Abstract
Growth at high temperatures is one of the desired features for industrial applications of microbes, as it results in decrease in contamination and enhanced solubility of certain substrates. In this study, it is demonstrated that heterologous expression of a wheat cyclophilin, TaCypA-1, confers thermotolerance to Escherichia coli. The TaCypA-1 possesses peptidyl-prolyl cis-trans isomerase (PPIase) activity that catalyses cis to trans isomerization of the peptidyl prolyl bonds, a rate limiting step in protein folding. Expression of deleted mutants of TaCypA-1, that lacked PPIase activity, resulted in abrogation of thermotolerance, providing the first evidence that this activity plays a key role in stress tolerance of cells and can be exploited for industrial applications. Further, we also demonstrate that TaCypA-1 interacts with calmodulin (CaM), and the CaM-binding domain is localized to amino acid residues 51–71 in the N-terminus region.
Keywords: Cyclophilin, Escherichia coli, Heat stress, Peptidyl-prolyl cis-trans isomerase activity, Wheat
Highlights
-
•
Heterologous expression of a wheat cyclophilin, TaCypA-1, confers thermotolerance to E. coli.
-
•
PPIase activity is essential for heat stress tolerance of E. coli cells.
-
•
TaCypA-1 binds calmodulin in vitro in a Ca2+ -dependent manner.
-
•
CaM-binding domain is localized to 51–71 amino acid residues in the N-terminus of TaCypA-1.
1. Introduction
Cyclophilins and FK506-binding proteins (FKBPs), identified as receptors of immunosuppressive drugs cyclosporin A (CsA) and FK506, respectively [1], [2] are also called immunophilins. Several members of the cyclophilin and FKBP sub-families catalyse cis-trans isomerisation of the peptidyl-prolyl bond, which is a rate limiting step in protein folding [3]. Cyclophilins are ubiquitous proteins present in a range of evolutionary diverse organisms, from bacteria to higher organisms, including humans and plants. The cyclophilins constitute a large family of proteins in plants, with 31 and 29 different genes identified in the genomes of Arabidopsis and rice, respectively [4]. Though some of the cylophilins have been implicated in a variety of cellular functions such as protein folding, chaperonic activity, RNA processing, apoptosis, assembly and maintenance of photosystem II, and stress tolerance, the precise role of most of these proteins is still a matter of conjecture in plants ([5] and references therein).
Expression of cyclophilins in plants is modulated by different abiotic stresses such as heat-, cold-, drought- and salt stress [4], [5], [6], [7], [8], [9], suggesting a role of these proteins in stress adaptation. The role of cyclophilins in abiotic stress tolerance is further supported by recent studies which demonstrated that ectopic expression of cyclophilin genes from pigeon pea (CcCyp) [9], rice (OsCyp2) [4], [5] and a xerophytic fungus Piriformospora indica (PiCypA) [10] in the transgenic plants resulted in enhanced tolerance to multiple abiotic stress conditions. Wheat is one of the most important grain crops, and our recent studies demonstrated that one of its cyclophilin, TaCypA-1, possesses PPIase activity [11]. The role of TaCypA-1 in stress tolerance has not been investigated yet. Therefore, in the present study, we explored the potential of this gene for enhancing the heat stress tolerance of microbial cells using Escherichia coli as a model organism. Heat stress tolerance is an important factor for industrial application of microbial cells, since growth at elevated temperature minimizes contamination and also enhances the solubility of substrates. This study is the first one to demonstrate that overexpression of TaCypA-1 in E. coli results in enhanced tolerance to heat stress, and PPIase activity is essential for the protective effect of this protein. Further, we also provide evidence that TaCypA-1 is a calmodulin (CaM)-interacting protein and the CaM-binding domain is localized to 51–70 amino acid residues in the N-terminus region.
