Summary:
As technology and interventional techniques continue to evolve, both the volume and complexity of cardiac catheterizations will increase, leading to a rise in the number of complications. One of the most morbid complications of cardiac catheterization is vascular injury. We report the case of a 31-day-old, 3.0-kg infant with hypoplastic left heart syndrome who experienced a left common iliac artery disruption during cardiac catheterization resulting in a retroperitoneal hemorrhage. The extent of the vascular injury combined with the vessel caliber posed a technically challenging surgical scenario. Ultimately, the vascular supply to the left lower extremity was reconstructed by the plastic surgery team with a reverse autologous vein graft. To our knowledge, this multidisciplinary approach with the involvement of plastic surgery represents a unique case.
Cardiac catheterization has transformed the management of pediatric cardiac disease, as the indications for pediatric catheterization are broad and far-reaching.1–3 Cardiac catheterization, however, is not without risk. In pediatric patients, the rate of complications ranges from 7.8% to 16.2%.1,5–9 The most common complication is vascular injury, which accounts for nearly one third of all complications.1,7
We report the surgical treatment of a 31-day-old infant with an injury to the left common iliac artery (CIA) leading to significant retroperitoneal hemorrhage (RPH) that occurred during a diagnostic cardiac catheterization.
CASE REPORT
A neonate was born at 39-week gestation with hypoplastic left heart syndrome. On day of life 3, a Norwood procedure with a Sano shunt was performed to establish a functional systemic circuit. On postoperative day 22, the patient decompensated requiring cardiopulmonary resuscitation. Because of an echocardiogram consistent with cardiac failure, the patient was placed on extracorporeal membrane oxygenation (ECMO).
Three days after placement on ECMO, an investigational cardiology catheterization was performed. During catheterization, an obstruction was experienced with catheter manipulation in the left groin region, and adherent tissue was noted upon catheter removal. Angiography demonstrated blood extravasation from the proximal left CIA (Figs. 1 and 2). The patient developed rapid abdominal distention and inadequate ECMO flow rates due to a significant retroperitoneal bleed. Cardiothoracic surgery was consulted, and the patient was taken urgently to the operating room for exploration. A large retroperitoneal hematoma was evacuated, and a vascular disruption of the left CIA was controlled with vascular clamps.
Fig. 1.
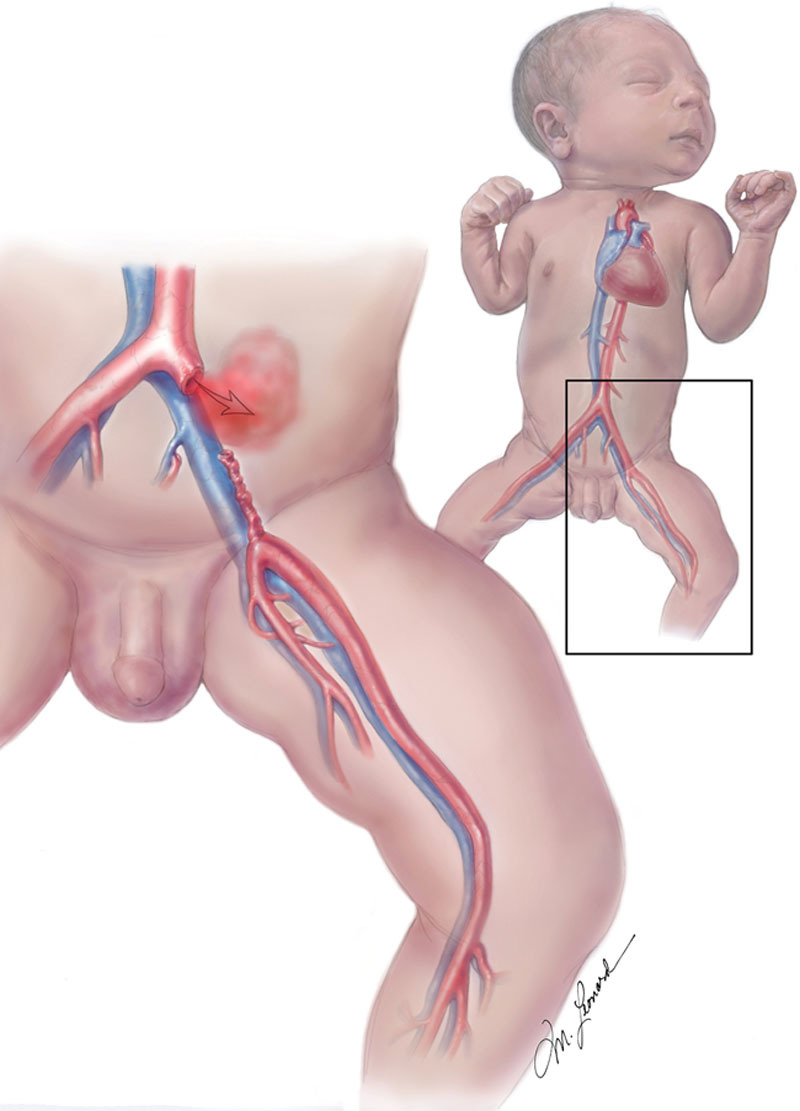
Initial injury with extravasation of blood from the CIA resulting in a large retroperitoneal hematoma. Illustrated by Michael Leonard.
Fig. 2.
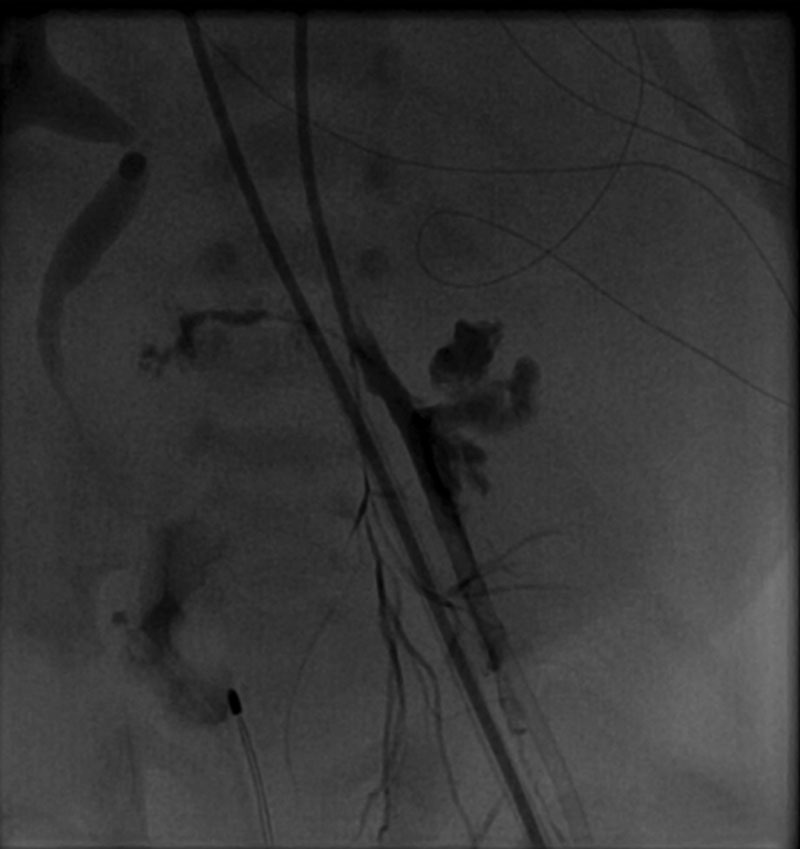
Arteriogram demonstrating extravasation of contrast into the retroperitoneum after the initial injury.
Despite improvement in systemic parameters, there was inadequate circulation to the left lower extremity as evidenced by significant cyanosis. Plastic surgery was then consulted to assist in vascular reconstruction.
