Abstract
Objective:
Even though injectable hyaluronic acid (HA)–based fillers are considered safe, rare complications, such as late-onset inflammatory reactions have been reported. Possible causes and effective treatments have not been formally described, so this work aims to discuss these and offer a formal protocol for treatment.
Methods:
This article presents 5 clinical cases of late-onset inflammatory response occurring at least 3 months after uneventful injection of HA dermal filler.
Results:
Inflammation appeared spontaneously, usually 4–5 months after the last injection, but in 1 patient, almost 14 months later. One patient was injected at the same time with fillers manufactured by 2 different technologies. In this case, all areas treated with the same filler showed diffuse swelling of inflammatory nature, whereas the lips, treated with the second filler brand, remained unaffected. Four patients reported a flu-like illness or gastrointestinal upset a few days before the onset of dermal filler inflammation.
Conclusion:
Late-onset inflammatory reactions to HA fillers may be self-limiting but are easily and rapidly treatable with oral steroids, and with hyaluronidase in the case of lumps. It is likely these reactions are due to a Type IV delayed hypersensitivity response. Delayed inflammation associated with HA fillers is nonbrand specific. However, the case where 2 different brands were injected during the same session, but only 1 brand triggered a hypersensitivity reaction, suggests that the technology used in the manufacturing process, and the subsequent differing products of degradation, may have an influence on potential allergic reactions to HA fillers.
INTRODUCTION
Aesthetic procedures with hyaluronic acid (HA) dermal fillers have been rated the second most popular nonsurgical procedure.1 They are favored for their ease of administration and achievement of the desired aesthetic improvement.2 Despite the renowned safety of the procedure, rare adverse events have been reported in the literature.2–7
The author is an ophthalmologist and a laser eye surgeon with over 20 years of experience in HA use, and with over 5,000 cosmetic injection procedures. Over these years, only transient swelling and bruising during injection have been observed as procedure-related expected side effects. The objective of this article was to discuss late-onset inflammatory reactions by describing 5 clinical cases encountered over the past 14 years in the author’s practice.
Hypersensitivity reactions can be classified as acute or delayed, depending on the time of onset.8 Type I hypersensitivity reactions occur within minutes or hours after injections due to an immunoglobulin E (IgE)-mediated immune response to the dermal filler.6 They may manifest as angioedema or anaphylactic reactions occurring after initial or repeated exposure.2,6,9,10
Delayed hypersensitivity reactions are characterized by induration, erythema, and edema and are mediated by T lymphocytes rather than antibodies. They typically occur 48–72 hours after injection but may be seen as late as several weeks postinjection and may persist for many months.5,11 Late-onset reactions occur at least 3 months after uneventful injection of a dermal filler. Even though the etiology of delayed hypersensitivity in relation to HA fillers is not completely understood, suggested influencing factors include previous infections and trauma, as well as the injection technique (eg, filler volume, repeated treatments, and intramuscular implantation) and different properties of the filler.3,6,12
CASE 1
A 44-year-old female Asian patient presented with a diffuse swelling and tenderness without lumps 4 months after receiving 1.6 mL injection of Hydrafill Softline® (Inamed Aesthetics, Wicklow, Ireland) in the labiomental corners and nasolabial folds Table 1. The injection was performed in the author’s clinic with a needle, in a deep dermal/subdermal plane, with a retrograde linear thread technique. As the filler was lidocaine-free, a topical EMLA® cream was applied before the treatment.
Table 1.
Patient Data
The patient’s medical history included 7 injections of products from Juvéderm® (Allergan Inc., Pringy, France) and Hydrafill® ranges administered over the previous 3 years (total volume = 5.6 mL) for treatment of the same areas. No known allergies or history of autoimmune diseases were reported. Approximately 1 week before the reported reaction onset, the patient had suffered a flu-like illness.
The reaction began with redness and firm swelling at the corners of the mouth. The patient massaged the area in an attempt to resolve the symptoms. Within 24 hours, the swelling has spread to the inferior nasolabial folds and was characterized by redness, tenderness, and inflammation. The patient self-administered antihistamines (oral cetirizine hydrochloride, 10 mg) for 2 days without improvement. The reaction resolved completely in 1 week with a 5-day treatment with oral steroids (soluble Prednisolone®) in reducing doses of 60, 40, 20, 10, and 5 mg. Hyaluronidase was not required. At the time of this case, the use of hyaluronidase as a reversal agent for HA was not widely established in the United Kingdom.
