Abstract
Anesthesia/surgery could be associated with cognitive impairment and Alzheimer’s disease neuropathogenesis. However, whether surgery under different anesthetics has different effects on cognitive function remains largely unknown. We therefore set out to compare effects of anesthetic isoflurane or desflurane plus surgery on cognitive function and hippocampus levels of synaptic marker (postsynaptic density-95 and synaptophysin) and ATP. Five-month-old AD Transgenic (Tg) (FAD5X) and wild-type male mice received isoflurane or desflurane plus abdominal surgery. We assessed cognitive function in Barnes maze, and measured hippocampus levels of postsynaptic density-95, synaptophysin, and ATP in the mice. We determined whether Vitamin K2 could mitigate these anesthesia/surgery-induced changes. Isoflurane, but not desflurane, plus surgery increased escape latency and escape distance in Barnes maze probe test and reduced postsynaptic density-95, synaptophysin and ATP levels as compared to control condition in AD Tg mice. Vitamin K2 attenuated the anesthesia/surgery-induced changes in the AD Tg mice. These findings suggest that isoflurane, but not desflurane, plus surgery might induce cognitive impairment via causing brain energy deficits. Pending confirmative studies in both animals and humans, desflurane could be a better choice for AD patients when surgery is needed. Moreover, Vitamin K2 could treat cognitive deficiency associated with anesthesia and surgery.
Keywords: Anesthesia/surgery, isoflurane, desflurane, cognitive deficiency, synapse, ATP, Alzheimer’s disease, transgenic mice
Introduction
Clinical investigations suggest potential association between anesthesia/surgery and cognitive impairment [1–5], and Alzheimer’s disease (AD) neuropathogenesis [6–9]. However, findings that anesthesia/surgery is not associated with dementia also exists [10–14]. Interestingly, the majority of these studies did not specifically determine the effects of different anesthetics (e.g., isoflurane versus desflurane) on the cognitive function. Answering the question whether surgery under certain anesthetics (e.g., desflurane) are less associated with cognitive impairment than other anesthetics (e.g., isoflurane) would be important in the ultimate development of strategy to prevent postoperative cognitive impairment in AD patients [reviewed in [15]].
Our previous studies have shown that anesthesia with isoflurane, but not with desflurane, can induce cognitive impairment in wild-type (WT) mice [16]. Moreover, such a difference could be due to the different effects of isoflurane and desflurane on mitochondrial function [16,17]. Other studies also assessed the effects of anesthetic isoflurane and halothane on cognitive function in AD transgenic (Tg) mice [18]. However, these studies did not include surgery, thus the outcomes may not be sufficient enough to promote clinical investigation to determine whether surgery under desflurane is less associated with cognitive impairment and/or dementia than surgery under isoflurane. Other studies show that surgery under brief general anesthesia [19–25] or under local anesthesia [26,27] can induce cognitive impairment in rodents. But these studies did not use AD Tg mice and did not compare the effects of surgery under different anesthetics (e.g., isoflurane versus desflurane) on cognitive function in the rodents.
Our recent studies have established the system of abdominal surgery under anesthesia in mice [28,29]. Thus, the objective of the current studies is to compare the effects of surgery under either isoflurane or desflurane on cognitive function in 5 month-old AD Tg (5XFAD) mice and age-matched WT mice to test a hypothesis that isoflurane, but not desflurane, plus surgery induces cognitive impairment in AD Tg mice.
Postsynaptic density 95 (PSD-95) [30,31] and synaptophysin (SVP) [32] are markers of synapse in hippocampus. Therefore, we compared the effects of isoflurane or desflurane plus surgery on the hippocampus levels of PSD-95 and SVP, as well as ATP levels, as parts of the mechanistic investigation.
Finally, Vitamin K2 is a mitochondrial electron carrier and energy enhancer [33], and has neuroprotective effects [33–35]. We therefore assessed whether Vitamin K2 could mitigate the effects of anesthesia plus surgery on cognitive function, hippocampus levels of synaptic markers and ATP in the mice.
Materials and Methods
Mice anesthesia/surgery and treatment
We performed all experiments in accordance with the National Institutes of Health guidelines and regulations. Massachusetts General Hospital (Boston, Massachusetts) Standing Committee on the Use of Animals in Research and Teaching has approved the animal protocol (Protocol number: 2006N000219). Efforts were made to minimize the number of animals used. The wild-type (WT) mice in the current studies were C57BL/6J mice (The Charles River Laboratories, Wilmington, MA). The AD transgenic mice were purchased from Jackson Lab (Bar Harbor, ME) (B6SJL Tg(APPSwFlLon,PSEN1*M146L*L286V)6799Vas/Mmjax; Stock Number: 006554), and maintained in our own lab. Standard genotype technique was used to confirm the condition of AD Tg mice. Both WT and AD Tg mice used in the studies were 5-month old male mice. The AD Tg mice are APP/PS1 double transgenic mice with coexpression of five familial Alzheimer’s disease (FAD) mutations (5XFAD) [36]. The amyloid deposition (and gliosis) in 5XFAD mice begins at 2 months of age and intraneuronal Aβ42 accumulation in these mice starts at 1.5 months of age [36]. Therefore, 5 month-old 5XFAD mice have developed sufficient AD neuropathogenesis. For the purpose of comparison, we employed same age (5 month-old) WT mice in the studies. We used male mice because there are no studies which exclusively used male mice to assess the effects of anesthesia and/or surgery on cognitive function. In addition, we had sufficient amounts of male AD Tg mice at the time of the experiments. We housed the mice in a controlled environment (20 – 22°C; 12 hours of light/dark on a reversed light cycle) for seven days prior to the studies. As demonstrated in Supplementary Figure 1, the mice were randomly assigned to isoflurane or desflurane anesthesia plus surgery (anesthesia/surgery) group or the control group. The anesthesia/surgery was started between 9:00 and 10:00 am. A simple laparotomy was performed under 1.4% isoflurane or 7.5% desflurane anesthesia. Specifically, anesthesia was induced and maintained with 1.4% isoflurane or 7.5% desflurane in 100% oxygen in a transparent acrylic chamber. Fifteen minutes after the induction, each of the mice was moved out of the chamber, and anesthesia (isoflurane or desflurane) was maintained via a cone device. One 16-gauge needle was inserted into the cone near the nose of the mouse to monitor the concentration of isoflurane. A longitudinal midline incision was made from the xiphoid to the 0.5 centimeter proximal pubic symphysis on the skin, abdominal muscles and peritoneum. Then, the incision was sutured layer by layer with 5-0 Vicryl thread. At the end of the procedure, EMLA cream (2.5% lidocaine and 2.5% prilocaine) was applied to the incision wound, and then three times daily for three days to treat the pain associated with the incision. Our previous studies [26] have shown that there was no significant difference of pain threshold between the control and surgery mice at 24 hours after the surgery. The procedure for each mouse lasted about ten minutes, and the mouse was put back into the anesthesia chamber for up to two hours to receive the rest of the same anesthesia (1.4% isoflurane or 7.5% desflurane in 100% oxygen). We used this combination of anesthesia and surgery in the studies because the abdominal surgery could potentiate the anesthesia neurotoxicity and this combination has been shown to induce cognitive impairment in our previous studies [29,28]. The temperature of the anesthetizing chamber was controlled (DC Temperature Control System; FHC, Bowdoinham, Maine) to maintain the rectal temperature of the mice at 37 ± 0.5 °C during the anesthesia/surgery. After recovering from the anesthesia, each mouse was returned to a home cage with food and water available ad libitum. The mice in the control group (food and water available ad libitum) were placed in their home cages with room air for two hours, which was consistent with the condition of humans without anesthesia and surgery. Our previous studies found that neither this type of surgery [27,26] nor anesthesia with 1.4% isoflurane [37] significantly disturbed the values of blood pressure and blood gas in the mice. EMLA was able to treat the pain associated with the surgery in the mice [27,26]. In the interventional studies, Vitamin K2 (Sigma-Aldrich Inc., Natick, MA, Cat. Number: V9378) or coil oil (vehicle of Vitamin K2) was administered to the AD Tg mice via intraperitoneal injection daily for 9 days (8 days before the anesthesia/surgery day and then 30 minutes before the anesthesia/surgery on the procedure day). The dosage of Vitamin K2 (100 mg/kg/d) was selected according to the previous studies [35].
Barnes maze test
We performed Barnes maze test using the methods described in other studies [38–41] with modifications. Barnes maze, a circular open platform (about 90 center meter diameter) with 20 equally spaced holes (one of these holes connects with a small dark recessed chamber called escape box), located in a quiet area and was surrounded by a dark curtain with 4 simple colored-paper shapes (square, circle, triangle and star) as markers (Stoelting, Wood Dale, IL). A video hangs right above the platform which can capture the entire platform. The movement parameters (escape latency, escape distance, escape speed, escape errors and time in target quadrant) of the mouse were monitored and analyzed via a video camera connected to the Any-Maze animal tracking system software (Stoelting Co., Wood Dale, IL) as described in previous studies [42,43,39]. The % of time in the time in target quadrant corresponds to the time spent in 1/4 of the maze. We used the hidden-target hole protocal in the studies according to the methods described in previous studies [44,45]. Specifically, the location of the target hole was fixed and was not marked by any intra-maze reference, which was located in the same position relative to the extramaze cues around the test room. This protocol refers the use of the spatial cues to find the hidden target, which is specific to the spatial reference-memory version of the Barnes-maze task [44,45]. We only measured the total errors and total latency in the current studies according to the methods described in the previous studies [46–50]. The Barnes maze test in the current studies included Barnes maze training test (day 1 to 7 before the anesthesia/surgery) and Barnes maze probe test (3, 7, 14, 21, 28, 35 and 42 days after the anesthesia/surgery, respectively) (Supplementary Figure 1). On day 8 before the anesthesia/surgery, all of the mice were habituated to the maze. During the habituation, the mouse was placed in the escape box for 2 minutes, then placed directly in the hole that led to the escape box and allowed to remain in the box for another 4 minutes. Finally, the mouse was placed under a bucket in the center of the circular platform and motivated to escape under the stimulation of bright light (200 Watt) and aversive noise (85 decibel), and gently guided to the hole connecting to the escape box. Immediately after the mouse entered the tunnel between the hole and the escape box, the buzzer should be turned off. Each mouse was allowed to remain in the escape box for 1 minute, and then removed and placed back to the home cage. The Barnes maze training test (day 7 to day 1 before the anesthesia/surgery) consisted of 2 trials (3 minutes each trial and 15 minutes between the trials) for 7 days. Our pilot studies showed that the AD Tg mice had not learnt to find the escape hole at the end of 4-day training period because the AD Tg mice had impaired learning ability. Previous studies also suggest that 7-day training period may increase the learning performance in Barnes maze as compared to 4-day training period [43]. Therefore, we used the 7-, but not 4-, day training period in the current studies to better illustrate the potential difference in Barnes maze performance between the mice in control group and the mice in anesthesia/surgery groups. In each trial, the mouse was placed under a bucket in the center of the circular platform for 10 seconds and was allowed to escape under the same stimulation of light and aversive noise. Once reaching the escape box, the mouse was allowed to remain in the escape box for 1 minute. The mouse was then removed and placed back to the home cage for 15 minutes of rest period before returning back for another trial. The values of the escape latency of the last training day served as the Barnes maze probe test baseline. After the training period, the mice had the anesthesia/surgery (day 0). Then the mice had the Barnes maze probe test on day 3, 7, 14, 21, 28, 35 and 42 after the anesthesia/surgery, respectively (Supplementary Figure 1). The total time to find the escape box was recorded as escape latency. Moreover, the total path length of distance traveled (escape distance), mean speed (escape speed), the total error holes searched (escape errors) and the percentage of time spent in the target quadrant [time in target quadrant (%)] before the mice found the escape box in both probe test and training test were recorded. The increase in escape latency, escape distance, escape errors and decrease in time in target quadrant in the Barnes maze suggests cognitive impairment of the mice [38–41]. The decreases in escape speed suggest impairment of locomotor activity. We used the percentage of time in the target quadrant rather than the target preference in the current studies according to the previous studies [51,42]. Between each test, the Barnes maze was cleaned with 75% alcohol solution to avoid olfactory cues.