Upon arrival, the plastic surgery team divided the inguinal ligament and exposed the regional anatomy. The zone of vascular injury extended from the proximal left CIA to the proximal superficial femoral artery (SFA) (Fig. 3). There was a “pseudo-sheath” of tissue that outlined the preexisting vascular anatomy, which demonstrated a 6-cm vascular defect.
Fig. 3.
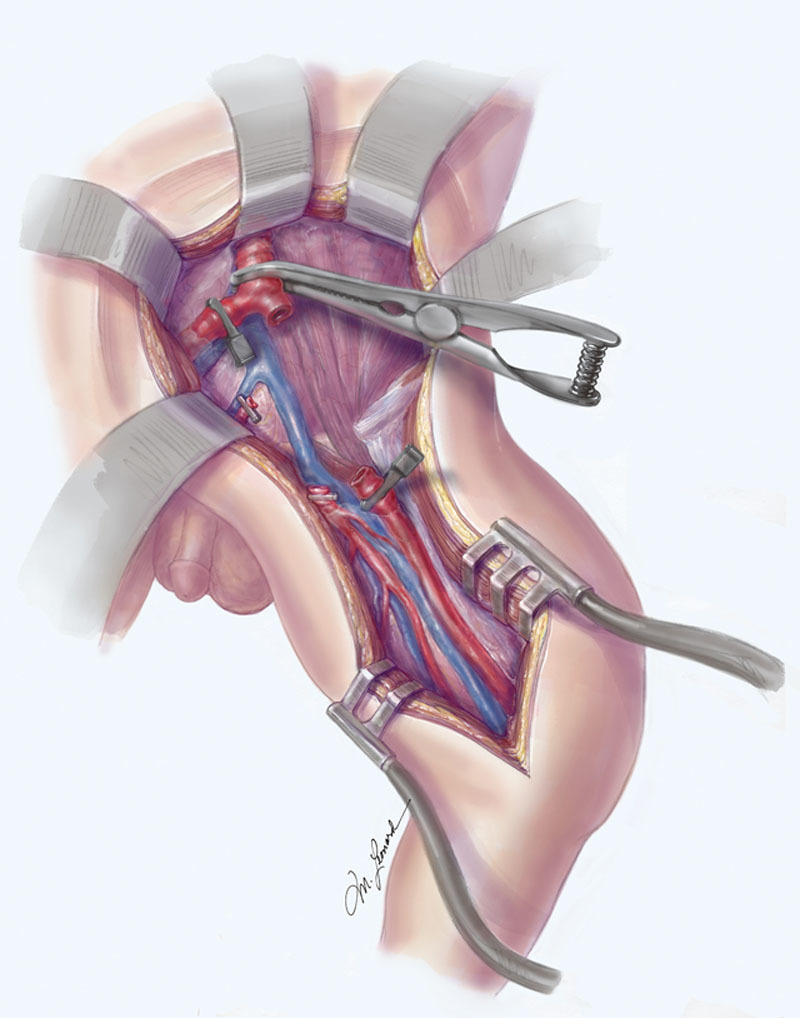
The zone of injury after surgical exposure extended from the proximal left CIA to the proximal SFA. Illustrated by Michael Leonard.
The vascular diameter was 2 mm proximally at the CIA and 1.5 mm distally at the SFA. Local exploration was performed for veins that would be of adequate length and caliber for autologous reverse vein grafting. The greater saphenous vein was located, but the vascular diameter measured less than 1 mm and was too small for the interpositional conduit. The superficial femoral venae (SFV) comitantes were identified and were significantly larger, measuring 2 mm at the level of the groin. The SFV was therefore harvested and reversed to be used as an interpositional autologous vein graft (Fig. 4). Because of the presence of a second venous comitante and substantial collateral venous outflow, the decision to harvest the SFV was not expected to impair vascular outflow from the lower extremity.
Fig. 4.