CASE 2
A 48-year-old female Caucasian patient presented to her treating practitioner with a localized redness and swelling without lumps in the nasolabial folds 5 months after receiving 1 mL injection of Restylane® (Q-Med AB, Uppsala, Sweden) in the same area. The injection was performed in a different clinic with a retrograde linear thread technique Table 1. The filler was lidocaine-free and the use of additional anesthetics is unknown.
A couple of weeks before the reaction onset, the patient reported a cold sore on the lip, which had fully healed before the reaction. No further medical history, including any previous treatments with HA fillers and known allergies, is available.
The patient had been treated with oral antihistamines for 5 days without improvement. The treating practitioner called the author, who advised to prescribe a 5-day course of steroids, commencing at 60 mg, with reducing doses, and to schedule a review appointment. The injecting practitioner did not call the author back after 5 days, but subsequent confirmation of full resolution was obtained.
CASE 3
A 54-year-old female Caucasian patient presented with a diffuse, reddish, painful swelling without lumps 4 months after receiving injections of 2.4 mL of Teosyal® Puresense Ultra Deep (Teoxane S.A., Geneva, Switzerland) in the cheeks, chin, and marionette lines and 1 mL of Belotero® Intense (Anteis S.A, Geneva, Switzerland; a wholly owned subsidiary of Merz Pharmaceuticals GmbH) in the lips on the same day Table 1. The injections were performed in the author’s clinic. Teosyal® Puresense Ultra Deep was injected with the supplied needle and a 27G cannula supraperiosteally and in the deep dermis. Belotero® Intense was injected with the supplied needle by subdermal and submuscular retrograde linear thread technique. The products contained lidocaine, and no additional anesthetics were applied.
The patient’s medical history included a 1 mL injection of Teosyal® Puresense Deep Lines to the nasolabial folds in the preceding year. No known allergies or history of autoimmune diseases were reported. A few days before the reaction onset, the patient had experienced a gastrointestinal upset.
The patient is a health care professional, so she was able to describe her reactions correctly over a phone consultation with the author. The patient was unaware of the injected filler brands, but unprompted, reported swelling in all treated areas, except for the lips. The photographs of her reactions were unfortunately lost over time. The patient administered oral antihistamines for 7 days and reported a gradual improvement. She refused intake of steroids and reported full resolution of her symptoms over a 2-week period.
Two months later, the patient experienced a second gastrointestinal upset, and subsequently the same facial areas flared up with red, tender swelling, but again the lips remained unaffected. The patient reported that the second episode was not as severe as the first one, and she self-administered antihistamines for 7 days with slow recovery of her symptoms.
CASE 4
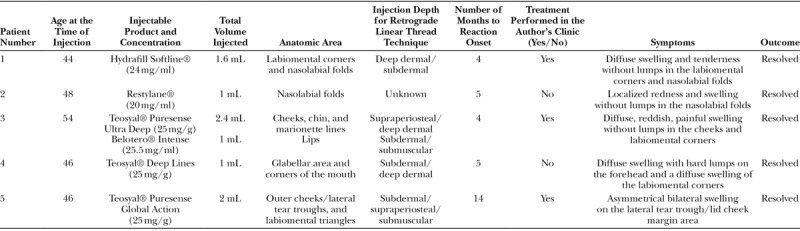
A 46-year-old female Caucasian patient presented with a diffuse swelling with hard lumps on the forehead and a diffuse swelling of the labiomental corners 5 months after receiving a 1 mL injections of Teosyal® Deep Lines in the glabellar area and corners of the mouth (Fig. 1) Table 1. The injection was performed in a different clinic, to subdermal/deep dermis with a retrograde linear thread technique. The filler was lidocaine-free.
Fig. 1.
Photographs of patient 4 (46 years old) taken at first presentation to the author after unsuccessful treatment with antihistamines (5 months after injection). A–D, immediate reaction onset; A, B, diffuse swelling with hard lumps on the forehead; C, D, diffuse swelling on the labiomental corners.