Brain tissue harvest, lysis and protein quantification
At the end of last behavior test on day 42, each of mice was killed by decapitation. The brain tissues (hippocampus) of the mice were harvested and subjected to Western blot analysis. The brain tissues (cortex) of the mice were also harvested and subjected to ELISA Aβ measurement. Different groups of mice were used for the studies to determine brain ATP levels. The brain tissues (hippocampus) of the mice were harvested immediately at the end of the anesthesia/surgery for the measurement of brain ATP levels [28]. We homogenized the harvested brain tissues on ice using immunoprecipitation buffer (10 mMTris-HCl, pH 7.4, 150 mMNaCl, 2 mM EDTA, and 0.5% Nonidet P-40) plus protease inhibitors (1 mg/ml aprotinin, 1mg/ml leupeptin, and 1 mg/ml pepstatin A). The lysates were then collected and centrifuged at 10,000 rpm for 5 minutes at 4° C. Total protein levels were quantified with bicinchoninicacid (BCA) protein assay kit (Pierce, Iselin, NJ) as described in our prior studies [16].
Western blot analysis
The Postsynaptic Density Protein 95 (PSD-95) antibody (95 kDa, 1:1,000, Cell Signaling, Danvers, MA) and synaptophysin (SVP) antibody (38kDa, 1:1,000, Abcam, Cambridge, MA) were used in the Western blot analysis to detect the levels of PSD-95 and SVP, respectively. The quantification of Western blot was performed as described in our previous studies [16,37]. Specifically, signal intensity was analyzed using image analysis program Quantity One (Bio-Rad, Hercules, CA). The Western blots were then quantified in two steps. First, we used β-actin levels to normalize protein levels (e.g., determining the ratio of PSD-95 to β-actin amount) and control for loading differences in the total protein amount. Second, we presented the changes in protein levels from the mice undergoing anesthesia/surgery as a percentage of those in the mice from control group. 100% changes of protein levels refer to control levels for the purpose of comparison with experimental conditions.
ATP measurement
The levels of ATP in the hippocampus of mice were determined by the ATP Colorimetric/Fluorometric Assay Kit following the protocol provided by the manufacturer (Biovision Inc, Milpitas, CA) and the methods described in previous studies [28,52–54]. Briefly, we homogenized the harvested hippocampus tissues on ice by using 100 μL ATP assay buffer, then we took 80 μL sample and 20 μL ice cold perchloric acid and put them in a new tube, placed the tube on ice for 5 minutes and mixed well by vortex. We centrifuged the samples for 2 minutes at the speed of 13,000 g. 76 μL of the supernatant were transferred to a new tube. Then, we added 4 μL ice-cold neutralization solution to the tube, mixed to neutralize the sample placed on ice, and the sample tube was opened to air for 5 minutes. We then centrifuged the tube at 13,000 g for 2 minutes for bicinchoninic acid assay. We diluted 10 μL of the ATP standard with 90 μL distilled water to generate 1 mM ATP standard and mixed it well. We added sample and ATP assay buffer (1 mM) to 50 μL/well in the 96-well plate. Finally, we added reaction mix of 50 μL to each well containing the ATP standard or test samples. The reaction mix includes 44 μL ATP assay buffer; 2 μL ATP probe; 2 μL ATP converter; and 2 μL developer. We mixed the samples and incubated them for 30 minutes at room temperature (avoiding light). Finally, we measured the absorbance (OD 570 nm) in a micro-plate reader to calculate the ATP level.
Enzyme-linked immunosorbent assay (ELISA) Aβ measurement
The levels of Aβ42 in the cortex of mice were measured by the mouse Aβ42 immunoassay Kits (Invitrogen, San Francisco, CA, Catalog number: KMB 3441) according to the manufacturer’s instructions and the methods described in our previous studies [27].
Statistics
Data of escape latency, escape distance, escape speed, escape errors and time in target quadrant were expressed as mean ± standard error of mean (SEM). Data of other variables were expressed as mean ± standard deviation. The number of samples was 10 – 12 per group for the behavior test and 6 per group for the biochemical studies. The power calculation was performed using information collected from a preliminary study that was conducted under the same conditions. Based on the preliminary data, assuming a two-sided Student’s t-test, samples of 6 and 10 for each control and treatment group for the biochemistry and behavior studies, respectively, would lead to 90% power at the 0.05 alpha level. In the Barnes maze studies, interaction between time and group factors in a two-way ANOVA with repeated measurements was used to analyze the difference of memory curves (e.g., based on escape latency). Post hoc analyses (Bonferroni) were used to compare the difference in escape latency, escape distance, escape speed, escape errors and time in target quadrant for each testing day. A two-way ANOVA was used to determine the interaction between group and treatment on the levels of PSD-95, SVP, and ATP, followed by Bonferroni test for post-hoc comparisons. PSD-95, SVP, and ATP levels were presented as a percentage of those of the control group. P values less than 0.05 were considered statistically significant. Prism 6 software (Graph Pad Software, Inc, La Jolla, CA) was used to analyze the data.
Results
Different effects between isoflurane plus surgery and desflurane plus surgery on cognitive function in AD Tg and WT mice
The experiments were performed as demonstrated in Supplementary Figure 1. The baseline data of escape latency, escape distance, escape speed, escape errors and time in target quadrant of probe test of Barnes maze among the control condition, isoflurane plus surgery and desflurane plus surgery were not significantly different (Supplemental Figure 2). Moreover, There were no significant differences in the escape latency, escape distance, escape speed, escape errors and time in target quadrant of between the AD Tg and WT mice in control and the mice in anesthesia/surgery group in the Barnes maze training test (Supplemental Figure 3).
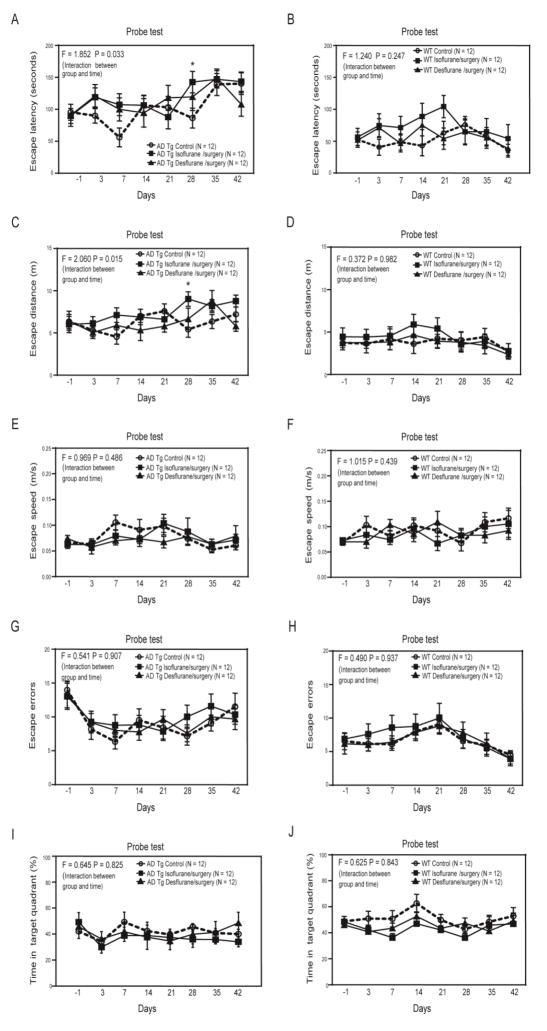
Two-way ANOVA with repeated measurement showed significant interaction of treatment (isoflurane plus surgery or desflurane plus surgery versus control condition) and time (days before and after the anesthesia/surgery) on escape latency of the AD Tg mice in the Barnes maze probe test (F = 1.852, P = 0.033, Figure 1A). Specifically, the isoflurane plus surgery, but not desflurane plus surgery, significantly increased the escape latency of the AD Tg mice in the Barnes maze probe test as compared to the control condition at 28 days (adjusted P = 0.034, left part of Supplementary Table 1) after the anesthesia/surgery (Figure 1A). Note that the AD Tg mice in both control group and isoflurane plus surgery group could have lost the memory obtained from Barnes maze training at 35 and 42 days after the anesthesia/surgery as evidenced by the longer escape latency the mice had. Neither the isoflurane plus surgery nor the desflurane plus surgery increased escape latency of the WT mice in the Barnes maze probe test as compared to the control condition (F = 1.240, P = 0.247, two-way ANOVA, Figure 1B).
Figure 1. Isoflurane plus surgery and desflurane plus surgery differently affected cognitive function of mice in AD Tg and WT mice.
