Abstract
We recently demonstrated that citrulline (CIT) reduced the expression of inflammatory genes in cultured explants from retroperitoneal (RET) white adipose tissue (WAT) from young (2–4 months) but not old (25 months) rats. Here we show that in RET WAT from old rats and high-fat-diet-fed (HFD) young rats, the basal expression of the leptin gene was increased (275–345%) whereas that of the adiponectin gene was decreased (48–60%), when compared to those from control-diet-fed (CD) young rats. We show also that in RET WAT from old rats, a diet supplemented with CIT for 3 months reduced macrophage (F4/80, CD68) and inflammation (interleukin-6, tumor necrosis factor-α) marker genes 23–97%. CIT supplementation lowered leptin mRNA 62% and increased adiponectin mRNA 232%. In cultured explants of RET WAT from 4 month-old CD, 4 month-old HFD and 25-month-old CD rats, the exposure to 2.5 mmol/L CIT for 24 h up-regulated adiponectin gene expression 151%, 362% and 216% respectively. In contrast, leptin gene expression was down-regulated 66% selectively in CIT-treated explants from 25-month-old CD rats. These results further support the proposed beneficial effect of CIT to counteract the deleterious effects of aging and overweight on the metabolic, inflammatory and endocrine functions of WAT.
Keywords: Adipose tissue, Citrulline, Adiponectin, Leptin, Aging, Obesity
Abbreviations: ASL, argininosuccinate lyase; ASS, argininosuccinate synthase; CD, control diet; CIT, citrulline; HFD, high-fat diet; IL, interleukin; INFγ, interferon gamma; NOS, nitric oxide synthase; NFκB, nuclear factor κ B; RET, retroperitoneal; TNF-α, tumor necrosis factor alpha; UCP1, uncoupling protein 1; WAT, white adipose tissue
Highlights
-
•
HFD and aging increase leptin mRNA and decrease adiponectin mRNA in rat adipose tissue.
-
•
In old rats a CIT diet reduces leptin mRNA and augments adiponectin mRNA.
-
•
In adipose tissue explants, CIT induces adiponectin mRNA whatever age and diet.
-
•
CIT exposure of adipose tissue explants reduces leptin mRNA selectively in old rats.
1. Introduction
Citrulline (CIT) has interesting nutritional benefits. In particular, a CIT-enriched diet administered to rats for 3 months induces an increase in muscle mass while decreasing fat mass [1], [2]. We showed recently that CIT acted directly in white adipose tissue (WAT) explants from young (2–4 month-old) rats whether fed a control (CD) or a high-fat (HFD) diet [1], [3]. Specifically, CIT induced lipolysis, beta-oxidation and the expression of uncoupling protein 1 (UCP1), while it reduced glyceroneogenesis and fatty acid re-esterification [3], [4]. In aged rats (25-month-old) CIT was also lipolytic and anti-glyceroneogenic but did not affect beta-oxidation and UCP1 [1], [4]. In addition to its metabolic effects, CIT also presented anti-inflammatory actions selectively in explants from HFD young rats [3].
Leptin and adiponectin play crucial roles in the regulation of inflammation [5], [6], [7]. Although WAT is heterogeneous, leptin and adiponectine are selectively produced in adipocytes [8], [9]. Leptin is also a pro-inflammatory cytokine that induces the expression of interferon gamma (INFγ), tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β) and interleukin-6 (IL-6), in immune cells such as macrophages, neutrophils and T cells [7], [10]. In contrast, adiponectin is an important anti-inflammatory adipokine that inhibits nuclear factor κB (NFκB) and is implicated in the switch of M1 macrophages towards M2 [11], [12]. The secretion of anti-inflammatory cytokines, including adiponectin, is associated with a lower level of adiposity, whereas the over expression of pro-inflammatory cytokines, like leptin, is linked to WAT mass expansion and frequently associated with insulin resistance, type 2 diabetes and atherosclerosis [9], [13], [14], [15].
Calorie restriction down-regulates WAT leptin levels in rats and in obese patients [16], [17], [18].