This was the patient’s first experience with HA injections. No known allergies were reported. During the week before the reaction onset, the patient had been abroad on holiday, where she had been systemically unwell and had suffered a gastrointestinal upset.
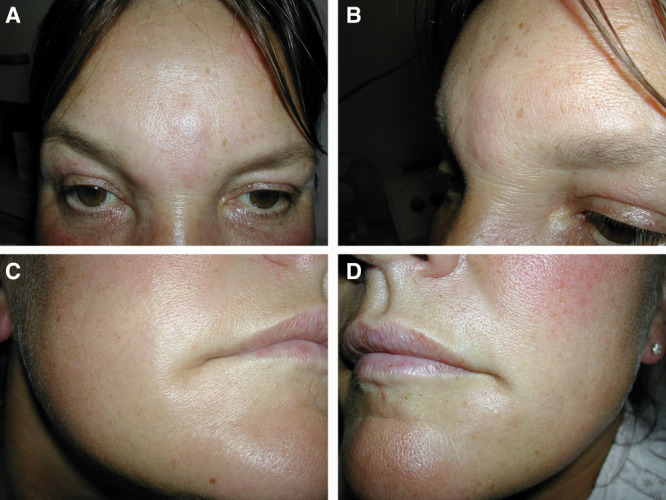
Before being referred to the author, the patient had taken oral antihistamines for 2 days without improvement. The author treated the affected areas with an injection of hyaluronidase (Hyalase®, Wockhardt UK Ltd.) 1,500 units in 1 mL, and the patient was given oral steroids (soluble Prednisolone®) for 5 days in reducing doses of 60, 40, 20, 10, and 5 mg. The erythema and swelling resolved, but a small palpable lump remained in the glabellar area, despite the overall improvement (Fig. 2). Three weeks following the first hyaluronidase administration, the patient was reviewed, and a further injection of Hyalase 1,500 units dissolved in 4 mL was injected into the residual glabellar lump. The patient subsequently called to report full resolution of her symptoms.
Fig. 2.
Full resolution at labiomental corners and improvement in the glabellar area following treatment with steroids and the first dose of hyaluronidase. A-D, Photographs of patient 4 taken at full resolution at labiomental corners and improvement in the glabellar area following treatment with steroids and the first dose of hyaluronidase.
CASE 5
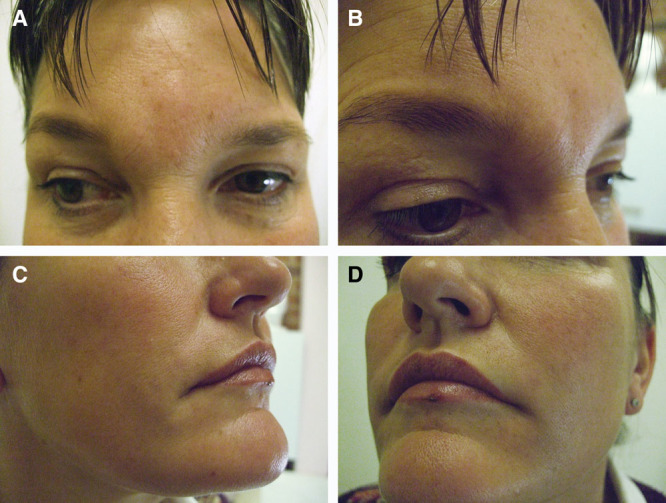
A 46-year-old female Caucasian patient presented with an asymmetrical bilateral swelling on the outer lid-cheek margins 14 months after receiving a 1 mL injection of Teosyal® Puresense Global Action in the outer cheeks and lateral tear troughs, as well as 1 mL of the same filler in the labiomental triangles (Fig. 3) Table 1. The injections were performed in the author’s clinic with subdermal retrograde linear technique with needle and cannula. A supraperiosteal bolus was deposited around the cheeks and the lid-cheek junction with a needle. A deep dermal bolus was deposited in the labiomental triangles with a cannula. The filler contained lidocaine, and no additional anesthetics were applied. The reaction was seen only in the lateral tear trough/lid cheek margin area; the labiomental triangles were unaffected.
Fig. 3.
