Fitzwater TL. Effects of stress on alcohol drinking: a review of animal studies. Psychopharmacology (Berl) 2011;218(1):131–156. doi: 10.1007/s00213-011-2443-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin I, Givry-Steiner L, Lecrubier Y, Puech AJ. Measures of anhedonia and hedonic responses to sucrose in depressive and schizophrenic patients in comparison with healthy subjects. Eur Psychiatry. 1998;13(6):303–309. doi: 10.1016/S0924-9338(98)80048-5. [DOI] [PubMed] [Google Scholar]
- Bigio B, Mathe AA, Sousa VC, Zelli D, Svenningsson P, McEwen BS, Nasca C. Epigenetics and energetics in ventral hippocampus mediate rapid antidepressant action: Implications for treatment resistance. Proc Natl Acad Sci U S A. 2016;113(28):7906–7911. doi: 10.1073/pnas.1603111113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder EB, Nemeroff CB. The CRF system, stress, depression and anxiety-insights from human genetic studies. Mol Psychiatry. 2010;15(6):574–588. doi: 10.1038/mp.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanova OV, Kanekar S, D’Anci KE, Renshaw PF. Factors influencing behavior in the forced swim test. Physiol Behav. 2013;118:227–239. doi: 10.1016/j.physbeh.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady KT, Sinha R. Co-occurring mental and substance use disorders: the neurobiological effects of chronic stress. Am J Psychiatry. 2005;162(8):1483–1493. doi: 10.1176/appi.ajp.162.8.1483. [DOI] [PubMed] [Google Scholar]
- Breese GR, Chu K, Dayas CV, Funk D, Knapp DJ, Koob GF, Le DA, O’Dell LE, Overstreet DH, Roberts AJ, Sinha R, Valdez GR, Weiss F. Stress enhancement of craving during sobriety: a risk for relapse. Alcohol Clin Exp Res. 2005;29(2):185–195. doi: 10.1097/01.alc.0000153544.83656.3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commons KG, Cholanians AB, Babb JA, Ehlinger DG. The Rodent Forced Swim Test Measures Stress-Coping Strategy, Not Depression-like Behavior. ACS Chem Neurosci. 2017;8(5):955–960. doi: 10.1021/acschemneuro.7b00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox BR, Olney JJ, Lowery-Gionta EG, Sprow GM, Rinker JA, Navarro M, Kash TL, Thiele TE. Repeated cycles of binge-like ethanol (EtOH)-drinking in male C57BL/6J mice augments subsequent voluntary EtOH intake but not other dependence-like phenotypes. Alcohol Clin Exp Res. 2013;37(10):1688–1695. doi: 10.1111/acer.12145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet ER, Molendijk ML. Coping with the Forced Swim Stressor: Towards Understanding an Adaptive Mechanism. Neural Plast. 2016;2016:6503162. doi: 10.1155/2016/6503162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Smoski MJ, Kampov-Polevoy AB, Gallop R, Garbutt JC. Unipolar depression does not moderate responses to the Sweet Taste Test. Depress Anxiety. 2010;27(9):859–863. doi: 10.1002/da.20690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Criado JR, Wills DN, Liu W, Crews FT. Periadolescent ethanol exposure reduces adult forebrain ChAT+IR neurons: correlation with behavioral pathology. Neuroscience. 2011;199:333–345. doi: 10.1016/j.neuroscience.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhardt M, Hansson AC, Spanagel R, Bilbao A. Chronic intermittent ethanol exposure in mice leads to an up-regulation of CRH/CRHR1 signaling. Alcohol Clin Exp Res. 2015;39(4):752–762. doi: 10.1111/acer.12686. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Herman MA, Roberto M. The central amygdala as an integrative hub for anxiety and alcohol use disorders. Biol Psychiatry. 2015;77(10):859–869. doi: 10.1016/j.biopsych.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Misra K, Herman MA, Cruz MT, Koob GF, Roberto M. Neuropeptide Y opposes alcohol effects on gamma-aminobutyric acid release in amygdala and blocks the transition to alcohol dependence. Biol Psychiatry. 2011;69(11):1091–1099. doi: 10.1016/j.biopsych.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RB, Gu H, Mitchell TD, Endale L, Russo M, Ryan DH. Increased glucocorticoid response to a novel stress in rats that have been restrained. Physiol Behav. 2004;81(4):557–568. doi: 10.1016/j.physbeh.2004.01.017. [DOI] [PubMed] [Google Scholar]
- Hatzigiakoumis DS, Martinotti G, Giannantonio MD, Janiri L. Anhedonia and substance dependence: clinical correlates and treatment options. Front Psychiatry. 2011;2:10. doi: 10.3389/fpsyt.2011.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedou G, Pryce C, Di Iorio L, Heidbreder CA, Feldon J. An automated analysis of rat behavior in the forced swim test. Pharmacol Biochem Behav. 2001;70(1):65–76. doi: 10.1016/s0091-3057(01)00575-5. [DOI] [PubMed] [Google Scholar]