In contrast, plasma adiponectin levels are reduced in obese rodents and humans while calorie restriction up-regulates its expression [14], [16], [18], [19]. This hormone exerts protective effects against the development of diabetes, atherosclerosis or inflammation [20], [21].
During aging, an alteration of WAT distribution is also frequently observed in humans and rodents. This redistribution is commonly characterized by the loss of subcutaneous fat mass, whereas intra-abdominal fat is augmented [22], [23]. As seen in overweight or obesity, the synthesis and function of leptin and adiponectin are altered with age, in addition to the common occurrence of a pro-inflammatory status [1], [16]. Aging is also associated with a status of leptin resistance and/or with a decrease in leptin receptors [24], [25]. In contrast, blood adiponectin level declines with age in rats and humans and its rise correlates with an increase in lifespan and a decrease in the prevalence of many obesity-related diseases [16], [26], [27], [28].
The objective of this study was to evaluate the efficacy of CIT treatment on the expression of leptin, adiponectin and inflammatory marker genes in retroperitoneal (RET) WAT explants from CD-fed and HFD-fed young rats together with old rats.
2. Materials and methods
2.1. Animals
The protocols for in vivo and ex vivo studies on male Sprague Dawley rats was conducted as previously described and in agreement with the French Guidelines for the care and use of experimental animals [1], [2], [3]. The anthropometric and biological parameters of each group were described in our previous studies [1], [2], [3].
2.2. Culture of explants
About 200 mg of tissues were cut into fragments of approximately 20 mg and cultured in Krebs Ringer buffer saline (KREBS) medium as previously described [1], [3]. Explants were exposed to 2.5 mmol/L CIT for 24 h then frozen before further studies.
2.3. RNA extraction, cDNA synthesis and real-time RT-PCR
Total RNA was extracted from RET WAT with Rneasy lipid tissue mini kit from Qiagen. Methods of RNA Extraction, cDNA Synthesis, Real-time RT-PCR and primer sequences were identical to those previously described [1], [3], [4]. Sequences of the rat sense and antisense nucleotides for leptin and adiponectin were: Leptin: 5′-aggtggaggtgaactggarcggg -3′ 5′-ggcccacaaagtcctctcagcac3′; Adiponectin: 5′- gcactggcaagttctactgcaa 3′ 5′-gtaggtgaagagaacggccttgt-3′.
2.4. Statistical analysis
Data are presented as means ± SEM (n = 7). Each independent experiment was carried out in triplicate. Statistical analysis was carried out using the nonparametric Mann & Whitney U test for pairwise comparisons, which was applied due to the small number of experiments (n < 10). A value of P < 0.05 was considered as significant.
∗P < 0.05 vs. control; ∗∗P < 0.01 vs. control.
3. Results
3.1. In vivo effects of diet and age on the expression of leptin and adiponectin genes
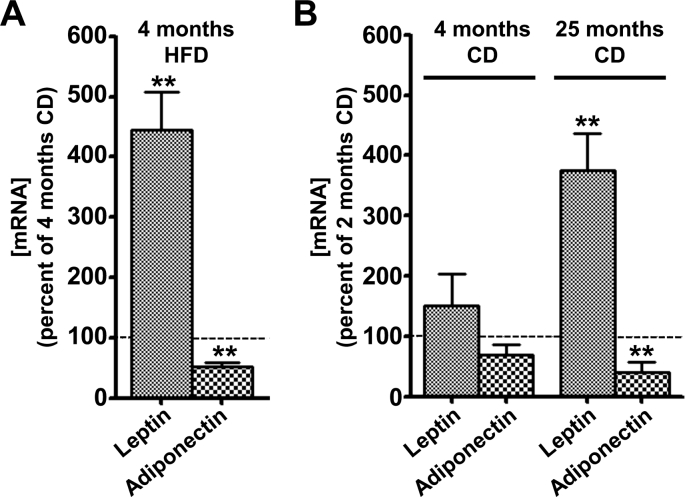
Overweight or obesity and aging induce changes in body composition with a dysregulation of leptin and adiponectine expression [9], [14]. We evaluated the effect of HFD and aging on the relative leptin and adiponectin gene expressions in RET WAT. HFD increased leptin gene expression 345% above control and decreased adiponectin mRNA 48% in comparison with 4-month-old CD animals taken as controls (Fig. 1A). Leptin and adiponectin mRNA levels were similar between 2-month-old and 4-month-old CD rats (Fig. 1B). In contrast, leptin mRNA was up-regulated 275% above control and adiponectin mRNA was down-regulated 60% in explants from 25-month-old CD rats (Fig. 1B).