A. Two-way ANOVA with repeated measurement showed significant interaction (F = 1.852, P = 0.033) of treatment (isoflurane plus surgery or desflurane plus surgery versus control condition) and time (days) on escape latency of Barnes maze probe test in the AD Tg mice. The isoflurane plus surgery, but not desflurane plus surgery, significantly increased the escape latency in Barnes maze probe test as compared to control condition on day 28 after the anesthesia/surgery in the AD Tg mice. B. Two-way ANOVA with repeated measurement showed no significant interaction (F = 1.240, P = 0.247) of treatment (isoflurane plus surgery or desflurane plus surgery versus control condition) and time (days) on escape latency of Barnes maze probe test in the WT mice. C. Two-way ANOVA with repeated measurement showed significant interaction (F = 2.060, P = 0.015) of treatment (isoflurane plus surgery or desflurane plus surgery versus control condition) and time (days) on escape distance of Barnes maze probe test in the AD Tg mice. The isoflurane plus surgery, but not desflurane plus surgery, significantly increased the escape distance in Barnes maze probe test as compared to control condition on day 28 after the anesthesia/surgery in the AD Tg mice. D. Two-way ANOVA with repeated measurement showed no significant interaction (F = 0.377, P = 0.980) of treatment (isoflurane plus surgery or desflurane plus surgery versus control condition) and time (days) on escape distance of Barnes maze probe test in the WT mice. E. Two-way ANOVA with repeated measurement showed no significant interaction (F = 0.969, P = 0.486) of treatment (isoflurane plus surgery or desflurane plus surgery versus control condition) and time (days) on escape speed of Barnes maze probe test in the AD Tg mice. F. Two-way ANOVA with repeated measurement showed no significant interaction (F = 1.015, P = 0.439) of treatment (isoflurane plus surgery or desflurane plus surgery versus control condition) and time (days) on escape speed of Barnes maze probe test in the WT mice. Two-way ANOVA with repeated measurement showed no significant interaction of treatment (isoflurane plus surgery or desflurane plus surgery versus control condition) and time (days) on escape errors (G and H) and time in target quadrant (I and J) in AD Tg (G and I) and WT (H and J) mice. ANOVA, analysis of variance; AD, Alzheimer’s disease; Tg, transgenic; WT, wild-type. N = 12 in each group.
Similarly, there was a significant interaction of treatment (isoflurane plus surgery or desflurane plus surgery versus control condition) and time (days before and after the anesthesia/surgery) on escape distance of the AD Tg mice in the Barnes maze probe test (F = 2.060, P = 0.015, two-way ANOVA, Figure 1C). Isoflurane plus surgery, but not desflurane plus surgery, increased escape distance of the AD Tg mice in the Barnes maze test (adjusted P = 0.008, right part of Supplemental Table 1). The isoflurane plus surgery or the desflurane plus surgery did not significantly change the escape distance of the WT mice in the Barnes maze probe test as compared to the control condition (F = 0.377, P = 0.980, two-day ANOVA, Figure 1D).
However, the two-way ANOVA with repeated measurement showed no significant interaction of treatment (isoflurane plus surgery or desflurane plus surgery versus control condition) and time (days before and after the anesthesia/surgery) on escape speed of the AD Tg mice (Figure 1E) and WT mice (Figure 1F) in the Barnes maze probe test. These data suggest that the anesthesia/surgery did not impair the locomotor activity in the mice. These treatments did not significantly affect the escape errors and time in target quadrant of both AD Tg and WT mice in the Barnes maze probe test (Figure 1G, 1H, 1I and 1J).
Finally, the AD Tg mice had longer baseline escape latency of Barnes maze probe test and higher brain Aβ levels as compared to WT mice (Supplementary Figure 4). Collectively, these data suggest that isoflurane plus surgery, but not the desflurane plus surgery, induced longer escape latency and escape distance, detected in Barnes maze probe test, in the AD Tg, but not the WT, mice, without impairment of locomotor activity. The isoflurane plus surgery selectively increased the escape latency and escape distance, but not escape errors or time in target quadrant, of the AD Tg mice in the Barnes maze probe test, but not training test.
Different effects between isoflurane plus surgery and desflurane plus surgery on levels of synaptic markers in hippocampus of AD Tg and WT mice
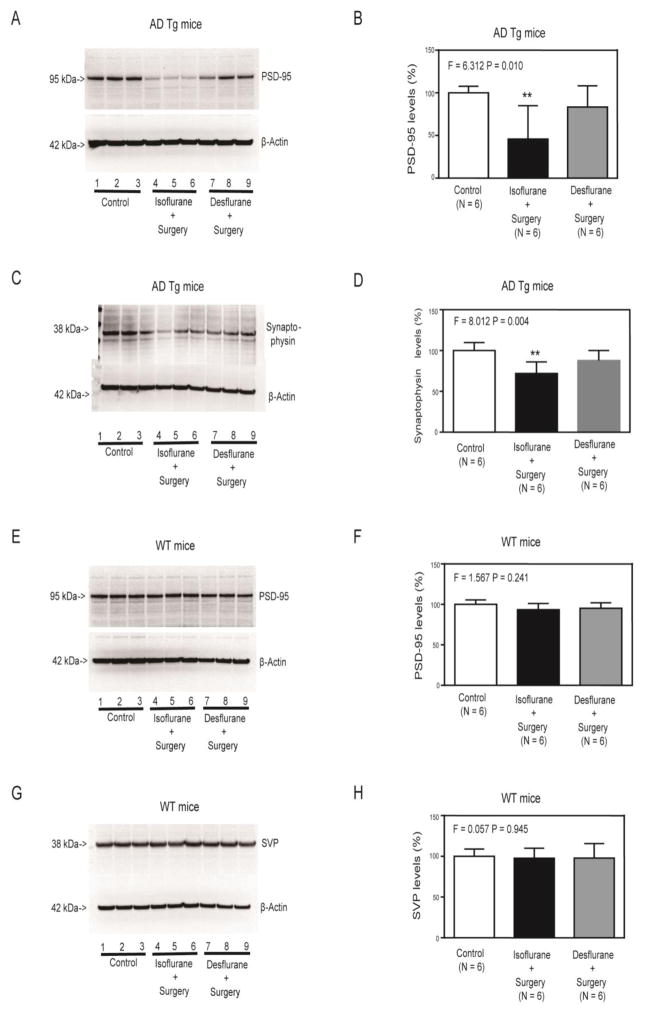
The immunoblotting of PSD-95 revealed visible decreases in density of the bands representing PSD-95 following isoflurane plus surgery (lanes 4 to 6), but not desflurane plus surgery (lanes 7 to 9), as compared to control condition (lanes 1 to 3) (Figure 2A). The quantification of the Western blot, based on the ratio of PSD-95 to β-Actin, showed that the isoflurane plus surgery (black bar), but not desflurane plus surgery (gray bar), significantly decreased the levels of PSD-95 in the hippocampus of AD Tg mice as compared to the control condition (white bar) (F = 6.312, P = 0.010, one-way ANOVA, Figure 2B). Similarly, the quantitative Western blot showed that the isoflurane plus surgery (lanes 4 to 6 in Figure 2C, black bar in Figure 2D), but not desflurane plus surgery (lanes 7 to 9 in Figure 2C, gray bar in Figure 2D), decreased the levels of SVP in the hippocampus of the AD Tg mice as compared to the control condition (lanes 1 to 3 in Figure 2C and white bar in Figure 2D) (F = 8.427, P = 0.004, one-way ANOVA).
Figure 2. Isoflurane plus surgery and desflurane plus surgery differently changed the levels of PSD-95 and SVP in hippocampus of AD Tg and WT mice.
Isoflurane plus surgery (lanes 4 to 6), but not desflurane plus surgery (lanes 7 to 9), decreased PSD-95 (A) or SVP (C) levels in hippocampus of AD Tg mice as compared to control condition (lanes 1 to 3). There was no significant difference in β-Actin levels among the various groups. Quantification of Western blot showed that the isoflurane plus surgery (black bar), but not desflurane plus surgery (gray bar), significantly decreased PSD-95 (B) or SVP (D) levels in the hippocampus of AD Tg mice as compared to control condition (white bar). Isoflurane plus surgery (lanes 4 to 6) or desflurane plus surgery (lanes 7 to 9) did not significantly decrease PSD-95 (E) or SVP (G) levels in the hippocampus of WT mice as compared to control condition (lanes 1 to 3). There was no significant difference in β-Actin levels among the various groups. Quantification of Western blot showed that isoflurane plus surgery (black bar) or desflurane plus surgery (gray bar) did not significantly decrease PSD-95 (F) or SVP (H) levels in the hippocampus of WT mice as compared to control condition (white bar). AD, Alzheimer’s disease; Tg, transgenic; WT, wild-type; PSD-95, postsynaptic density-95; SVP, synaptophysin. N = 6 in each group. The experiments to assess the effects of anesthesia and surgery on the levels of PSD-95 and SVP were performed on day 42 after the anesthesia plus surgery.
However, neither isoflurane plus surgery (lanes 4 to 6, black bar) nor desflurane plus surgery (lanes 7 to 9, gray bar) significantly altered the levels of PSD-95 (Figure 2E and Figure 2F) or SVP (Figure 2G and Figure 2H) in the hippocampus of WT mice.
Different effects between isoflurane plus surgery and desflurane plus surgery on ATP levels in hippocampus of AD Tg and WT mice
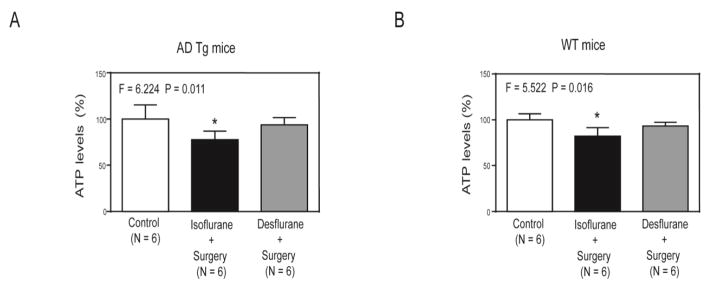
ELISA showed that isoflurane plus surgery (black bar in Figure 3A), but not desflurane plus surgery (gray bar in Figure 3A), decreased ATP levels in hippocampus of AD Tg mice as compared to control condition (white bar in Figure 3A) (F = 6.224, P = 0.011, one-way ANOVA). Interestingly, the isoflurane plus surgery (black bar in Figure 3B), but not desflurane plus surgery (gray bar in Figure 3B), also decreased ATP levels in hippocampus of WT mice as compared to control condition (white bar in Figure 3B) (F = 5.522, P = 0.016, one-way ANOVA).
Figure 3. Isoflurane plus surgery and desflurane plus surgery differently changed the ATP levels in the hippocampus of AD Tg and WT mice.
A. ELISA showed that the isoflurane plus surgery (black bar), but not desflurane plus surgery (gray bar), significantly decreased ATP levels in hippocampus of AD Tg mice as compared to control condition (white bar) at the end of the anesthesia/surgery. B. ELISA showed that the isoflurane plus surgery (black bar), but not desflurane plus surgery (gray bar), significantly decreased ATP levels in hippocampus of WT mice as compared to control condition (white bar) at the end of the anesthesia/surgery. AD, Alzheimer’s disease; Tg, transgenic; WT, wild-type; ATP, adenosine triphosphate. N = 6 in each group. The experiments to assess the effects of anesthesia and surgery on the levels of ATP were performed immediately at the end of the anesthesia plus surgery.
