Abstract
Background:
Breast augmentation with autologous fat has been performed in Japan for over 30 years. However, complications include breast lumps and oil cysts. Such breast lumps greatly reduce patient satisfaction, and are currently difficult to diagnose and treat for many cosmetic surgery clinics. This study aimed to elucidate the effectiveness of ultrasound diagnosis and treatment of patients with breast lumps after breast augmentation with autologous fat grafting.
Methods:
We used diagnostic and therapeutic ultrasound to examine 256 patients with breast lumps between April 2012 and April 2017. We determined the nature, size, and location of the maximal lump. Breast lumps were classified into five types: cystic, complex, solid, calcification, and unclassifiable. The method of treatment (including fine-needle aspiration, VASER liposuction, lumpectomy, and extended lumpectomy) was selected according to the lump type, and the efficacy of treatment was determined by postoperative palpation and ultrasound.
Results:
A total of 198 patients (198/256, 77%) requested treatment. Cystic lumps (79/256, 31%) were treated by fine-needle aspiration. VASER liposuction was used to treat complex (64/256, 25%) and solid lumps (50/256, 19%). Calcification (58/256, 23%) and unclassifiable lumps (5/256, 2%) were removed via periareolar incision. There were no serious complications. In all cases, the lumps were no longer palpable after treatment, and ultrasound showed that they had either contracted or disappeared.
Conclusions:
The appropriate treatment for breast lumps after breast augmentation with autologous fat grafting must be selected according to the nature of the lumps. Ultrasound is essential for diagnosing the breast lump type and determining the best treatment.
INTRODUCTION
Breast augmentation with liposuction was first reported by Bircoll1 in 1987, but in the same year the American Society of Plastic Surgeons (ASPS) prohibited breast augmentation by autologous fat grafting because of the potential for scarring or calcification to interfere with mammographic breast cancer diagnosis after augmentation surgery.2 Subsequently, Coleman and Saboeiro3 and Spear et al.4 reported that autologous fat grafting was safe and achieved good results, and in 2009 the ASPS proposed recommendations for autologous fat grafts.5,6 Today, breast augmentation by autologous fat grafting is widely performed both in cosmetic surgery clinics and as reconstructive surgery, including in Japan.7
However, the increasing number of patients undergoing this procedure has resulted in a range of reported complications, including fat necrosis, oil cysts, and calcification.8–10 Unfortunately, many cosmetic surgery clinics in Japan currently do not know the risk and incidence of lumps after fat grafting to the breast is technique and volume dependent. These clinics have also difficulty diagnosing and treating breast lumps after breast augmentation and so the lumps are left untreated. Breast lumps after breast augmentation greatly reduce patient satisfaction and are a cause of mental stress.
Extracorporeal ultrasound is widely known as a medical examination in a variety of diseases. Ultrasound carries absolutely no risk for any patient and can be carried out easily in comparison with computed tomography or magnetic resonance imaging (MRI). Furthermore, ultrasound is a noninvasive and convenient method for screening and also diagnosis and treatment of breast, liver, and pancreatic cancer.11–13 However, there are no detailed reports about treatment strategies for breast lumps after breast augmentation with autologous fat grafting.
In this study, we investigated the effectiveness of ultrasound diagnosis and treatment of breast lumps after autologous fat grafting.
SUBJECTS AND METHODS
This study was approved by our institutional review board, and all subjects provided written, informed consent before study inclusion. The study subjects were 259 patients who had noticed lumps after autologous fat grafting between April 2012 and April 2017. The location, size, and nature of the lumps were diagnosed by doctors specializing in breast augmentation or breast cancer and some radiologists. The ultrasound (HIVISION Avius with an EUP-L74M probe, Hitachi Medical Systems, Northamptonshire, UK) was used, and lumps were classified into five types: cystic, complex, solid, calcification, and unclassifiable. Liposuction was performed with an ultrasound device (VASER Lipo System; Sound Surgical Technologies, Louisville, Colo.).
Characteristics of Each Lump Type
Cystic lumps contained only oily components, whereas complex lumps consisted of mixed oily and solid components, and solid lumps contained almost all solid components. Calcification lumps were defined as those with widespread calcification of the capsule. Unclassifiable lumps involved inflammatory change of the mammary gland and pectoralis major muscle; this invasiveness was unable to be diagnosed with only ultrasound and so MRI was used.
Anesthesia
Surgery was performed using a combination of local anesthesia (1.0% lidocaine with 0.01% epinephrine), intravenous anesthesia (1.0% diprivan), and liposuction with the tumescent technique (1 mL of epinephrine, 20 mL of 8.4% sodium hydrogen carbonate, and 50 mL of 1.0% lidocaine per 1000 mL of saline solution).3
Treatment for Each Lump Type
Cystic lumps were treated by fine-needle aspiration, whereas VASER liposuction (V-mode 50%, 1 minute) was used to break down and aspirate fat from complex and solid lumps. Calcification and unclassifiable lumps were removed via a periareolar incision. All types of lumps were treated under ultrasound guidance. Treatment efficacy was determined by postoperative palpation and ultrasound.
