Abstract
Objective
To examine the effect of a lifestyle behavior intervention (SystemCHANGE) on physical activity and diet quality among sedentary people living with HIV (PLHIV). All participants expressed a desire to improve lifestyle health behaviors.
Methods
One-hundred and six HIV+ adults were randomized to either the intervention (six, in-person, standardized group sessions focusing on improving lifestyle behaviors) or a control condition (general advice on AHA diet and exercise guidelines). All participants wore an ActiGraph accelerometer and completed 24-hour dietary recalls at baseline, 3 and 6 months. Generalized estimating equations were used to examine intervention effects. The primary activity outcome was time spent in moderate-to-vigorous physical activity, and the primary dietary outcome was Healthy Eating Index.
Results
Mean age was 53 years; 65% were male and 86% African American. Approximately 90% attended at least half of the sessions and 60% attended 5 or more sessions. The intervention did not significantly improve our primary lifestyle behavior endpoints (p ≥ 0.05); however, intervention participants consumed fewer carbohydrates—primarily sugar-sweetened beverages—per day and lost 0.732 kg body weight compared to a 0.153 weight gain in the control group (p=0.03).
Conclusion
Among sedentary PLHIV at high risk for CVD, the SystemCHANGE intervention reduced daily carbohydrate intake and body weight, but did not increase physical activity or improve overall diet quality. Future work should identify fundamental personal, interpersonal, and contextual factors that will increase physical activity and improve overall diet quality among this population, and integrate these factors into tailored, lifestyle interventions for aging PLHIV.
Keywords: HIV, exercise, diet, cardiovascular disease, clinical trial
Over the past two decades, as people living with HIV (PLHIV) have aged, there has been corresponding need to develop interventions to prevent and mitigate cardiovascular disease (CVD). While much of the focus has been on pharmacological strategies, there is a growing need for effective behavioral CVD self-management strategies (e.g., physical activity and eating a healthy diet). 1 Though behavioral strategies are recommended, there is limited evidence on how to help PLHIV initiate and maintain changes in CVD self-management behaviors in their everyday lives. 1–5
The effects of physical activity and eating a healthy diet on reducing diabetes, hypertension, obesity, and CVD events are indisputable. 6–11 Yet, for PLHIV to benefit from these behaviors they must, over the long term, adopt a self-management approach that includes regular physical activity and eating a healthy diet. 12–14 Recently, Willig et al., (2016) found that 68% of PLHIV in the United States engaged in low amounts of physical activity, which was associated with increased diabetes, hypertension, obesity, CVD, and cerebrovascular disease.15 Vancampfort et al. (2016) reported that only an estimated 50% of PLHIV worldwide met the recommendations of 150 minutes of moderate-to-vigorous physical activity (MVPA) per week, 16 and further found that 30% of PLHIV who consented and enrolled in physical activity interventions dropped out, a higher rate than other chronically ill populations. 17 A study examining diet quality in PLHIV in a high resource setting reported an average Healthy Eating Index of 50 out of 100, indicating that PLHIV conformed with half of the daily dietary recommendations; 18 slightly lower than the average in American adults of 55. 19 These studies suggest that most PLHIV do not meet physical activity and diet quality recommendations and that increasing their engagement in these behaviors is likely to reduce CVD. Given this, new interventions to improve physical activity and dietary quality for PLHIV are needed.
SystemCHANGE is an innovative self-management intervention that draws on social-ecological and process-improvement theories to promote behavior change. 20–22 Individuals redesign their interpersonal environmental systems related to health behavior (i.e. daily routines linked to others in the household or interpersonal network) to help build habitual lifestyle behaviors into one’s daily routine. 23–27 We used the SystemCHANGE framework to help PLHIV change their daily interpersonal environmental systems to promote healthy behavior. We tailored specific content to address common challenges faced by PLHIV, such as being immunocompromised, receiving conflicting recommendations about diet and exercise, limited opportunities and venues in which to exercise, medication adherence; and high levels of fatigue, neuropathy and other distressing symptoms.26 Feasibility pilot testing of the intervention suggested it was acceptable and feasible to PLHIV and would increase physical activity. 26 However, a fully powered randomized clinical trial was necessary to evaluate its efficacy to improve CVD self-management in PLHIV.
The purpose of this study was to evaluate the 3- and 6-month effects of SystemCHANGE on physical activity and dietary quality in PLHIV at high risk for developing CVD. We hypothesized that the SystemCHANGE intervention would lead to improvements in physical activity and diet quality compared to enhanced usual care.
Methods
Design
We conducted a randomized clinical trial to test the effect of the SystemCHANGE intervention on physical activity and dietary quality in adults living with HIV(NCT02553291).
Sample and Recruitment
One-hundred and nine (n=109) PLHIV were recruited via letters sent to an HIV research registry and flyers posted in HIV care organizations in Cleveland, Ohio. Our sample size was based on our prior work indicating that at three months, the SystemCHANGE participants would engage in an additional 2.24 hours per month of MVPA.28 With 109 participants we had 90% power to detect a statistically significant difference in MVPA between the groups.
Potential participants telephoned a Research Assistant who screened them for study eligibility. To be included in the study, participants had to be 1) >18 years of age, 2) at high risk for developing CVD (Framingham 30-year CVD risk score>20% for females and >30% for males), 3) if prescribed a statin medication, taking it for at least 6 months, and 4) on antiretroviral therapy with suppressed HIV-1 viremia (<400 copies/mL) for at least one year prior to enrollment. Potential participants were excluded if they 1) had a medical contraindication for exercise, 29 2) already met weekly physical activity recommendations of 150 minutes of MVPA 30 (assessed with the 7-day physical activity recall 31), 3) did not understand spoken English, 4) expected to move out of the immediate area, have surgery, were pregnant or planned on becoming pregnant in the next 6 months, 6) had a hgA1c>7%, or 7) were enrolled in a weight loss program.
The Institutional Review Board at University Hospitals, Cleveland Medical Center approved this study. Eligible participants were invited to a baseline visit in which a research assistant reviewed an informed consent document that outlined the study’s scope, purpose, risks and potential benefits. After confirming understanding, the research assistant obtained written informed consent, and the participant had his or her blood drawn and, if a woman of childbearing age, a urine pregnancy test. After completing all baseline measures, participants were randomized 1:1 using randomly generated numbers from uniform distribution in Stata 14.0, stratified on gender and race, to either the SystemCHANGE intervention or a one-time cardiovascular risk reduction counselling condition. All study procedures occurred between November, 2014 and Ma,y 2017.
