Abstract
Background:
Acellular dermal matrices (ADMs) are an integral component of breast reconstruction. The ideal matrix would be relatively immuno-inert, allow rapid vascularization, and be affordable. The purpose of this study was to histologically compare 2 commonly used ADM products.
Methods:
This is a prospective histological study of 17 patients (20 breasts) following prosthetic-based breast reconstruction with ADM: Alloderm (LifeCell Corp, Branchburg, N.J.) or Cortiva (RTI Surgical, Alachua Fla.). Biopsies were taken from the dermal matrix and natural capsules surrounding the expander/implant during secondary surgery [Range, 72—694 days (mean, 217 days)]. Biopsy specimens were prepared via hematoxylin and eosin, Masson's trichrome, elastin, and transforming growth factor (TGF)-1 stains. Quantitative analysis of staining was performed with ImageJ software. The clinical outcome of each patient is analyzed in relation to capsule architecture and ADM performance.
Results:
There were 7 breasts in the Alloderm group and 13 in the Cortiva group. Both groups had similar demographic, aesthetic results, and complication profiles. The TGF-1 staining demonstrated significantly lower levels in the Cortiva capsules (P = 0.0139). The percentage of elastin and collagen are similar in the Cortiva, Alloderm, and natural peri-implant capsules. The native capsules show a significantly greater number of blood vessels when compared with Cortiva and Alloderm (P = 0.0371 and P = 0.0347, respectively); however, there is no difference in vascular pattern between the 2 dermal matrices.
Discussion:
Postoperatively, Cortiva demonstrates equal vascularity with less TGF-1 activation compared with Alloderm. The clinical success and complication profile were similar between the Alloderm and Cortiva patients.
INTRODUCTION
Tissue expanders or implants were used in an estimated 75% of the 109,000 breast reconstructions in 2016.1 The large majority of these cases also use acellular dermal matrices (ADMs). Tissue biologics have become an integral part of breast reconstruction, whether dual plane or prepectoral, due to their ease of use and relatively low complication profile. The benefits of ADM in reconstruction were originally defined as: (1) an improved contour of the inframammary fold and lateral breast margin; (2) an increase in intraoperative fill volumes; and (3) a lack of donor-site morbidity. In addition, the popularization of prepectoral breast reconstruction has only increased the use of these products.
ADMs are prepared in a variety of ways to remove cellular contents and improve sterility; however, issues unique to these products could potentially increase complications in breast reconstruction. Seroma continues to be a concern in prosthetic-based reconstruction either with or without ADM products. The addition of foreign bodies to the postinflammatory milieu from a mastectomy could have deleterious effects. Kim et al.2 reviewed the available data for the adjusted risk ratio of developing a seroma with ADM and found an increase of 1.28–6.21 fold.3 Infection, whether resulting from a seroma or overt from surgery, is reported to be 1.4 times higher with ADM use in a large national database review.4 Finally, most large studies have found a significant or near significant increase in reconstruction failure when adding ADM to the algorithm.5–7 Given the many benefits of using ADM in breast reconstruction, surgeons need to minimize their potential added complication through appropriate technique, patient selection, and product choice.
Surgeons now have a multitude of dermal matrix products from which to choose. The manufacturers tout unique harvest methods, differing processing techniques, and a variety of sterilization methods to differentiate products. Focus has been placed on minimal manipulation and fenestrations to decrease seroma rates as well as terminal sterility practices to reduce the potential for infection. Early incorporation with rapid vascularization is most likely to reduce these complications by limiting the chronic inflammatory response.8
The clinical similarities between Alloderm and Cortiva have previously been reported in a large series.9 The purpose of this study was to examine the architectural differences and histology between the same 2 commercially available dermal matrix products compared with the native capsule surrounding a device.
METHODS
This is a prospective cohort of 20 patients who underwent skin-sparing mastectomies followed by immediate prosthetic breast reconstruction with ADM. Patients were reconstructed between January 2014 and December 2015 at Emory University Hospital and Northside Hospital in Atlanta, Georgia, by 2 surgeons. The type of ADM placed during the initial operation was determined by institutional availability of product at the time of expander placement and included Cortiva ADM (RTI Surgical Inc.) initially (0.8–1.8 mm with a target of 1.3 mm), Cortiva 1 mm (range, 0.8–1.2 mm with a target of 1 mm) and Alloderm RTU (Lifecell Corp). Data were pooled from clinic notes, operative notes, pathology records, and the oncology summary. Minor complications were defined as those not requiring operative intervention, whereas major complications required a return trip to the operating room.
