Abstract
Aims
Denervation of the bladder is a detrimental consequence of bladder outlet obstruction (BOO). We have previously shown that, during BOO, inflammation triggered by the NLRP3 inflammasome in the urothelia mediates physiological bladder dysfunction and downstream fibrosis in rats. The aim of this study was to assess the effect of NLRP3-mediated inflammation on bladder denervation during BOO.
Methods
There were 5 groups of rats: 1) Control (no surgery), 2) Sham-operated, 3) BOO rats given vehicle, 4) BOO rats given the NLRP3 inhibitor glyburide, and 5) BOO rats given the IL-1 receptor antagonist anakinra. BOO was constructed by ligating the urethra over a 1 mm catheter and removing the catheter. Medications were given prior to surgery and once daily for 12 days. Bladder sections were stained for PGP9.5, a pan neuronal marker. Whole transverse sections were used to identify and count nerves while assessing cross-sectional area. For in vitro studies, pelvic ganglion neurons were isolated and treated with IL-1β. 48h later apoptosis, neurite length and branching were assessed.
Results
In obstructed bladders, the number of nerves decreased while total area increased, indicating a loss of cell number and/or branching. The decrease in nerve density was blocked by glyburide or anakinra, clearly implicating the NLRP3 pathway in denervation. In vitro analysis demonstrated that IL-1β, a product of the inflammasome, induced apoptosis in pelvic ganglion neurons, suggesting one mechanism of BOO-induced denervation is NLRP3/IL-1β triggered apoptosis.
Conclusions
The NLRP3/IL-1β-mediated inflammation pathway plays a significant role in denervation during BOO.
Keywords: Urinary Bladder, Bladder Outlet Obstruction, Interleukin, Interleukin-1beta, Glyburide, Interleukin-1 Receptor Antagonist, Cytokines, Inflammasomes, NLRP3, Neuron, Benign Prostatic Hyperplasia, Neuroscience, Urology
Introduction
Bladder outlet obstruction (BOO) is a prevalent condition with long-term consequences. The most common cause of BOO is benign prostatic hyperplasia (BPH) in older men. Enlargement of the prostate creates a partial urethral obstruction restricting urine flow and leading to repeated bouts of high pressure voiding (and eventual storage) and excessive stretch. Additionally, there are oxidative stresses caused by ischemia/hypoxia as blood vessels within the bladder wall are compressed during filling, and reperfusion as the ischemia is relieved during micturition. In the early stages, a healthy detrusor compensates for restricted outflow and patients notice little difference in voiding patterns. However, over time symptoms progress toward bladder overactivity (increased frequency and urgency) and urge incontinence, defined as an irritative voiding pattern.1 Previous studies suggested that bladder inflammation underlies these irritative voiding symptoms.2–4 Although symptomatic relief is often possible with medications (alpha blockers, 5-alpha reductase inhibitors, antimuscarinics and β3- agonists), these treatments do not fully alleviate the outflow resistance and the repeated physical/chemical insults associated with it. The result is low-level chronic inflammation3,4 leading to detrimental changes such as fibrosis5 and denervation which can be difficult or even impossible to reverse with medical or surgical intervention.6–10 Over time, they can lead to bladder decompensation11,12, an end-stage event characterized by detrusor underactivity/acontractility, overflow incontinence and even renal failure in severe cases.
Interestingly, while the association of fibrosis to decompensation appears direct –fibrosis confers worse contractility– the association of denervation is not. Early denervation contributes to detrusor overactivity whereas, over time, extensive neuron loss results in underactivity.13 Loss of nerve density and/or responsiveness has been found in many different species including man14, guinea pig9, sheep6, rabbit8 and rat7. While the mechanism of denervation remains unknown, it has been speculated to result from hypoxia and reperfusion with production of reactive oxygen species (ROS).9,10 ROSs can be an activator of the NLRP3 inflammasome and our lab has been exploring the importance of NLRP3 to the underlying inflammation and pathological changes in the bladder during BOO.2,5 NLRP3 is a member of the NOD-like family of intracellular receptors. NLRP3 responds to intracellular molecules released following damage, known as damage or danger-associated molecular patterns (DAMPs), and forms an inflammasome, a multimeric complex whose formation activates caspase-1. Caspase-1 cleaves pro-IL-1β and pro-IL-18 into their mature forms which are released through a lytic process known as pyroptosis. IL-1β and IL-18 are pro-inflammatory cytokines which trigger the inflammatory response. We have shown in rats that an inhibitor of NLRP3, glyburide,15 not only blocks BOO-induced inflammation2,5 but also the irritative voiding symptoms2 and downstream fibrosis.5 In this study we explore a role for NLRP3-mediated inflammation in triggering the denervation of the bladder that occurs following BOO.6–10 In addition to glyburide to inhibit NLRP3, we have employed anakinra, an FDA-approved recombinant version of the naturally occurring interleukin-1 receptor antagonist that will compete with NLRP3-derived IL-1β for its receptor but have no effect on IL-18. Thus, anakinra will not only further confirm the involvement of NRRP3 pathway but also differentiate between the effects of IL-1β and IL-18.