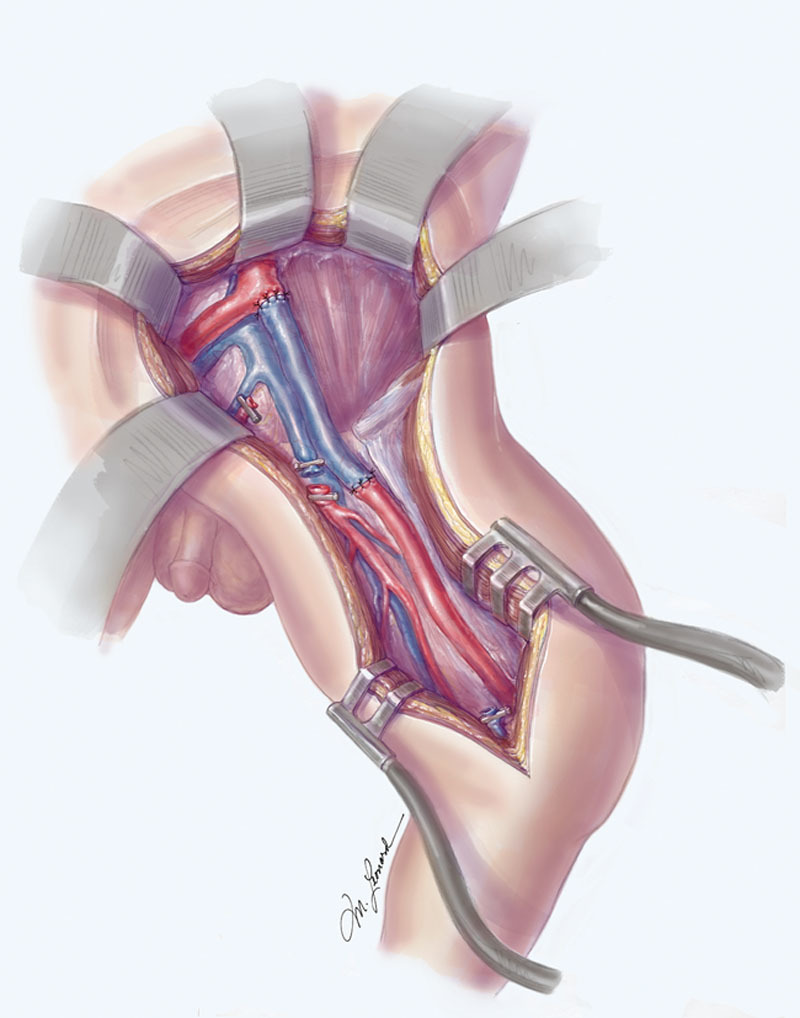
The SFV comitantes were utilized for an autologous reverse vein for primary repair. Illustrated by Michael Leonard.
Vascular repairs were performed with standard microsurgical techniques utilizing 8-0 nylon sutures with the aid of an operating microscope. Intraoperative Doppler examination showed biphasic pulsatile vascular flow in the dorsalis pedis and posterior tibial vessels. The total time from vascular injury to reperfusion was approximately 2.5 hours. Because of expeditious vascular repair, the risk of compartment syndrome after revascularization was low, and the surgical team decided to proceed with serial examinations rather than a prophylactic fasciotomy.
Bilateral lower extremity Doppler signals and clinical signs of perfusion of the leg were maintained throughout the hospitalization. The patient remained on ECMO for 5 days after the procedure. Approximately 1 month after the procedure, the patient received a heart transplant. He was discharged from the hospital on posttransplant day 17 with perfusion to the distal lower extremity. There was no impairment of venous outflow from the lower extremity.
