Abstract
In patients with sepsis, liver metabolism and its capacity to provide other organs with energetic substrates are impaired. This and many other pathophysiological changes seen in human patients are reproduced in mice injected with purified endotoxin (lipopolysaccharide, LPS). In the present study, down-regulation of genes involved in hepatic fatty acid oxidation (FAOx) and gluconeogenesis in mice exposed to LPS was challenged by nutritional intervention with Argan oil. Mice given a standard chow supplemented or not with either 6% (w/w) Argan oil (AO) or 6% (w/w) olive oil (OO) prior to exposure to LPS were explored for liver gene expressions assessed by mRNA transcript levels and/or enzyme activities. AO (or OO) food supplementation reveals that, in LPS-treated mice, hepatic expression of genes involved in FAOx and gluconeogenesis was preserved. This preventive protection might be related to the recovery of the gene expressions of nuclear receptors peroxisome proliferator-activated receptor α (PPARα) and estrogen related receptor α (ERRα) and their coactivator peroxisome proliferator-activated receptor gamma coactivator-1α, (PGC-1α). These preventive mechanisms conveyed by AO against LPS-induced metabolic dysregulation might add new therapeutic potentialities in the management of human sepsis.
Keywords: Argan oil, Beta-oxidation, Coactivator, Gluconeogenesis, Nuclear receptor
Abbreviations: ACADS, acyl CoA dehydrogenase short-chain; ACADM, acyl CoA dehydrogenase medium-chain; ACADL, acyl CoA dehydrogenase long-chain; AO, Argan oil; ACOX1, acyl-CoA oxidase 1; ERRα, estrogen related receptor α; G6PH, glucose-6-phosphatase; Glut2, glucose transporter 2; Glut4, glucose transporter 4; HNF-4α, hepatic nuclear factor-4α; OO, olive oil; LPS, lipopolysaccharide; PGC-1α, peroxisome proliferator-activated receptor γ coactivator-1α; PEPCK, phospoenolpyruvate carboxykinase; PPARα, peroxisome proliferator-activated receptor α
Highlights
-
•
Argan oil prevents LPS-treated mice from liver dysregulation of FAOx and gluconeogenesis.
-
•
Argan oil improves hepatic expression of PPARα and ERRα, and their coactivators PGC-1α and Lipin-1.
-
•
New preventive mechanisms conveyed by Argan oil against LPS-induced metabolic dysregulation.
1. Introduction
Bacterial infection is a common cause of sepsis, a pathological state inducing a severe organ dysfunction and a high mortality rate, and requiring intensive care [1], [2], [3]. This acute syndrome is associated with systemic inflammation and disturbed metabolism [4], [5]. During bacterial infection, release in the host of endotoxins (lipopolysaccharides, LPS) from gram-negative bacteria membrane generates a potent inflammatory cytokine response and severely impairs lipid metabolism, inducing reduced serum high density lipoprotein (HDL), increased plasma free fatty acids and triglycerides levels [3]. These metabolic changes are mainly accounted for by enhanced hepatic triglyceride synthesis and adipose tissue lipolysis combined with a drop in fatty acid oxidation (FAOx) in several tissues including heart, kidney, liver and skeletal muscle [3], [6], [7], [8], [9], [10]. The downregulation of FAOx by LPS is correlated with decreased expressions of the nuclear receptor Peroxisome Proliferator-Activated Receptor (PPAR)α and its coactivator PPARγ Coactivator (PGC)-1α, which physiologically work in concert to regulate FAOx-related gene expressions [11], [12]. In this respect, ligand-dependent activation of the nuclear receptor PPARα prompts its heterodimerization with Retinoid X Receptor (RXR)α [13], [14]. The PPARα/RXRα complex binds to PPARα-response elements (PPRE) of target genes which may code for mitochondrial and peroxisomal enzymes involved in fatty acid β-oxidation pathways such as carnitine palmitoyl transferase 1 (CPT1a and CPT1b), short-, medium-, long- and very long-chain acyl CoA dehydrogenases (ACADS, ACADM, ACADL and ACADVL) [15], [16], [17], [18], [19], acyl-CoA oxidase 1 (ACOX1) [17], [20], [21] and other proteins [12], [22]. On the other hand, Lipin-1, a phosphatidate phosphatase, has arisen as an additional transcriptional co-regulator of PPARα–PGC-1α-directed gene expression [23]. Its interaction with PPARα-PGC1α complex promotes the induction of FAOx genes [24]. Beside PPARα, estrogen related receptor (ERR) α or (ESRRα), an orphan nuclear receptor, has been also shown to regulate energy metabolism gene expression [25], [26], particularly genes involved in FAOx [27], [28]. This transcriptional regulation involves interaction with PCG-1α coactivator through a protein motif specifically dedicated to ERRα [29], [30]. In liver, another interaction of PGC-1α is also observed for hepatic nuclear factor-4α (HNF-4α) to control genes coding gluconeogenesis proteins (phospoenolpyruvate carboxykinase (PEPCK), glucose-6-phosphatase (G6PH)) and glucose transporter 4 (Glut4) [31].
More than 20% of patients with sepsis develop liver dysfunction [1] and hence dysregulation of hepatic metabolism and reduced energy supply for other organs. In mouse models of sepsis, injection of purified LPS triggers many pathophysiological changes resembling those described in human patients [32]. Though down-regulatory mechanisms by which LPS impacts FAOx have been extensively studied, little attention has been actually paid to mechanisms capable of preserving normal FAOx and inflammation status. Interestingly, supplementation of parenteral nutrition with fish oil to patients, during the postoperative period, revealed lowest levels of circulating inflammatory mediators [33], [34], [35]. Accordingly, polyunsaturated fatty acid-rich diet has been reported to reduce acute inflammation and to promote anti-inflammatory process in mice [36]. Therefore, lipid nutritional support might help the prevention of not only inflammatory damages but also disrupted lipid homeostasis.
Argan edible oil (AO) is obtained by cold-pressure of roasted kernels from Argania spinosa [L.] Skeels, a singular Mediterranean species growing in the southwestern region of Morocco. Argan oil is used as a traditional food ingredient in the ‘Amazigh diet’, bringing almost 25% of total diet fat intake to indigenous consumers [37]. Accordingly, early clinical studies on Argan oil reported a decrease in plasma low density lipoprotein-cholesterol (LDL-cholesterol) and lipid hydroperoxides along with a rise in plasma tocopherol concentration [38]. Health benefits of this delectable virgin oil have been highlighted by several studies documenting its cardiovascular protective potential including hypocholesterolemic and hypotriglyceridemic properties in consumer populations [39], [40], [41]. AO has been also shown to reduce circulating LDL-cholesterol and ApoB and, in AO consumers, to increase HDL and ApoAI [40], [41] whereas in human macrophages it increases HDL-mediated cholesterol efflux and reduces LDL-lipid peroxidation [38], [39].