Interventions
The SystemCHANGE Intervention is based on an existing, manualized self-management intervention emphasizing interpersonal environmental systems,27 as opposed to self-management interventions based on cognitive behavioral skill-building. SystemCHANGE was effective in improving lifestyle behaviors in adults with cardiovascular disease,28 and is currently being tested in stroke survivors and kidney transplant recipients.25,32 The SystemCHANGE Intervention has two content components: behavior-change techniques and healthy lifestyle behavior education. The intervention was administered in six, weekly face-to-face group sessions. Each session consisted of ~30 minutes of interactive education on behavioral-change techniques and ~30 minutes of physical activity and nutrition education (Supplemental Digital Material 1). The interventionist was a public high school teacher who had previously served as the interventionist in a study testing the effect of SystemCHANGE on behavioral obesity management in urban families.34
Behavior-Change Techniques
The group sessions included techniques for changing a system to achieve a specific, participant-defined goal to improve lifestyle behaviors (physical activity and diet). These techniques helped participants (1) examine the system processes surrounding goal attainment, (2) engage in a series of experiments to test the best ideas to improve the process, (3) track outcomes of these trials, (4) implement the most successful ideas based on data from experiences, and (5) monitor the system to maintain the gains. Thus, participants were taught how to modify their immediate environments so that they could succeed despite wavering motivation. 23
Healthy Lifestyle Behavior Education
Our healthy lifestyle content was based on the 2013 Guidelines on Lifestyle Management to Reduce Cardiovascular Risk and emphasized a diet consisting of low-energy-density foods through increased fresh fruits, vegetables, and whole grains.35 The interventionist discussed the types of physical activity, explained issues that may interfere with sufficient activity, taught techniques to modify the participants’ physical environment to encourage activity and eating a healthy diet, and discussed how to incorporate healthy eating and physical activity into the participant’s daily routine (e.g., after taking medications). Throughout the intervention, participants were encouraged to increase their activity amount and intensity and improve diet quality as their goals were met. 36,37
Enhanced Usual Care Intervention
All participants continued receiving usual care at their primary health care facility. Participants randomized to enhanced usual care received an American Heart Association pamphlet that contained information on healthy eating and physical activity. A research assistant reviewed this with each participant. Study staff also telephoned participants in this group at the same time points as the SystemCHANGE intervention group sessions. These 5–10 minute calls were primarily social/generic in content to provide an equivalent number of contacts as the SystemCHANGE group. They also served to maintain involvement and promote retention of the enhanced usual care group.
Procedures and Measures
Demographic, Anthropometric, and HIV Characteristics
At baseline, participants completed a self-reported demographic survey on a private computer.38 A research assistant assisted those who were unable to complete the survey. After completing the survey, participants had standardized weight, height, and hip and waist circumference measures assessed by trained staff at the University Hospitals, Cleveland Medical Center Dahms Clinical Research Unit (DCRU). Participants also consented to medical chart abstraction from which team members abstracted relevant medical data including the number of years the participant had been living with HIV, current CD4+ T cell count, and CD4+ T cell nadir at baseline.
Physical Activity
A research assistant gave all participants an ActiGraph wGT3X-BT accelerometer (ActiGraph, LLC, Fort Walton Beach, FL) 39–41 and instructed them to wear the accelerometer during waking hours for 7 consecutive days, except for when showering and swimming. A research assistant then affixed the accelerometer to elastic belts, placed it over the non-dominant hip, and counseled participants on the importance of wearing it every day. A research assistant called each participant two days after they received the devices to ensure they were wearing it correctly, address concerns, and remind them to return it in one week. Upon return, a research assistant checked that the data met the minimum quality standards for valid wear time (at least 4 days and at least 10 hours per day). 42–44 Participants not meeting standards were asked to re-wear the accelerometer for 7 days. All accelerometer data were sampled at 30Hz, using 60 second epochs, and the normal filter. 45 MVPA was defined as activity ≥ 2690 counts per minute for ≥ 10 minutes. 46 We used the ActiLife software to calculate the amount of time spent in MVPA per valid day using the Sasaki, John, and Freedson (2011) adult cutpoints for tri-axial accelerometers. 47 We used the same parameters to classify sedentary time when participants recorded less than 100 counts per minute ≥ 10 consecutive minutes. 47 Subjects also completed a daily physical activity diary where they self-reported the type physical activity and the average intensity of all bouts of physical activity.
Dietary Quality
After receiving the accelerometer, participants completed a 24-hour dietary recall with a trained nutritionist at the DCRU. During this interview, a nutritionist conducted a standardized interview in which participants were asked to recall what they consumed in the previous 24 hours. Responses were simultaneously entered into the Nutrition Data System for Research software 48–50 which calculated nutrient values consumed. At the baseline diet interview, participants were taught to estimate portion size using a standard food amounts booklet, which guided the interviews at three and six months. Our primary endpoint was the Healthy Eating Index, a summary measure of diet quality assessing conformance to dietary guidelines in the U.S. 48–53
With the exception of the demographic measures, all measures were assessed at baseline and 3 and 6 months after randomization. All participants were called the day before their visits to remind them to attend the visit. Upon return of the Actigraph, participants were compensated $50 at the first visit, $60 at the second, and $70 at the third visit (total of $180).
Intervention Fidelity
To ensure standard application of the intervention, we examined fidelity according to the National Institutes of Health Behavior Change Consortium recommendations. 54 To assess intervention delivery, a research assistant audited approximately 80% of all intervention sessions and, using a fidelity checklist, tracked session length, key concept content, and attendance. The interventionist also tracked intervention receipt and enactment of each participant by completing an individualized, weekly form (Supplemental Digital Material 2) documenting attendance, weight, current experiment(s), any purchases to carry out the experiment, hospitalizations, and perceived engagement in the intervention sessions.
Analysis
We checked all variables distributions, presented in Table 1, by running frequency analyses and inspecting graphs. Data were cleaned before any statistical analysis, and we performed necessary transformation for modeling outcome variables. We checked distributional assumptions for all outcome variables prior to studying relationship between outcome of interest and the intervention variable. We analyzed baseline demographic, HIV, cardiometabolic, and CVD self-management characteristics by group. Categorical variables were summarized using frequencies and percentages. Continuous variables, depending on their distribution, were summarized by either means and standard deviations or medians and interquartile ranges.
Table 1.