Materials and Methods
Animals
Female Sprague Dawley rats (≈200 g, 40–50 days, Envigo, USA) were used for BOO studies. Female rats are the standard for BOO based on ease of surgery, lack of tortuosity and short length of the urethra. Male Sprague Dawley rats (≈350 g, 70–80 days, Envigo, USA) were used for in vitro experiments. Animal use protocols were approved by the Institutional Animal Care and Use Committees (IACUC) at either Duke University Medical Center or East Carolina University.
In Vivo Experimental Design
There were five groups: 1) Control (no treatment), 2) sham-operated (Sham), 3) BOO plus vehicle (BOO + Veh; obstructed rats given 1 ml of 40% ethanol (p.o.) at each dosing interval), 4) BOO plus glyburide (BOO + Gly; obstructed rats given 1 ml of 40% ethanol (p.o.) containing 10 mg/kg glyburide at each dosing interval), and 5) BOO plus anakinra (BOO + Ana; obstructed rats given 25 mg/kg anakinra (i.p.) in 0.2 ml at each dosing interval). Glyburide (Enzo Life Sciences Inc., Farmingdale, NY) was prepared and used as previously described.2,16 Anakinra was obtained from Duke University Medical Center Pharmacy as prefilled syringes (Swedish Orphan Biovitrum AB, Stockholm, Sweden) and diluted 1:6 with saline prior to injection. Animals were given medication the day prior and again 30–60 min before surgery (Day 0). Thereafter they were administered a dose daily for 11 days and sacrificed on day 12.
Surgery
Rats were anesthetized with ketamine hydrochloride (90 mg/kg) and xylazine (10 mg/kg) (i.p.).2 For BOO and Sham, a catheter (PE50 tubing, O.D. 1 mm) was inserted transurethrally. The abdominal cavity was opened and a 5-0 silk suture passed around the urethra and tied securely in BOO and loosely in Sham rats. The catheter was removed and the abdominal wall closed (5-0 PGA).
Histology and Image Analysis
Bladders were weighed, fixed (formalin), embedded in paraffin and sectioned (5µm). Transverse sections from the lower third of the bladder were stained with a rabbit anti-PGP9.5 antibody (1:200; cat# 381000; ThermoFisher Novex, Waltham, MA) using standard methods and citrate antigen retrieval when necessary. HRP development was accomplished with the Vectastain ABC Staining Kit (Vector Laboratories, Burlingame, CA) using a secondary antibody provided. Sections were imaged (10X; Zeiss Axio Imager 2, Carl Zeiss AG, Oberkochen, Germany) with Zen software (Zeiss). The entire section was imaged using tiling micrographs stitched into a continuous image by the software. Calibration bars were inserted and images exported as TIFF files. Files were imported into NIS-Elements (Nikon Co., Tokyo, Japan) and calibrated. The outer bladder wall was identified using edge detection algorithms and confirmed visually. A hue spectrum was used to identify and exclude the urothelium and luminal space creating a region of interest (ROI) equivalent to the bladder wall. The software then provided the area of the ROI in µm2. The autodetect function was then used to define and count black/brown spots within the ROI, radially covered by non-adventitial tissue. Zhong et al.17 found small-medium size neurons in the bladder to be 15.5 ± 3.5 µm in radius, or ≈240 µm2 in cross-sectional area, assuming a roughly circular shape. In our analysis a size cut off of 50 µm2 was used to insure small neurons were counted while precipitates and staining artifacts were excluded. Neuronal density in a given section was calculated by dividing the number of nerves by the µm2 of the ROI.
Neuronal Isolation, Culture, Branching and Apoptosis Assay
Major pelvic ganglia were carefully dissected and dissociated with collagenase (2 mg/ml) and dispase (2.5 mg/ml) for 45 min. They were then cultured (37 °C/5% CO2) on poly-L-ornithine and laminin coated coverslips (Invitrogen Co., Carlsbad, CA, USA) in neurobasal medium containing B27 supplement, GlutaMax, penicillin/streptomycin, glial cell derived neutrophic factor (GDNF; 2ng/ml) and neurotropin-4 (NT-4, 10ng/ml). Media and supplements were from Invitrogen Co. (Carlsbad, CA, USA). After 24 h, pelvic neurons were administered IL-1β (0, 100, 250 and 500 ng/ml) and cultured an additional 48 h. Apoptosis was assessed using the In Situ Cell Death Detection Kit, Fluorescein (Roche, Penzberg, Germany) followed by immunofluorescent staining for neuron-specific class III beta-tubulin (TUJ1, 1:200, BioLegend; San Diego, CA, USA) with a secondary antibody conjugated with Alexa Fluor 594 (Thermo Fisher Scientific, Waltham, MA, USA) to identify neurons. Samples were visualized with an Olympus IX81 fluorescent microscope and images captured with cellSens Dimension software (Olympus America Inc., Center Valley, PA, USA). Neurite length, number of branches per neurite, number of total neurons and number of apoptotic neurons were measured using ImageJ software (NIH, USA). For each coverslip, 15–30 images were taken. From each image several neurons, which were completely distinguishable from neighboring cells, were measured. The longest axon for each neuron was measured and recorded. Branches at least two times longer than the diameter of the cell body were counted.
Statistical analysis
Data are reported as the mean ± standard error (SEM). Statistical analyses were performed using GraphPad InStat software (GraphPad Software, inc., La Jolla, CA, USA) and a one-way ANOVA with a Tukey’s post-hoc test. Results were considered statistically significant at P<0.05.
