Abstract
BACKGROUND
Clinical reports suggest that, rather than directly driving cocaine use, stress may create a biological context within which other triggers for drug use become more potent. We hypothesize that stress-induced increases in corticosterone “set the stage” for relapse by promoting endocannabinoid-induced attenuation of inhibitory transmission in the prelimbic cortex (PL).
METHODS
We have established a rat model for these stage-setting effects of stress. In this model, neither a stressor (electric footshock) nor stress-level corticosterone treatment alone reinstates cocaine seeking following self-administration and extinction, but each treatment potentiates reinstatement in response to an otherwise subthreshold cocaine priming dose (2.5 mg/kg, ip). The contributions of endocannabinoid signaling in the PL to the effects of stress-level corticosterone on PL neurotransmission and cocaine seeking were determined using intra-PL micro-infusions. Endocannabinoid-dependent effects of corticosterone on inhibitory synaptic transmission in the rat PL were determined using whole-cell recordings in layer V pyramidal neurons.
RESULTS
Corticosterone application attenuated inhibitory synaptic transmission in the PL via CB1R- and 2-arachidonoylglycerol (2-AG)-dependent inhibition of GABA release without altering postsynaptic responses. The ability of systemic stress-level corticosterone treatment to potentiate cocaine-primed reinstatement was recapitulated by intra-PL injection of corticosterone, the CB1R agonist WIN 55-212,2, or the monoacylglycerol lipase inhibitor URB602. Corticosterone effects on reinstatement were attenuated by intra-PL injections of either the CB1R antagonist, AM251, or the diacylglycerol lipase inhibitor, DO34.
CONCLUSIONS
These findings suggest that stress-induced increases in corticosterone promote cocaine seeking by mobilizing 2-AG in the PL, resulting in CB1R-mediated attenuation of inhibitory transmission in this brain region.
Keywords: Corticosterone, endocannabinoids, addiction, cocaine, prelimbic cortex, self-administration
INTRODUCTION
Despite efforts to identify effective treatment strategies for patients with substance use disorders (SUD), relapse rates remain high. This prevalence is due in part to the complex interactions among factors that promote drug craving and seeking. One factor, stress, is particularly problematic, as it is prevalent in SUD populations and unavoidable in daily life. Although there is a well-demonstrated influence of stress on drug craving in individuals with SUDs (1; 2), the effects of stress on drug-seeking behavior can be complex. Emerging evidence indicates that stress does not always directly trigger craving but instead can increase reactivity to other relapse-inducing stimuli (3–6). This understudied influence of stress can also be observed in rats (7–10). While it has been established that both stress (11) and high dose priming injections of the drug (12) can trigger reinstatement, under conditions where stress does not directly trigger reinstatement, it can potentiate cocaine seeking in response to an otherwise subthreshold priming injection of cocaine following self-administration and extinction (8; 10).
Footshock-induced potentiation of cocaine seeking is glucocorticoid-dependent and corticosterone administration, at a dose that reproduces stress-induced levels, is sufficient to potentiate reinstatement (8). A likely site of corticosterone regulation of cocaine use is the medial prefrontal cortex. The prelimbic region of the medial prefrontal cortex has been extensively implicated in cocaine-seeking behavior (13–15), and is highly regulated by stress and glucocorticoids (16; 17). Taken together, these data provide strong evidence that the prefrontal cortex is a key site for corticosterone actions that mediate stress regulation of cocaine-seeking behavior.
Corticosterone may regulate prefrontal cortical function through interactions with the endocannabinoid system. In the prefrontal cortex, cannabinoid receptor type-1 (CB1R) is predominantly located on perisomatically-targeting GABAergic interneurons (18), positioning activation of this system to regulate output of prefrontal pyramidal neurons. In mice, stress can increase levels of the endocannabinoid 2-arachidonoylglycerol (2-AG) in the prefrontal cortex in a glucocorticoid-dependent manner (18). Furthermore, bath application of corticosterone to mouse prefrontal slices attenuates inhibitory neurotransmission in a CB1R-dependent manner (18). Importantly, the same stressor, electric footshock, which potentiates reinstatement of cocaine seeking also elevates levels of endocannabinoids in subregions of the prefrontal cortex in drug-naïve rats (10). Taken together, these data suggest that stress, and corticosterone, potentiate reinstatement through increased CB1R activation in the prelimbic cortex. Importantly, we and others have demonstrated that systemic CB1R antagonism blocks stress-potentiated but not stress- (19; 20) or cocaine-induced reinstatement (10; 20).
In the current study, we test the hypothesis that, in rats, corticosterone exerts effects in the prelimbic subregion of the medial prefrontal cortex to potentiate reinstatement of cocaine seeking through a CB1R-dependent attenuation of inhibitory neurotransmission. We demonstrate that corticosterone acts in the prelimbic cortex to potentiate cocaine seeking, and that corticosterone attenuates inhibitory neurotransmission in prelimbic slices in a CB1R-dependent manner. Furthermore, we show that CB1R activation in the prelimbic cortex is necessary for stress- and corticosterone-potentiated reinstatement as well as sufficient to reproduce the potentiating effects of stress or corticosterone administration. Finally, we determine that prelimbic cortical CB1R activation involves 2-AG signaling, as inhibition of 2-AG production blocks and attenuation of 2-AG breakdown reproduces corticosterone-potentiated reinstatement.
MATERIALS AND METHODS
Subjects
102 Male Sprague-Dawley rats (275–300g at arrival; Envigo, Indianapolis, IN), were individually housed in a 12h/12h reverse light/dark cycle (0700–1900 lights off) as described in the supplemental information. All behavioral procedures were conducted in the dark phase. Of the rats designated for behavioral testing, 14 were excluded from the study because of misplaced cannulation or they did not complete all behavioral testing.
Surgery
For intravenous self-administration and reinstatement testing, rats were anesthetized with ketamine HCl (100 mg/kg, i.p.; Henry Schein, Melville, NY) and xylazine (2 mg/kg, i.p.; Henry Schein, Melville, NY) and surgically implanted with indwelling venous catheters. Rats that received intra-cranial micro-infusions prior to reinstatement testing also had bilateral guide cannula targeting the prelimbic cortex. All procedures are described in detail in the supplemental information.
Cocaine self-administration, extinction and reinstatement
Rats were trained to press a lever to self-administer cocaine (0.5 mg/kg/inf) under a fixed-ratio (FR) 4 schedule of reinforcement during daily 2-h sessions. Once trained, rats underwent daily self-administration for 14 days prior to extinction training, during which the cocaine solution was replaced with saline. Each rat underwent extinction training until extinction criterion was met (<10 lever presses/2-h) at which point reinstatement testing was conducted. Procedures are described in detail in the supplemental information.