Baseline Characteristics of the Participants by Group
| Intervention (n=54) | Control (n=53) | Difference | |
|---|---|---|---|
| Demographics Characteristics | |||
| Age in years (±SD) | 52.3 (7.39) | 53.3 (7.36) | 0.521 |
| Gender | |||
| Male (%) | 34 (65) | 35 (66) | 0.742 |
| Transgender (%) | 3 (6) | 1 (2) | |
| Race (%) | |||
| African American/Black (%) | 48 (89) | 44 (83) | 0.242 |
| Caucasian/White/Other (%) | 6 (9) | 9 (17) | |
| Marital Status (%) | |||
| Married or Domestic Partnership (%) | 7 (13) | 8 (15) | 0.922 |
| Single/Separated/Divorced (%) | 43 (80) | 45 (85) | |
| Education (%) | |||
| High school, GED or less (%) | 26 (48) | 30 (57) | 0.292 |
| Some college or technical school training (%) | 20 (37) | 14 (27) | |
| College or higher (%) | 7 (13) | 9 (17) | |
| Monthly Income (%) | |||
| Less than $200 | 8 (15) | 6 (11) | 0.512 |
| $200-$799 | 24 (45) | 21 (40) | |
| $800-or more | 20 (37) | 26 (49) | |
| Have Health Insurance (%) | 48 (89) | 49 (94) | 0.732 |
| Have Children (%) | 29 (54) | 28 (54) | 0.922 |
| Currently Working (%) | 4 (7) | 5 (10) | 0.732 |
| Have Permanent Housing (%) | 49 (91) | 48 (92) | 0.782 |
| Adherent to HIV medications (>90%) (%) | 51 (94) | 52 (98) | 0.562 |
| Cardiometabolic Measures | |||
| 10-year AVSCD risk (±SD) | 10.7 (7.2) | 10.1 (8.5) | 0.73 |
| Waist-to-Hip Ratio (±SD) | 0.95 (0.07) | 0.94 (0.09) | 0.69 |
| Weight (kg) (±SD) | 88.9 (21.8) | 82.2 (20.9) | 0.11 |
| Body Mass Index (kg/m2) (±SD) | 29.9 (7.9) | 28.3 (8.2) | 0.31 |
| Systolic Blood Pressure (mmHg) (±SD) | 122.6 (15.7) | 125.4 (14.9) | 0.36 |
| Diastolic Blood Pressure (mmHg) (±SD) | 78.4 (11.0) | 81.4 (8.6) | 0.12 |
| HOMA-IR (±SD) | 3.69 (2.63) | 3.88 (3.52) | 0.74 |
| Total Cholesterol (±SD) | 169.7 (36.8) | 178.4 (37.8) | 0.21 |
| HDL (±SD) | 55.0 (15.0) | 54.7 (20.0) | 0.93 |
| LDL (±SD) | 90.2 (35.5) | 92.6 (27.7) | 0.70 |
| Triglycerides (±SD) | 124.9 (97.7) | 148.9 (82.7) | 0.18 |
| IL-6 (±SD) | 2.89 (1.94) | 3.35 (2.76) | 0.33 |
| Current Smoker (%) | 23 (43) | 20 (38) | 0.552 |
| Past Smoker (%) | 33 (61) | 41 (77) | 0.062 |
| Lifestyle Behavior Characteristics | |||
| Time in MVPA (minutes per week) (±SD) | 63.96 (100.82) | 56.65 (73.45) | 0.69 |
| Sedentary time (minutes per day) (±SD) | 230.45 (140.1) | 244.81(139.0) | 0.62 |
| Step Count per Day (SD) | 7066 (3609) | 6227 (2660) | 0.21 |
| Healthy Eating Index (±SD) | 49.0 (11.6) | 48.3 (12.6) | 0.75 |
| Fruit Servings | 1.17 (2.53) | 1.18 (2.33) | 0.98 |
| Vegetable Servings | 3.55 (3.18) | 3.39 (3.40) | 0.37 |
| Calories per day | 2460 (1747) | 2147 (1234) | 0.29 |
| Calories from carbohydrates (%) (±SD) | 48.1 (13.3) | 45.6 (10.8) | 0.29 |
| Servings of Sugary Drinks | 2.16 (3.25) | 1.44 (1.68) | 0.15 |
| HIV Characteristics | |||
| Years since diagnosis(±SD) | 15.9 (7.5) | 15.7 (7.8) | 0.88 |
| Visited ED in last year (%) | 14 (26) | 19 (36) | 0.282 |
| Hospitalized in last year (%) | 11(20) | 8 (15) | 0.422 |
| CD4+ count cells/ μL (±SD) | 632 (327) | 726 (405) | 0.22 |
| CD4+ Nadir cells/ μL (±SD) | 236 (197) | 179 (136) | 0.12 |
| Retained care in last year(%) | 41 (76) | 40 (76) | 0.77 |
Differences between groups analyzed using t-test unless otherwise noted;
Differences between groups analyzed using rank sum test; GED: General Equivalency Diploma, ASCVD: Atherosclerotic Cardiovascular Disease, HOMA-IR: Homeostatic model assessment-Insulin Resistance, HDL: High-Density Lipoprotein; LDL: Low-Density Lipoprotein; MVPA: Moderate-to-Vigorous Physical Activity; ED: Emergency Department
The co-primary outcomes of this analysis were time spent in MVPA and Healthy Eating Index. Secondary outcomes were sedentary time per day, steps per day and weight.
We used generalized estimating equations (GEE) with an identity link function and an unstructured covariance matrix to examine the effect of the intervention on our physical activity and diet endpoints across the three time points. To assess the moderating effect of the intervention over time, we created a group by time interaction variable. In addition to the primary treatment effect of the intervention, we examined the independent effects of age, gender, race, and for the MVPA outcome season of enrollment, by adding these covariates to the GEE models. All statistical analyses were performed using Stata version 14.0 (College Station, Texas) and p-values <0.05 were considered statistically significant.
Results
Sample Description
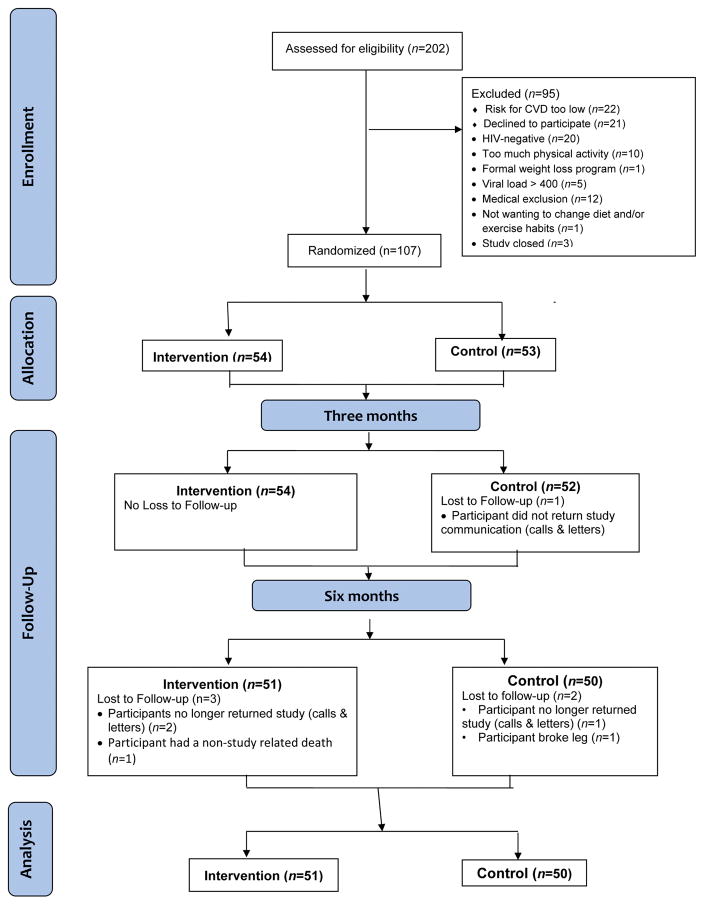
One-hundred and seven PLHIV were randomized to either the intervention or the enhanced usual care condition. Of those, 106 (99%) PLHIV were retained at 3 months and 101 (94%) at 6 months. These 101 participants were included in the analysis (Figure 1).