Results
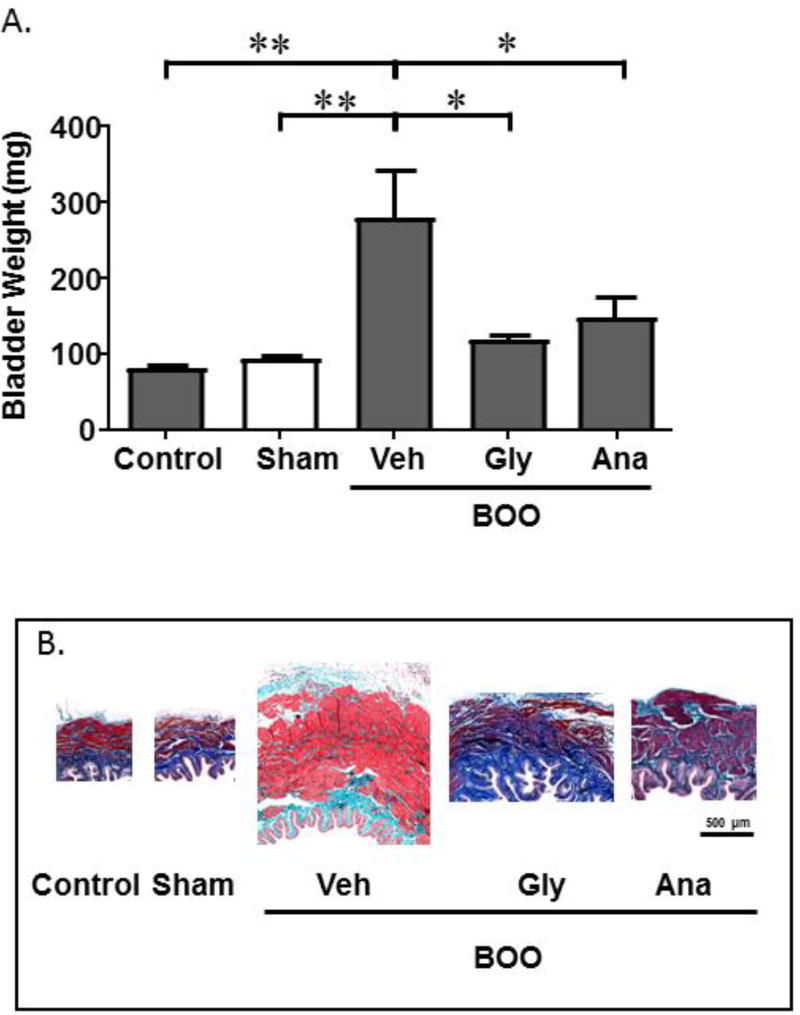
To ensure BOO triggered inflammation in the current cohort of animals and that blocking NLRP3 reduced this inflammation, as we have previously shown,2,5 we recorded bladder weights. Figure 1A demonstrates that bladders from BOO rats had significantly increased weights compared to Sham bladders. This increase was blunted by glyburide to levels not significantly different from Sham. A similar result was seen with anakinra. In addition, hypertrophy of the bladder’s smooth muscle has long been associated with BOO and has recently been shown to be mediated through the NLRP3/IL-1β pathway.18 As shown in Figure 1B, there was clear muscle hypertrophy in the BOO animals given vehicle, demonstrating there was a sufficient obstruction produced in those animals. Importantly, the hypertrophy was reduced in the presence of either inhibitor demonstrating that our treatment regime was effective at blocking the NLRP3/IL-1β pathway.
Figure 1.
Bladder weights and smooth muscle hypertrophy are increased in BOO and this increase is blocked by glyburide (Gly) and anakinra (Ana). A. Bladder weights of the various experimental conditions are shown. Data are presented as mean ± SEM. n = 4 for Control, 6 for Sham, 6 for BOO + Veh, 5 for BOO + Gly, 4 for BOO + Ana. * indicates P<0.05, ** indicates P<0.01 for the comparisons indicated. Any between-group comparisons that are not indicated were not significant. B. Mason’s trichrome staining of tissue sections from the various groups indicated in B.