RESULTS
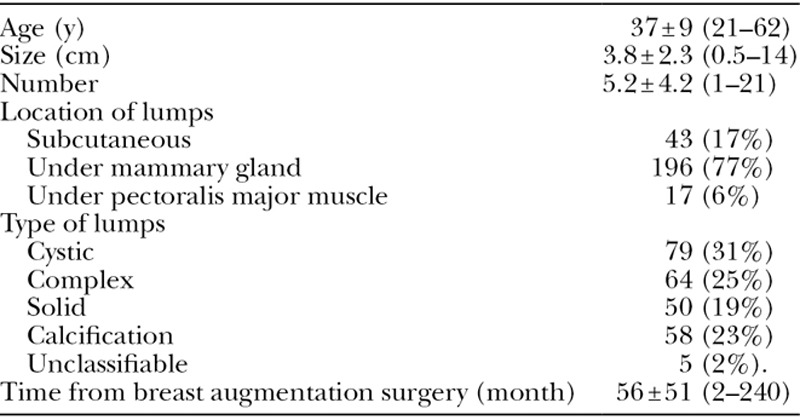
All 259 patients who had noticed lumps after breast augmentation with autologous fat had undergone fat grafting for cosmetic reasons. Three patients were suspected to have a malignant tumor, and we therefore referred them to a specialist hospital. Hence, we examined a final total of 256 patients. Mean patient age was 37 ± 9 years, mean lump size was 3.8 ± 2.3 cm, and mean number of lumps was 5.2 ± 4.2. The prevalence of each lump type was as follows: cystic in 79 patients (31%), complex in 64 patients (25%), solid in 50 patients (19%), calcification in 58 patients (23%), and unclassifiable in five patients (2%). Lumps were located subcutaneously in 43 patients (17%), beneath the mammary glands in 196 (77%), and beneath the pectoralis major muscle in 17 (6%). The mean time from breast augmentation surgery to lump diagnosis was 56 ± 51 months (Table 1).
Table 1.
Patient Characteristics (n = 256)
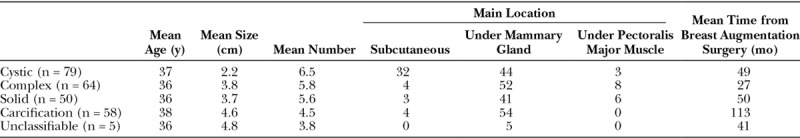
The mean lump size was 2.2 cm for the cystic type, 3.8 cm for the complex type, 3.7 cm for the solid type, 4.6 cm for the calcification type, and 4.8 cm for the unclassifiable type. Cystic lumps tended to be smaller than the other types. The mean number of lumps per patient was 6.5 for the cystic type, 5.8 for the complex type, 5.6 for the solid type, 4.5 for the calcification type, and 3.8 for the unclassifiable type. The majority of all types were found under mammary gland and the mean time from breast augmentation surgery to lump diagnosis for calcification type lumps were longest (Table 2).
Table 2.
Characteristics of Each Type of Breast Lump (n = 256)
Of the 256 patients, 198 (77%) requested treatment, whereas 58 declined treatment. The main reasons for declining treatment were non-malignancy, cost, desire to preserve breast shape and volume, and scar avoidance. Fine-needle aspiration was used to treat 56 patients with cystic lumps. VASER liposuction was used to treat 49 patients with complex lumps and 44 with solid lumps. Lumpectomy was performed in 47 patients with calcification lumps, and three patients with unclassifiable lumps underwent extended lumpectomy. All cystic lumps were resolved by fine-needle aspiration alone. Almost all complex and solid lumps were resolved by VASER liposuction. However, the results were not good for eight patients; two of these patients had big complex lesions, and six had big solid lesions with thick capsules that were partially calcified. Although their lump contents were aspirated, these lumps recurred because fluid accumulated within the capsules. The complex lesions were subsequently resolved after a single aspiration, and the solid lesions resolved after lumpectomy. All calcification lumps were resolved by lumpectomy, and unclassifiable lumps were by extended lumpectomy alone (Fig. 1). There were no serious complications. In all cases, the lumps were no longer palpable after treatment, and ultrasound showed that they had either contracted or disappeared.
Fig. 1.
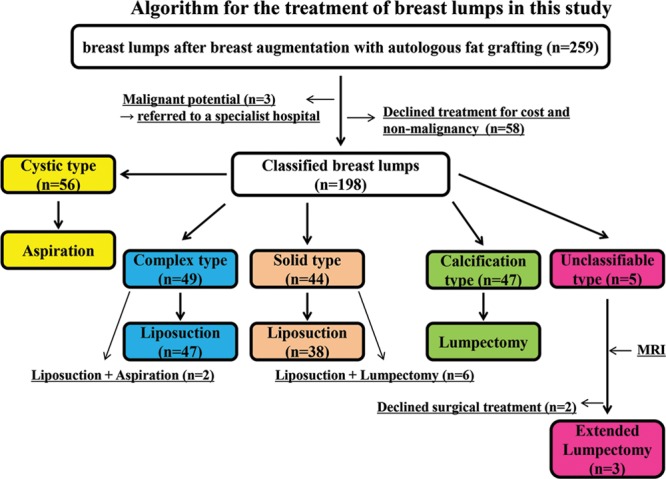
Algorithm for the treatment of breast lumps after breast augmentation with autologous fat grafting. Breast lumps (n = 198) were classified into five types: cystic, complex, solid, calcification, and unclassifiable. Cystic types were treated with fine-needle aspiration. Complex and solid types underwent liposuction by VASER. Calcification types were treated by lumpectomy, and unclassifiable types by extended lumpectomy.