DISCUSSION
Vascular injury is the most common complication of all cardiac catheterizations with rates of injury ranging from 0.3% to 3.2%.10–14 Cardiac catheterization causes iatrogenic arterial trauma at the access point that is typically managed through direct pressure or closure devices. This trauma is often insignificant and does not result in obstruction of flow. In rare cases, however, a vascular injury may occur distantly, or complications including hemorrhage, thrombosis, pseudoaneurysm, and arterial dissection may arise.12 These complications occasionally require immediate surgical intervention to restore circulation.10,13
RPH is a well-described hemorrhagic complication of catheterization and occurs in 0.15–6% of adult procedures.15–21 In children, however, the rate of RPH is lower. In a study evaluating 4,952 pediatric cardiac catheterizations, there was only one reported case of RPH after a tear in the iliac artery.7 However, RPH is a severe and potentially fatal complication, as the retroperitoneum can accommodate a significant volume of blood.15,17,19,20 The survival of patients is dependent on early recognition and surgical or interventional management.
After immediate vascular control in the case of an arterial injury leading to RPH, the vascular supply to the lower extremity must be evaluated. An injury to the common femoral artery can be devastating as it is the sole supply of blood to the lower extremity and requires urgent surgical or interventional management in both adults and children. Pediatric cases, however, can be more complex as repair of small caliber vessels requires great technical skill and may require microvascular techniques. Furthermore, the management of limb ischemic differs in children and adults as limb growth may be impaired by ischemia. Both growth restriction and limb length discrepancies have been reported in the literature after iatrogenic vascular injury.22,23 Thus, the threshold to perform vascular reconstruction is far lower in children. Both the increased urgency and technical skill required to perform a complex repair in a pediatric arterial injury necessitate adequate preparation as the scope of pediatric catheterization continues to expand.
CONCLUSIONS
Vascular injuries are the most common complication of cardiac catheterization and can lead to life- and/or limb-threatening events. As interventions are performed on younger and more complex patients, clinicians should be aware of the risk for adverse events and should be prepared with a multidisciplinary team should they arise.
ACKNOWLEDGMENTS
The authors would like to thank Michael Leonard for his work in illustrating the figures.
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Mehta R, Lee K, Chaturvedi R, et al. Complications of pediatric cardiac catheterization: a review in the current era. Catheter Cardiovasc Interv. 2008;72:278–285.. [DOI] [PubMed] [Google Scholar]
- 2.Feltes TF, Bacha E, Iii RHB, et al. Indications for cardiac catheterization and intervention in pediatric cardiac disease. Circulation 2011;123:2607–2652.. [DOI] [PubMed] [Google Scholar]
- 3.Allen HD, Beekman RH, 3rd, Garson A, Jr, et al. Pediatric therapeutic cardiac catheterization: a statement for healthcare professionals from the Council on Cardiovascular Disease in the Young, American Heart Association. Circulation 1998;97:609–625.. [DOI] [PubMed] [Google Scholar]
- 4.de Bono D. Complications of diagnostic cardiac catheterisation: results from 34,041 patients in the United Kingdom confidential enquiry into cardiac catheter complications. The Joint Audit Committee of the British Cardiac Society and Royal College of Physicians of London. Br Heart J. 1993;70:297–300.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stanger P, Heymann MA, Tarnoff H, et al. Complications of cardiac catheterization of neonates, infants, and children. A three-year study. Circulation 1974;50:595–608.. [DOI] [PubMed] [Google Scholar]