Figure 1.
The intervention and enhanced usual care groups were demographically equivalent at baseline (Table 1). Participants were approximately 53 years old, 65% male (n=70), 86% African American (n=93). On average, participants had a 10.4% 10-year atherosclerotic cardiovascular event risk using the pooled-cohort equations, 55 had a mean Body Mass Index (BMI) of 29.1, and 43 (41%) currently smoked cigarettes. Participants had been living with HIV for a mean of 16 years and had a current CD4+ T cell count of 671 cells/μL.
At baseline, participants engaged in approximately 60.5 (±88) minutes of MVPA per week, 237 (±139) minutes of sedentary time per day, and took 6,656 (±3,191) steps per day. They consumed a mean of 2,297.5 calories (±1,514) per day, 47% of which were calories from carbohydrates. The mean Healthy Eating Index was 48.6 (±12.1), indicating that on average, participant’s conformed with less than half of the recommended dietary quality.
Intervention Characteristics
Approximately 90% of the participants randomized to the intervention attended at least half of the sessions and 60% attended at least 5 sessions. Five participants were hospitalized during the intervention for non-study related medical issues. Each participant designed their own behavior change experiments; a total of 220 experiments were conducted. Of these, 44% focused on dietary changes; 37% focused on increasing exercise; and 19% included both diet and exercise goals. Guided by the interventionist, participants chose a range of experiments from reducing sugar-sweetened beverage consumption to taking the stairs in their apartment building (Table 2).
Table 2.
Intervention Fidelity Characteristics
| Interventionist Training | Interventionist received standard manualized training; periodic supervision and re-training; and 1 interventionist delivered the intervention across groups | ||
|---|---|---|---|
| Intervention Dose | Median number of Sessions Attended | Number of Subjects Missing 2 or more consecutive Sessions | Subjects Hospitalized During the Intervention |
| 5 (3, 5) | 17 (33 %) | 5 (10%) subjects; 2 subjects hospitalized twice | |
| Intervention Receipt and Enactment | Number of subjects who purchased equipment | Mean weight change from week 1 to week 6 | |
| 0 | Men | Women | |
| −0.42kg | −1.61kg | ||
| Experiment Goals | Mean Perceived Engagement: Range 0 (no engagement) to 10 (very engaged) | ||
|
7.35 (2.22) | ||
Examples:
| |||
Primary physical activity and diet outcomes
Our primary behavioral outcomes were MVPA per week and healthy eating index. We did not observe any effect of the intervention on change in these outcomes over time (p for group*time interactions >0.05) (Table 3). However, being female, older, and enrolling in the study in the fall were consistently associated with less physical activity. Only education level was significantly associated with improved Healthy Eating Index. The physical activity diary revealed that, on average, participants mostly engaged in low intensity physical activity including walking, climbing stairs, and stretching (Supplemental Digital Material 3).
Table 3.
Generalized Estimating Equation of the Effect of the SystemCHANGE Intervention Physical Activity and Diet Quality, adjusted for age, gender, and race
| β | SE(β) | p-value | 95% Confidence Interval | ||
|---|---|---|---|---|---|
| Minutes of Moderate-to-Vigorous Physical Activity per week (Square root) | |||||
| SystemCHANGE Intervention | −0.040 | 0.938 | 0.966 | −1.879 | 1.798 |
| Time | −0.037 | 0.346 | 0.916 | −0.715 | 0.642 |
| Intervention x Time | −0.514 | 0.476 | 0.281 | −1.447 | 0.420 |
| Season Enrolled | |||||
| Spring | −1.054 | 1.107 | 0.341 | −3.224 | 1.116 |
| Summer | −0.922 | 1.265 | 0.466 | −3.401 | 1.558 |
| Fall | −3.050 | 1.673 | 0.068 | −6.329 | 0.229 |
| Healthy Eating Index | |||||
| SystemCHANGE Intervention | 0.804 | 2.095 | 0.701 | −3.303 | 4.911 |
| Time | −0.235 | 1.048 | 0.822 | −2.290 | 1.819 |
| Intervention x Time | 1.219 | 1.462 | 0.405 | −1.647 | 4.084 |
| Education | |||||
| 11th Grade or Less | 3.139 | 2.218 | 0.157 | −1.208 | 7.485 |
| High School Diploma or GED | 3.243 | 2.327 | 0.163 | −1.317 | 7.804 |
| Some College or Technical Training | 0.932 | 2.768 | 0.736 | −4.493 | 6.358 |
| Associates Degree or Equivalent | 4.827 | 2.825 | 0.078 | −0.710 | 10.363 |
| Bachelor’s Degree or Higher | 20.952 | 4.660 | <0.001* | 11.819 | 30.085 |
| Percent of Daily Calories from Carbohydrates | |||||
| SystemCHANGE Intervention | 4.017 | 2.200 | 0.068 | −0.294 | 8.327 |
| Time | 1.309 | 1.186 | 0.269 | 1.014 | 3.633 |
| Intervention x Time | −3.815 | 1.654 | 0.021* | −7.056 | −0.573 |
| Servings of Sugary Drinks Per Day | |||||
| SystemCHANGE Intervention | 0.606 | 0.351 | 0.084 | −0.082 | 1.293 |
| Time | 0.018 | 0.192 | 0.926 | −0.539 | 0.394 |
| Intervention x Time | −0.602 | 0.270 | 0.026* | −1.131 | −0.073 |
Secondary outcomes
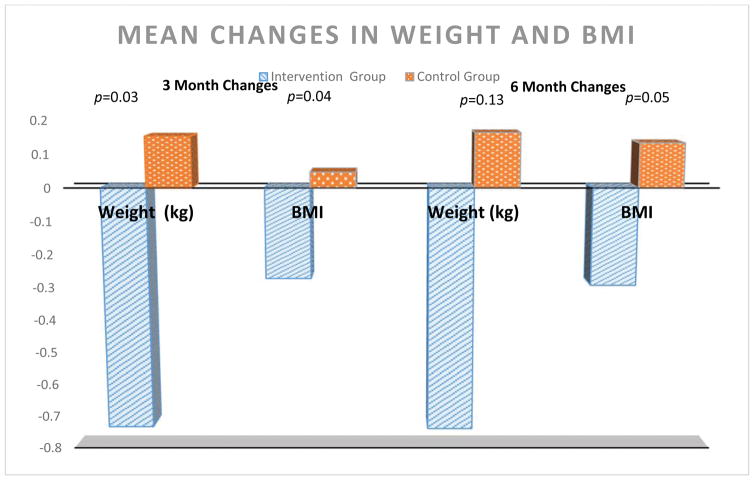
Our secondary outcomes were sedentary time per day, steps per day, and weight. There was no effect of the intervention on sedentary time per day and steps per day (p>0.05) Participants in the intervention group lost 0.732 (±3.66) kg over 3 months and maintained it over 6 months compared to a gain of 0.153 (±3.42) kg at 3 months (p=0.03) in the control group (Figure 2). BMI changes were similar. Although the effect of the intervention on our behavioral outcomes was null, we considered additional behaviors that may have contributed to weight loss. Our qualitative fidelity data indicated that many experiments focused on dietary changes, namely reducing carbohydrate intake through surgery drinks. In GEE models, the intervention was associated with an almost 4% decrease in daily calories from carbohydrates and 0.60 fewer servings of sugar-sweetened beverages per day (Table 3). There was a dose-response to the intervention in that among those who consumed the most sugar-sweetened drinks at baseline (top quartile) decreased their intake by 1.78 servings per day (p for group*time interactions =0.02).