Therefore, in an attempt to test our hypothesis regarding the preventive effects of Argan oil against LPS-induced FAOx downregulation, mice pretreated with AO were subsequently injured by LPS to determine whether an experimental support may be or not given to this working hypothesis. The effects of AO against sepsis-associated liver hyperlipidemia are compared to those of olive oil (OO), a more usual ingredient in Mediterranean diets. We report here that, in fact, AO-enriched diet prevents LPS-associated hyperlipidemic effect through the induction of the hepatic expressions of PPARα, ERRα and their coactivator PGC-1α along with the up-regulation of their mitochondrial (ACADS, ACADM, ACADVL) and peroxisomal (ACOX1) target genes.
2. Material and methods
2.1. Argan oil treatment
Swiss OF1 mice (12–16 week-old) were obtained from IFFA CREDO (Casablanca). They were acclimatized in the laboratory for 10 days at 22 ± 2 °C with standard chow and water ad libitum. Animal studies were conducted in accordance with the protocols of Animal Use and Care of the University of Hassan 1st, Settat, Morocco. The virgin Argan oil used in this work was obtained from the Aklim area in the northeast of Morocco. Six groups of mice (5 mice/group) received during 25 days: a standard chow (2 groups, control); a standard chow supplemented with 6% (w/w) of Argan oil (2 groups, AO) or a standard chow supplemented with 6% (w/w) of olive oil (2 groups, OO). Oils were included in the diets by direct mixing with the standard animal chow. Sixteen hours before euthanasia and during the fed state, one group from control (+LPS), AO (AO + LPS) and OO (OO + LPS) respectively received (5 mg/kg) intraperitoneal injections of 100 µg of Escherichia coli 0111:B4 LPS (Sigma) resuspended in phosphate-buffered saline (PBS) or an equal volume of PBS alone.
2.2. Composition of oils
Both Argan and olive oils (AO and OO) contain mono and polyunsaturated fatty acids. However, Argan oil has 35% of C18:2n-6 and 45% of C18:1n-9 while olive oil shows only 6% of C18:2n-6 and more than 75% of C18:1n-9, leading to a higher unsaturation index of AO (120.4) versus OO (108.3) [42].
2.3. Quantitative PCR analysis
Total RNA from liver was extracted using the RNeasy Mini kit (Qiagen) following the manufacturer's instructions. cDNA was generated by reverse transcription using Moloney Murine Leukemia Virus Reverse Transcriptase (Promega) according to the manufacturer's protocol and analyzed by quantitative PCR using the GoTaq® qPCR Master Mix (Promega), and a StepOnePlus Real-Time PCR System (Applied Biosystem). The primer sequences were chosen using the Beacon Designer Software (Bio-Rad). Oligonucleotide sequences are shown in the Supplementary Table 1. PCR reactions were carried out in duplicate in a final volume of 12.5 µL containing 6.25 µL of MESA Green qPCR Master mix (Eurogentec), 2.5 µL of cDNA and forward and reverse primers at 300 nM. The PCR enzyme (Taq DNA polymerase) was heat-activated at 95 °C for 10 min, and the DNA was amplified for 40 cycles at 95 °C for 15 s, 60 °C for 30 s, and 72 °C for 30 s, followed by a melting curve analysis to control the absence of nonspecific products. For each transcript, the amplification efficiency was determined by the slope of the standard curve generated from two fold serial dilutions of cDNA. Gene expression was quantified using cycle to threshold (Ct) values and normalized by the reference gene, 36B4 encoding the acidic ribosomal phosphoprotein P0. To this end, the quantitative gene expression was determined according to 2−ΔΔCt with ΔCt = (Ct of the gene studied) – (Ct of the 36B4 gene).
2.4. Enzymatic activity measurements
One hundred mg of liver tissue were homogenized by a Potter-Elvehjm homogenizer in 0.2 ml of a buffer containing 250 mmol/L sucrose, 20 mmol/L Tris–HCl pH 7.5 and 2 mmol/L EDTA. After centrifugation at 600 g for 5 min at 4 °C, the supernatant was collected and stored at −80 °C until use. Peroxisomal acyl-CoA oxidase (ACOX1) activity was measured by the fluorometric assay using palmitoyl-CoA as a substrate as described previously [43]. Catalase activity was monitored at 240 nm as described elsewhere [44]. Mitochondrial acyl-CoA dehydrogenases activities were followed at 600 nm on acyl-CoAs of different chain lengths (for experimental details, see legends to figures) [45].
2.5. Statistical analysis
Statistical analyses to compare two experimental groups were performed with, an unpaired, two-tailed, Student-t test (Excel software) for calculating the probability values and data were considered statistically different at a P-value of 0.05 or less.
3. Results
3.1. Body weight under experimental diets
Fig. S1 shows the time-course of body weights evolution during the nutritional intervention. Each group of mice was weighted at four different time points during the 3 weeks that precede the LPS administration; no significant differences in body weight have been found between the different experimental groups.