Photographs of patient 5 (46 years old) taken at first presentation to the author after unsuccessful treatment with antihistamines (14 months after injection). A–C, immediate reaction onset. The larger swelling on the left lid-cheek margin, where the reaction is more pronounced due to a slightly larger injected volume, and the smaller swelling on the right.
The patient’s previous medical history included 6 injections with Juvéderm® 18, Succeev® One (Sanofi Aventis, Paris, France) and Teosyal® Puresense Global Action administered over the previous 7 years (total volume = 4.8 mL). The patient reported a long-term history of hay fever and atopy, without mentioning any prior systemic illness.
At the reaction onset, the patient did not relate the symptoms to the filler, and thus first contacted her general practitioner. Upon prescription, the patient administered oral antihistamines for a week without improvement. Then the patient presented to the author and was treated with oral steroids (soluble Prednisolone®) for 5 days in reducing doses of 60, 40, 20, 10, and 5 mg leading to full resolution of the symptoms (Fig. 4).
Fig. 4.
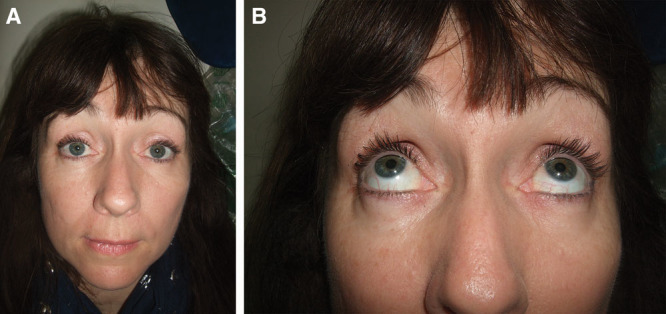
Full resolution following steroid intake. A, B, Photographs of patient 5 at full resolution following steroid intake.
DISCUSSION
Late-onset inflammatory reactions are rare complications, which may occur following injection of HA dermal fillers. Their cause may be infectious or immune-mediated in origin, and their outbreak can be triggered, for example, by a flu-like illness.2,3,5,6,13,14 Nevertheless, the latter events may be coincidental.
If an infection is suspected, steroids should not be prescribed.15 All presented cases are believed to be immunological in nature, specifically as all injected areas were affected simultaneously, except in cases 3 and 5 discussed below. In the event of infection, symptoms would be localized or restricted to a discrete area. Moreover, at presentation, the patients were systemically well and had been asymptomatic in the injection area for months between the last treatment and reaction onset. Even the associated lump reported in patient 4 was hard and nonfluctuant and atypical of infection.7 Therefore, there was no need for an empirical antibiotic treatment. Microbiological analysis for detection of a quiescent biofilm was not performed.
Delayed type IV hypersensitivity following HA implantation is the most likely explanation of the observed late-onset events. This rare systemic response is initiated by T lymphocytes and mediated by CD4+ cells. The reaction manifests as a persistent facial edema in the treated area, at times accompanied by inflammatory nodules.4,5,16 A foreign body granuloma can be suspected in patient 4 due to the presence of a nonfluctuant lump. Based on data from early 2000s, the rates of delayed hypersensitivity vary between 0.02% and 4%.17–19 As the number of fillers, performed procedures, and the associated complications have increased in the last decade,9 a newer estimate would be of interest.