Reinstatement conditions
The 2-h reinstatement sessions were preceded by footshock, corticosterone administration, and/or drug delivery, as described in the Results, and were otherwise identical to extinction conditions. A within-subject testing design was utilized in which each rat received all treatments in a given experiment in a counterbalanced order. Nine rats did not complete all tests and were excluded from the study. Reinstatement was defined as significantly increased responding on the lever previously reinforced by cocaine relative to the preceding extinction session and compared to control conditions. Responding on a second lever that was inactive through self-administration training was also recorded during the sessions. There were no significant changes in inactive lever responding between extinction and reinstatement testing days (Supplemental Figure S3). Potentiated cocaine seeking was defined as significant reinstatement in response to low-dose cocaine (2.5 mg/kg, ip) following shock or drug pretreatment under conditions where neither low-dose cocaine nor the drug pretreatment alone produced reinstatement.
Intra-cranial drug administration
Intra-prelimbic cortical (PL) infusions were given at various time points prior to reinstatement testing as described in the Results. All infusions were a volume of 0.3 μL at a rate of 0.3 μL/min. Placement of guide cannula was verified as described in the supplemental information. Albeit in only five rats, injections outside of the prelimbic cortex did not have effects on cocaine-seeking behavior.
Slice electrophysiology
Whole cell voltage clamp recordings were made from pyramidal neurons in the layer V of the prelimbic cortex of drug-naïve rats as described in detail in the supplemental information. For recording of evoked inhibitory post-synaptic currents (IPSCs), layer V pyramidal neurons were voltage-clamped at −60 mV, and IPSCs were evoked at 0.05 Hz by a tungsten stimulation electrode placed near the apical dendrites. Spontaneous miniature IPSCs (mIPSCs) were recorded from the pyramidal neurons at a holding potential of −70 mV. Action potential generation was blocked with tetrodotoxin (TTX; 0.5 μM). Glutamate receptor antagonists 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 10 μM) and D-2-amino-5-phosphonovaleric acid (D-AP-5, 20 μM) were present in the ACSF throughout the experiments. Series resistance (15–30 MΩ) was monitored throughout the recordings, and data were discarded if the resistance changed by more than 20%. All recordings were performed at 32 ± 1°C.
Mass spectrometry
To determine if the diacylglycerol lipase (DAGL) inhibitor DO34 attenuates corticosterone-induced 2-arachidonoylglycerol (2-AG) levels, rats received intra-PL infusions of DO34 (1 μg/0.3 μL) or vehicle (70% DMSO) 30-min prior to a corticosterone (2 mg/kg, ip) or vehicle (10% EtOH) injection. Rats were rapidly decapitated 45-min following the injection and brains were removed within 90-sec, frozen in liquid nitrogen, and stored at −80°C. Prelimbic cortex was dissected and endocannabinoids extracted as previously described (10). N-arachidonoylethanolamine (AEA) and 2-AG, were isolated and quantified as described in the supplemental information using isotope dilution, liquid chromatography/mass spectrometry.
Statistical Analysis
Statistical analyses were conducted using one- or two-way repeated measures ANOVA, followed by Bonferroni post-hoc tests when appropriate for behavioral and mass spectrometry experiments and Student’s t-test for electrophysiology experiments (see supplemental information for more detail).
RESULTS
Corticosterone acts in the prelimbic cortex to potentiate reinstatement
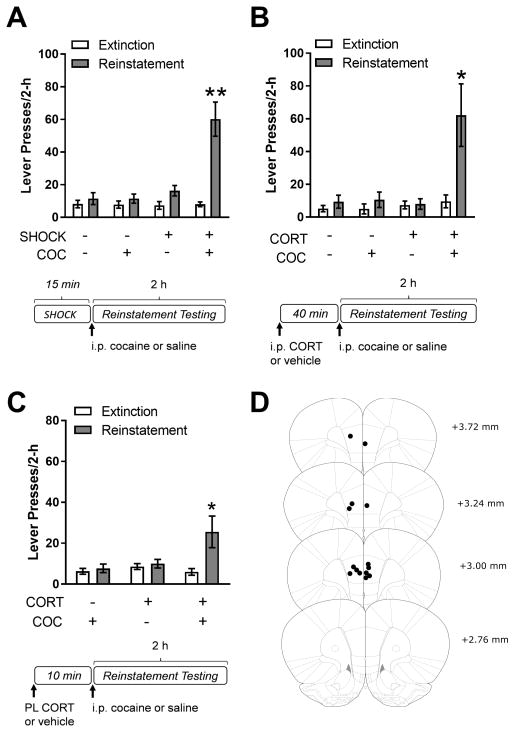
After 14 days of stable cocaine self-administration, responding was extinguished and rats were tested for reinstatement of cocaine seeking. All self-administration and extinction data are displayed in Supplementary Table S1. As previously reported under these conditions (8; 10), electric footshock stress (3 × 0.5 mA, 200-msec duration, mean intershock interval 40-sec, range 10–70 sec over a 15-min period) alone did not induce reinstatement but potentiated cocaine seeking in response to an otherwise sub-threshold priming dose of cocaine (2.5 mg/kg, i.p.; Fig. 1A; 2-way repeated measure (RM) ANOVA, reinstatement condition x day interaction, F(3,15)=17.11, p<.001; post-hoc comparison, shock/cocaine combination vs. extinction, p<.05). The ability of footshock to potentiate reinstatement was reproduced by systemic administration of corticosterone (2 mg/kg, i.p.) at a dose that established footshock-induced levels of plasma corticosterone (Supplementary Figure S1). As with footshock, corticosterone alone was insufficient to induce reinstatement. However, when corticosterone was combined with an otherwise subthreshold priming injection of cocaine, significant reinstatement was observed (Fig. 1B; 2-way RM ANOVA, reinstatement condition x day, F(3,12)=10.30, p<.001; post-hoc comparison, corticosterone/cocaine combination vs. extinction, p<.05). To localize corticosterone action in the brain, soluble 2-hydroxypropyl-β-cyclodextrin (HBC)-conjugated corticosterone (50 ng/0.3 μL), was micro-infused directly into the prelimbic cortex (PL) 10 minutes prior to low-dose cocaine. As was the case with systemic administration, intra-PL administration of corticosterone alone did not increase cocaine seeking but induced significant reinstatement when given prior to administration of the subthreshold priming dose of cocaine (Fig. 1C; 2-way RM ANOVA, reinstatement condition x day interaction, F(2, 12)=4.89, p<.05; post-hoc comparison, corticosterone/cocaine combination vs. extinction, p<.05). These findings identify the PL as an important site for corticosterone effects on cocaine seeking.
Figure 1.