Figure 2.
Discussion
Our results demonstrated that among PLHIV at high risk for developing CVD, the SystemCHANGE intervention did not improve physical activity or the Healthy Eating Index. However, we also found that those randomized to the intervention lost 0.732 kg over 3 months and maintained those changes at 6 months. By incorporating our qualitative fidelity data, we were able to determine that this weight loss resulted from reduced daily carbohydrate and sugar-sweetened beverage intake. These mixed results suggest that aspects of the SystemCHANGE intervention can improve some CVD self-management behaviors leading to improved markers of cardiometabolic health, but that additional strategies are needed to improve its effectiveness.
Our findings are similar to studies aiming to improve diet and physical activity behaviors. There are challenges to promoting and sustaining increases in healthy diet and physical activity through behavioral interventions, particularly among individuals living with chronic diseases.56 Recent studies in PLHIV and other populations have shown that many factors influence readiness for change and the success of behavior-change interventions.56,57 There are many factors that influence readiness to change, as seen in studies framed by the Transtheoretical Model (TTM).56 Individuals may choose not to engage in physical activity or diet behavior change because they don’t perceive that change as important, do not feel they have adequate support, or are uncertain about the impact of such behaviors on their health.56,57 While we asked about readiness for behavior change during our screening process for this study, we may have benefited from a more thorough assessment of readiness at baseline to guide intervention content, intensity, and duration.57
While our intervention had several notable strengths supported by recent research, including face-to-face meetings, a hands-on approach to education, and integration of group and peer support,56 there are areas that may warrant revision. These revisions include integration of content expertise in diet and exercise, group exercise sessions and exercise demonstration, and exploration of increased duration or intensity may improve our outcomes.56,58 Our session duration was found to be acceptable by participants. Future research should consider increasing the intervention intensity by increasing the number of sessions (e.g. more than one during a given week; greater total number of contacts), or increasing the overall duration of intervention contacts (e.g. greater than six weeks long). Such changes, however, must be congruent with feasibility data and cost-effectiveness.
These findings also reveal the difficulty in engaging sedentary, aging PLHIV in regular MVPA and suggest that behavioral strategies alone may not sufficient to change habits. Vancampfort (2016) examined factors associated with increased retention with physical activity interventions among PLHIV and suggested that supervised exercise and experiencing gains early on the intervention is likely to keep PLHIV engaged in physical activity. Potential interventions can incorporate initial supervised exercise training, tailored to the individual’s exercise capacity and goals, coupled with behavioral and environmental strategies to support a lifelong commitment to physical activity. A similar model is used in phase III cardiac rehabilitation and may work with this population. 59 For those who have undergone a cardiac event (e.g., Myocardial Infarction), cardiac rehabilitation is a low cost, community-based activity program. Extending it to other high-risk groups will utilize existing clinical infrastructure, and may allow for a more efficient use of clinical resources. However, such a model should be adapted to this population and tested before implementation.
We also made several important observations relevant to clinical practice. First, by documenting the experiments participants chose, we saw that reducing carbohydrate intake and sugar-sweetened beverages were a priority for this population. Limiting consumption of sugar-sweetened beverages is widely recommended as part of a healthy diet. 60 This may be a resonate, simple behavior to begin assessing and intervening to improve the diet quality of PLHIV. Second, our analyses illustrate that among PLHIV in the United States, physical activity varies by season. Similar variations have been found in self-reported, adult leisure-time physical activity in the general population. 61–63 These data suggest that interventions to increase physical activity need to overcome seasonal barriers to activity and that future research should account for seasonal variations in physical activity. Intervention strategies incorporating indoor activity options, maybe with technology-enhanced strategies, 64 may help increase activity. Third, older women engaged in the least physical activity and this subgroup can be targeted with more intense, frequent, tailored interventions to increase physical activity. This is particularly important since this population tends to be more obese, leading to increased risk of CVD and reduced quality of life.
Our findings also have several research implications. Recent results from several clinical trials to improve physical activity in PLHIV did not achieve their intended effect. Jaggers et al (2016) conducted a 9-month clinical trial of a home-based exercise intervention that incorporated self-monitoring, exercise equipment, and telephone behavioral counselling. 65 The intervention did not increase MVPA among PLHIV, and they concluded that a home-based exercise approach with coaching might not be feasible for increase physical activity in this population. Additionally, Couterno et al (2016) enrolled PLHIV in a supervised 4x/week aerobic and resistance training program in a community-based center (designed to overcome barriers of access) and found that 55% of participants did not comply with the moderate-to-vigorous exercise program. 66 These interventions targeted individual factors by providing resources to overcome barriers and incorporated cognitive strategies such as goal-setting and self-monitoring. SystemCHANGE also included environmental strategies such as building diet and exercise changes into one’s daily routine in their home environment, but similarly failed to improve long-term exercise behavior in PLHIV. However, our participants did change aspects of their diet quality and maintained these changes for six months. Our findings suggest that PLHIV can change critical CVD health behaviors, but that new strategies are needed to increase the effectiveness of these interventions. Building on this, more fundamental research is needed to understand how HIV, contextual, and cognitive factors influence daily CVD self-management behaviors in this population. Such evidence could help us to develop more effective CVD self-management interventions, adequately targeted to PLHIV.
