Abstract
Background:
Congenital and acquired chest wall deformities represent a significant challenge to functional reconstruction and may impact feasibility of heart transplantation for patients with end-stage organ failure. In the recent past, the concept of replacing like-with-like tissue by using vascularized composite allografts (VCA) has been enthusiastically employed for reconstruction of complex tissue defects.
Methods:
In this study, we introduce a novel murine model for en bloc chest wall, heart, and thymus transplantation and thereby the use of complex tissue allografts for reconstruction of both chest wall defects and also end-stage organ failure. Additionally, this model allows us to study the features of combined vascularized bone marrow (VBM), thymus, and heart transplantation on allograft survival and function. Heterotopic chest wall, thymus, and heart transplants were performed in untreated syngeneic and allogeneic combinations and in allogeneic combinations treated with costimulation blockade (CTLA4-Ig and MR-1).
Results:
Indefinite (ie, 150 d, N = 3) graft survival was observed in syngeneic controls. In untreated recipients of allogeneic grafts, the skin component was rejected after 10 (±1) days, whereas rejection of the heart occurred after 13 (± 1) days (N = 3). Costimulation blockade treatment prolonged survival of the heart and chest wall component (130 d, N = 3) as well as the VBM niche as evidenced by donor-specific chimerism (average: 2.35 ± 1.44%), whereas interestingly, the skin component was rejected after 13 (±1) days.
Conclusion:
Thus, this novel microsurgical model of VCA combined with solid organ transplantation is technically feasible and results in split tolerance when treated with costimulatory blockade.
INTRODUCTION
Solid organ transplantation (SOT) is the gold standard for treatment of end-stage organ failure. However, patient and allograft survival hinges on the life-long use of pharmacological immunosuppression associated with significant side effects, such as increased risk of infection, malignancies, and also drug toxicities. In reconstructive transplantation, the use of vascularized composite allografts (VCA), such as hand and face, has become a viable alternative for reconstruction of complex tissue defects compared with conventional reconstructive means.1 Since the first successful hand transplantation in 1998, over 120 procedures2 have been performed with highly encouraging functional and aesthetic outcomes. As a nonlife saving procedure, however, in stark contrast to SOT, the need for life-long immunosuppression to maintain graft survival has hindered the wide-spread application of VCA. It is therefore of utmost importance, not only for reconstructive transplantation but also for transplantation medicine in general, to develop strategies for minimizing or avoiding immunosuppression altogether.
To date, the most promising clinical concepts for the induction of transplantation tolerance rely on the cotransplantation or coadministration of donor-derived bone marrow, which have been shown to prolong graft survival while decreasing the immunosuppression necessary for preventing chronic allograft rejection.3–7 In that regard, certain VCAs may present unique immunological features and advantages as they inherently contain donor-derived vascularized bone marrow (VBM), a continuous source of bone marrow progenitor cells. Combining SOT with VCA could therefore have a beneficial effect on tolerance induction and immunosuppression minimization in SOT. Furthermore, thymus-dependent mechanisms are also involved in the induction and maintenance of donor-specific tolerance and may be further exploited in the scenario of a thymus containing allograft.8,9
Combining VCA with heart transplantation may help address challenges in heart-transplanted patients in need for another transplant because preceding corrective surgeries often result in complex regional anatomy.10,11 Specifically in the pediatric setting, chest wall deformities and scar tissue encroaching on mediastinal volume may limit the possible size of a donor heart. Gottlieb et al.12 have designed a concept for an en bloc chest wall, thymus, and heart VCA in the cadaveric setting. Although this shows the feasibility of clinical application of such a procedure,13 we herein present the first mouse model for a heterotopic, en bloc chest wall, thymus, and heart transplantation. Using this murine model system, we investigate immunological features inherent to a bone marrow and thymus containing VCA combined with SOT.
MATERIALS AND METHODS
Animals
Male 8 to 10 week old Balb/C (H-2d), C57BL/6 (B6; H-2b) mice were purchased specific pathogen free from The Jackson Laboratories. Mice were housed at Johns Hopkins University, Baltimore, Md., in individually ventilated caging, 1–3 mice per cage, with autoclave sterilized caging and on Teklad corncob bedding and received Teklad 2018 diet and bottled water ad libitum. All procedures were approved by the Johns Hopkins Animal Care and Use Committee (ACUC) under protocol #MO13M490.