Corticosterone acts in the prelimbic cortex to potentiate reinstatement of cocaine seeking. A) Electric footshock stress (SHOCK; n=6) potentiates reinstatement when given in combination with a low dose of cocaine (COC; 2.5 mg/kg, i.p). B) Systemic corticosterone (CORT; 2 mg/kg, i.p.; n=5) reproduces the effect of footshock and potentiates reinstatement when given 40 minutes prior to an injection of low dose cocaine (2.5 mg/kg, i.p). C) 2-hydroxypropyl-β-cyclodextrin HBC-conjugated corticosterone (CORT; 50 ng/0.3 μL; n=7) administered directly into the prelimbic cortex (PL) 10 minutes prior to a low dose cocaine injection (2.5 mg/kg, i.p) induces significant reinstatement whereas either administered alone does not (p<.05; **p<.01, *p<.05, compared to extinction). Data are presented as mean ± SEM.
Corticosterone attenuates prelimbic cortical inhibitory neurotransmission in a CB1R-dependent manner
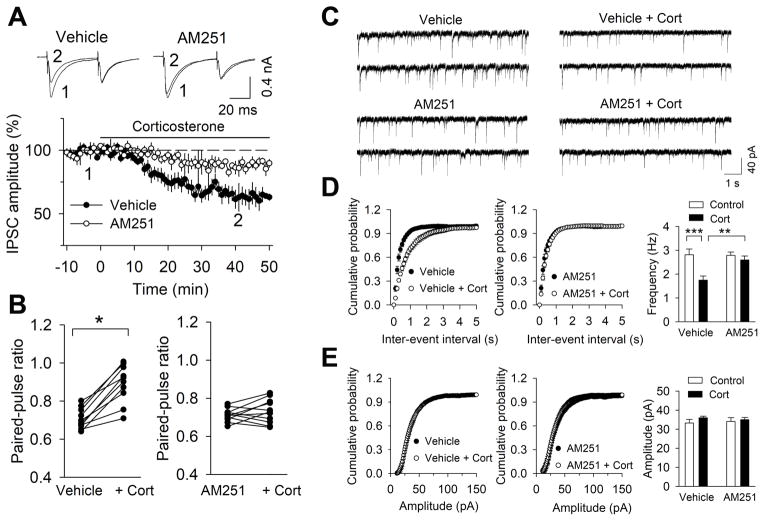
To investigate corticosterone effects on prelimbic cortical neurotransmission in rats, whole-cell recordings were made in visually-identified layer V pyramidal neurons in rat PL. We initially examined the effects of corticosterone on evoked IPSCs. Following stable baseline recordings, bath application of corticosterone (1 μM) for 30-min gradually decreased the amplitude of evoked IPSCs with the depression having a rapid onset (~5-min) and peaking in 30 minutes (Fig. 2A). This depression was accompanied by an increase in the paired-pulse ratio (PPR), suggesting a presynaptic mechanism (Fig. 2B). The depression of evoked IPSC amplitude by corticosterone was prevented by the continuous presence of CB1R antagonist AM251 (2 μM; Fig. 2A). Moreover, in the presence of AM251, paired-pulse ratio (PPR) was not altered by corticosterone (Fig. 2B). Thus, these results suggest that corticosterone depresses IPSCs in rat prelimbic cortex through a presynaptic CB1R-dependent mechanism. To further test this, we examined the effects of corticosterone on miniature IPSCs (mIPSCs) in layer V pyramidal neurons in rat PL slices. A change in mIPSC frequency indicates a presynaptic mechanism, whereas a change in mIPSC amplitude signifies a likely change in postsynaptic responsiveness (21). PL slices were perfused with either vehicle or corticosterone (1 μM) for 20–60 minutes and mIPSCs were recorded from layer V pyramidal neurons. Corticosterone perfusion significantly decreased the frequency of mIPSCs (Fig. 2D) as shown by both a decrease in the mean frequency of mIPSCs (p<.01) and a rightward shift in the cumulative probability plot for inter-event intervals. By contrast, corticosterone did not alter the mean amplitude of mIPSCs (Fig. 2E; p>.05) or the cumulative amplitude distribution (p>.05). To investigate if corticosterone depressed mIPSCs through a CB1R-dependent mechanism, we repeated the above experiments in the presence of the CB1R antagonist, AM251. PL slices were perfused with either AM251 (2 μM) alone or AM251 with corticosterone (1 μM) for 20–60 minutes. In the presence of AM251, corticosterone did not significantly alter mIPSCs (Fig. 2D; p> 05) as there were no significant changes in the mean frequency of mIPSCs (p>.05) or the cumulative inter-event interval probability plot (p>.05). In the presence of AM251, corticosterone did not significantly alter the mean amplitude of mIPSCs (Fig. 2E; p>.05) or cumulative amplitude distribution (p>.05). These results suggest that corticosterone-induced depression of mIPSCs in the PL is dependent upon presynaptic CB1R-mediated suppression of GABAergic transmission.
Figure 2.
Corticosterone depressed evoked and miniature inhibitory post-synaptic currents through a cannabinoid receptor type 1 (CB1R)-dependent mechanism in rat prelimbic slices. A) Bath application of corticosterone (Cort; 1 μM) caused rapid depression of evoked IPSCs (n=10 neurons; n=2–3 rats) in layer V pyramidal neurons in the prelimbic cortex (PL) which were blocked by the CB1R antagonist AM251 (2 μM, n=11 neurons; n=2–3 rats). B) Bath application of corticosterone increased the paired-pulse ratio (PPR) which was blocked by AM251 (n=11 neurons; n=2–3 rats, p<.05). C) Representative traces of mIPSCs recorded in layer V pyramidal neurons in PLC slices that were treated with vehicle (control), Cort (1 μM), AM251 (2 μM) or AM251 plus Cort (1 μM). D) Corticosterone treatment decreased the mean frequency of mIPSCs (n=9–11 neurons; n=2–3 rats, p<.01) and caused a right shift of the inter-event intervals of the cumulative probability plot. However, in the presence of AM251, corticosterone did not alter the mean frequency of mIPSCs (n=10–11 neurons; n=2–3 rats, p>.05) or inter-event intervals of the cumulative probability plot. E) Corticosterone did not alter the mean amplitude of mIPSCs (p>.05) and cumulative amplitude distribution. In addition, in the presence of AM251, corticosterone had no significant effect on the mean amplitude of mIPSCs (p>.05) or on the cumulative amplitude distribution of mIPSCs.