2. Materials and methods
2.1. Cloning of TaCypA-1 and its deletion variants
The cloning of 516 bp cDNA encoding full length TaCypA-1 in pET-28a(+) was described earlier [11]. The deleted mutants viz., TaCypA-1(64-513), TaCypA-1(151-513) and TaCypA-1(211-513) encoding the truncated proteins TaCyPA-1(22-171), TaCyPA-1(51-171) and TaCypA-1(71-171), respectively, were cloned using the forward and reverse primers that carried the EcoRI and XhoI restriction sites, respectively (Table 1). Amplification of different cDNA fragments was carried out by polymerase chain reaction (PCR) using the following conditions: initial denaturation (98 °C for 30 s) followed by 35 cycles of denaturation (98 °C for 10 s), primer annealing (57 °C for 30 s) and extension (72 °C for 30 s), with a final extension of 10 min at 72 °C. The PCR reaction mix contained primers (1 μM each), dNTPs (1 mM) and Pfu polymerase (0.5 U/μl). The amplified fragments were analysed on 1.4% agarose gel (Sigma–Aldrich Co., USA), extracted using commercial gel extraction kit (Bangalore GeNei Pvt. Ltd., India) and cloned into the plasmid pET-28a(+) after digesting with EcoRI and XhoI. Cloning of the different cDNA fragments was confirmed by sequencing (Bioserve Biotechnologies Pvt. Ltd., India).
Table 1.
Forward and reverse primer sequences used for cloning of the deleted mutants of TaCypA-1. The EcoRI and XhoI restriction sites are underlined.
| cDNA | Forward primer | Reverse primer |
|---|---|---|
| TaCypA-1(22-171) | CGGAATTCATGGGACTTTTCGGCAAGGC | CCGCTCGAGTCACAGCGGCACTTCACCGCTGT |
| TaCypA-1(51-171) | GACGAATTCAAGCCGCTGCACTACAAGGGC | GACCTCGAGCTAGAGCTGGCCGCAGTC |
| TaCypA-1(71-171) | CGGAATTCGGCGGCGACTTCACCAGGGG | CCGCTCGAGCTAGAGCTGGCCGCAGTCGGC |
2.2. Heterologous expression of recombinant proteins
Following transformation of the E. coli strain BL21(DE3)pLysS with the recombinant vectors, the 6xHis-tagged proteins were expressed after induction of the bacterial cells with 0.5 mM isopropyl-β-d-1-thiogalactopyranoside (IPTG) when absorbance at 600 nm reached 0.4–0.5, followed by further incubation for 16 h at 15 °C for TaCypA-1(51-171), and for 4 h at 25 °C for TaCypA-1, TaCypA-1(22-171) and TaCypA-1(71-171), with shaking at 180 rpm. The induced proteins were analysed by 15% SDS-PAGE followed by coomassie brilliant blue (CBB) staining [12]. The recombinant proteins were purified by suspending the cells in lysis buffer [50 mM Tris–HCl, 200 mM NaCl, 0.5% Triton X-100, 1 mM protease inhibitor cocktail (PIC), 80 μg/ml lysozyme] followed by sonication (time: 30 s, pulse on/off: 1.0 s) and centrifugation for 20 min at 14,000 rpm. The supernatants containing the recombinant proteins were incubated with Ni-NTA slurry (G Biosciences) in binding buffer [50 mM NaH2PO4 (pH 8.0), 300 mM NaCl, 0.25% Triton X-100, 1 mM PIC, 10% glycerol, 10 mM imidazole] for 2 h at 4 °C and applied to the column. The columns were washed with three bed-volumes of wash buffer [50 mM NaH2PO4 (pH 8.0), 300 mM NaCl, 0.25% Triton X-100, 1 mM PIC, 10% glycerol, 50 mM imidazole], followed by centrifugation at 4000 rpm for 4 min. The matrix-bound proteins were eluted thrice by addition of one bed-volume of elution buffer each [50 mM NaH2PO4 (pH 8.0), 300 mM NaCl, 0.25% Triton X-100, 1 mM PIC, 10% glycerol, 250 mM imidazole] and analysed by 15% SDS-PAGE.
2.3. Calmodulin (CaM)-binding assays
Gel-overlay assays were performed to study the CaM-binding property of the recombinant cyclophilins. The CaM gel-overlay assays were carried out as described earlier [13], except that the membrane was incubated with biotinylated CaM (Calbiochem, USA) at 25 °C instead of 4 °C.
