Abstract
Background:
Wound healing problems at the donor site in free abdominal flap breast reconstruction cause significant morbidity to patients. No studies have investigated what impact the use of the internal mammary artery in free abdominal flap breast reconstruction has on abdominal skin perfusion. We hypothesized that harvesting the internal mammary vessels (IMV) has a negative effect on abdominal skin perfusion.
Methods:
The abdomen and anterior thoracic wall of 17 patients scheduled for secondary free abdominal flap breast reconstruction using IMV was pre-, intra-, and postoperatively examined with dynamic infrared thermography. Qualitative and quantitative analyses of the rate and pattern of recovery in Huger’s vascular zones were made with each patient being its own control. Zone III on the side where IMV were used was numbered zone IV. The contralateral zone III was used as reference.
Results:
Postoperative abdominal skin perfusion in zone IV was always significantly reduced compared with zone III (1-tailed t test, P < 0.05). The difference between zones II and III was statistically significant for day 1 and 3, but not for day 6 (2-tailed t test, P < 0.05). Skin perfusion in zones II and IV increased during consecutive postoperative days with an increase of hot spots in these areas.
Conclusions:
Using the IMV in free abdominal flap breast reconstruction had a significant effect on abdominal skin perfusion and may contribute to abdominal wound healing problems. The reperfusion of the abdominal skin was a dynamic process showing an increase in perfusion in the affected areas during the postoperative days.
INTRODUCTION
Breast reconstruction with a free abdominal flap can produce excellent aesthetic results with great patient satisfaction. However, unlike implant breast reconstruction, free abdominal flap breast reconstruction can cause complications at the recipient site and donor site. Mirzabeigi et al.1 reported a rate as high as 13.7% of delayed abdominal wound healing after free abdominal flap breast reconstruction. Although closure of these wounds usually can be obtained with local wound care, wound healing problems are often associated with longer hospital stays, intensive wound care, and more outpatient visits.1,2 Wound treatment not only causes psychological stress to a patient but is also an economic burden for health-care systems.2 Wound breakdown in itself has been associated with an increased risk for hernia formation.3
Adequate blood perfusion is crucial for normal wound healing. A better understanding of abdominal skin perfusion after free abdominal flap breast reconstruction may contribute to reduce wound healing problems at the donor site. In breast reconstruction with a free abdominal flap, either the internal mammary vessels (IMV) or the thoracodorsal vessels are selected as recipient vessels. Unlike the thoracodorsal vessels, the IMV are in direct continuity with the superior epigastric vessels and contribute to the perfusion of the epigastric arcade, which forms the dominant blood supply to the rectus abdominis muscles and the overlying skin.
Infrared thermography is a well-established, noninvasive technique that provides real-time information on skin surface temperature and skin blood perfusion. Studies have shown a good correlation between thermographic and laser Doppler volumetric results and also between thermographic results and perfusion monitored with indocyaninegreen videoangiography.4–7 Dynamic infrared thermography (DIRT) is based on the relationship between skin perfusion and the change in rate and pattern of skin surface temperature after a transient thermal challenge. The value of DIRT in monitoring flap perfusion and in preoperative perforator mapping for flap surgery has been reported in a number of studies.8–10
The purpose of this study was to evaluate what impact the use of IMV in free abdominal flap breast reconstruction has on abdominal skin perfusion as monitored with DIRT.
MATERIALS AND METHODS
This prospective, clinical study was approved by the regional ethical committee, and the principles outlined in the Declaration of Helsinki were followed. The inclusion criteria were patients scheduled for secondary unilateral free abdominal flap breast reconstructions who were nonsmokers or had stopped smoking at least 3 months before surgery and had no abdominal scar from previous surgery. Seventeen patients, mean age 51 years (range: 38–64) and mean body mass index 25.7 kg/m2 (range: 20.6–31.2), were included in the study. Of the 17 flaps, 9 were deep inferior epigastric perforator (DIEP) flaps and 8 were muscle-sparing transverse rectus abdominis musculocutaneous (ms-TRAM) flaps.
DIRT was performed on the day before surgery, intraoperatively, and on the first, third, and sixth postoperative day with all DIRT examinations performed in the same supine position. Using a specially designed camera stand, an infrared (IR) camera (FLIR ThermaCAM S65 HS FLIR Systems; FLIR Systems AB, Boston, Mass.) was positioned ca 1.0 m directly above the exposed anterior thorax and abdomen. This camera can produce sequences of high-definition digital IR images with an accuracy of 0.1°C. Thermal emissivity was set to 0.98, and the accuracy of the camera was regularly checked against a black body with a traceable temperature source (Model IR-2103/301; Infrared Systems Development Corp., Winter Park, Florida). IR images were taken at regular intervals to register the rate and pattern of skin rewarming for 3 minutes after a mild cold challenge. Images were electronically stored and afterward processed using image analysis software ThermaCAM Researcher Pro 2.8 SR-1 (FLIR Systems AB).