CB1R activation in the prelimbic cortex is necessary and sufficient for stress- and corticosterone-potentiated reinstatement
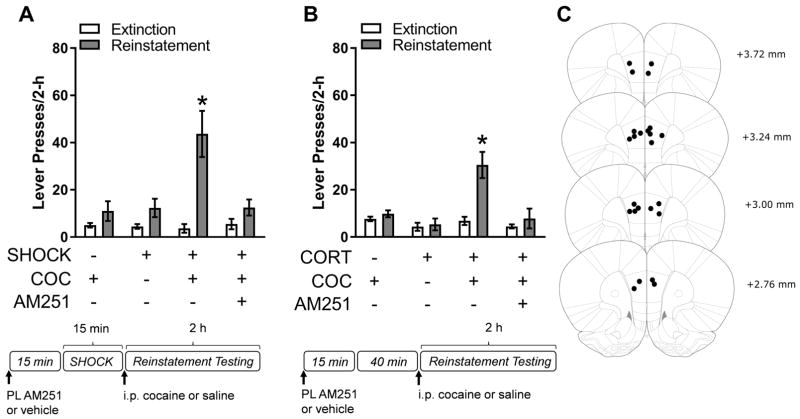
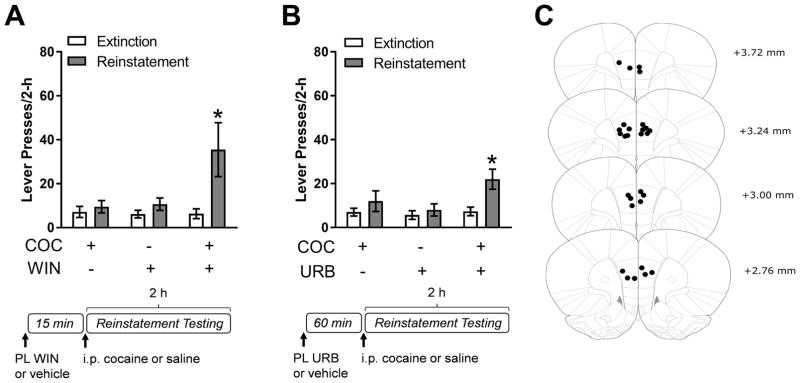
We have previously shown that stress-potentiated reinstatement is prevented by systemic administration of the CB1R antagonist, AM251 (10). These data, in combination with the observed CB1R-dependent corticosterone attenuation of PL inhibitory neurotransmission, led us to hypothesize that stress-potentiated reinstatement is mediated by corticosterone-regulated endocannabinoid signaling and PL CB1R activation. Consistent with this possibility, intra-PL administration of AM251 (300 ng/0.3 μL) 15 minutes prior to footshock blocked stress-potentiated reinstatement (Fig. 3A; 2-way RM ANOVA, reinstatement condition x day interaction, F(3, 15)=15.71, p<.001; post-hoc comparison, Veh/Shock/Coc combination vs extinction, p<.05; AM251/Shock/Coc combination vs. extinction, p>.05). Moreover, intra-PL administration of AM251 (300 ng/0.3 μL) 15 minutes prior to systemic corticosterone administration (2.5 mg/kg, i.p) also blocked corticosterone-potentiated reinstatement (Fig. 3B; 2-way RM ANOVA, reinstatement condition x day interaction, F(3,15)=6.94, p<.01; post-hoc comparison, Veh/Cort/Coc combination vs. extinction, p<.05; AM251/Cort/Coc combination vs. extinction, p>.05). These data provide compelling evidence that stress, likely through corticosterone, promotes cocaine seeking via increased endocannabinoid signaling in the PL. To determine if CB1R activation in the PL is sufficient to potentiate cocaine-primed reinstatement, we tested rats for the effects of the intra-PL delivery of the CB1R agonist, WIN 55-212,2, on reinstatement. Neither intra-PL WIN 55-212,2 (50 ng/0.3 μL; 15-min pretreatment) nor 2.5 mg/kg (ip) cocaine alone increased cocaine seeking. However, when intra-PL WIN 55-212,2 preceded the cocaine injection, significant reinstatement was observed (Fig. 4A; 2-way RM ANOVA, reinstatement condition x day interaction, F(2,10)=7.09, p<.05; post-hoc comparison WIN/Coc combination vs. extinction, p<.05).
Figure 3.
Stress- and corticosterone-potentiated reinstatement is blocked by cannabinoid receptor type 1 (CB1R) antagonism in the prelimbic cortex (PL). A) Electric footshock stress (SHOCK)-potentiated reinstatement to low-dose cocaine (COC; 2.5 mg/kg, i.p.) is blocked by pre-treatment with an intra-PL infusion of the CB1R antagonist AM251 (300 ng/0.3 μL) 15 minutes prior to the reinstatement test (n=6). B) Corticosterone (CORT; 2.0 mg/kg, i.p.)-potentiated reinstatement to low-dose cocaine is blocked by pre-treatment with an intra-PL infusion of AM251 15-min prior to the reinstatement test (n=6, *p<.05, compared to extinction). Data are presented as mean ± SEM.
Figure 4.
Cannabinoid receptor type 1 (CB1R) activation in the prelimbic cortex (PL) is sufficient to potentiate reinstatement. A) Intra-PL administration of the CB1R agonist, WIN 55,212-2 (50 ng/0.3 μL) 15 minute prior to a low-dose cocaine injection (COC; 2.5 mg/kg, i.p.) induces significant reinstatement of cocaine-seeking behavior while either administered alone does not. (n=6, *p<.05, compared to extinction). B) Intra-PL administration of a monoacylglycerol lipase (MAGL) inhibitor URB602 (300 pmol/0.3 μL), which elevates 2-arachidonoylglycerol (2-AG) levels and acts an indirect agonist of the CB1R receptor, 60 minutes prior to a low-dose cocaine injection (2.5 mg/kg, i.p.) induces significant reinstatement while administration of either alone does not (n=6). Data are presented as mean ± SEM.
There is evidence that stress can mobilize the endocannabinoid, 2-AG, in the PL (16), suggesting that CB1R-dependent effects of corticosterone may be mediated through 2-AG signaling. To determine if elevated 2-AG levels are sufficient to potentiate reinstatement, we tested rats for the effect of intra-PL administration of URB602, an inhibitor of the enzyme monoacylglycerol lipase (MAGL) inhibitor which is responsible for the breakdown of 2-AG. As was the case with corticosterone and WIN 55-212,2, neither intra-PL URB602 (300 pmol/0.3 μL; 60-min pretreatment) nor 2.5 mg/kg (ip) cocaine alone increased cocaine seeking, but significant reinstatement was observed when URB602 preceded the cocaine injection (Fig. 4B; 2-way RM ANOVA, reinstatement condition x day interaction, F(2,10)=5.89, p<.05; post-hoc comparison URB/Coc combination vs. extinction, p<.05). Collectively, these data demonstrate that endocannabinoid signaling in the PL is necessary and sufficient for stress- and corticosterone-potentiated reinstatement, and suggest that corticosterone may potentiate reinstatement through increased 2-AG signaling.