Late-onset hypersensitivity may manifest from weeks to many months after HA injection. It is impossible to predict, and it may occur in both previously injected and first time patients. Several case reports have been published attempting to understand the etiology in relation to HA dermal fillers.12,13,17,19–32 Suggested influencing factors include previous infections and trauma, as well as the injection technique (eg, filler volume, repeated treatments, and intramuscular implantation) and different properties of the filler.3,6,12
Although severe adverse events may occur with any HA filler, the rates seem to vary among different products.22 The fillers cited in this report are characterized as nonimmunogenic, biocompatible, and nontoxic implants, composed of sodium hyaluronate from nonanimal origin cross-linked with 1,4-butanediol diglycidyl ether.33–37 The technology of the cross-linking varies among different manufacturers. Based on preclinical data, it is advisable not to modify the HA molecule to such a large extent that it would no longer be recognized as HA, and thus potentially lead to foreign body reactions.38,39 The limit of accepted modifications remains unknown. Among the cited fillers, Restylane® products have the lowest and Teosyal® the highest degree of modification.38 Additional filler-related factors suggested to influence inflammatory activities include presence of impurities from the cross-linking and biofermentation processes, higher concentration of HA, and characteristics of the filler particles (eg, size, surface, and charge).5,18,19,23,37,40 However, as the manufacturing procedures remain confidential, one can only speculate on the technology-related factors associated with filler complications.22
In almost all the described cases, the patients experienced a systemic illness 1–2 weeks before the reaction onset. The proposed hypothesis involves macrophages remembering stimulations such as a severe systemic infection, and their activation then triggering giant cell formation and foreign body granuloma.19,41,42 Beleznay et al.13 proposed a different mechanism, by which VYCROSS technology HA may contribute to the inflammatory activities and thus exhibit immunologic properties. The suggested mechanism involves release of proinflammatory low molecular weight HA fragments during an accelerated breakdown of HA gels, triggered by a systemic inflammatory response to an unknown antigen.13
In all true type IV granulomatous processes, all sites that were originally injected with filler material would be expected to be adversely affected simultaneously, as observed in this report.4 Patient 3 is a particularly interesting case because all sites injected with 1 brand were inflamed with the exception of the lips, which were injected with another brand. The fact that 2 different brands of filler were injected simultaneously in the same patient, but only 1 brand appears to have triggered a hypersensitivity response, suggests that the technology used in the manufacturing process of these brands can have an influence on possible side effects in the patient, even months after treatment. Published clinical data show that products from the second range of fillers (Belotero®) preserve the structural integrity of the surrounding tissues and have a favorable safety profile.43–45
In case of patient 5, inflammation was observed approximately 14 months postinjection of the lid-cheek margins, but the labiomental triangles were unaffected. Normally, by this time, it would be expected that the dermal filler would have degraded and been eliminated, but published literature suggests that fillers injected in the tear troughs do not degrade as quickly as in other areas.46,47 This evidence may explain why the delayed reactions were observed only in the 2 tear troughs, after the other areas were free of injected HA.
Delayed complications are particularly difficult to diagnose and treat due to the time lapse from the last procedure.24 As observed in the described cases, type IV hypersensitivity reactions are unresponsive to antihistamines.5 Steroids are required to alleviate the inflammatory signs.15,48 In case of patient 3, if steroids had been used when the first reaction occurred, the second flare-up might have been avoided. Type IV foreign body reactions are however generally self-limiting until the foreign body is destroyed.2,15,49 Therefore, patient 3 achieved slow and full recovery even without steroids and attributed it to the antihistamines. Hyaluronidase may be injected to remove the allergen in case of lumps,5,15,48,50 as shown in patient 4.
Inflamed areas should not be massaged, as done by patient 1. The patient felt the need to massage, as the reaction appeared as if the filler was freshly injected and needed to be dispersed. However, manipulation aggravated the condition, induced tenderness and increased the edema.
The main limitation of this article is the absence of histological analysis for detection of macrophages and thus confirmation of the granulomatous reaction to HA. Biopsies were not performed due to the patients’ desire to have minimally invasive resolutions to their symptoms as quickly as possible. As this is a retrospective data review, and 2 patients were referred to the clinic by different practitioners, the medical history is at times incomplete and exact doses of prescribed medication are also unknown. Only available nonstandardized patients’ photographs are presented in this article.
CONCLUSIONS
Late-onset inflammatory response occurs at least 2 months after HA injection, and presents as diffuse, firm, red, nonfluctuant inflammation of all areas containing the dermal filler. Patients are otherwise systemically well. Very late presentation, over a year after the last injection, can occur in some cases, depending on the location of the injected product and the speed of degradation in that area. Such reactions may occur with any HA dermal filler, but their incidence may vary depending on the manufacturing technology.
Prompt identification and correct treatment allow successful resolution of inflammatory symptoms within a few days. In the absence of lumps, the reactions may settle over time without intervention, but will need oral steroids in most cases for rapid and sustained improvement, and to reduce the risk of recurrence. Treatment of persistent lumps additionally requires injection of hyaluronidase for optimal resolution. Patients should be correctly informed of all possible rare adverse reactions before treatment to avoid fear, disappointment, or litigation and to ensure that they seek prompt and correct medical intervention when necessary.