The pre- and postoperative images were taken in a dedicated laboratory (room temperature: 21–23°C) before and during rewarming following a cold challenge after an acclimatization period of 10 minutes with the thoracic and abdominal wall exposed. The thermal challenge was delivered by blowing air at room temperature over the skin surface for 2 minutes with a desktop fan. The intraoperative examination was performed with the patient in anesthesia just before and at the end of surgery. The thermal cold challenge was performed by washing the thorax and abdomen evenly for 1 minute with gauze soaked in saline at room temperature (22–23°C). The IMV were used as recipient vessels in all reconstructions. The abdomen was divided into vascular zones as defined by Huger but with modifications. The lateral zone III on the side where the IMV were harvested was numbered zone IV, and zones III and IV were subdivided into upper halves (subzones IIIA and IVA) and lower halves (subzones IIIB and IVB), respectively (Fig. 1). Zone III is the reference zone as this zone is not involved in surgery. Each case served as its own control.
Fig. 1.
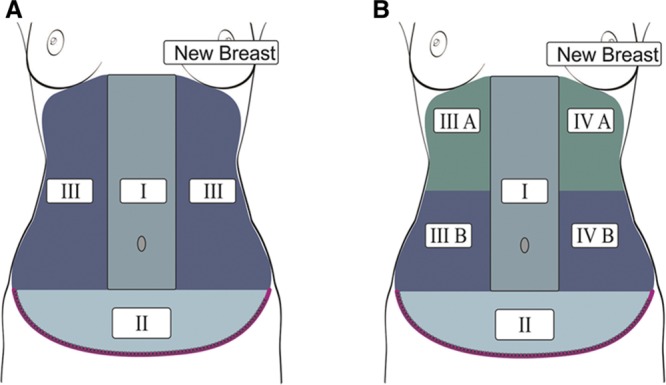
A, Illustration of Huger’s vascular zones of the abdominal wall. B, The authors’ modification of Huger’s vascular zones in which zone III on the side where the IMVs are harvested is numbered zone IV, and zones III and IV are subdivided into upper halves (subzones IIIA and IVA) and lower halves (subzones IIIB and IVB), respectively. Zone III is the reference zone.
The mean skin temperature was calculated for each zone in the postoperative phase before cooling, at end cooling, and at 1-, 2-, and 3-minute rewarming. A qualitative analysis of the changes of pattern and rate of rewarming of hot spots within each zone was made and compared with the results from the other zones. A 1-tailed t test for paired variables was used to see whether the mean temperatures of zones II and IV were lower compared with reference zone III. A 2-tailed t test of paired variables was used to see whether there was a statistically significant difference in mean zone temperatures between zone II and zone III at precooling, at end cooling, and at 1-, 2-, and 3-minute rewarming for postoperative days 1, 3, and 6 and also to see whether there was a statistically significant difference between the mean temperatures of all zones together at 3-minute rewarming after cooling. Statistical significance was defined as P < 0.05.
The study was conducted in compliance with the recognized international standards and the principles of the Declaration of Helsinki.
RESULTS
All flaps survived. Two patients had wound healing problems located at the center of the transverse suture line. These wounds healed with local wound care.
Qualitative Analysis
Qualitative analysis of the pre-, intra-, and postoperative DIRT examinations showed a large variability in distribution of hot spots between patients. In all patients, surgery caused a similar effect on abdominal skin perfusion. The pattern of hot spots at the end of surgery had become less clearly visible or had disappeared in zones I and II but also in subzone IVA caudal to the reconstructed breast. This effect was most pronounced in zone II and subzone IVA (Fig. 2). Postoperative examinations revealed a gradual increase in the number of hot spots in these zones. Hot spots in zone III and subzone IVB showed a hyperemic state with a more rapid rate of rewarming at the hot spots during the first postoperative day compared with the preoperative examinations. Both the hyperemia and rate of rewarming at the hot spots in zone III and subzone IVB decreased on days 3 and 6. During the same period, hot spots increased in number in zones I and II and subzone IVA, and the rate of rewarming at the appearing hot spots increased.
Fig. 2.