2.4. Estimation of peptidyl prolyl cis-trans isomerase (PPIase) activity
Total soluble proteins from the induced E. coli cells were extracted as described earlier. The concentration of proteins was estimated according to Bradford [14] using BSA as a standard. The PPIase activity in the crude extracts was assayed at 15 °C for 360 s in a coupled reaction with chymotrypsin [15]. The p-nitroanilide residue from the peptide is cleaved by chymotrypsin only from trans-isomer of peptide containing proline but not the cis-isomer of peptide substrate. The release of p-nitroanilide from test peptide leads to a concomitant absorption increase at 390 nm. The 1 ml assay mixture contained 80 μM succinyl-ala-ala-pro-phe-p-nitroanilidine as a test peptide, assay buffer [50 mM HEPES (pH 8.0), 150 mM NaCl, 0.05% Triton X-100] and total soluble proteins (10 μg). The reaction was initiated by addition of chymotrypsin (300 μg/ml), and change in absorbance at 390 nm was monitored by using a spectrophotomer equipped with peltier temperature control system (Perkin Elmer, Lambda Bio 25). The effect of CsA, a specific inhibitor of cyclophilins [1], was studied by determining the extent of inhibition of the reaction. The CsA (10 μM) was added to the assay mix and incubated at 4 °C for 30 min before the start of reaction by addition of the test peptide (80 μM) and chymotrypsin (12 μM). The PPIase activity was calculated as a product of difference in catalysed and uncatalysed first order rate constants (derived from kinetics of absorbance change at 390 nm) and amount of substrate in each reaction [16]. The data obtained were analysed using software Grafit 4.0 (http://www.erithacus.com/grafit).
2.5. Stress tolerance assays
The E. coli BL21(DE3)pLysS cells, transformed with the recombinant pET-28a(+) plasmids encoding full length (TaCypA-1) and truncated cyclophilins [TaCypA-1(22-171), TaCypA-1 (51-171) and TaCypA-1(71-171) ], were grown in 25 ml Luria broth (LB) containing kanamycin (50 ug/ml) for 2 h (A600 ∼ 0.4–0.5). The cells transformed with non-recombinant plasmid were used as control. One ml of this culture was transferred to nine ml of LB containing IPTG (0.5 mM) in a 50 ml tube and incubated for 1 h at 37 °C before being subjected to heat stress at 47 °C (selected after evaluating a range of temperature conditions). The effect of cyclophilin inhibitor, CsA, on growth of cells expressing full length TaCypA-1 protein was studied by adding 1 μM CsA (selected after evaluating different concentrations) to the culture medium. The effect of high temperature on E. coli growth was monitored at regular intervals by recording the absorbance at 600 nm, and also by determining the colony counts after 2 h, 3 h and 5 h of heat stress. Parallel cultures (1 ml) were collected at these time points for studying the induction of recombinant proteins and estimation of PPIase activity. Data were subjected to two way analysis of variance (ANOVA) and the statistical level of significance was validated by Fisher's LSD method.
3. Results and discussion
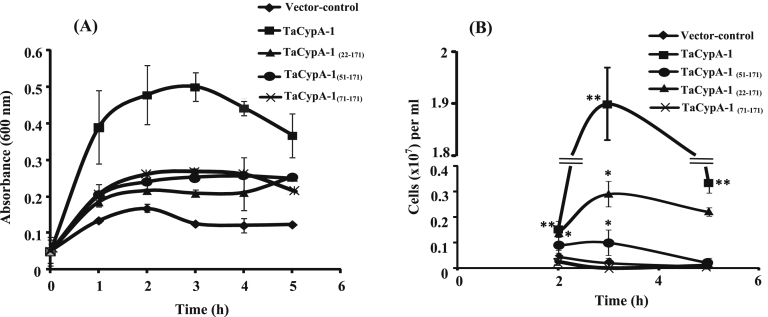
Few of the cyclophilins have been implicated in abiotic stress tolerance of plants [9], [10], [17] but the role of wheat cyclophilin, TaCypA-1, is still a matter of conjecture. Our previous studies demonstrated that TaCypA-1 is an enzymatically active protein and can catalyse cis-trans isomerisation of peptidyl-prolyl bond in vitro [11]. As PPIase activity is essential for protein folding in the cells, we used E. coli as a model organism to analyse the role of TaCypA-1 in heat stress tolerance. TaCypA-1 was expressed as a recombinant fusion protein and induced in E. coli with IPTG. The growth of E. coli cultures at 37 °C was not affected significantly by heterologous expression of full length or deleted wheat cyclophilins. The protective role of TaCypA-1 against heat stress in E. coli was studied by following the growth of cultures at 47 °C by taking absorbance at 600 nm, and also by determining colony counts (colony forming units or cfu/ml). It was observed that as compared to cells transformed with empty vector, the cells expressing full-length TaCypA-1 showed significantly higher growth under heat stress (Fig. 1A). The colony count was also significantly higher for the cells that expressed TaCypA-1 (Fig. 1B).
Fig. 1.