ACKNOWLEDGMENTS
The author would like to thank Maria Kravtsov, MSc, for providing editorial assistance.
Footnotes
Disclosure: Dr. Bhojani-Lynch is currently a consultant for Merz Pharmaceuticals GmbH and Teoxane UK Limited. She has previously performed consulting services for Q-med AB and Allergan Inc. The Article Processing Charge was paid for by Merz Pharmaceuticals GmbH, Frankfurt am Main, Germany.
Supported by Merz Pharmaceuticals GmbH, Frankfurt am Main, Germany.
REFERENCES
- 1.Surgery ASoAP. Cosmetic surgery national data bank statistics. 2015. Available at http://www.surgery.org/media/statistics. Accessed July 27, 2015. [DOI] [PubMed]
- 2.Ozturk CN, Li Y, Tung R, et al. Complications following injection of soft-tissue fillers. Aesthet Surg J. 2013;33:862–877.. [DOI] [PubMed] [Google Scholar]
- 3.Alijotas-Reig J, Fernández-Figueras MT, Puig L. Inflammatory, immune-mediated adverse reactions related to soft tissue dermal fillers. Semin Arthritis Rheum. 2013;43:241–258.. [DOI] [PubMed] [Google Scholar]
- 4.DeLorenzi C. Complications of injectable fillers, part I. Aesthet Surg J. 2013;33:561–575.. [DOI] [PubMed] [Google Scholar]
- 5.Funt D, Pavicic T. Dermal fillers in aesthetics: an overview of adverse events and treatment approaches. Clin Cosmet Investig Dermatol. 2013;6:295–316.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Funt D, Pavicic T. Dermal fillers in aesthetics: an overview of adverse events and treatment approaches. Plast Surg Nurs. 2015;35:13–32.. [DOI] [PubMed] [Google Scholar]
- 7.Lowe NJ, Maxwell CA, Patnaik R. Adverse reactions to dermal fillers: review. Dermatol Surg. 2005;31:1616–1625.. [PubMed] [Google Scholar]
- 8.Rzany B, DeLorenzi C. Understanding, avoiding, and managing severe filler complications. Plast Reconstr Surg. 2015;136:196S–203S.. [DOI] [PubMed] [Google Scholar]
- 9.Alijotas-Reig J, Fernández-Figueras MT, Puig L. Late-onset inflammatory adverse reactions related to soft tissue filler injections. Clin Rev Allergy Immunol. 2013;45:97–108.. [DOI] [PubMed] [Google Scholar]
- 10.Dayan SH. Complications from toxins and fillers in the dermatology clinic: recognition, prevention, and treatment. Facial Plast Surg Clin North Am. 2013;21:663–673.. [DOI] [PubMed] [Google Scholar]
- 11.Arron ST, Neuhaus IM. Persistent delayed-type hypersensitivity reaction to injectable non-animal-stabilized hyaluronic acid. J Cosmet Dermatol. 2007;6:167–171.. [DOI] [PubMed] [Google Scholar]
- 12.Rongioletti F. Complications granulomateuses des techniques de comblement. Annales de Dermatologie et de Venerologie. 2008;135:59–65.. [DOI] [PubMed] [Google Scholar]
- 13.Beleznay K, Carruthers JD, Carruthers A, et al. Delayed-onset nodules secondary to a smooth cohesive 20 mg/mL hyaluronic acid filler: cause and management. Dermatol Surg. 2015;41:929–939.. [DOI] [PubMed] [Google Scholar]
- 14.Lemperle G, Gauthier-Hazan N. Foreign body granulomas after all injectable dermal fillers: part 2. Treatment options. Plast Reconstr Surg. 2009;123:1864–1873.. [DOI] [PubMed] [Google Scholar]