3.2. Argan oil modulates the expression of PPARα, PGC-1α and related target genes
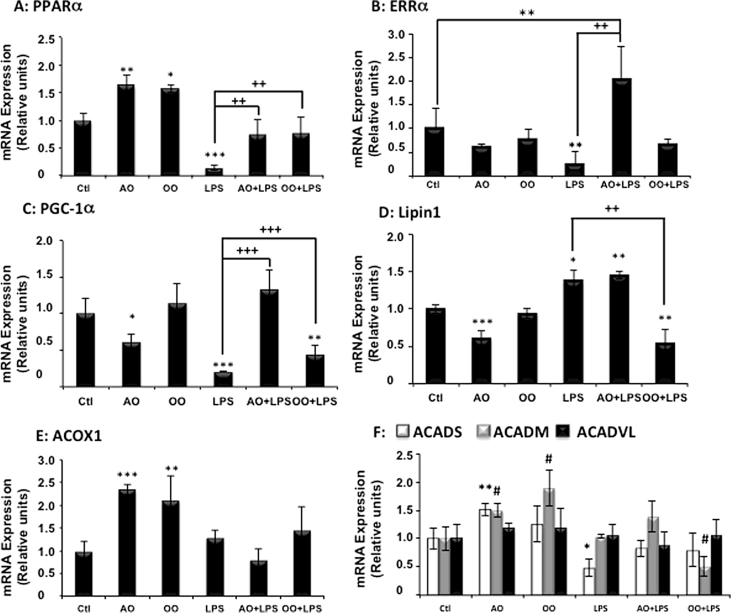
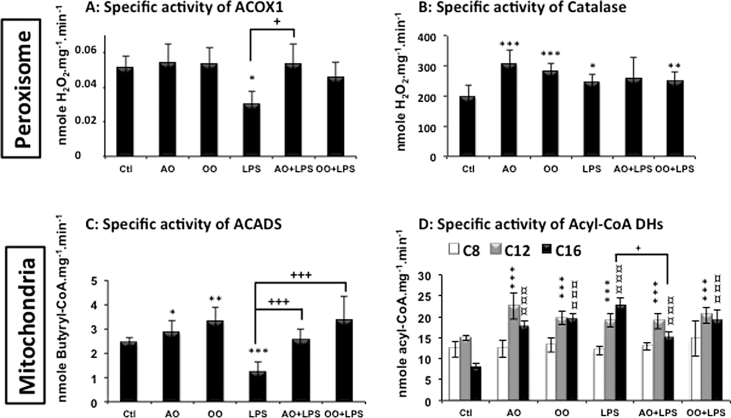
Interestingly, polyunsaturated fatty acids are known to be activators of PPARα, a nuclear receptor which governs lipid metabolism and fatty acid oxidation [46]. Here we report (Fig. 1A) that mice treated with AO or OO showed a significant increase in hepatic PPARα mRNA, while the mRNA expression of ERRα displayed no significant variation (Fig. 1B). Intriguingly, the expression of PGC-1α mRNA was specifically down regulated by AO (Fig. 1C). However, the expression of PPARα target genes (i.e. ACOX1 and ACADM) was clearly induced by both AO and OO, while ACADS mRNA level was only increased by AO (Fig. 1E, F). Regarding the mitochondrial fatty acid beta-oxidation activities, only short (C4:0)-, long (C12:0)- and very long (C16:0)-acyl-CoA mitochondrial dehydrogenases were increased but not the medium (C:8) acyl-CoA dehydrogenase activity (Fig. 2C, D). By contrast to mitochondria, peroxisomal palmitoyl-CoA oxidase was not changed and only peroxisomal catalase activity was induced by AO or OO (Fig. 2A). These results support experimentally that AO and OO up-regulate specifically mitochondrial fatty acid β-oxidation (except for the medium chain) and peroxisomal catalase activities (Fig. 2B).
Fig. 1.
Argan oil preserves hepatic mRNA expressions of nuclear receptors PPARα and ERRα, coactivator PGC-1α and target genes during exposure of mice to LPS. Real-time PCR was used to quantify the hepatic mRNA levels of PPARα (A) and ERRα (B), coactivators PGC-1α (C) and lipin-1 (D) their target genes: Acox1 (E), Acads, Acadm and Acadvl (F). All real-time PCR reactions were performed in duplicate. All values are means ± SEM (n = 5/group) and are normalized to control mice. Symbols (∗, # and +) correspond to a statistical significance of higher mean signal intensity, (p < 0.01 for ∗∗∗ and +++, p < 0.02 for ∗∗ and ++, p < 0.05 for ∗ and #), compared with the control (∗) or with the LPS-treated mice (+). Mice received for 25 days a standard chow (control); a standard chow supplemented with 6% (w/w) of Argan oil (AO) or a standard chow supplemented with 6% (w/w) of olive oil (OO). Sixteen hours before euthanasia, one group from control (+LPS), AO (AO + LPS) and OO (OO + LPS) respectively received intraperitoneal injection of 100 µg LPS.
Fig. 2.
Argan oil protects hepatic mitochondrial and peroxisomal fatty acid oxidation during exposure of mice to LPS. The specific activities of mitochondrial acyl-CoA dehydrogenases (SCAD, MCAD, LCAD and VLCAD) and peroxisomal enzymes (ACOX1 and Catalase) were measured in liver homogenates as described in “Material and Methods” section. All values are means ± SEM (n = 5/group). Symbols (∗, # and +) correspond to a statistical significance of higher mean signal intensity (p < 0.01 for ∗∗∗, ¤¤¤ and ###; p < 0.02 for ∗∗, ## and ++; p < 0.05 for ∗, ¤ and #), compared with the control mice (∗) or with the LPS-treated mice (+). Mice received for 25 days a standard chow (control); a standard chow supplemented with 6% (w/w) of Argan oil (AO) or a standard chow supplemented with 6% (w/w) of olive oil (OO). Sixteen hours before euthanasia, one group from control (+LPS), AO (AO + LPS) and OO (OO + LPS) respectively received intraperitoneal injection of 100 µg LPS.
3.3. LPS induces selective changes in mitochondrial and peroxisomal FAOx gene expression
Consistent with previous studies [47], [48], treatment with LPS strongly decreased the expression of nuclear receptors, PPARα and ERRα, mRNA levels in mouse liver (Fig. 1A, B). LPS also decreased the hepatic expression of mRNA level of PGC-1α. However in these conditions, lipin-1, which may interact with PGC-1α [24], showed enhanced mRNA levels (Fig. 1D). Measurements of the expression of mitochondrial and peroxisomal FAOx gene products at two stages (mRNA levels and enzyme activities) and 16 h after LPS injection showed a selective decrease of ACADS without changes in ACOX1, ACADM and ACADVL mRNA expressions (Fig. 1E, F). In addition, LPS treatment led to a reduction of mitochondrial ACADS enzyme activity (Fig. 2C) and also, as further discussed, of peroxisomal ACOX1 activity (Fig. 2A), while the activity of peroxisomal catalase was enhanced (Fig. 2B).
3.4. Argan oil protects against the drops induced by LPS in hepatic expression of PPARα, ERRα and coactivator PGC-1α
To evaluate the direct therapeutic benefit of Argan oil (AO), mice were pretreated with AO for 24 days before LPS injection taking place 16 h prior to euthanasia. The effects of AO were compared to those of OO, and as illustrated by Fig. 1A, preserved levels of PPARα mRNA were observed in AO + LPS-treated mouse livers, being comparable to protection observed in livers from OO + LPS-treated mice (Fig. 1A). By contrast, only AO + LPS induced substantial significant increases in liver expression of ERRα and PGC-1α mRNA when compared to LPS alone, while OO + LPS induced only a modest significant increase in PGC-1α mRNA level (Fig. 1B and C). At the opposite, pretreatment with AO + LPS showed an equal increase in levels of Lipin-1 mRNA as in LPS group, revealing no effect of AO. However, combined OO pretreatment and LPS injury reduced significantly the expression of Lipin-1 mRNA (Fig. 1D). Evaluation of mRNA levels of PPARα target genes involved in mitochondrial and peroxisomal FAOx (Fig. 1E, F) showed that LPS provoked only a decrease in ACADS mRNA, being without significant effects on mRNA levels of ACADM, ACADVL and ACOX1. Measurements of FAOx enzyme activities revealed that the selective reduction by LPS treatment of ACOX1 and ACADS (C4:0) activities were prevented by AO or OO (AO + LPS or OO + LPS), while for the other dehydrogenase activities, no effects of the oils were observed except for C16:0 with AO + LPS versus LPS (Fig. 2A, C and D). The enhanced activity of peroxisomal catalase, after LPS treatment, was preserved by OO pretreatment (OO + LPS) and to a lesser extent by AO (AO + LPS) (Fig. 2B).