- Juszczak GR, Lisowski P, Sliwa AT, Swiergiel AH. Computer assisted video analysis of swimming performance in a forced swim test: simultaneous assessment of duration of immobility and swimming style in mice selected for high and low swim-stress induced analgesia. Physiol Behav. 2008;95(3):400–407. doi: 10.1016/j.physbeh.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Juszczak GR, Sliwa AT, Wolak P, Tymosiak-Zielinska A, Lisowski P, Swiergiel AH. The usage of video analysis system for detection of immobility in the tail suspension test in mice. Pharmacol Biochem Behav. 2006;85(2):332–338. doi: 10.1016/j.pbb.2006.08.016. [DOI] [PubMed] [Google Scholar]
- Kiefer SW, Bice PJ, Orr MR, Dopp JM. Similarity of taste reactivity responses to alcohol and sucrose mixtures in rats. Alcohol. 1990;7(2):115–120. doi: 10.1016/0741-8329(90)90071-j. [DOI] [PubMed] [Google Scholar]
- Kliethermes CL, Cronise K, Crabbe JC. Anxiety-like behavior in mice in two apparatuses during withdrawal from chronic ethanol vapor inhalation. Alcohol Clin Exp Res. 2004;28(7):1012–1019. doi: 10.1097/01.alc.0000131976.40428.8f. [DOI] [PubMed] [Google Scholar]
- Koob GF. Alcoholism: allostasis and beyond. Alcohol Clin Exp Res. 2003;27(2):232–243. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- Lee KM, Coehlo M, McGregor HA, Waltermire RS, Szumlinski KK. Binge alcohol drinking elicits persistent negative affect in mice. Behav Brain Res. 2015;291:385–398. doi: 10.1016/j.bbr.2015.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery-Gionta EG, Navarro M, Li C, Pleil KE, Rinker JA, Cox BR, Sprow GM, Kash TL, Thiele TE. Corticotropin releasing factor signaling in the central amygdala is recruited during binge-like ethanol consumption in C57BL/6J mice. J Neurosci. 2012;32(10):3405–3413. doi: 10.1523/JNEUROSCI.6256-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery EG, Spanos M, Navarro M, Lyons AM, Hodge CW, Thiele TE. CRF-1 antagonist and CRF-2 agonist decrease binge-like ethanol drinking in C57BL/6J mice independent of the HPA axis. Neuropsychopharmacology. 2010;35(6):1241–1252. doi: 10.1038/npp.2009.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery EG, Sparrow AM, Breese GR, Knapp DJ, Thiele TE. The CRF-1 receptor antagonist, CP-154,526, attenuates stress-induced increases in ethanol consumption by BALB/cJ mice. Alcohol Clin Exp Res. 2008;32(2):240–248. doi: 10.1111/j.1530-0277.2007.00573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall SA, Casachahua JD, Rinker JA, Blose AK, Lysle DT, Thiele TE. IL-1 receptor signaling in the basolateral amygdala modulates binge-like ethanol consumption in male C57BL/6J mice. Brain Behav Immun. 2016a;51:258–267. doi: 10.1016/j.bbi.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall SA, McKnight KH, Blose AK, Lysle DT, Thiele TE. Modulation of Binge-like Ethanol Consumption by IL-10 Signaling in the Basolateral Amygdala. J Neuroimmune Pharmacol. 2016b doi: 10.1007/s11481-016-9709-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall SA, Rinker JA, Harrison LK, Fletcher CA, Herfel TM, Thiele TE. Assessment of the Effects of 6 Standard Rodent Diets on Binge-Like and Voluntary Ethanol Consumption in Male C57BL/6J Mice. Alcohol Clin Exp Res. 2015 doi: 10.1111/acer.12773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinotti G, Nicola MD, Reina D, Andreoli S, Foca F, Cunniff A, Tonioni F, Bria P, Janiri L. Alcohol protracted withdrawal syndrome: the role of anhedonia. Subst Use Misuse. 2008;43(3–4):271–284. doi: 10.1080/10826080701202429. [DOI] [PubMed] [Google Scholar]
- McCall NM, Sprow GM, Delpire E, Thiele TE, Kash TL, Pleil KE. Effects of sex and deletion of neuropeptide Y2 receptors from GABAergic neurons on affective and alcohol drinking behaviors in mice. Front Integr Neurosci. 2013;7:100. doi: 10.3389/fnint.2013.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintick JN, McBride WJ, Bell RL, Ding ZM, Liu Y, Xuei X, Edenberg HJ. Gene expression changes in serotonin, GABA-A receptors, neuropeptides and ion channels in the dorsal raphe nucleus of adolescent alcohol-preferring (P) rats following binge-like alcohol drinking. Pharmacol Biochem Behav. 2015;129:87–96. doi: 10.1016/j.pbb.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Allostasis and allostatic load: implications for neuropsychopharmacology. Neuropsychopharmacology. 2000;22(2):108–124. doi: 10.1016/S0893-133X(99)00129-3. [DOI] [PubMed] [Google Scholar]
- Mickey BJ, Zhou Z, Heitzeg MM, Heinz E, Hodgkinson CA, Hsu DT, Langenecker SA, Love TM, Pecina M, Shafir T, Stohler CS, Goldman D, Zubieta JK. Emotion processing, major depression, and functional genetic variation of neuropeptide Y. Arch Gen Psychiatry. 2011;68(2):158–166. doi: 10.1001/archgenpsychiatry.2010.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC. Guide for the care and use of laboratory animals. 8. National Academies Press; Washington, D.C: 2011. [PubMed] [Google Scholar]