Fig. 1.
In vivo effects of high-fat-diet feeding (A) and aging (B) on the expression of leptin and adiponectin genes in RET WAT from rats. RET WAT explants from 4-month-old CD, 4-month-old HFD and 25-month-old CD rats were cultured for 24 h as described in materials and methods. Leptin and adiponectin mRNA levels were evaluated by RT-qPCR. Results were normalized to RPL13 mRNA and expressed in % of control. Results represent the mean ± SEM of independent experiments (n = 7) carried out in triplicate from different explants. ∗P < 0.05 vs. Control; ∗∗P < 0.01 vs. Control. (A) Effects of diet on leptin and adiponectin gene expressions. Histograms represent the ratio of the values obtained from 4-month-old HFD rats to the values obtained from 4-month-old CD rats, taken as controls (100%). (B) Effects of age on leptin and adiponectin gene expressions. Histograms represent the ratio of the values obtained from 4-month-old CD or 25-month-old CD rats to the values obtained from 2-month-old CD rats, taken as controls (100%).
3.2. Effects of CIT on the expression of genes coding leptin, adiponectin and inflammatory markers
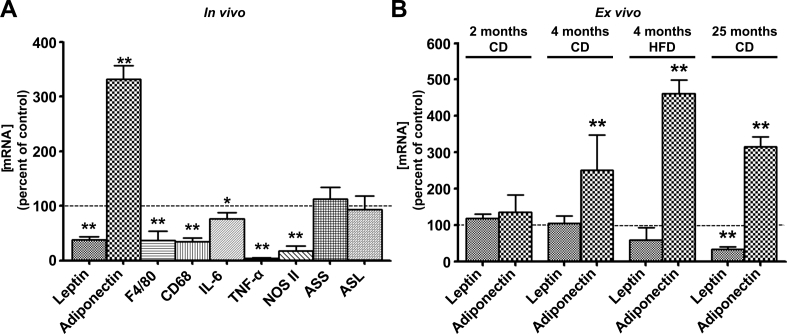
In RET WAT from old rats that were fed a CIT-supplemented diet for 3 months, leptin mRNA was down-regulated (62%) whereas adiponectin mRNA was up-regulated (232% above control) (Fig. 2A). This CIT-supplementation reduced significantly the expression of F4/80 (64%) and CD68 (66%) macrophage markers together with a decrease in that of inflammatory markers such as IL-6 (23%), TNF-α (97%) and NOS II (82%) (Fig. 2A). In contrast, ASS and ASL mRNA remained unaffected by CIT-supplementation (Fig. 2A).
Fig. 2.
Effects of CIT in vivo (A) or ex vivo (B) on the expression of leptin, adiponectin and inflammatory marker genes in RET WAT explants from rats. (A) Leptin, adiponectin, F4/80 Cd68, Il-6, TNF-α, NOS II, ASS and ASL mRNA levels in RET WAT explants from 25-month-old CD rats fed the CIT supplemented diet for 3 months were evaluated by RT-qPCR as described in materials and methods. Results were normalized to RPL13 mRNA and expressed in % of control. Control values (100%) were obtained from 25-month-old CD rats in the absence of CIT. Results represent the mean ± SEM of independent experiments (n = 7) carried out in triplicate from different explants. (B) Leptin and adiponectin mRNA levels in RET WAT explants from 2-month-old CD rats, 4-month-old CD rats, 4-month-old HFD rats and 25-month-old CD rats incubated without or with 2.5 mmol/L CIT, as indicated, for 24 h. mRNA levels were evaluated by RT-qPCR as described in materials and methods. Results were normalized to RPL13 mRNA and expressed in % of control. Control values (100%) correspond to those obtained in untreated explants from rats of the corresponding age. Results represent the mean ± SEM of independent experiments (n = 7) carried out in triplicate from different explants. ∗P < 0.05 vs. Control; ∗∗P < 0.01 vs. Control.