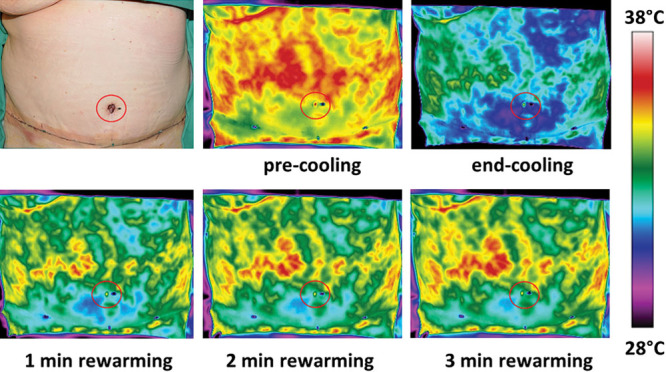
Day 1 postoperative photograph and DIRT images of the abdominal wall before and after a cold challenge. The thermal images during the rewarming show clearly the hypoperfused area in subzone IVA caudal to the reconstructed breast and in zone II (above the transverse suture line). Skin perfusion is clearly best on the patient’s right side (zone III). The red circle indicates the position of the navel.
Quantitative Analysis
In general, the quantitative results reflected the qualitative findings. A statistical significant difference in mean temperatures was found between zone III (reference zone) and zone II at end cooling and at 1-, 2-, and 3-minute rewarming on days 1 and 3, but not on day 6 with zone II being cooler. The precooling showed no statistically difference between zones III and II. Analyzes for the difference of mean temperatures between zones III and IV showed a statistical significant difference on days 1, 3, and 6 for end cooling and 1, 2, and 3 minutes with zone IV being cooler. An overview of the quantitative results for days 1, 3, and 6 is presented in Figures 3 and 6. Figure 4 shows how the mean skin temperature in zone III decreases during the postoperative days, whereas the mean skin temperature in zone II increases at the same time supporting the qualitative findings seen in zones II and III.
Fig. 3.
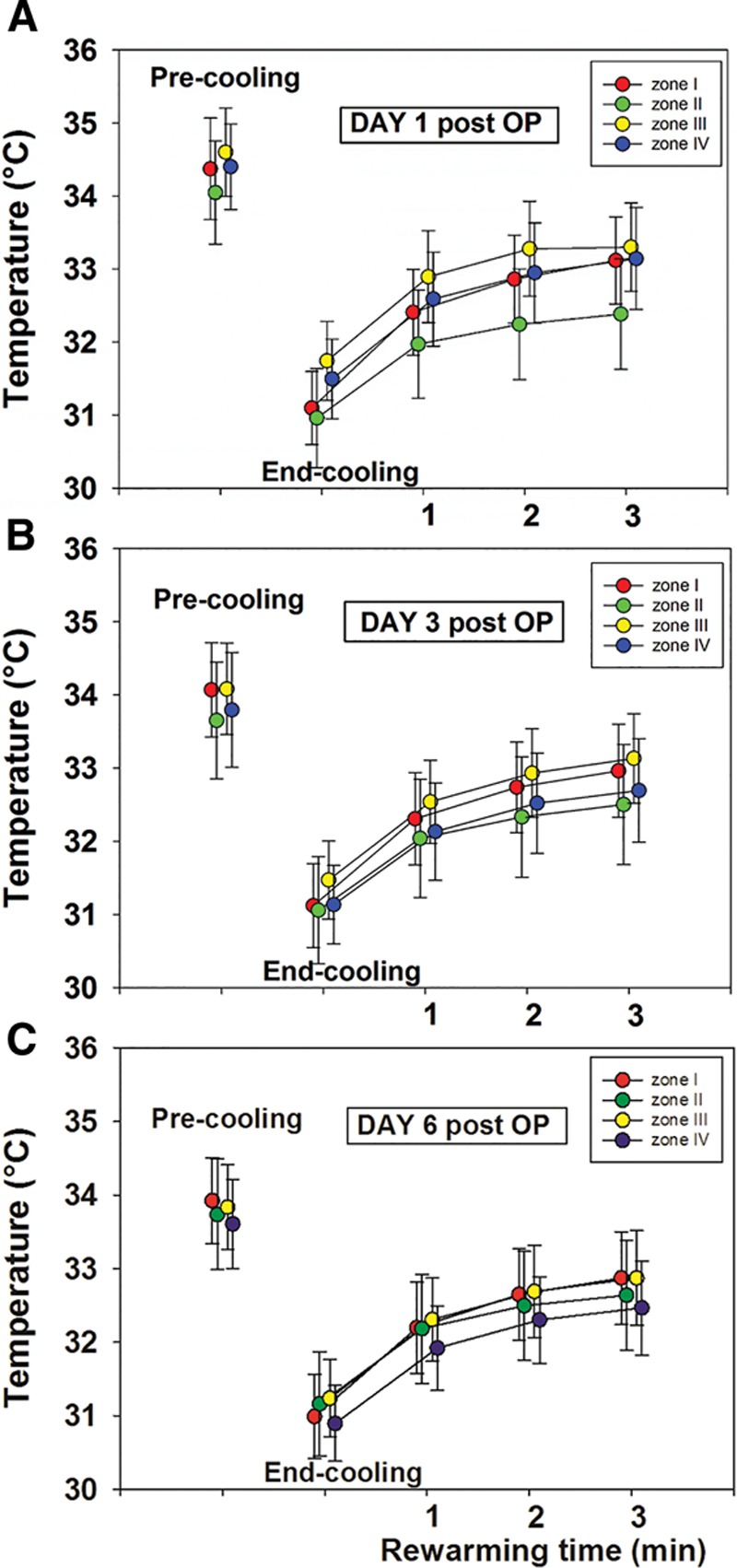
An overview of the mean skin temperatures of zones I–IV before, immediately after, and during a 3-minute rewarming after a mild cold challenge on postoperative day 1 (A), day 3 (B), and day 6 (C).