CASE PRESENTATIONS
Representative cases of each type are described below.
Case 1: Cystic Type
A 32-year-old woman had undergone breast augmentation with autologous fat 10 months previously. A 1.3-cm subcutaneous cystic lesion was evident in the lateral upper part of the left breast. This disappeared after fine-needle aspiration with an 18-G syringe needle under local anesthesia. The aspirated content was a white oily substance (Figs. 2, 3).
Fig. 2.
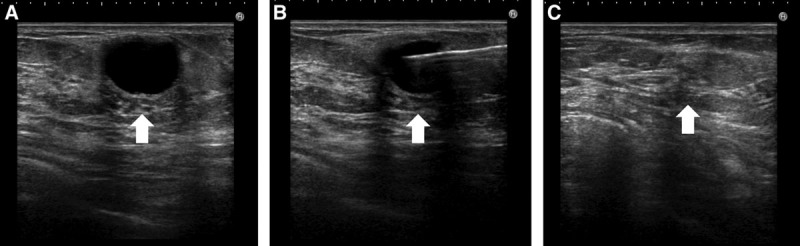
Cystic type lump in a 32-year-old woman had undergone breast augmentation 10 months previously. A, Ultrasound revealed a subcutaneous, 1.3-cm, round, anechoic shadow. Posterior echo enhancement was also evident, and an oil cyst was diagnosed (arrow). B, Ultrasound-guided fine-needle aspiration was performed with an 18-G syringe needle (arrow). C, After aspiration, the oil cyst had completely disappeared (arrow).
Fig. 3.
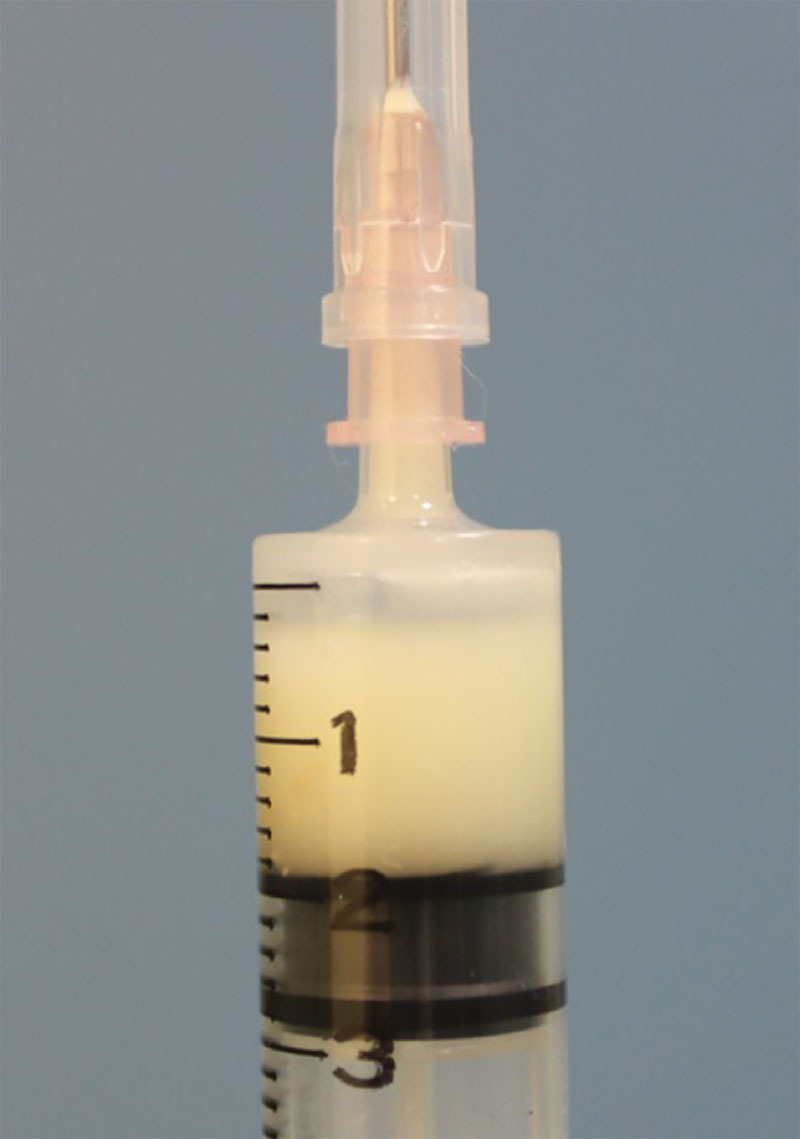
The aspirated content was a white, opaque fluid.
Case 2: Complex Type
A 24-year-old woman had undergone breast augmentation with autologous fat 14 months previously. A 10-cm complex lesion was evident beneath the right mammary gland. A 5-mm skin port was inserted into the right axillary region and VASER liposuction was performed, after which the contents were aspirated via a 3.7-mm cannula. The aspirated contents were a white oily substance and bloody or necrotic fat (Figs. 4, 5).
Fig. 4.