Additionally, our results underscore the importance of using rigorous, detailed data collection tools and fidelity measures. We were able to observe changes in daily carbohydrate intake because we collected both detailed fidelity enactment data and used a comprehensive, 24-hour diet recall. These assessments allowed us to not only observe these changes but also to contextualize them in the larger study. Future intervention and observational studies should consider not only a priori outcomes, but should measure the mechanisms that lead to their outcomes of interest. Such examinations will require refined, qualitative and quantitative data extending beyond self-report measures. Technological advancements such as sensor-enabled devices and ecological momentary assessments can help efficiently collect these data. 67,68
Our study had several strengths including using a randomized controlled trial design, having a high intervention fidelity and study retention, using objective measures of physical activity, and integrating qualitative and quantitative fidelity data. Our study also had several limitations. The study occurred in only one site in the Midwestern United States. The enrolled population is reflective of the HIV epidemic in that region, but may not reflect the national and international HIV epidemic. We only included participants at high risk for CVD and who were sedentary. It is possible those at a lower risk for CVD may have been more responsive to the intervention. For example, a lower risk group that engages in some physical activity may only need a short behavioral intervention to increase their physical activity and diet quality up to recommended levels. Those who are more sedentary and at higher risk may need a more intense, frequent, multicomponent intervention to improve their CVD self-management behavior and maintain those changes. To standardize data collection, data were collected at 3 months’ post-randomization instead immediately after the conclusion of the intervention. This may have diminished the effect of the intervention on our outcomes. Future work should consider including assessments immediately post-intervention and at a standard time interval to determine if there is a durable effect. Finally, while accelerometers have many advantages over self-reported physical activity data they are unable to reliably measure swimming and cycling on a stationary bike.69 We examined our physical activity diaries and found that seven participants (4 in the intervention group and 3 in the control group) reported swimming or using a stationary bike throughout the study duration. We conducted a sensitivity analysis excluding these cases from the GEEs and the results did not change (p>0.05). Future studies using accelerometers should consider this limitation in their study design.
In conclusion, among sedentary PLHIV at high risk for CVD, the SystemCHANGE intervention reduced daily carbohydrate intake resulting in weight loss, but did not improve our primary outcomes of overall diet quality and physical activity. Future work should identify fundamental factors that will facilitate a sustained increase in physical activity and improve overall diet quality among this population.
Supplementary Material
Acknowledgments
We wish to acknowledge the substantial and invaluable contributions to this study by Shyla Urban, Nate Schreiner, and Marianne Vest. These data will be presented at the American Heart Association Scientific Sessions in November, 2017 in Anaheim, CA.
Source of Funding: This project was funded by grants from the American Heart Association (14CRP20380259) and a developmental grant from the University Hospitals/Case Western Reserve University Center for AIDS Research (National Institutes of Health Grant # P30 AI036219). It was also supported by National Institutes of Health Grants # UL1 RR024989 and # T32NR014213.
Footnotes
Conflicts of Interest: The authors have no relevant conflicts of interest do declare.
References
- 1.NHLBI NHLBI AIDS Working Group. Advancing HIV/AIDS Research in Heart, Lung, and Blood Diseases. Bethesda, MD: 2012. [Google Scholar]
- 2.Artinian NT, Fletcher GF, Mozaffarian D, et al. Interventions to Promote Physical Activity and Dietary Lifestyle Changes for Cardiovascular Risk Factor Reduction in Adults: A Scientific Statement From the American Heart Association. Circulation. 2010;122(4):406–441. doi: 10.1161/CIR.0b013e3181e8edf1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drozd DR, Kitahata MM, Althoff KN, et al. Increased Risk of Myocardial Infarction in HIV-Infected Individuals in North America Compared to the General Population. J Acquir Immune Defic Syndr. 2017 doi: 10.1097/QAI.0000000000001450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lake JE, Stanley TL, Apovian CM, et al. Practical Review of Recognition and Management of Obesity and Lipohypertrophy in Human Immunodeficiency Virus Infection. Clin Infect Dis. 2017;64(10):1422–1429. doi: 10.1093/cid/cix178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamitani E, Sipe TA, Higa DH, Mullins MM, Soares J. Evaluating the Effectiveness of Physical Exercise Interventions in Persons Living With HIV: Overview of Systematic Reviews. AIDS Education and Prevention. 2017;29(4):347–363. doi: 10.1521/aeap.2017.29.4.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee IM, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet. 2012;380(9838):219–229. doi: 10.1016/S0140-6736(12)61031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fletcher GF, Balady G, Blair SN, et al. Statement on exercise: benefits and recommendations for physical activity programs for all Americans. A statement for health professionals by the Committee on Exercise and Cardiac Rehabilitation of the Council on Clinical Cardiology, American Heart Association. Circulation. 1996;94(4):857–862. doi: 10.1161/01.cir.94.4.857. [DOI] [PubMed] [Google Scholar]
- 8.Kokkinos P, Myers J. Exercise and Physical Activity: Clinical Outcomes and Applications. Circulation. 2010;122(16):1637–1648. doi: 10.1161/CIRCULATIONAHA.110.948349. [DOI] [PubMed] [Google Scholar]
- 9.Department of Health and Human Services; DHHS, editor. Physical Activity Guidelines Advisory Committee Report. Washington, DC: 2008. [Google Scholar]