Experimental Design
To assess feasibility of the surgical model, en bloc chest wall, thymus, and heart transplants were first performed in syngeneic mice. Subsequently, fully H2-mismatched transplants were performed from Balb/c (donor) into C57BL6 (recipient) mice. Graft survival was assessed daily by visual inspection of skin and palpation of the heartbeat. Full rejection was defined as Grade 3 skin rejection with epidermolysis and focal necrosis and cessation of a palpable heart beat. Analysis of donor-derived chimerism was used to evaluate the viability of the donor bone marrow and chest wall component and also to measure donor bone marrow cell engraftment.
Surgical Technique
The surgery was performed using a technique described by Oh et al.14 (Fig. 1). After transplantation, skin survival was monitored daily. Equally, heart grafts were monitored by daily palpation.
Fig. 1.
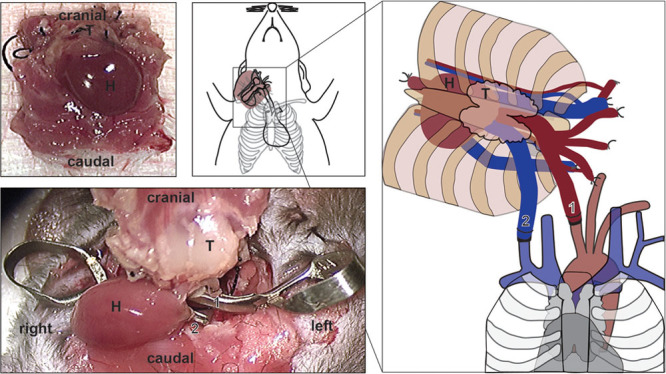
En bloc chest wall transplantation. Intrathoracic perspective of the VCA containing chest wall, thymus, and heart, which was obtained by dissection of the cervical region bilaterally to identify, ligate, and divide the external jugular veins. Next, the internal jugular veins and common carotid arteries were exposed bilaterally disected and divided. The animal was injected with heparin and IV Euro-collins solution injection for cardioplegia before opening the thoracic cavity. The descending aorta and the pulmonary trunk were cut. Pulmonary trunk and heart were flushed with 2 ml cold Euro-Collins. The inferior vena cava (IVC), confluence of pulmonary veins, and accessory branches of the superior vena cava were ligated and divided using a 6-0 silk suture. After dissecting attachments, chest wall, thymus, and heart were cauterized and completely liberated from the donor mouse (A). Schematic depiction of graft placement in the lateral right cervical aspect of the recipient mouse (B). Schematic depiction of graft inset and orientation using the nonsuture cuff technique anastomosing recipient common carotid artery with the distal aortic arch of the donor and the recipient internal jugular vein with the pulmonary truck of the donor heart (C, D). H indicates heart; T, thymus; 1, arterial anastomosis; 2, venous anastomosis.
Imaging
To obtain representative information on both graft position and graft component viability, micro-CT scans (Perkin Elmer IVIS Spectrum/CT optical imaging device, PerkinElmer, Waltham, WA) and MR-imaging (Bruker 11.7T system, Brunker, Belgium) were performed.
Treatment Protocol—Costimulation Blockade (CoB)
Hamster antimouse CD154 mAb (MR-1, BioXcell, Lebanon, N.H.) and CTLA4-Ig (Bristol-Myers Squibb, Princeton, N.J.) were administered on days 0, 2, 4, and 6 (500 µg/dose intra peritoneal) after transplantation.