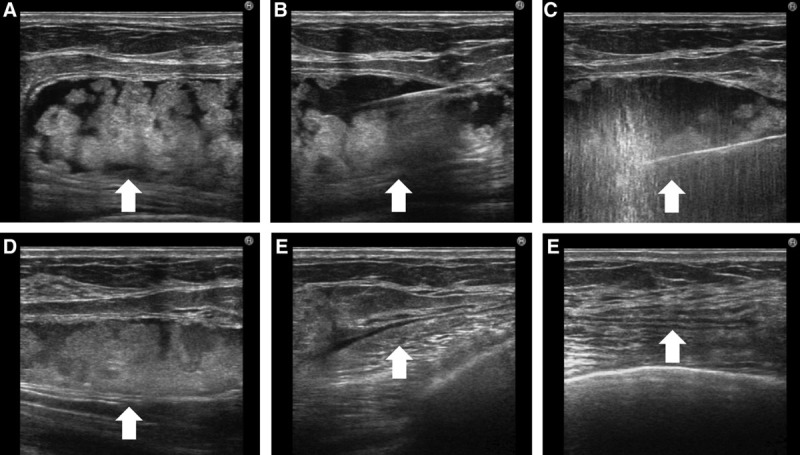
Complex type lump in a 24-year-old woman who had undergone breast augmentation 14 months previously. A, A 10-cm complex lump was evident beneath the right mammary gland (arrow). B, Liposuction with the tumescent technique in the capsule was performed (arrow). C, The solid component was broken down by VASER (arrow). D, At the conclusion of VASER treatment, the oily component had decreased (arrow). E, The contents were aspirated via a 3.7-mm cannula, leaving a small amount of liquid (arrow). F, After treatment, the contents had completely disappeared (arrow).
Fig. 5.
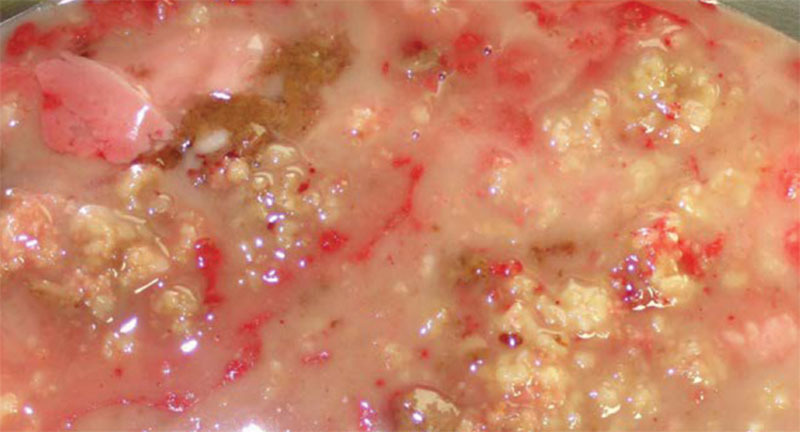
The aspirated contents revealed a large amount of necrotic fat.
Case 3: Solid Type
A 43-year-old woman had undergone breast augmentation with autologous fat 14 months previously. A 4.8-cm solid lesion was present beneath the left mammary gland. A 5-mm skin port was inserted into the right axillary region and VASER liposuction was performed, after which the contents were aspirated via a 3.7-mm cannula. Pathological examination of the aspirated contents revealed degenerated and necrotic fat cells (Figs. 6, 7).
Fig. 6.
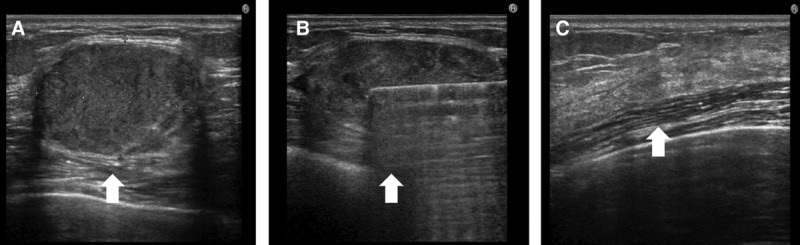
Solid type lump in a 43-year-old woman who had undergone breast augmentation 14 months previously. A, A 4.8-cm solid lump was evident beneath the left mammary gland (arrow). B, Liposuction with the tumescent technique in the capsule was performed, and VASER was used to break down the solid component (arrow). C, After treatment, the contents had completely disappeared (arrow).
Fig. 7.
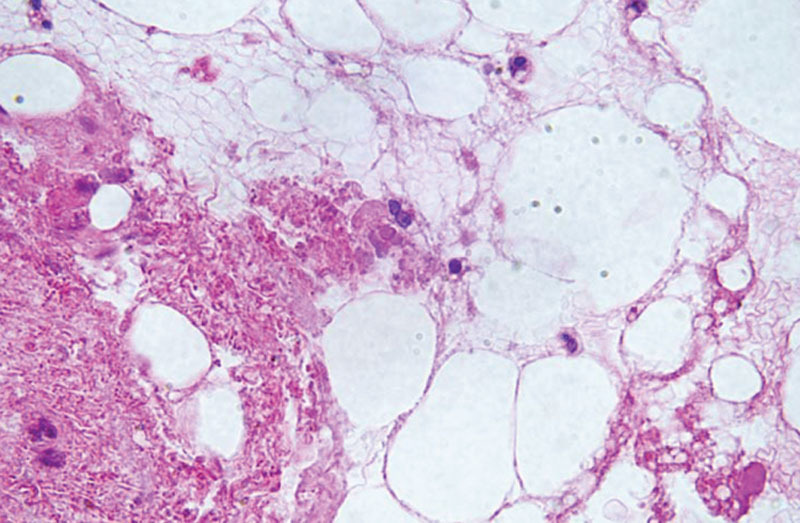
Pathological examination (H-E stain) revealed degenerated and necrotic fat cells.