Effect of heat stress (47 °C) on growth of E. coli BL21(DE3)pLysS cells expressing different variants of wheat cyclophilin TaCypA-1. The cells transformed with non-recombinant pET-28a(+) were used as control. The growth of cultures was monitored by recording absorbance at 600 nm (A) and by determining the colony forming units (cfu/ml) (B). The starting viable cell density was similar for all cultures. Data represent mean ± S.E of three independent biological replicates. Asterisks represent level of significance determined after two way analysis of variance. ** = statistically significant at P < .001 (LSD = 0.050); * = statistically significant at P = .011 at 2 h and P = .001 at 3 h and 5 h (LSD = 0.050).
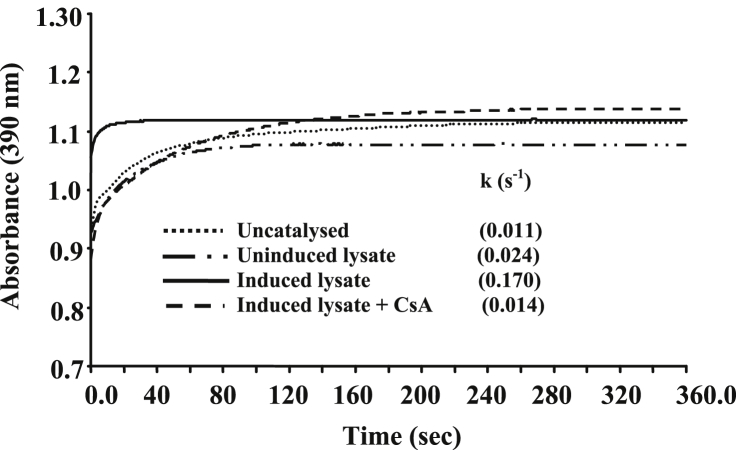
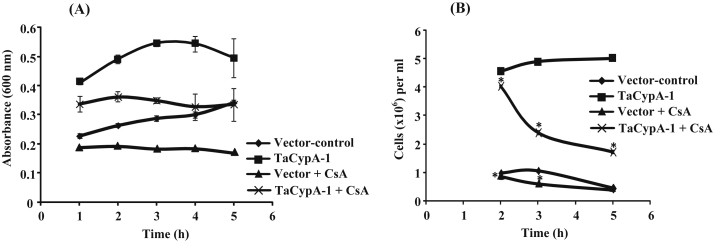
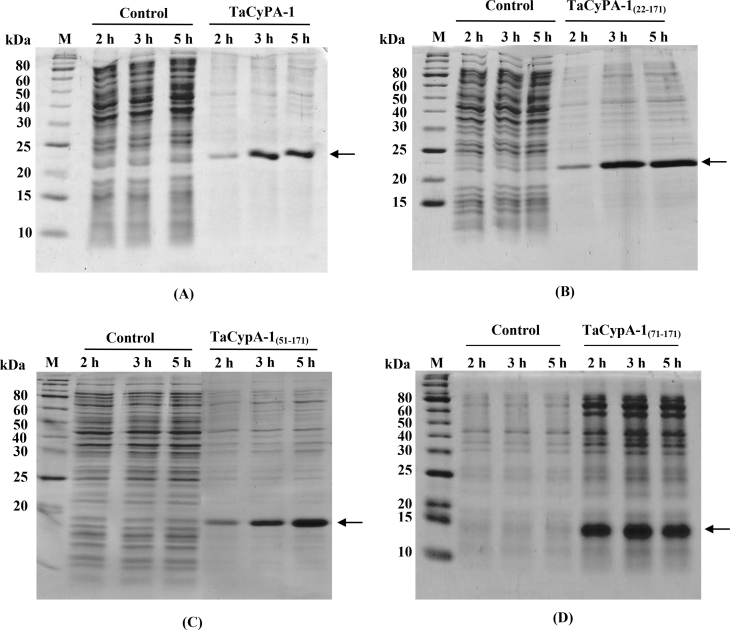
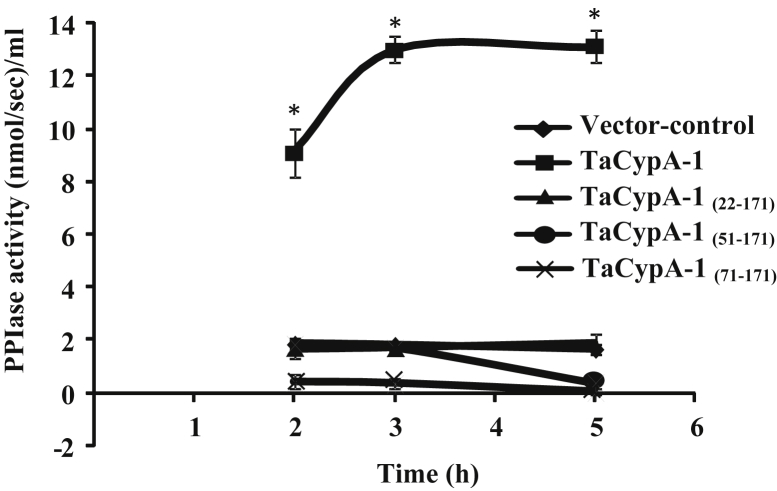
To further understand the basis of TaCypA-1-induced stress tolerance, we estimated PPIase activity in the lysates of different E. coli cells (Fig. 2). The first order rate constant (0.024 s−1) in the presence of crude proteins extracted from the control cells carrying empty vector was almost 2-fold higher than the uncatalysed control (0.011 s−1). These results are consistent with the earlier studies that reported the presence of PPIases in E. coli [18]. In comparison to the control cells carrying empty vector, the PPIase activity of the cells that over-expressed full-length TaCypA-1 was approximately 7-fold higher (0.170 s−1) (Fig. 2). This activity was, however, completely inhibited by CsA since the rate constant decreased to 0.014 s−1 (Fig. 2), implying the role of cyclophilin. Furthermore, addition of CsA (1 μM) to the recombinant cultures resulted in a substantial decrease in the growth of cells under heat stress, indicating decrease in thermotolerance (Fig. 3A, B). Since CsA is a specific inhibitor of cyclophilins [1], these results imply that TaCypA-1-associated PPIase activity was responsible for enhanced protection against elevated temperatures, thereby, enhancing the viability of TaCypA-1-expressing E. coli cells. Further validation for the role of PPIase activity in heat stress tolerance was carried out by expressing the deleted TaCypA-1 variants, that lacked N-terminal amino acid residues 21 [TaCypA-1(22-171)], 50 [TaCypA-1(51-171)] and 70 [TaCypA-1(71-171)], in the E. coli cells. The different recombinant proteins were induced, purified