Flow Cytometry Analysis of Mixed Chimerism and T Cell Receptor (TCR) Vβ TCR Families
Four-color flow cytometry was performed to distinguish donor from host mononuclear leukocytes on a FACS Calibur flow cytometer (BD Biosciences) and subsequently analyzed with FlowJo software. The percentage of donor cells circulating in host peripheral blood was calculated as described previously.15 Mouse peripheral blood was incubated after red blood cell (RBC) lysis with fluorescein isothiocyanate (FITC)-conjugated mAb directed against H-2d (SF1-1.1), PerCP-Cy5.5-conjugated antimouse CD3e (145-2C11), PE-conjugated B220 (RA3-6B2), and Alexa Flour 647-conjugated CD11b (M1/70) were used (BD Pharmingen, BioLegend and eBioscience) and non-Ag-specific FcγR-related binding blocker (2.4G2).
Histopathology
Euthanasia was performed by CO2 inhalation and then exsanguination and blood collection by cardiocentesis. Mice were perfused with heparinized saline, followed by 10% neutral buffered formalin, via the left ventricle of the host heart. The grafts were dissected en bloc to permit assessment of all graft components. Fixed tissues were processed routinely to paraffin in graded alcohols, sectioned at 5 u, and stained with hematoxylin and eosin (H&E). Specimens were assessed by a veterinary pathologist.
Statistical Analysis
Results are expressed as means. Survival curves were generated with Prism Software package (GraphPad Software, GraphPad, San Diego, Calif.), and Kaplan–Meier analysis was used to determine significance of differences in graft survival between groups.
RESULTS
Graft Survival
Syngeneic B6 grafts demonstrated long-term survival that exceeded 150 days (Figs. 2A, C), attesting to the technical feasibility of the model. In untreated allogeneic recipients, the skin component was rejected after an average of 10 ± 1 days, whereas rejection of the solid organ graft occurred after 13 ± 1 days (Fig. 2A). Clinically, this rejection profile is similar to previous results of hind limb allograft rejection in 4 distinct rejection stages—beginning with skin loss.16 In allogeneic recipients under costimulation blockade treatment, skin rejection was observed after 13 ± 1 days. However, we observed long-term survival of all other allograft components, exceeding 130 days (Fig. 2B).
Fig. 2.
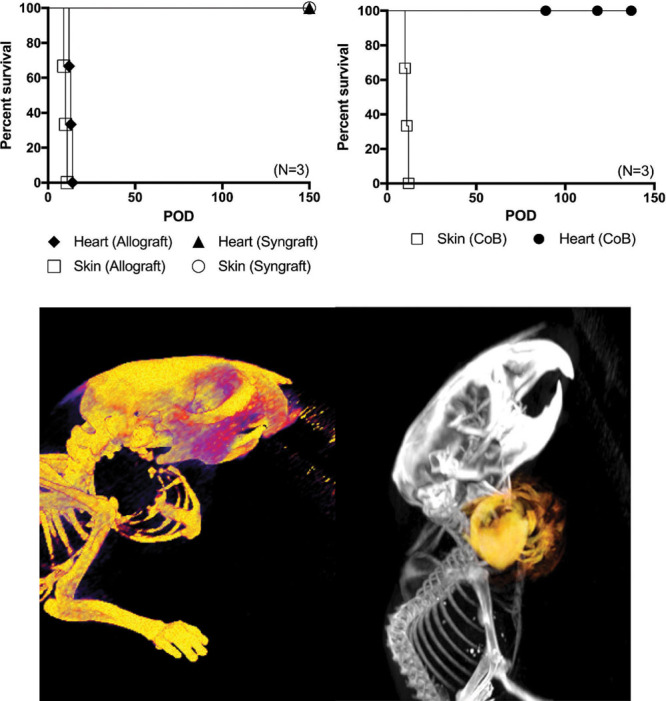
Kaplan–Meier survival curve. Syngeneic (N = 5) versus allogeneic (N = 3) en bloc chest wall, thymus, and heart VCA (A). Although syngeneic grafts show long-term survival of both skin and heart components clinically and histologically, rejection of fully H2-mismatched grafts appear early with skin component rejection at an average of 10 days and heart component at an average of 13 days after transplantation. Survival of en bloc chest wall, thymus, and heart allograft components using costimulation blockade (N = 3). Skin component rejection occurs as early as 9 days after transplantation (average: 10 ± 1 d), whereas survival of the heart component and other tissue components is prolonged with costimulation blockade (B). Micro-computed tomography (CT) image of the chest wall recipient from a lateral–dorsal perspective showing graft inset location at the lateral aspect of the right neck ([C], left). Overlay of micro-CT and magnetic resonance imaging (MRI) showing both contours of the chest wall and the heart as a VCA ([C], right).