- 10.Dufour C, Marquine M, Fazeli P, et al. Physical exercise is associated with less neurocognitive impairment among HIV-infected adults. J Neurovirol. 2013;19(5):410–417. doi: 10.1007/s13365-013-0184-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith PJ, Blumenthal JA, Hoffman BM, et al. Aerobic Exercise and Neurocognitive Performance: A Meta-Analytic Review of Randomized Controlled Trials. Psychosomatic Medicine. 2010;72(3):239–252. doi: 10.1097/PSY.0b013e3181d14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foster C, Hillsdon M, Thorogood M. Interventions for promoting physical activity. Cochrane Database Syst Rev. 2005:1. doi: 10.1002/14651858.CD003180.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grinspoon SK, Grunfeld C, Kotler DP, et al. State of the Science Conference: Initiative to Decrease Cardiovascular Risk and Increase Quality of Care for Patients Living With HIV/AIDS: Executive Summary. Circulation. 2008;118(2):198–210. doi: 10.1161/CIRCULATIONAHA.107.189622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Brien K, Tynan AM, Nixon S, Glazier R. Effectiveness of aerobic exercise for adults living with HIV: systematic review and meta-analysis using the Cochrane Collaboration protocol. BMC Infectious Disease. 2016;16:182. doi: 10.1186/s12879-016-1478-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Willig A, Westfall A, Crane H, et al. The beneficial effects of physical activity in the setting of HIV infection. Paper presented at. Antiviral Therapy. 2016 [Google Scholar]
- 16.Vancampfort D, Mugisha J, De Hert M, et al. Global physical activity levels among people living with HIV: a systematic review and meta-analysis. Disability and Rehabilitation. 2016:1–10. doi: 10.1080/09638288.2016.1260645. [DOI] [PubMed] [Google Scholar]
- 17.Vancampfort D, Mugisha J, Richards J, et al. Dropout from physical activity interventions in people living with HIV: a systematic review and meta-analysis. AIDS care. 2017;29(5):636–643. doi: 10.1080/09540121.2016.1248347. [DOI] [PubMed] [Google Scholar]
- 18.Anema A, Fielden SJ, Shurgold S, et al. Association between food insecurity and procurement methods among people living with HIV in a high resource setting. PloS one. 2016;11(8):e0157630. doi: 10.1371/journal.pone.0157630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pieroth R, Radler DR, Guenther PM, Brewster PJ, Marcus A. The Relationship between Social Support and Diet Quality in Middle-Aged and Older Adults in the United States. Journal of the Academy of Nutrition and Dietetics. 2017 doi: 10.1016/j.jand.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 20.Kok G, Gottlieb NH, Commers M, Smerecnik C. The ecological approach in health promotion programs: a decade later. Am J Health Promot. 2008;22(6):437–442. doi: 10.4278/ajhp.22.6.437. [DOI] [PubMed] [Google Scholar]
- 21.Hamid T. Thinking In Circles About Obesity: Applying Systems Thinking to Weight Managment. Springer; 2009. [Google Scholar]
- 22.Alemi FND, Ardito S, Headrick L, Moore S, Hekelman F, Norman L. Continuous self-improvement: systems thinking in a personal context. The Joint Commission journal on quality improvement. 2000;26(2):74–86. doi: 10.1016/s1070-3241(00)26006-9. [DOI] [PubMed] [Google Scholar]
- 23.Moore SM, Borawski EA, Cuttler L, Ievers-Landis CE, Love TE. IMPACT: A multi-level family and school intervention targeting obesity in urban youth. Contemp Clin Trials. 2013;36(2):574–586. doi: 10.1016/j.cct.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moore SM, Charvat JM, Gordon NH, et al. Effects of a CHANGE intervention to increase exercise maintenance following cardiac events. Ann Behav Med. 2006;31(1):53–62. doi: 10.1207/s15324796abm3101_9. [DOI] [PubMed] [Google Scholar]
- 25.Plow M, Moore SM, Kirwan JP, et al. Randomized controlled pilot study of a SystemCHANGE weight management intervention in stroke survivors: rationale and protocol. Trials. 2013;14:130. doi: 10.1186/1745-6215-14-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Webel AR, Moore SM, Hanson JE, RAS The Rationale, Design, and Initial Efficacy of SystemCHANGE™ -HIV: A Systems-Based Intervention to Improve Physical Activity in People Living with HIV. Journal of AIDS Clinical Research. 2013;4(200) doi: 10.4172/2155-6113.1000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moore SM, Jones L, Alemi F. Family self-tailoring: Applying a systems approach to improving family healthy living behaviors. Nurs Outlook. 2016;64(4):306–311. doi: 10.1016/j.outlook.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moore SMCJ, Alemi F, Gordon N, Ribisl P, Rocco M. Improving Lifestyle Exercise in Cardiac Patients: Results of the SystemCHANGE Trial. 530 Walnut St, Philadelphia, PA 19106–3621 USA: Lippincott Williams & Wilkins; 2011. [Google Scholar]
- 29.Gibbons RJ, Balady GJ, Beasley JW, et al. ACC/AHA Guidelines for Exercise Testing: Executive Summary. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Exercise Testing) 1997;96(1):345–354. doi: 10.1161/01.cir.96.1.345. [DOI] [PubMed] [Google Scholar]
- 30.Webel AR, Perazzo J, Decker M, Horvat-Davey C, Sattar A, Voss J. Physical activity is associated with reduced fatigue in adults living with HIV/AIDS. Journal of Advanced Nursing. 2016;72(12):3104–3112. doi: 10.1111/jan.13084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hayden-Wade HA, Coleman KJ, Sallis JF, Armstrong C. Validation of the telephone and in-person interview versions of the 7-day PAR. Medicine and Science in Sports and Exercise. 2003;35(5):801–809. doi: 10.1249/01.MSS.0000064941.43869.4E. [DOI] [PubMed] [Google Scholar]
- 32.Russell CL, Moore S, Hathaway D, Cheng A-L, Chen G, Goggin K. MAGIC Study: Aims, Design and Methods using SystemCHANGE™ to Improve Immunosuppressive Medication Adherence in Adult Kidney Transplant Recipients. BMC Nephrology. 2016;17(1):84. doi: 10.1186/s12882-016-0285-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Webel AR, Moore SM, Hanson JE, Salata RA. The Rationale, Design, and Initial Efficacy of SystemCHANGE(™) -HIV: A Systems-Based Intervention to Improve Physical Activity in People Living with HIV. Journal of AIDS & clinical research. 2013;4(3):1000200. doi: 10.4172/2155-6113.1000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moore SM, Borawski EA, Cuttler L, Ievers-Landis CE, Love T. IMPACT: A Multi-Level Family and School Intervention Targeting Obesity in Urban Youth. Contemporary clinical trials. 2013;36(2) doi: 10.1016/j.cct.2013.1008.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC Guideline on Lifestyle Management to Reduce Cardiovascular RiskA Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology. 2013 doi: 10.1016/j.jacc.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 36.Hudson A, Portillo C, Lee K. Sleep Disturbances in Women With HIV or AIDS: Efficacy of a Tailored Sleep Promotion Intervention. Nursing Research. 2008;57(5):360–366. doi: 10.1097/01.NNR.0000313501.84604.2c. doi:310.1097/1001.NNR.0000313501.0000384604.0000313502c. [DOI] [PubMed] [Google Scholar]
- 37.Reid S, Dwyer J. Insomnia in HIV Infection: A Systematic Review of Prevalence, Correlates, and Management. Psychosom Med. 2005;67(2):260–269. doi: 10.1097/01.psy.0000151771.46127.df. [DOI] [PubMed] [Google Scholar]
- 38.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strath SJ, Kaminsky LA, Ainsworth BE, et al. Guide to the assessment of physical activity: clinical and research applications: a scientific statement from the american heart association. Circulation. 2013;128(20):2259–2279. doi: 10.1161/01.cir.0000435708.67487.da. [DOI] [PubMed] [Google Scholar]