Vitamin K2 mitigated the longer escape latency and distance of Barnes maze probe test and reductions in the levels of hippocampus synaptic markers and ATP induced by isoflurane plus surgery in AD Tg mice
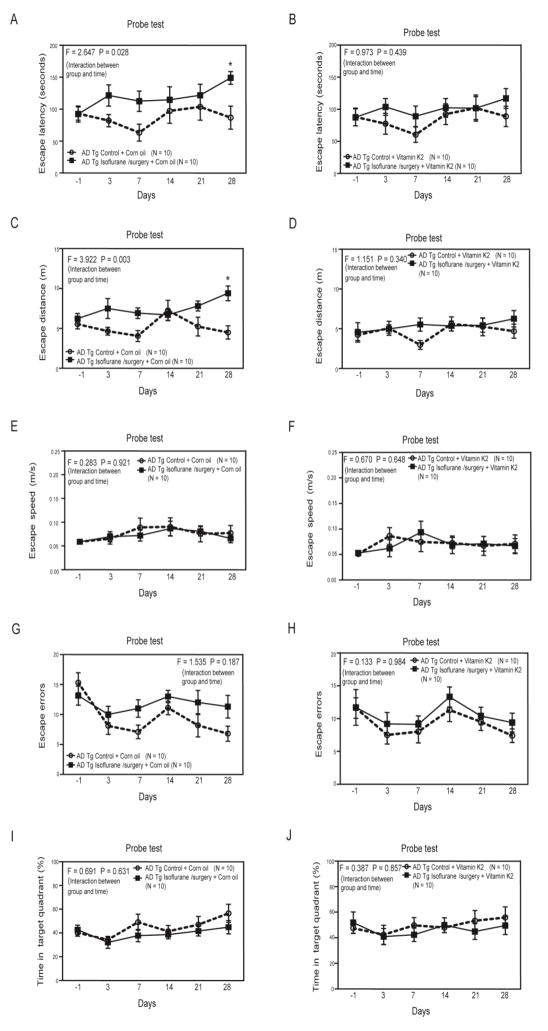
We found that the isoflurane plus surgery still induced longer escape latency of Barnes maze probe test as compared to control condition on day 28 after the anesthesia/surgery) in a different group of AD Tg mice pretreated with corn oil (the vehicle of Vitamin K2) (Figure 4A, F = 2.547, P = 0.028, two-way ANOVA). However, the isoflurane plus surgery no longer increased escape latency in the AD Tg mice pretreated with Vitamin K2 (Figure 4B, F = 0.973, P = 0.439, two-way ANOVA). Consistently, the isoflurane plus surgery increased escape distance of Barnes maze probe test as compared to control condition in the AD Tg mice pretreated with corn oil (Figure 4C, F = 3.922, P = 0.003, two-way ANOVA), but not Vitamin K2 (Figure 4D, F = 1.151, P = 0.340, two-way ANOVA). In addition, these treatments did not significantly affect the escape speed of Barnes maze probe test (Figure 4E and Figure 4F). Finally, the isoflurane plus surgery did not significantly alter the escape errors and time in target quadrant of Barnes maze probe test as compared to the control condition in the AD Tg mice with pretreatment of corn oil or Vitamin K2 (Figure 4G, 4H, 4I and 4J).
Figure 4. Vitamin K2 mitigated the cognitive deficiency induced by isoflurane plus surgery in AD Tg mice.
A. Two-way ANOVA with repeated measurement showed significant interaction (F = 2.647, P = 0.028) of treatment (isoflurane plus surgery versus control condition) and time (days) on escape latency of Barnes maze probe test in AD Tg mice pretreated with corn oil (the vehicle of Vitamin K2). Specifically, the isoflurane plus surgery significantly increased the escape latency of Barnes maze probe test as compared to control condition in the AD Tg mice on day 28 after the anesthesia/surgery. B. Two-way ANOVA with repeated measurement showed no significant interaction (F = 0.973, P = 0.439) of treatment (isoflurane plus surgery versus control condition) and time (days) on escape latency of Barnes maze probe test in the AD Tg mice pretreated with Vitamin K2. C. Two-way ANOVA with repeated measurement showed significant interaction (F = 3.922, P = 0.003) of treatment (isoflurane plus surgery versus control condition) and time (days) on escape distance of Barnes maze probe test in AD Tg mice pretreated with corn oil (the vehicle of Vitamin K2). Specifically, the isoflurane plus surgery significantly increased the escape distance of Barnes maze probe test as compared to control condition in the AD Tg mice on day 28 after the anesthesia/surgery. D. Two-way ANOVA with repeated measurement showed no significant interaction (F = 1.151, P = 0.340) of treatment (isoflurane plus surgery versus control condition) and time (days) on escape distance of Barnes maze probe test in the AD Tg mice pretreated with Vitamin K2. E. Two-way ANOVA with repeated measurement showed no significant interaction (F = 0.283, P = 0.921) of treatment (isoflurane plus surgery versus control condition) and time (days) on escape speed of Barnes maze probe test in AD Tg mice pretreated with corn oil (the vehicle of Vitamin K2). F. Two-way ANOVA with repeated measurement showed no significant interaction (F = 0.670, P = 0.648) of treatment (isoflurane plus surgery versus control condition) and time (days) on escape speed of Barnes maze probe test in the AD Tg mice pretreated with Vitamin K2. Two-way ANOVA with repeated measurement showed no significant interaction of treatment (isoflurane plus surgery versus control condition) and time (days) on escape errors (G and H) and time in target quadrant (I and J) in AD Tg mice with pretreatment of corn oil (G and I) or Vitamin K2 (H and J). ANOVA, analysis of variance; AD, Alzheimer’s disease; Tg, transgenic. N = 10 in each group.
There were no differences in the escape latency, escape distance, escape speed, escape errors and time in target quadrant between the mice in control group and the mice in the anesthesia/surgery group in the Barnes maze training test (Supplemental Figure 5). These data demonstrated again that the isoflurane plus surgery was able to induce longer escape latency and distance of Barnes maze probe test in AD Tg mice and suggest that Vitamin K2 may mitigate the cognitive changes induced by isoflurane plus surgery in the AD Tg mice. In the interaction studies, we only assessed the cognitive function up to 28 days in the mice because isoflurane plus surgery increased neither escape latency nor escape distance at 35 or 42 days after the anesthesia/surgery (Figure 1).
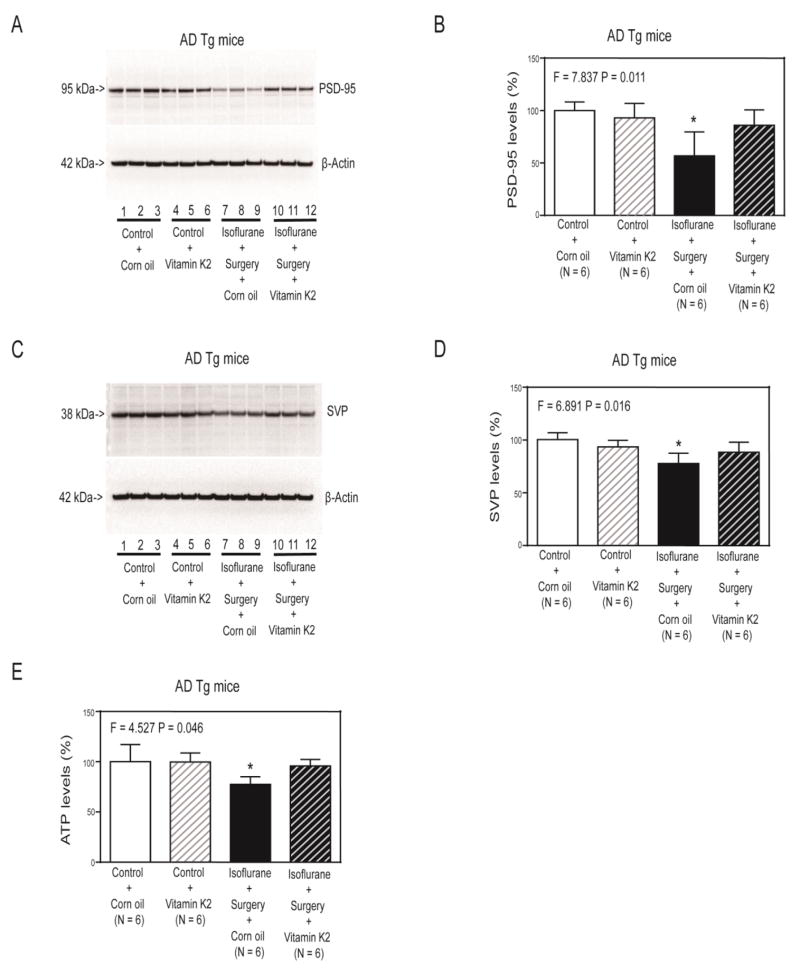
Next, we found that Vitamin K2 (lanes 10 to 12 in Figure 5A and striped black bar in Figure 5B) specifically mitigated the isoflurane plus surgery-induced reduction in hippocampus PSD-95 levels (lanes 7 to 9 in Figure 5A and black bar in Figure 5B) in the AD Tg mice (F = 7.837, P = 0.011, two-way ANOVA). The isoflurane plus surgery plus corn oil (lanes 7 to 9 in Figure 5A and black bar in Figure 5B) decreased the hippocampus PSD-95 levels of AD Tg mice as compared to the control condition plus corn oil (lanes 1 to 3 in Figure 5A and white bar in Figure 5B). Similarly, Vitamin K2 mitigated the isoflurane plus surgery-induced reduction in hippocampus SVP levels in the AD Tg mice (Figure 5C and Figure 5D).
Figure 5. Vitamin K2 mitigated the reductions in PSD-95, SVP and ATP levels induced by isoflurane plus surgery in AD Tg mice.
A. Corn oil plus isoflurane plus surgery (lanes 7 to 9) decreased levels of PSD-95 (A) or SVP (C) as compared to corn oil plus control condition (lanes 1 to 3) in hippocampus of AD Tg mice. Vitamin K2 plus control condition (lanes 4 to 6) did not significantly alter levels of PSD-95 (A) or SVP (C) as compared to corn oil plus control condition (lanes 1 to 3). Vitamin K2 plus isoflurane plus surgery (lanes 10 to 12) led to lesser reductions of levels of PSD-95 (A) or SVP (C) as compared to corn oil plus isoflurane plus surgery (lanes 7 to 9). Quantification of Western blot showed that Vitamin K2 (striped and black bar) inhibited the reduction in levels of PSD-95 (B) or SVP (D) induced by the isoflurane plus surgery (black bar). E. ELISA showed that corn oil plus isoflurane plus surgery (black bar) significantly decreased ATP levels in hippocampus of AD Tg mice as compared to corn oil plus control condition (white bar) at the end of the anesthesia/surgery. Treatment with Vitamin K2 alone (white and striped bar) did not significantly change ATP levels as compared to control condition plus corn oil (white bar). However, there was a significant interaction of Vitamin K2 and isoflurane plus surgery on ATP levels, and Vitamin K2 mitigated the reductions in ATP levels induced by the isoflurane plus surgery in the AD Tg mice. AD, Alzheimer’s disease; Tg, transgenic; ATP, adenosine triphosphate. N = 6 in each group. The experiments to assess the effects of anesthesia and surgery on the levels of PSD-95 and SVP were performed on day 28 after the anesthesia plus surgery. The experiments to assess the effects of anesthesia and surgery on the levels of ATP were performed immediately at the end of the anesthesia plus surgery.