Pathology
In the syngeneic graft recipient after >130 days, the graft tissues were viable clinically and perfused successfully. The graft heart features were similar to the host heart, thymus morphology was similar to the host, and marrow cellularity and hematopoiesis were similar to the host (Fig. 3). In the allogeneic graft recipient at >130 days, the graft tissues were viable clinically and perfused successfully. The graft heart showed inflammation and adhesions to adjacent tissues, graft thymus was identified, and there was hematopoiesis in graft bone marrow (Fig. 3).
Fig. 3.
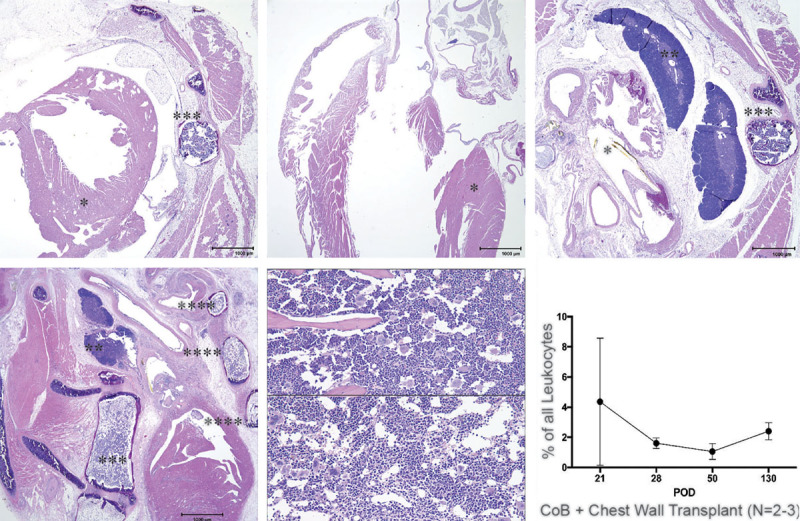
H&E histology and mixed chimerism. A–C, Graft and host tissue components in a syngeneic transplant combination (>130 d): syngeneic (B6) graft heart (*), cross-section including both ventricles, with associated body wall, including sternum with cellular hematopoietic bone marrow (***), attached muscles have (expected) myofiber loss (atrophy), and scattered mineralized fibers (A); host B6 heart (*) longitudinal (sagittal) section including right and left ventricles (B); graft heart base (rostral to A) polyethylene cuff tube- (yellow (*)), graft thymus (**) with distinct medulla and cortex (C), attached sternum (***) and musculature. (D–F) Graft (BALB/c) heart and host (B6) tissue components in an allogeneic CoB-treated transplant combination (>130 d): graft (BALB/c) heart with great vessels and cuff, attached graft sternum (****) and ribs (***) with hematopoietic bone marrow, graft thymus with distinct medulla and cortex (D); graft sternum bone marrow (E) and host sternum bone marrow (F); active hematopoiesis in both indicates viability of the VBM component. (G) Donor-specific chimerism is observed in peripheral blood mononuclear cells (>130 d) of C57BL6 recipients of a Balb/c allograft treated with CoB indicating viability of the VBM component of the chest wall allograft (data obtained from 2 to 3 animals).
Chimerism
Using flow cytometry analysis, donor-specific multilineage mixed chimerism was observed in peripheral blood mononuclear cells of long-term survivors (average: 2.35 ± 1.44%, range: 1.0–4.36%) (3G) indicating a viable donor bone marrow compartment after costimulation blockade–based treatment. Although levels of donor-type leukocytes diminished with time in some animals, their presence persisted in all animals for the duration of the analysis.