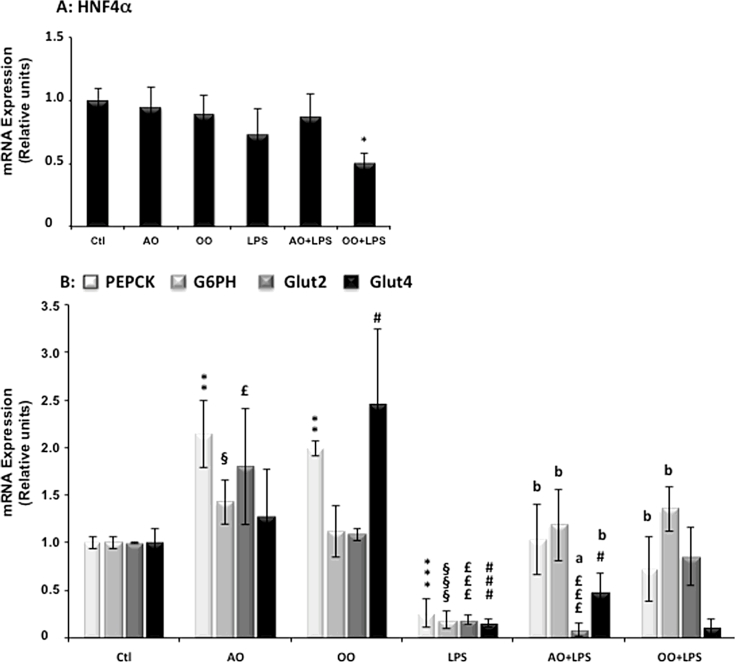
3.5. Argan oil preserves hepatic gluconeogenesis gene expressions during LPS-induced liver dysfunction
HNF4α is a critical nuclear receptor of PGC-1α-mediated gluconeogenesis and controls the expression of gluconeogenic genes (PEPCK, G6PH) [31]. Here we showed that AO and OO treatments had no effect on the mRNA level of HNF4α, and 16 h after LPS administration, there was still no change in the expression of HNF4α (Fig. 3A), though the expression of its known target genes were induced to different extents by AO and OO treatments. Indeed, AO up-regulated the expression of PEPCK, G6PH and Glut2 mRNA, while OO, PEPCK and Glut4 mRNA levels (Fig. 3B). Finally, administration of LPS deeply reduced the mRNA expression of these four target genes (Fig. 3B). Interestingly, AO + LPS-treated mice exhibited preservation of liver gluconeogenic gene expressions, particularly PEPCK, G6PH and Glut4, which were kept quasi-normal. However, the expression of Glut2, the highly expressed glucose transporter in liver, was more deceased in AO + LPS-treated compared to LPS-treated mice (Fig. 3B). OO + LPS-treated mice exhibited also prevention towards LPS-downregulation of liver gluconeogenic genes and no protection for Glut4 was induced by OO (Fig. 3B).
Fig. 3.
Argan oil maintains hepatic gluconeogenesis during exposure of mice to LPS- Real-time PCR was used to quantify the hepatic mRNA levels of HNF-4α (A) and PEPCK, G6PH and Glut4 (B). All real-time PCR reactions were performed in duplicate. All values are means ± SEM (n = 5/group) and are normalized to control mice. Symbols (∗, §, £ and a) correspond to a statistical significance of higher mean signal intensity, (p < 0.01 for ∗∗∗, £££ and §§§; p < 0.02 for ∗∗, §§ and b; p < 0.05 for ∗, £ and §), compared with the control mice (∗, §, £) or with the LPS-treated mice (a and b). Mice received for 25 days a standard chow (control); a standard chow supplemented with 6% (w/w) of Argan oil (AO) or a standard chow supplemented with 6% (w/w) of olive oil (OO). Sixteen hours before euthanasia, one group from control (+LPS), AO (AO + LPS) and OO (OO + LPS) respectively received intraperitoneal injection of 100 µg LPS.
4. Discussion
The present work provides evidence that mice fed with AO have enhanced expressions of several hepatic FAOx and gluconeogenesis transcripts and this AO-mediated upregulation persists during endotoxic LPS shock. This protective effect appears to associate coregulations of hepatic nuclear receptors PPARα, ERRα and HNF-4α and their coactivator PGC-1α [31]. In addition, AO seems to have specific effects on the activities of mitochondrial acyl-CoA dehydrogenases and peroxisomal catalase.
4.1. AO and OO vs control diets
The body weight of mice fed dietary Argan oil or olive oil did not show any significant difference in comparison to the body weight found in mice fed the control diet. Although data on mice fed Argan oil are absents in the literature, 10% diet supplementation by AO or OO in rat during 4 weeks also showed no significant differences [49].
In the absence of LPS, AO increases mRNA expression of PPARα and of its mitochondrial (ACADS, ACADM) and peroxisomal (ACOX1) target genes. Regarding OO treatment, we obtained similar results as with AO for ACADM and ACOX1 mRNA. Even if these oils have different fatty acid compositions, AO and OO induced almost a similar induction of PPARα and its target genes. As mentioned above, OO contains mainly about 70% of oleic acid and only 6–9% of linoleic acid, while AO harbors 35% of linoleic acid and 45% of oleic acid, indicating that AO is richer in polyunsaturated fatty acids [42]. FAOx induction by OO has been shown to be dependent on PPARα, since induction by OO or fish oil of hepatic ACOX1 mRNA is abrogated in Pparα null mice [50]. In this respect, several fatty acids and their polyunsaturated derivatives have been shown to activate responsive element of PPARα target genes and the generation of Pparα null mice established that PPARα coordinates transcriptional activation of the genes coding for proteins catalyzing FAOx pathways [14], [51], [52], [53]. Furthermore, at the energetic level fatty acids are more essential than glucose to the adaptation-phase responses in acute or chronic systemic inflammatory diseases [54]. Thus, it will be of interest to compare in the future the potential preventive effect of supplementing AO to curative properties of its parenteral administration during a septic shock.