Case 4: Calcification Type
A 34-year-old woman had undergone breast augmentation with autologous fat 10 years and 9 months previously. A 4.0-mm calcified mass was evident beneath the left mammary gland. The lump was removed via a periareolar incision. The enucleated mass was necrotic fat with a hard, calcified capsule (Fig. 8).
Fig. 8.
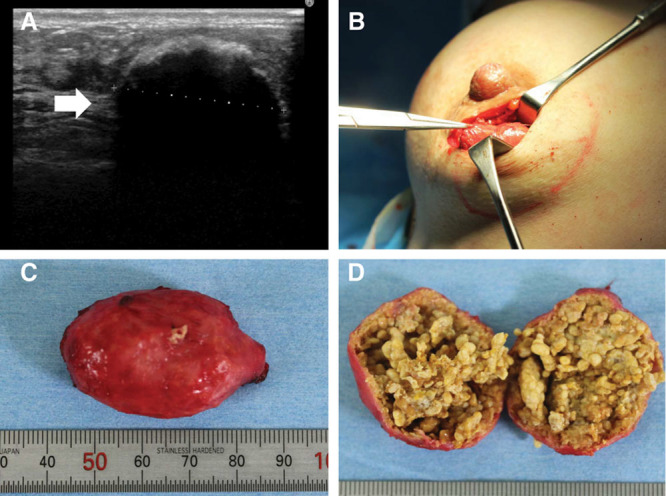
Calcification type lump in a 34-year-old woman who had undergone breast augmentation 10 years and 9 months previously. A, A 4-cm calcified mass was evident beneath the left mammary gland (arrow). B, The calcified mass was removed via a periareolar incision. C, The resected lump measured 4.2 × 3.2 cm and its smooth surface was very hard. D, In cross section, the lump had a thick calcified capsule and was full of necrotic fat with no oily content.
Case 5: Unclassifiable Type
A 30-year-old woman had undergone breast augmentation with autologous fat 33 months previously. A 3.5-cm cystic mass was evident beneath the internal part of the right mammary gland. There were extended adhesive and inflammatory changes of the mammary gland and pectoralis major muscle. The lump and surrounding hard tissues were removed via a periareolar incision. Postoperatively, the right breast was deformed. The surrounding hard tissues had necrotic changes (Figs. 9, 10).
Fig. 9.
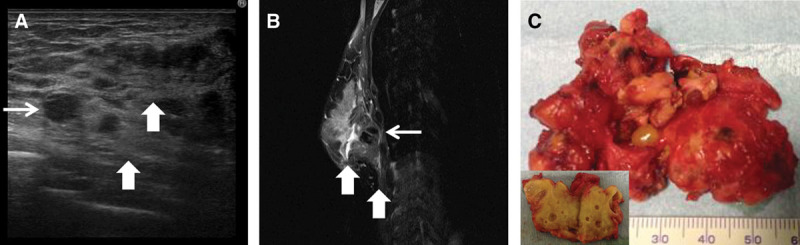
Unclassifiable type lump in a 30-year-old woman who had undergone breast augmentation 33 months previously. A, B, A 3.5-cm cystic mass was evident beneath the right mammary gland (small arrow). This lump had caused extended adhesive and inflammatory changes of the mammary gland and pectoralis major muscle (big arrow). C, The resected lump measured 6.1 × 4.3 cm and was hard. In cross section, the lump and surrounding tissue was very hard and had inflammatory changes.
Fig. 10.
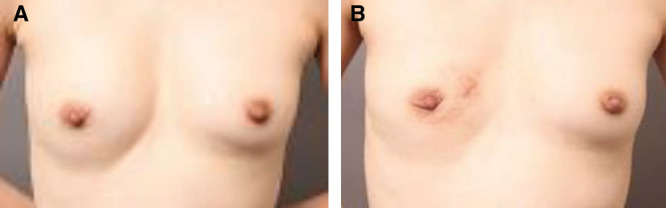
The lump and surrounding hard tissues were removed via a periareolar incision. The right breast was deformed (A) postoperatively, and (B) preoperatively.
DISCUSSION
Mechanism of Lumps
Although the process whereby the injected fat forms lumps is not well understood, lumps after autologous fat grafting are thought to be formed as a result of inflammatory reactions and fibrosis of the injected fat, followed by the occurrence of fat necrosis. Histopathologically, phagocytosis by foam cells occurs in response to the breakdown and vacuolization of fat cells, and unprocessed necrotic substances are surrounded by proliferated fibroblasts.14,15 The progression of fibrosis is considered to vary depending on the status of fat necrosis, and may be accompanied by calcification.10,14,15
In this study, the breast lumps after breast augmentation with autologous fat grafting comprised a variety of forms. It is conceivable that if necrotic fat is solid and not completely absorbed, a hard capsule may form around it as a result of inflammation and fibrosis to create a solid lesion16; if the central part then becomes necrotic and liquefies, this may result in the formation of a complex or cystic lesion.16
Diagnosis of Lumps
Chala et al.14 carried out a detailed investigation of fat necrosis using mammography, ultrasound, computed tomography, and MRI, and reported that mammography is useful for identifying microcalcifications, whereas MRI is useful for identifying inflammation and fibrosis. Costantini et al.17 used mammography, ultrasound, and MRI to examine women before breast augmentation by autologous fat grafting and 6 and 12 months after the procedure, and found that calcifications were best diagnosed by mammography, oil cysts by ultrasound, and fat necrosis by MRI. We consider that ultrasound is best for evaluating breast augmentation by autologous fat grafting for the following reasons. Mammography does not indicate the layer in which a lump is located, whereas ultrasound is capable of evaluating the size, nature, location, and perfusion of lumps, making it easy to differentiate them from breast cancer. Furthermore, ultrasound is better than computed tomography and MRI for the initial diagnosis of breast lumps in terms of cost, simplicity, and invasiveness.