Finally, Two-way ANOVA showed significant interaction of treatment (corn oil and Vitamin K2) and group (control condition versus isoflurane plus surgery), and Vitamin K2 (striped and black bar in Figure 5E) specifically mitigated the reduction in hippocampus ATP levels induced by isoflurane plus surgery (black bar in Figure 5E) (F = 4.527, P = 0.046, two-way ANOVA) in the AD Tg mice.
Discussion
Isoflurane plus surgery, but not desflurane plus surgery, induced longer escape latency and distance of Barnes maze probe test in AD Tg, but not WT, mice (Figure 1 and Figure 4). The findings that the anesthesia/surgery did not significantly affect escape speed suggest that the anesthesia/surgery did not impair the locomotor activity and that the observed longer escape latency and distance of Barnes maze probe test following the anesthesia/surgery was not the result of the changes in locomotor activity (Figure 1 and Figure 4). These results could promote clinical investigation to determine whether there are different cognitive outcomes between the patients who have surgery under isoflurane anesthesia and the patients who have surgery under desflurane anesthesia. Ultimately, these efforts could identify better anesthetic (e.g., desflurane versus isoflurane) for AD patients and the senior patients (who are more vulnerable to develop cognitive deficiency) when surgery becomes necessary.
Interestingly, we did not demonstrate that isoflurane plus surgery induced cognitive deficiency in adult WT male mice. These data were different from the findings that surgery under general or local anesthesia could induce cognitive impairment in rodents [19–25,27,26]. The potential explanations include that the mice used in the current experiments were male adult mice. It is possible that the anesthesia/surgery only induces cognitive impairment in female but not male WT mice, which is supported by our recent findings [55].
Furthermore, our previous studies demonstrated that surgery under local anesthesia only induced cognitive impairment in WT aged mice (18 month-old), but not WT adult mice (9 month-old mice) [27,26], which were consistent with the findings observed in the current studies that anesthesia/surgery did not induce cognitive changes in WT adult mice (5 month-old). Nevertheless, the data obtained from the current studies demonstrated that desflurane plus surgery is associated with less cognitive changes than isoflurane plus surgery in both AD Tg mice and WT mice, as evidenced that the isoflurane plus surgery, but not desflurane plus surgery, induced a borderline increase of escape latency in Barnes maze probe test at 14 days (adjusted P = 0.088, Supplementary Table 1) after the anesthesia/surgery in the WT mice.
Interestingly, the observed longer escape latency and distance of Barnes maze probe test in the AD Tg mice following the isoflurane plus surgery did not occur until 28 days after the anesthesia/surgery (Figure 1). The reason why the anesthesia/surgery may induce late-onset cognitive changes in the AD Tg mice is unknown at the present time. The possible explanations include that the anesthesia/surgery could initially reduce brain energy levels, which then induce a time-dependent reduction in synapse, leading to cognitive changes. We will use the established system in the current studies to perform a timecourse study to assess whether the anesthesia/surgery induces a maximal reduction of synapse at 28 days after the anesthesia/surgery.
Future studies may also include investigation of potential longer-term effects of anesthesia plus surgery on cognitive function in both AD Tg and WT mice. Specifically, we may need to study whether exposure of different anesthesia (e.g., isoflurane versus desflurane) plus surgery in different age (e.g., 6, 60 and 270 day-old) of AD Tg or WT mice can cause cognitive changes and promote AD neuropathogenesis in the mice when they grow older (e.g., 540 day-old). Ultimately, these efforts would lead to facilitate the development of strategic approach to reduce the incidence of AD and to lessen the severity of AD symptoms.
Previous studies have shown that isoflurane and desflurane have different effects on intracellular calcium levels [56], mitochondrial function [17,16] and behavioral changes [16]. These differences may also contribute to the observed changes in the current studies that the surgery under isoflurane, but not desflurane, induces longer escape latency and distance of Barnes maze test in the AD Tg mice.
Bianchi et al. reported that anesthesia with 0.8% to 1% isoflurane in 30% oxygen for two hours daily for 5 days did not induce cognitive impairment, assessed in Morris Water Maze, in 12 month-old AD Tg mice (Tg2576) [18]. These studies were different from the current studies in anesthesia (0.8% to 1% isoflurane in 30% oxygen for two hours daily for 5 days versus 1.4% isoflurane 100% oxygen for two hours), AD Tg mice (Tg2576 versus 5XFAD), behavioral test (Morris Water Maze versus Barnes maze) and surgery in the mice (absence versus presence). These differences could contribute to the different behaviors (no cognitive changes versus cognitive changes) observed between the studies by Bianchi et al. and the current studies.
We found that the isoflurane plus surgery, but not desflurane plus surgery, decreased the levels of synaptic marker PSD-95 [30,31] and SVP [32] in the hippocampus of AD Tg mice, but not WT mice (Figure 2). These cellular changes further suggest the different effects of isoflurane plus surgery versus desflurane plus surgery. However, the underlying mechanisms why isoflurane, but not desflurane, plus surgery decreased the hippocampus levels of PSD-95 and SVP of AD Tg mice remain to be determined in the future.
Consistently, the isoflurane plus surgery, but not desflurane plus surgery, reduced the hippocampus ATP levels in the mice. Taken together, these data suggest the potential mechanistic cascade that anesthesia/surgery induced ATP reduction, which may then caused reduction in synapse, consequently leading to cognitive changes. More importantly, the current studies have established a system to dissect the cascade in the future investigations. However, we did not examine the dynamic changes of brain ATP levels in the current studies, thus the correlation between brain energy and cognitive function in the mice needs to be further tested in the future.
Interestingly, the isoflurane plus surgery decreased hippocampus ATP levels but did not cause cognitive changes in the WT mice. These observations could be due to the fact that WT mice may have more brain or cognitive reserve and the initial reduction in hippocampus ATP levels may not cause sufficient reduction in hippocampus synapse, thus not causing cognitive changes in the WT mice. At the present time, we only showed that the combination of anesthesia and surgery reduced ATP levels in brain tissues (hippocampus) in the mice. Our future studies will assess the extent to which the anesthesia/surgery reduces ATP levels in neurons or microglia via mitochondrial dysfunction.
Finally, Vitamin K2 was able to mitigate the isoflurane plus surgery-induced longer escape latency and distance of Barnes maze probe test and reduction in the levels of synaptic markers and ATP in AD Tg mice (Figure 4 and 5). These data further support the hypothesized pathway of the anesthesia/surgery-induced changes in cognitive function. More importantly, these results may promote clinical investigation to determine whether Vitamin K2 can treat the anesthesia/surgery-induced cognitive impairment in AD patients.
Vitamin K2 has been shown to have neuroprotective effects [33–35] through serving as a mitochondrial electron carrier and energy enhancer [33]. The anesthesia/surgery reduced ATP levels and induced longer escape latency and distance of Barnes maze probe test in the AD Tg mice. Collectively, the findings that Vitamin K2 mitigated the anesthesia/surgery-induced both ATP reduction and longer escape latency and distance of Barnes maze probe test suggest that brain energy deficits may contribute to the longer escape latency and distance of Barnes maze probe test and Vitamin K2 may improve cognitive function through enhancing brain energy levels, pending further investigation.
The isoflurane plus surgery induced longer escape latency and distance of Barnes maze probe test at 28 days after the anesthesia/surgery. But the effects of anesthesia/surgery on the levels of PSD-95 and SVP were determined at the 42 days after the anesthesia/surgery. This is because AD Tg mice are expensive and we only had limited supply of AD Tg mice, thus, we did not use separate group of AD Tg mice for behavior and Western blot studies. Rather, we performed the Barnes maze probe test every 7 days after the anesthesia/surgery up to 42 days and then harvested the brain tissues of these AD Tg mice at the end of the behavior studies for the Western blot analysis (Figure 2). Nevertheless, in the rescue studies of Vitamin K2, we indeed demonstrated that isoflurane plus surgery reduced levels of PSD-95 and SVP at the 28 days after the anesthesia/surgery (Figure 5), which was accompanied by the findings the same isoflurane plus surgery induced longer escape latency and distance of Barnes maze probe test at the 28 days after the anesthesia/surgery (Figure 4).
The levels of SVP and PSD-95 decreased to the same extent at day 28 and day 42 following isoflurane plus surgery, but the isoflurane plus surgery only induced behavioral changes at day 28, but not day 42. The exact reason underlying this observation is unknown at the present time. However, the escape latency and escape distance of the AD Tg mice on day 28 and 42 following isoflurane plus surgery were at similar levels (Figure 1A and Figure 1C), which was consistent with the findings that the levels of SVP and PSD-95 decreased to the same extent at day 28 and day 42 following the isoflurane plus surgery. At the day 42 after the control condition, the AD Tg mice had increased escape latency and escape distance as compared to those at baseline, which contributed to the fact that there was no significant difference on escape latency and distance between control condition and isoflurane plus anesthesia on day 42 after the control condition or isoflurane plus anesthesia. The increases of escape latency and escape distance in AD Tg mice following the control condition could be due to the fact that the AD Tg mice forgot the initial training in the Barnes maze. The future studies should test this hypothesis to further adjust our established system to better assess the effects of anesthesia plus surgery on cognitive function in AD Tg mice.
Interestingly, the isoflurane plus surgery only increased the escape latency and escape distance in the AD Tg mice (Figure 1) without significant changes in escape errors and the time in target quadrant (Figure 1). Increased escape latency and escape distance may reflect an increased exploratory behavior or avoidance of closed areas [57]. Therefore, the limitation of the present study is that the findings that isoflurane plus surgery increased escape latency and escape distance might not be sufficient enough to demonstrate that the isoflurane plus surgery induced cognitive impairment. However, many other studies suggest that increased escape latency and escape distance without significant alterations in escape errors and the time in target quadrant also indicate cognitive impairment in rodents [46,58,47,59–63,48]. Moreover, the increased exploratroy behavior described in the literature did not appear in the 5xFAD mice until 12 month-old [57], and we only used 5 month-old 5xFAD mice in the current studies. Finally, the major objective of the current study was to compare the effects of isoflurane plus surgery versus desflurane plus surgery on animal behavior changes. Nevertheless, the future studies should include the experiments to assess the effects of anesthesia plus surgery on cognitive function in AD Tg mice employing other behavior models (e.g., Fear Conditioning System and Morris Water Maze) in addition to Barnes maze.