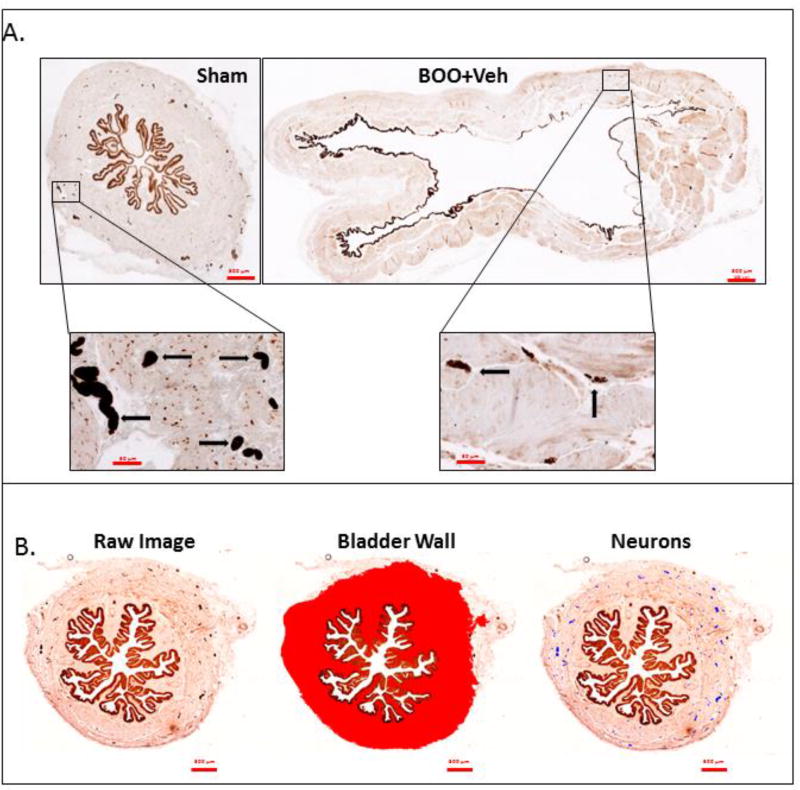
To assess innervation, the bladders used in Figure 1 were processed, sectioned and stained for the pan-neuronal marker PGP9.5. For quantitation, entire cross-sections from the lower third of the bladder were scanned. Figure 2A shows a representative scan of bladders from a Sham and a BOO rat while illustrating the much larger size of the bladder from the BOO rat. Interestingly, the anti PGP9.5 antibody strongly stained the urothelium as well. While the significance of urothelial staining is unknown, it has been reported previously.19 The inserts display the staining of neurons. Using NIS-Elements image analysis software (Nikon Co., Tokyo, Japan) the bladder wall was identified (Figure 2B, middle panel) and the cross-sectional area of the wall was quantitated. Individual neurons were then identified by a hue spectrum (Figure 2B, right panel – neurons falsely colored blue) and counted. Neuronal density was calculated as the number of nerves per µm2 of tissue.
Figure 2.
Representative histological sections of bladders stained for PGP9.5 that illustrate the quantitation method. A. Representative staining of Sham and BOO + Veh sections that illustrate both the dramatic increase in size (upper panels) as well as the decrease in neuronal numbers (brown spots in the lower panels, several of which are marked with arrows) of the BOO + Veh bladder compared to Sham. B. Representative quantitation of a Sham bladder. The left panel demonstrates the actual staining (brown). There is strong staining of both neurons and the urothelia. The middle panel demonstrates how the bladder wall was chosen to exclude adjacent non-bladder tissue, the lumen and the urothelia. The chosen ROI is indicated in red and this is the ROI used to calculate bladder cross-sectional area by the NIS-Elements image analysis software. The right panel illustrates how stained neurons in the ROI were identified by the software and false colored blue. The number of neurons (blue spots) was then calculated by the Elements software.
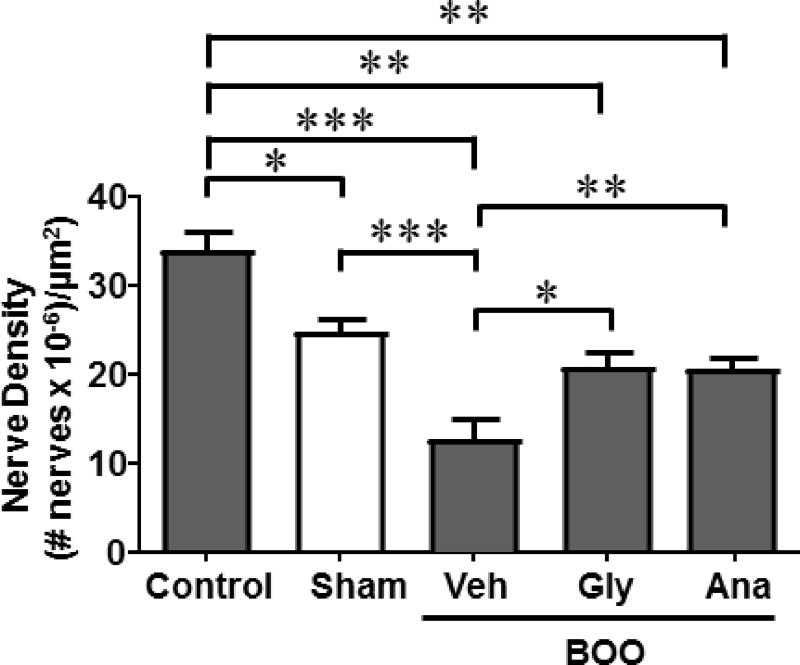
As shown in Figure 3, there was a slight, but significant, decrease in nerve density in response to sham surgery alone This decrease was considerably more substantial in the BOO rats. Both glyburide and anakinra prevented the loss of nerve density resulting in numbers that were lower than controls but were not significantly different from sham.
Figure 3.
Denervation during BOO is blocked by glyburide or anakinra. Nerve density was measured in whole bladder cross-sections, as described in the Material and Methods section, in the various experimental groups. The nerves were identified and counted and the area of the ROI, as illustrated in Figure 2, were all determined using the Elements software. The number of nerves was then divided by the area to get a measurement of nerve density. Data are presented as mean ± SEM. n = 4 for Control, 6 for Sham, 6 for BOO + Veh, 5 for BOO + Gly, 4 for BOO + Ana. * indicates P<0.05, ** indicates P<0.01 and *** indicates P<0.005 for the comparisons indicated. Any between-group comparisons that are not indicated were not significant.