Corticosterone potentiates reinstatement through increased 2-AG signaling in the prelimbic cortex
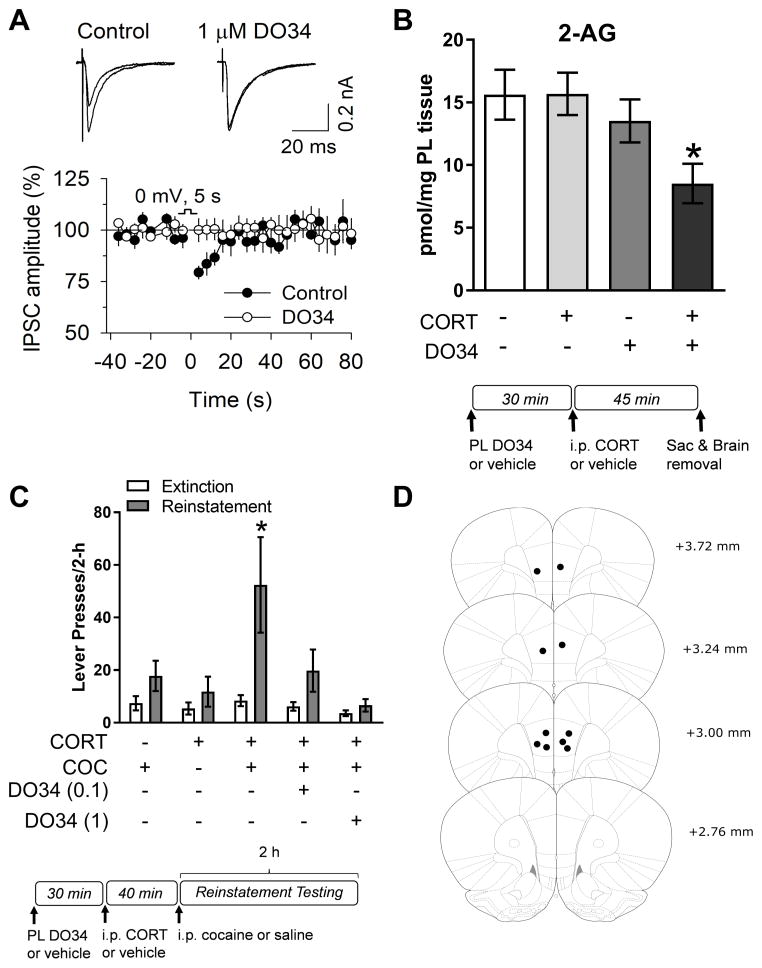
To further test if PL 2-AG signaling mediates corticosterone effects on reinstatement, DO34 (provided by the Cravatt lab), a novel inhibitor of diacylglycerol lipase (DAGL), the synthetic enzyme for 2-AG, was used. Bath application of DO34 (1 μM) to PL cortical slices blocked depolarization-induced suppression of inhibition (n=7–8, p<.01), which is CB1R-dependent (18), suggesting that 2-AG signaling plays a critical role in endocannabinoid effects on PL neurotransmission (Fig. 5A). Furthermore, intra-PL administration of DO34 (1 μg/0.3 μL; 30-min pretreatment) prior to a systemic injection of corticosterone (2 mg/kg, i.p.) significantly reduced PL 2-AG content in a corticosterone-dependent manner (Fig. 5B; two-way ANOVA with DO34 and corticosterone treatments as between factors, main effect of DO34 F(1,26)=6.97, p<.01; main effect of corticosterone F(1,26)=1.98, p>.05; DO34 x corticosterone F(1,26)=2.09, p>.05), while having no effect on levels of anandamide (Supplementary Figure S2; p>.05). Confirming a role for PL 2-AG in the corticosterone-dependent stress-induced potentiation of cocaine seeking, intra-PL administration of DO34 (0.1, 1 μg/0.3 μL; 30-min pretreatment) prior to systemic corticosterone dose-dependently inhibited the ability of stress-level corticosterone to potentiate cocaine seeking when combined with low-dose cocaine (Fig. 5C; 2-way RM ANOVA, reinstatement condition x day interaction, F(4,16)=3.84, p<.05; post-hoc comparisons, Veh/Cort/Coc combination vs extinction, p<.05; DO34 0.1/Cort/Coc or DO34 1.0/Cort/Coc vs. extinction, p>.05). Taken together these data suggest that, during stress, corticosterone-induced PL 2-AG signaling acting via CB1Rs attenuates inhibitory neurotransmission to increase the excitability of cortical outputs mediating cocaine seeking, thereby promoting relapse vulnerability to ordinarily subthreshold triggers.
Figure 5.
Corticosterone-potentiated reinstatement is dependent upon 2-arachidonoylglycerol (2-AG) signaling in the prelimbic cortex (PL). A) Bath application of the diacylglycerol lipase (DAGL) inhibitor DO34 (1 μM) to PL slices blocks depolarization-induced suppression of inhibition (n=7–8, p<.01). B) Direct administration of the DAGL inhibitor DO34 (1 μg/0.3 μL) into the PL 30 minutes prior to a systemic injection of corticosterone (2 mg/kg, i.p.) attenuates PL 2-AG in the presence of corticosterone (CORT; n=7–8; *p<.05, main effect of DO34). C) Corticosterone-potentiated reinstatement is dose-dependently blocked by intra-PL administration of DO34 (0.1, 1.0 μg/0.3 μL) 30 minutes prior to the corticosterone injection (n=5; *p<.05, compared to extinction). Data are presented as mean ± SEM.
DISCUSSION
Stress is a powerful determinant of drug seeking in individuals with substance use disorders (SUDs). This is problematic for relapse prevention, as stress is prevalent and unavoidable in the daily lives of drug addicts. Although the general idea that stress contributes to drug seeking is well-established (22–24), the nature of this contribution appears to be more complex than previously thought. While it is true that, in some cases, stress can serve as a direct trigger for drug craving (2; 11), supported by findings in rodent models (11), recent evidence points to a more opportunistic role of stress that involves interactions with other factors that promote relapse [e.g., cues, drug re-exposure; (4; 5; 25)]. Consistent with reports from individuals with SUDs, these findings imply that stress may function by augmenting the ability of other stimuli to elicit drug seeking, thereby “setting the stage” for relapse. We have recently established a pre-clinical model for these stage-setting effects of stress wherein stress can potentiate, but not directly trigger, reinstatement of cocaine seeking in response to an otherwise sub-threshold priming injection of cocaine (8; 10). Similarly, it has been reported that stress can potentiate the reinstatement of drug seeking upon the presentation of drug-associated cues (7; 9; 26). Importantly, evidence suggests that the neurobiological processes that mediate stress-potentiated reinstatement are distinct from those that mediate stress-triggered cocaine seeking. Specifically, shock-potentiated cocaine seeking, observed in rats with a history of shorter daily access to cocaine, requires acute elevation of corticosterone (8) and CB1R activation (10), while shock-triggered cocaine seeking, observed in rats with a history of longer daily access to cocaine, does not (10, 26).