and validated by the presence of bands corresponding to 22.22 kDa, 20.1 kDa, 16.9 kDa and 14.6 kDa, respectively after 15% SDS-PAGE (Fig. 4), which is in accordance with the predicted molecular weights of 18.32 kDa, 16.2 kDa, 13 kDa and 10.70 kDa for TaCypA-1 and its deleted versions [TaCypA-1(22-171)], [TaCypA-1(51-171)] and [TaCypA-1(71-171)], respectively. Enzymatic analysis revealed that none of the purified deleted cyclophilins had any significant effect on chymotrypsin-mediated cleavage of the substrate peptide, as indicated by the first order rate constants, implying that deletion of N-terminus amino acid residues resulted in abrogation of PPIase activity in TaCypA-1 (Table 2). On the contrary, addition of full length TaCypA-1 to the reaction resulted in approximately 6-fold increase in the rate constant (0.089 s−1) as compared to the uncatalysed (0.015 s−1) (Table 2). Stress tolerance studies demonstrated that cells expressing the deleted TaCypA-1 proteins showed significantly lesser growth as compared to the cells expressing full-length TaCypA-1 (Fig. 1A, B). SDS-PAGE analysis of the crude extracts after 2 h, 3 h and 5 h of induction demonstrated that the recombinant TaCypA-1, TaCypA-1(22-171), TaCypA-1(51-171) and TaCypA-1(71-171) proteins were expressed in all the respective E. coli cultures (Fig. 4). These observations indicate that the heat stress-induced decrease in viability of the cells that expressed different deleted TaCypA-1 proteins was not due to the lack of expression of recombinant proteins. Furthermore, the cells expressing different truncated TaCypA-1 proteins exhibited significantly lower PPIase activity under heat stress than the cells expressing intact cyclophilin (Fig. 5). It is, thus, evident that higher growth of E. coli cells, that expressed native TaCypA-1, at elevated temperature was also associated with higher PPIase activity. These results, therefore, provide the first evidence that PPIase activity of TaCypA-1 is essential for imparting tolerance to E. coli cells towards heat stress and, therefore, potential of this gene to develop industrially important microbial strains, that are able to withstand stressful conditions, need to be explored further by carrying out fermenter scale studies. Further, the potential of TaCypA-1 gene to enhance stress tolerance also needs to be validated using microbes other than E. coli so as to determine its industrial applications.