Fig. 6.
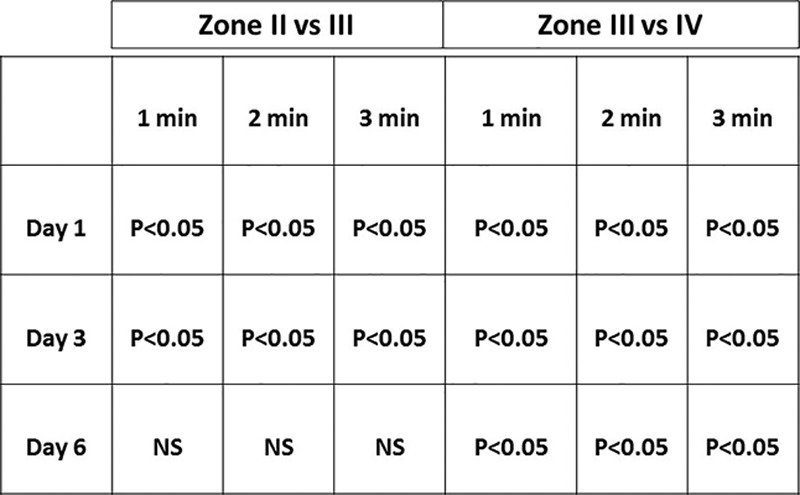
A statistical comparison (2-tailed t test for paired variables) between the mean skin temperatures of zones II and III and between the mean skin temperatures of zones III and IV at the first, second, and third minute during rewarming after a mild cold challenge on postoperative days 1, 3, and 6. NS, not statistically significantly different.
Fig. 4.
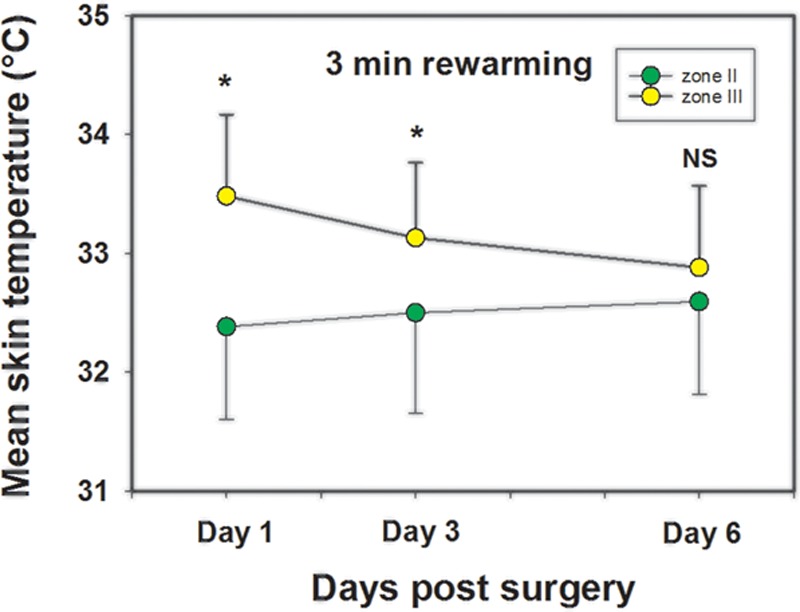
A comparison of the mean skin temperature of zones II and III 3 minutes after rewarming after a mild cold challenge on postoperative day 1, day 3, and day 6. The figure shows how the mean skin temperature in zone III settles down during the postoperative days, whereas the mean skin temperature in zone II increases at the same time supporting the qualitative findings seen in these two zones. Statistically significant differences indicated by *. NS, not statistically different.
The mean temperature of all zones together taken at 3-minute recovery decreased from day 1 to day 6. This difference was only statistically significant between day 1 and day 6 (Fig. 5).