On the other hand, though AO or OO increases mRNA expression of mitochondrial acyl-CoA dehydrogenases and peroxisomal ACOX1, only mitochondrial oxidation of C4:0, C12:0 and C16:0 acyl-CoA esters and not peroxisomal palmitoyl-CoA oxidase activity were increased. Acyl-CoA oxidase is long known to bind weakly its flavine adenine dinucleotide (FAD) [50], [55] and this might contribute to intraperoxisomal dissociation of holoenzyme into FAD and apoenzyme (less stable than holoenzyme). Mitochondrial matrix contains a FAD synthetase [56] and, therefore, might better secure protein need in FAD, and hence a better stability of acyl-CoA dehydrogenases by favoring holoenzyme vs apoenzyme forms. In addition, absence of the induction of mitochondrial octanoyl-CoA (C8:0) dehydrogenase activity by different treatments may be related to the process of fatty acid degradations in mitochondria and peroxisomes respectively [53]. Thus, the already known incomplete chain shortening of fatty acyl-CoA in peroxisomes would participate to the octanoyl-CoA export for replenishment of the mitochondrial pool [53].
By contrast to OO, gene expressions of both PGC-1α and Lipin-1 were down regulated by AO. Such effects might explain the observation of Berrougui et al. [57] that AO diminished both LDL and TG according to the key role of Lipin-1 in the assembly and secretion of hepatic very low-density lipoprotein and the increase of TG synthesis as well [58].
4.2. LPS + AO and LPS + OO vs control + LPS diets
In rodents, previous studies have shown that LPS (after a single bolus) induced similar cytokines profiles in either fed or 48 h-fasted rats [59]. Accordingly, given that 24 h-fasting per se significantly enhances hepatic PPARα mRNA expression and activity [60] and due to its potent anorexigenic effect, LPS has been injected during the fed state [61].
For the first time, AO is shown to enhance gene expressions of hepatic FAOx and gluconeogenesis in a way persisting during endotoxic LPS shock. This protective mechanism appears to involve coregulation by PGC-1α of hepatic nuclear receptors PPARα, ERRα and HNF-4α [27]. The present work also reports that AO and OO increase liver expression of gluconeogenic genes. Hepatic gluconeogenesis is regulated by PGC-1α through the coactivation of HNF-4α [31]. Prevention of the LPS-associated downregulation of gluconeogenic genes is better accounted for by PGC-1α than by HNF-4α without ruling out lipin-1 activation. Under Lipin-1 RNAi, PEPCK and G6PH are indeed down regulated [62]. Dysregulation of lipid metabolism by LPS injection is characterized by a dramatic decrease in mitochondrial fatty acid oxidative enzymes [63] and hence fatty acid oxidation (FAOx) in several tissues, including liver [3], [6], [7], [8], [9], [10]. Proposed underlying mechanisms include a reduction in PPARα and its coactivator PGC-1α expressions and hence in mRNA levels of FAOx genes [3], [47], [48]. Additionally, we showed a strong decrease of ERRα mRNA and increase in liver Lipin-1 transcripts after LPS treatment. Interestingly, both ERRα and Lipin-1 play key roles in the expression of FAOx genes [48], [64]. LPS decreases Lipin-1 mRNA in mouse adipose tissue but not skeletal muscle [64] and ERRα in liver, heart, and kidney of mice markedly during the LPS-induced acute-response phase [48]. Intriguingly, AO supplementation in LPS treated mice here has no effect on the level of Lipin-1 mRNA, in contrast to OO supplementation which prevents enhancement of Lipin-1 mRNA by LPS. Such differential effect between AO and OO is similar to what we obtained for PGC-1α transcripts. Knowing that Lipin-1 RNAi has been shown to mediate reduction of PGC-1α mRNA [62], we could suggest that increase in Lipin-1 may participate to the preservation of PGC-1α mRNA by AO. Our results corroborate and extend previous studies demonstrating that suppression by LPS of FAOx addresses, particularly in liver, peroxisomal ACOX1 and ACADS genes. Such LPS suppressive effect is prevented by nutritional supplementation with AO, which also preserves the gene expressions of ERRα, PPARα and their coactivator PGC-1α.
LPS treatment exhibited an opposite effect on peroxisomal beta-oxidation and antioxidant activities, which were decreased for ACOX1 and increased for catalase respectively. This may be related to the fact that ACOX1 is an H2O2-generating enzyme, while catalase is an H2O2-degrading enzyme [52], [53]. The LPS-associated generation of reactive oxygen species is believed to play a key role during the pathogenesis of sepsis [65]. Accordingly, the inhibition of catalase was associated with the progression of LPS/d-galactosamine-induced fulminant liver injury [66]. Thus, the preservation of catalase activity by AO may have a protective effect against the exacerbation of liver injury during LPS-induced endotoxemia.
5. Conclusion
In conclusion, the present work showed that Argan oil protects against the decreased expression of genes involved in hepatic FAOx and gluconeogenesis usually observed during the acute response phase associated with LPS administration. This preventive protection might be related to the recovery of the gene expressions of nuclear receptors PPARα and ERRα as well as of their coactivator PGC-1α. Such recovery may explain the preservations of mitochondrial and peroxisomal enzymatic activities in parallel to an improvement of gluconeogenic gene expressions.
Conflict of interests
The authors have declared no conflict of interest.
Acknowledgments
This work was supported by the Action Intégrée of the Comité Mixte Inter-universitaire Franco-Marocain (CMIFM, AIMA/14/310, CampusFrance) from the PHC Volubilis/Toubkal program (No30293PA), Ministère des Affaires Etrangères, The Centre National Pour la Recherche Scientifique et Technique (CNRST)- Morocco, the Conseil Régional de Bourgogne (PARI 2012:A324; PARI2013:B135; CP S0009) and the Ministère de l'enseignement et de la Recherche (crédits récurrents).