Fig. 2.
Effect of CsA (10 μM) on peptidyl-prolyl cis-trans isomerase (PPIase) activity of E. coli lysates overexpressing TaCypA-1 after exposure to heat stress at 47 °C for 5 h. Total PPIase activity in the cell cultures was estimated by studying the effect of 10 μg of crude proteins, harvested from the induced and uninduced E. coli cultures, on the chymotrypsin-mediated cleavage of the test peptide (N-succinyl-ala-ala-pro-phe-p-nitroanilidine). The uncatalysed reaction signifies the chymotrypsin-mediated cleavage of the test peptide in the absence of E. coli lysate. The PPIase activity is expressed as first order rate constant (s−1).
Fig. 3.
Effect of CsA (1 μM) on growth of E. coli BL21(DE3)pLysS cells transformed with wheat cyclophilin TaCypA-1 gene. The cells transformed with non-recombinant pET-28a(+) were used as control. The growth of cultures was monitored by recording the absorbance at 600 nm (A) and by determining the colony forming units (cfu/ml) (B). The starting viable cell density was similar for all cultures. Asterisks represent level of significance determined after two way analysis of variance by using Fischer LSD method. * = statistically significant at P < .001 (LSD = 0.050).
Fig. 4.
15% SDS-PAGE analysis of total proteins extracted from the recombinant E. coli cells over-expressing TaCypA-1 (A), TaCypA-1 (22-171) (B) TaCypA-1 (51-171) (C) and TaCypA-1 (71-171) (D) after exposure to heat stress at 47 °C. The E. coli lysates were analysed after induction with 0.5 mM of isopropyl β-d-1-thiogalactopyranoside after 2 h, 3 h and 5 h of growth. The cells transformed with non-recombinant pET28a (+) were used as control. The induced proteins are indicated by arrows. M: molecular weight markers.
Table 2.
Effect of purified wheat cyclophilin, TaCypA-1, and its deleted mutants on the rate of reaction of chymotrypsin-mediated hydrolysis of N-succinyl-ala-ala-pro-phe-p-nitroanilidine (peptidyl-prolyl cis-trans isomerase activity). The uncatalysed control consisted of the reaction carried out in the absence of cyclophilin. Data represent the mean of three replicates ±SE.
| Recombinant protein | First order rate constant (s−1) |
|---|---|
| Uncatalysed | 0.015 ± 0.0068 |
| TaCypA-1 | 0.089 ± 0.0012 |
| TaCypA-1 (22–171) | 0.022 ± 0.0072 |
| TaCypA-1 (51–171) | 0.019 ± 0.0026 |
| TaCypA-1 (71–171) | 0.0185 ± 0.0057 |
Fig. 5.
Effect of heat stress on peptidyl-prolyl cis-trans isomerase (PPIase) activity of the E. coli cells transformed with non-recombinant pET-28a(+) (vector control) and recombinant vector containing cDNAs encoding different variants of TaCypA-1. The cells were grown at 47 °C and harvested after 2 h, 3 h, and 5 h of induction with 0.5 mM of isopropyl β-d-1-thiogalactopyranoside. Total PPIase activity in the cell cultures was estimated by studying the effect of 10 μg of crude proteins, harvested from the induced E. coli cultures, on the chymotrypsin-mediated cleavage of the test peptide (N-succinyl-ala-ala-pro-phe-p-nitroanilidine). The PPIase activity is expressed as first order rate constant (s−1). Data represent the mean ±S.E of three replicates. Asterisks represent level of significance by using Fisher's LSD method after two way analysis of variance of this data. * = Statistically significant at P < .05.