One patient had an implant placed immediately after completion of the oncologic extirpation. The dual-plane reconstruction was performed in the standard fashion as described previously. A single nonperforated piece of ADM measuring approximately 8 cm by 16 cm was hydrated appropriately and secured to the inframammary fold and lateral chest wall using 2-0 PROLENE (Ethicon, San Angelo, Tex.) interrupted sutures. No antibiotics were used in the irrigation for either ADM. The expander (when used) was then sutured to the chest wall, and the ADM and pectoralis muscle were sewn together over the device using a running 2-0 PROLENE suture. Expanders were filled with saline until the pocket was protuberant but not taught. A drain was placed between the subcutaneous tissue and tissue biologic, and the skin tacked with staples. A cutaneous angiogram was performed (Spy, Novadaq, Bonita Springs, Fla.) to assess the mastectomy skin viability with fluid volumes in the expander adjusted accordingly.10 Drains were removed in clinic based on the axiom of drainage less than 30 cc/d for successive days.
Secondary surgeries including exchange from the expanders to permanent implants were planned once completion of any adjuvant therapy and once the patient and surgeon were satisfied with the expansion volume. A portion of the original skin incision was opened and the capsule accessed via direct dissection. The expander was removed and weighed. One square centimeter of tissue was then excised sharply from the dermal matrix capsule inferiorly where there was no superficial muscle coverage. A second specimen was taken from the superior pole native capsule. Each piece of tissue was placed in 10% formalin for permanent sectioning. Capsulotomy was performed as needed and a permanent silicone gel implant inserted (Mentor LLC, Santa Barbara, Calif. and Allergan Inc., Irvine, Calif.).
Histology specimens were paraffin embedded and tangentially sectioned to create 10 µm sections.
Hematoxylin and eosin, Masson’s trichrome, and elastin staining was performed using standard laboratory protocols. Additional TGF-ß1 (profibrotic growth factor) immunohistochemistry stains were optimized and detection assured using control kidney specimens. Images were captured at 100× and 400× via light Microscopy (Carl Zeiss, Heidenheim, Germany) and quantified staining was measured with ImageJ software (National Institute of Health, Bethesda, Md.).
Data were queried with Microsoft Excel (Microsoft Corporation, Redmond, Wash.), and statistics were analyzed using StatPlus software (AnalystSoft, Alexandria, Va.). A 2-tailed Student’s t test set for a type I error of 5% (alpha = 0.05) was used to calculate significance between variables.
RESULTS
Seventeen patients were included in the analysis for a total of 20 breasts (14 unilateral and 3 bilateral). Breasts were categorized into 3 groups based upon the biopsy specimens as: (1) Native capsule (n = 15); (2) Cortiva capsule (n = 13), and (3) Alloderm capsule (n = 7). The native capsule group comprised patients from both the Cortiva and Alloderm group. The cohort has an average age of 62 years (range, 33–77) and BMI of 25.6 (range, 19.5–33.1). Of note, the average age of patients receiving Cortiva matrix placement was significantly younger than those that underwent Alloderm implantation (Table 1). Body mass index, smoking status, radiation therapy, and the use of neoadjuvent chemotherapy were similar among the 3 groups.
Table 1.
Different Variable Including Body Mass Index, Smoking Status Are Detailed in a Chart

Nineteen tissue expanders were used: five 133SV-T expanders (Allergan Inc., Irvine, Calif.), 14 DermaSpan Full Height expanders (Specialty Surgical Products, Victor, Mont.), and 1 Allergan SRM-350 silicone implant. Initially, the expanders were filled to an average volume of 261 cc (range, 50–500 cc). The implant placed was a 350 cc device. The Cortiva group was initially expanded 10% more than the Alloderm cohort; however, this was not significant (P = 0.588 and 0.539, respectively). Implant exchange occurred on average 7 months later (range, 72–694 days). There was no difference in sampling time between the 3 groups (P = 0.448, 0.436, and 0.809).