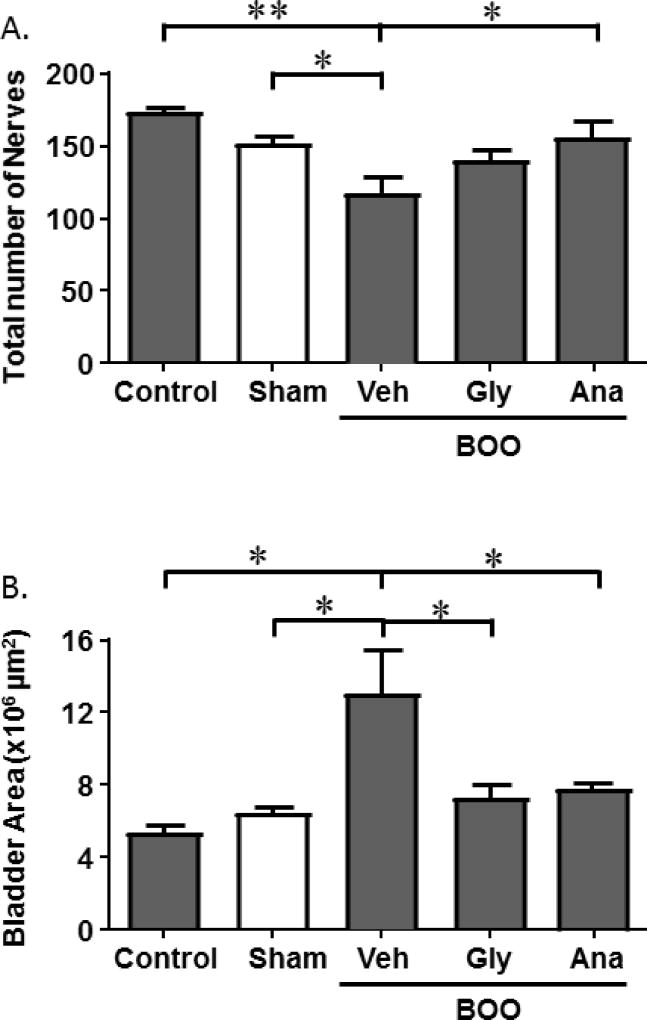
A decrease in nerve density can be accomplished by either a reduction in the total number of nerves or an increase in bladder area without commensurate nerve production or branching, or both. Figure 4A shows that the total number of nerves in the bladder sections decreased with BOO and this decrease was blocked by both glyburide and anakinra, although the increase by glyburide did not rise to the level of significance. In addition, the bladder wall cross-sectional area was significantly increased by BOO and this increase was again blocked by both glyburide and anakinra (Figure 4B). Thus, both a decrease in actual cell number and an increase in total bladder area contribute to the decrease in nerve density and both were mediated, in large part, through the NLRP3 inflammasome.
Figure 4.
BOO-induced decrease in nerve density is produced by both a decrease in nerve number and an increase in bladder area, both of which are blocked by glyburide or anakinra. A.) Total number of nerves in a transverse bladder section. B.) Area (in µm2) of the region of interest in a transverse bladder section, as defined in the Materials and Methods section and illustrated in Figure 1. Data are presented as mean ± SEM. N = 4 for Control, 6 for Sham, 6 for BOO + Veh, 5 for BOO + Gly, 4 for BOO + Ana. * indicates P<0.05, ** indicates P<0.01 for the comparisons indicated. Any between-group comparisons that are not indicated were not significant.
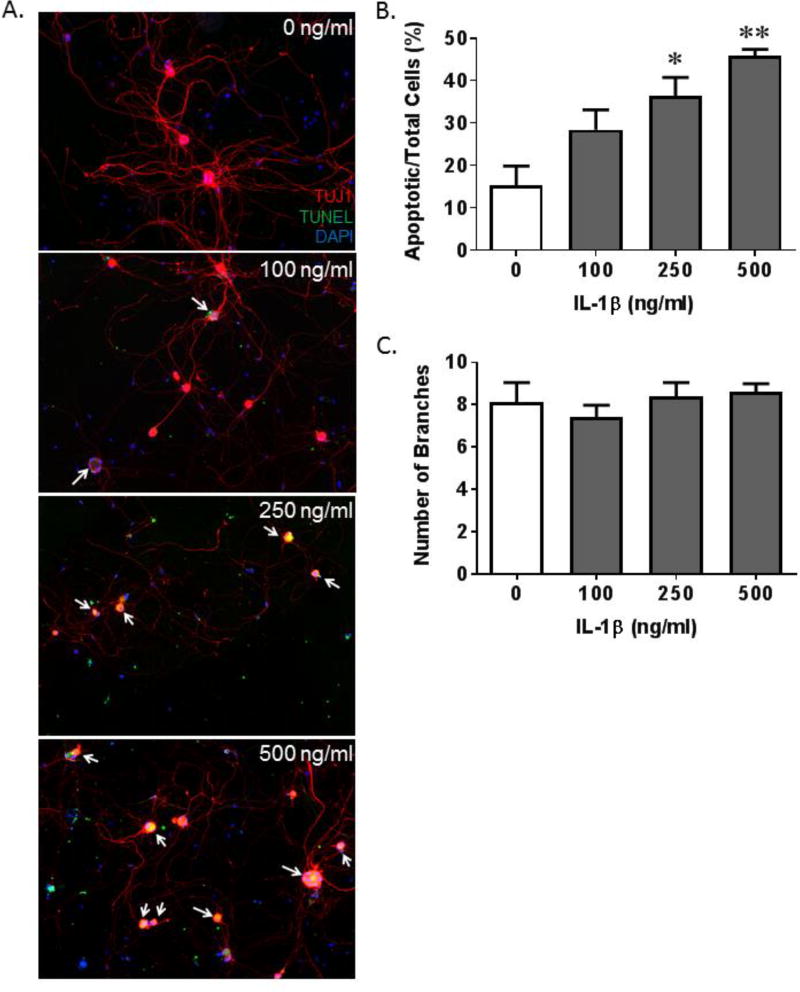
The decrease in the number of nerves reported in Figure 4A could be accounted for simply by a retraction of neuronal branching or through cell death mechanisms (likely apoptosis). Of course, apoptosis would involve both mechanisms for when cells undergo apoptosis they retract appendages (i.e. branches) and detach from the substratum before dying. To gain insight into the mechanism leading to the decrease in the number of nerves we treated cultured neurons from the pelvic ganglia with increasing doses of recombinant IL-1β. We then measured apoptosis, neuronal branching and neurite length concomitantly, as shown in Figure 5A. Quantitation clearly showed that the relative number of apoptotic cells increased with increasing doses of IL-1β (Figure 5B) while the number of neurite branches remained unchanged (Figure 5C).