DISCUSSION
Animal models have provided valuable insights into transplant immunology research. Multiple murine transplant models for either organ transplantation or VCA are available.16,17 In contrast, models are needed for an investigation of combined SOT and VCA. The major advantage of the en bloc chest wall, thymus, and heart transplant murine model is that it includes both types of transplant and also a thymic component that has the potential to support T-cell development. Heterotopic heart transplantation to the neck, using a nonsuture cuff technique, is a well-established procedure in mice. To study VBM transplantation, Santiago et al.18 developed a heterotopic sternal transplantation model in rats. In 2013, Bozkurt et al. extended the heterotopic sternal transplantation model to include thymus and the entire osteomyocutaneous portion of the chest wall. Of note, Bozkurt et al.19 did not include a solid organ component into their allograft. In line with our results, Bozkurt et al. concluded that donor-derived thymus, transplanted with donor-derived bone marrow, is beneficial to induction and maintenance of chimerism in a heterotopic sternal transplant setting. Similarly, a superior outcome of heart allografts was shown in a swine model when the thymus was cotransplanted.5 The long-term viability of hematopoietic allograft components and thus continuous hematopoietic output could provide the basis for strategies with the potential to reduce maintenance immunosuppression needed to sustain allograft survival. To date, no in vivo studies have reported combined solid organ, thymus, and vascularized bone marrow transplantation. Despite being technically challenging, the model presented in this study has applications for in vivo studies of immunological properties of combined SOT and VCA.
Evident from the robust allograft rejection in untreated recipients in our study, the presence of thymus and VBM alone does not prolong survival. Interestingly, CoB treatment extended heart allograft survival beyond 130 days, whereas rejection of the skin component occurred after an average of 13 days (Fig. 2B). This is reminiscent of split tolerance, the acceptance of one donor-derived tissue while rejecting another one from the same donor.20 One explanation for the failure of CoB to induce acceptance of the skin component may be the tissue’s higher immunogenicity when compared with musculoskeletal tissues—a result of the skin’s unique immunological defense functions. As the first barrier to pathogen invasion, the skin has a large population of Langerhans’ cells and other dendritic cells that allow for efficient antigen presentation and T-cell costimulation.21 Interestingly, the application of CoB in a comparable model of hind limb transplantation promotes long-term survival of all allograft components equally22 with only minor mononuclear cell infiltration present at post operative day (POD) 50. Nonetheless, epidermal mononuclear cell infiltration had progressed to be more pronounced by POD 70, highlighting increased immunogenicity of the skin component (data not shown). Tung et al.23 observed an accelerated rejection of the skin component in a murine model of limb allotransplantation. Although rejection of the allograft skin occurred at an increased pace in our study, additional factors, such as size of the target organ24 and the presence of ischemia-reperfusion injury,25 also have an influence on the rejection dynamic. Compared with Tung et al., our graft was smaller in size, and the technical complexity of the surgery leads to a longer ischemia time. In contrast to our results, previous studies were able to induce prolonged survival of the skin allograft component using the same treatment protocol. However, the Emory group used a different pairing of donor and recipient mouse strain,26 where costimulation blockade was more efficacious. When the same group published another article more recently,27 using a Balb/c to C57BL/6 combination, the time of alloskin survival was closer to the results presented here. In this study, histopathologic and flow cytometry–based analysis of the bone marrow compartment of the chest wall showed high cellularity and trilineage cell differentiation with higher levels of donor leukocytes after POD 50 (Fig. 3). The percentage of mixed hematopoietic chimerism is comparable to our hind limb allografts where multilineage mixed chimerism (in T, B, and myeloid cells) ranged between 1% and 5% after CoB only (data not shown). We found no difference in the number of T regulatory cells present between the groups (data not shown). In future studies, testing of myeloid-derived suppressor cells in the periphery could provide additional valuable insight into the underlying immune mechanisms. To advance the field of transplantation, novel treatment modalities are needed that prolong allograft survival, while minimizing or even abandoning the need for immunosuppression. The induction of mixed chimerism, even if only transiently,28,29 has established immunosuppression-free donor-specific tolerance. Additionally, tolerance induction may also prevent chronic rejection, a major cause of graft loss in SOT.20 However, the extensive preconditioning regimens necessary for bone marrow transfusion in combination with solid organ30 or VCA transplantation31,32 represent a major disadvantage for this approach. The potentially advantageous feature of VCAs containing VBM could overcome this limitation. As shown by our results, the unaltered bone marrow microenvironment of VBM may provide continuous hematopoietic output, rendering VBM superior to conventional cellular bone marrow transplantation for tolerance induction and immunomodulation.33–35 The presence of thymic tissue in addition to VBM could facilitate chimerism induction and maintenance. However, tolerance induction was not achieved in our model.