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.biopen.2015.10.002.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Vincent J.L., Sakr Y., Sprung C.L., Ranieri V.M., Reinhart K., Gerlach H., Moreno R., Carlet J., Le Gall J.R., Payen D. Sepsis in European intensive care units: results of the SOAP study. Crit. Care Med. 2006;34:344–353. doi: 10.1097/01.ccm.0000194725.48928.3a. [DOI] [PubMed] [Google Scholar]
- 2.Maeder M., Fehr T., Rickli H., Ammann P. Sepsis-associated myocardial dysfunction: diagnostic and prognostic impact of cardiac troponins and natriuretic peptides. Chest. 2006;129:1349–1366. doi: 10.1378/chest.129.5.1349. [DOI] [PubMed] [Google Scholar]
- 3.Khovidhunkit W., Kim M.S., Memon R.A., Shigenaga J.K., Moser A.H., Feingold K.R., Grunfeld C. Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J. Lipid Res. 2004;45:1169–1196. doi: 10.1194/jlr.R300019-JLR200. [DOI] [PubMed] [Google Scholar]
- 4.Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420:885–891. doi: 10.1038/nature01326. [DOI] [PubMed] [Google Scholar]
- 5.Sriskandan S., Altmann D.M. The immunology of sepsis. J. Pathol. 2008;214:211–223. doi: 10.1002/path.2274. [DOI] [PubMed] [Google Scholar]
- 6.Liu M.S., Spitzer J.J. In vitro effects of E. coli endotoxin on fatty acid and lactate oxidation in canine myocardium. Circ. Shock. 1977;4:181–190. [PubMed] [Google Scholar]
- 7.Wang X., Evans R.D. Effect of endotoxin and platelet-activating factor on lipid oxidation in the rat heart. J. Mol. Cell. Cardiol. 1997;29:1915–1926. doi: 10.1006/jmcc.1997.0430. [DOI] [PubMed] [Google Scholar]
- 8.Johnson A.C., Stahl A., Zager R.A. Triglyceride accumulation in injured renal tubular cells: alterations in both synthetic and catabolic pathways. Kidney Int. 2005;67:2196–2209. doi: 10.1111/j.1523-1755.2005.00325.x. [DOI] [PubMed] [Google Scholar]
- 9.Zager R.A., Johnson A.C., Hanson S.Y. Renal tubular triglyercide accumulation following endotoxic, toxic, and ischemic injury. Kidney Int. 2005;67:111–121. doi: 10.1111/j.1523-1755.2005.00061.x. [DOI] [PubMed] [Google Scholar]
- 10.Feingold K.R., Staprans I., Memon R.A., Moser A.H., Shigenaga J.K., Doerrler W., Dinarello C.A., Grunfeld C. Endotoxin rapidly induces changes in lipid metabolism that produce hypertriglyceridemia: low doses stimulate hepatic triglyceride production while high doses inhibit clearance. J. Lipid Res. 1992;33:1765–1776. [PubMed] [Google Scholar]
- 11.Handschin C., Spiegelman B.M. Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr. Rev. 2006;27:728–735. doi: 10.1210/er.2006-0037. [DOI] [PubMed] [Google Scholar]
- 12.Rakhshandehroo M., Knoch B., Muller M., Kersten S. Peroxisome proliferator-activated receptor alpha target genes. PPAR Res. 2010;2010:20–39. doi: 10.1155/2010/612089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vluggens A., Andreoletti P., Viswakarma N., Jia Y., Matsumoto K., Kulik W., Khan M., Huang J., Guo D., Yu S., Sarkar J., Singh I., Rao M.S., Wanders R.J., Reddy J.K., Cherkaoui-Malki M. Reversal of mouse Acyl-CoA oxidase 1 (ACOX1) null phenotype by human ACOX1b isoform [corrected] Lab. Invest. 2010;90:696–708. doi: 10.1038/labinvest.2010.46. [DOI] [PubMed] [Google Scholar]
- 14.Vluggens A., Reddy J.K. Nuclear receptors and transcription factors in the development of fatty liver disease. Curr. Drug Metab. 2012;13:1422–1435. doi: 10.2174/138920012803762710. [DOI] [PubMed] [Google Scholar]
- 15.Brandt J.M., Djouadi F., Kelly D.P. Fatty acids activate transcription of the muscle carnitine palmitoyltransferase I gene in cardiac myocytes via the peroxisome proliferator-activated receptor alpha. J. Biol. Chem. 1998;273:23786–23792. doi: 10.1074/jbc.273.37.23786. [DOI] [PubMed] [Google Scholar]
- 16.Kersten S., Seydoux J., Peters J.M., Gonzalez F.J., Desvergne B., Wahli W. Peroxisome proliferator-activated receptor alpha mediates the adaptive response to fasting. J. Clin. Invest. 1999;103:1489–1498. doi: 10.1172/JCI6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leone T.C., Weinheimer C.J., Kelly D.P. A critical role for the peroxisome proliferator-activated receptor alpha (PPARalpha) in the cellular fasting response: the PPARalpha-null mouse as a model of fatty acid oxidation disorders. Proc. Natl. Acad. Sci. U. S. A. 1999;96:7473–7478. doi: 10.1073/pnas.96.13.7473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mascaro C., Acosta E., Ortiz J.A., Marrero P.F., Hegardt F.G., Haro D. Control of human muscle-type carnitine palmitoyltransferase I gene transcription by peroxisome proliferator-activated receptor. J. Biol. Chem. 1998;273:8560–8563. doi: 10.1074/jbc.273.15.8560. [DOI] [PubMed] [Google Scholar]
- 19.Rakhshandehroo M., Hooiveld G., Muller M., Kersten S. Comparative analysis of gene regulation by the transcription factor PPARalpha between mouse and human. PLoS One. 2009;4:e6796. doi: 10.1371/journal.pone.0006796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aoyama T., Peters J.M., Iritani N., Nakajima T., Furihata K., Hashimoto T., Gonzalez F.J. Altered constitutive expression of fatty acid-metabolizing enzymes in mice lacking the peroxisome proliferator-activated receptor alpha (PPARalpha) J. Biol. Chem. 1998;273:5678–5684. doi: 10.1074/jbc.273.10.5678. [DOI] [PubMed] [Google Scholar]
- 21.Varanasi U., Chu R., Huang Q., Castellon R., Yeldandi A.V., Reddy J.K. Identification of a peroxisome proliferator-responsive element upstream of the human peroxisomal fatty acyl coenzyme A oxidase gene. J. Biol. Chem. 1996;271:2147–2155. doi: 10.1074/jbc.271.4.2147. [DOI] [PubMed] [Google Scholar]
- 22.Cherkaoui-Malki M., Meyer K., Cao W.Q., Latruffe N., Yeldandi A.V., Rao M.S., Bradfield C.A., Reddy J.K. Identification of novel peroxisome proliferator-activated receptor alpha (PPARalpha) target genes in mouse liver using cDNA microarray analysis. Gene Expr. 2001;9:291–304. doi: 10.3727/000000001783992533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sugden M.C., Caton P.W., Holness M.J. PPAR control: it's SIRTainly as easy as PGC. J. Endocrinol. 2010;204:93–104. doi: 10.1677/JOE-09-0359. [DOI] [PubMed] [Google Scholar]