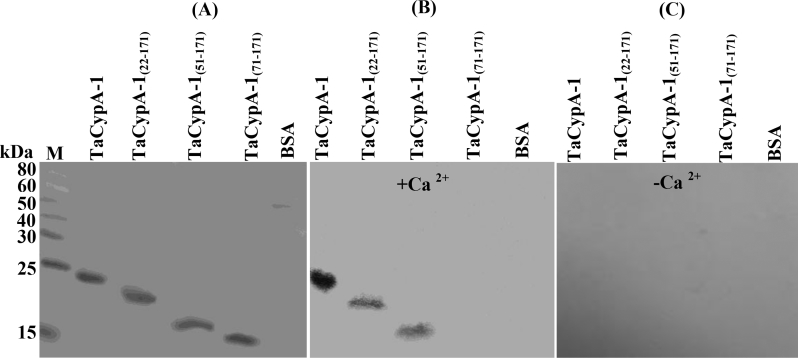
Several studies have shown that besides cis-trans isomerization, members of cyclophilin family also perform additional functions in the cell [5]. We recently demonstrated that an Arabidopsis cyclophilin, AtCyp19-3, which is 72.67% identical to TaCypA-1, interacts with CaM in a Ca2+-dependent manner [19]. CaM is one of the most well characterized sensors of Ca2+, and it consists of four EF-hands that bind to Ca2+ cooperatively [20]. After binding with Ca2+, the CaM undergoes conformation change and regulates activities of a diverse group of proteins. As information on this aspect is lacking for TaCypA-1, we studied the interaction of this cyclophilin with CaM by performing gel-overlay assays in the presence and absence of Ca2+ (Fig. 6). These studies showed the binding of CaM with native TaCypA-1, TaCypA-1(22-171) and TaCypA-1(51-171) but not with TaCypA-1(71-171), implying that CaM-binding property in this cyclophilin is determined by the N-terminus 50–71 amino acid residues. It was also observed that binding of CaM with TaCypA-1, TaCypA-1(22-171) and TaCypA-1(51-171) was observed only in the presence of Ca2+. Thermotolerance of the cells expressing TaCypA-1(22-171) and TaCypA-1(51-171), that contained CaM binding domain but lacked PPIase activity, was significantly attenuated as compared to cells expressing native TaCypA-1 protein, thus suggesting that CaM-interacting domain is not essential for survival of cells under heat shock. Further studies, which are underway, will elucidate the physiological significance of interaction of TaCyPA-1 with CaM.
Fig. 6.
Calmodilin (CaM) gel overlay assays to identify CaM-binding domain of TaCypA-1. One μg of the purified recombinant cyclophilin proteins was resolved by 15% SDS-PAGE, followed by transfer to Hybond-C nitrocellulose membrane and staining with Ponceau S (A). The membrane was incubated with biotinylated-CaM in the presence (B) and absence of Ca2+ (C) followed by probing with streptavidin-alkaline phosphate conjugate. The CaM-binding proteins were visualized using nitro-blue tetrazolium/5-bromo-4-chloro-3-indoly phosphate as substrate. Bovine serum albumin (BSA) was used as a negative control.
4. Conclusions
To conclude, the present study is the first to demonstrate that heterologous expression of the wheat cyclophilin, TaCypA-1, confers protection against thermal stress in E. coli cells and PPIase activity is imperative for the same. Further, this cyclophilin also interacts with CaM in a Ca2+-dependent manner, and the CaM-binding domain is localized to 51–71 amino acid residues in the N-terminus. Further studies, employing large-scale cultures, are required to validate the potential of TaCypA-1 in imparting thermotolerance to commercially useful microbial strains.
Conflict of interest
There is no conflict of interest.
Acknowledgements
Financial assistance for this work from Department of Biotechnology, Govt. of India is thankfully acknowledged. GK is thankful to University Grants Commission, Govt. of India for the award of Maulana Azad National fellowship. HK and TD are grateful to Council of Scientific and Industrial Research, Govt. of India for the grant of Senior Research Fellowships.