- 6.Cassidy SC, Schmidt KG, Van Hare GF, et al. Complications of pediatric cardiac catheterization: a 3-year study. J Am Coll Cardiol. 1992;19:1285–1293.. [DOI] [PubMed] [Google Scholar]
- 7.Vitiello R, McCrindle BW, Nykanen D, et al. Complications associated with pediatric cardiac catheterization. J Am Coll Cardiol. 1998;32:1433–1440.. [DOI] [PubMed] [Google Scholar]
- 8.Lee KE, Seo YJ, Kim GB, et al. Complications of cardiac catheterization in structural heart disease. Korean Circ J. 2016;46:246–255.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang YC, Chang JS, Lai YC, et al. Importance of prevention and early intervention of adverse events in pediatric cardiac catheterization: a review of three years of experience. Pediatr Neonatol. 2009;50:280–286.. [DOI] [PubMed] [Google Scholar]
- 10.Filis K, Arhontovasilis F, Theodorou D, et al. Management of early and late detected vascular complications following femoral arterial puncture for cardiac catheterization. Hellenic J Cardiol. 2007;48:134–142.. [PubMed] [Google Scholar]
- 11.Fransson SG, Nylander E. Vascular injury following cardiac catheterization, coronary angiography, and coronary angioplasty. Eur Heart J. 1994;15:232–235.. [DOI] [PubMed] [Google Scholar]
- 12.Messina LM, Brothers TE, Wakefield TW, et al. Clinical characteristics and surgical management of vascular complications in patients undergoing cardiac catheterization: interventional versus diagnostic procedures. J Vasc Surg. 1991;13:593–600.. [DOI] [PubMed] [Google Scholar]
- 13.Babu SC, Piccorelli GO, Shah PM, et al. Incidence and results of arterial complications among 16,350 patients undergoing cardiac catheterization. J Vasc Surg. 1989;10:113–116.. [DOI] [PubMed] [Google Scholar]
- 14.Johnson LW, Lozner EC, Johnson S, et al. Coronary arteriography 1984-1987: a report of the Registry of the Society for Cardiac Angiography and Interventions. I. Results and complications. Cathet Cardiovasc Diagn. 1989;17:5–10.. [DOI] [PubMed] [Google Scholar]
- 15.Sajnani N, Bogart DB. Retroperitoneal hemorrhage as a complication of percutaneous intervention: report of 2 cases and review of the literature. Open Cardiovasc Med J. 2013;7:16–22.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ellis SG, Bhatt D, Kapadia S, et al. Correlates and outcomes of retroperitoneal hemorrhage complicating percutaneous coronary intervention. Catheter Cardiovasc Interv. 2006;67:541–545.. [DOI] [PubMed] [Google Scholar]
- 17.Kent KC, Moscucci M, Mansour KA, et al. Retroperitoneal hematoma after cardiac catheterization: prevalence, risk factors, and optimal management. J Vasc Surg. 1994;20:905–910.; discussion 910. [DOI] [PubMed] [Google Scholar]
- 18.Sreeram S, Lumsden AB, Miller JS, et al. Retroperitoneal hematoma following femoral arterial catheterization: a serious and often fatal complication. Am Surg. 1993;59:94–98.. [PubMed] [Google Scholar]
- 19.Tiroch KA, Arora N, Matheny ME, et al. Risk predictors of retroperitoneal hemorrhage following percutaneous coronary intervention. Am J Cardiol. 2008;102:1473–1476.. [DOI] [PubMed] [Google Scholar]
- 20.Farouque HM, Tremmel JA, Raissi Shabari F, et al. Risk factors for the development of retroperitoneal hematoma after percutaneous coronary intervention in the era of glycoprotein IIb/IIIa inhibitors and vascular closure devices. J Am Coll Cardiol. 2005;45:363–368.. [DOI] [PubMed] [Google Scholar]
- 21.Waksman R, King SB, 3rd, Douglas JS, et al. Predictors of groin complications after balloon and new-device coronary intervention. Am J Cardiol. 1995;75:886–889.. [DOI] [PubMed] [Google Scholar]
- 22.Taylor LM, Jr, Troutman R, Feliciano P, et al. Late complications after femoral artery catheterization in children less than five years of age. J Vasc Surg. 1990;11:297–304.; discussion 304. [PubMed] [Google Scholar]
- 23.Flanigan DP, Keifer TJ, Schuler JJ, et al. Experience with iatrogenic pediatric vascular injuries. Incidence, etiology, management, and results. Ann Surg. 1983;198:430–442.. [DOI] [PMC free article] [PubMed] [Google Scholar]