Here we identify the prelimbic sub-region (PL) of the medial prefrontal cortex as a critical site of action for the corticosterone-dependent stage-setting effects of stress on cocaine seeking. We demonstrate that the ability of stress and stress-level corticosterone to potentiate cocaine-induced reinstatement is reproduced by intra-PL corticosterone delivery. The prelimbic cortex has been previously implicated in drug seeking in response to multiple reinstating stimuli (13–15) and is critical for the control of goal-directed behavior (27). Moreover, the medial prefrontal cortex is highly responsive to stress, as it contains a high density of glucocorticoid receptors (28; 29) and is heavily regulated by corticosterone which acts in the region to exert negative feedback on the HPA axis (18; 30) and modify neuronal morphology (31; 32), gene transcription (33; 34), synaptic physiology (18; 35–38) and behavior (39–42).
In the PL, corticosterone likely regulates cocaine seeking through a CB1R-dependent attenuation of GABAergic neurotransmission. Here we report that both stress- and corticosterone-potentiated reinstatement are prevented by intra-PL administration of the CB1R antagonist AM251, while intra-PL administration of the CB1R agonist WIN 55,212-2 is sufficient to potentiate cocaine-induced reinstatement. PL CB1Rs are located predominately on GABAergic interneurons (18) and activation of CB1Rs in the prefrontal cortex attenuates inhibitory neurotransmission (43). In mice, ex vivo bath application of corticosterone to mPFC slices produces a CB1R-dependent reduction in inhibitory neurotransmission (18). Furthermore, we demonstrate that bath application of corticosterone to rat PL slices attenuates both spontaneous and evoked inhibitory neurotransmission via a CB1R-dependent pre-synaptic mechanism in rats.
The effects of stress and corticosterone on reinstatement likely involve mobilization of 2-AG in the PL. Prior studies have suggested that stress and glucocorticoids mobilize 2-AG (44; 45), particularly in the prefrontal cortex (18). Here we report that intra-PL delivery of a MAGL inhibitor, URB602, which elevates 2-AG brain content (46; 47), is sufficient to potentiate reinstatement in response to low-dose cocaine, similar to what is observed with stress, corticosterone and intra-PL CB1R agonist administration. However, a role for AEA signaling was not examined in the current study and cannot be ruled out. To determine if corticosterone potentiates reinstatement through 2-AG mobilization, we utilized a novel DAGL inhibitor, DO34, which reduces 2-AG brain content (48). Bath application of DO34 blocks depolarization-induced suppression of inhibition in the PL, which is known to be CB1R-dependent (18), suggesting that mobilization of 2-AG is critical for endocannabinoid-regulated synaptic plasticity, while intra-PL DO34 both prevents corticosterone-potentiated reinstatement and reduces PL 2-AG content in corticosterone-treated rats without altering anandamide levels. Interestingly, while intra-PL administration of the DAGL inhibitor DO34 reduced 2-AG content in corticosterone-treated rats, a corticosterone-induced elevation of PL 2-AG was not observed, in contrast to earlier studies demonstrating glucocorticoid-dependent stress-induced mobilization of 2-AG (16). However, these findings are consistent with reports that systemic administration of corticosterone alone does not elevate AEA or 2-AG in the prefrontal cortex as observed with bulk tissue dissection (49). Given that a majority of brain tissue 2-AG content is not involved in signaling (50), tissue concentration of 2-AG may not be sensitive to an increase in 2-AG mobilization at the synapse. The mechanism through which corticosterone regulates PL endocannabinoid signaling is unclear and will require further investigation.
Our data suggest that corticosterone potentiates reinstatement through an endocannabinoid-mediated, likely 2-AG, attenuation of inhibitory neurotransmission, which should increase the excitability of outputs mediating drug seeking. This is supported by findings that acute stress recruits endocannabinoid signaling, specifically 2-AG, to attenuate inhibitory neurotransmission in the basolateral amygdala (44). While the current study did not directly examine specific prelimbic cortical projection fields, the projection pathway from the prelimbic cortex to the nucleus accumbens core is one likely to be affected as it has been established as a key pathway for cocaine seeking (13–15; 51–53). Specifically, we hypothesize that corticosterone mobilizes 2-AG, thereby activating CB1Rs that regulate GABA release from cortical interneurons, to attenuate inhibitory regulation of cortico-accumbens pyramidal projection neurons. This disinhibition would render the cortico-accumbens pathway more responsive to convergent PL excitatory inputs, including those activated by stimuli that trigger cocaine seeking. This hypothesis is consistent with a previous report that attenuated GABAergic neurotransmission in the mPFC facilitates cue-induced reinstatement to nicotine seeking (54). However, this requires further study as it is unknown whether corticosterone-potentiated reinstatement results in increased activation of the cortico-accumbens pathway and there are several possible prelimbic cortical projection pathways that can regulate potentiated cocaine seeking. Furthermore, while CB1Rs are predominantly on GABAergic terminals in the PL, it is not currently known whether corticosterone can potentially regulate neurotransmission in a CB1R-dependent manner through non-GABAergic mechanisms.
While our findings demonstrate that the PL is an important site for corticosterone effects, it is not the only site at which corticosterone can regulate cocaine seeking. This may contribute to the differential magnitude of reinstatement observed with intraperitoneal vs intra-PL administration of corticosterone. We previously reported that corticosterone delivery into the nucleus accumbens can also potentiate cocaine-induced reinstatement in rats. However, the effects of corticosterone in the nucleus accumbens involve its inhibition of organic cation transporter 3 (OCT3), a secondary uptake mechanism for monoamines, and the resulting decrease in dopamine clearance (8). However, there is likely interplay between the two brain regions, and others, during reinstatement. Furthermore, it is possible that OCT3 and endocannabinoid mechanisms may work in tandem within the PL itself. Ultimately, the ability of corticosterone to regulate behavior at multiple sites suggests that, during periods of stress, glucocorticoids may impose a brain state that produces system-wide changes in synaptic transmission to promote adaptive behavioral response patterns. The ubiquitous nature of endocannabinoid signaling suggests that glucocorticoid mobilization of 2-AG may serve as a key mechanism through which stress shifts neuronal excitability and therefore influence behavior at the network level.
The complexity of the contribution of stress to drug seeking has limited our ability to develop effective strategies for relapse prevention aimed at targeting stress-responsive mechanisms. Here we report that stress, under conditions where it does not directly trigger responding, can potentiate cocaine-induced reinstatement in a rodent model, thereby setting the stage for cocaine seeking. This approach is consistent with many clinical reports and therefore may offer greater validity for studying the contribution of stress to relapse. Importantly, the observation that the neurobiological mechanisms underlying these “stage-setting” effects of stress are distinct from those through which stress triggers cocaine seeking, suggests that this model has the potential to reveal new targets for relapse prevention.
Finally, while our findings have direct implications for understanding SUDs, the ability of stress to modify synaptic transmission and neuronal excitability in the prelimbic cortex via glucocorticoid regulation of endocannabinoid signaling has important implications for a range of stress-related pathological conditions that have been associated with prefrontal cortical dysregulation.