The current studies have other limitations. First, we did not systematically investigate the effects of different treatment of isoflurane and desflurane (dosage and time) plus surgery on cognitive deficiency and the associated cellular mechanism. However, the data demonstrated the difference between the clinical relevant concentrations of isoflurane plus surgery and desflurane plus surgery. Second, we did not determine the effects of the anesthesia/surgery on brain levels of Aβ and phosphorylated Tau protein. This is because previous studies have revealed that anesthesia and/or surgery can increase brain levels of Aβ and phosphorylated Tau protein [26,37,23]. Third, we only used male AD Tg mice in the studies, thus the outcomes may not imply for female AD patients. Finally, we did not know whether the reduction in brain ATP levels could directly lead to decreased levels of PSD-95 and SVP. However, the findings that Vitamin K2 attenuated the anesthesia/surgery-induced reduction in both ATP levels and levels of PSD-95 and SVP, as well as behavioral changes suggest the potential association of ATP, synapse and cognitive changes.
In conclusion, we showed that the commonly used inhalation anesthetic isoflurane plus surgery could induce cognitive impairment (e.g., longer escape latency and distance of Barnes maze probe test), reductions in hippocampus levels of ATP and synaptic markers in male AD Tg mice, but not in male WT mice. However, another commonly used inhalation anesthetic desflurane plus surgery did not induce these changes in the mice. Moreover, Vitamin K2 could mitigate the longer escape latency and distance of Barnes maze probe test and reduction in hippocampus levels of ATP and synaptic markers induced by the isoflurane plus surgery. These findings should promote clinical investigation to determine whether desflurane plus surgery is less associated with cognitive impairment than isoflurane plus surgery in AD patients, and whether Vitamin K2 can prevent or treat postoperative cognitive dysfunction in AD and senior patients. Pending further animals and clinical investigations, these findings would suggest that desflurane could be a better choice for AD patients and other senior patients who are vulnerable to the development of postoperative cognitive impairment.
Supplementary Material
Supplementary Figure 1. Diagram of the experimental design. Part 1. The mice received Barnes Maze training from day 7 to day 1 (−7 to −1) before isoflurane or desflurane anesthesia plus the abdominal surgery (anesthesia/surgery). The values of the escape latency, escape distance, escape speed, escape errors and the time in target quadrant on the last day of Barnes Maze training serve as the baseline of Barnes Maze probe test. Then, the mice received Barnes Maze probe test on day 3, 7, 14, 21, 28, 35 and 42 after the anesthesia/surgery, respectively. Western blot analysis was performed at the end of the behavior tests on day 42. ATP measurement was performed immediately after the anesthesia/surgery on day 0. Part 2. Vitamin K2 treatment (100 mg/kg, daily) started 8 days before the anesthesia/surgery. Western blot analysis was performed after the behavior tests on day 28. ATP measurement was performed immediately after the anesthesia/surgery on day 0.
Supplementary Figure 2. Effects of isoflurane or desflurane plus surgery on baseline escape latency, escape distance, escape speed, escape errors and time in quadrant of Barnes Maze test in AD Tg or WT mice. One-way ANOVA showed no significant difference between isoflurane plus surgery or desflurane plus surgery versus control condition on escape latency (A and B), escape distance (C and D), escape speed (E and F), escape errors (G and H) and time in target quadrant (I and J) of Barnes maze baseline performance in AD Tg (A, C, E, G and I) or WT (B, D, F, H and J) mice. ANOVA, analysis of variance; AD, Alzheimer’s disease; Tg, transgenic; WT, wild-type. N = 12 in each group.
Supplementary Figure 3. Escape latency, escape distance, escape speed, escape errors and time in target quadrant of Barnes Maze training test in AD Tg and WT mice. Two-way ANOVA with repeated measurement showed that no significant interaction of mice in the group (isoflurane plus surgery or desflurane plus surgery or control condition) and time (days) on escape latency (A and B), escape distance (C and D), escape speed (E and F), escape errors (G and H) and time in target quadrant (I and J) in AD Tg (A, C, E, G and I) and WT (B, D, F, H and J) mice. ANOVA, analysis of variance; AD, Alzheimer’s disease; Tg, transgenic; WT, wild-type. N = 12 in each group.
Supplementary Figure 4. The comparison of the baseline of escape latency in Barnes Maze probe test and Aβ42 levels in the brain tissues between AD Tg control and WT control mice. A. The baseline of escape latency in Barnes Maze probe test of AD Tg control mice was significant longer than that of WT control mice at one day (day -1) before the anesthesia/surgery (P = 0.034). B. The Aβ42 levels of cortex of AD Tg control mice were significantly higher than that of WT control mice (P < 0.001). AD, Alzheimer’s disease; Tg, transgenic; WT, wild-type; Aβ, β-amyloid protein. N = 12 in each group.
Supplementary Figure 5. Effects of isoflurane plus surgery on escape latency, escape distance, escape speed, escape errors and time in target quadrant of Barnes Maze training test in AD Tg mice with pretreatment of corn oil or Vitamin K2. Two-way ANOVA with repeated measurement showed no significant interaction of group (isoflurane plus surgery versus control condition) and time (days) on escape latency (A), escape distance (B), escape speed (C), escape errors (D) and time in target quadrant (E) of Barnes maze training test in AD Tg mice with pretreatment of corn oil or Vitamin K2. ANOVA, analysis of variance; AD, Alzheimer’s disease; Tg, transgenic; WT, wild-type. N = 10 in each group.
Supplementary Table 1. Effects of isoflurane or desflurane plus surgery on cognitive function in both AD Tg and WT mice. Left part: Isoflurane plus surgery increased the escape latency in the Barnes Maze probe test as compared to control condition on day 28 after the anesthesia/surgery in AD Tg mice (P = 0.034). Isoflurane plus surgery induced a borderline increase in escape latency in the Barnes Maze probe test as compared to control condition on day 7 after the anesthesia/surgery in AD Tg mice (P = 0.059). Finally, Isoflurane plus surgery induced a borderline increase in escape latency in the Barnes Maze probe test as compared to control condition on day 14 in WT mice (P = 0.088). Desflurane plus surgery did not increase the escape latency as compared to the control condition in either AD Tg or WT mice. Right part: Isoflurane plus surgery increased the escape distance in the Barnes Maze probe test as compared to control condition on day 28 after the anesthesia/surgery in AD Tg mice (P = 0.011). Desflurane plus surgery did not increase the escape distance as compared to the control condition in either AD Tg or WT mice. AD, Alzheimer’s disease; Tg, transgenic; WT, wild-type. N = 12 in each group.
Acknowledgments
This research was supported by R01GM088801, R01AG041274 and R01HD 086977 from National Institutes of Health, Bethesda, Maryland (to Z. X.). Dr. Edward Marcantonio was funded by the following grants from the National Institute on Aging: P01 AG031720, R01AG030618, K24AG035075, and R01AG051658. Dr. Greg Crosby was funded by the following grants from the National Institute on Aging: R21AG048637 and RO1AG051812. Dr. Yuan Shen was funded by 81571034 from the National Natural Science Foundation of China. The costs of isoflurane, desflurane and EMLA cream (2.5% lidocaine and 2.5% prilocaine) were generously provided by the Department of Anesthesia, Critical Care and Pain Medicine at Massachusetts General Hospital. The studies were performed in the Geriatric Anesthesia Research Unit in the Department of Anesthesia, Critical Care and Pain Medicine at Massachusetts General Hospital, Boston, MA. These works should be attributed to the Department of Anesthesia, Critical Care and Pain Medicine at Massachusetts General Hospital and Harvard Medical School.
Footnotes
AUTHOR CONTRIBUTION: Z.X., H.M., Y.Z., Y.S., G.C., D.J.C., and E.M. conceived and designed the project. H.M., Y.D., Y.Z. H.Z. performed all the experiments, analyzed the data and prepared the figures. Z.X. and H.M. wrote the manuscript. All authors reviewed the manuscript.
COMPETING FINANCIAL INTERESTS: The authors declare no competing financial interests.