Two patients (2 breasts) experienced minor complications: 1 Grade III capsular contracture and 1 partial mastectomy skin loss. The capsular contracture developed 14 months postimplant exchange, and the patient did not receive radiation therapy or chemotherapy. This patient had undergone Alloderm placement with a tissue expander filled to 300 cc. In the patient with partial mastectomy, skin necrosis wounds were managed with serial clinic debridements and dressing changes. This patient had Cortiva placed atop an expander filled to 200 cc. She was treated with postoperative ionizing radiation. She has healed well and completed her reconstruction. The 1 major complication (1 breast) occurred in an obese female who had Alloderm placement and an expander filled to 500 cc intraoperative. Despite Spy angiography confirmation, she progressed to partial full-thickness skin loss. The left expander was removed, and the biopsy specimen for this patient was taken from the right side at a later reoperation.
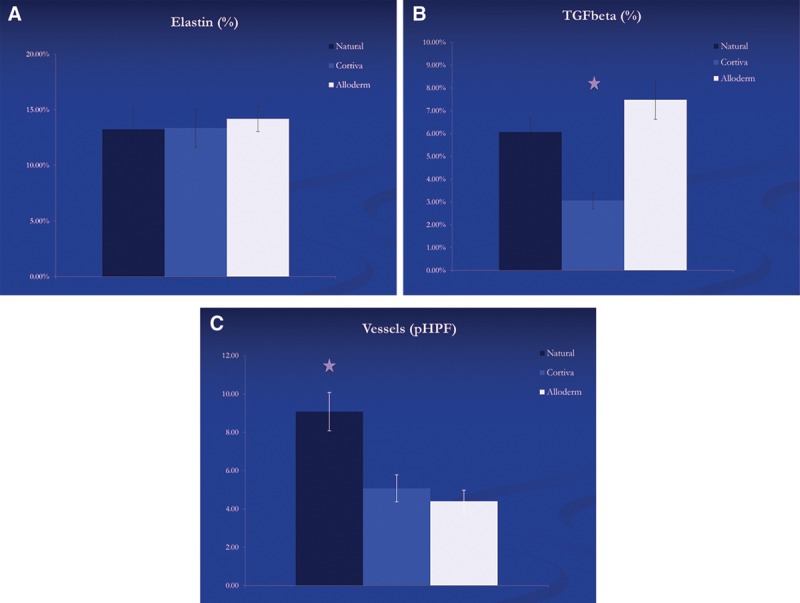
Histologic examination demonstrated little difference in cellular counts, collagen content, and elastin staining among the Alloderm, Cortiva, and native capsule tissues (Fig. 1). The Cortiva capsules were significantly thicker than both the Alloderm and native tissue specimens (Cortiva = 1.63 mm ± 0.50, Alloderm = 0.97 mm ± 0.17, Native = 0.96 mm ± 0.38; P = 0.0033 and 0.00010, respectively); however, the percentage of collagen in relation to total thickness was similar in all groups (Fig. 2). Elastin content was also similar in all specimens, and a majority of staining was noted in the inner, cellular layer, and around blood vessels in the outer, collagen capsule layer.
Fig. 1.
Histology specimens at 400× magnification showing native (1a-1c), Alloderm (1d-1f), and Cortiva (1h-1j) capsules. Samples were prepared with Mason’s trichrome (A) and elastin (B) and TGF-beta1 (C) immunohistologic stains.
Fig. 2.

Percentage of elastin (A), TGF-B (B), and number of blood vessels per high power field (C) for each capsule type.
The capsules that formed from Cortiva implantation showed significantly less transforming growth factor (TGF) β1 staining than both the Alloderm and native capsules (P = 0.0139 and 0.00501, respectively). The TGF staining was primarily centered on areas of focal inflammation, but we also noted staining around centers of neovascularization. However, the decreased TGF staining in the Cortiva cohort is independent of blood vessel formation.
At 400× magnification, specimens excised from the upper pole (native) demonstrated close to twice as many vessels within the capsule compared with the both dermal matrix products (P = 0.0347 for Cortiva and 0.0371 for Alloderm). New blood vessels were spread throughout the entire depth of the outer, collagen layer of the native capsules, whereas most new blood vessel formation was noted in the outer 50% of the collagen layer in the dermal matrix specimens, independent of manufacturer.
DISCUSSION
The perfect ADM would be inexpensive, immuno-inert, and immediately vascularize. Unfortunately, the latter 2 characteristics are mutually exclusive as neovascularization is dependent on a coordinated immune response. Thus, the ideal product would elicit a limited and highly ordered immune reaction leading to early vascularization and incorporation. A tempered immune response should limit seroma formation and decrease the potential of chronic inflammation leading to contracture. Several reports have described a decrease in inflammation around and within dermal matrix capsules compared with non-ADM capsules;11–13 however, these articles examine inflammation months after implantation and not in the initial period. This is likely why this fact has not translated into a reduced complication rate when using ADM.5 Clearly, the rate and amount of neovascularization also plays a predominate role in minimizing complications when ADM is used.