- 40.Berntsen S, Hageberg R, Aandstad A, et al. Validity of physical activity monitors in adults participating in free-living activities. Br J Sports Med. 2010;44(9):657–664. doi: 10.1136/bjsm.2008.048868. [DOI] [PubMed] [Google Scholar]
- 41.Anastasopoulou P, Tubic M, Schmidt S, Neumann R, Woll A, Hartel S. Validation and comparison of two methods to assess human energy expenditure during free-living activities. PLoS One. 2014;9(2):e90606. doi: 10.1371/journal.pone.0090606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hitz MM, Conway PG, Palcher JA, McCarty CA. Using PhenX toolkit measures and other tools to assess urban/rural differences in health behaviors: recruitment methods and outcomes. BMC Res Notes. 2014;7(1):847. doi: 10.1186/1756-0500-7-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haskell WL, Troiano RP, Hammond JA, et al. Physical activity and physical fitness: standardizing assessment with the PhenX Toolkit. Am J Prev Med. 2012;42(5):486–492. doi: 10.1016/j.amepre.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hamilton CM, Strader LC, Pratt JG, et al. The PhenX Toolkit: get the most from your measures. Am J Epidemiol. 2011;174(3):253–260. doi: 10.1093/aje/kwr193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Migueles JH, Cadenas-Sanchez C, Ekelund U, et al. Accelerometer Data Collection and Processing Criteria to Assess Physical Activity and Other Outcomes: A Systematic Review and Practical Considerations. Sports Medicine. 2017:1–25. doi: 10.1007/s40279-017-0716-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100(2):126–131. [PMC free article] [PubMed] [Google Scholar]
- 47.Sasaki JE, John D, Freedson PS. Validation and comparison of ActiGraph activity monitors. Journal of Science and Medicine in Sport. 2011;14(5):411–416. doi: 10.1016/j.jsams.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 48.Dennis B, Ernst N, Hjortland M, Tillotson J, Grambsch V. The NHLBI nutrition data system. Journal of the American Dietetic Association. 1980;77(6):641–647. [PubMed] [Google Scholar]
- 49.Buzzard M, Feskanich D. Maintaining a Food Composition Data Base for Multiple Research Studies: The NCC Food Table. In: Rand W, Wyse CWB, YV, editors. Food Composition Data: A User’s Perspective. The United Nations University; 1987. [Google Scholar]
- 50.Sievert YA, Schakel SF, Buzzard IM. Maintenance of a nutrient database for clinical trials. Controlled Clin Trials. 1989;10:416–425. doi: 10.1016/0197-2456(89)90006-8. [DOI] [PubMed] [Google Scholar]
- 51.Guenther PM, Reedy J, Krebs-Smith SM. Development of the Healthy Eating Index-2005. J Am Diet Assoc. 2008;108(11):1896–1901. doi: 10.1016/j.jada.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 52.Guenther PM, Reedy J, Krebs-Smith SM, Reeve BB. Evaluation of the Healthy Eating Index-2005. J Am Diet Assoc. 2008;108(11):1854–1864. doi: 10.1016/j.jada.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 53.Freedman LS, Guenther PM, Krebs-Smith SM, Kott PS. A population’s mean Healthy Eating Index-2005 scores are best estimated by the score of the population ratio when one 24-hour recall is available. J Nutr. 2008;138(9):1725–1729. doi: 10.1093/jn/138.9.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bellg AJ, Borrelli B, Resnick B, et al. Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH Behavior Change Consortium. Health Psychology. 2004;23(5):443. doi: 10.1037/0278-6133.23.5.443. [DOI] [PubMed] [Google Scholar]
- 55.Goff DC, Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S49–73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 56.Cradock KA, ÓLaighin G, Finucane FM, Gainforth HL, Quinlan LR, Ginis KAM. Behaviour change techniques targeting both diet and physical activity in type 2 diabetes: A systematic review and meta-analysis. International Journal of Behavioral Nutrition and Physical Activity. 2017;14(1):18. doi: 10.1186/s12966-016-0436-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Simonik A, Vader K, Ellis D, et al. Are you ready? Exploring readiness to engage in exercise among people living with HIV and multimorbidity in Toronto, Canada: a qualitative study. BMJ open. 2016;6(3):e010029. doi: 10.1136/bmjopen-2015-010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Melnyk B, Morrison-Beedy D. Intervention research: Designing, conducting, analyzing, and funding. Springer Publishing Company; 2012. [Google Scholar]
- 59.Williams MA, Kaminsky LA. Healthy Lifestyle Medicine in the Traditional Healthcare Environment-Primary Care and Cardiac Rehabilitation. Prog Cardiovasc Dis. 2017;59(5):448–454. doi: 10.1016/j.pcad.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 60.Malik VS. Sugar sweetened beverages and cardiometabolic health. Curr Opin Cardiol. 2017;32(5):572–579. doi: 10.1097/HCO.0000000000000439. [DOI] [PubMed] [Google Scholar]
- 61.Pivarnik JM, Reeves MJ, Rafferty AP. Seasonal variation in adult leisure-time physical activity. Med Sci Sports Exerc. 2003;35(6):1004–1008. doi: 10.1249/01.MSS.0000069747.55950.B1. [DOI] [PubMed] [Google Scholar]
- 62.Merrill RM, Shields EC, White GL, Jr, Druce D. Climate conditions and physical activity in the United States. Am J Health Behav. 2005;29(4):371–381. doi: 10.5993/ajhb.29.4.9. [DOI] [PubMed] [Google Scholar]
- 63.Tucker P, Gilliland J. The effect of season and weather on physical activity: a systematic review. Public Health. 2007;121(12):909–922. doi: 10.1016/j.puhe.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 64.Becofsky K, Wing EJ, McCaffery J, Boudreau M, Wing RR. A Randomized Controlled Trial of a Behavioral Weight Loss Program for Human Immunodeficiency Virus–Infected Patients. Clinical Infectious Diseases. 2017;65(1):154–157. doi: 10.1093/cid/cix238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jaggers JR, Sneed JM, Lobelo RF, et al. Results of a nine month home-based physical activity intervention for people living with HIV. International Journal of Clinical Trials. 2016;3(3):106–119. [Google Scholar]
- 66.Cutrono SE, Lewis JE, Perry A, Signorile J, Tiozzo E, Jacobs KA. The Effect of a Community-Based Exercise Program on Inflammation, Metabolic Risk, and Fitness Levels Among Persons Living with HIV/AIDS. AIDS Behav. 2016;20(5):1123–1131. doi: 10.1007/s10461-015-1245-1. [DOI] [PubMed] [Google Scholar]
- 67.Dunton GF. Ecological momentary assessment in physical activity research. Exercise and sport sciences reviews. 2017;45(1):48–54. doi: 10.1249/JES.0000000000000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Higgins JP. Smartphone Applications for Patients’ Health and Fitness. The American Journal of Medicine. 2016;129(1):11–19. doi: 10.1016/j.amjmed.2015.05.038. [DOI] [PubMed] [Google Scholar]
- 69.Herman Hansen B, Bortnes I, Hildebrand M, Holme I, Kolle E, Anderssen SA. Validity of the ActiGraph GT1M during walking and cycling. Journal of sports sciences. 2014;32(6):510–516. doi: 10.1080/02640414.2013.844347. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.