In cultured RET WAT explants from 2-month-old and 4-month-old rats under either CD or HFD, a 24 h treatment with 2.5 mmol/L CIT did not affect leptin gene expression (Fig. 2B). However, CIT diminished leptin mRNA 66% selectively in explants from 25-month-old rats (Fig. 2B). In contrast, adiponectin gene expression was up-regulated (151%, 362% and 216%) by CIT in RET WAT explants from 4-month-old CD or HFD rats and 25-month-old CD rats respectively, while it remained unaffected in explants from 2-month-old CD rats (Fig. 2B).
4. Discussion
We show that overweight and aging up-regulate leptin gene expression and reduce adiponectin gene expression in RET WAT from rats, in accordance with previous studies using epididymal WAT from rodents [16], [19], [29]. In addition, the expression of leptin, macrophage and inflammatory marker genes is down-regulated while adiponectin mRNA is up-regulated in RET WAT from 25-month-old rats fed a CIT-supplemented diet for 3 months. We show also that a 24 h treatment of RET WAT explants from 4-month-old CD or HFD rats and 25-month-old rats with 2.5 mmol/L CIT augments adiponectin mRNA. However, CIT diminishes leptin gene expression selectively in explants from 25-month-old rats.
Our previous results established that CIT induced direct, rapid and deep modifications of inflammation status and NEFA metabolism in RET WAT explants from rats, with different mechanisms depending on the status of the animals [1], [3]. Leptin exerts positive effect on NEFA metabolism, particularly on β-oxidation [30]. In RET WAT from 25-month-old rats, the decrease in leptin mRNA could explain why CIT cannot increase β-oxidation while such an increase is observed in 4-month-old HFD rats in link with the maintained level of leptin mRNA.
Obesity or overweight, and aging can be considered as similar situations when it comes to fat mass, since they share an augmentation of the ratio of fat to lean mass, more specifically in favor of visceral WAT [26]. In both cases, serum adiponectin level is decreased whereas inflammatory markers and leptin level are increased [5], [6], [7]. In our study, a CIT-supplemented diet administered to 25-month-old rats produced a reduction of F4/80 and CD68 mRNA, suggesting a decrease in macrophages in RET WAT. This is consistent with the CIT-induced down-regulation of IL-6, TNF-α and NOS II mRNA leading to a reversal of the pro-inflammatory status linked to aging. In previous studies the expression of leptin and adiponectin genes have not been always approached [5], [6], [7]. It can however be assumed that the level of expression of adiponectin and leptin genes would reflect the variations of protein levels observed in serum [19], [29]. In that context, CIT, whether given in vivo as food supplement or ex vivo on WAT explants, tends to normalize leptin and adiponectin gene expressions to values similar to those obtained from young lean rats. It tends also to attenuate inflammation, as we show here in vivo, in agreement with our previous ex vivo results [3].
Other in vivo studies showed that natural compound that can be taken as food supplements can have effects similar to those of CIT. For instance, arginine-supplemented diet in humans provokes an increase in plasma adiponectin and an anti-inflammatory effect [31], [32], [33]. A lycopene-supplemented HFD in young rats reduces leptin mRNA and protein in epididymal WAT and serum [29]. Like CIT, lycopene possesses anti-inflammatory and antioxidant properties [2], [29], [34]. Whether or not the antioxidant effect of CIT could be related to its action on WAT remains an opened question.