- 24.Finck B.N., Gropler M.C., Chen Z., Leone T.C., Croce M.A., Harris T.E., Lawrence J.C., Jr., Kelly D.P. Lipin 1 is an inducible amplifier of the hepatic PGC-1alpha/PPARalpha regulatory pathway. Cell Metab. 2006;4:199–210. doi: 10.1016/j.cmet.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 25.Villena J.A., Kralli A. ERRalpha: a metabolic function for the oldest orphan, trends endocrinol. Metab. 2008;19:269–276. doi: 10.1016/j.tem.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giguere V. Transcriptional control of energy homeostasis by the estrogen-related receptors. Endocr. Rev. 2008;29:677–696. doi: 10.1210/er.2008-0017. [DOI] [PubMed] [Google Scholar]
- 27.Schreiber S.N., Knutti D., Brogli K., Uhlmann T., Kralli A. The transcriptional coactivator PGC-1 regulates the expression and activity of the orphan nuclear receptor estrogen-related receptor alpha (ERRalpha) J. Biol. Chem. 2003;278:9013–9018. doi: 10.1074/jbc.M212923200. [DOI] [PubMed] [Google Scholar]
- 28.Mootha V.K., Handschin C., Arlow D., Xie X., St Pierre J., Sihag S., Yang W., Altshuler D., Puigserver P., Patterson N., Willy P.J., Schulman I.G., Heyman R.A., Lander E.S., Spiegelman B.M. Erralpha and Gabpa/b specify PGC-1alpha-dependent oxidative phosphorylation gene expression that is altered in diabetic muscle. Proc. Natl. Acad. Sci. U. S. A. 2004;101:6570–6575. doi: 10.1073/pnas.0401401101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huss J.M., Kopp R.P., Kelly D.P. Peroxisome proliferator-activated receptor coactivator-1alpha (PGC-1alpha) coactivates the cardiac-enriched nuclear receptors estrogen-related receptor-alpha and -gamma. Identification of novel leucine-rich interaction motif within PGC-1alpha. J. Biol. Chem. 2002;277:40265–40274. doi: 10.1074/jbc.M206324200. [DOI] [PubMed] [Google Scholar]
- 30.Gaillard S., Dwyer M.A., McDonnell D.P. Definition of the molecular basis for estrogen receptor-related receptor-alpha-cofactor interactions. Mol. Endocrinol. 2007;21:62–76. doi: 10.1210/me.2006-0179. [DOI] [PubMed] [Google Scholar]
- 31.Rhee J., Inoue Y., Yoon J.C., Puigserver P., Fan M., Gonzalez F.J., Spiegelman B.M. Regulation of hepatic fasting response by PPARgamma coactivator-1alpha (PGC-1): requirement for hepatocyte nuclear factor 4alpha in gluconeogenesis. Proc. Natl. Acad. Sci. U. S. A. 2003;100:4012–4017. doi: 10.1073/pnas.0730870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin W.J., Yeh W.C. Implication of toll-like receptor and tumor necrosis factor alpha signaling in septic shock. Shock. 2005;24:206–209. doi: 10.1097/01.shk.0000180074.69143.77. [DOI] [PubMed] [Google Scholar]
- 33.Calder P.C. Long-chain n-3 fatty acids and inflammation: potential application in surgical and trauma patients. Braz. J. Med. Biol. Res. 2003;36:433–446. doi: 10.1590/s0100-879x2003000400004. [DOI] [PubMed] [Google Scholar]
- 34.Morlion B.J., Torwesten E., Lessire H., Sturm G., Peskar B.M., Furst P., Puchstein C. The effect of parenteral fish oil on leukocyte membrane fatty acid composition and leukotriene-synthesizing capacity in patients with postoperative trauma. Metab. Clin. Exp. 1996;45:1208–1213. doi: 10.1016/s0026-0495(96)90237-1. [DOI] [PubMed] [Google Scholar]
- 35.Roulet M., Frascarolo P., Pilet M., Chapuis G. Effects of intravenously infused fish oil on platelet fatty acid phospholipid composition and on platelet function in postoperative trauma. JPEN J. Parenter. Enter. Nutr. 1997;21:296–301. doi: 10.1177/0148607197021005296. [DOI] [PubMed] [Google Scholar]
- 36.Sadeghi S., Wallace F.A., Calder P.C. Dietary lipids modify the cytokine response to bacterial lipopolysaccharide in mice. Immunology. 1999;96:404–410. doi: 10.1046/j.1365-2567.1999.00701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chafchaouni-Moussaoui I., Charrouf Z., Guillaume D. Triterpenoids from Argania spinosa: 20 years of research. Nat. Prod. Commun. 2013;8:43–46. [PubMed] [Google Scholar]
- 38.Berrougui H., Cloutier M., Isabelle M., Khalil A. Phenolic-extract from Argan oil (Argania spinosa L.) inhibits human low-density lipoprotein (LDL) oxidation and enhances cholesterol efflux from human THP-1 macrophages. Atherosclerosis. 2006;184:389–396. doi: 10.1016/j.atherosclerosis.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 39.Cherki M., Derouiche A., Drissi A., El Messal M., Bamou Y., Idrissi-Ouadghiri A., Khalil A., Adlouni A. Consumption of Argan oil may have an antiatherogenic effect by improving paraoxonase activities and antioxidant status: intervention study in healthy men. Nutr. Metab. Cardiovasc. Dis. 2005;15:352–360. doi: 10.1016/j.numecd.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 40.Derouiche A., Cherki M., Drissi A., Bamou Y., El Messal M., Idrissi-Oudghiri A., Lecerf J.M., Adlouni A. Nutritional intervention study with Argan oil in man: effects on lipids and apolipoproteins. Ann. Nutr. Metab. 2005;49:196–201. doi: 10.1159/000087072. [DOI] [PubMed] [Google Scholar]
- 41.Drissi A., Girona J., Cherki M., Godas G., Derouiche A., El Messal M., Saile R., Kettani A., Sola R., Masana L., Adlouni A. Evidence of hypolipemiant and antioxidant properties of Argan oil derived from the Argan tree (Argania spinosa) Clin. Nutr. 2004;23:1159–1166. doi: 10.1016/j.clnu.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 42.El Kebbaj R., El Kamouni S., El Hajj H.I., Andreoletti P., Gresti J., Latruffe N., El Kebbaj M.S., Vamecq J., Lizard G., Nasser B., Cherkaoui-Malki M. Modulation of peroxisomes abundance by Argan oil and lipopolysaccharides in acyl-CoA oxidase 1-deficient fibroblasts. Health. 2013;5:62–69. [Google Scholar]
- 43.Oaxaca-Castillo D., Andreoletti P., Vluggens A., Yu S., van Veldhoven P.P., Reddy J.K., Cherkaoui-Malki M. Biochemical characterization of two functional human liver acyl-CoA oxidase isoforms 1a and 1b encoded by a single gene. Biochem. Biophys. Res. Commun. 2007;360:314–319. doi: 10.1016/j.bbrc.2007.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ni J., Sasaki Y., Tokuyama S., Sogabe A., Tahara Y. Conversion of a typical catalase from Bacillus sp. TE124 to a catalase-peroxidase by directed evolution. J. Biosci. Bioeng. 2002;93:31–36. doi: 10.1016/s1389-1723(02)80050-0. [DOI] [PubMed] [Google Scholar]