Fig. 5.
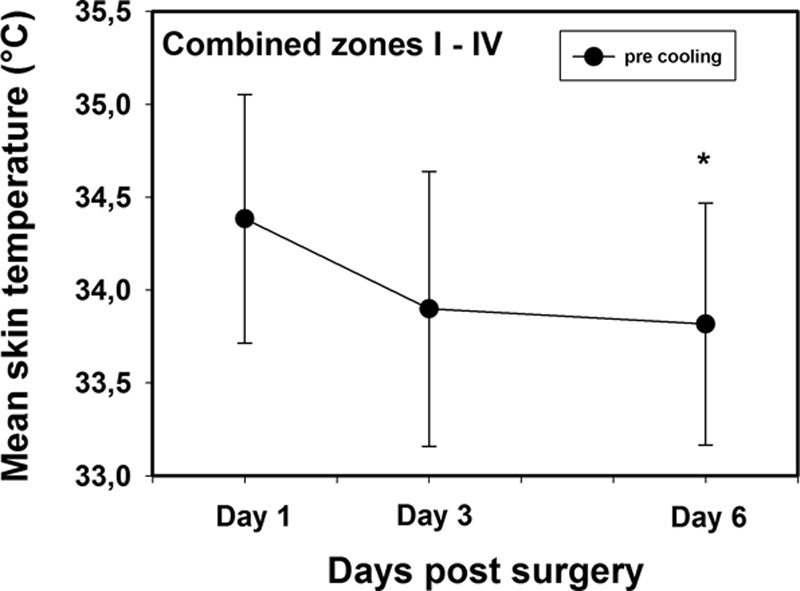
A comparison of the combined mean skin temperatures of all zones (I–IV) immediately before application of a mild cold challenge on postoperative day 1, day 3, and day 6. The * symbol indicates statistically significant difference between day 1 and day 6.
DISCUSSION
This study shows that free abdominal flap breast reconstruction using IMV causes considerable changes in abdominal skin perfusion during the first postoperative week. These changes in skin perfusion are best explained using Huger’s vascular zones and the angiosome theory.11,12 It is important to realize that although the anatomic landmarks for the division of the abdomen in Huger’s zones have not changed, closure as in an abdominoplasty results in an inferior-medial advancement of skin and subcutaneous tissue into Huger’s zones I and II.
The qualitative results of this study showed a dynamic change in the pattern of hot spots after surgery. The patterns of hot spots on the IR images of the abdominal skin have been previously described.9,10 Heat radiation from the skin is registered with the IR camera with a higher IR emission at the hot spots. These hot spots are associated with locations where perforators, transporting warm blood to the skin, are connecting with the subdermal plexus. Studies on the use of DIRT in the planning of perforator flaps have shown that bright hot spots are associated with arterial Doppler sounds and clearly visible perforators on computed tomography angiography (CTA).10,13 Chubb et al.10 showed how DIRT also can be used to identify the robustness of interconnections between perforators.
The IR images at the end of surgery showed that hot spots had disappeared in zones I and II but also in subzone IVA caudal to the reconstructed breast. Closure after flap harvest proceeds as in an abdominoplasty and includes undermining in zones I and II, and most perforators in these zones are severed and, as a result, do not produce hot spots. The zones I and II become hypoperfused compared with the preoperative DIRT and the reference zone III. This explanation cannot be used for the disappearance of hot spots in the area beneath the reconstructed breast (zone IVA).
Skin perfusion in the submammary area is normally not affected by an abdominoplasty as shown by Mayr et al.14 using intraoperative indocyaninegreen videoangiography. However, in contrast to a formal abdominoplasty, in free abdominal flap breast reconstruction using IMV, the internal mammary artery (IMA) angiosome is involved. Ingvaldsen et al.15 studied the microcirculatory abdominal skin circulation after DIEP breast reconstruction using laser Doppler perfusion imaging, but they did not evaluate the effect of the IMV on abdominal skin perfusion. A plausible explanation for the changes in zone IVA may be found in the angiosome theory.12 The IMA has its own angiosome and divides at the sixth intercostal space into the superior epigastric artery (SEA) and musculophrenic artery (MPA).16,17 In free abdominal flap breast reconstruction, the IMA is transected at the third or fourth intercostal space. As a result, blood perfusion to the IMA angiosome distal to this level and also to the angiosomes of the SEA and MPA becomes drastically reduced. Perforators no longer transport blood to the skin and, as a result, hot spots disappear and the zone becomes hypoperfused. The hypoperfusion at the end of surgery was most pronounced in the infraumbilical region of Huger’s zone II and in subzone IVA.