A low-grade inflammatory status was reported to be involved in the development of pathological disorders linked to overweight or obesity and to aging [35], [36]. These disorders are also associated with leptin resistance [24], [25]. The involvement of leptinemia and leptin receptors for potential leptin resistance in the present study remains to be determined. A link between low adiponectine levels and increased risk for the development of type 2 diabetes and atherosclerosis was observed in obese humans, compared to their leaner counterparts, whether adjustment was made or not with body mass index [21], [37]. In our study, the animals do not develop diabetes neither with aging nor HFD, as demonstrated by the lack of difference in insulinemia and glycemia [1], [3]. However, CIT treatment could prevent the deleterious metabolic effects linked to obesity and aging, that could develop on a longer-term basis.
5. Conclusion
Our results show that CIT has the capacity to restore levels of expression of adiponectin and leptin genes close to those found in healthy young rats. These observations, together with the previously described impact of this amino-acid on WAT metabolism, reinforce the idea that CIT exerts an important beneficial action for overweight and aged subjects. This natural compound could thus represent a new strategy to reduce WAT mass and the associated inflammation, in relation with obesity and aging.
Conflicts of interests
No conflict of interest.
Acknowledgements
We thank Dr. Brigitte Potier for providing us with a series of old rats. We acknowledge the Institut National de la Santé et de la Recherche Médicale and the Université Paris Descartes for their financial support.
References
- 1.Joffin N., Jaubert A.-M., Durant S., Bastin J., De Bandt J.-P., Cynober L. Citrulline induces fatty acid release selectively in visceral adipose tissue from old rats. Mol. Nutr. Food Res. 2014;58:1765–1775. doi: 10.1002/mnfr.201400053. [DOI] [PubMed] [Google Scholar]
- 2.Moinard C., Le Plénier S., Noirez P., Morio B., Bonnefont-Rousselot D., Kharchi C. Citrulline supplementation induces changes in body composition and limits metabolic alterations in healthy aged rats. J. Nutr. 27 May 2015 doi: 10.3945/jn.114.200626. pii:jn200626. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 3.Joffin N., Jaubert A.-M., Durant S., Bastin J., De Bandt J.-P., Cynober L. Citrulline reduces glyceroneogenesis and induces fatty acid release in visceral adipose tissue from overweight rats. Mol. Nutr. Food Res. 2014;58:2320–2330. doi: 10.1002/mnfr.201400507. [DOI] [PubMed] [Google Scholar]
- 4.Joffin N., Jaubert A.-M., Bamba J., Barouki R., Noirez P., Forest C. Acute induction of uncoupling protein 1 by citrulline in cultured explants of white adipose tissue from lean and high-fat-diet-fed rats. Adipocyte. 2015;4:129–134. doi: 10.4161/21623945.2014.989748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grunfeld C., Zhao C., Fuller J., Pollack A., Moser A., Friedman J. Endotoxin and cytokines induce expression of leptin, the ob gene product, in hamsters. J. Clin. Invest. 1996;97:2152–2157. doi: 10.1172/JCI118653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J. Allergy Clin. Immunol. 2005;115:911–919. doi: 10.1016/j.jaci.2005.02.023. quiz 920. [DOI] [PubMed] [Google Scholar]
- 7.Carbone F., La Rocca C., Matarese G. Immunological functions of leptin and adiponectin. Biochimie. 2012;94:2082–2088. doi: 10.1016/j.biochi.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 8.Scherer P.E., Williams S., Fogliano M., Baldini G., Lodish H.F. A novel serum protein similar to C1q, produced exclusively in adipocytes. J. Biol. Chem. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 9.Frühbeck G., Jebb S.A., Prentice A.M. Leptin: physiology and pathophysiology. Clin. Physiol. Oxf. Engl. 1998;18:399–419. doi: 10.1046/j.1365-2281.1998.00129.x. [DOI] [PubMed] [Google Scholar]