When conducting an ultrasound examination of breast lumps after breast augmentation by autologous fat grafting, the most important point is their differentiation from breast cancer. In this study, three patients had indicative signs of breast cancer and were referred to a hospital specializing in breast cancer.
Frequency of Lumps
Numerous recent reports have described the efficacy and safety of breast augmentation by autologous fat grafting. The reported incidence of palpable lumps varies, but a review of 36 studies found that palpable lumps occurred in 7% of cases,8 and a review of 18 studies reported rates of 0%–8%.9 Wang et al.18 also found lumps in 34 out of 41 patients who underwent autologous fat grafting (cystic lesions in 61.9%, complex lesions in 16.1%, and solid lesions in 22%). In this study, the lumps were cystic in 31%, complex in 25%, solid in 19%, calcification in 23%, and unclassifiable in 2%; similar to previous studies, the cystic type was the most common (Table 1).
Size and Number of Each Type of Lump
Several studies have reported that larger volumes of injected fat result in a greater size and number of lumps.19,20 The volumes of autologous fat injected in the all patients of this study were unknown, as the fat injections were done at other clinics. However, there was a possibility of an inverse correlation between the number and size of the lumps; the bigger the lump size, the lesser the number of lumps (Table 2).
Location of Each Lump Type
A study of 66 patients reported liponecrotic cysts in 11 patients (16.7%), with the majority located beneath the mammary gland.21 The authors suggested that the reason for the large number of lumps at this location may have been that contractions of the pectoralis major muscle caused the injected fat to accumulate in one place, where it became necrotic.21 Another reason may be that perfusion beneath the mammary gland is poorer than under the skin or pectoralis major muscle.22 In this study, all types of lumps were also more commonly found under the mammary gland than subcutaneously and/or under the pectoralis major muscle. Although the injection sites were unknown (as all the present patients underwent breast augmentation at other clinics), most lumps were located beneath the mammary gland, consistent with previous studies (Table 2).
Progress of Each Lump Type
Soo et al.23 used ultrasound to evaluate fat necrosis, and reported that complex lesions transformed into solid or cystic lesions. Wang et al.18 also reported that four solid lesions and one complex lesion transformed into cystic lesions, suggesting that lumps may change in nature. In this study, the duration from first operation to lump formation in cases with complex type lumps was much shorter than in cases with other lump types, and the duration from first operation to formation of cystic and solid type lumps was similar. Moreover, some complex type lumps were very oily (similar to the cystic type), and some had almost solid components like solid type lumps. We speculate that there is a tendency for complex type lumps to change into the cystic type if necrotic fat is absorbed, and into the solid type if it is not. Furthermore, several of the solid type lumps had a partially calcified capsule, indicating that the solid type may gradually change into the calcification type (Table 2).
Unclassifiable type lumps were very rare in this study. Normally, breast lumps caused by breast augmentation with autologous fat do not infiltrate the mammary gland or pectoralis major muscle.16–19 However, there have recently been a few patients with inflammatory changes in the surrounding tissue. These cases were not reported, so we consider that these cases were likely the unclassifiable type. Breast augmentation by autologous fat grafting is now widely performed both in cosmetic surgery clinics and as reconstructive surgery. Recently, especially in Japan, the importance of breast cancer screening has become widely recognized, and mammography for detecting breast cancer has been gradually increasing. We speculate that strongly pressing the breast lumps during mammography causes breast lumps to rupture, and necrotic fat infiltrates the surrounding tissue. We think that these lumps change over time and become the unclassifiable type.
Treatment of Lumps
The most important aspects of treatment are to diagnose the condition of the lump and completely remove the necrotic tissue. Ultrasound is essential to this process, as it enables monitoring of the lump condition in real time. At our clinic, we treat breast lumps after breast augmentation with autologous fat by fine-needle aspiration, liposuction, lumpectomy, and extended lumpectomy. Cystic lumps are treated with fine-needle aspiration, complex and solid types with VASER liposuction, calcification types are enucleated, and unclassifiable types are resected with the surrounding tissues via a periareolar incision. Ultrasound guidance is indispensable in the treatment of breast lumps after breast augmentation for avoiding damaging the surrounding tissue while accurately treating lumps.
Cystic Type
All 56 patients with cystic lumps underwent ultrasound-guided aspiration with a 16-G or 18-G needle, and in all cases the lumps disappeared after a single treatment. Maillard et al. reported increased calcification some months after oil cyst aspiration.24 This likely resulted from the leakage of oily components during aspiration, which caused inflammation and calcification. We did not find this to be an issue, as we carried out aspiration under ultrasound guidance and carefully ensured that all contents were aspirated.