Supplementary Material
Acknowledgments
This research was funded by NIH grant DA015758 to JR Mantsch, NIH grant DA038663 to JR Mantsch, CJ Hillard and QS Liu and NIH grant DA035217 to QS Liu.
Footnotes
FINANCIAL DISCLOSURES
JR Mantsch and DA Baker are consultants for and stakeholders in Promentis Pharmaceuticals Inc. CJ Hillard is on the Scientific Advisory Board for Phytecs, Inc. The remaining authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sinha R, Shaham Y, Heilig M. Translational and reverse translational research on the role of stress in drug craving and relapse. Psychopharmacology (Berl) 2011;218:69–82. doi: 10.1007/s00213-011-2263-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sinha R. Modeling stress and drug craving in the laboratory: implications for addiction treatment development. Addict Biol. 2009;14:84–98. doi: 10.1111/j.1369-1600.2008.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Preston KL, Epstein DH. Stress in the daily lives of cocaine and heroin users: relationship to mood, craving, relapse triggers, and cocaine use. Psychopharmacology (Berl) 2011;218:29–37. doi: 10.1007/s00213-011-2183-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Furnari M, Epstein DH, Phillips KA, Jobes ML, Kowalczyk WJ, Vahabzadeh M, et al. Some of the people, some of the time: field evidence for associations and dissociations between stress and drug use. Psychopharmacology (Berl) 2015;232:3529–37. doi: 10.1007/s00213-015-3998-7. [DOI] [PubMed] [Google Scholar]
- 5.Preston K, Vahabzadeh M, Schmittner J, Lin J-L, Gorelick D, Epstein D. Cocaine craving and use during daily life. Psychopharmacology. 2009;207:291–301. doi: 10.1007/s00213-009-1655-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moran-Santa Maria MM, McRae-Clark A, Baker NL, Ramakrishnan V, Brady KT. Yohimbine administration and cue-reactivity in cocaine-dependent individuals. Psychopharmacology (Berl) 2014;231:4157–65. doi: 10.1007/s00213-014-3555-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feltenstein MW, See RE. Potentiation of cue-induced reinstatement of cocaine-seeking in rats by the anxiogenic drug yohimbine. Behav Brain Res. 2006;174:1–8. doi: 10.1016/j.bbr.2006.06.039. [DOI] [PubMed] [Google Scholar]
- 8.Graf EN, Wheeler RA, Baker DA, Ebben AL, Hill JE, McReynolds JR, et al. Corticosterone acts in the nucleus accumbens to enhance dopamine signaling and potentiate reinstatement of cocaine seeking. J Neurosci. 2013;33:11800–10. doi: 10.1523/JNEUROSCI.1969-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buffalari DM, See RE. Footshock stress potentiates cue-induced cocaine-seeking in an animal model of relapse. Physiol Behav. 2009;98:614–7. doi: 10.1016/j.physbeh.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McReynolds JR, Doncheck EM, Vranjkovic O, Ganzman GS, Baker DA, Hillard CJ, Mantsch JR. CB1 receptor antagonism blocks stress-potentiated reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2016;233:99–109. doi: 10.1007/s00213-015-4092-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mantsch JR, Baker DA, Funk D, Lê AD, Shaham Y. Stress-Induced Reinstatement of Drug Seeking: 20 Years of Progress. Neuropsychopharmacology. 2016;41:335–56. doi: 10.1038/npp.2015.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- 13.McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21:8655–63. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McFarland K, Davidge S, Lapish C, Kalivas P. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J Neurosci Official J Soc Neurosci. 2004;24:1551–60. doi: 10.1523/JNEUROSCI.4177-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stefanik MT, Moussawi K, Kupchik YM, Smith KC, Miller RL, Huff ML, et al. Optogenetic inhibition of cocaine seeking in rats. Addict Biol. 2013;18:50–3. doi: 10.1111/j.1369-1600.2012.00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Popoli M, Yan Z, McEwen BS, Sanacora G. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci. 2011;13:22–37. doi: 10.1038/nrn3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McKlveen JM, Myers B, Herman JP. The medial prefrontal cortex: coordinator of autonomic, neuroendocrine and behavioural responses to stress. J Neuroendocrinol. 2015;27:446–56. doi: 10.1111/jne.12272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hill MN, McLaughlin RJ, Pan B, Fitzgerald ML, Roberts CJ, Lee TT, et al. Recruitment of prefrontal cortical endocannabinoid signaling by glucocorticoids contributes to termination of the stress response. J Neurosci. 2011;31:10506–15. doi: 10.1523/JNEUROSCI.0496-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Vries TJ, Shaham Y, Homberg JR, Crombag H, Schuurman K, Dieben J, et al. A cannabinoid mechanism in relapse to cocaine seeking. Nat Med. 2001;7:1151–4. doi: 10.1038/nm1001-1151. [DOI] [PubMed] [Google Scholar]
- 20.Kupferschmidt DA, Klas PG, Erb S. Cannabinoid CB1 receptors mediate the effects of corticotropin-releasing factor on the reinstatement of cocaine seeking and expression of cocaine-induced behavioural sensitization. Br J Pharmacol. 2012;167:196–206. doi: 10.1111/j.1476-5381.2012.01983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pan B, Hillard CJ, Liu QS. D2 dopamine receptor activation facilitates endocannabinoid-mediated long-term synaptic depression of GABAergic synaptic transmission in midbrain dopamine neurons via cAMP-protein kinase A signaling. J Neurosci. 2008;28:14018–30. doi: 10.1523/JNEUROSCI.4035-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci. 2008;1141:105–30. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sinha R, Fox HC, Hong K-IAI, Hansen J, Tuit K, Kreek MJ. Effects of adrenal sensitivity, stress- and cue-induced craving, and anxiety on subsequent alcohol relapse and treatment outcomes. Arch Gen Psychiatry. 2011;68:942–52. doi: 10.1001/archgenpsychiatry.2011.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sinha R. New findings on biological factors predicting addiction relapse vulnerability. Curr Psychiatry Rep. 2011;13:398–405. doi: 10.1007/s11920-011-0224-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Regier PS, Monge ZA, Franklin TR, Wetherill RR, Teitelman A, Jagannathan K, et al. Emotional, physical and sexual abuse are associated with a heightened limbic response to cocaine cues. Addict Biol. 2016 doi: 10.1111/adb.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Banna KM, Back SE, Do P, See RE. Yohimbine stress potentiates conditioned cue-induced reinstatement of heroin-seeking in rats. Behav Brain Res. 2010;208:144–8. doi: 10.1016/j.bbr.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gourley SL, Taylor JR. Going and stopping: dichotomies in behavioral control by the prefrontal cortex. Nat Neurosci. 2016;19:656–64. doi: 10.1038/nn.4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meaney MJ, Sapolsky RM, Aitken DH, McEwen BS. [3H]dexamethasone binding in the limbic brain of the fetal rat. Brain Res. 1985;355:297–300. doi: 10.1016/0165-3806(85)90054-9. [DOI] [PubMed] [Google Scholar]