- 10.Zhao Y., Sun R., You L., Gao C., Tian Z. Expression of leptin receptors and response to leptin stimulation of human natural killer cell lines. Biochem. Biophys. Res. Commun. 2003;300:247–252. doi: 10.1016/s0006-291x(02)02838-3. [DOI] [PubMed] [Google Scholar]
- 11.Ouchi N., Kihara S., Arita Y., Okamoto Y., Maeda K., Kuriyama H. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation. 2000;102:1296–1301. doi: 10.1161/01.cir.102.11.1296. [DOI] [PubMed] [Google Scholar]
- 12.Ohashi K., Parker J.L., Ouchi N., Higuchi A., Vita J.A., Gokce N. Adiponectin promotes macrophage polarization toward an anti-inflammatory phenotype. J. Biol. Chem. 2010;285:6153–6160. doi: 10.1074/jbc.M109.088708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hotamisligil G.S., Arner P., Caro J.F., Atkinson R.L., Spiegelman B.M. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J. Clin. Invest. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arita Y., Kihara S., Ouchi N., Takahashi M., Maeda K., Miyagawa J. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem. Biophys. Res. Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 15.Myers M.G., Cowley M.A., Münzberg H. Mechanisms of leptin action and leptin resistance. Annu. Rev. Physiol. 2008;70:537–556. doi: 10.1146/annurev.physiol.70.113006.100707. [DOI] [PubMed] [Google Scholar]
- 16.Zhu M., Lee G.D., Ding L., Hu J., Qiu G., de Cabo R. Adipogenic signaling in rat white adipose tissue: modulation by aging and calorie restriction. Exp. Gerontol. 2007;42:733–744. doi: 10.1016/j.exger.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Luis D.A., Aller R., Izaola O., Sagrado M.G., Conde R., Bellido D. Influence of insulin resistance and adipocytokines on elevated serum alanine aminotransferase in obese patients. Arch. Med. Res. 2008;39:110–114. doi: 10.1016/j.arcmed.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 18.Bôas Huguenin G.V., Kimi Uehara S., Nogueira Netto J.F., Gaspar de Moura E., Rosa G., da Fonseca Passos M.C. Short term low-calorie diet improves insulin sensitivity and metabolic parameters in obese women. Nutr. Hosp. 2014;30:53–59. doi: 10.3305/nh.2014.30.1.7464. [DOI] [PubMed] [Google Scholar]
- 19.Combs T.P., Berg A.H., Rajala M.W., Klebanov S., Iyengar P., Jimenez-Chillaron J.C. Sexual differentiation, pregnancy, calorie restriction, and aging affect the adipocyte-specific secretory protein adiponectin. Diabetes. 2003;52:268–276. doi: 10.2337/diabetes.52.2.268. [DOI] [PubMed] [Google Scholar]
- 20.Okada-Iwabu M., Yamauchi T., Iwabu M., Honma T., Hamagami K., Matsuda K. A small-molecule AdipoR agonist for type 2 diabetes and short life in obesity. Nature. 2013;503:493–499. doi: 10.1038/nature12656. [DOI] [PubMed] [Google Scholar]
- 21.Wannamethee S.G., Lowe G.D.O., Rumley A., Cherry L., Whincup P.H., Sattar N. Adipokines and risk of type 2 diabetes in older men. Diabetes Care. 2007;30:1200–1205. doi: 10.2337/dc06-2416. [DOI] [PubMed] [Google Scholar]
- 22.Hughes V.A., Roubenoff R., Wood M., Frontera W.R., Evans W.J., Singh M.A.F. Anthropometric assessment of 10-y changes in body composition in the elderly. Am. J. Clin. Nutr. 2004;80:475–482. doi: 10.1093/ajcn/80.2.475. [DOI] [PubMed] [Google Scholar]
- 23.Escrivá F., Gavete M.L., Fermín Y., Pérez C., Gallardo N., Alvarez C. Effect of age and moderate food restriction on insulin sensitivity in Wistar rats: role of adiposity. J. Endocrinol. 2007;194:131–141. doi: 10.1677/joe.1.07043. [DOI] [PubMed] [Google Scholar]