The different human ADM products are processed differently, which likely affects their incorporation and eventual histologic architecture. Alloderm is produced by LifeCell via a proprietary processing technique. The current product used in this study, labeled ready-to-use, is processed in compliance with the requirements of United States Pharmacopeia <71> Sterility tests and is terminally sterilized by electron beam radiation for a sterility assurance level of 10–3. The product is shipped hydrated, and the surgeon is instructed to rinse the matrix in sterile saline for 2 minutes to remove any residual packaging fluid. Cortiva is produced by RTI Surgical via the Tutoplast processing protocol. This technique has a proven 95% removal of donor DNA particulate. The tissue is handled in compliance with the requirements of United States Pharmacopeia <71> Sterility tests and is terminally sterilized by gamma irradiation for a sterility assurance level of 10–6. This product is shipped dehydrated and requires a 30-second hydration before implantation.
The Cortiva pieces used at the initiation of this were thicker; however, this did not result in increased complications in our series. The authors now use the thinner Cortiva 1 mm thickness pieces that have easy handling and are similar to Alloderm in terms of thickness. Although the complication profiles were similar between our groups, Rose et al.13 recently published data showing greater complications when using ADM greater than 1.2 mm in thickness. The patients reviewed experienced significantly more hematomas, seromas, and reoperations. The rationale proposed by the authors was the thicker material led to prolongation of the inflammatory milieu, delay to neovascularization, and ultimate impediment to incorporation.
TGF-ß1 is a regulatory cytokine known to induce periprosthetic fibrosis via the SMAD signaling pathway.2,15 The TGF-ß family of cytokines (TGF-ß1, TGF-ß2, and TGF-ß3) is released initially by platelets and macrophages to induce and home fibroblasts to a wound. As part of the normal response, TGF-ß increases the production of collagen and matrix proteins, activates fibroblasts to become myofibroblasts, and inhibits the secretion of proteases.16 Unfortunately, TGF-ß1 is profibrotic and appears to act as the “master switch” initiating the cascade leading to capsular contracture.17,18 In addition, the ß1 cytokine is also a known chronic inflammatory marker in skin conditions and wound healing models.2,8,19 The decrease in TGF-B1 staining in the Cortiva specimens at an average of 7 months postimplantation was surprising. One hypothesis for the decrease in this chronic inflammatory marker is the significantly lower amount of donor DNA found in Cortiva matrix compared with Alloderm (Fig. 2). The implantation of allogeneic DNA is known to mobilize both the antibody and cell-mediated anti-major histocompatability compex (MHC) immune response.20
Any dermal matrix is a foreign body until revascularized. Until this occurs, the product will incite an inflammatory reaction that can potentiate fluid exudate, host bacteria, and disrupt the healing of the mastectomy skin. The time to complete vascularization is important. Wong et al.20 implanted Alloderm underneath a superficial inferior epigastric flap in rats and studied the material at successive weeks. They found epithelial cells present at 7 days and both rudimentary blood vessels and presumptive lymphatic channels at 14 days.21 Lynch et al.21 analyzed the results of Alloderm inferior pole coverage versus dermal autograft coverage harvested from the patients abdomen. The authors report a lower incidence of major complications and delayed wound healing in the autograft group, and they contribute this in part to a 3-fold increase in the number of blood vessels per high power field in the autograft capsules.22 Unfortunately, there is a dearth of data directly comparing the rate of blood vessel formation for the available products. We cannot comment on the rate of blood vessel formation, but we can report a similar vascular pattern and number of vessels between capsules formed in Cortiva and Alloderm grafts at an average of 7 months. The majority of blood vessels were found at the outermost portion of the capsule signifying neovascularization originates from the mastectomy flap/matrix junction. This supports the findings of Degeorge et al.1 of a less uniform vascular pattern in ADM capsules.23 The native capsules had nearly twice as many blood vessels per high-powered field; however, it is important to note that these capsules form between the device and the pectoralis major muscle. This environment is likely more favorable for vascular in-growth as the muscle and fascia are less traumatized and innately more vascularized than the mastectomy flap.