Complex and Solid Types
Given that complex and solid types contain necrotic or degenerated adipose tissue within a fibrous capsule, we think that as long as the contents are removed they will disappear without the need to remove the capsule. Although the VASER is an ultrasound device originally designed for use in liposuction,25 we used it to remove necrotic tissue from 47 patients with complex type lumps and 38 with solid type lumps. After using ultrasound, we performed liposuction and used the VASER to break down the capsule and suck out the contents. We have been using VASER liposuction to treat complex and solid breast lumps for about 10 years, and regard it as both safe and effective. It provides minimally invasive treatment for complex and solid type lumps that were formerly removed by lumpectomy, leaving only a scar around 5 mm long under the armpit. Ultrasound guidance is important to ensure that all necrotic substances inside the capsule are completely aspirated. If aspiration is incomplete, the lump will persist and the patient will be dissatisfied.
However, if the capsule is big, heavily fibrous, or partially calcified, it may be difficult to remove the lesion entirely and it may still be evident on ultrasound. In two patients with big, complex type lumps who were treated with VASER, the lumps disappeared immediately after treatment but reappeared as cystic lesions several weeks later; these lesions were resolved with fine-needle aspiration. Furthermore, there were six patients with solid type lumps with a heavily fibrous or partially calcified capsule that were difficult to remove completely with VASER treatment; in such cases, lumpectomy is the best treatment (similar to calcification type lumps). Therefore, informed consent about combination therapies for such patients is very important.
Calcification Type
Calcification type lumps have a widespread calcified capsule surrounding the lump. Hence, currently they can be treated only by complete lumpectomy. Kim et al.26 treated 5 cm calcified pseudocysts by resection via an inframammary incision. Similarly, in Japan, Hyakusoku et al.27 reported the treatment of palpable lumps with lumpectomy although almost all lumps were removed via a periareolar incision. We also use this technique as it leaves a less obvious scar.
Unclassifiable Type
In cases with unclassifiable type lumps, it was impossible to clearly diagnose the spread of lesions using ultrasound alone and so MRI was necessary. We had to perform extended lumpectomy via a periareolar incision to remove the lumps and surrounding hard tissue, meaning that their removal caused obvious changes in breast shape. Therefore, two patients declined surgical treatment in our study.
Treatment for All Lump Types
Once lumps are covered by a capsule, they are not spontaneously resorbed, and calcification may occur as the capsule thickens over time28; hence, the risk of rupture increases. Therefore, early diagnosis and treatment is required.
Risk Factors for Lump Formation
The quality of fat, method or location of injection, and injection volume are all important for reducing the risk of breast lumps. Adipose-derived stem/progenitor cells play an important role in improving the quality of injected fat, and Coleman’s technique of injecting small amounts at a time should be followed, as breast lumps may be formed if a large volume is injected into a single site.1–4,7,10,29,30 Yoshimura et al.31 performed surgery to switch from implants to autologous fat grafting by cell-assisted lipotransfer, and reported that injecting fat into several different layers with fine 16–18G needles resulted in no palpable lumps, cysts of size 5 mm or larger, or calcification.
All of the patients in this study had undergone breast augmentation at other clinics, and details of the amounts and methods of fat injection were therefore unknown. It is possible that large or multiple lesions were the result of an injection of a large volume of fat at a single location, especially under the mammary gland.
CONCLUSIONS
Ultrasound is essential for the diagnosis and classification of breast lumps after breast augmentation by autologous fat grafting, and can be used to select the appropriate treatment in accordance with the condition of the lumps.
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Bircoll M. Cosmetic breast augmentation utilizing autologous fat and liposuction techniques. Plast Reconstr Surg. 1987;79:267–271.. [DOI] [PubMed] [Google Scholar]
- 2.ASPRS Ad-Hoc Committee on New Procedures. Report on autologous fat transplantation. Plast Surg Nurs. 1987;7:140–141.. [PubMed] [Google Scholar]
- 3.Coleman SR, Saboeiro AP. Fat grafting to the breast revisited: safety and efficacy. Plast Reconstr Surg. 2007;119:775–85.; discussion 786. [DOI] [PubMed] [Google Scholar]
- 4.Spear SL, Wilson HB, Lockwood MD. Fat injection to correct contour deformities in the reconstructed breast. Plast Reconstr Surg. 2005;116:1300–1305.. [DOI] [PubMed] [Google Scholar]
- 5.American Society of Plastic Surgeons. American Society of Plastic Surgeons guiding principle for fat transfer/fat graft and fat injection. 2009. Available at http://www.plasticsurgery.org/Documents/medical-professionals/health-policy/guiding-principles/ASPS-Fat-Transfer-Graft-Guiding-Principles.pdf. Accessed July 20, 2017.