- 45.Hryb D.J., Hogg J.F. Chain length specificities of peroxisomal and mitochondrial beta-oxidation in rat liver. Biochem. Biophys. Res. Commun. 1979;87:1200–1206. doi: 10.1016/s0006-291x(79)80034-0. [DOI] [PubMed] [Google Scholar]
- 46.Desvergne B., Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr. Rev. 1999;20:649–688. doi: 10.1210/edrv.20.5.0380. [DOI] [PubMed] [Google Scholar]
- 47.Beigneux A.P., Moser A.H., Shigenaga J.K., Grunfeld C., Feingold K.R. The acute phase response is associated with retinoid X receptor repression in rodent liver. J. Biol. Chem. 2000;275:16390–16399. doi: 10.1074/jbc.M000953200. [DOI] [PubMed] [Google Scholar]
- 48.Kim M.S., Shigenaga J.K., Moser A.H., Feingold K.R., Grunfeld C. Suppression of estrogen-related receptor alpha and medium-chain acyl-coenzyme A dehydrogenase in the acute-phase response. J. Lipid Res. 2005;46:2282–2288. doi: 10.1194/jlr.M500217-JLR200. [DOI] [PubMed] [Google Scholar]
- 49.Benzaria A., Meskini N., Dubois M., Croset M., Nemoz G., Lagarde M., Prigent A.F. Effect of dietary Argan oil on fatty acid composition, proliferation, and phospholipase D activity of rat thymocytes. Nutrition. 2006;22:628–637. doi: 10.1016/j.nut.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 50.Ren B., Thelen A.P., Peters J.M., Gonzalez F.J., Jump D.B. Polyunsaturated fatty acid suppression of hepatic fatty acid synthase and S14 gene expression does not require peroxisome proliferator-activated receptor alpha. J. Biol. Chem. 1997;272:26827–26832. doi: 10.1074/jbc.272.43.26827. [DOI] [PubMed] [Google Scholar]
- 51.Forman B.M., Chen J., Evans R.M. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta. Proc. Natl. Acad. Sci. U. S. A. 1997;94:4312–4317. doi: 10.1073/pnas.94.9.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vamecq J., Cherkaoui-Malki M., Andreoletti P., Latruffe N. The human peroxisome in health and disease: the story of an oddity becoming a vital organelle. Biochimie. 2014;98:4–15. doi: 10.1016/j.biochi.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 53.Cherkaoui-Malki M., Surapureddi S., El-Hajj H.I., Vamecq J., Andreoletti P. Hepatic steatosis and peroxisomal fatty acid beta-oxidation. Curr. Drug Metab. 2012;13:1412–1421. doi: 10.2174/138920012803762765. [DOI] [PubMed] [Google Scholar]
- 54.Liu T.F., Brown C.M., El Gazzar M., McPhail L., Millet P., Rao A., Vachharajani V.T., Yoza B.K., McCall C.E. Fueling the flame: bioenergy couples metabolism and inflammation. J. Leukoc. Biol. 2012;92:499–507. doi: 10.1189/jlb.0212078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Inestrosa N.C., Bronfman M., Leighton F. Properties of fatty acyl-CoA oxidase from rat liver, a peroxisomal flavoprotein. Life Sci. 1979;25:1127–1135. doi: 10.1016/0024-3205(79)90134-6. [DOI] [PubMed] [Google Scholar]
- 56.Barile M., Brizio C., Valenti D., De Virgilio C., Passarella S. The riboflavin/FAD cycle in rat liver mitochondria. Eur. J. Biochem. 2000;267:4888–4900. doi: 10.1046/j.1432-1327.2000.01552.x. [DOI] [PubMed] [Google Scholar]
- 57.Berrougui H., Ettaib A., Herrera Gonzalez M.D., Alvarez de Sotomayor M., Bennani-Kabchi N., Hmamouchi M. Hypolipidemic and hypocholesterolemic effect of Argan oil (Argania spinosa L.) in Meriones shawi rats. J. Ethnopharmacol. 2003;89:15–18. doi: 10.1016/s0378-8741(03)00176-4. [DOI] [PubMed] [Google Scholar]
- 58.Bou Khalil M., Sundaram M., Zhang H.Y., Links P.H., Raven J.F., Manmontri B., Sariahmetoglu M., Tran K., Reue K., Brindley D.N., Yao Z. The level and compartmentalization of phosphatidate phosphatase-1 (lipin-1) control the assembly and secretion of hepatic VLDL. J. Lipid Res. 2009;50:47–58. doi: 10.1194/jlr.M800204-JLR200. [DOI] [PubMed] [Google Scholar]
- 59.Inoue W., Somay G., Poole S., Luheshi G.N. Immune-to-brain signaling and central prostaglandin E2 synthesis in fasted rats with altered lipopolysaccharide-induced fever. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;295:R133–R143. doi: 10.1152/ajpregu.90335.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Patsouris D., Mandard S., Voshol P.J., Escher P., Tan N.S., Havekes L.M., Koenig W., Marz W., Tafuri S., Wahli W., Muller M., Kersten S. PPARalpha governs glycerol metabolism. J. Clin. Invest. 2004;114:94–103. doi: 10.1172/JCI20468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Volkoff H., Peter R.E. Effects of lipopolysaccharide treatment on feeding of goldfish: role of appetite-regulating peptides. Brain Res. 2004;998:139–147. doi: 10.1016/j.brainres.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 62.Ryu D., Oh K.J., Jo H.Y., Hedrick S., Kim Y.N., Hwang Y.J., Park T.S., Han J.S., Choi C.S., Montminy M., Koo S.H. TORC2 regulates hepatic insulin signaling via a mammalian phosphatidic acid phosphatase, LIPIN1. Cell Metab. 2009;9:240–251. doi: 10.1016/j.cmet.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 63.Feingold K.R., Wang Y., Moser A., Shigenaga J.K., Grunfeld C. LPS decreases fatty acid oxidation and nuclear hormone receptors in the kidney. J. Lipid Res. 2008;49:2179–2187. doi: 10.1194/jlr.M800233-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lu B., Lu Y., Moser A.H., Shigenaga J.K., Grunfeld C., Feingold K.R. LPS and proinflammatory cytokines decrease lipin-1 in mouse adipose tissue and 3T3-L1 adipocytes. Am. J. Physiol. Endocrinol. Metab. 2008;295:E1502–E1509. doi: 10.1152/ajpendo.90323.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Singh N., Li L. Reduced oxidative tissue damage during endotoxemia in IRAK-1 deficient mice. Mol. Immunol. 2012;50:244–252. doi: 10.1016/j.molimm.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jia M., Jing Y., Ai Q., Jiang R., Wan J., Lin L., Zhou D., Che Q., Li L., Tang L., Shen Y., Zhang L. Potential role of catalase in mice with lipopolysaccharide/D-galactosamine-induced fulminant liver injury. Hepatol. Res. 2014;44:1151–1158. doi: 10.1111/hepr.12220. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.