Interestingly, hot spots reappeared in zones I and II and subzone IVA during the postoperative period. This may again be explained using the angiosome theory. In zones III and IV, the angiosomes of the intercostal, subcostal, and lumbar arteries lie adjacent to the angiosomes of zones I and II, which are the angiosomes of the paired internal mammary-epigastric systems and the angiosomes of the superficial inferior epigastric and iliac circumflex arteries. Because of undermining of the abdominoplasty flap, perforators of the angiosomes incorporated in zones I and II are no longer perfused by their source vessels but depend for their perfusion on the source vessels of adjacent angiosomes. This situation is comparable to that seen in the delay phenomenon of flaps. Dhar and Taylor18 reported on the anatomic changes that occur at the level of the reduced-caliber choke vessels between adjacent vascular territories of a pedicled flap. The results from their animal study on the delay phenomenon showed an initial vasoconstriction, which lasted for up to 3 hours. Between 3 and 24 hours, the choke vessels returned to a diameter comparable to the control and, thereafter, underwent progressive sequential dilation that was most dramatic between 48 and 72 hours. Other studies support these findings.19,20 With the opening of choke vessels, the adjacent angiosomes become reperfused. Our results show a reappearance of hot spots in the angiosomes incorporated in Huger’s vascular zones I and II and subzone IVA during the postoperative period. Further support for the reperfusion of zones I and II and subzone IVA from the adjacent angiosomes can be found in the change in direction of the rewarming pattern seen with DIRT. The intercostal, subcostal, and lumbar arteries follow the nerves and their dermatomes. These dermatomes have a nearly transverse course. In an abdominoplasty, closure of the skin defect results in skin advancement in an inferior-medial direction. The direction in the postoperative rewarming pattern seen with DIRT changed compared with the preoperative DIRT. Postoperatively, rewarming started laterally and proceeded inferiorly and medially.
This dynamic process of reperfusion with reappearances of hot spots in angiosomes was also seen in a DIRT study on the postoperative reperfusion of deep inferior epigastric artery (DIEA) and superficial inferior epigastric artery (SIEA) flaps.21
The number of hot spots in zone III and subzone IVB did not change. However, hot spots in these zones showed a more rapid rewarming at the end of surgery and the first postoperative day creating a state of hyperemia compared with the preoperative DIRT. This hyperemia subsided during the following days.
The quantitative analysis showed that the mean temperature difference between zones III and IV was significant for days 1, 3, and 6, whereas the difference between zones III and II was only significant for days 1 and 3 (Fig. 6). The mean temperature of all zones combined indicated a hyperemia on day 1, which disappeared during the following days. Although the hyperemia in zone III decreased, zone II showed an increase in mean skin temperature during the postoperative period (Fig. 4). We anticipate that the changes in hyperemia are a result of a redistribution of blood flow after an increase in the diameter of choke vessel lumen in the subcutaneous tissue and skin.
The change in skin perfusion in zone II, particularly the hypoperfusion during the first 3 postsurgical days, is an interesting finding. Wound break down usually occurs in zone II near the center of the suture line and was seen in 2 of our patients.1–3 Although tension at the suture line after wound closure can be responsible for hypoperfusion, it is also reasonable to assume that it is caused by delayed adequate reperfusion due to the long distances to the adjacent angiosomes. The IMA angiosome on the side the IMV are harvested does not contribute to the reperfusion of zone II. In addition, the transverse incision line prevents vascular territories in the suprapubic area to contribute to the reperfusion of zone II across the suture line. The area of hypoperfusion corresponds also with the area of hyposensitivity on the abdomen after DIEP breast reconstruction as described by Tindholdt et al. and Visconti et al. which is in agreement with the finding of Taylor and Palmer that blood vessels are accompanied by nerves.12,22,23
In unilateral DIEP breast reconstruction, one can select either an ipsilateral or a contralateral pedicle. Skin reperfusion of the abdominoplasty flap relies on the reperfusion of the involved zones from adjacent lateral zones and on the IMV vessels that are not used as recipient vessels as there is no blood supply coming from both deep inferior epigastric arteries. In such, we anticipate that an ipsilateral and a contralateral pedicle will have the same impact on abdominal skin perfusion. However, in bilateral DIEP breast reconstruction, one could speculate that the risk for abdominal wound problems increases as reperfusion of the abdominoplasty flap relies then mainly on adjacent lateral angiosomes as the IMV and DIEA on both sides have been used. Johnson et al.24 reported abdominal wall necrosis after harvest of both IMAs and deep inferior epigastric arteries.