- 6.Gutowski KA; ASPS Fat Graft Task Force. Current applications and safety of autologous fat grafts: a report of the ASPS fat graft task force. Plast Reconstr Surg. 2009;124:272–280.. [DOI] [PubMed] [Google Scholar]
- 7.Ohashi M, Yamakawa M, Chiba A, et al. Our experience with 131 cases of Simultaneous Breast Implant Exchange with Fat (SIEF). Plast Reconstr Surg Glob Open. 2016;4:e691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Largo RD, Tchang LA, Mele V, et al. Efficacy, safety and complications of autologous fat grafting to healthy breast tissue: a systematic review. J Plast Reconstr Aesthet Surg. 2014;67:437–448.. [DOI] [PubMed] [Google Scholar]
- 9.Leopardi D, Thavaneswaran P, Mutimer KL, et al. Autologous fat transfer for breast augmentation: a systematic review. ANZ J Surg. 2014;84:225–230.. [DOI] [PubMed] [Google Scholar]
- 10.Claro F, Jr, Figueiredo JC, Zampar AG, et al. Applicability and safety of autologous fat for reconstruction of the breast. Br J Surg. 2012;99:768–780.. [DOI] [PubMed] [Google Scholar]
- 11.Gordon PB, Goldenberg SL. Malignant breast masses detected only by ultrasound. A retrospective review. Cancer. 1995;76:626–630.. [DOI] [PubMed] [Google Scholar]
- 12.Sharma C, Eltawil KM, Renfrew PD, et al. Advances in diagnosis, treatment and palliation of pancreatic carcinoma: 1990-2010. World J Gastroenterol. 2011;17:867–897.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trinchet JC, Chaffaut C, Bourcier V, et al. ; Groupe d’Etude et de Traitement du Carcinome Hépatocellulaire (GRETCH). Ultrasonographic surveillance of hepatocellular carcinoma in cirrhosis: a randomized trial comparing 3- and 6-month periodicities. Hepatology. 2011;54:1987–1997.. [DOI] [PubMed] [Google Scholar]
- 14.Chala LF, de Barros N, de Camargo Moraes P, et al. Fat necrosis of the breast: mammographic, sonographic, computed tomography, and magnetic resonance imaging findings. Curr Probl Diagn Radiol. 2004;33:106–126.. [DOI] [PubMed] [Google Scholar]
- 15.Rosen PP. Rosen PP. Inflammatory and reactive tumors. In: Rosen’s Breast Pathology. 1997:1st ed Philadelphia: Lippincott-Raven; 23–56.. [Google Scholar]
- 16.Mu DL, Luan J, Mu L, et al. Breast augmentation by autologous fat injection grafting: management and clinical analysis of complications. Ann Plast Surg. 2009;63:124–127.. [DOI] [PubMed] [Google Scholar]
- 17.Costantini M, Cipriani A, Belli P, et al. Radiological findings in mammary autologous fat injections: a multi-technique evaluation. Clin Radiol. 2013;68:27–33.. [DOI] [PubMed] [Google Scholar]
- 18.Wang H, Jiang Y, Meng H, et al. Sonographic identification of complications of cosmetic augmentation with autologous fat obtained by liposuction. Ann Plast Surg. 2010;64:385–389.. [DOI] [PubMed] [Google Scholar]
- 19.Mandrekas AD, Zambacos GJ, Kittas C. Cyst formation after fat injection. Plast Reconstr Surg. 1998;102:1708–1709.. [DOI] [PubMed] [Google Scholar]
- 20.Castelló JR, Barros J, Vázquez R. Giant liponecrotic pseudocyst after breast augmentation by fat injection. Plast Reconstr Surg. 1999;103:291–293.. [DOI] [PubMed] [Google Scholar]
- 21.Zheng DN, Li QF, Lei H, et al. Autologous fat grafting to the breast for cosmetic enhancement: experience in 66 patients with long-term follow up. J Plast Reconstr Aesthet Surg. 2008;61:792–798.. [DOI] [PubMed] [Google Scholar]
- 22.Illouz YG. Body Sculpturing by Lipoplasty. 1989:London: Churchill Livingstone; 390–394.. [Google Scholar]
- 23.Soo MS, Kornguth PJ, Hertzberg BS. Fat necrosis in the breast: sonographic features. Radiology. 1998;206:261–269.. [DOI] [PubMed] [Google Scholar]
- 24.Maillard GF. Liponecrotic cysts after augmentation mammaplasty with fat injections. Aesthetic Plast Surg. 1994;18:405–406.. [DOI] [PubMed] [Google Scholar]
- 25.de Souza Pinto EB, Abdala PC, Maciel CM, et al. Liposuction and VASER. Clin Plast Surg. 2006;33:107–115, vii.. [DOI] [PubMed] [Google Scholar]
- 26.Kim H, Yang EJ, Bang SI. Bilateral liponecrotic pseudocysts after breast augmentation by fat injection: a case report. Aesthetic Plast Surg. 2012;36:359–362.. [DOI] [PubMed] [Google Scholar]
- 27.Hyakusoku H, Ogawa R, Ono S, et al. Complications after autologous fat injection to the breast. Plast Reconstr Surg. 2009;123:360–370.; discussion 371. [DOI] [PubMed] [Google Scholar]
- 28.Srinivas RP, Thomas P, Eleftherios PM. Long-term clinical and radiologic results with autologous fat transplantation for breast augmentation: case reports and review of the literature. Breast J. 2006;12:63–65.. [DOI] [PubMed] [Google Scholar]
- 29.Yoshimura K, Suga H, Eto H. Adipose-derived stem/progenitor cells: roles in adipose tissue remodeling and potential use for soft tissue augmentation. Regen Med. 2009;4:265–273.. [DOI] [PubMed] [Google Scholar]
- 30.Coleman SR. Long-term survival of fat transplants: controlled demonstrations. Aesthetic Plast Surg. 1995;19:421–425.. [DOI] [PubMed] [Google Scholar]
- 31.Yoshimura K, Asano Y, Aoi N, et al. Progenitor-enriched adipose tissue transplantation as rescue for breast implant complications. Breast J. 2010;16:169–175.. [DOI] [PubMed] [Google Scholar]