Figure 5.
IL-1β induces apoptosis and an increase in neurite length in pelvic neurons in vitro. A. Representative images of pelvic neurons stained for TUJ1 (red; neuron specific class III β-tubulin), TUNEL (green, apoptotic marker) and DAPI (blue; nuclear stain). Apoptotic neurons are indicated by the white arrows. B. The number of apoptotic pelvic neurons, normalized to total neurons, per area of interest in cultures treated with increasing concentrations of IL-1β. C. The number of branches of the neurons visualized for B. Data in all graphs are presented as the mean ± SEM. n=4 for all groups. * indicates p<0.05 and ** indicates p<0.01 versus the 0 ng/ml IL-1β sample.
Discussion
Previously, we have shown that NLRP3 is activated in urothelia during BOO and activation is responsible for inflammatory changes such as edematous bladder weight gain and increased vascular permeability.2 Inhibiting NLRP3 diminished the inflammatory response and prevented deleterious functional effects such as increased frequency and decreased voiding volumes. Inflammation can affect storage and voiding physiology through direct effects on the detrusor smooth muscle, influencing innervation to the bladder, altering blood flow, and/or disrupting the urothelium. In this study we specifically focused on how the anatomic integrity of bladder nerves may be jeopardized due to NLRP3-mediated inflammation during BOO.
Since at least the 1980s it has been known that men with BOO secondary to BPH develop a decrease in bladder nerve density.14 In those studies Harrison et al. removed strips of muscle from BPH patients undergoing transurethral resection of the prostate (TURP) and measured contractile responses to carbachol and nerve (electrical) stimulation. Since their patients underwent urodynamics prior to surgery, they were able to compare men with demonstrated detrusor overactivity to those without. They found that specimens from men with bladder instability were hypersensitive to carbachol but had a diminished response to nerve stimulation. Thus, irritative symptoms such as bladder overactivity correlated with denervation in the obstructed bladder, although it should be noted that many non-neuronal factors play a role including muscle gene expression, muscle hypertrophy and state of fibrosis. While irritative voiding due to denervation may, at first, seem counterintuitive, it is well-established that initial, relatively slight, denervation provokes overactivity whereas more severe denervation leads to detrusor underactivity and decompensation. Drake et al.13 proposed that the initial overactivity was due to a patchy loss of efferent inhibitory input while in the latter stages denervation is so severe that the bladder is simply unable to respond to neuronal signals emanating from higher order nervous structures. The present study was performed on day 12 following BOO, a time at which our previous study had documented significant detrusor overactivity. Since both denervation and detrusor overactivity were prevented by inhibition of the NLRP3/IL-1β pathway one would predict this pathway may be acting, at least initially, on the efferent inhibitory neurons. Assessing inhibitory versus excitatory efferent neurons at this time point, and at chronic periods, will provide exciting fodder for future studies. In addition, for a long time the impairment of detrusor contractility in BOO was thought to be a result only of defective efferent input. However, more recently, there has been a greater appreciation of the contribution from the afferent pathways. During voiding, sensory input to the CNS from afferent neurons helps to maintain the voiding contraction and, in its absence, voiding efficiency is diminished. Thus, selective removal/apoptosis of afferent neurons could contribute to the poor voiding efficiency and increased post-void residual volumes that plague BPH patients. The current study does not differentiate between afferent and efferent nerves, which will also constitute interesting future studies. Regardless, preservation of either, or both, groups of neurons could slow or halt progression of bladder damage in BOO patients and the present results suggest that the NLRP3/IL-1β pathway is a prime target for intervention. Clinically, this could result in suppressing the development of bladder instability in patients who are not yet surgical candidates for TURP, attenuating the emergence of bladder decompensation and improving surgical outcomes for patients who ultimately undergo de-obstructing procedures.