References
- 1.Handschumacher R.E., Harding M.W., Rice J., Drugge R.J., Speicher D.W. Cyclophilin: a specific cytosolic binding protein for cyclosporin A. Science. 1984;226:544–547. doi: 10.1126/science.6238408. [DOI] [PubMed] [Google Scholar]
- 2.Harding M.W., Gallat A., Uehling D.E., Schreiber S.L. A receptor for the immunosuppressant FK506 is a cis-trans peptidyl-prolyl isomerase. Nature. 1989;341:758–760. doi: 10.1038/341758a0. [DOI] [PubMed] [Google Scholar]
- 3.Fischer G., Bang H. The refolding of urea-denatured ribonuclease A is catalyzed by peptidyl-prolyl cis-trans isomerase. Biochim. Biophys. Acta. 1985;828:39–42. doi: 10.1016/0167-4838(85)90006-8. [DOI] [PubMed] [Google Scholar]
- 4.Kumari S., Joshi R., Singh K., Roy S., Tripathi A.K., Singh P. Expression of a cyclophilin OsCyp2-P isolated from a salt-tolerant landrace of rice in tobacco alleviates stress via ion homeostasis and limiting ROS accumulation. Funct. Integr. Genomics. 2015;15:395–412. doi: 10.1007/s10142-014-0429-5. [DOI] [PubMed] [Google Scholar]
- 5.Kumari S., Roy S., Singh P., Singla-Pareek S.L., Pareek A. Cyclophilins: proteins in search of function. Plant Signal Behav. 2013;8:e22734. doi: 10.4161/psb.22734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marivet J., Frendo P., Bukard G. Effects of abiotic stresses on cyclophilin gene expression in maize and bean and sequence analysis of bean cyclophilin cDNA. Plant Sci. 1992;84:171–178. [Google Scholar]
- 7.Sharma A.D., Singh P. Effect of water stress on expression of a 20 kD cyclophilin-like protein in drought susceptible and tolerant cultivars of sorghum. J. Plant Biochem. Biotech. 2003;12:77–80. [Google Scholar]
- 8.Sharma A.D., Singh P. Comparative studies on drought-induced changes in peptidyl prolyl cis-trans isomerase activity in drought-tolerant and susceptible cultivars of Sorghum bicolor. Curr. Sci. 2003;84:912–917. [Google Scholar]
- 9.Sekhar K., Priyanka B., Reddy V.D., Rao K.V. Isolation and characterization of a pigeonpea cyclophilin (CcCYP) gene, and its over-expression in Arabidopsis confers multiple abiotic stress tolerance. Plant Cell Environ. 2010;33:1324–1338. doi: 10.1111/j.1365-3040.2010.02151.x. [DOI] [PubMed] [Google Scholar]
- 10.Trivedi D.K., Ansari M.W., Dutta T., Singh P., Tuteja N. Molecular characterization of cyclophilin A-like protein from Piriformospora indica for its potential role to abiotic stress tolerance in E. coli. BMC Res. Notes. 2013;23:555. doi: 10.1186/1756-0500-6-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sekhon S.S., Kaur H., Dutta T., Singh K., Kumari S., Kang S. Structural and biochemical characterization of the cytosolic wheat cyclophilin TaCypA-1. Acta. Crystallogr. D. Biol. Crystallogr. 2013;69:555–563. doi: 10.1107/S0907444912051529. [DOI] [PubMed] [Google Scholar]
- 12.Laemmli U.K. Cleavage of structural protein during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 13.Virdi A.S., Thakur A., Dutt S., Kumar S., Singh P. A sorghum 85 kDa heat stress-modulated protein shows calmodulin-binding property and cross-reactivity to anti-Neurospora crassa Hsp 80 antibodies. FEBS Lett. 2009;583:767–770. doi: 10.1016/j.febslet.2009.01.025. [DOI] [PubMed] [Google Scholar]
- 14.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;2:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 15.Fischer G., Bang H., Mech C. Determination of enzymatic catalysis for the cis-trans isomerisation of peptide binding in proline containing peptides. Biomed. Biochim. Acta. 1984;43:1101–1111. [PubMed] [Google Scholar]
- 16.Breiman A., Fawcett T.W., Ghirardi M.I., Mattoo A.K. Plant organelles contain distinct peptidyl prolyl cis-trans isomerases. J. Biol. Chem. 1992;267:21293–21296. [PubMed] [Google Scholar]
- 17.Kumari S., Singh P., Singla-Pareek S.L., Pareek A. Heterologous expression of a salinity and developmentally regulated rice cyclophilin gene (OsCyp2) in E. coli and S. cerevisiae confers tolerance towards multiple abiotic stresses. Mol. Biotechnol. 2009;43:195–204. doi: 10.1007/s12033-009-9153-0. [DOI] [PubMed] [Google Scholar]
- 18.Compton L.A., Davis J.M., MacDonald R., Bachinger H.P. Structural and functional characterization of Escherichia coli peptidyl-prolyl cis-trans isomerases. Eur. J. Biochem. 1992;206:927–934. doi: 10.1111/j.1432-1033.1992.tb17002.x. [DOI] [PubMed] [Google Scholar]
- 19.Kaur G., Singh S., Singh H., Chawla M., Dutta T., Kaur H. Characterization of peptidyl-prolyl cis-trans isomerase- and calmodulin-binding activity of a cytosolic Arabidopsis thaliana cyclophilin AtCyp19-3. PLoS One. 2015;10:1–21. doi: 10.1371/journal.pone.0136692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Snedden W.A., Fromm H. The calcium and calmodulin signaling networks in plants. New Phytol. 2001;51:35–66. doi: 10.1046/j.1469-8137.2001.00154.x. [DOI] [PubMed] [Google Scholar]