The literature comparing different dermal matrix products is increasing.9,24–30 In this series, we report a similar complication profile between Cortiva and Alloderm-assisted breast reconstructions. This is in addition to and in correlation with our earlier clinical report comparing these 2 products. In that study of 298 breast reconstructions, there was no difference in complications between the products.9 The weakness of the current report is the small sample size and relatively low power, although the clinical results of this series closely resemble the previous, larger report. Additionally, the cohorts were closely matched, but the Cortiva group was significantly younger. Younger age has not been shown to be an independent predictor of morbidity; however, our previous article showed a trend (P = 0.093) toward a reduction in complications.
As a follow-up to our previous study, we now report similar histology architecture between implanted Cortiva and Alloderm at an average of 7 months, with the only difference being capsule thickness and a reduction in profibrotic staining in Cortiva specimens. It is important to understand the histological behavior of the various ADM products we choose as they are likely to directly impact complication profiles and outcomes.
CONCLUSIONS
At an average of 7 months postimplantation, Cortiva dermal matrix demonstrates equal vascularity with less TGF-β1 activation when compared directly with Alloderm. The clinical success and complication profile were similar between the Alloderm and Cortiva patients.
Footnotes
Disclosure: The authors have the following to disclose: Dr. Losken is a speaker for RTI. Dr. Moyer was paid a research stipend from RTI surgical to conduct this study. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.DeGeorge BR, Jr, Ning B, Salopek LS, et al. Advanced imaging techniques for investigation of acellular dermal matrix biointegration. Plast Reconstr Surg. 2017;139:395–405.. [DOI] [PubMed] [Google Scholar]
- 2.Kim S, Ahn M, Piao Y, et al. Effect of Botulinum toxin type A on TGF-β/Smad pathway signaling: implications for silicone-induced capsule formation. Plast Reconstr Surg. 2016;138:821e–829e.. [DOI] [PubMed] [Google Scholar]
- 3.Jordan SW, Khavanin N, Kim JY. Seroma in prosthetic breast reconstruction. Plast Reconstr Surg. 2016;137:1104–1116.. [DOI] [PubMed] [Google Scholar]
- 4.Winocour S, Martinez-Jorge J, Habermann E, et al. Early surgical site infection following tissue expander breast reconstruction with or without acellular dermal matrix: national benchmarking using National Surgical Quality Improvement Program. Arch Plast Surg. 2015;42:194–200.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim JY, Davila AA, Persing S, et al. A meta-analysis of human acellular dermis and submuscular tissue expander breast reconstruction. Plast Reconstr Surg. 2012;129:28–41.. [DOI] [PubMed] [Google Scholar]
- 6.Lee KT, Mun GH. Updated evidence of acellular dermal matrix use for implant-based breast reconstruction: a meta-analysis. Ann Surg Oncol. 2016;23:600–610.. [DOI] [PubMed] [Google Scholar]
- 7.Zhao X, Wu X, Dong J, et al. A meta-analysis of postoperative complications of tissue expander/implant breast reconstruction using acellular dermal matrix. Aesthetic Plast Surg. 2015;39:892–901.. [DOI] [PubMed] [Google Scholar]
- 8.Carruthers CA, Dearth CL, Reing JE, et al. Histologic characterization of acellular dermal matrices in a porcine model of tissue expander breast reconstruction. Tissue Eng Part A. 2015;21:35–44.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keifer OPJ, Page EK, Hart A, et al. A complication analysis of 2 acellular dermal matrices in prosthetic-based breast reconstruction. Plast Reconstr Surg. 2016;4:e800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Basu CB, Leong M, Hicks MJ. Acellular cadaveric dermis decreases the inflammatory response in capsule formation in reconstructive breast surgery. Plast Reconstr Surg. 2010;126:1842–1847.. [DOI] [PubMed] [Google Scholar]
- 11.Leong M, Basu CB, Hicks MJ. Further evidence that human acellular dermal matrix decreases inflammatory markers of capsule formation in implant-based breast reconstruction. Aesthet Surg J. 2015;35:40–47.. [DOI] [PubMed] [Google Scholar]