Although denervation is clearly a major factor in the progressive deterioration during BOO,6–10 surprisingly little is known about the mechanism underlying it. Results from the vehicle-treated BOO animals in this study confirm reports that nerve density decreases after obstruction.6–10 We also found that the decreased density was conferred by both an increase in the overall mass of the bladder and a decrease in the total number of nerves. Thus, there appears to be a real loss of nerve number and/or branching during BOO. Furthermore, we demonstrated in the in vivo experiments that the loss of bladder density was diminished by blocking either the activation of NLRP3 with glyburide or the downstream action of IL-1β with anakinra. The in vitro experiments reflected a possible mechanism of neuronal loss by demonstrating a concentration-dependent increase in apoptosis of rat pelvic ganglia neurons in response to IL-1β. These data parallel evidence in Alzheimer’s disease in which IL-1β is elevated with injury and negatively regulates cell survival in the CNS.20 Taken together our results suggest BOO-induced denervation takes place by a hypertrophy of bladder mass with a concomitant death of neurons through IL-1β-stimulated apoptosis.
When tissues are damaged they release DAMPs that activate NLRP3. Tissue damage from BOO may occur as the result of elevated pressure, stretch and/or a sustained state of hypoxia followed by cyclical reperfusion with voiding. In our in vivo study, we cannot separate out these factors and all three conditions are present. Evidence of hypoxic involvement comes from a rabbit model of ischemia where hypoxia is the sole factor and denervation is a prominent feature.21 NLRP3/IL-1β-mediated nerve damage in response to hypoxia has also been found in post-mortem analyses of stroke victims as well as a mouse model of ischemia.22 Consideration of hypoxia as a triggering mechanism may also shed light on the usefulness of PDE5 inhibitors in the clinic. These agents improve bladder blood flow and urinary function in obstructed rat models23 which would relieve hypoxia. Our finding that NLRP3-mediated denervation in BOO is consistent with a major role for hypoxia, although effects of stretch and pressure cannot be ruled out. The results of studies on these stressors could provide a host of targets towards a multimodal strategy for the treatment of BOO-induced bladder dysfunction.
A limitation of this study is the use of female rats for the in vivo study and male neurons for the in vitro study. Certainly males and females perceive pain differently and sex hormones can have observable effects on neuroinflammation and neurodegeration.24 Moreover, there are significant differences in the inflammatory potential of male versus female urothelia which is illustrated by a more severe infection in males compared to females when anatomic barriers are bypassed.25
Conclusion
Denervation in response to BOO was blocked by inhibition of the NLRP3/IL-1β pathway and IL-1β directly induced apoptosis in pelvic ganglion neurons in vitro. Together the results suggest that one mechanism of BOO-induced denervation is NLRP3/IL-1β induced apoptosis. Inhibition of this pathway is a promising strategy as an adjunct therapy for treating patients with BOO.
Acknowledgments
The authors would like to thank Julie Fuller and the Substrate Services Core and Research Support Services (SCRSS) in the Department of Surgery as well as the Histology Core in the Department of Pathology for their help with histological embedding and sectioning. They would also like to thank the Light Microscopy Core Facility and Yasheng Gao for their help obtaining images. Both core facilities are at Duke University Medical Center.
Funding
This research was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health, USA (Award Number R01DK103534 to JTP) and intramural funds from Duke University Medical Center, Department of Surgery, Division of Urology.
Footnotes
Work was performed at Duke University Medical Center and Brody School of Medicine.
References
- 1.Macey MR, Raynor MC. Medical and Surgical Treatment Modalities for Lower Urinary Tract Symptoms in the Male Patient Secondary to Benign Prostatic Hyperplasia: A Review. Semin Intervent Radiol. 2016;33:217–223. doi: 10.1055/s-0036-1586142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hughes FM, Jr, Hill HM, Wood CM, et al. The NLRP3 Inflammasome Mediates Inflammation Produced by Bladder Outlet Obstruction. J Urol. 2016;195:1598–1605. doi: 10.1016/j.juro.2015.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Metcalfe PD, Wang J, Jiao H, et al. Bladder outlet obstruction: progression from inflammation to fibrosis. BJU Int. 2010;106:1686–1694. doi: 10.1111/j.1464-410X.2010.09445.x. [DOI] [PubMed] [Google Scholar]
- 4.Oka M, Fukui T, Ueda M, Tagaya M, Oyama T, Tanaka M. Suppression of bladder oxidative stress and inflammation by a phytotherapeutic agent in a rat model of partial bladder outlet obstruction. J Urol. 2009;182:382–390. doi: 10.1016/j.juro.2009.02.104. [DOI] [PubMed] [Google Scholar]