Nonetheless, these immunological findings may be relevant to the field of pediatric heart transplantation, as increasing long-term survival of a heart transplant is of utmost importance for this patient population because of the greater expected longevity and limited donor organ availability.36 Current immunosuppressive protocols seem inadequate to prevent chronic rejection and delayed appearance of cardiac allograft vasculopathy (CAV) in heart transplantation. In a recent report, the Pediatric Heart Transplant Study concluded that 48% of recipients will experience rejection during the first 5 years.37 The incidence of CAV in pediatric patients 10 and 15 years after transplantation was found to be 25% and 54%, respectively.38 Both, chronic rejection and CAV are thus major factors necessitating retransplantation,10 which is associated with a significant increase in mortality compared with primary transplantation.39 A mismatch in size between donor and recipient heart is another frequently encountered problem in pediatric heart transplantation. Twenty-five percent of pediatric heart transplants are performed with hearts from an adult donor (>18 y), owing to the severe donor shortage.40 Although proposing the integration of chest wall, thymus, and heart as a VCA may not address the shortage of suitable organs, the application of a more advanced reconstructive protocol may benefit selected patients with a size mismatch or those with the need for extensive chest reconstruction after multiple redo sternotomies. Most importantly, however, using a VCA containing VBM and thymus may contribute to the overall goal of reducing the need for long-term maintenance immunosuppression. As such a large transplant involves the transfer of a considerable amount of donor cells, the question of graft-versus-host disease needs to be addressed. In this study, there was no evidence of graft versus host disease (GVHD) despite the large load of donor immune cells transferred. In accordance with our findings, long-term survivors of a composite “thymoheart” transplant did not develop any signs of GVHD under cyclosporine A treatment.5
This proof-of-concept study has a number of limitations. First, the use of anti-CD154 as costimulatory blockade is not directly translatable into the clinical setting. However, the used regimen represents an established approach to establishment of long-term survival in various murine transplant models and allowed a comparison with previous studies and historic data from our own group on hind limb transplantation. Second, albeit we observed consistent results within our small group sizes, an increase in experimental animals will certainly be necessary for future mechanistic studies.
In conclusion, results obtained in this study show evidence of VBM and thymus promoting allograft survival of all components, except the skin, in a fully mismatched murine model of chest wall, thymus, and heart transplantation.
ACKNOWLEDGMENTS
We would like to thank the American Association of Plastic Surgery for their generous support with this project.
Footnotes
Oh and Furtmüller contributed equally to this work.
Drs. Brandacher and Dorafshar are co-senior authors.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Dorafshar AH, Bojovic B, Christy MR, et al. Total face, double jaw, and tongue transplantation: an evolutionary concept. Plast Reconstr Surg. 2013;131:241–251.. [DOI] [PubMed] [Google Scholar]
- 2.MacKay BJ, Nacke E, Posner M. Hand transplantation—a review. Bull Hosp Jt Dis (2013) 2014;72:76–88.. [PubMed] [Google Scholar]
- 3.Barth RN, Rodriguez ED, Mundinger GS, et al. Vascularized bone marrow-based immunosuppression inhibits rejection of vascularized composite allografts in nonhuman primates. Am J Transplant. 2011;11: 1407–1416.. [DOI] [PubMed] [Google Scholar]