- 12.Moyer HR, Pinell-White X, Losken A. The effect of radiation on acellular dermal matrix and capsule formation in breast reconstruction: clinical outcomes and histologic analysis. Plast Reconstr Surg. 2014;133:214–221.. [DOI] [PubMed] [Google Scholar]
- 13.Rose JF, Zafar SN, Ellsworth Iv WA. Does acellular dermal matrix thickness affect complication rate in tissue expander based breast reconstruction? Plast Surg Int. 2016;2016:2867097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujio K, Komai T, Inoue M, et al. Revisiting the regulatory roles of the TGF-β family of cytokines. Autoimmun Rev. 2016;15:917–922.. [DOI] [PubMed] [Google Scholar]
- 15.Reinke JM, Sorg H. Wound repair and regeneration. Eur Surg Res. 2012;49:35–43.. [DOI] [PubMed] [Google Scholar]
- 16.Katzel EB, Koltz PF, Tierney R, et al. The impact of Smad3 loss of function on TGF-β signaling and radiation-induced capsular contracture. Plast Reconstr Surg. 2011;127:2263–2269.. [DOI] [PubMed] [Google Scholar]
- 17.Kuhn A, Singh S, Smith PD, et al. Periprosthetic breast capsules contain the fibrogenic cytokines TGF-beta1 and TGF-beta2, suggesting possible new treatment approaches. Ann Plast Surg. 2000;44:387–391.. [DOI] [PubMed] [Google Scholar]
- 18.Han G, Li F, Singh TP, et al. The pro-inflammatory role of TGFβ1: a paradox? Int J Biol Sci. 2012;8:228–235.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dela Cruz CS, Chamberlain JW, MacDonald KS, et al. Xenogeneic and allogeneic anti-MHC immune responses induced by plasmid DNA immunization. Vaccine. 1999;17:2479–2492.. [DOI] [PubMed] [Google Scholar]
- 20.Wong AK, Schonmeyr B, Schonmyer BH, et al. Histologic analysis of angiogenesis and lymphangiogenesis in acellular human dermis. Plast Reconstr Surg. 2008;121:1144–1152.. [DOI] [PubMed] [Google Scholar]
- 21.Lynch MP, Chung MT, Rinker BD. Dermal autografts as a substitute for acellular dermal matrices (ADM) in tissue expander breast reconstruction: a prospective comparative study. J Plast Reconstr Aesthet Surg. 2013;66:1534–1542.. [DOI] [PubMed] [Google Scholar]
- 22.DeGeorge BR, Jr, Olenczak JB, Cottler PS, et al. Evaluation of sidestream darkfield microscopy for real-time imaging acellular dermal matrix revascularization. Ann Plast Surg. 2016;76:S255–S259.. [DOI] [PubMed] [Google Scholar]
- 23.Becker S, Saint-Cyr M, Wong C, et al. AlloDerm versus DermaMatrix in immediate expander-based breast reconstruction: a preliminary comparison of complication profiles and material compliance. Plast Reconstr Surg. 2009;123:1–6.; discussion 107. [DOI] [PubMed] [Google Scholar]
- 24.Brooke S, Mesa J, Uluer M, et al. Complications in tissue expander breast reconstruction: a comparison of AlloDerm, DermaMatrix, and FlexHD acellular inferior pole dermal slings. Ann Plast Surg. 2012;69:347–349.. [DOI] [PubMed] [Google Scholar]
- 25.Chauviere MV, Schutter RJ, Steigelman MB, et al. Comparison of AlloDerm and AlloMax tissue incorporation in rats. Ann Plast Surg. 2014;73:282–285.. [DOI] [PubMed] [Google Scholar]
- 26.Cheng A, Saint-Cyr M. Comparison of different ADM materials in breast surgery. Clin Plast Surg. 2012;39:167–175.. [DOI] [PubMed] [Google Scholar]
- 27.Eichler C, Vogt N, Brunnert K, et al. A head-to-head comparison between SurgiMend and Epiflex in 127 breast reconstructions. Plast Reconstr Surg Glob Open. 2015;3:e439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pittman TA, Fan KL, Knapp A, et al. Comparison of different acellular dermal matrix (ADM) in breast reconstruction: the 50/50 study. Plast Reconstr Surg. 2016. [DOI] [PubMed] [Google Scholar]
- 29.Ricci JA, Treiser MD, Tao R, et al. Predictors of complications and comparison of outcomes using SurgiMend fetal bovine and AlloDerm human cadaveric acellular dermal matrices in implant-based breast reconstruction. Plast Reconstr Surg. 2016;138:583e–91e.. [DOI] [PubMed] [Google Scholar]
- 30.Zenn MR, Salzberg CA. A direct comparison of Alloderm-ready to use (RTU) and DermACELL in immediate breast implant reconstruction. Eplasty. 2016;16:e23. [PMC free article] [PubMed] [Google Scholar]