References
- 1.Moller JT, Cluitmans P, Rasmussen LS, Houx P, Rasmussen H, Canet J, Rabbitt P, Jolles J, Larsen K, Hanning CD, Langeron O, Johnson T, Lauven PM, Kristensen PA, Biedler A, van Beem H, Fraidakis O, Silverstein JH, Beneken JE, Gravenstein JS. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post-Operative Cognitive Dysfunction. Lancet. 1998;351(9106):857–861. doi: 10.1016/s0140-6736(97)07382-0. [DOI] [PubMed] [Google Scholar]
- 2.Schenning KJ, Murchison CF, Mattek NC, Silbert LC, Kaye JA, Quinn JF. Surgery is associated with ventricular enlargement as well as cognitive and functional decline. Alzheimer’s & dementia: the journal of the Alzheimer’s Association. 2016;12(5):590–597. doi: 10.1016/j.jalz.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inouye SK, Marcantonio ER, Kosar CM, Tommet D, Schmitt EM, Travison TG, Saczynski JS, Ngo LH, Alsop DC, Jones RN. The short-term and long-term relationship between delirium and cognitive trajectory in older surgical patients. Alzheimer’s & dementia: the journal of the Alzheimer’s Association. 2016;12(7):766–775. doi: 10.1016/j.jalz.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saczynski JS, Marcantonio ER, Quach L, Fong TG, Gross A, Inouye SK, Jones RN. Cognitive trajectories after postoperative delirium. The New England journal of medicine. 2012;367(1):30–39. doi: 10.1056/NEJMoa1112923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pandharipande PP, Girard TD, Jackson JC, Morandi A, Thompson JL, Pun BT, Brummel NE, Hughes CG, Vasilevskis EE, Shintani AK, Moons KG, Geevarghese SK, Canonico A, Hopkins RO, Bernard GR, Dittus RS, Ely EW Investigators B-IS. Long-term cognitive impairment after critical illness. The New England journal of medicine. 2013;369(14):1306–1316. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bohnen N, Warner MA, Kokmen E, Kurland LT. Early and midlife exposure to anesthesia and age of onset of Alzheimer’s disease. Int J Neurosci. 1994;77(3–4):181–185. doi: 10.3109/00207459408986029. [DOI] [PubMed] [Google Scholar]
- 7.Chen CW, Lin CC, Chen KB, Kuo YC, Li CY, Chung CJ. Increased risk of dementia in people with previous exposure to general anesthesia: A nationwide population-based case-control study. Alzheimer’s & dementia: the journal of the Alzheimer’s Association. 2013 doi: 10.1016/j.jalz.2013.05.1766. [DOI] [PubMed]
- 8.Liu Y, Pan N, Ma Y, Zhang S, Guo W, Li H, Zhou J, Liu G, Gao M. Inhaled Sevoflurane May Promote Progression of Amnestic Mild Cognitive Impairment: A Prospective, Randomized Parallel-Group Study. Am J Med Sci. 2013 doi: 10.1097/MAJ.0b013e31825a674d. [DOI] [PubMed] [Google Scholar]
- 9.Chen PL, Yang CW, Tseng YK, Sun WZ, Wang JL, Wang SJ, Oyang YJ, Fuh JL. Risk of dementia after anaesthesia and surgery. The British journal of psychiatry: the journal of mental science. 2013 doi: 10.1192/bjp.bp.112.119610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bohnen NI, Warner MA, Kokmen E, Beard CM, Kurland LT. Alzheimer’s disease and cumulative exposure to anesthesia: a case-control study. Journal of the American Geriatrics Society. 1994;42(2):198–201. doi: 10.1111/j.1532-5415.1994.tb04952.x. [DOI] [PubMed] [Google Scholar]
- 11.Knopman DS, Petersen RC, Cha RH, Edland SD, Rocca WA. Coronary artery bypass grafting is not a risk factor for dementia or Alzheimer disease. Neurology. 2005;65(7):986–990. doi: 10.1212/01.wnl.0000171954.92119.c7. 01.wnl.0000171954.92119.c7 [pii] [DOI] [PubMed] [Google Scholar]
- 12.Avidan MS, Searleman AC, Storandt M, Barnett K, Vannucci A, Saager L, Xiong C, Grant EA, Kaiser D, Morris JC, Evers AS. Long-term cognitive decline in older subjects was not attributable to noncardiac surgery or major illness. Anesthesiology. 2009;111(5):964–970. doi: 10.1097/ALN.0b013e3181bc9719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sprung J, Jankowski CJ, Roberts RO, Weingarten TN, Aguilar AL, Runkle KJ, Tucker AK, McLaren KC, Schroeder DR, Hanson AC, Knopman DS, Gurrieri C, Warner DO. Anesthesia and incident dementia: a population-based, nested, case-control study. Mayo Clinic proceedings. 2013;88(6):552–561. doi: 10.1016/j.mayocp.2013.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sprung J, Roberts RO, Knopman DS, Olive DM, Gappa JL, Sifuentes VL, Behrend TL, Farmer JD, Weingarten TN, Hanson AC, Schroeder DR, Petersen RC, Warner DO. Association of Mild Cognitive Impairment With Exposure to General Anesthesia for Surgical and Nonsurgical Procedures: A Population-Based Study. Mayo Clinic proceedings. 2016;91(2):208–217. doi: 10.1016/j.mayocp.2015.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vutskits L, Xie Z. Lasting impact of general anaesthesia on the brain: mechanisms and relevance. Nature reviews Neuroscience. 2016;17(11):705–717. doi: 10.1038/nrn.2016.128. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Xu Z, Wang H, Dong Y, Shi HN, Culley DJ, Crosby G, Marcantonio ER, Tanzi RE, Xie Z. Anesthetics isoflurane and desflurane differently affect mitochondrial function, learning, and memory. Annals of neurology. 2012;71(5):687–698. doi: 10.1002/ana.23536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y, Dong Y, Wu X, Lu Y, Xu Z, Knapp A, Yue Y, Xu T, Xie Z. The mitochondrial pathway of anesthetic isoflurane-induced apoptosis. The Journal of biological chemistry. 2010;285(6):4025–4037. doi: 10.1074/jbc.M109.065664. M109.065664 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bianchi SL, Tran T, Liu C, Lin S, Li Y, Keller JM, Eckenhoff RG, Eckenhoff MF. Brain and behavior changes in 12-month-old Tg2576 and nontransgenic mice exposed to anesthetics. Neurobiology of aging. 2008;29(7):1002–1010. doi: 10.1016/j.neurobiolaging.2007.02.009. S0197-4580(07)00047-4 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cibelli M, Fidalgo AR, Terrando N, Ma D, Monaco C, Feldmann M, Takata M, Lever IJ, Nanchahal J, Fanselow MS, Maze M. Role of interleukin-1beta in postoperative cognitive dysfunction. Annals of neurology. 2010;68(3):360–368. doi: 10.1002/ana.22082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Terrando N, Monaco C, Ma D, Foxwell BM, Feldmann M, Maze M. Tumor necrosis factor-alpha triggers a cytokine cascade yielding postoperative cognitive decline. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(47):20518–20522. doi: 10.1073/pnas.1014557107. 1014557107 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Terrando N, Rei Fidalgo A, Vizcaychipi M, Cibelli M, Ma D, Monaco C, Feldmann M, Maze M. The impact of IL-1 modulation on the development of lipopolysaccharide-induced cognitive dysfunction. Crit Care. 2010;14(3):R88. doi: 10.1186/cc9019. cc9019 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wan Y, Xu J, Ma D, Zeng Y, Cibelli M, Maze M. Postoperative impairment of cognitive function in rats: a possible role for cytokine-mediated inflammation in the hippocampus. Anesthesiology. 2007;106(3):436–443. doi: 10.1097/00000542-200703000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Wan Y, Xu J, Meng F, Bao Y, Ge Y, Lobo N, Vizcaychipi MP, Zhang D, Gentleman SM, Maze M, Ma D. Cognitive decline following major surgery is associated with gliosis, beta-amyloid accumulation, and tau phosphorylation in old mice. Critical care medicine. 2010 doi: 10.1097/CCM.0b013e3181f17bcb. [DOI] [PubMed] [Google Scholar]
- 24.Zhang J, Dong Y, Zhou C, Zhang Y, Xie Z. Anesthetic Sevoflurane Reduces Levels of Hippocalcin and Postsynaptic Density Protein 95. Molecular neurobiology. 2014 doi: 10.1007/s12035-014-8746-1. [DOI] [PubMed] [Google Scholar]
- 25.Zhang J, Jiang W, Zuo Z. Pyrrolidine dithiocarbamate attenuates surgery-induced neuroinflammation and cognitive dysfunction possibly via inhibition of nuclear factor kappaB. Neuroscience. 2014;261:1–10. doi: 10.1016/j.neuroscience.2013.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu Z, Dong Y, Wang H, Culley DJ, Marcantonio ER, Crosby G, Tanzi RE, Zhang Y, Xie Z. Age-dependent postoperative cognitive impairment and Alzheimer-related neuropathology in mice. Scientific reports. 2014;4:3766. doi: 10.1038/srep03766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu Z, Dong Y, Wang H, Culley DJ, Marcantonio ER, Crosby G, Tanzi RE, Zhang Y, Xie Z. Peripheral surgical wounding and age-dependent neuroinflammation in mice. PloS one. 2014;9(5):e96752. doi: 10.1371/journal.pone.0096752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peng M, Zhang C, Dong Y, Zhang Y, Nakazawa H, Kaneki M, Zheng H, Shen Y, Marcantonio ER, Xie Z. Battery of behavioral tests in mice to study postoperative delirium. Scientific reports. 2016;6:29874. doi: 10.1038/srep29874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ren Q, Peng M, Dong Y, Zhang Y, Chen M, Yin N, Marcantonio ER, Xie Z. Surgery plus anesthesia induces loss of attention in mice. Frontiers in cellular neuroscience. 2015;9:346. doi: 10.3389/fncel.2015.00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hunt CA, Schenker LJ, Kennedy MB. PSD-95 is associated with the postsynaptic density and not with the presynaptic membrane at forebrain synapses. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1996;16(4):1380–1388. doi: 10.1523/JNEUROSCI.16-04-01380.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cho KO, Hunt CA, Kennedy MB. The rat brain postsynaptic density fraction contains a homolog of the Drosophila discs-large tumor suppressor protein. Neuron. 1992;9(5):929–942. doi: 10.1016/0896-6273(92)90245-9. [DOI] [PubMed] [Google Scholar]
- 32.Buckley K, Kelly RB. Identification of a transmembrane glycoprotein specific for secretory vesicles of neural and endocrine cells. The Journal of cell biology. 1985;100(4):1284–1294. doi: 10.1083/jcb.100.4.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vos M, Esposito G, Edirisinghe JN, Vilain S, Haddad DM, Slabbaert JR, Van Meensel S, Schaap O, De Strooper B, Meganathan R, Morais VA, Verstreken P. Vitamin K2 is a mitochondrial electron carrier that rescues pink1 deficiency. Science. 2012;336(6086):1306–1310. doi: 10.1126/science.1218632. [DOI] [PubMed] [Google Scholar]
- 34.Li J, Lin JC, Wang H, Peterson JW, Furie BC, Furie B, Booth SL, Volpe JJ, Rosenberg PA. Novel role of vitamin k in preventing oxidative injury to developing oligodendrocytes and neurons. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2003;23(13):5816–5826. doi: 10.1523/JNEUROSCI.23-13-05816.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Onodera K, Shinoda H, Zushida K, Taki K, Kamei J. Antinociceptive effect induced by intraperitoneal administration of vitamin K2 (menatetrenone) in ICR mice. Life Sci. 2000;68(1):91–97. doi: 10.1016/s0024-3205(00)00917-6. [DOI] [PubMed] [Google Scholar]