- 24.Scarpace P.J., Matheny M., Moore R.L., Tümer N. Impaired leptin responsiveness in aged rats. Diabetes. 2000;49:431–435. doi: 10.2337/diabetes.49.3.431. [DOI] [PubMed] [Google Scholar]
- 25.Ryan A.S., Berman D.M., Nicklas B.J., Sinha M., Gingerich R.L., Meneilly G.S. Plasma adiponectin and leptin levels, body composition, and glucose utilization in adult women with wide ranges of age and obesity. Diabetes Care. 2003;26:2383–2388. doi: 10.2337/diacare.26.8.2383. [DOI] [PubMed] [Google Scholar]
- 26.Vilarrasa N., Vendrell J., Maravall J., Broch M., Estepa A., Megia A. Distribution and determinants of adiponectin, resistin and ghrelin in a randomly selected healthy population. Clin. Endocrinol. (Oxf.) 2005;63:329–335. doi: 10.1111/j.1365-2265.2005.02346.x. [DOI] [PubMed] [Google Scholar]
- 27.Arai Y., Takayama M., Abe Y., Hirose N. Adipokines and aging. J. Atheroscler. Thromb. 2011;18:545–550. doi: 10.5551/jat.7039. [DOI] [PubMed] [Google Scholar]
- 28.McKee Alderman J., DePetrillo M.A., Gluesenkamp A.M., Hartley A.C., Verhoff S.V., Zavodni K.L. Calorie restriction and dwarf mice in gerontological research. Gerontology. 2010;56:404–409. doi: 10.1159/000235720. [DOI] [PubMed] [Google Scholar]
- 29.Luvizotto R.deA.M., Nascimento A.F., Imaizumi E., Pierine D.T., Conde S.J., Correa C.R. Lycopene supplementation modulates plasma concentrations and epididymal adipose tissue mRNA of leptin, resistin and IL-6 in diet-induced obese rats. Br. J. Nutr. 2013;110:1803–1809. doi: 10.1017/S0007114513001256. [DOI] [PubMed] [Google Scholar]
- 30.Carter S., Caron A., Richard D., Picard F. Role of leptin resistance in the development of obesity in older patients. Clin. Interv. Aging. 2013;8:829–844. doi: 10.2147/CIA.S36367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bogdanski P., Suliburska J., Grabanska K., Musialik K., Cieslewicz A., Skoluda A. Effect of 3-month L-arginine supplementation on insulin resistance and tumor necrosis factor activity in patients with visceral obesity. Eur. Rev. Med. Pharmacol. Sci. 2012;16:816–823. [PubMed] [Google Scholar]
- 32.Lucotti P., Setola E., Monti L.D., Galluccio E., Costa S., Sandoli E.P. Beneficial effects of a long-term oral L-arginine treatment added to a hypocaloric diet and exercise training program in obese, insulin-resistant type 2 diabetic patients. Am. J. Physiol. Endocrinol. Metab. 2006;291:E906–E912. doi: 10.1152/ajpendo.00002.2006. [DOI] [PubMed] [Google Scholar]
- 33.De Luis D., Izaola O., De la Fuente B., Aller R. Effect of L-arginine supplmentation on insulin resistance and adipocitokines levels in head and neck cancer non diabetic patients after surgery. Nutr. Hosp. 2014;30:870–875. doi: 10.3305/nh.2014.30.4.7864. [DOI] [PubMed] [Google Scholar]
- 34.Akashi K., Miyake C., Yokota A. Citrulline, a novel compatible solute in drought-tolerant wild watermelon leaves, is an efficient hydroxyl radical scavenger. FEBS Lett. 2001;508:438–442. doi: 10.1016/s0014-5793(01)03123-4. [DOI] [PubMed] [Google Scholar]
- 35.Salminen A., Huuskonen J., Ojala J., Kauppinen A., Kaarniranta K., Suuronen T. Activation of innate immunity system during aging: NF-kB signaling is the molecular culprit of inflamm-aging. Ageing Res. Rev. 2008;7:83–105. doi: 10.1016/j.arr.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 36.Hotamisligil G.S., Shargill N.S., Spiegelman B.M. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 37.Ohashi K., Ouchi N., Matsuzawa Y. Anti-inflammatory and anti-atherogenic properties of adiponectin. Biochimie. 2012;94:2137–2142. doi: 10.1016/j.biochi.2012.06.008. [DOI] [PubMed] [Google Scholar]