- 15.Signorini M, Liew S, Sundaram H, et al. ; Global Aesthetics Consensus Group. Global aesthetics consensus: avoidance and management of complications from hyaluronic acid fillers-evidence- and opinion-based review and consensus recommendations. Plast Reconstr Surg. 2016;137:961e–971e.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Shraim M, Jaragh M, Geddie W. Granulomatous reaction to injectable hyaluronic acid (Restylane) diagnosed by fine needle biopsy. J Clin Pathol. 2007;60:1060–1061.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.André P. Evaluation of the safety of a non-animal stabilized hyaluronic acid (NASHA – Q-Medical, Sweden) in European countries: a retrospective study from 1997 to 2001. J Eur Acad Dermatol Venereol. 2004;18:422–425.. [DOI] [PubMed] [Google Scholar]
- 18.Friedman PM, Mafong EA, Kauvar AN, et al. Safety data of injectable nonanimal stabilized hyaluronic acid gel for soft tissue augmentation. Dermatol Surg. 2002;28:491–494.. [DOI] [PubMed] [Google Scholar]
- 19.Lee JM, Kim YJ. Foreign body granulomas after the use of dermal fillers: pathophysiology, clinical appearance, histologic features, and treatment. Arch Plast Surg. 2015;42:232–239.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hatcher JL, Goldman ND. Recognition and treatment of non-infectious hyaluronic acid reactions. J Dermatolog Treat. 2014;25:513–515.. [DOI] [PubMed] [Google Scholar]
- 21.Mamelak AJ, Katz TM, Goldberg LH, et al. Foreign body reaction to hyaluronic acid filler injection: in search of an etiology. Dermatol Surg. 2009;35:1701–1703.. [DOI] [PubMed] [Google Scholar]
- 22.Artzi O, Loizides C, Verner I, et al. Resistant and recurrent late reaction to hyaluronic acid-based gel. Dermatol Surg. 2016;42:31–37.. [DOI] [PubMed] [Google Scholar]
- 23.Curi MM, Cardoso CL, Curra C, et al. Late-onset adverse reactions related to hyaluronic acid dermal filler for aesthetic soft tissue augmentation. J Craniofac Surg. 2015;26:782–784.. [DOI] [PubMed] [Google Scholar]
- 24.Tseng CHW, Chen AM, Chang JYF. Hyaluronic acid injection-induced delayed-onset foreign body granuloma. J Dental Sci. 2015;10:341–343.. [Google Scholar]
- 25.Ghislanzoni M, Bianchi F, Barbareschi M, et al. Cutaneous granulomatous reaction to injectable hyaluronic acid gel. Br J Dermatol. 2006;154:755–758.. [DOI] [PubMed] [Google Scholar]
- 26.Lupton JR, Alster TS. Cutaneous hypersensitivity reaction to injectable hyaluronic acid gel. Dermatol Surg. 2000;26:135–137.. [DOI] [PubMed] [Google Scholar]
- 27.O’Reilly P, Malhotra R. Delayed hypersensitivity reaction to Restylane ® SubQ. Orbit. 2011;30:54–57.. [DOI] [PubMed] [Google Scholar]
- 28.Bardazzi F, Ruffato A, Antonucci A, et al. Cutaneous granulomatous reaction to injectable hyaluronic acid gel: another case. J Dermatolog Treat. 2007;18:59–62.. [DOI] [PubMed] [Google Scholar]
- 29.Bitterman-Deutsch O, Kogan L, Nasser F. Delayed immune mediated adverse effects to hyaluronic acid fillers: report of five cases and review of the literature. Dermatol Reports. 2015;7:5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edwards PC, Fantasia JE, Iovino R. Foreign body reaction to hyaluronic acid (Restylane): an adverse outcome of lip augmentation. J Oral Maxillofac Surg. 2006;64:1296–1299.; discussion 1299. [DOI] [PubMed] [Google Scholar]
- 31.Sanchis-Bielsa JM, Bagán JV, Poveda R, et al. Foreign body granulomatous reactions to cosmetic fillers: a clinical study of 15 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:237–241.. [DOI] [PubMed] [Google Scholar]
- 32.Alijotas-Reig J, Garcia-Gimenez V. Delayed immune-mediated adverse effects related to hyaluronic acid and acrylic hydrogel dermal fillers: clinical findings, long-term follow-up and review of the literature. J Eur Acad Dermatol Venereol. 2008;22:150–161.. [DOI] [PubMed] [Google Scholar]