The changes seen in skin perfusion may also reflect what has occurred with the blood supply to the rectus abdominis muscle. This muscle has a type III pattern of circulation according to the classification of Mathes and Nahai and has the DIEA and SEA as its dominant pedicles for blood supply, whereas the subcostal and six or seven intercostal arteries are minor pedicles.25 Harvesting the IMV and deep inferior epigastric vessels on the same side in DIEP and ms-TRAM flap breast reconstruction reduces the blood supply to this muscle and its overlying fascia layer drastically. We postulate that abdominal wall bulging or hernia formation after DIEP and ms-TRAM flap breast reconstruction is a result of impaired wound healing at the fascia layer because of the loss of its dominant blood supply when using an ipsilateral pedicle. In bilateral DIEP breast reconstructions, in which both DIEAs and IMAs are used, Vyas et al. reported a significant risk for hernia and bulge formation and also other abdominal complications.26
In DIEP breast reconstructions, donor-site morbidity may be reduced by using the thoracodorsal vessels as recipient vessels or using an IMA preserving approach by doing end-to-side anastomosis or anastomosing to a perforator of the IMV. Such would preserve the perfusion of zone IVA.
The limitation of this prospective and clinical perfusion study is the use of a method that provides only indirect information on skin perfusion. Based on the results of other studies, providing scientific support for the use of skin temperature to measure skin perfusion, reliable information was obtained on dynamic changes that occurred in abdominal skin perfusion after free abdominal flap breast reconstruction using IMV as recipient vessels.4–7,27–29
This study provides for the first time scientific information on the impact free abdominal flap breast reconstruction using IMV has on abdominal skin perfusion. DIRT showed in vivo the reperfusion of the abdominoplasty flap over time as a dynamic process, quite similar to that seen in flap delay.
ACKNOWLEDGMENT
The authors wish to thank Mr. Knut Steinnes at the Department of Medical Physiology, Faculty of Health Sciences, University of Tromsø, for his assistance in drawing the figures.
Footnotes
Clinical trial registration in 2017, www.clinicaltrials.gov under NCT03057249.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the publication fund of UiT, The Arctic University of Norway.
REFERENCES
- 1.Mirzabeigi MN, Wilson AJ, Fischer JP, et al. Predicting and managing donor-site wound complications in abdominally based free flap breast reconstruction: improved outcomes with early reoperative closure. Plast Reconstr Surg. 2015;135:14–23.. [DOI] [PubMed] [Google Scholar]
- 2.Losken A, Carlson GW, Jones GE, et al. Importance of right subcostal incisions in patients undergoing TRAM flap breast reconstruction. Ann Plast Surg. 2002;49:115–119.. [DOI] [PubMed] [Google Scholar]
- 3.Cleveland EC, Fischer JP, Nelson JA, et al. Optimizing the fascial closure: an analysis of 1261 abdominally based free flap reconstructions. Ann Plast Surg. 2013;71:255–260.. [DOI] [PubMed] [Google Scholar]
- 4.Mercer JB, de Weerd L. The effect of water-filtered infrared (IR)-A (wIRA) irradiation on skin temperature and skin blood flow as evaluated by infrared thermography and scanning laser Doppler imaging. Thermol Int. 2005;15:89–94.. [Google Scholar]
- 5.Merla A, Di Romualdo S, Di Donato L, et al. Combined thermal and laser Doppler imaging in the assessment of cutaneous tissue perfusion. Conf Proc IEEE Eng Med Biol Soc. 2007;2007:2630–2633.. [DOI] [PubMed] [Google Scholar]
- 6.Miland ÅO, de Weerd L, Weum S, Mercer JB. Visualizing vascular perfusion in isolated human abdominal skin flaps using dynamic infrared thermography (DIRT) and Indocyanine green fluorescence videoangiogrphy (ICG-FA). Eur J Plast Surg. 2008;31:235–242.. [Google Scholar]
- 7.Pauling JD, Shipley JA, Raper S, et al. Comparison of infrared thermography and laser speckle contrast imaging for the dynamic assessment of digital microvascular function. Microvasc Res. 2012;83:162–167.. [DOI] [PubMed] [Google Scholar]
- 8.Zetterman E, Salmi A, Suominen S, et al. Effect of cooling and warming on thermographic imaging of the perforating vessels of the abdomen. Eur J Plast Surg. 1999;22:58–61.. [Google Scholar]