- Odlaug BL, Gual A, DeCourcy J, Perry R, Pike J, Heron L, Rehm J. Alcohol Dependence, Co-occurring Conditions and Attributable Burden. Alcohol Alcohol. 2016;51(2):201–209. doi: 10.1093/alcalc/agv088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney JJ, Navarro M, Thiele TE. Binge-like consumption of ethanol and other salient reinforcers is blocked by orexin-1 receptor inhibition and leads to a reduction of hypothalamic orexin immunoreactivity. Alcohol Clin Exp Res. 2015;39(1):21–29. doi: 10.1111/acer.12591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol. 1978;47(4):379–391. doi: 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- Rinker JA, Marshall SA, Mazzone CM, Lowery-Gionta EG, Gulati V, Pleil KE, Kash TL, Navarro M, Thiele TE. Extended Amygdala to Ventral Tegmental Area Corticotropin-Releasing Factor Circuit Controls Binge Ethanol Intake. Biol Psychiatry. 2016 doi: 10.1016/j.biopsych.2016.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soleimani L, Oquendo MA, Sullivan GM, Mathe AA, Mann JJ. Cerebrospinal fluid neuropeptide Y levels in major depression and reported childhood trauma. Int J Neuropsychopharmacol. 2014;18(1) doi: 10.1093/ijnp/pyu023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somkuwar SS, Vendruscolo LF, Fannon MJ, Schmeichel BE, Nguyen TB, Guevara J, Sidhu H, Contet C, Zorrilla EP, Mandyam CD. Abstinence from prolonged ethanol exposure affects plasma corticosterone, glucocorticoid receptor signaling and stress-related behaviors. Psychoneuroendocrinology. 2017;84:17–31. doi: 10.1016/j.psyneuen.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer WH, Rimondini R, Hansson AC, Hipskind PA, Gehlert DR, Barr CS, Heilig MA. Upregulation of voluntary alcohol intake, behavioral sensitivity to stress, and amygdala crhr1 expression following a history of dependence. Biol Psychiatry. 2008;63(2):139–145. doi: 10.1016/j.biopsych.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Sparrow AM, Lowery-Gionta EG, Pleil KE, Li C, Sprow GM, Cox BR, Rinker JA, Jijon AM, Pena J, Navarro M, Kash TL, Thiele TE. Central neuropeptide Y modulates binge-like ethanol drinking in C57BL/6J mice via Y1 and Y2 receptors. Neuropsychopharmacology. 2012;37(6):1409–1421. doi: 10.1038/npp.2011.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparta DR, Hopf FW, Gibb SL, Cho SL, Stuber GD, Messing RO, Ron D, Bonci A. Binge ethanol-drinking potentiates corticotropin releasing factor R1 receptor activity in the ventral tegmental area. Alcohol Clin Exp Res. 2013;37(10):1680–1687. doi: 10.1111/acer.12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprow GM, Rinker JA, Lowery-Gointa EG, Sparrow AM, Navarro M, Thiele TE. Lateral hypothalamic melanocortin receptor signaling modulates binge-like ethanol drinking in C57BL/6J mice. Addict Biol. 2015 doi: 10.1111/adb.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swendsen JD, Merikangas KR, Canino GJ, Kessler RC, Rubio-Stipec M, Angst J. The comorbidity of alcoholism with anxiety and depressive disorders in four geographic communities. Compr Psychiatry. 1998;39(4):176–184. doi: 10.1016/s0010-440x(98)90058-x. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Navarro M. “Drinking in the dark” (DID) procedures: A model of binge-like ethanol drinking in non-dependent mice. Alcohol. 2014;48(3):235–241. doi: 10.1016/j.alcohol.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Zald DH. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci Biobehav Rev. 2011;35(3):537–555. doi: 10.1016/j.neubiorev.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuli JS, Smith JA, Morton DB. Corticosterone, adrenal and spleen weight in mice after tail bleeding, and its effect on nearby animals. Lab Anim. 1995;29(1):90–95. doi: 10.1258/002367795780740339. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, Koob GF. Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcohol Clin Exp Res. 2002;26(10):1494–1501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- Walker BM, Drimmer DA, Walker JL, Liu T, Mathe AA, Ehlers CL. Effects of prolonged ethanol vapor exposure on forced swim behavior, and neuropeptide Y and corticotropin-releasing factor levels in rat brains. Alcohol. 2010;44(6):487–493. doi: 10.1016/j.alcohol.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology (Berl) 1987;93(3):358–364. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Weiss F, Zorrilla EP. Remission and resurgence of anxiety-like behavior across protracted withdrawal stages in ethanol-dependent rats. Alcohol Clin Exp Res. 2007;31(9):1505–1515. doi: 10.1111/j.1530-0277.2007.00456.x. [DOI] [PubMed] [Google Scholar]