- 33.Hamilton RG, Strobos J, Adkinson NF., Jr. Immunogenicity studies of cosmetically administered nonanimal-stabilized hyaluronic acid particles. Dermatol Surg. 2007;33:S176–S185.. [DOI] [PubMed] [Google Scholar]
- 34.Sánchez O, Rodríguez-Sureda V, Domínguez C, et al. Study of biomaterial-induced macrophage activation, cell-mediated immune response and molecular oxidative damage in patients with dermal bioimplants. Immunobiology. 2012;217:44–53.. [DOI] [PubMed] [Google Scholar]
- 35.Larsen NE, Pollak CT, Reiner K, et al. Hylan gel biomaterial: dermal and immunologic compatibility. J Biomed Mater Res. 1993;27:1129–1134.. [DOI] [PubMed] [Google Scholar]
- 36.Richter AW, Ryde EM, Zetterström EO. Non-immunogenicity of a purified sodium hyaluronate preparation in man. Int Arch Allergy Appl Immunol. 1979;59:45–48.. [DOI] [PubMed] [Google Scholar]
- 37.Romagnoli M, Belmontesi M. Hyaluronic acid-based fillers: theory and practice. Clin Dermatol. 2008;26:123–159.. [DOI] [PubMed] [Google Scholar]
- 38.Edsman K, Nord LI, Ohrlund A, et al. Gel properties of hyaluronic acid dermal fillers. Dermatol Surg. 2012;38:1170–1179.. [DOI] [PubMed] [Google Scholar]
- 39.Tezel A, Fredrickson GH. The science of hyaluronic acid dermal fillers. J Cosmet Laser Ther. 2008;10:35–42.. [DOI] [PubMed] [Google Scholar]
- 40.Bentkover SH. The biology of facial fillers. Facial Plast Surg. 2009;25:73–85.. [DOI] [PubMed] [Google Scholar]
- 41.Lemperle G, Gauthier-Hazan N, Wolters M, et al. Foreign body granulomas after all injectable dermal fillers: part 1. Possible causes. Plast Reconstr Surg. 2009;123:1842–1863.. [DOI] [PubMed] [Google Scholar]
- 42.Lemperle G, Rullan PP, Gauthier-Hazan N. Avoiding and treating dermal filler complications. Plast Reconstr Surg. 2006;118:92S–107S.. [DOI] [PubMed] [Google Scholar]
- 43.Micheels P, Besse S, Flynn TC, et al. Superficial dermal injection of hyaluronic acid soft tissue fillers: comparative ultrasound study. Dermatol Surg. 2012;38:1162–1169.. [DOI] [PubMed] [Google Scholar]
- 44.Flynn TC, Sarazin D, Bezzola A, et al. Comparative histology of intradermal implantation of mono and biphasic hyaluronic acid fillers. Dermatol Surg. 2011;37:637–643.. [DOI] [PubMed] [Google Scholar]
- 45.Tran C, Carraux P, Micheels P, et al. In vivo bio-integration of three hyaluronic acid fillers in human skin: a histological study. Dermatology. 2014;228:47–54.. [DOI] [PubMed] [Google Scholar]
- 46.Lambros VS. Hyaluronic acid injections for correction of the tear trough deformity. Plast Reconstr Surg. 2007;120:74S–80S.. [DOI] [PubMed] [Google Scholar]
- 47.Dayan SH, Arkins JP, Somenek M. Restylane persisting in lower eyelids for 5 years. J Cosmet Dermatol. 2012;11:237–238.. [DOI] [PubMed] [Google Scholar]
- 48.Alijotas-Reig J, Fernández-Figueras MT, Puig L. Pseudocystic encapsulation: a late noninflammatory complication of hyaluronic acid filler injections. Dermatol Surg. 2013;39:1726–1728.. [DOI] [PubMed] [Google Scholar]
- 49.Adams DO. The granulomatous inflammatory response. A review. Am J Pathol. 1976;84:164–192.. [PMC free article] [PubMed] [Google Scholar]
- 50.Brody HJ. Use of hyaluronidase in the treatment of granulomatous hyaluronic acid reactions or unwanted hyaluronic acid misplacement. Dermatol Surg. 2005;31:893–897.. [DOI] [PubMed] [Google Scholar]