- 4.Siemionow M, Izycki D, Ozer K, et al. Role of thymus in operational tolerance induction in limb allograft transplant model. Transplantation 2006;81:1568–1576.. [DOI] [PubMed] [Google Scholar]
- 5.Menard MT, Schwarze ML, Allan JS, et al. Composite “thymoheart” transplantation improves cardiac allograft survival. Am J Transplant. 2004;4:79–86.. [DOI] [PubMed] [Google Scholar]
- 6.Chen YB, Kawai T, Spitzer TR. Combined bone marrow and kidney transplantation for the induction of specific tolerance. Adv Hematol. 2016;2016:6471901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawai T, Cosimi AB, Spitzer TR, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2008;358:353–361.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamada K, Choo JK, Allan JS, et al. The effect of thymectomy on tolerance induction and cardiac allograft vasculopathy in a miniature swine heart/kidney transplantation model. Transplantation 1999;68:485–491.. [DOI] [PubMed] [Google Scholar]
- 9.Yamada K, Gianello PR, Ierino FL, et al. Role of the thymus in transplantation tolerance in miniature swine. I. Requirement of the thymus for rapid and stable induction of tolerance to class I-mismatched renal allografts. J Exp Med. 1997;186:497–506.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tjang YS, Stenlund H, Tenderich G, et al. Pediatric heart transplantation: current clinical review. J Card Surg. 2008;23:87–91.. [DOI] [PubMed] [Google Scholar]
- 11.Stehlik J, Edwards LB, Kucheryavaya AY, et al. The registry of the International Society for Heart and Lung Transplantation: twenty-eighth adult heart transplant report—2011. J Heart Lung Transplant. 2011;30:1078–1094.. [DOI] [PubMed] [Google Scholar]
- 12.Schultz BD, Mohan R, Dorafshar AH, et al. Chest wall, thymus, and heart vascularized composite allograft proof of concept cadaveric model for heart transplantation. Ann Plast Surg. 2014;73:102–104.. [DOI] [PubMed] [Google Scholar]
- 13.Torregrossa G, Sosin M, Gerosa G, et al. Chest wall, thymus, and heart transplant: pushing the boundary of solid organ and vascularized composite allotransplantation. Vascularized Composite Allotransplantation. 2015;2: 29–36.. [Google Scholar]
- 14.Oh B, Furtmuller GJ, Sosin M, et al. A novel microsurgical model for heterotopic, en bloc chest wall, thymus, and heart transplantation in mice. J Vis Exp. 2016;107:e53442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pree I, Wekerle T. Inducing mixed chimerism and transplantation tolerance through allogeneic bone marrow transplantation with costimulation blockade. Methods Mol Biol. 2007;380:391–403.. [DOI] [PubMed] [Google Scholar]
- 16.Furtmuller GJ, Oh B, Grahammer J, et al. Orthotopic hind limb transplantation in the mouse. J Vis Exp. 2016;108:53483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brandacher G, Grahammer J, Sucher R, et al. Animal models for basic and translational research in reconstructive transplantation. Birth Defects Res C Embryo Today 2012;96:39–50.. [DOI] [PubMed] [Google Scholar]
- 18.Santiago SF, de Faria W, Khan TF, et al. Heterotopic sternum transplant in rats: a new model of a vascularized bone marrow transplantation. Microsurgery 1999;19:330–334.. [DOI] [PubMed] [Google Scholar]
- 19.Bozkurt M, Klimczak A, Nasir S, et al. Composite osseomusculocutaneous sternum, ribs, thymus, pectoralis muscles, and skin allotransplantation model of bone marrow transplantation. Microsurgery 2013;33:43–50.. [DOI] [PubMed] [Google Scholar]
- 20.Leonard DA, Cetrulo CL, Jr, McGrouther DA, et al. Induction of tolerance of vascularized composite allografts. Transplantation 2013;95:403–409.. [DOI] [PubMed] [Google Scholar]
- 21.Larsen CP, Steinman RM, Witmer-Pack M, et al. Migration and maturation of Langerhans cells in skin transplants and explants. J Exp Med. 1990;172:1483–1493.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin CH, Wang YL, Anggelia MR, et al. Combined anti-CD154/CTLA4Ig costimulation blockade-based therapy induces donor-specific tolerance to vascularized osteomyocutaneous allografts. Am J Transplant. 2016;16:2030–2041.. [DOI] [PubMed] [Google Scholar]