- 9.de Weerd L, Mercer JB, Weum S. Dynamic infrared thermography. Toolbox for autologous breast reconstruction. Clin Plast Surg. 2011;38:277–292.. [DOI] [PubMed] [Google Scholar]
- 10.Chubb DP, Taylor GI, Ashton MW. True and ‘choke’ anastomoses between perforator angiosomes: part II. Dynamic thermographic identification. Plast Reconstr Surg. 2013;132:1457–1464.. [DOI] [PubMed] [Google Scholar]
- 11.Huger WE., Jr. The anatomic rationale for abdominal lipectomy. Am Surg. 1979;45:612–617.. [PubMed] [Google Scholar]
- 12.Taylor GI, Palmer JH. The vascular territories (angiosomes) of the body: experimental study and clinical applications. Br J Plast Surg. 1987;40:113–141.. [DOI] [PubMed] [Google Scholar]
- 13.de Weerd L, Weum S, Mercer JB. The value of dynamic infrared thermography (DIRT) in perforator selection and planning of free DIEP flaps. Ann Plast Surg. 2009;63:274–279.. [DOI] [PubMed] [Google Scholar]
- 14.Mayr M, Holm C, Höfter E, et al. Effects of aesthetic abdominoplasty on abdominal wall perfusion: a quantitative evaluation. Plast Reconstr Surg. 2004;114:1586–1594.. [DOI] [PubMed] [Google Scholar]
- 15.Ingvaldsen CA, Tønseth KA, Pripp AH, et al. Microcirculatory evaluation of the abdominal skin in breast reconstruction with deep inferior epigastric artery perforator flap. Plast Reconstr Surg Glob Open 2016;4:e616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palmer JH, Taylor GI. The vascular territories of the anterior chest wall. Br J Plast Surg. 1986;39:287–299.. [DOI] [PubMed] [Google Scholar]
- 17.Paes EC, Schellekens PP, Hage JJ, et al. A cadaver study of the vascular territories of dominant and nondominant internal mammary artery perforators. Ann Plast Surg. 2011;67:68–72.. [DOI] [PubMed] [Google Scholar]
- 18.Dhar SC, Taylor GI. The delay phenomenon: the story unfolds. Plast Reconstr Surg. 1999;104:2079–2091.. [DOI] [PubMed] [Google Scholar]
- 19.Aydin MA, Mavili ME. Examining microcirculation improves the angiosome theory in explaining the delay phenomenon in a rabbit model. J Reconstr Microsurg. 2003;19:187–194.. [DOI] [PubMed] [Google Scholar]
- 20.Morris SF, Taylor GI. The time sequence of the delay phenomenon: when is a surgical delay effective? An experimental study. Plast Reconstr Surg. 1995;95:526–533.. [DOI] [PubMed] [Google Scholar]
- 21.de Weerd L, Miland AO, Mercer JB. Perfusion dynamics of free DIEP and SIEA flaps during the first postoperative week monitored with dynamic infrared thermography. Ann Plast Surg. 2009;62:42–47.. [DOI] [PubMed] [Google Scholar]
- 22.Tindholdt TT, Tønseth KA. Donor site sensitivity after breast reconstruction with deep inferior epigastric artery perforator flap. Ann Plast Surg. 2009;63:143–147.. [DOI] [PubMed] [Google Scholar]
- 23.Visconti G, Tomaselli F, Monda A, et al. Deep inferior epigastric artery perforator flap donor-site closure with cannula-assisted, limited undermining, and progressive high-tension sutures versus standard abdominoplasty: complications, sensitivity, and cosmetic outcomes. Plast Reconstr Surg. 2015;135:1–12.. [DOI] [PubMed] [Google Scholar]
- 24.Johnson DY, Johnson FE, Barner HB. Abdominal wall necrosis after harvest of both internal thoracic and inferior epigastric arteries. Ann Thorac Surg. 2011;91:38–41.. [DOI] [PubMed] [Google Scholar]
- 25.Mathes SJ, Nahai F. Classification of the vascular anatomy of muscles: experimental and clinical correlation. Plast Reconstr Surg. 1981;67:177–187.. [PubMed] [Google Scholar]
- 26.Vyas RM, Dickinson BP, Fastekjian JH, et al. Risk factors for abdominal donor-site morbidity in free flap breast reconstruction. Plast Reconstr Surg. 2008;121:1519–1526.. [DOI] [PubMed] [Google Scholar]
- 27.Ring FE, Jones BF. Diakides NA, Bronzino JD. The historical development of thermometry and thermal imaging in medicine. In: Medical Infrared Imaging. 2008:Boca Raton: CRC Press, Taylor & Francis Group; 2-1–2-5.. [Google Scholar]
- 28.Jiang LJ, Ng EY, Yeo AC, et al. A perspective on medical infrared imaging. J Med Eng Technol. 2005;29:257–267.. [DOI] [PubMed] [Google Scholar]
- 29.Jones BF. A reappraisal of the use of infrared thermal image analysis in medicine. IEEE Trans Med Imaging. 1998;17:1019–1027.. [DOI] [PubMed] [Google Scholar]