- 29.Reul JM, de Kloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985;117:2505–11. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- 30.Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J Neurosci. 1993;13:3839–47. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wellman CL. Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. J Neurobiol. 2001;49:245–53. doi: 10.1002/neu.1079. [DOI] [PubMed] [Google Scholar]
- 32.Anderson RM, Glanz RM, Johnson SB, Miller MM, Romig-Martin SA, Radley JJ. Prolonged corticosterone exposure induces dendritic spine remodeling and attrition in the rat medial prefrontal cortex. J Comp Neurol. 2016;524:3729–3746. doi: 10.1002/cne.24027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mikkelsen JD, Larsen MH. Effects of stress and adrenalectomy on activity-regulated cytoskeleton protein (Arc) gene expression. Neurosci Lett. 2006;403:239–43. doi: 10.1016/j.neulet.2006.04.040. [DOI] [PubMed] [Google Scholar]
- 34.McReynolds JR, Holloway-Erickson CM, Parmar TU, McIntyre CK. Corticosterone-induced enhancement of memory and synaptic Arc protein in the medial prefrontal cortex. Neurobiol Learn Mem. 2014;112:148–57. doi: 10.1016/j.nlm.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu R-JJ, Aghajanian GK. Stress blunts serotonin- and hypocretin-evoked EPSCs in prefrontal cortex: role of corticosterone-mediated apical dendritic atrophy. Proc Natl Acad Sci USA. 2008;105:359–64. doi: 10.1073/pnas.0706679105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yuen EY, Wei J, Liu W, Zhong P, Li X, Yan Z. Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron. 2012;73:962–77. doi: 10.1016/j.neuron.2011.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Musazzi L, Milanese M, Farisello P, Zappettini S, Tardito D, Barbiero VS, et al. Acute stress increases depolarization-evoked glutamate release in the rat prefrontal/frontal cortex: the dampening action of antidepressants. PLoS ONE. 2010;5:e8566. doi: 10.1371/journal.pone.0008566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Treccani G, Musazzi L, Perego C, Milanese M, Nava N, Bonifacino T, et al. Stress and corticosterone increase the readily releasable pool of glutamate vesicles in synaptic terminals of prefrontal and frontal cortex. Mol Psychiatry. 2014;19:433–43. doi: 10.1038/mp.2014.5. [DOI] [PubMed] [Google Scholar]
- 39.Roozendaal B, McReynolds JR, McGaugh JL. The basolateral amygdala interacts with the medial prefrontal cortex in regulating glucocorticoid effects on working memory impairment. J Neurosci. 2004;24:1385–92. doi: 10.1523/JNEUROSCI.4664-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, et al. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006;26:7870–4. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yuen EY, Liu W, Karatsoreos IN, Feng J, McEwen BS, Yan Z. Acute stress enhances glutamatergic transmission in prefrontal cortex and facilitates working memory. Proc Natl Acad Sci USA. 2009;106:14075–9. doi: 10.1073/pnas.0906791106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koot S, Koukou M, Baars A, Hesseling P, van ’t Klooster J, Joëls M, van den Bos R. Corticosterone and decision-making in male Wistar rats: the effect of corticosterone application in the infralimbic and orbitofrontal cortex. Front Behav Neurosci. 2014;8:127. doi: 10.3389/fnbeh.2014.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chiu CQ, Puente N, Grandes P, Castillo PE. Dopaminergic modulation of endocannabinoid-mediated plasticity at GABAergic synapses in the prefrontal cortex. J Neurosci. 2010;30:7236–48. doi: 10.1523/JNEUROSCI.0736-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Di S, Itoga CA, Fisher MO, Solomonow J, Roltsch EA, Gilpin NW, Tasker JG. Acute Stress Suppresses Synaptic Inhibition and Increases Anxiety via Endocannabinoid Release in the Basolateral Amygdala. J Neurosci. 2016;36:8461–70. doi: 10.1523/JNEUROSCI.2279-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Di S, Malcher-Lopes R, Halmos KC, Tasker JG. Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: a fast feedback mechanism. J Neurosci. 2003;23:4850–7. doi: 10.1523/JNEUROSCI.23-12-04850.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.King AR, Duranti A, Tontini A, Rivara S, Rosengarth A, Clapper JR, et al. URB602 inhibits monoacylglycerol lipase and selectively blocks 2-arachidonoylglycerol degradation in intact brain slices. Chem Biol. 2007;14:1357–65. doi: 10.1016/j.chembiol.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wiskerke J, Irimia C, Cravatt BF, De Vries TJ, Schoffelmeer AN, Pattij T, Parsons LH. Characterization of the effects of reuptake and hydrolysis inhibition on interstitial endocannabinoid levels in the brain: an in vivo microdialysis study. ACS Chem Neurosci. 2012;3:407–17. doi: 10.1021/cn300036b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ogasawara D, Deng H, Viader A, Baggelaar MP, Breman A, den Dulk H, et al. Rapid and profound rewiring of brain lipid signaling networks by acute diacylglycerol lipase inhibition. Proc Natl Acad Sci USA. 2016;113:26–33. doi: 10.1073/pnas.1522364112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hill MN, Karatsoreos IN, Hillard CJ, McEwen BS. Rapid elevations in limbic endocannabinoid content by glucocorticoid hormones in vivo. Psychoneuroendocrinology. 2010;35:1333–8. doi: 10.1016/j.psyneuen.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Buczynski MW, Parsons LH. Quantification of brain endocannabinoid levels: methods, interpretations and pitfalls. Br J Pharmacol. 2010;160:423–42. doi: 10.1111/j.1476-5381.2010.00787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McFarland K, Lapish C, Kalivas P. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci Official J Soc Neurosci. 2003;23:3531–7. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stefanik MT, Kupchik YM, Kalivas PW. Optogenetic inhibition of cortical afferents in the nucleus accumbens simultaneously prevents cue-induced transient synaptic potentiation and cocaine-seeking behavior. Brain Struct Funct. 2016;221:1681–9. doi: 10.1007/s00429-015-0997-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McGlinchey EM, James MH, Mahler SV, Pantazis C, Aston-Jones G. Prelimbic to Accumbens Core Pathway Is Recruited in a Dopamine-Dependent Manner to Drive Cued Reinstatement of Cocaine Seeking. J Neurosci. 2016;36:8700–11. doi: 10.1523/JNEUROSCI.1291-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lubbers BR, van Mourik Y, Schetters D, Smit AB, De Vries TJ, Spijker S. Prefrontal gamma-aminobutyric acid type A receptor insertion controls cue-induced relapse to nicotine seeking. Biol Psychiatry. 2014;76:750–8. doi: 10.1016/j.biopsych.2014.02.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.