- 23.Tung TH, Mackinnon SE, Mohanakumar T. Long-term limb allograft survival using anti-CD40L antibody in a murine model. Transplantation 2003;75:644–650.. [DOI] [PubMed] [Google Scholar]
- 24.He C, Schenk S, Zhang Q, et al. Effects of T cell frequency and graft size on transplant outcome in mice. J Immunol. 2004;172:240–247.. [DOI] [PubMed] [Google Scholar]
- 25.Fuller BJ. Ischaemia/reperfusion injury and inflammation. Transplantation 2000;69:327–328.. [DOI] [PubMed] [Google Scholar]
- 26.Larsen CP, Elwood ET, Alexander DZ, et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature 1996;381:434–438.. [DOI] [PubMed] [Google Scholar]
- 27.Ford ML, Wagener ME, Gangappa S, et al. Antigenic disparity impacts outcome of agonism but not blockade of costimulatory pathways in experimental transplant models. Am J Transplant. 2007;7:1471–1481.. [DOI] [PubMed] [Google Scholar]
- 28.Quaini F, Urbanek K, Beltrami AP, et al. Chimerism of the transplanted heart. N Engl J Med. 2002;346:5–15.. [DOI] [PubMed] [Google Scholar]
- 29.Laflamme MA, Myerson D, Saffitz JE, et al. Evidence for cardiomyocyte repopulation by extracardiac progenitors in transplanted human hearts. Circ Res. 2002;90:634–640.. [DOI] [PubMed] [Google Scholar]
- 30.Leventhal J, Abecassis M, Miller J, et al. Chimerism and tolerance without GVHD or engraftment syndrome in HLA-mismatched combined kidney and hematopoietic stem cell transplantation. Sci Transl Med. 2012;4:124ra28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Talmor M, Steinman RM, Codner MA, et al. Bone marrow-derived chimerism in non-irradiated, cyclosporin-treated rats receiving microvascularized limb transplants: evidence for donor-derived dendritic cells in recipient lymphoid tissues. Immunology 1995;86:448–455.. [PMC free article] [PubMed] [Google Scholar]
- 32.Arslan E, Klimczak A, Siemionow M. Chimerism induction in vascularized bone marrow transplants augmented with bone marrow cells. Microsurgery 2007;27:190–199.. [DOI] [PubMed] [Google Scholar]
- 33.Page EK, Dar WA, Knechtle SJ. Tolerogenic therapies in transplantation. Front Immunol. 2012;3:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lukomska B, Durlik M, Cybulska E, et al. Comparative analysis of immunological reconstitution induced by vascularized bone marrow versus bone marrow cell transplantation. Transpl Int. 1996;9:S492–S496.. [DOI] [PubMed] [Google Scholar]
- 35.Brandacher G, Lee WP, Schneeberger S. Minimizing immunosuppression in hand transplantation. Expert Rev Clin Immunol. 2012;8:673–683.; quiz 684. [DOI] [PubMed] [Google Scholar]
- 36.Kirk R, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: thirteenth official pediatric heart transplantation report—2010. J Heart Lung Transplant. 2010;29:1119–1128.. [DOI] [PubMed] [Google Scholar]
- 37.Dipchand AI, Kirk R, Mahle WT, et al. Ten yr of pediatric heart transplantation: a report from the Pediatric Heart Transplant Study. Pediatr Transplant. 2013;17:99–111.. [DOI] [PubMed] [Google Scholar]
- 38.Kobayashi D, Du W, L’ecuyer TJ. Predictors of cardiac allograft vasculopathy in pediatric heart transplant recipients. Pediatr Transplant. 2013;17:436–440.. [DOI] [PubMed] [Google Scholar]
- 39.Mahle WT, Vincent RN, Kanter KR. Cardiac retransplantation in childhood: analysis of data from the United Network for Organ Sharing. J Thorac Cardiovasc Surg. 2005;130:542–546.. [DOI] [PubMed] [Google Scholar]
- 40.Benden C, Goldfarb SB, Edwards LB, et al. ; International Society for Heart and Lung Transplantation. The registry of the International Society for Heart and Lung Transplantation: seventeenth official pediatric lung and heart-lung transplantation report—2014; focus theme: retransplantation. J Heart Lung Transplant. 2014;33:1025–1033.. [DOI] [PubMed] [Google Scholar]