- 36.Oakley H, Cole SL, Logan S, Maus E, Shao P, Craft J, Guillozet-Bongaarts A, Ohno M, Disterhoft J, Van Eldik L, Berry R, Vassar R. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: potential factors in amyloid plaque formation. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26(40):10129–10140. doi: 10.1523/JNEUROSCI.1202-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xie Z, Culley DJ, Dong Y, Zhang G, Zhang B, Moir RD, Frosch MP, Crosby G, Tanzi RE. The common inhalation anesthetic isoflurane induces caspase activation and increases amyloid beta-protein level in vivo. Annals of neurology. 2008;64(6):618–627. doi: 10.1002/ana.21548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barnes CA. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol. 1979;93(1):74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- 39.Nunes MA, Schowe NM, Monteiro-Silva KC, Baraldi-Tornisielo T, Souza SI, Balthazar J, Albuquerque MS, Caetano AL, Viel TA, Buck HS. Chronic Microdose Lithium Treatment Prevented Memory Loss and Neurohistopathological Changes in a Transgenic Mouse Model of Alzheimer’s Disease. PloS one. 2015;10(11):e0142267. doi: 10.1371/journal.pone.0142267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin D, Cao L, Wang Z, Li J, Washington JM, Zuo Z. Lidocaine attenuates cognitive impairment after isoflurane anesthesia in old rats. Behavioural brain research. 2012;228(2):319–327. doi: 10.1016/j.bbr.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang J, Tan H, Jiang W, Zuo Z. Amantadine alleviates postoperative cognitive dysfunction possibly by increasing glial cell line-derived neurotrophic factor in rats. Anesthesiology. 2014;121(4):773–785. doi: 10.1097/ALN.0000000000000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoder RM, Kirby SL. Otoconia-deficient mice show selective spatial deficits. Hippocampus. 2014;24(10):1169–1177. doi: 10.1002/hipo.22300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosenfeld CS, Ferguson SA. Barnes maze testing strategies with small and large rodent models. J Vis Exp. 2014;(84):e51194. doi: 10.3791/51194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li J, Deng J, Sheng W, Zuo Z. Metformin attenuates Alzheimer’s disease-like neuropathology in obese, leptin-resistant mice. Pharmacol Biochem Behav. 2012;101(4):564–574. doi: 10.1016/j.pbb.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reiserer RS, Harrison FE, Syverud DC, McDonald MP. Impaired spatial learning in the APPSwe + PSEN1DeltaE9 bigenic mouse model of Alzheimer’s disease. Genes Brain Behav. 2007;6(1):54–65. doi: 10.1111/j.1601-183X.2006.00221.x. [DOI] [PubMed] [Google Scholar]
- 46.Agrawal R, Gomez-Pinilla F. ‘Metabolic syndrome’ in the brain: deficiency in omega-3 fatty acid exacerbates dysfunctions in insulin receptor signalling and cognition. The Journal of physiology. 2012;590(10):2485–2499. doi: 10.1113/jphysiol.2012.230078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Corrigan F, Vink R, Blumbergs PC, Masters CL, Cappai R, van den Heuvel C. sAPPalpha rescues deficits in amyloid precursor protein knockout mice following focal traumatic brain injury. Journal of neurochemistry. 2012;122(1):208–220. doi: 10.1111/j.1471-4159.2012.07761.x. [DOI] [PubMed] [Google Scholar]
- 48.Tan H, Cao J, Zhang J, Zuo Z. Critical role of inflammatory cytokines in impairing biochemical processes for learning and memory after surgery in rats. Journal of neuroinflammation. 2014;11:93. doi: 10.1186/1742-2094-11-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Agrawal R, Noble E, Tyagi E, Zhuang Y, Ying Z, Gomez-Pinilla F. Flavonoid derivative 7,8-DHF attenuates TBI pathology via TrkB activation. Biochimica et biophysica acta. 2015;1852(5):862–872. doi: 10.1016/j.bbadis.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O’Leary TP, Brown RE. Visuo-spatial learning and memory deficits on the Barnes maze in the 16-month-old APPswe/PS1dE9 mouse model of Alzheimer’s disease. Behavioural brain research. 2009;201(1):120–127. doi: 10.1016/j.bbr.2009.01.039. [DOI] [PubMed] [Google Scholar]
- 51.Flinn JM, Bozzelli PL, Adlard PA, Railey AM. Spatial memory deficits in a mouse model of late-onset Alzheimer’s disease are caused by zinc supplementation and correlate with amyloid-beta levels. Frontiers in aging neuroscience. 2014;6:174. doi: 10.3389/fnagi.2014.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tota S, Kamat PK, Shukla R, Nath C. Improvement of brain energy metabolism and cholinergic functions contributes to the beneficial effects of silibinin against streptozotocin induced memory impairment. Behavioural brain research. 2011;221(1):207–215. doi: 10.1016/j.bbr.2011.02.041. [DOI] [PubMed] [Google Scholar]
- 53.Berendsen TA, Izamis ML, Xu H, Liu Q, Hertl M, Berthiaume F, Yarmush ML, Uygun K. Hepatocyte viability and adenosine triphosphate content decrease linearly over time during conventional cold storage of rat liver grafts. Transplant Proc. 2011;43(5):1484–1488. doi: 10.1016/j.transproceed.2010.12.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kamat PK, Tota S, Shukla R, Ali S, Najmi AK, Nath C. Mitochondrial dysfunction: a crucial event in okadaic acid (ICV) induced memory impairment and apoptotic cell death in rat brain. Pharmacol Biochem Behav. 2011;100(2):311–319. doi: 10.1016/j.pbb.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 55.Zhang C, Zhang Y, Shen Y, Zhao G, Xie Z, Dong Y. Anesthesia/Surgery Induces Cognitive Impairment in Female Alzheimer’s Disease Transgenic Mice. Journal of Alzheimer’s disease: JAD. 2017;57(2):505–518. doi: 10.3233/JAD-161268. [DOI] [PubMed] [Google Scholar]
- 56.Yang H, Liang G, Hawkins BJ, Madesh M, Pierwola A, Wei H. Inhalational anesthetics induce cell damage by disruption of intracellular calcium homeostasis with different potencies. Anesthesiology. 2008;109(2):243–250. doi: 10.1097/ALN.0b013e31817f5c47. 00000542-200808000-00013 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jawhar S, Trawicka A, Jenneckens C, Bayer TA, Wirths O. Motor deficits, neuron loss, and reduced anxiety coinciding with axonal degeneration and intraneuronal Abeta aggregation in the 5XFAD mouse model of Alzheimer’s disease. Neurobiology of aging. 2012;33(1):196e129–140. doi: 10.1016/j.neurobiolaging.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 58.Chrzaszcz M, Venkatesan C, Dragisic T, Watterson DM, Wainwright MS. Minozac treatment prevents increased seizure susceptibility in a mouse “two-hit” model of closed skull traumatic brain injury and electroconvulsive shock-induced seizures. Journal of neurotrauma. 2010;27(7):1283–1295. doi: 10.1089/neu.2009.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Provencio JJ, Altay T, Smithason S, Moore SK, Ransohoff RM. Depletion of Ly6G/C(+) cells ameliorates delayed cerebral vasospasm in subarachnoid hemorrhage. Journal of neuroimmunology. 2011;232(1–2):94–100. doi: 10.1016/j.jneuroim.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smithason S, Moore SK, Provencio JJ. Low-dose lipopolysaccharide injection prior to subarachnoid hemorrhage modulates Delayed Deterioration associated with vasospasm in subarachnoid hemorrhage. Acta neurochirurgica Supplement. 2013;115:253–258. doi: 10.1007/978-3-7091-1192-5_45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smithason S, Moore SK, Provencio JJ. Systemic administration of LPS worsens delayed deterioration associated with vasospasm after subarachnoid hemorrhage through a myeloid cell-dependent mechanism. Neurocrit Care. 2012;16(2):327–334. doi: 10.1007/s12028-011-9651-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nanou E, Scheuer T, Catterall WA. Calcium sensor regulation of the CaV2.1 Ca2+ channel contributes to long-term potentiation and spatial learning. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(46):13209–13214. doi: 10.1073/pnas.1616206113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Raffo E, Coppola A, Ono T, Briggs SW, Galanopoulou AS. A pulse rapamycin therapy for infantile spasms and associated cognitive decline. Neurobiol Dis. 2011;43(2):322–329. doi: 10.1016/j.nbd.2011.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Diagram of the experimental design. Part 1. The mice received Barnes Maze training from day 7 to day 1 (−7 to −1) before isoflurane or desflurane anesthesia plus the abdominal surgery (anesthesia/surgery). The values of the escape latency, escape distance, escape speed, escape errors and the time in target quadrant on the last day of Barnes Maze training serve as the baseline of Barnes Maze probe test. Then, the mice received Barnes Maze probe test on day 3, 7, 14, 21, 28, 35 and 42 after the anesthesia/surgery, respectively. Western blot analysis was performed at the end of the behavior tests on day 42. ATP measurement was performed immediately after the anesthesia/surgery on day 0. Part 2. Vitamin K2 treatment (100 mg/kg, daily) started 8 days before the anesthesia/surgery. Western blot analysis was performed after the behavior tests on day 28. ATP measurement was performed immediately after the anesthesia/surgery on day 0.
Supplementary Figure 2. Effects of isoflurane or desflurane plus surgery on baseline escape latency, escape distance, escape speed, escape errors and time in quadrant of Barnes Maze test in AD Tg or WT mice. One-way ANOVA showed no significant difference between isoflurane plus surgery or desflurane plus surgery versus control condition on escape latency (A and B), escape distance (C and D), escape speed (E and F), escape errors (G and H) and time in target quadrant (I and J) of Barnes maze baseline performance in AD Tg (A, C, E, G and I) or WT (B, D, F, H and J) mice. ANOVA, analysis of variance; AD, Alzheimer’s disease; Tg, transgenic; WT, wild-type. N = 12 in each group.
Supplementary Figure 3. Escape latency, escape distance, escape speed, escape errors and time in target quadrant of Barnes Maze training test in AD Tg and WT mice. Two-way ANOVA with repeated measurement showed that no significant interaction of mice in the group (isoflurane plus surgery or desflurane plus surgery or control condition) and time (days) on escape latency (A and B), escape distance (C and D), escape speed (E and F), escape errors (G and H) and time in target quadrant (I and J) in AD Tg (A, C, E, G and I) and WT (B, D, F, H and J) mice. ANOVA, analysis of variance; AD, Alzheimer’s disease; Tg, transgenic; WT, wild-type. N = 12 in each group.
Supplementary Figure 4. The comparison of the baseline of escape latency in Barnes Maze probe test and Aβ42 levels in the brain tissues between AD Tg control and WT control mice. A. The baseline of escape latency in Barnes Maze probe test of AD Tg control mice was significant longer than that of WT control mice at one day (day -1) before the anesthesia/surgery (P = 0.034). B. The Aβ42 levels of cortex of AD Tg control mice were significantly higher than that of WT control mice (P < 0.001). AD, Alzheimer’s disease; Tg, transgenic; WT, wild-type; Aβ, β-amyloid protein. N = 12 in each group.
Supplementary Figure 5. Effects of isoflurane plus surgery on escape latency, escape distance, escape speed, escape errors and time in target quadrant of Barnes Maze training test in AD Tg mice with pretreatment of corn oil or Vitamin K2. Two-way ANOVA with repeated measurement showed no significant interaction of group (isoflurane plus surgery versus control condition) and time (days) on escape latency (A), escape distance (B), escape speed (C), escape errors (D) and time in target quadrant (E) of Barnes maze training test in AD Tg mice with pretreatment of corn oil or Vitamin K2. ANOVA, analysis of variance; AD, Alzheimer’s disease; Tg, transgenic; WT, wild-type. N = 10 in each group.
Supplementary Table 1. Effects of isoflurane or desflurane plus surgery on cognitive function in both AD Tg and WT mice. Left part: Isoflurane plus surgery increased the escape latency in the Barnes Maze probe test as compared to control condition on day 28 after the anesthesia/surgery in AD Tg mice (P = 0.034). Isoflurane plus surgery induced a borderline increase in escape latency in the Barnes Maze probe test as compared to control condition on day 7 after the anesthesia/surgery in AD Tg mice (P = 0.059). Finally, Isoflurane plus surgery induced a borderline increase in escape latency in the Barnes Maze probe test as compared to control condition on day 14 in WT mice (P = 0.088). Desflurane plus surgery did not increase the escape latency as compared to the control condition in either AD Tg or WT mice. Right part: Isoflurane plus surgery increased the escape distance in the Barnes Maze probe test as compared to control condition on day 28 after the anesthesia/surgery in AD Tg mice (P = 0.011). Desflurane plus surgery did not increase the escape distance as compared to the control condition in either AD Tg or WT mice. AD, Alzheimer’s disease; Tg, transgenic; WT, wild-type. N = 12 in each group.