- 5.Hughes FM, Govada V, Sexton SJ, Purves J. Fibrosis in the Bladder in Response to Outlet Obstruction is Triggered through the NLRP3 Inflammasome and the Production of IL-1β. Am J Physiol-Renal. 2017 doi: 10.1152/ajprenal.00128.2017. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nyirady P, Thiruchelvam N, Fry CH, et al. Effects of in utero bladder outflow obstruction on fetal sheep detrusor contractility, compliance and innervation. J Urol. 2002;168:1615–1620. doi: 10.1016/S0022-5347(05)64530-2. [DOI] [PubMed] [Google Scholar]
- 7.Barendrecht MM, Chichester P, Michel MC, Levin RM. Effect of short-term outlet obstruction on rat bladder nerve density and contractility. Auton Autacoid Pharmacol. 2007;27:47–53. doi: 10.1111/j.1474-8673.2006.00393.x. [DOI] [PubMed] [Google Scholar]
- 8.Levin RM, Levin SS, Zhao Y, Buttyan R. Cellular and molecular aspects of bladder hypertrophy. Eur Urol. 1997;32(Suppl 1):15–21. [PubMed] [Google Scholar]
- 9.de Jongh R, Dambros M, Haenen GR, et al. Partial bladder outlet obstruction reduces the tissue antioxidant capacity and muscle nerve density of the guinea pig bladder. Neurourol Urodyn. 2009;28:461–467. doi: 10.1002/nau.20677. [DOI] [PubMed] [Google Scholar]
- 10.Nomiya M, Andersson KE, Yamaguchi O. Chronic bladder ischemia and oxidative stress: new pharmacotherapeutic targets for lower urinary tract symptoms. Int J Urol. 2015;22:40–46. doi: 10.1111/iju.12652. [DOI] [PubMed] [Google Scholar]
- 11.Tubaro A, Carter S, Trucchi A, Punzo G, Petta S, Miano L. Early treatment of benign prostatic hyperplasia: implications for reducing the risk of permanent bladder damage. Drugs Aging. 2003;20:185–195. doi: 10.2165/00002512-200320030-00003. [DOI] [PubMed] [Google Scholar]
- 12.Zderic SA, Wein A, Rohrman D, et al. Mechanisms of bladder smooth-muscle hypertrophy and decompensation: lessons from normal development and the response to outlet obstruction. World J Urol. 1998;16:350–358. doi: 10.1007/s003450050079. [DOI] [PubMed] [Google Scholar]
- 13.Drake MJ, Kanai A, Bijos DA, et al. The potential role of unregulated autonomous bladder micromotions in urinary storage and voiding dysfunction; overactive bladder and detrusor underactivity. BJU Int. 2017;119:22–29. doi: 10.1111/bju.13598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harrison SC, Hunnam GR, Farman P, Ferguson DR, Doyle PT. Bladder instability and denervation in patients with bladder outflow obstruction. Br J Urol. 1987;60:519–522. doi: 10.1111/j.1464-410x.1987.tb05033.x. [DOI] [PubMed] [Google Scholar]
- 15.Lamkanfi M, Mueller JL, Vitari AC, et al. Glyburide inhibits the Cryopyrin/Nalp3 inflammasome. J Cell Biol. 2009;187:61–70. doi: 10.1083/jcb.200903124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hughes FM, Jr, Vivar NP, Kennis JG, et al. Inflammasomes are important mediators of cyclophosphamide-induced bladder inflammation. Am J Physiol Renal Physiol. 2014;306:F299–308. doi: 10.1152/ajprenal.00297.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhong Y, Banning AS, Cockayne DA, Ford AP, Burnstock G, McMahon SB. Bladder and cutaneous sensory neurons of the rat express different functional P2X receptors. Neuroscience. 2003;120:667–675. doi: 10.1016/s0306-4522(03)00243-4. [DOI] [PubMed] [Google Scholar]
- 18.Haldar S, Dru C, Choudhury D, et al. Inflammation and pyroptosis mediate muscle expansion in an interleukin-1beta (IL-1beta)-dependent manner. J Biol Chem. 2015;290:6574–6583. doi: 10.1074/jbc.M114.617886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guan NN, Svennersten K, de Verdier PJ, Wiklund NP, Gustafsson LE. Receptors involved in the modulation of guinea pig urinary bladder motility by prostaglandin D2. Br J Pharmacol. 2015;172:4024–4037. doi: 10.1111/bph.13174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu KT, Wang YW, Yang JT, Yang YL, Chen HI. Effect of interleukin-1 on traumatic brain injury-induced damage to hippocampal neurons. J Neurotrauma. 2005;22:885–895. doi: 10.1089/neu.2005.22.885. [DOI] [PubMed] [Google Scholar]
- 21.Yoshida M, Masunaga K, Nagata T, Satoji Y, Shiomi M. The effects of chronic hyperlipidemia on bladder function in myocardial infarction-prone Watanabe heritable hyperlipidemic (WHHLMI) rabbits. Neurourol Urodyn. 2010;29:1350–1354. doi: 10.1002/nau.20843. [DOI] [PubMed] [Google Scholar]
- 22.Fann DY, Lee SY, Manzanero S, et al. Intravenous immunoglobulin suppresses NLRP1 and NLRP3 inflammasome-mediated neuronal death in ischemic stroke. Cell Death Dis. 2013;4:e790. doi: 10.1038/cddis.2013.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lythgoe C, McVary KT. The use of PDE-5 inhibitors in the treatment of lower urinary tract symptoms due to benign prostatic hyperplasia. Curr Urol Rep. 2013;14:585–594. doi: 10.1007/s11934-013-0373-2. [DOI] [PubMed] [Google Scholar]
- 24.Massa MG, David C, Jorg S, et al. Testosterone Differentially Affects T Cells and Neurons in Murine and Human Models of Neuroinflammation and Neurodegeneration. Am J Pathol. 2017;187:1613–1622. doi: 10.1016/j.ajpath.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 25.Olson PD, Hruska KA, Hunstad DA. Androgens Enhance Male Urinary Tract Infection Severity in a New Model. J Am Soc Nephrol. 2016;27:1625–1634. doi: 10.1681/ASN.2015030327. [DOI] [PMC free article] [PubMed] [Google Scholar]